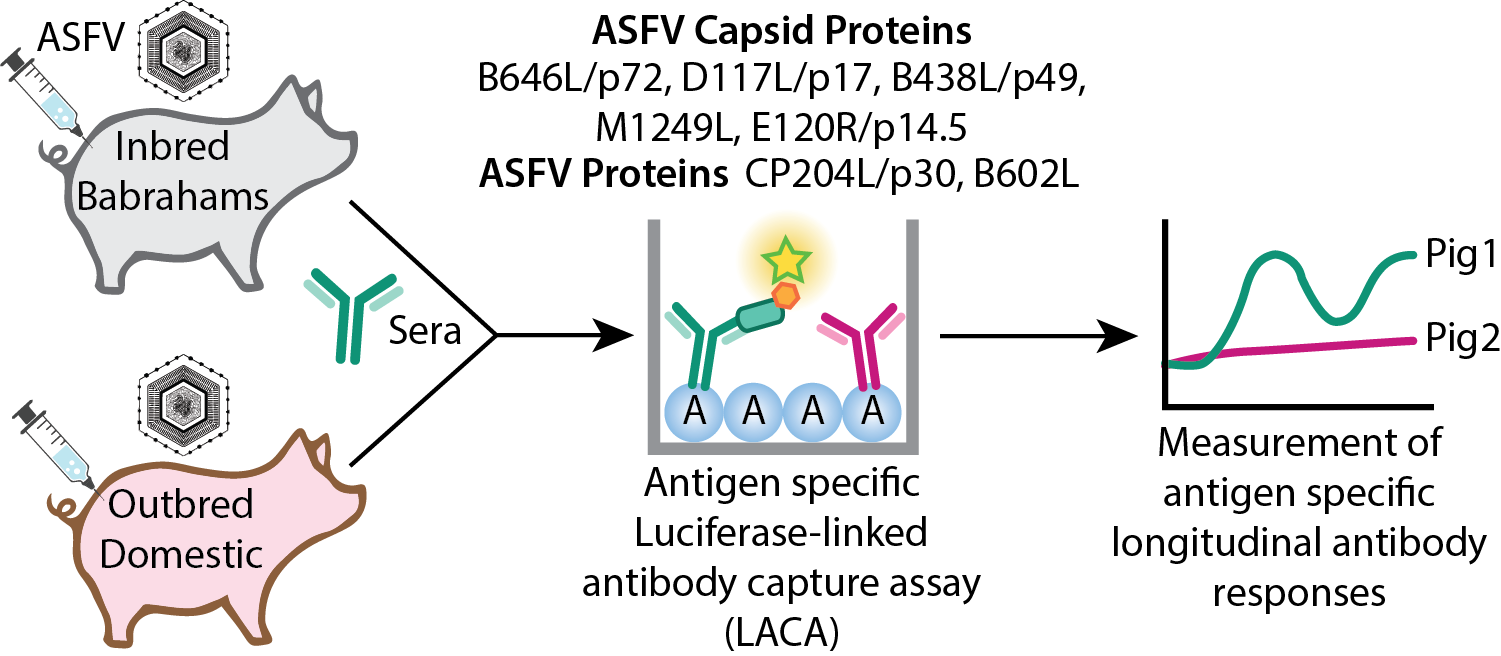
1. Introduction
The on-going African swine fever (ASF) panzootic in domestic pigs and wild boar is caused by the ASF virus (ASFV) [
1,
2]. It is a contagious and lethal hemorrhagic disease that is of international concern. Due to the absence of approved and effective vaccines or treatments, main control measures involve slaughter and movement control of swine [
3], resulting in high economic losses and impact on global food security. ASFV is a large double stranded virus that has a 170 to 193kb genome encoding over 150 genes [
4,
5], many of which are uncharacterized [
1,
5].
Protective immune responses after recovery from ASFV infection are poorly understood. Vaccine development efforts are mostly focused on the development of live attenuated viruses (LAV) [
6,
7,
8,
9,
10]. Although these afford good protection, they are not DIVA (differentiating infected from vaccinated animals) compliant and there are potential safety concerns [
9,
11]. Unlike LAVs, subunit vaccines only encode for selected viral antigens and have an inherently safe design that is DIVA compliant. However, combinations that have been developed and tested so far offer varied protection [
12,
13,
14,
15]. Generating good T-cell responses has generally been the focus of ASFV vaccine development efforts [
12,
14,
16] although both cellular and humoral immune responses are important for robust protection against ASFV. Despite studies demonstrating the importance of anti-ASFV antibodies in disease protection [
17,
18,
19], antigen specific antibody responses to ASFV have remained largely uncharacterized due to the difficulties in detecting neutralizing antibodies [
20,
21] and the lack of tools.
Antibody responses are typically measured with ELISAs, but ELISAs using fixed virus infected cells or lysates only provide a broad overview of the antibody responses and few ASFV antigen specific ELISAs are commercially available. Moreover, development of ASFV antigen specific ELISAs has mostly focused on diagnostic purposes with highly immunogenic antigens [
22,
23,
24,
25,
26,
27]. Recombinant protein production and purification is a core prerequisite for ELISA development and due to structure and the immunomodulatory nature of many ASFV proteins [
28], high yields in mammalian expression systems with proper post-translational modifications can prove difficult to achieve.
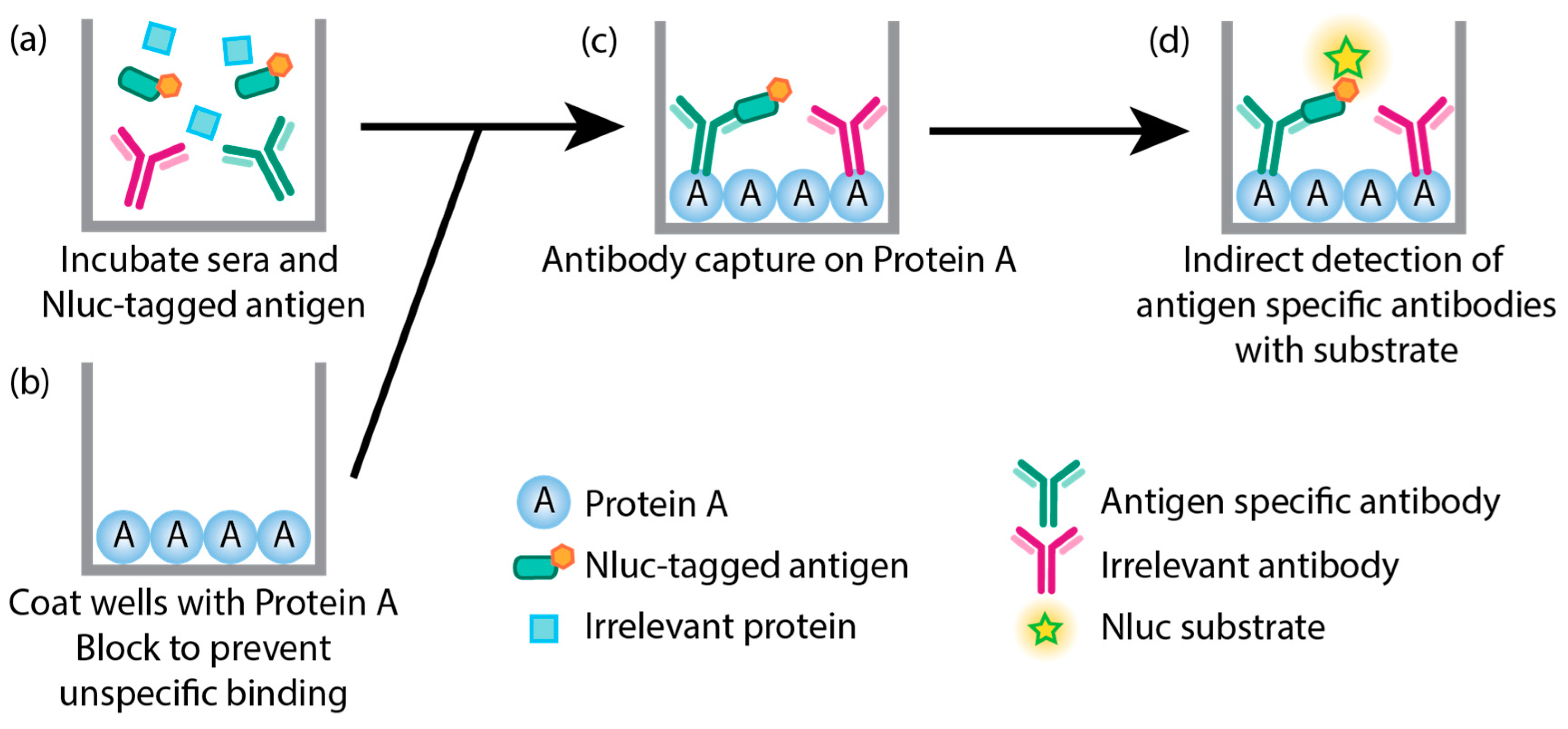
Luciferase based antibody diagnostics has previously been reported for porcine diseases using luciferase immunoprecipitation systems (LIPS) [
29,
30] and luciferase-linked antibody capture assays (LACAs) [
31]. Both LIPS and LACAs detect and quantify antigen specific antibodies indirectly through the capture of antibodies that are bound to recombinant luciferase tagged proteins of interest [
31,
32]. Capture of antibodies is typically achieved with protein A. Furthermore, unlike ELISAs, these assays do not require protein purification and allow the use of crude cell lysates [
32]. Compared to LIPS, LACAs are a more cost-effective approach for screening a large number of samples with multiple antigens [
31].
