1. Introduction
Currently, much attention is given to producing safe and clean food. Although there has been a change in diets in developed countries, causing a decrease in meat consumption per capita, global meat consumption is still significantly increasing due to the development of under-developed countries and population increase [
1]. This increased demand for food, including chicken eggs and meat, has led to a high specialization of chicken breeds (i.e., genetic lines providing high food quantities with the least resources possible) and extensive poultry industry mechanization [
2]. However, male chicks have no use in the industry since they do not lay eggs, and their meat is not appreciated by typical consumers [
3]. Hence, male chicks are killed upon hatching (known as male chick culling) and used for other purposes, e.g., zoo animal feed [
1]. Nevertheless, male culling raises ethical and economic issues among consumers, who prefer food from animal-friendly methods [
4], and among the industry who sees the culling as an economic burden since half of the incubators (culled males) hardly generates any revenue [
1,
2]. Therefore, in some European countries (e.g., Germany [
5], France [
6], and Italy [
7]), there is an agenda for a more environmentally and animal-friendly poultry industry. In Germany, culling has been prohibited since the beginning of 2022 [
5].
In this context, two solutions were proposed to tackle the chick culling issue: 1) raising males from layer breeds or dual-purpose chickens with males and females being used for respectively meat and eggs, although this results in a higher environmental impact and mainly serves niche market purposes since most consumers do not appreciate the product [
8]; and 2)
in ovo sexing – methods used to determine the embryo's sex before hatching. Even though the technology cost can influence the final price for consumption eggs, surveys showed that this latter solution pleases the consumers (when performed in an early incubation stage, i.e. before the onset of embryonic pain perception) and the industry the most, allowing them to use male eggs for animal feed and to fill the incubators with only females [
4]). Regarding pain perception, it is accepted that this might start after day 7 when the multisynaptic reflex arches are closed, making the embryo sensitive to mechanical stimuli [
