1. Introduction
Beef from the Maronesa cattle has high commercial value due to its autochthonous breed, environmentally sustainable production system and it has Protected Designation of Origin (PDO) [1,2]. However, in order to exploit the full potential of meat products it is important to ensure that products reach the consumer in the best condition [3]. The quality of the meat can be affected by intrinsic and extrinsic parameters, such as age, sex, breed [4], feeding [5], enzyme activity [6], meat modified atmospheres packaging (MAP) [7], or meat contamination [8]. The hygienic quality, which is a result of beef processing and handling, is particularly important because it has direct implications on the consumer’s confidence [9]. Contamination of beef occurs throughout slaughtering, deboning, cutting, packaging, and storage, during which, time, temperature and hygiene are important parameters affecting the shelf-life of fresh meat for industrial use or retail [10,11].
Microbial pathogens are usually associated with the most serious meat safety issues in product recalls and foodborne disease [12,13,14]. Nowadays, besides the considerable knowledge about the microbiota responsible for foodborne diseases, a high number of outbreaks and incidents keep occurring, many of them associated with meat and meat products’ which in 2021 represented 11.9% of positive units reported by MS of EU [15]. Beef, in particular, has been associated with foodborne diseases, causing tragic outcomes, involving death in some cases. An aiding fact of foodborne infections due to beef consumption may be related to its preparation methods, in which these meats are many times only subjected to mild heat treatments, commonly referred to as undercooked or rare [16]. Enterohemorrhagic E. coli O157:H7 is a highly pathogenic subset of Shiga toxin-producing E. coli [17]. E. coli belongs to the enteric microflora of many healthy animals being cattle the main reservoirs of E. coli O157:H7 [18,19]. E. coli O157:H7 has emerged as an important foodborne pathogen that causes diarrhea, hemorrhagic colitis, and hemolytic-uremic syndrome in humans [17] and, in severe cases, can cause death [20]. On the other hand, L. monocytogenes has an ubiquitous distribution and a great ability to grow in a wide range of conditions such as refrigeration [21,22] and has been regarded as the pathogen of concern in ready-to-eat (RTE) meat [23]. L. monocytogenes is also one of the most serious agent of foodborne diseases under EU surveillance and, infection in humans still high, some of them with death [15]. Unsafe practices including high storage durations and abusive temperatures have a potential impact on the human listeriosis risk [24]. The temperature of domestic refrigerators was shown to be very variable through a review of survey studies from 1991 to 2016. It was observed that mean temperatures ranged from <5 to 8.1°C, the minimum temperatures from -7.9 to 3.8°C and maximum from 11.4 to 20.7°C [24].
The gaseous composition of the packaging and pHu values are parameters that can affect the growth of microorganisms in meat [25,26]. In red meat products, one of the most common methods of packaging is the use of MAP, usually through mixtures of CO2, O2 and N2, each one with a specific function. The MAP generally inhibits microbial spoilage of fresh meat, minimizing the loss of products and maintaining a higher quality in perishable food during its normal shelf-life or extending it [27]. Nevertheless, MAP should be associated with strict temperature control to achieve maximum microbial inhibition [11,28]. The pHu of meat higher than the normal values (pHu: 5.5-5.8), as in dark, firm and dry (DFD) (pHu: >6.1) or moderate DFD (pHu: 5.9-6.1) meat, can be responsible for the meat spoilage [29,30]. The production system of Maronesa beef is known for the occurrence of DFD condition [32], consequently reducing the product’s shelf-life therefore less acceptable to the consumer [32]. According to Silva el al. [32], during two years of measurements of pHu, it was noticed that in longissimus dorsi about 25% of the cases had a final pH equal or superior to 6.2 and were classified as DFD. Thus, the results revealed that the occurrence of high pH meat is dependent on the muscle. Other muscles as gracillis (8%) and psoas major (≤2%) muscles presented less percentage of DFD condition. DFD meat is related with animal stress and transport with multifactorial origin and has been reported in many countries, with variable occurrence rates of 1.3% in Canada, 3.2% in USA, 4.5% in Brazil or 13.45% in Mexico [29,33,34,35]. The incidence of DFD cases was also dependent on the sex of the animal, with the higher occurrence of DFD cases observed in males [32].
Considering the lack of studies related to the quality of autochthonous breeds meat associated with the occurrence of DFD beef in Maronesa breed, the aim of this study was to evaluate the influence of pHu (Normal and High) and meat packaging (air, vacuum and three MAP with gas) on the behaviour of E. coli O157:H7 and L. monocytogenes inoculated on beef of Maronesa breed and stored at 4±0.5ºC and 9±0.5ºC, during 28 days of storage.
2. Materials and Methods
2.1. Experimental Design
2.1.1. Sampling
Beef longissimus dorsi muscle was obtained from eight Portuguese autochthonous Maronesa, 9 to 11 month old bulls whose carcass weighed from 90 to 150 kg. Longissimus dorsi was excised from the carcasses between the 6th thoracic and the 2th lumbar vertebra at 24h post mortem (pm). Based on pHu measured at 24h pm directly in the muscle using a combined glass electrode with a pH-meter Crison 2002, muscles were divided into two pH groups: Normal (pH≤5.8, n=4) and DFD (pH≥6.2, n=4). After that, muscles were cut into pieces of approximately 200g, packed in vacuum and stored at -80 °C until the beginning of the experiment. In the day 1 of the experiment, cuts of muscles were kept at 2ºC during 2h. After this time, approximately 1 cm of external surface of meat was aseptically removed and cuts were sliced. At the end, pieces of meat (0.5 cm thick, surface 2x2.5cm, ≈5g) were obtained. Immediately after this preparation, meat samples were analysed (24h pm), for meat characterisation and discarding L. monocytogenes and E. coli O157:H7 contamination, prior to inoculation. Experiments were performed using four animals in each experimental unit per each pH group and a control sample was prepared in all conditions of the experimental design.