ASFV is a highly complex virus with many structural proteins involved in the assembly of its multi-layered structure. Recently the ASFV capsid structure has been resolved by three separate groups highlighting the proteins involved in capsid construction [
33,
34,
35]. B646L/p72 is the major capsid protein and is the most abundant protein within the capsid [
36]. It is highly immunogenic and conserved, hence its use in routine serological diagnostics [
37] and genotyping [
38]. To obtain B646L/p72 proteins that have a native conformation co-expression of the virally encoded B602L chaperone is required [
39,
40]. B602L is not present in virus replication sites and antibodies against B602L can be detected in recovered pigs [
41,
42]. D117L/p17 a minor capsid protein that is also a component of the internal envelope [
34] – is also essential for virus morphogenesis [
43]. D117L/p17 is immunogenic [
44] and may have immunomodulatory abilities [
45]. B438L/p49 is required for the assembly of icosahedral virions and is located at the vertices of the capsid [
35,
46]. Previous work has associated B438L/p49 with low immunogenicity [
47]. M1249L is a large structural protein that may be involved in building the framework of the capsid [
33,
34]. E120R/p14.5 is a minor capsid protein that is associated with B646L/p72 and is essential for virus dissemination [
48]. It has been suggested that E120R/p14.5 may have DNA binding properties [
49]. Similar to other capsid proteins, host immunomodulatory functions have been associated with E120R [
50]. To our knowledge, porcine antibody responses to M1249L and E120R have not been described before.
In this study, we produced a CP204L/p30 LACA to confirm the performance of LACAs for measurement of antibody responses. We then sought to develop LACAs for a panel of ASFV capsid and associated proteins: (1) B646L/p72 (with and without co-expression of B602L); (2) D117L/p17; (3) B438L/p49; (4) M1249L; (5) E120R/p14.5 and (6) the chaperone B602L. We used the LACAs to profile longitudinal humoral responses using samples from our previous study [
51] where we immunized inbred Babraham pigs and outbred domestic pigs with the low virulent OURT88/3 (genotype I) isolate. We were able to identify antigen specific antibodies against most of the antigens tested and distinguish responses associated with recovery from virulent ASFV challenge.
3. Results
We previously reported on the humoral responses of inbred Babraham animals and outbred domestic pigs that were immunized with low virulent OURT88/3 before challenge with virulent ASFV [
51]. Using whole ASFV fixed cell- and commercial ELISAs we found the general humoral responses of inbred Babraham animals were weakly associated with recovery after challenge with virulent ASFV OURT88/1. We did not, however, identify crucial antigens that were associated with protection due to the lack of available tools. To probe the antigen specific antibody responses of samples collected from our previous study, we developed a modified version of LACAs [
31] (
Figure 1). ASFV capsid proteins of interest were fused to Nluc, which is a smaller luciferin that is able to metabolize coelenterazine - similar to
Renilla luciferase – but with higher levels of luminescence [
54]. The smaller size of Nluc (approximately 19.1 kDa), compared to
Renilla luciferase (36 kDa), reduces the possibility of steric hindrance of potential epitopes. Since ASFV replicates in porcine cells, we expressed Nluc-tagged recombinant ASFV proteins in mammalian cell lines to ensure that mammalian post-translational modifications were applied appropriately. Recombinant ASFV protein expression was confirmed with confocal imaging (
Supplementary Figure 1 and
Figure 2) and western blot (
Supplementary Figure 3). Two assays were developed for the major capsid protein B646L/p72: one where B646L/p72 is co-expressed with an untagged chaperone B602L, which has been described to be essential for proper conformation of B646L/p72 [
36,
39,
40], and one where B646L/p72 is expressed alone. The co-transfection ratio of B646L/p72 and B602L to develop an assay using recombinant B646L/p72 with a conformation that is reminiscent of its native conformation was determined empirically with confocal imaging; binding of the conformation dependent 4H3 antibody [
55] was only observed at a ratio of 1:1 (B646L/p72: B602L,
Supplementary Figure 4). In this work, we refer to antibodies that target B646L/p72 co-expressed with B602L as B646L/p72-B602L antibodies and antibodies that bind to non-conformational B646L/p72 (no co-expression of B602L) targets as B646L/p72 antibodies.
3.1. Antigen specific antibody responses of inbred Babraham pigs
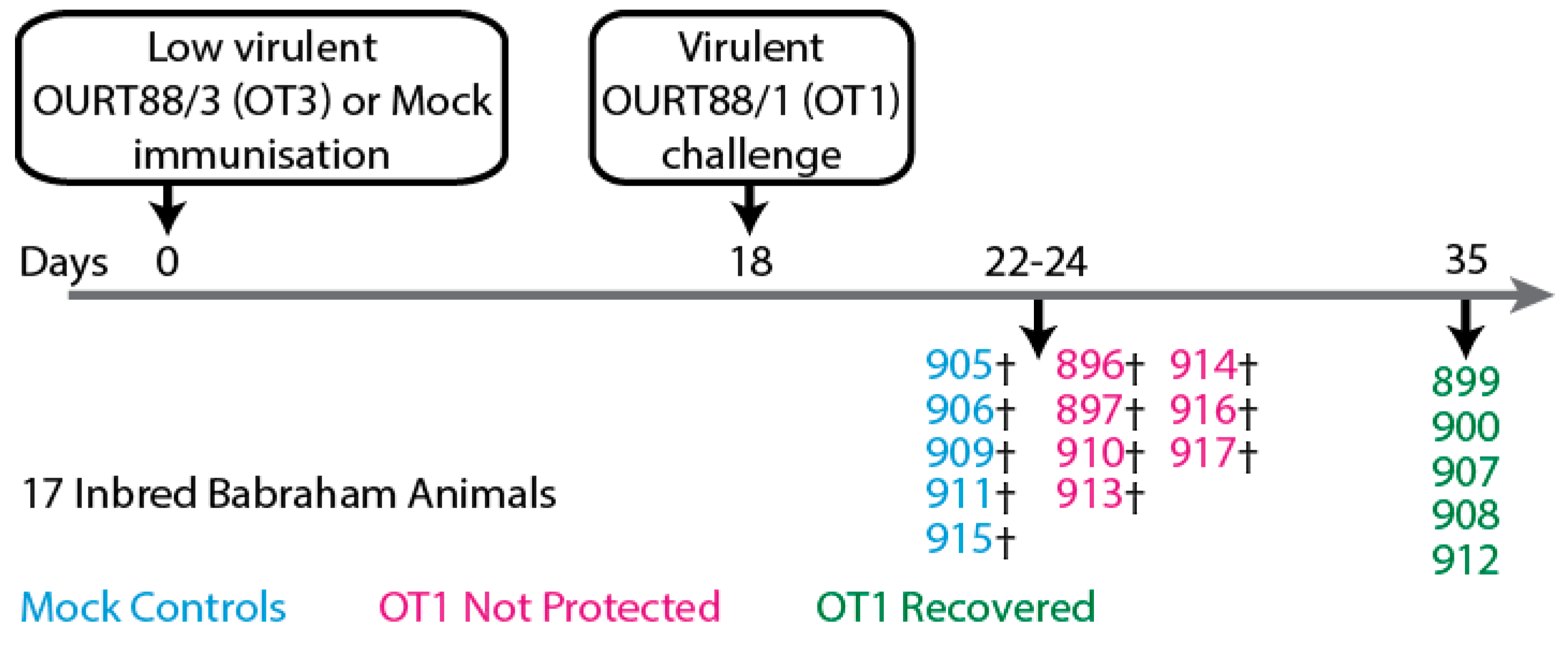
Sera samples collected from inbred Babraham animals [
51] (
Figure 2) were assayed with the CP204L/p30 and ASFV capsid protein specific LACAs. Antigen specific antibody responses were detected against CP204L/p30 (
Figure 3a, b), the combination of B646L/p72-B602L (
Figure 3c, d), B646L/p72 (
Figure 3e, f), the chaperone B602L (
Figure 3g, h), D117L/p17 (
Figure 4a, b), M1249L (
Figure 4e, f) and E120R/p14.5 (
Figure 4g, h). Antibody responses for B438L (
Figure 4c, d) were not observed, potentially due to lower expression of B438L, and low or non-immunogenicity of this protein.