9]. However, encephalogram signals are visible only after day 14, and it is expected that chicken embryos will start feeling pain from that moment onwards [
10].
Nevertheless, to gain broad market acceptance,
in ovo sexing technologies must possess the following characteristics: 1) work with all colors of eggs (e.g., white and brown eggs), 2) present accuracies higher than 98.5 %, (i.e., the same accuracy of a sexing expert), 3) identify the sex at the earliest possible stage (preferably before possible onset of pain), 4) have high throughput (20,000 to 30,000 eggs per hour), 5) avoid disturbing the embryos or their hatchability, and 6) have a low impact on the production cost [
1,
11]. Currently, only a few technologies are being used in the industry, even though they do not comply with all the mentioned requirements.
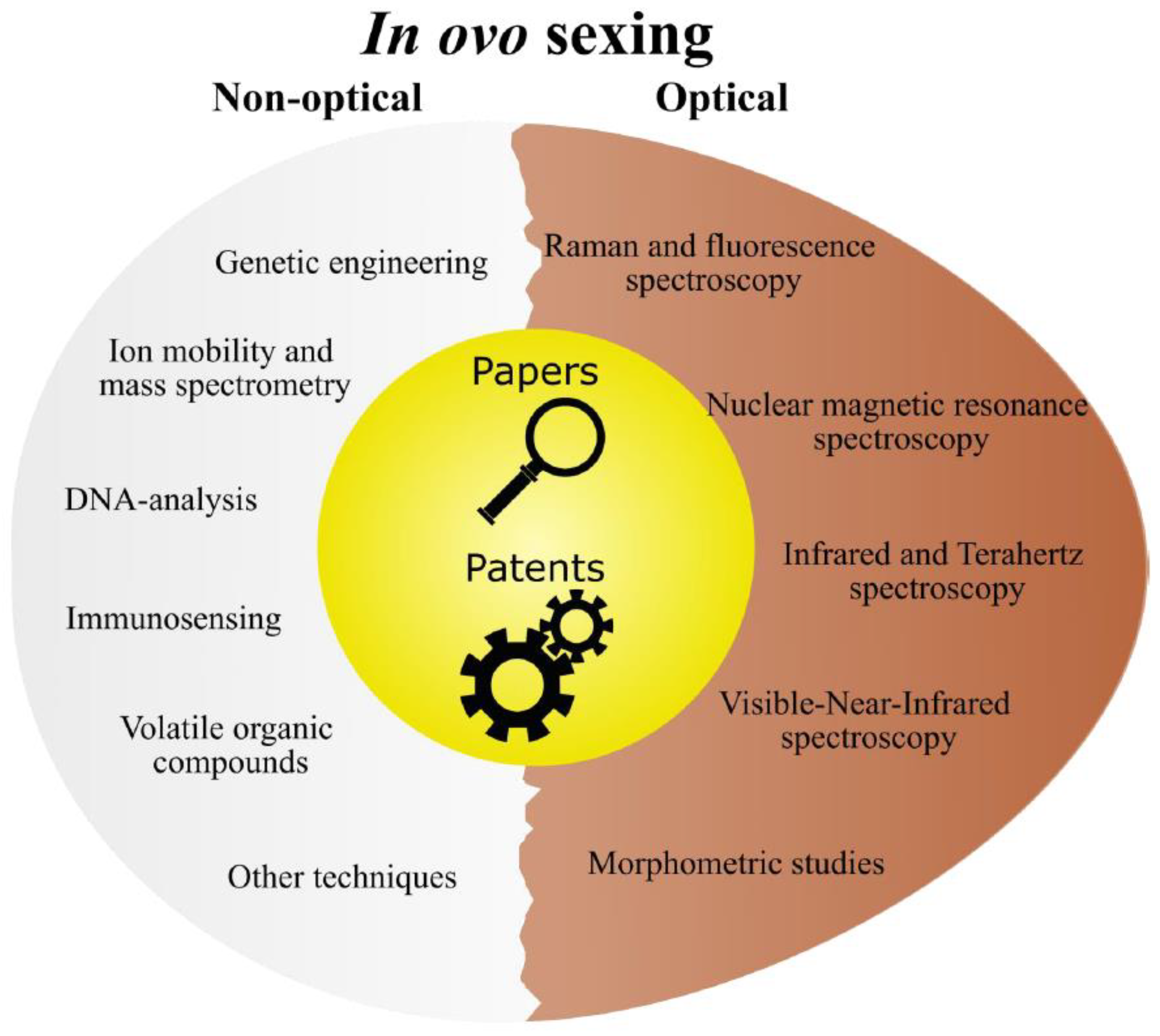
This review intends to give an overview of the history and current trends of
in ovo sex identification techniques. Unlike previous reviews, this work combines the paper and patent literature and aims to reveal the in-depth relationship between innovations from the academic and industrial side. Conventional qualitative analysis based on scientific content is followed by quantitative analysis on origin, submission year, and current status. The search method and final search keys can be found in Section S1 of the Supplementary materials. Similar to the latest review on
in ovo sexing techniques by Krautwald-Junghanns et al. [
12], we grouped our 11 technology categories into six non-optical and five optical techniques (
Figure 1).
2. Non-optical in ovo sexing methods
The non-optical in ovo sexing technologies (summarized in Table 2) mainly comprise invasive approaches, such as sample extraction, marker insertion, or shell windowing for vessel screening. This section includes a discussion on 1) DNA analysis and 2) immunosensing, both being standard in ovo sexing methods, 3) ion mobility spectrometry (IMS) and mass spectrometry (MS; measuring the presence of hormones or metabolites), an overview of 4) genetically modified organisms (GMO) and 5) Volatile Organic Compounds (VOC) as gases emitted through the eggshell, next to a compilation of 6) other techniques not included in the previous five categories.