2.1.2. Microorganisms and Growth Conditions
Pieces of meat were inoculated with 20 µl of a suspension of E. coli O157:H7 (NCTC 12900) and L. monocytogenes (ATCC 7973), for an inoculation level of 2-3 log (CFU/g) per strain and package.
Inoculates used in this study were prepared at growth suspension of L. monocytogenes (30ºC, 24h) and E. coli O157:H7 (37ºC, 24h) in brain heart infusion broth (Oxoid CM225). Cells were centrifugated (5000xg, 15 min, 4ºC) and washed three times in 0.85% sterile physiologic saline and compared to a 0.5 McFarland turbidity standard. Serial (10-fold) dilutions were performed to yield, approximately 1x103 cells/cm2. To verify the number of viable L. monocytogenes and E. coli O157:H7 in the suspension, dilutions were spread on Compass L. monocytogenes Agar (Biokar BM06508) and CT-SMAC (Biokar BK147+BS037), respectively.
2.1.3. Packaging
Inoculated and control samples were packed in five different types of packaging namely: air; vacuum; 70%O2/20%CO2/10%N2 (MAP70/20); 50%O2/40%CO2/10%N2 (MAP50/40); and 30%O2/60%CO2/10%N2 (MAP30/60).
In air packaging, meat pieces were tray-packaged in air overwrapped with polyetilen film and in vacuum they were individually vacuum packaged in COMBITHERM bags (WIPAK Walsrode, HAFRI) which have an oxygen transmission rate (OTR) of 63 cm3 m-2d-1atm-1 at 23ºC, 0% RH and water vapor transmission (WVT) of 1g m-2d-1 at 23ºC, 85% RH. For MAP, pieces of meat were individually placed in COMBITHERM XX bags (WIPAK Walsrode, HAFRI) 0.115 mm thick and OTR of 1 cm3 m-2d-1atm-1 at 23ºC, 0% RH and WVT of 1g m-2d-1 at 23ºC, 85% RH. The atmosphere in the MAP was first removed and then flushed with the appropriate gas mixture (Praxair, Portugal) using a SAMMIC V-420 SGA. The final ratio between gas and meat was approximately of 3:1.
Samples were stored at 4±0.5ºC and 9±0.5ºC and examined for microbiological counts on days 1 (2h after packaging), 3, 7, 10, 14 pm. For air packaging, microbiological counts were not carried out at 14 days pm. On the contrary, on vacuum packaging microbiological counts were also carried out at 21 and 28 days pm.
2.2. Microbial Analysis
Meat pieces were aseptically collected at each interval. Samples were homogenized with sterile buffered peptone water for 90s in a Stomacher (IUL, Barcelona, Spain). Serial decimal dilutions were prepared in the same solution and were plated on CT-SMAC (Biokar BK147+BS037) for E. coli O157:H7 counts (37ºC for 24h) and Compass L. mono agar (Biokar BM06508) in case of L. monocytogenes (37ºC for 24-48h). After incubation, typical colonies were counted and results were expressed in log CFU/g.
2.3. Data Analysis
One-way analysis of variance (ANOVA) was conducted to test the effect of pHu (Normal and DFD) and of temperature of storage (4±0.5ºC and 9±0.5ºC), for each day of microbiological counts (1, 3, 7, 10, 14, 21, 28 days pm) using the Systat programme 10.2 (Systat Software Inc., 2002) at 5% level of probability.