Figure 2.
Schematic representation of experiment involving large-white inbred Babraham pigs that were immunized with low virulent OURT88/3 (OT3) before challenge with virulent OURT88/1 (OT1) as published previously [
51]. Partial protection from challenge with related ASFV was observed.
Figure 2.
Schematic representation of experiment involving large-white inbred Babraham pigs that were immunized with low virulent OURT88/3 (OT3) before challenge with virulent OURT88/1 (OT1) as published previously [
51]. Partial protection from challenge with related ASFV was observed.
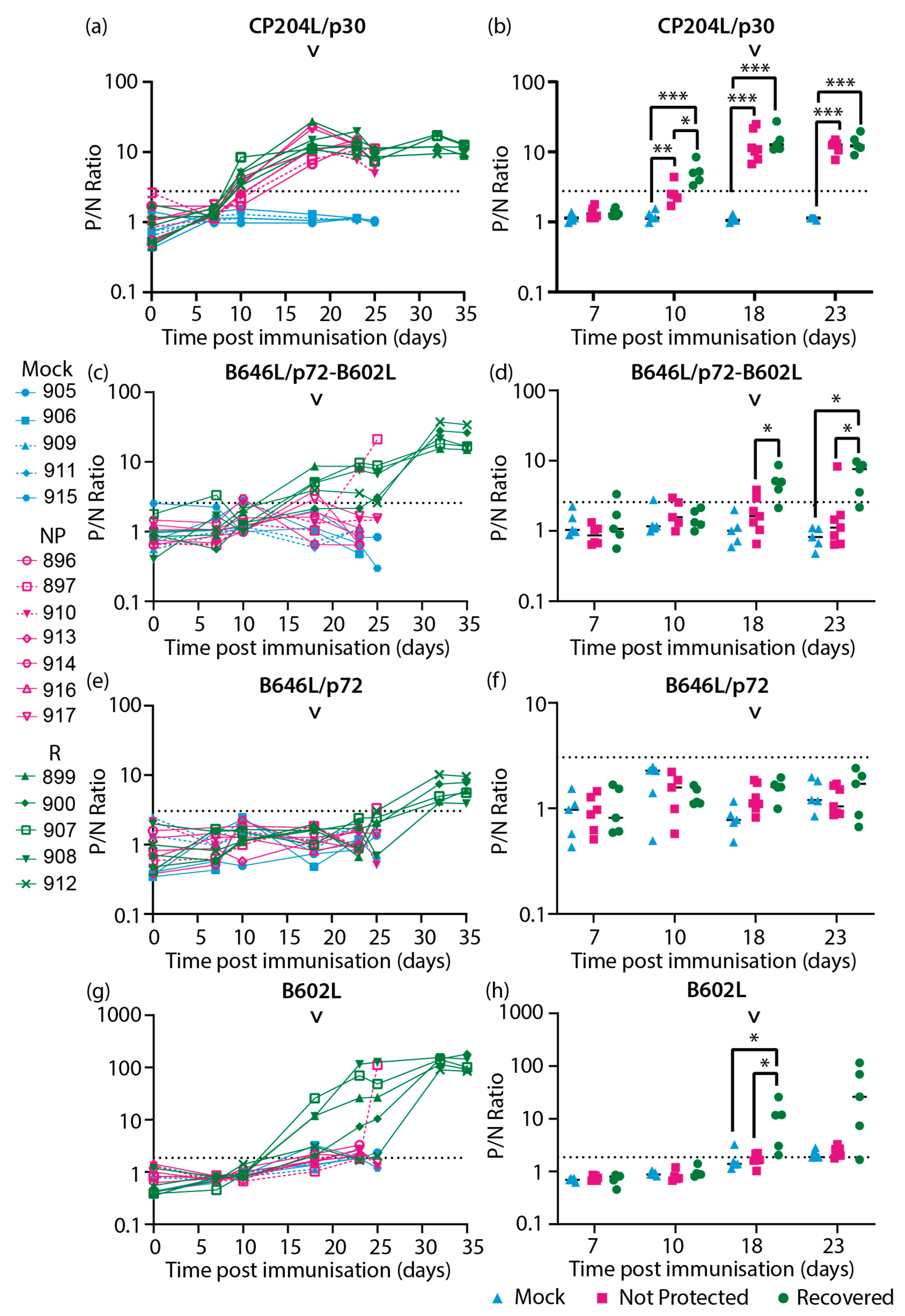
CP204L/p30 antibodies were detected in most of the animals by 10 days post immunization (10 dpi,
Figure 3a) and interestingly, were higher in the recovered group in comparison to the not protected group at this time point (
Figure 3b). However, by the day of challenge similar levels of CP204L/p30 antibodies were observed in both recovered and not protected animals (18 dpi,
Figure 3a, b) and at subsequent timepoints. Antibodies that recognized B646L/p72 when co-expressed with B602L (
Figure 3d) as well as B602L itself (
Figure 3h) were detected at challenge (18 dpi) in most of the animals that recovered from OURT88/1 challenge. In contrast, animals that were not protected had lower levels of B646L/p72 and B602L antibodies (
Figure 3d) at challenge. By the time the non-protected animals reached their humane endpoints, only one animal (pig 897) had an observable increase in antibodies against B646L/p72-B602L and B602L (
Figure 3c and g, respectively). Antibodies targeting B602L independent B646L/p72 conformational epitopes were not quantifiable in the assay with B646L/p72 until 32 dpi in recovered animals (
Figure 3e). In most of the animals that recovered from challenge, anti-B646L/p72 and B602L antibody levels increased between 25 and 32 dpi (7- and 14-days post challenge, dpc,
Figure 3e and g) and plateaued between 32 dpi to the end of the study (35dpi/17dpc). These results indicated that recovery from OURT88/1 infection may be associated with anti-B646L/p72 and B602L antibody levels at the point of challenge.
Figure 3.
Longitudinal antibody responses of inbred Babraham animals to ASFV recombinant proteins (a-b) CP204L/p30, (c-d) B646L/p72 co-expressed with chaperone B602L, (e-f) B646L/p72 and (g-h) B602L chaperone detected with antigen specific LACAs at selected days post-immunization (dpi). (a, c, e, g) Antigen specific antibody kinetics of each animal are plotted. (b, d, f, h) Antibody responses consolidated as a group at each relevant time point are plotted. Only time points where there were four data sets or more were included in this analysis. Lines indicate the mean. Each data point corresponds to a single animal. The point of challenge with virulent OURT88/1 (OT1, 18 dpi) is denoted by the arrowhead in each graph. P/N Ratio: ratio of luciferase activity of each sample to the luciferase activity of the negative control. Dashed line indicates the cutoff determined from the mean and 3x standard deviation of all negative sera samples in each experiment. Blue, Mock: mock control animals immunized with PBS, Pink, NP: OURT88/3 immunized animals that were not protected from OURT88/1, Green, R: OURT88/3 immunized animals that recovered from OURT88/1. * p<0.05, ** p<0.01, *** p<0.005, mixed effects model.
Figure 3.
Longitudinal antibody responses of inbred Babraham animals to ASFV recombinant proteins (a-b) CP204L/p30, (c-d) B646L/p72 co-expressed with chaperone B602L, (e-f) B646L/p72 and (g-h) B602L chaperone detected with antigen specific LACAs at selected days post-immunization (dpi). (a, c, e, g) Antigen specific antibody kinetics of each animal are plotted. (b, d, f, h) Antibody responses consolidated as a group at each relevant time point are plotted. Only time points where there were four data sets or more were included in this analysis. Lines indicate the mean. Each data point corresponds to a single animal. The point of challenge with virulent OURT88/1 (OT1, 18 dpi) is denoted by the arrowhead in each graph. P/N Ratio: ratio of luciferase activity of each sample to the luciferase activity of the negative control. Dashed line indicates the cutoff determined from the mean and 3x standard deviation of all negative sera samples in each experiment. Blue, Mock: mock control animals immunized with PBS, Pink, NP: OURT88/3 immunized animals that were not protected from OURT88/1, Green, R: OURT88/3 immunized animals that recovered from OURT88/1. * p<0.05, ** p<0.01, *** p<0.005, mixed effects model.
