1. Introduction
We have recently reviewed literature focusing on calories, carbohydrates, lipids and proteins/amino acids ratios provided by food to cancer bearing patients [
1]. We also suggested the existence of a possible energy dependent relationship (ruling reciprocal dimensions of synthesis) between high energy consuming processes, and autophagy, whose activation lowers ATP levels thus increasing AMP concentration. The attendant AMP-kinase (AMPK) activation [
1] blunts mTORC1 (mammalian target of rapamycin) dependent protein synthesis and activates the autophagic machinery, promoting
ATP refueling in continuous synchrony [
2]. We previously showed
in vitro that autophagy is triggered
by providing to cancer cells an excess of EAA [
3].
Other experimental reports have formerly described the effects on cancer cells of heightened EAA concentration [
4,
5], although in Methods it was not openly recognized that by subtracting from tested formulations some non-EAA (serine and glycine), obviously EAA percentages would have been increased, as lately acknowledged [
6] and discussed elsewhere [
7]. In the present study, we investigated whether altering significantly EAA/non-EAA ratios in diets, would influence
in vivo cancer development. Furthermore, we investigated the role of serine in cancer including such amino acid in the non-EAA smaller fraction [
7].
2. Results
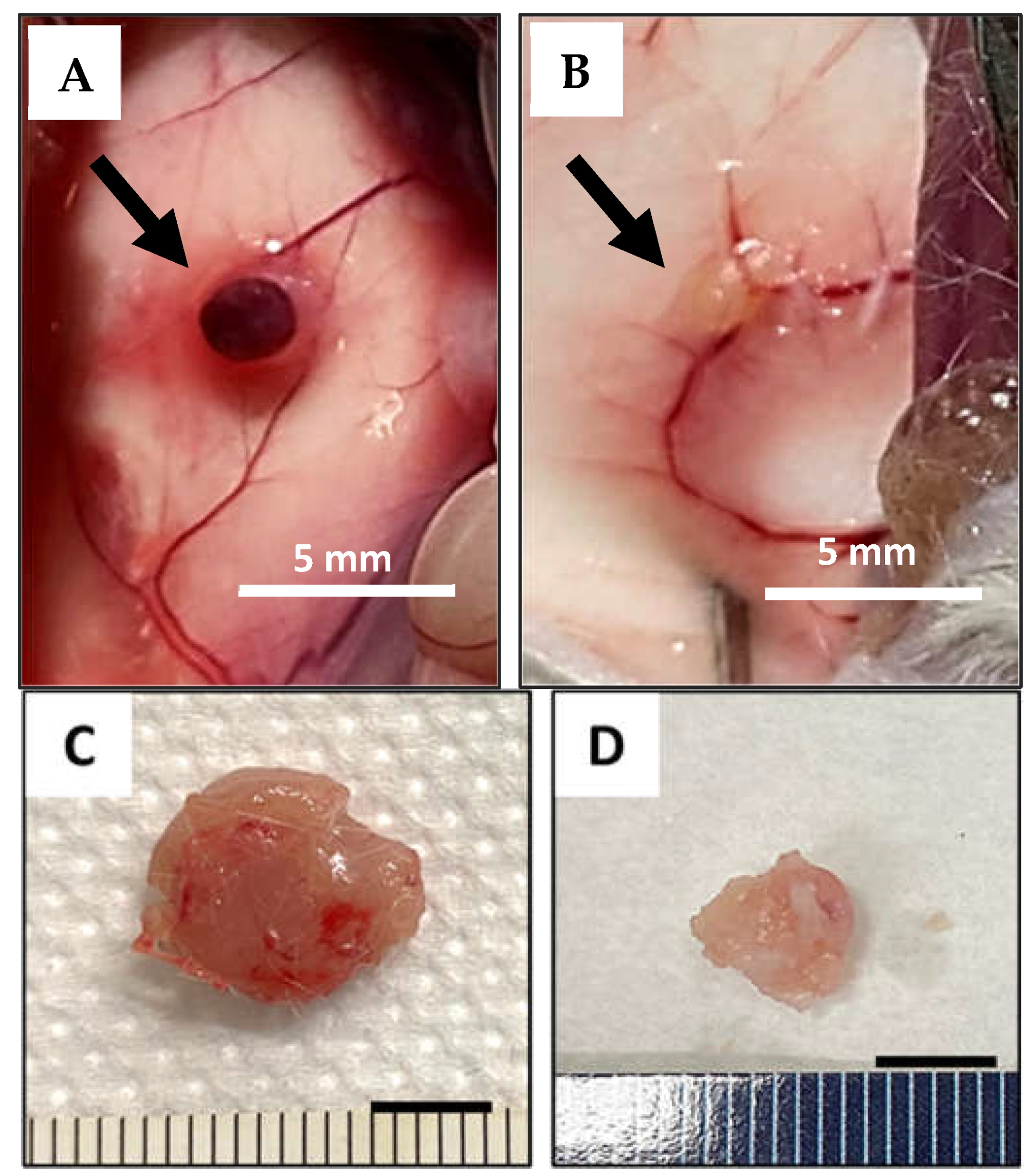
These preliminary data suggest that, among macronutrients, AA “quality” is the most determinant factor in cancer growth promoting a conspicuous slowdown in the growth of the subcutaneous tumor in animals fed with the EAArmd. Photos at the site of injection and explanted tumor mass in one sample of any group are presented in
Figure 1.