3. Results
3.1. Behaviour of Escherichia coli O157:H7
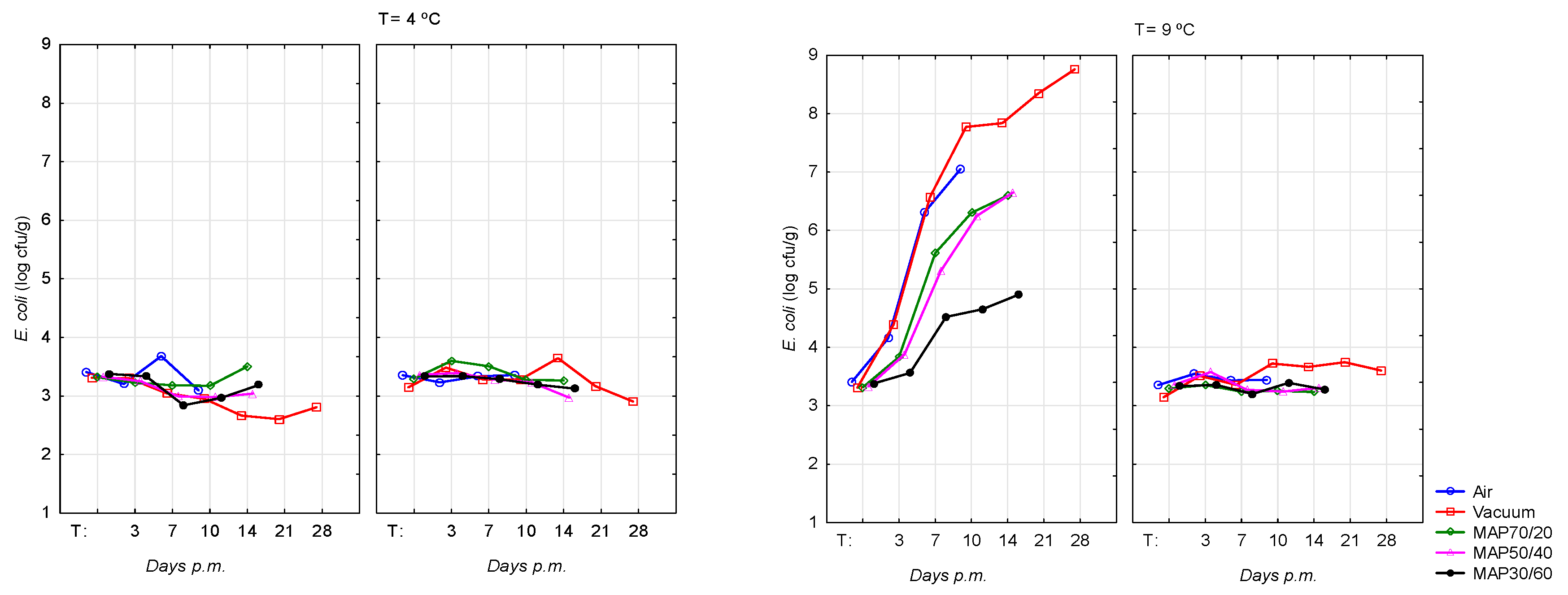
The results of counts of
E. coli O157:H7 in inoculated beef samples, according to pHu (Normal and DFD) and type of packaging for each storage temperature are presented in
Table 1. Two hours after inoculation the Normal and DFD samples had a similar count for
E. coli O157:H7. These counts were slightly greater than the programmed level of inoculation, though this was a very slight excess, never attained a logarithmic unit. The final pH of the meat was not decisive on the growth of
E. coli O157:H7 in beef stored at 4±0.5°C. The highest
E. coli O157:H7 counts for DFD meat and temperature of 9°C were observed in vacuum packaging for all storage times (4.39±0.65 log CFU/g at 3 days
pm; 6.57±0.26 log CFU/g at 7 days
pm; 7.77±0.32 log CFU/g at 10 days
pm and 8.35±0.21 log CFU/g at 21 days
pm), however no significant differences were observed between Normal and DFD pHu at 3 days
pm. At 10 days
pm the largest difference between the DFD and Normal meat were observed on vacuum atmosphere (4.05 log CFU/g;
P <0.001) followed by air atmosphere (3.61 log CFU/g;
P <0.01). In the MAP70/20, differences between the DFD and Normal meat reached 3.04 log CFU/g (
P <0.001). The MAP packaging with more concentration of CO
2, namely MAP30/60 showed the lowest counts with no significant differences between pH groups at 10 and 14 days
pm.
The counts of
E. coli O157:H7 on Normal and DFD beef were similar at temperature of 4 ºC during all the storage period, maintaining the initial levels. However, at 9 ºC were achieved growth levels for DFD meat of ~8 log CFU/g in vacuum from the day 10
pm, reaching at day 28
pm the highest value of 8.76±0.64 log CFU/g. In the MAP with the high CO
2 concentration (MAP30/60) counts were not higher than 5 log CFU/g, which appears to reveal the inhibitory effect of CO
2 in the development of
E. coli O157:H7 (
Figure 1). The pathogen
E. coli O157:H7 packed in MAP70/40 and MAP30/60 and stored at mild abusive temperature (9ºC) followed the same pattern and showed similar counts over time.
3.2. Behaviour of Listeria monocytogenes
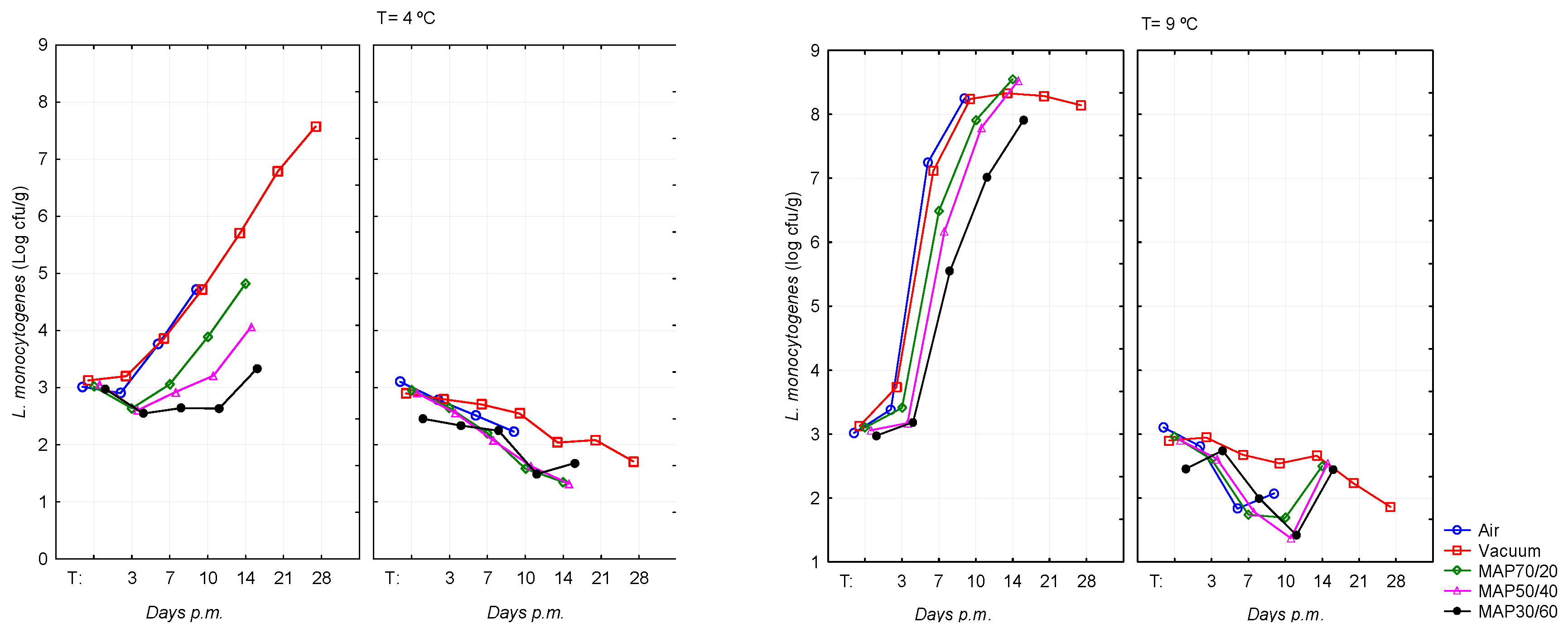
The results of counts of
L. monocytogenes in inoculated beef samples, according to pHu (Normal and DFD) and type of packaging for each storage temperature are presented in
Table 2. At 1 day
pm, in the five packaging, DFD and Normal samples had a similar count for
L. monocytogenes with a score closer to that desired in all packaging. Two more days after storage, the consequences of temperature started to show that