In recovered animals, antibodies targeting D117L/p17 (
Figure 4a) and M1249L (
Figure 4e) were generally observed to increase by 32 dpi/14 dpc and stabilize between 32 dpi/14 dpc and 35 dpi/17 dpc. Increases in anti-E120R/p14.5 antibodies were only detected in two animals in the recovered group (pigs 899 and 900,
Figure 4g) and this was only evident at termination on 35 dpi/17 dpc. In the non-protected group, pig 897 had increased antibody levels against D117/p17 (
Figure 4a), M1249L (
Figure 4e) and E120R/p14.5 (
Figure 4g) at the point of termination (24 dpi/6 dpc), in contrast to the other animals that were not protected.
To relate the antigen specific antibody responses measured in this study with the clinical (
Supplementary Figure 5), virological (
Figure 5a) and anti-ASFV antibody (
Figure 5b) data previously collected [
51], heatmaps of the different data sets were plotted (
Figure 5) to determine if trends were observable. In the previous study [
51] antibody levels against the highly immunogenic CP204L/p30 were detected with a commercially available blocking ELISA. Here, the CP204L/p30 LACA (
Figure 5c) broadly confirmed our previous CP204L/p30 ELISA results [
51]. The higher anti-ASFV antibody responses in recovered animals with the fixed cell ELISA (
Figure 5b) generally corresponded to the kinetics observed with B646L/p72-B602L (
Figure 5d) and B602L (
Figure 5f).
Of all the proteins tested, CP204L/p30 had the strongest responses in the not protected group (
Figure 5c). Within the animals that were not protected, pig 897 raised antibodies against B646L/p72-B602L (
Figure 5d), B602L (
Figure 5f), D117L/p17 (
Figure 5g), M1249L (
Figure 5h) and E120R/p14.5 (
Figure 5i) by the time it was culled, and its end point temperature (40.6°C,
Supplementary Figure 5b) was the lowest within its group. Pig 896 had higher ASFV antibody titers in the fixed cell ELISA (
Figure 5b), but antibodies to the panel of antigens (
Figure 5d-i) were not detected in this animal, so there may be antibodies to other antigens besides CP204L/p30 (
Figure 5c) that are contributing to the response measured by the fixed cell ELISA in this animal.
Figure 4.
Longitudinal antibody responses of inbred Babraham pigs to ASFV recombinant capsid proteins (a-b) D117L/p17, (c-d) B438L/p49, (e-f) M1249L and (g-h) E120R/p14.5 detected with antigen specific LACAs at selected dpi. (a, c, e, g) Antigen specific antibody kinetics of each animal are plotted. (b, d, f, h) Antibody responses consolidated as a group at each relevant time point are plotted. Only time points where there were four data points or more were included in this analysis. Lines indicate the mean. Each data point corresponds to a single animal. The point of challenge with virulent OURT88/1 (OT1, 18 dpi) is denoted by the arrowhead in each graph. P/N Ratio: ratio of luciferase activity of each sample to the luciferase activity of the negative control. Dashed line indicates the cutoff determined from the mean and 3 × standard deviation of all negative sera samples in each experiment. Blue, Mock: mock control animals immunized with PBS, Pink, NP: OURT88/3 immunized animals that were not protected from OURT88/1, Green, R: OURT88/3 immunized animals that recovered from OURT88/1. * p<0.05, ** p<0.01, mixed effects model.
Figure 4.