Rough data of cancers volumes (mm
3) [(maximal diameter x smallest diameter
2)/2], and weights (grams, in brackets) are presented in
Table 1.
Statistical analysis was performed by Student’s T test for paired data, two tail calculation, type 1, and standard regression test by Microsoft Office Excel. In both groups, tumor volume and weight are correlated (r = 0,83 and 0,94 respectively). Sum of volumes (1784.1 in controls and 383.8 in EAArmd) showed a ratio (4.65) strikingly most elevated in control (low EAA/non-EAA) animals. By T-test, differences between volumes in the two groups were confirmed (p < 0.0012) as well as those between weights (p < 0.03).
3. Discussion
Our preliminary data suggest that, among macronutrients, AA “quality” is a most determinant factor in cancer growth. Indeed, as already observed
in vitro [
3], altering EAA/non-EAA ratios (which is regularly <0.9) may have a deep impact on cancer biology [
7]. Such ratio can only be efficiently maintained
in vivo by proteolysis, whose intervention releases an excess of non-EAA, matching the EAA supplied by EAArmd diet. Such compensatory mechanism cannot be established
in vitro where EAA supplied to cells keep dominating the nutritional fluid composition without any chance to compensate such imbalance. Also, this study suggests that AA ratios provided by food are a most efficient determinant in eliciting modifications of metabolism in cells. Hence, macronutrients should not be considered just passive molecules used by cells according to their metabolic needs. Indeed, food quality appear to modify cell behaviors actively and consistently. Thus, epigenetic modifications, such as changes in EAA/non-EAA ratios, can be implemented and maintained as long as necessary by engineering food compositions. Conversely, subtraction of non-EAA (single or pairs) such as serine and glutamine would be an inefficient strategy unless resulting in a rise of EAA/non-EAA ratio [
7].
4. Materials and Methods
The experimental protocol was approved and conducted in accordance with laws of the Italian Ministry of Health and complied with the ‘The National Animal Protection Guidelines’. The Ethical Committee for animal experiments of the University of Brescia (OPBA) and the Italian Ministry of Health had approved the procedures (decree n. 539/2021-PR). 8 BALB/c mice were randomized to two different diets: normal diet and EAArmd diet, following already published studies on lifespan [
8]. Both diets (Dottori Piccioni s.r.l., Gessate, Milano-Italy) perfectly matched the same total macronutrients and micronutrients contents and provide same amounts of calories. Main difference was in type of nitrogen content, which was near 20% in weight, in both diets: EAA to non-EAA ratio (EAA/non-EAA) was <<0,9 in control diet, while EAArmd diet provided 84% of EAA, EAA/non-EAA <5). Small differences in nitrogen content evaluated at the end of production were linked to technical reasons in preparation of pellets and dehydration necessary to long term conservation. While normal diet respected strictly AIN76-A/NIH7 requirements, EAArmd (Nutrixam, Named S.r.i. Lesmo, Italy) provided nitrogen as free AA according the formulation presented in
Table 2.
After15 days of nutrition randomly assigned to mice, 1x105 CT26 cells, ATCC code CRL-2638™ strain Balb/c, suspended in 100μl of physiological solution were subcutaneously injected in the same position (right hip) in a group of 8 animals, 4 controls and 4 EAArmd fed. After 21 days animals were sacrificed and tumors accurately isolated, measured (latitude, length, depth) and weighted. Autopsy was performed in any animal and organs (skeletal muscle, adipose tissue, heart, kidneys, liver….) sampled and stored appropriately.
5. Conclusions
A main target of altered EAA/non-EAA is protein synthesis and autophagy balance. As we discussed elsewhere, protein synthesis and autophagy are not in opposition, but they work in a synchrony whose rhythms and intensities are dictated by ATP availability: the more ATP, the more would be implemented synthesis of proteins, which, being extremely expensive in terms of energy, would produce AMP in amounts proportional to the starting ATP concentration. The rise of AMP, in turn, would activate AMPK, which would blunt protein synthesis and activate autophagy allowing to restore ATP reserves and preventing mortal energy defaults [
1].