2.1. DNA analysis
DNA analysis using polymerase chain reaction (
PCR) followed by band observation with gel electrophoresis has been used as an
in ovo sexing technique relying on the sexual gametes heterogeneity between males (ZZ) and females (ZW) [
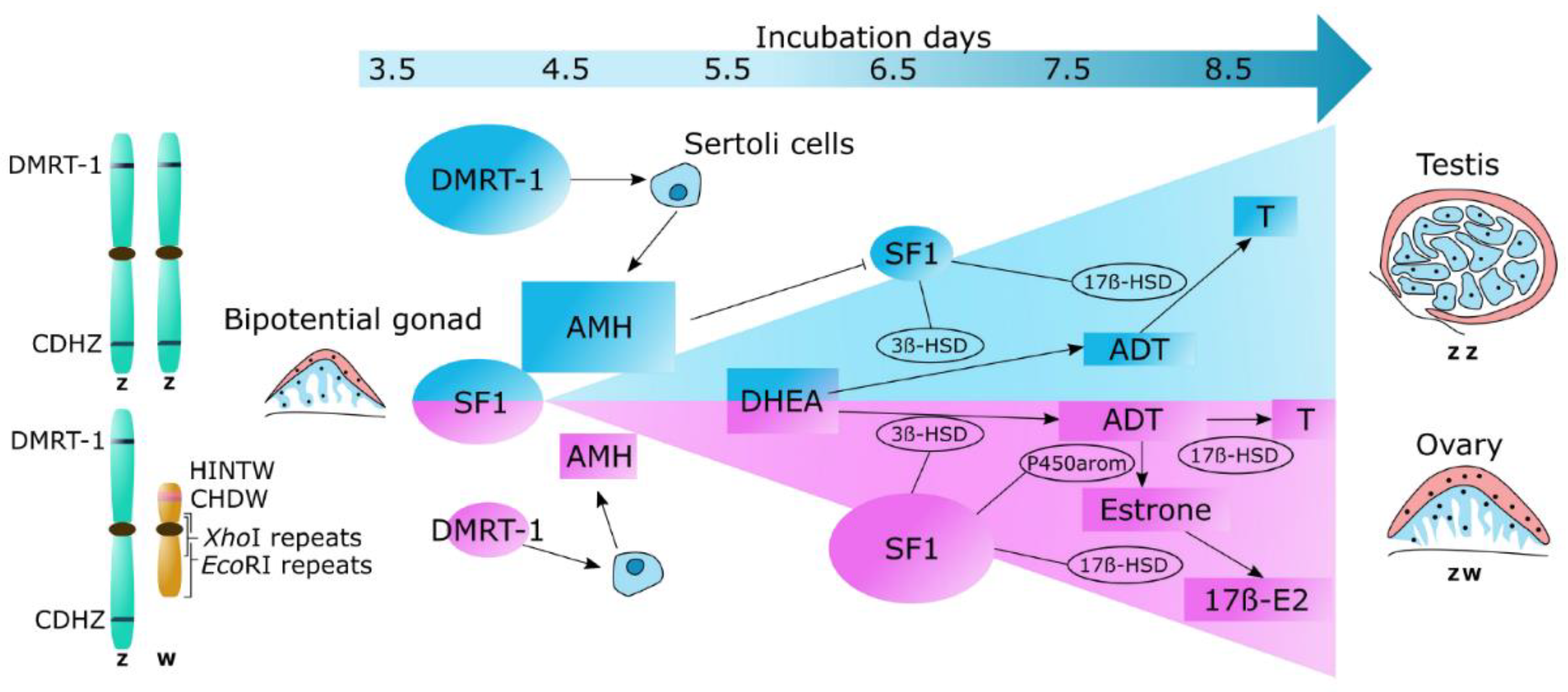
13]. Due to this heterogeneity, it is possible to perform sexual detection using W chromosome genes. In the literature, six distinct genes have been reported (
Figure 2): doublesex and mab-3 related transcription factor 1 (
DMRT-1) and chromodomain helicase DNA (
CHD)-Z from the Z-chromosome, and
CHD-W, W-protein kinase C iota (
WPKCI),
XhoI family, and
SWIM from the W-chromosome. Interestingly, this detection method has been applied using distinct types of tissues from the embryo (e.g., blood, muscle, feathers, or brain).