L. monocytogenes increased considerably in DFD compared to Normal meat and, these differences, were more pronounced particularly in MAP70/20 compared to the other two MAP.
L. monocytogenes achieved at 3 days
pm and 7 days
pm average counts of 3.41±0.92 log CFU/g and 6.49±0.83 log CFU/g in DFD meat and 2.61±0.65 log CFU/g and 1.74±0.76 log CFU/g in Normal meat which generally maintained the same pattern during the remaining storage time.
In Normal pH, the effect of abusive storage temperature was not noticed in the development of L. monocytogenes, contrarily to DFD meat which presented very significant differences in most situations in which the meat was in abusive temperature.
The MAP30/60 with the higher content of CO2 seems to inhibit a little more this microorganism, showing inferior counts for all storage times when compared with MAP70/20 and MAP50/40.
At temperature of storage of 4ºC, the growth of L. monocytogenes was not observed for Normal meat. On DFD meat, the counts achieved levels of 6 log CFU/g, 7 log CFU/g and 8 log CFU/g in vacuum at day 14 pm, 21 pm and 28 pm, respectively. The lowest counts were obtained on meat packaged in the MAP30/60 along time.
At abusive temperature (9ºC) and Normal pHu L. monocytogenes presented similar counts during the storage period. On contrary, for DFD meat L. monocytogenes developed very fast after day 3 pm achieving values higher than 8 log CFU/g in air and vacuum at day 10 pm. These levels were attained in MAPs later at day 14 pm.
4. Discussion
The pHu and storage temperature influenced significantly the growth of both E. coli O157:H7 and L. monocytogenes.
At 4ºC of storage, the counts of
L. monocytogenes for all packages in DFD beef were below 5 log CFU/g (10 day
pm) with the higher counts observed in air (4.47 log CFU/g;
P <0.05) and vacuum (4.55 log CFU/g;
P <0.05) packages (
Table 2.). On contrary, at 4ºC the counts of
E. coli H157:H7 were about 3 log CFU/g during all times of storage (3, 7, 10, 14, 21 and 28 days
pm) in all types of packaging showing no significant differences (
Table 1). Thus, the low storage temperature (4ºC) associated to the DFD condition was not enough to produce an extensive inhibition of
L. monocytogenes as observed in
E. coli O157:H7. These results can be justified by the fact that
L. monocytogenes being a psychrotrophic bacterium, capable of survive and multiply at low temperatures, both under aerobic and anaerobic conditions, and adhere to various surfaces [36]. Moreover,
L. monocytogenes has the ability of grow at pH of 6 or higher, as observed in DFD meat [37] and has a high tolerance to low pH and high salt concentration [38]. According to Nissen et al. [39] at 4ºC,
L. monocytogenes and
Y.