Thus, both the balance among activation/inactivation of the upstream and downstream components of mTORCs and AMPK pathways should be examined in the attempt to understand a) which pathway is predominant in normally fed cancer cells, and b) what epigenetic level makes EAArmd fed cancer cells metabolically fragile.
We are interested in observing whether EAArmd diet increases frequency and/or amplitude of phases of AMPK driven autophagy, and/or whether some component of mTORCs is peculiarly regulated by altered EAA/non-EAA ratio. BCL2 anti- and pro-autophagy branches should also be studied, with a focus on BH3 only BIM/BID and BAD/BAX dependent paths [
10]. Furthermore, calpains [
11] should be investigated as likely involved as epigenetic drivers of the observed differences in growth. Of interest, membrane lipid synthesis inhibition triggered by AMPK, may be modulated by EAArmd: peculiarly PLD 1 and 2 [
12] would be a main focus of our studies. Feeding cancer bearing patients with very high doses of EAA may unveil specific frailties of cancer cells, opening new options for cancer treatments.
Author Contributions
Conceptualization, F.S.D. and G.C.; methodology, G.C., C.R.; software, G.C. and C.R.; investigation, GC, CR; resources, GC; data curation, G.C. and C.R.; writing—original draft preparation, F.S.D. and G.C.; writing—review and editing, E.P., G.C., TS and C.CS; supervision, FSD; project administration, GC; funding acquisition, GC. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grant provided by NAMED S.p.a. (Milan, Italy) and by grants provided by Dolomite-Franchi S.p.a. (Marone, Brescia, Italy) to G.C.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Ethics Committee of University of Brescia, Animal Welfare Committee (OPBA) and by the Italian Ministry of Health, authorization number 539/2021-PR.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Dioguardi, F.S.; Chen-Scarabelli, C.; Pasini, E.; Corsetti, G.; Scarabelli, T.M. Diet, Muscle Protein Synthesis and Autophagy Rela-tionships in Cancer. An Attempt to Understand Where Are We Going, and Why. Adv in Nutri and Food Sci 2022, 238. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. MTOR signaling in growth, metabolism and disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Bonfili, L.; Cecarini, V.; Cuccioloni, M.; Angeletti, M.; Flati, V.; Corsetti, G.; Pasini, E.; Dioguardi, F.S.; Eleuteri, A.M. Essential amino acid mixtures drive cancer cells to apoptosis through proteasome inhibition and autophagy activation. FEBS J. 2017, 284 (11), 1726-1737. [CrossRef]
- Maddocks, O.D.K.; Berkers, C.R.; Mason, S.M.; Zheng, L.; Blyth, K.; Gottlieb, E.; Vousden, K.H. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature 2013. 493 (7433) :542-6. [CrossRef]
- Newman, A.C.; Maddocks, O.D.K. One-carbon metabolism in cancer. Br J Cancer 2017, 116, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Maddocks, O.D.K.; Athineos, D.; Cheung, E.C.; Lee, P.; Zhang, T.; van den Broek, N.J.F.; Mackay, G.M.; Labuschagne, C.F.; Gay, D.; Kruiswijk, F.; Blagih, J.; Vincent, D.F.; Campbell, K.J.; Ceteci, F.; Sansom, O.J.; Blyth, K.; Vousden, K.H. Corrigendum: Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature 2017, 548 (7665): 122. Erratum for: Nature. 2017, 544(7650): 372-376. [CrossRef]
- Dioguardi, F.S.; Flati, V.; Corsetti, G.; Pasini, E.; Romano, C. Is the Response of Tumours Dependent on the Dietary Input of Some Amino Acids or Ratios among Essential and Non-Essential Amino Acids? All That Glitters Is Not Gold. Int J Mol Sci. 2018, 19 (11): 3631. [CrossRef]
- Romano, C.; Corsetti, G.; Flati, V.; Pasini, E.; Picca, A.; Calvani, R.; Marzetti, E.; Dioguardi, F.S. Influence of Diets with Varying Essential/Nonessential Amino Acid Ratios on Mouse Lifespan. Nutrients 2019, 11 (6) :1367. [CrossRef]
- Dioguardi, F.S. Clinical use of amino acids as dietary supplement: pros and cons. J Cachexia Sarcopenia Muscle 2011, 2, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Pawloski, J.; Kraft, A.S. Bax-induced apoptotic cell death. PNAS 2000, 97, 2: 529-531. [CrossRef]
- Shapovalov, I.; Harper, D.; Greer, P.A. Calpain as a therapeutic target in cancer. Expert Opinion on Therapeutic Targets, 2022. 26, 3, 217-231. [CrossRef]
- Foster, D.A.; Salloum, D.; Menon, D.; Frias, M.A. Phospholipase D and the Maintenance of Phosphatidic Acid Levels for Regula-tion of Mammalian Target of Rapamycin (mTOR). J Biol Chem. 2014, 289, 33: 22583–22588. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).