Griffiths Richard [
14] patented the first female-specific gene for
in ovo sex identification (discontinued in 2004), using CHD from genomic DNA (
gDNA) (the patent also included antibodies use for the encoded proteins, which lays outside of this section’s scope). A paper by Li et al. [
15] aligns with the claims in the previous patent using the sequences
CHDZ and
CHDW with several duck species’ samples such as blood and feathers (adults), chorioallantoic membrane (day 13), and allantoic fluid (
AF) (days 8, 10 and 13). The report’s main outcome was an assay with a 100 % correct identification rate based on 256 female and 256 male analyzed samples.
Another example of a commonly used gene is the
XhoI repetition family. Petitte and Kegelmever [
16] published one of the first papers on this, showing that
XhoI was specific for female samples, enabling DNA detection as low as two cells per sample. Further, other
XhoI repetition family examples were presented by Haunshi et al. [
17] and Clinton et al. [
18], the latter using an isothermal PCR-free approach and fluorescence resonance energy transfer (
FRET) method in blood samples, with real-time results observation in 15 minutes.
Another possibility was presented when Michael Clinton [
19] protected a new method claiming a new nucleic acid probe to detect
WPKCI in AF or amniotic fluid, tissue, and organs. Furthermore, in He et al. [
20], quantitative PCR (
qPCR) and a new female-specific gene sequence (i.e.,
SWIM) were used for the first time for
in ovo sexing on day 9. The results showed no misidentification and a low detection limit (as low as 1 ng of total DNA) with crude brain samples.
In conclusion, so far, the methods in papers rely on highly invasive samples (i.e., inadequate for
in ovo sexing). Contrary to this, AF is an extra-embryonic sample offering relatively easy sampling, low invasiveness and low hatchability effects. Furthermore, the industry has higher demand for non-invasive methods, compared to research. Thus, except for one paper [
15], DNA analysis from AF was only reported in patents. Despite that, DNA analysis methods have a high accuracy (≈ 100 %) and are applicable across several breeds due to the presence of conserved genes. However, although presently the company PLANTegg applies PCR for
in ovo sexing (PlantEgg, GmbH, 2022), its industrial application is restricted because of the lengthy process time (> 50 minutes) and prices (≈ 4 – 5 €/day-old-chick) [
21].
2.2. Immunosensing
Hormones play an essential role in chickens' sexual differentiation, with concentrations changing during incubation and growth of the fetus. A simplified cascade of these main hormones is depicted in
Figure 2. These hormones are mainly gauged
via competitive enzyme-linked immunosorbent assay (
ELISA) or radioimmunoassay (
RIA) due to the high accuracy and sensitivity of these assays. Competitive ELISA or RIA assays are based on the competition between the ssample target and the same labeled target for specific antibodies, whereas the label for a target can be either a fluorescent enzyme (ELISA) or radioisotope (RIA).
Table 1 gives a brief overview of papers and patents with the hormones analyzed using immunoassays.
The first paper regarding the use of immunoassays dates back to 1979 when Tanabe et al. reported detecting hormones from embryos (day 17 and 20) [
23]. Moreover, a paper from Gill et al. [
24] showed the possibility of detecting several hormones from day 10 and 14 (
Table 1), while showing the hormonal level increase during the incubation stages. Furthermore, in 1998, Patricia Phelps protected [
25] and published [
11] a method measuring estrogens and androgens in extra-embryonic fluid (e.g., AF). Tyczkowski [
26] patented another method in 2006, describing an assay type to detect estrogenic steroid compounds using microparticles and a specific fluorescently labeled binding protein.
Furthermore, Butt and Tran [
27] patented, in 2002, a new yeast-based assay type for detecting E2 in AF on day 17, claiming higher hormone concentrations in females. Results supporting this patent's claims can be found in a paper from 2010 [
28]. However, in 2017 Einspanier [
29] protected a method that would allow
in ovo sexing based on the E1S concentration in AF extracted from the egg on days 9 and 10. The patent was corroborated by Weissman et al. [
30], which showed significant concentration differences for E1S at day 9 and E2 at day 10. This scientific report is currently considered a standard golden method for AF extraction and sex determination through hormone concentration measurement in the AF sample.