enterocolitica are considered the most serious pathogens in meat. Low temperatures induce enzymes such as RNA helicase which improves the activity of
L. monocytogenes, as well as replication at low temperatures. Moreover, the capacity to produce biofilms enhances
L. monocytogenes ability to survive harsh environments and also use flagella at lower temperatures which enables the ability to propel itself [42]. Elevated CO
2 and reduced O
2 levels are commonly used to extend shelf-life of food products through inhibition of microbial growth and oxidative changes [42]. Use of O
2-free atmospheres in packages has been suggested for different meat products [42]. In the present study, MAPs inhibited, compared to the air and vacuum atmospheres, the development of
L. monocytogenes mainly in the MAP with the highest concentration of CO
2. The lower OTR (1.0 cm
3m
-2day
-1) of the packaging film used in this experiment for MAP, in comparison to films of greater O
2 permeability (OTR=4.5 cm
3m
2day
-1) used in the study conducted by Tsigarida et al. [43] can justify these results. Saraiva et al. [41] using the same packaging film of the present study found that
L. monocytogenes in beef may be reduced by ~1.0 log in vacuum packaging and by ~1.5 log on average in the MAPs. Generally, under anaerobic modified atmosphere the LAB compete with the support microflora and have shown to be effective in inhibiting the growth of pathogenic bacteria such as
L. monocytogenes in meat products [42]. The combination of selected LAB strains with antimicrobial compounds, for instance, acid and sodium lactate or the use of active packaging could be the next step strategy for eliminating risk of
L. monocytogenes in meat and dairy-ripened products [42]. Many food-spoiling LAB are facultative aerobic and quite resistant to CO
2, this contributes to the fact that LAB can be found as main spoilers on high oxygen packaged meat [41]. Therefore,
L. monocytogenes can represent a risk when stored at temperatures higher than 0°C and the efficacy of thermal treatments is limited by the ability to survive and actively replicate at temperatures between -0.4 and 45ºC [42]. Air and vacuum atmospheres had similar counts for this pathogen (
Figure 2) which could be related to the O
2 permeability rate of the packaging film used for vacuum atmosphere (OTR=63cm
3m
-2day
-1), not so efficient in inhibiting the
L. monocytogenes [41].
A higher proportion of CO2 in MAP50/40 and even more in MAP30/60 have led to a lower growth of L. monocytogenes compared to other types of packaging. This result is consistent with other researches which results confirmed that the growth of L. monocytogenes was inhibited with increasing concentrations of CO2 [42]. According to Saraiva et al. [41] L. monocytogenes requires CO2 levels of 40% v/v or higher for an effective inhibition. In view to maintain the cytoplasmic pH within a range that is consistent with growth and survival the decarboxylation reaction need to occur helping maintain the cytoplasmic pH, though high concentration of CO2 can inhibit the decarboxylation reaction by which CO2 is released through feedback mechanisms [42].
At temperature of storage of 4ºC, the counts of
E. coli O157:H7 on Normal and DFD, as well, for abusive temperature on Normal beef, were similar during all the storage period, maintaining the initial levels (
Figure 1). Regarding DFD meat and temperature of storage of 9ºC, the
E. coli O157:H7 achieved the highest growth levels in vacuum atmosphere (~8 log CFU/g), showing statist differences from Normal meat (
P< 0.001) and from temperature of storage of 4ºC (
P< 0.001) at 7, 10, 14, 21 and 28 days
pm.
The pathogen E. coli O157:H7 in air packaging multiplied rapidly; however, MAP30/60 showed the lower growth levels during all the storage period. In this study, this was the atmosphere with high CO2 content (60%) and E. coli O157:H7 counts do not attained 5 log CFU/g at 14 days pm. These results can be due to the inhibitory effect of CO2. In previous experiments, E. coli O157:H7 in atmospheres with high concentrations of CO2 (ratio of 30%CO2:70% O2 or 0.4%CO:60%CO2:39.6%N2) have been reported as being inhibitory to the multiplication of E. coli O157:H7 at an abusive storage temperature of 10°C [39]. As mention above for L. monocytogenes, E. coli uses the same mechanism of decarboxylation systems to protect the cell from a precipitous drop in pH. These systems depend on the activity of cytoplasmic pyridoxal-5’-phosphate (PLP)-containing amino acid decarboxylases which consume one proton and release one CO2 for every molecule of substrate amino acid, thus helping maintain the cytoplasmic pH [42]. At refrigeration temperatures from 0 to 2°C, high concentrations of CO2 (ratio of 20%CO2:80%O2 or 0.4%CO:30%CO2:69.6%N2) can led to a reduction of one logarithmic unit in the levels from E. coli O157:H7 [50]. In accordance with the present study Nissen el al. [39] reported that E. coli O157:H7 could be inhibited at 10 ºC at high CO2 concentration and pHu inferior of 6. Moreover, even in MAP with high CO2 and low CO mixture meat acquires a stable color and the shelf-life can be extended.
The behaviour of L. monocytogenes in abusive temperatures differed between DFD and Normal pHu meats, for all types of packaging after 7 days pm. For instance, at 10 day pm, Normal and DFD meat showed, respectively counts of 2.07 log CFU/g and 8.25 log CFU/g in air (P< 0.001); 2.54 log CFU/g and 8.24 log CFU/g (P< 0.001) on Vacuum; 1.69 log CFU/g and 7.91 log CFU/g in MAP70/20 (P< 0.001); 1.37 log CFU/g and 7.79 log CFU/g in MAP50/40 (P< 0.001); and 1.42 log CFU/g and 7.01 log CFU/g in MAP30/60 (P< 0.001). However, regarding the stored temperatures, for instance at 14 day pm, count obtained at 4ºC and 9ºC of storage for DFD meat were respectively 5.71 log CFU/g and 8.33 log CFU/g (P<0.001) in Vacuum; 4.82 log CFU/g and 8.55 log CFU/g in MAP70/20 (P<0.001); and 4.07 log CFU/g and 8.53 log CFU/g in MAP50/40 (P< 0.001). Thus, even with meat stored at temperatures within recommended limits, the development of L. monocytogenes occurred markedly in meat with a high pHu, though when comparing both temperatures, the effect was not so intense as was the effect of pHu. However, unlike E. coli O157:H7 where the effect of pH was not felt when storage was carried out at the appropriate temperature, also in this pathogen, at 9±0.5ºC of storage; a significantly greater growth was noted in the DFD meat samples.