Longitudinal antibody responses of inbred Babraham pigs to ASFV recombinant capsid proteins (a-b) D117L/p17, (c-d) B438L/p49, (e-f) M1249L and (g-h) E120R/p14.5 detected with antigen specific LACAs at selected dpi. (a, c, e, g) Antigen specific antibody kinetics of each animal are plotted. (b, d, f, h) Antibody responses consolidated as a group at each relevant time point are plotted. Only time points where there were four data points or more were included in this analysis. Lines indicate the mean. Each data point corresponds to a single animal. The point of challenge with virulent OURT88/1 (OT1, 18 dpi) is denoted by the arrowhead in each graph. P/N Ratio: ratio of luciferase activity of each sample to the luciferase activity of the negative control. Dashed line indicates the cutoff determined from the mean and 3 × standard deviation of all negative sera samples in each experiment. Blue, Mock: mock control animals immunized with PBS, Pink, NP: OURT88/3 immunized animals that were not protected from OURT88/1, Green, R: OURT88/3 immunized animals that recovered from OURT88/1. * p<0.05, ** p<0.01, mixed effects model.
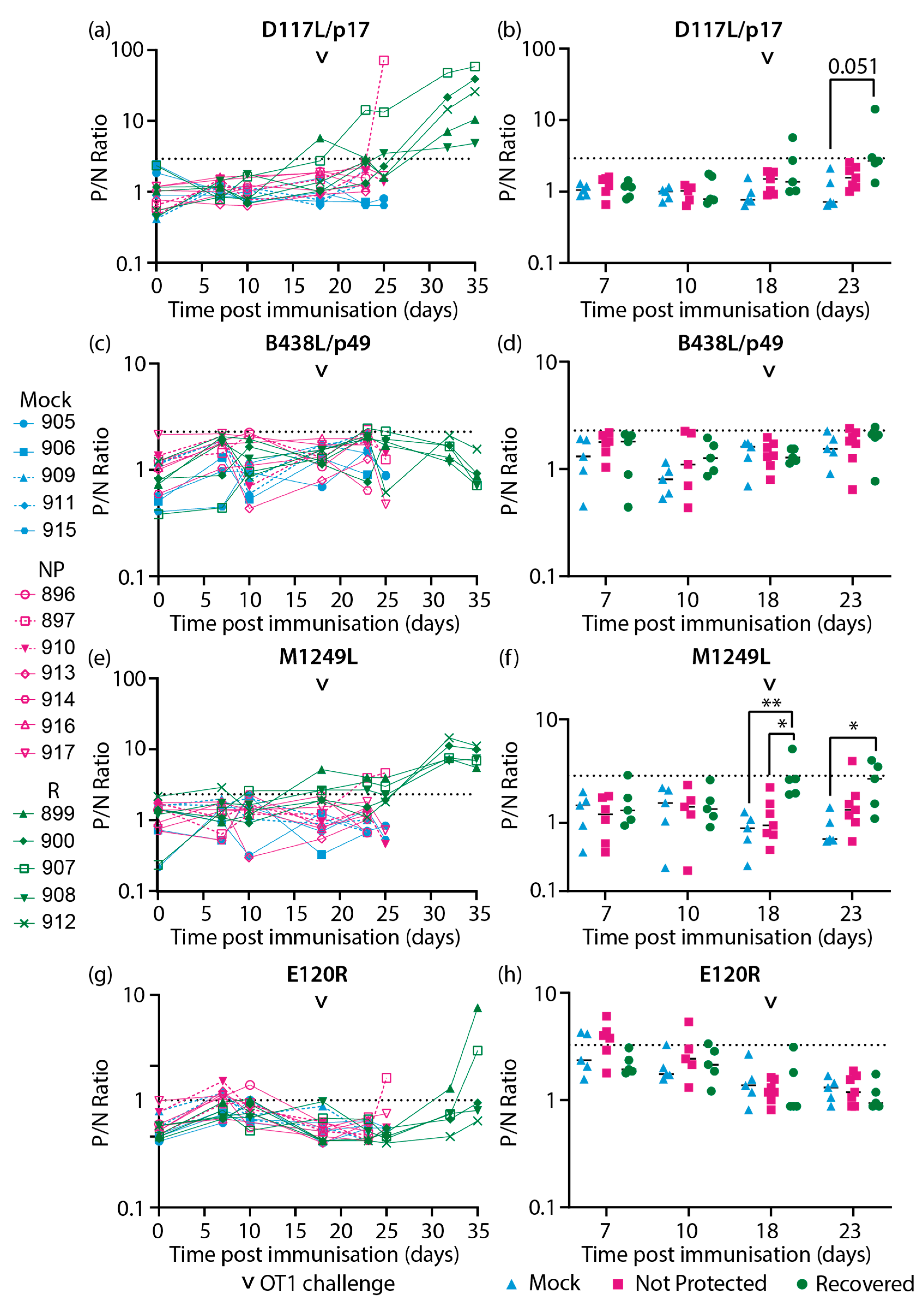
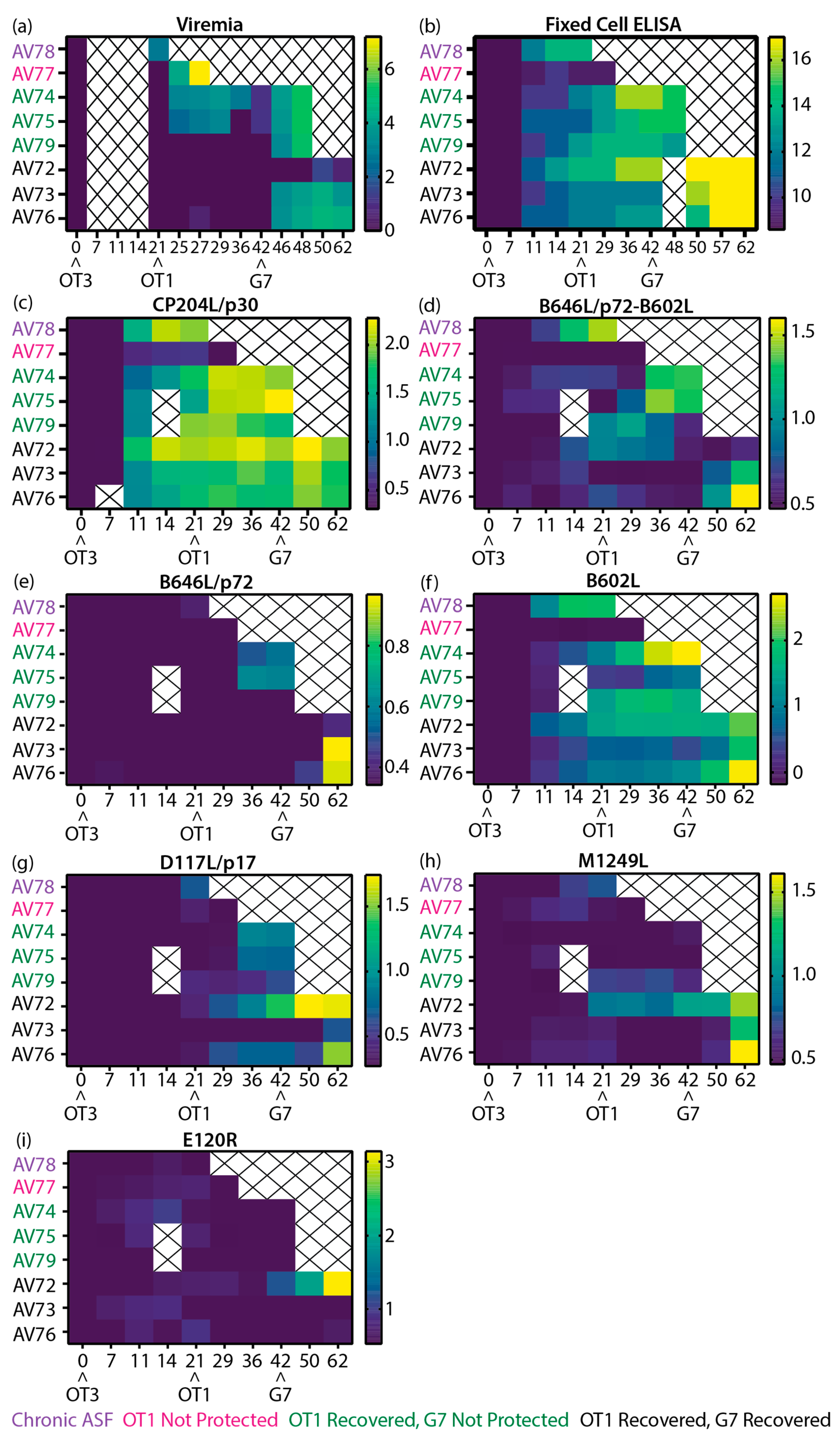
Figure 5.
Heatmaps of the virological and immunological parameters of inbred Babraham animals. (a-b) Data reported previously [
51], (a) viremia, (b) anti-ASFV antibody titer as determined by fixed cell ELISA on BA71V infected Vero cells [
51], and (c-i) recombinant ASFV protein specific LACAs targeting (c) CP204L/p30, (d) B646L/p72 co-expressed with B602L chaperone, (e) B646L/p72, (f) B602L chaperone, (g) D117L/p17, (h) M1249L and (i) E120R/p14.5. Data plotted as (a) Log10 genome copy numbers/ml, (b) Log2 antibody titer, (c-i) Log10 of P/N ratio. Each row denotes the responses of a single animal. The negative cutoff for each protein specific LACA was determined from the mean and 3x standard deviation of all negative sera samples in each experiment. Animal numbers are indicated on the y-axis and the time post-immunization is denoted on the x-axis. Crosses indicate samples that were not available for analysis. Arrowheads denote the immunization and ASFV challenge time points. Blue, Mock: mock control animals immunized with PBS, Pink, NP: OURT88/3 immunized animals that were not protected from OURT88/1, Green, R: OURT88/3 immunized animals that recovered from OURT88/1.
Figure 5.
Heatmaps of the virological and immunological parameters of inbred Babraham animals. (a-b) Data reported previously [
51], (a) viremia, (b) anti-ASFV antibody titer as determined by fixed cell ELISA on BA71V infected Vero cells [
51], and (c-i) recombinant ASFV protein specific LACAs targeting (c) CP204L/p30, (d) B646L/p72 co-expressed with B602L chaperone, (e) B646L/p72, (f) B602L chaperone, (g) D117L/p17, (h) M1249L and (i) E120R/p14.5. Data plotted as (a) Log10 genome copy numbers/ml, (b) Log2 antibody titer, (c-i) Log10 of P/N ratio. Each row denotes the responses of a single animal. The negative cutoff for each protein specific LACA was determined from the mean and 3x standard deviation of all negative sera samples in each experiment. Animal numbers are indicated on the y-axis and the time post-immunization is denoted on the x-axis. Crosses indicate samples that were not available for analysis. Arrowheads denote the immunization and ASFV challenge time points. Blue, Mock: mock control animals immunized with PBS, Pink, NP: OURT88/3 immunized animals that were not protected from OURT88/1, Green, R: OURT88/3 immunized animals that recovered from OURT88/1.