Some other studies show the presence of hormones in yolk [
31,
32] and serum [
33]. First, in 2002, Müller et al. [
31] studied the relationship between maternal social status and the deposited concentrations of androgens (see
Table 1) in freshly laid eggs, concluding that the androgens concentrations in yolk were insufficient for sex differentiation. Second, in 2013, Aslam et al. [
32] showed that hormone yolk concentrations were correlated with glucose concentration and unincubated egg dimensions and weight. Finally, Wang et al. [
33] showed differences from days 8 to 16, concluding that T, dihydrotestosterone (
DHT) and E1 were correlated with sex, and the differences between hormone concentrations varied with the incubation days.
Moreover, a strategy that stands out from the standard ELISA or RIA, while using antibodies, was used by Decuypere and Fey [
34], who filed a US patent related to fluorescently labeled antibodies inserted in the egg albumen before incubation. The antibody was specific for several genes encoded proteins from the W-chromosome (e.g.,
CHDIW,
ASW), or the Z-chromosome (e.g.,
DMRT-1), or hormones, e.g., anti-Mullerian hormone. However, as discussed in the genetic engineering section, the consumers and industry still question approaches that need the introduction of extra components in consumption products [
35].
In summary, the present section showed that day 9 was the earliest day on which it was possible to make this differentiation with E1S (the most promising hormonal biomarker) [
21,
30]. Moreover, the company Seleggt (Seleggt Gmbh, Cologne, Germany) applies ELISA for
in ovo sexing. Considering market application, the two challenges for this technology are the considerable cost of ELISA or RIA (~4 €/day-old-chick) and the duration of analysis (> 45 min), excluding egg handling and sample extraction.
Table 1.
Overview of papers and patents related to immunosensing strategies for in ovo sexing with hormones.
Table 1.
Overview of papers and patents related to immunosensing strategies for in ovo sexing with hormones.
| Hormone |
Sample |
Detection (days) |
Remarks |
Reference |
| Papers |
| Luteinizing hormone, T, E2, P4 |
Blood |
17 and 20 |
Detection with RIA
Progesterone and estradiol with the bigger differences between males and females |
Tanabe et al. [23] |
|
E1, E2-17β, E2-17αsulphate, E1, E2-17β, E2-17αglucuronide
|
AF |
8 to 17 |
Detection with RIA |
Gill et al. [24] |
| T, A4 |
Yolk |
|
Differences were not sufficient for sex determination |
Müller et al. [31] |
| E2 |
AF |
17 |
Detection with RIA
Significant differences |
Phelps et al. [11] |
| E2-17ss |
AF |
17 |
Use of a yeast transactivation assay
Significant differences |
Tran et al. [28] |
| T, E2, A4, P4, DHT |
Yolk |
|
Differences were not sufficient for sex determination |
Aslam et al. [32] |
| E1S, E2, T |
AF |
9 and 10 |
Detection with ELISA
Significant differences for E1S on day 9 and for E2 on day 10 |
Weissmann et al. [30] |
| E2, T, E1, A4, DHT |
Serum |
8 to 16 |
Detection with ELISA
Significant differences with T, DHT, and E1 |
Wang et al. [33] |
| Patents |
| E2, E2-17ss, estriol, E1, T, DHT |
AF |
11 to 19 |
|
Phelps [25] |
| E2, E2-17ss, estriol, E1, T, DHT |
AF |
11 to 19 |
|
Tyczkowski et al. [26] |
| E2 |
AF |
17 |
|
Butt and Tran [27] |
| E1S |
AF |
9 and 10 |
|
Einspanier [29] |
2.3. Ion mobility- and mass spectrometry
Analytical techniques such as IMS and MS for hormones and metabolites have also been described as potential
in ovo sexing methods. Both techniques require sample vaporization, ionization, and, subsequently, analyte separation based on mass and charge [
36]. In 2002, a patent was filed for detecting E2 concentration differences in AF of five males and females using IMS [
37]. However, the inventors were not certain whether these signals were E2-specific since they could not be related to the fingerprint signal of E2 in a standard solvent (i.e., methanol or acetone). Furthermore, the inventors admitted that the dataset was limited and required more measurements to prove the technique's robustness. Suggested improvements for future experiments were cleaning with a blank solvent between the egg fluid measurements (risking lengthening the analysis time) and combining the IMS with an MS. Usually, coupling of the two devices (i.e., hyphenation) is performed to deliver better results for more complex matrices [
36].