5. Conclusions
The present study highlights the importance of maintaining the cold chain under strict surveillance conditions is confirmed to avoid abuses, which can have consequences, even more serious in the case of DFD meat. As referred, the occurrence of DFD condition was more frequently observed in L. dorsi muscle of Maronesa breed, mainly in males. The results revealed that this condition associated to abusive storage temperatures allowed the growth of E. coli O157:H7 at higher levels, specially more evident in air and vacuum packages. For L. monocytogenes the low storage temperature (4ºC) associated to the DFD condition was not enough to inhibit the growth of L. monocytogenes as observed in E. coli O157:H7. This is due to the character psychotropic of L. monocytogenes.
The use of special packing conditions such as vacuum or MAP in order to increase the shelf-life of meat, or for technological and sensorial reasons, can have consequences in terms of development of some of the pathogens present in meat. Overall, the MAP were the most effective in controlling the development of E. coli O157:H7 and L. monocytogenes. In MAP, the effect of the CO2 level was observed, being even more noticeable in pH that better supported microbial growth.
Author Contributions
Conceptualization C.S. and M.C.F.; methodology C.S. and M.C.F.; software C.S. and L.P.; validation C.S. and M.C.F.; formal analysis C.S., S.S. and L.P.; investigation C.S., S.S. and M.C.F.; resources C.S. and C.M.; data curation C.S.; writing—original draft preparation C.S., S.S. and M.C.F.; writing—review and editing C.S. and S.S.; visualization C.S.; supervision C.S. and C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by national funds by FCT- Portuguese Foundation for Science and Technology, under the PEst-OE/AGR/UI0772/2014 and UIDB/CVT/00772/2020 projects.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank CECAV-UTAD.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. The State of Food and Agriculture 2019. Moving forward on Food Loss and Waste Reduction; FAO: Italy, Rome, 2019; pp. 1–182. [Google Scholar]
- Coutinho, P.; Simões, M.; Pereira, C.; Paiva, T. Sustainable Local Exploitation and Innovation on Meat Products Based on the Autochthonous Bovine Breed Jarmelista. Sustainability 2021, 13, 2515. [Google Scholar] [CrossRef]
- Pethick, D.; Hocquette, J.-F.; Scollan, N.; Dunshea, F. Review: Improving the nutritional, sensory and market value of meat products from sheep and cattle. Animal 2021, 15, 100356. [Google Scholar] [CrossRef] [PubMed]
- Guillemin, N.; Meunier, B.; Jurie, C.; Cassar-Malek, I.; Hocquette, J.F.; Leveziel, H.; Picard, B. Validation of a Dot-Blot quantitative technique for large scale analysis of beef tenderness biomarkers. J. Physiol. Pharmacol. 2009, 60, 91–97. [Google Scholar] [PubMed]
- Gracey, J. , Collins D.S., Huey R. Meat Hygiene, 10th ed.; W.B. Saunders Co. Ltd.: New York, USA, 1999. [Google Scholar]
- Chulayo, A.-Y.; Muchenje, V. Activities of some stress enzymes as indicators of slaughter cattle welfare and their relationship with physico-chemical characteristics of beef. Animal 2017, 11, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Kandeepan, G.; Tahseen, A. Modified Atmosphere Packaging (MAP) of Meat and Meat Products: A Review. J Package Technol Res 2022, 6, 137–148. [Google Scholar] [CrossRef]
- Geletu, U.S.; Usmael, M.A. , Mummed, Y.Y.; Ibrahim, A.M. Quality of Cattle Meat and Its Compositional Constituents. Vet Med Int. 2021, 2021, 7340495. [Google Scholar] [CrossRef]
- Gutema, F.D.; Agga, G.E.; Abdi, R.D.; Jufare, A.; Duchateau, L.; De Zutter, L.; Gabriël, S. Assessment of Hygienic Practices in Beef Cattle Slaughterhouses and Retail Shops in Bishoftu, Ethiopia: Implications for Public Health. Int J Environ Res Public Health 2021, 18, 2729. [Google Scholar] [CrossRef]
- Chai, C.; Lee, S.-Y.; Oh, S-W. Shelf-life charts of beef according to level of bacterial contamination and storage temperature. LWT-Food Sci. Technol. 2017, 81, 50–57. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, L.; Zhang, Y.; Liang, R.; Lu, X. Microbial community dynamics analysis by high-throughput sequencing in chilled beef longissimus steaks packaged under modified atmospheres. Meat Sci. 2018, 141, 94–102. [Google Scholar] [CrossRef]
- Sofos, J.N. Challenges to meat safety in the 21st century. Meat Sci. 2008, 78, 3–13. [Google Scholar] [CrossRef]