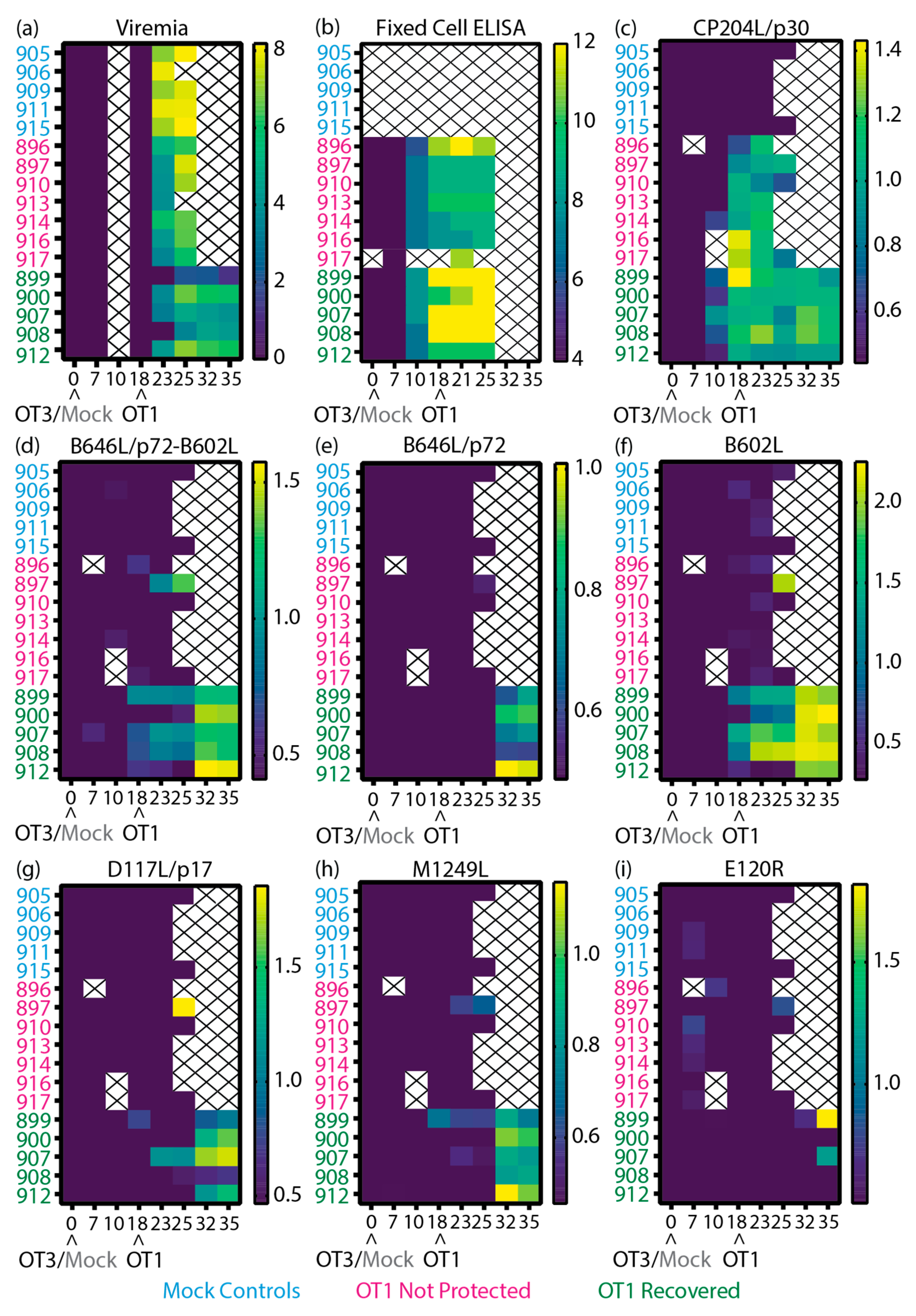
Amongst the recovered animals, pigs 900 and 912 had higher viremia on 25 dpi/7 dpc (
Figure 5a) and displayed clinical signs around the same time as the animals that were not protected (
Supplementary Figures 5a, b). Both 900 and 912 displayed the lowest amount of anti-ASFV (
Figure 5b), B646L/p72-B602L (
Figure 5d) and B602L (
Figure 5f) antibodies within the recovered animals on the day of challenge (18 dpi). As part of the secondary humoral response from pig 900, increased ASFV antibody titers were measured from 21 dpi/3 dpc (
Figure 5b), possibly with contribution from anti-B602L antibodies (
Figure 5f) due to the similar kinetics. Antibodies targeting the ASFV capsid proteins assessed in this panel were not detected in sera of pig 912 until 32 dpi/14 dpc, so antibodies against other ASFV antigens may play a contributing role to the recovery of this animal. By the end of the study, both animals displayed the highest viremia within the group, which corresponded with higher B646L/p72-B602L (
Figure 5d), B646L/p72 (
Figure 5e) and M1249L (
Figure 5h) antibody levels.
Pigs 899 and 908 displayed delayed clinical signs (
Supplementary Figures 5a, b) and viremia (
Figure 5a), whilst pig 907 showed milder clinical signs (
Supplementary Figure 5a). All three animals produced detectable levels of B646L/p72-B602L (
Figure 5d) and B602L (
Figure 5f) antibodies from 18 dpi/0 dpc until the end of the study, which may have contributed to the delay of viremia and/or milder clinical signs. In contrast to the rest of the group, levels of D117L/p17 antibody increased in animal 907 after challenge (
Figure 5g) after challenge, whilst animal 908 maintained higher levels of B602L (
Figure 5f) antibodies. Pig 899 had the lowest viremia detected in the study and this was complemented with higher CP204L and B646L/p72-B602L antibody levels (in comparison to the rest of the group,
Figure 5c-d), detectable antibody levels to D117L/p17 (
Figure 5g), and production of anti-M1249L antibodies (
Figure 5h) on 18 dpi/0 dpc. After peak viremia (25-32 dpi,
Figure 5a), pig 899 had high levels of B602L (
Figure 5f) and E120R/p14.5 (
Figure 5i) antibodies.
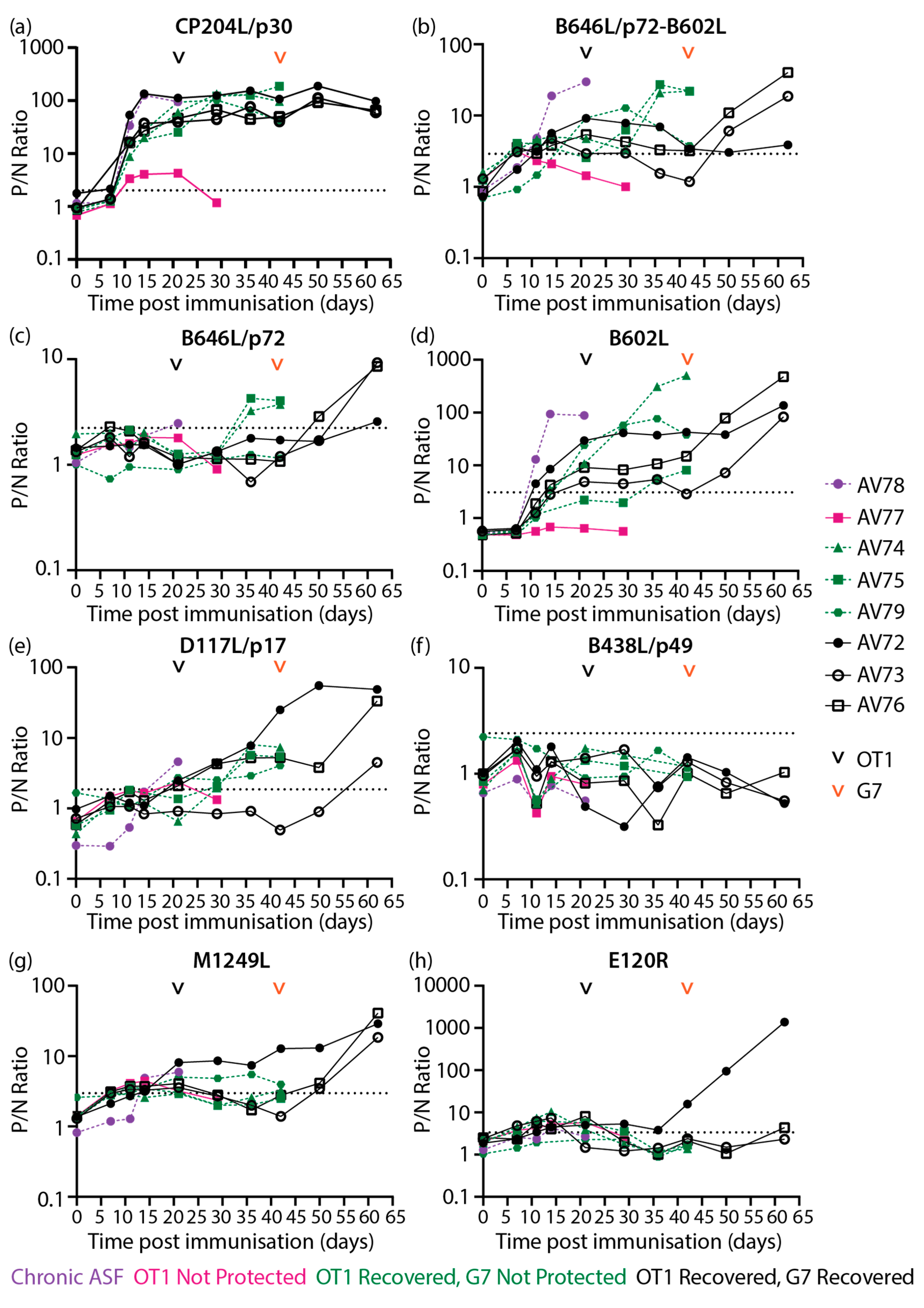
3.2. Antigen specific antibody respones of outbred domestic pigs
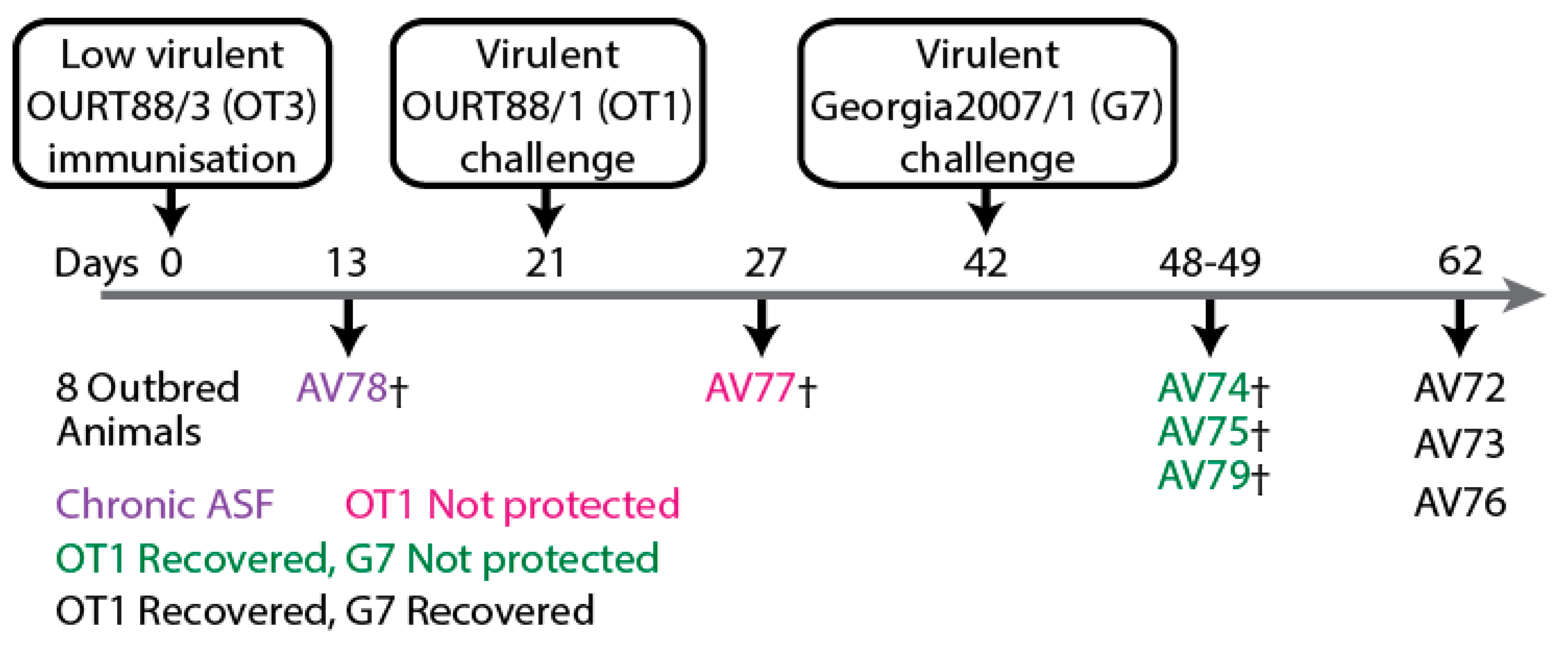
Sera samples collected from outbred domestic animals [
51] (
Figure 6) were also subjected to LACAs with ASFV capsid proteins and CP204L/p30. Antibody responses were detected against CP204L/p30 (
Figure 7a), B646L/p72-B602L (
Figure 7b), B646L/p72 (
Figure 7c), B602L (
Figure 7d), D117L/p17 (
Figure 7e), M1249L (
Figure 7g) and E120R/p14.5 (
Figure 7h). Antibodies were not quantifiable with the B438L/p49 assay (
Figure 7f), similar to observations with inbred Babraham animals (
Figure 4c).
High levels of CP204L/p30 antibodies were detected in most animals from 11 dpi and remained high throughout the study (
Figure 7a), broadly confirming previous CP204L/p30 blocking ELISA results [
51]. The differences in antibody levels identified with the B646L/p72-B602L assay (
Figure 7b) in comparison to the B602L independent B646L/p72 assay (
Figure 7c) were largely similar to results observed with inbred Babraham animals (
Figure 3c, e), where higher levels of B646L/p72 antibodies were detected when co-expressed with B602L. B646L/p72-B602L (
Figure 7b) and B602L (
Figure 7d) antibody levels increased strongly in AV78, which developed chronic ASF, and also in two of the animals that did not survive challenge with Georgia 2007/1 from 36 dpi/15 dpc OT1 (days post-challenge with OURT88/1). Antibodies against B602L independent epitopes of B646L/p72 (
Figure 7c) also increased after challenge in pigs AV74 and AV75 in a similar pattern to the B602L dependent assay (
Figure 7b). In animals that survived challenge with Georgia 2007/1, B646L/p72-B602L (
Figure 7b) and B602L (
Figure 7d) antibodies were measured on 21 dpi/0 dpc OT1. Higher B602L antibody levels (
Figure 7d) were generated in all animals within this group from 50 dpi/8 dpc G7 (days post-challenge with Georgia 2007/1) onwards, whilst elevation in B646L/p72-B602L (