Next, an MS approach was developed, and the inventors identified a sexual-discriminating, non-proteinogenic metabolite named 3-[(2-aminoethyl) sulfanyl] butanoic acid [
38], with higher concentrations in females and more than 90 % accuracy on day 9. In combination with another non-specified biomarker, this accuracy improved to more than 95 % on day 10. The inventors also recommended using hyphenated systems and thereby managed to analyze a sample in less than 10 seconds [
38]. Next to the DNA analysis and the immunosensing technique, this MS method is one of the three commercial techniques used on day 9 by the company In Ovo (In Ovo B.V., Leiden, The Netherlands).
The analyzed literature shows that IMS can operate with air or nitrogen ambient pressure and temperature. In contrast, the MS operates in a high vacuum and uses special carrier gases such as helium. However, MS instruments are more accurate but also have a higher operational cost [
36]. Compared to DNA analysis and immunosensing (see
Table 2), IMS and MS do not require sample preparation or incubation, allowing direct sample measurement resulting in a shorter analysis time. These features allow fast acquisition of sex-specific compounds (e.g. hormones or metabolites) in AF of chicken embryos. Furthermore, consumable costs are lower since no primers or antibodies are required compared to the previously described methodologies. An amelioration for the IMS and MS approach would be the investment in scientific research to identify and improve understanding of the measured physiological substances.
2.4. Genetic engineering
There are still several controversies around the use of GMOs for consumption products. Genetic engineering methods modify the animal (or plant) genome by introducing, eliminating, or rearranging specific genes [
39]. In the context of
in ovo sexing, the primary strategy is detecting a label targeting male embryos' genomes. However, GMOs gather different opinions from country to country. It is possible to classify countries into 1) allowing GMOs, 2) not allowing production but allowing imported GMO products, and 3) blocking both production and import [