- Lianou, A.; Panagou, E.Z.; Nychas, G.J.E. Meat Safety—I Foodborne Pathogens and Other Biological Issues. Lawrie´s Meat Science 2017, 2017, 521–552. [Google Scholar] [CrossRef]
- Desai, A.N.; Anyoha, A.; Madoff, L.C.; Lassmann, B. Changing epidemiology of Listeria monocytogenes outbreaks, sporadic cases, and recalls globally: A review of ProMED reports from 1996 to 2018. Int. J. Infect. Dis. 2019, 84, 48–53. [Google Scholar] [CrossRef] [PubMed]
- EFSA; ECDC. The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, e07666. [Google Scholar] [CrossRef]
- Lien, K.W.; Yang, M.X.; Ling, M.P. Microbial Risk Assessment of Escherichia coli O157:H7 in Beef Imported from the United States of America to Taiwan. Microorganisms 2020, 8, 676. [Google Scholar] [CrossRef]
- Chekabab, S.M.; Daigle, F.; Charette, S.J.; Dozois, C.M.; Harel, J. Shiga toxins decrease enterohaemorrhagic Escherichia coli survival within Acanthamoeba castellanii. FEMS Microbiol Lett. 2013, 344, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Bacon, R.T.; Sofos, J.N. Food hazards: Biological food; characteristics of biological hazards in foods. In Food safety handbook; Schmidt, R.H., Rodrick, G.E., Eds.; Wiley Interscience: New York, USA, 2003; pp. 157–195. [Google Scholar]
- Bekele, T.; Zewde, G.; Tefera, G.; Feleke, A.; Zerom, K. Escherichia coli O157:H7 in Raw Meat in Addis Ababa, Ethiopia: Prevalence at an Abattoir and Retailers and Antimicrobial Susceptibility. Int. J. Food Contam. 2014, 1, 1–9. [Google Scholar] [CrossRef]
- Bedasa, S.; Shiferaw, D.; Abraha, A.; Moges, T. Occurrence and antimicrobial susceptibility profile of Escherichia coli O157:H7 from food of animal origin in Bishoftu town, Central Ethiopia. Int. J. Food Contam. 2018, 5, 1–8. [Google Scholar] [CrossRef]
- Ryser, E.T.; Marth, E.H. Listeria, listeriosis and food safety, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- Buchanan, R.L.; Gorris, L.G.M.; Hayman, M.M.; Jackson, T.C.; Whiting, R.C. A review of Listeria monocytogenes: An update on outbreaks, virulence, dose-response, ecology, and risk assessments. Food Control 2017, 75, 1–13. [Google Scholar] [CrossRef]
- Gómez, D.; Iguácel, L.P.; Rota, M.C.; Carramiñana, J.J.; Ariño, A.; Yangüela, J. Occurrence of Listeria monocytogenes in Ready-to-Eat Meat Products and Meat Processing Plants in Spain. Foods 2015, 4, 271–282. [Google Scholar] [CrossRef]
- EFSA. Listeria monocytogenes contamination of ready-to-eat foods and the risk for human health in the EU. EFSA J. 2018, 16, 5134. [Google Scholar] [CrossRef]
- Zakrys, P.I.; Hogan, S.A.; O'Sullivan, M.G.; Allen, P.; Kerry, J.P. Effects of oxygen concentration on the sensory evaluation and quality indicators of beef muscle packed under modified atmosphere. Meat Sci. 2008, 79, 648e655. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, J.; Holman, B.W.B.; Liang, R.; Chen, X.; Luo, X.; Zhu, L.; Hopkins, D.L.; Zhang, Y. Investigation of the physicochemical, bacteriological, and sensory quality of beef steaks held under modified atmosphere packaging and representative of different ultimate pH values. Meat Sci. 2021, 174, 108416. [Google Scholar] [CrossRef] [PubMed]
- Conte-Junior, C.A.; Monteiro, M.L.G.; Patrícia, R.; Mársico, E.T.; Lopes, M.M.; Alvares, T.S.; Mano, S.B. The Effect of Different Packaging Systems on the Shelf Life of Refrigerated Ground Beef. Foods 2020, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- McMillin, K.W. Where is MAP Going? A review and future potential of modified atmosphere packaging for meat. Meat Sci. 2008, 80, 43e65. [Google Scholar] [CrossRef] [PubMed]
- Loredo-Osti, J.; Sánchez-López, E.; Barreras-Serrano, A.; Figueroa-Saavedra, F.; Pérez-Linares, C.; Ruiz-Albarrán, M.; Domínguez-Muñoz, M.A. An evaluation of environmental, intrinsic and pre- and post-slaughter risk factors associated to dark-cutting beef in a Federal Inspected Type slaughter plant. Meat Sci. 2019, 150, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.; Li, X.; Zhang, D.; Hussain, Z.; Ren, C.; Bai, Y.; Zheng, X. Association between meat color of DFD beef and other quality attributes. Meat Sci. 2020, 161, 107954. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.A.; Saraiva, C.; Payan, R.; Alves, V.; Bruno Soares, A.; Martins, C. Incidência e causas de carnes DFD em bovinos autóctones da raça Maronesa. Influência na tenrura e na qualidade higiénica da carne. Final Report of AGRO 165 2005. UTAD, ISA – UTL, DRATM, ACM and CA of Vila Real –Producers “Carne Maronesa – DOP”. 140 Pp.