Figure 7b) and B646L/p72 (
Figure 7c) antibodies were only observed in two animals within the group.
Low levels of D117L/p17 antibodies (
Figure 7e) were measured in most of the animals that survived OURT88/1 challenge and in the lone animal with chronic ASF. These levels increased in two of the animals that survived to the end of the study after challenge with Georgia 2007/1. Likewise, M1249L antibodies increased in animals that recovered from Georgia 2007/1 (
Figure 7g). Low levels of M1249L antibodies were detected in the animal that developed chronic ASF and in one of the animals that did not survive Georgia 2007/1 challenge (
Figure 7g). High levels of E120R/p14.5 antibodies (
Figure 7h) was produced in one of the animals that survived till the end of the study upon challenge with Georgia 2007/1 (42 dpi).
To facilitate comparison of clinical (
Supplementary Figure 6), virological (
Figure 8a) and antibody (
Figure 8b) data from the previous study [
51] to the results in this work, heatmaps of all relevant data sets were plotted (
Figure 8). Similar to the inbred Babraham sera samples, samples from this experiment were subjected to LACA analysis with Nluc-tagged CP204L/p30 (
Figure 8c). All animals produced CP204L/p30 antibodies by 11 dpi and antibody levels remained stable thereafter for most of the animals. AV78 developed chronic ASF after immunization with low virulent OURT88/3 and this was accompanied by an increase in ASFV antibodies at 11 dpi (
Figure 8b) and detectable viremia at 21 dpi/0 dpc OT1 (
Figure 8a). Results from the LACAs indicated that antibodies targeting CP204L/p30 (
Figure 8c), B646L/p72-B602L (
Figure 8d), and B602L (
Figure 8f) probably contributed to the increase in antibody titers in this animal. Of the animals that were challenged with OURT88/1, AV77 was the only animal that did not survive this challenge, and this can be attributed to the general poor immunological response of this animal. It had poor cellular responses as previously reported [
51] and here it demonstrated little to no antibody responses to CP204L (
Figure 8c) and any of the ASFV capsid antigens tested (
Figure 8d-i). The absence of B646L/p72-B602L and B602L antibodies in this animal (
Figure 8d, f) corresponded to observations in inbred Babraham animals, where B646L/p72-B602L (
Figure 5d) and B602L (
Figure 5f) antibodies were associated with recovery from OURT88/1 infection.
The fixed cell ELISA results (
Figure 8b) demonstrated the heterogeneity in ASFV antibody responses amongst the animals that survived OURT88/1 challenge. Of these six animals, two developed viremia (
Figure 8a) and mild clinical signs that resolved quickly (Supplementary
Figure 7). Differences between the antibody responses of the two animals (AV74 and AV75) and the rest of the animals that survived could not be identified between 21 dpi/0 dpc OT1 to 29 dpi/8 dpc OT1 with fixed cell ELISA (
Figure 8b), but B646L/p72-B602L (
Figure 8d), B646L/p72 (
Figure 8e) and B602L (only in AV74,
Figure 8f) were observed to increase to higher levels in comparison to the other survivors and this increase was detected as viremia was decreasing, corresponding to trends observed with viremic Babraham animals (
Figure 5d-f). Elevation of D117L/p17 antibodies was measured in all but one (AV73) of the survivors after OURT88/1 challenge (
Figure 8g).
Expression of B646L/p72-B602L (
Figure 8d), B646L/p72 (
Figure 8e), B602L (
Figure 8f) and D117L/p17 (
Figure 8g) antibodies did not protect three of the animals (AV74, AV75 and AV79) from Georgia 2007/1 challenge. From the current panel of ASFV capsid antigens, there are no clear differences between the animals that did and did not recover. Similar to animals that developed viremia after OURT88/1 challenge, the two animals that developed moderate viremia after Georgia 2007/1 infection and survived to the end of the study also had increased levels of B646L/p72-B602L (
Figure 8d), B646L/p72 (
Figure 8e) and B602L (only in AV76,
Figure 8f) antibodies after peak viremia was reached (
Figure 8a).