- Hughes, J.; Clarke, F.; Purslow, P.; Warner, R. High pH in beef longissimus thoracis reduces muscle fibre transverse shrinkage and light scattering which contributes to the dark colour. Food Res. Int. 2017, 101, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.C.; Gray, G.D.; Hale, D.S.; Kerth, C.R.; Griffin, D.B.; Savell, J.W.; Raines, C.R.; Belk, K.E.; Woerner, D.R.; Tatum, J.D.; Igo, J.L.; VanOverbeke, D.L.; Mafi, G.G.; Lawrence, T.E.; Delmore, R.J.; Christensen, L.M.; Shackelford, S.D.; King, D.A.; Wheeler, T.L.; Meadows, L.R.; O'Connor, M.E. National Beef Quality Audit–2011: In-plant survey of targeted carcass characteristics related to quality, quantity, value, and marketing of fed steers and heifers. J. Anim. Sci. 2012, 90, 5143–5151. [Google Scholar] [CrossRef]
- Holdstock, J.; Aalhus, J.L.; Uttaro, B.A.; López-Campos, Ó.; Larsen, I.L.; Bruce, H.L. The impact of ultimate pH on muscle characteristics and sensory attributes of the longissimus thoracis within the dark cutting (Canada B4) beef carcass grade. Meat Sci. 2014, 98, 842–849. [Google Scholar] [CrossRef]
- Rosa, A.; Fonseca, R.; Balieiro, J.C.; Poleti, M.D.; Domenech-Pérez, K.; Farnetani, B.; Eler, J. Incidence of DFD meat on Brazilian beef cuts. Meat Sci. 2016, 112, 132–133. [Google Scholar] [CrossRef]
- Arevalos-Sánchez, M.; Regalado, C.; Martin, S.E.; Domínguez-Domínguez, J.; García-Almendárez, B.E. Effect of neutral electrolyzed water and nisin on Listeria monocytogenes biofilms, and on listeriolysin O activity. Food Control 2012, 24, 116–122. [Google Scholar] [CrossRef]
- Grau, F.H.; Vanderlinde, P.B. Growth of Listeria monocytogenes on Vacuum-packaged Beef. J. Food Prot. 1990, 53, 739–741. [Google Scholar] [CrossRef]
- Meloni, D. Presence of Listeria monocytogenes in Mediterranean-Style Dry Fermented Sausages. Foods. 2015, 4, 34–50. [Google Scholar] [CrossRef] [PubMed]
- Nissen, H.; Alvseike, O.; Bredholt, S.; Holck, A.; Nesbakken, T. Comparison between the growth of Yersinia enterocolitica, Listeria monocytogenes, Escherichia coli O157:H7 and Salmonella spp. in ground beef packed by three commercially used packaging techniques. I. J. Food Microb. 2000, 59, 211–220. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Zhang, P.; Niu, Y.; Chen, Q.; Ma, X. Genomic Characterization of Clinical Listeria monocytogenes Isolates in Beijing, China. Front. Microbial. 2021, 12, 751003. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, C; Fontes, M. C.; Patarata, L.; Martins, C.; Cadavez, V.; Gonzales-Barron, U. Modelling the kinetics of Listeria monocytogenes in refrigerated fresh beef under different packaging atmospheres, LWT - Food Sci.Technol. 2016, 66, 664–671. [CrossRef]
- Narasimha Rao, D.N.; Sachindra, N.M. Modified atmosphere and vacuum packaging of meat and poultry products. Food Rev. Int. 2002, 18, 263–293. [Google Scholar] [CrossRef]
- Tsigarida, E.; Skandamis, P.; Nychas, G.-J.E. Behaviour of Listeria monocytogenes and autochthonous flora on meat stored under aerobic, vacuum and modified atmosphere packaging conditions with or without the presence of oregano essential oil at 5ºC. J. Appl. Microbiol. 2000, 89, 901–909. [Google Scholar] [CrossRef]
- Egan, K.; Field, D.; Rea, M.C.; Ross, R.P.; Hill, C.; Cotter, P.D. Bacteriocins: Novel solutions to age old spore-related problems? Front. Microbiol. 2016, 7, 461. [Google Scholar] [CrossRef]
- Martín, I.; Rodríguez, A.; Delgado, J.; Córdoba, J.J. Strategies for Biocontrol of Listeria monocytogenes Using Lactic Acid Bacteria and Their Metabolites in Ready-to-Eat Meat and Dairy-Ripened Products. Foods 2022, 11, 542. [Google Scholar] [CrossRef]
- Chan, Y.C.; Wiedmann, M. Physiology and genetics of Listeria monocytogenes survival and growth at cold temperatures. Crit. Rev. Food Sci. Nutr. 2009, 49, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Zhang, Y.; Tu, S.; Huang, Y.; Tu, K. Model for the Effect of Carbon Dioxide on Listeria Monocytogenes in Fresh-cut Iceberg Lettuce Packaged Under Modified Atmosphere, Food Sci. Technol. Res. 2018, 24, 1021–1027. [Google Scholar] [CrossRef]
- Arcari, T.; Feger, M.-L.; Guerreiro, D.N.; Wu, J.; O’Byrne, C.P. Comparative Review of the Responses of Listeria monocytogenes and Escherichia coli to Low pH Stress. Genes 2020, 11, 1330. [Google Scholar] [CrossRef] [PubMed]
- Feehily, C.; Karatzas, K.A. Role of glutamate metabolism in bacterial responses towards acid and other stresses. J. Appl. Microbial. 2013, 114, 11–24. [Google Scholar] [CrossRef]
- Brooks, J.C.; Alvarado, M.; Stephens, T.P.; Kellermeier, J.D.; Tittor, A.W.; Miller, M.F.; Brashears, M.M. Spoilage and Safety Characteristics of Ground Beef Packaged in Traditional and Modified Atmosphere Packages. Journal of Food Protection 2008, 71, 293–301. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).