Interestingly, of the six animals challenged with Georgia 2007/1, pig AV72 had the lowest viremia and displayed delayed clinical signs (Supplementary
Figure 6). These were associated with the appearance of D117L/p17, M1249L and E120R/p14.5 antibodies. AV72 expressed D117L/p17 antibodies (albeit at low levels,
Figure 8g) as early as 21 dpi/0 dpc OT1. By the time it received Georgia 2007/1, it had the highest level of D117L/p17 antibodies amongst the challenged animals. Similar antibody kinetics were observed with M1249L (
Figure 8h). Furthermore, AV72 was the only animal to express detectable amounts of E120R/p14.5 at 42dpi (day of Georgia 2007/1 challenge,
Figure 8i) and to increase production of E120R/p14.5 antibodies in a tertiary humoral immune response.
4. Discussion
In this study we sought to resolve the ASFV antigen specific antibody responses of our previous study with inbred Babraham animals and outbred domestic animals that were immunized with low virulent OURT88/3. Using a panel of modified LACAs targeting specific recombinant ASFV capsid proteins and the known chaperone B602L, we were able to characterize a small section of the complex humoral responses in the animals against ASFV.
The earliest antibody responses identified in animals were targeting CP204L/p30 (
Figure 3,
Figure 7a), B602L (
Figure 3,
Figure 7d) and to some extent B646L/p72 (when expressed in combination with B602L,
Figure 3c and
Figure 7b) and these antibodies potentially contributed to titers observed from 11 dpi in the fixed cell ELISA data collected previously [
51] (
Figure 5b and
Figure 8b). Of the antigen specific antibody responses probed, CP204L/p30 specific antibody levels were the highest in the Babraham animals that were not protected (
Figure 3a and
Figure 5c), confirming the strong immunogenicity of CP204L/p30 [
23,
56,
57]. This is in contrast to the other antigens in the panel (
Figure 5) suggesting a contributory role of anti-CP204L/p30 antibodies to the fixed cell ELISA antibody titers observed in these animals (
Figure 5b). CP204L/p30 antibody levels were higher at 10dpi in Babraham animals that recovered from OURT88/1 in comparison to animals that were not protected (
Figure 3b and Figure 5c). Similarly, early expression of CP204L/p30 antibodies was observed in outbred animals that recovered from OURT88/1 challenge (
Figure 7a and Figure 8c), indicating that earlier expression of anti-CP204L/p30 antibodies may contribute to the reduction of OURT88/1 induced clinical signs and viremia. B438L/p49 was observed to be low or non-immunogenic in both Babrahams and outbred pigs (
Figure 4c and
Figure 7f), which is consistent with data from Lokhandwala et al. [
47]. This could be attributed to improper folding of the antigen – potentially a requirement for co-expression of a chaperone similar to B646L/p72 – or, the potential location of B438L/p49 in the overall structure of the capsid as resolved by Wang et al. [
34] with cryo-electron microscopy methods. They postulated that B438L/p49 is positioned on the inner shell of the capsid vertices, connecting the overlying penton proteins to the inner membrane. It is possible that such a location and potential steric hindrance by penton proteins hinders the development of a strong antibody response to this protein. Furthermore, antibody responses were generally slower to develop against the minor capsid proteins D117L/p17 and M1249L (
Figure 5 and
Figure 8) possibly due to their frequency and location within the virion.
The animal that developed chronic ASF (AV78,
Figure 8), increased production of B602L and B646L/p72-B602L antibodies was evident as the disease progressed. These observations are consistent with the data obtained by Reis et al. [
58] in their analysis of antigen specific antibody responses from animals that developed chronic disease after infection with the low virulent ASFV/NH/p68. Separately, the presence of B646L/p72 and B602L antibodies (
Figure 5 and Figure 8) was associated with recovery from OURT88/1 challenge in both inbred Babrahams and outbred pigs, but these were not sufficient to prevent viremia induced by OURT88/1. Furthermore, expression of B646L/p72 and B602L antibodies did not protect against Georgia 2007/1 in outbred animals (
Figure 8). Due to the small number of outbred animals and the limited panel of ASFV antigens in this study, it was not possible to differentiate antigen specific antibody responses between animals that did and did not recover from Georgia 2007/1. However, the observations of antibody responses against M1249L and E120R/p14.5 in recovered animals with reduced clinical signs and viremia after OURT88/1 and Georgia 2007/1 challenge warrant further investigation.
Similar to our previous findings with regards to the cellular responses of these animals [
51], there were marked differences in the antibody responses between Babraham animals and the outbred animals that may account for differences in clinical outcomes. Over half of the immunized Babraham animals were unable to mount a response to B646L/p72-B602L and B602L (
Figure 5d, f), despite the ability to develop CP204L/p30 antibodies (
Figure 5c). In contrast, majority of the outbred animals produced antibodies to B646L/p72-B602L and B602L (
Figure 8d, f) and the poor outcome of AV77 can be attributed to a generally poor immune response as observable in its low levels of CP204L/p30 antibodies (
Figure 8c), in comparison to the other animals in both experiments.
In these experiments, we utilized the protein sequences of the genotype II Georgia 2007/1 strain even though all the animals were primed and boosted with genotype I ASFV strains, while the outbreds were also challenged with genotype I and II ASFV. The results we and others have published previously [
6,
51,
59] demonstrated the potential of cross-protection between genotype I and II ASFV strains and here we sought to identify if genotype I trained antibody responses would be able to bind to genotype II antigens, since most of the capsid proteins are highly conserved (
Supplementary Figure 7, Figure 8, Figure 9, Figure 10, Figure 11, Figure 12 and Figure 13). In both experiments antibody responses could be detected with the genotype II recombinant proteins even against B602L which had the lowest homology between genotypes due to the presence of a central variable region [
41] (
Supplementary Figure 8). Here we confirm that cross-reactive antibodies are present for all capsid proteins assessed to be immunogenic in this work.
Vaccine research efforts have mainly focused on cellular responses [
6,
12,
14] even though robust protection against ASFV involves both humoral and cellular immune pathways [
16]. More recently, the action of antibodies induced by live attenuated vaccines has been associated with survival and protection after virulent Georgia 2007/1 challenge and the presence of ASFV neutralizing antibodies [
19]– a point of contention for decades [
20] – was described. Our work presented here demonstrate the contributions of antibody responses to protection from fatal disease and identify a subset of the contributing antigens in inbred animals and, to a lesser extent, outbred pigs. The tools presented here expand the ability to decipher the antibody responses of non-protected and convalescent animals after immunization and ASFV challenge.
In contrast to antigen specific ELISAs that have been developed using recombinant ASFV antigens in bacterial [
22,
23,
24,
26,
27] or insect cells [
27,
60,
61], the assays described here use recombinant proteins produced in mammalian cells, similar to the EP402R/CD2v ELISA developed by Lv et al. [
25] and the LIPS assay developed by Luong et al. to a panel of ASFV antigens (O61R/p12, CP204L/p30, E183L/p54, CP530R/pp62, EP153R/C-type lectin and EP402R/CD2v) [
29] to ensure that the recombinant proteins receive mammalian post-translational modifications resembling their native conformation. The ability to utilize unpurified recombinant proteins in cell lysates for the assay simplifies the process [
31,
32] and enables the development of assays using antigens that are typically difficult to purify, like membrane bound proteins or hard to express proteins with low yields, which is typical of many ASFV proteins that have immunomodulatory properties.
Unlike LIPS, LACA has a lower dynamic range due to the use of white plates coated with protein A instead of protein A resin, but this setup drastically reduces the cost and requirements for specialized equipment and filter plates. Furthermore, the use of white plates provides the option to use isotype specific antibodies for antibody capture as described by Duong et al. in their development of a LACA for antibody responses from chickens [
62], since protein A has different affinities for antibodies from different species and subclasses. Further discrimination of antigen specific antibody responses at the subclass level will help direct studies into other antibody directed innate effector functions [
63] that may contribute to protection.
Whilst improving cellular responses to vaccination is important for protection against ASFV, increased emphasis needs to be placed on exploring the repertoire of ASFV antigen specific antibody responses of vaccinated and convalescent animals to identify protective antigens and inform vaccine design for the development of a safe and efficacious ASFV vaccine.
Author Contributions
Conceptualization, PYT, CLN; methodology, PYT, JH, CLN; formal analysis, PYT; investigation, PYT, LA-A, EP, JH; writing—original draft preparation, PYT; writing—review and editing, PYT, LA-A, EP, JH, CLN; visualization, PYT; resources, CLN; project administration, PYT, CLN; supervision, PYT, CLN; funding acquisition, PYT, CLN. All authors have read and agreed to the published version of the manuscript.