35]. Section S2 of the supplementary materials presents a list of these countries.
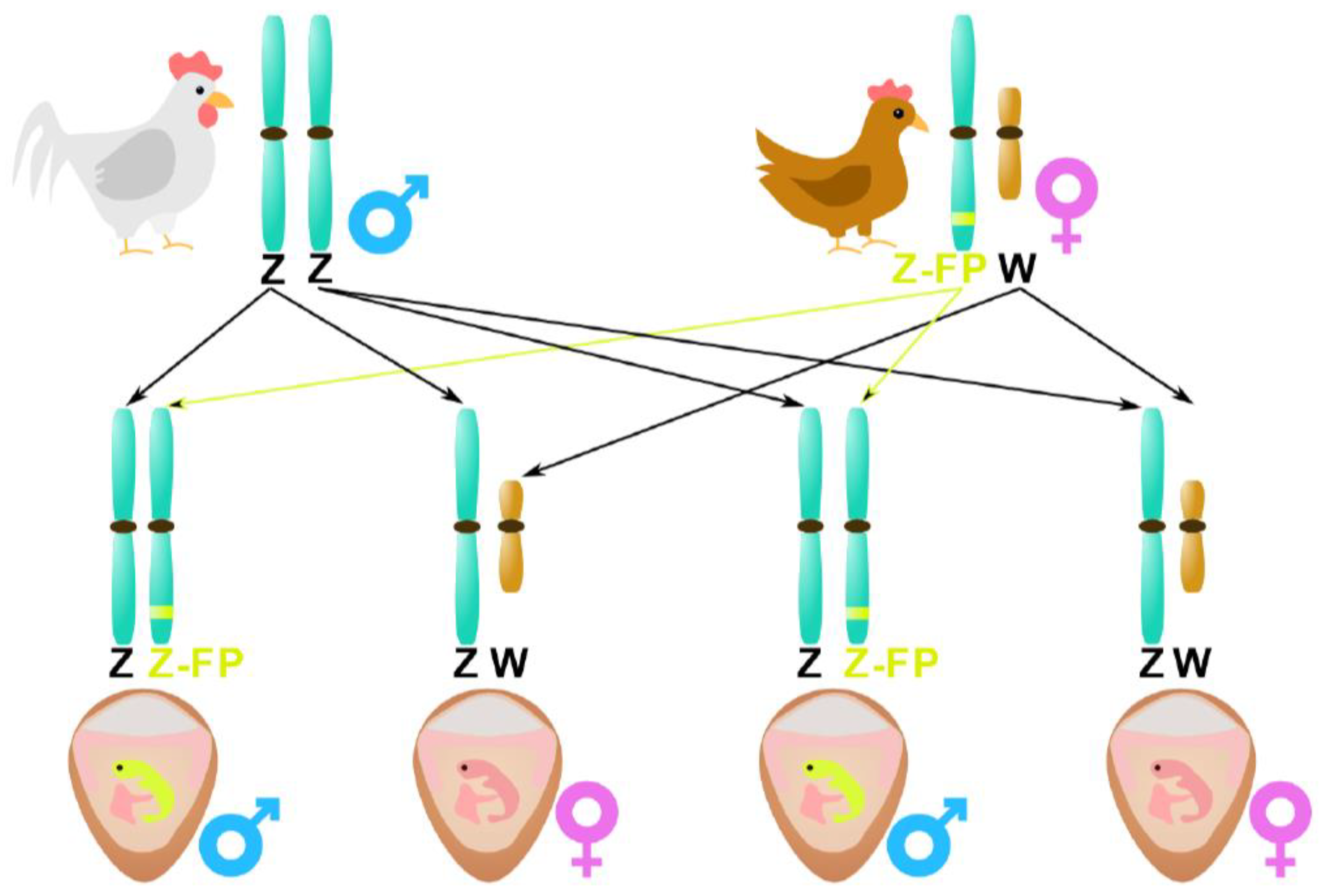
In Doran et al. [
40], the fluorescent gene was inserted into the Z-chromosome of the mother hen. The authors performed
in vivo transfection of primordial germ cells (
PGCs; i.e., gamete precursors showing unique migration activity) into the mother hen at an embryonal stage. Because the target was the mother Z-chromosome, only male embryos from that hen contained the tagged gene, as depicted in
Figure 3 In this context, inventor Offen [
41] patented a similar method by the transfection of PGCs with a fluorescently labeled reporter gene. In this case, the PGCs were inserted in the egg on day 2.5, and the reporter gene was incorporated into the embryo genome with a CRISPR/Cas9 gRNA (i.e., injected guide RNA) method. This technique is commercialized by the company eggXYt (eggXYt Ltd., Jerusalem, Israel). Some other techniques were also patented, such as a gene introduction in the broiler hen’s Z-chromosome, leading to the production of a specific protein lethal to the embryos steering to the “natural” death of male embryos [
42].
From the available data, only one patent was abandoned until now, whereas the remaining are still active or in the filing phase. This shows that, even though questioned by consumers and prohibited in some countries, GMOs remain a proper alternative to male chick culling [
1]. However, a large imbalance exists between the number of patents and scientific papers, with only one paper to date. Hence, more research should be performed on these practices, including the effects of adding fluorescent markers on chickens and consumers. Despite that, as shown in
Table 2, genetically engineered cells allow sexual sorting on day 2.5 whilst maintaining an accuracy close to 100 %.
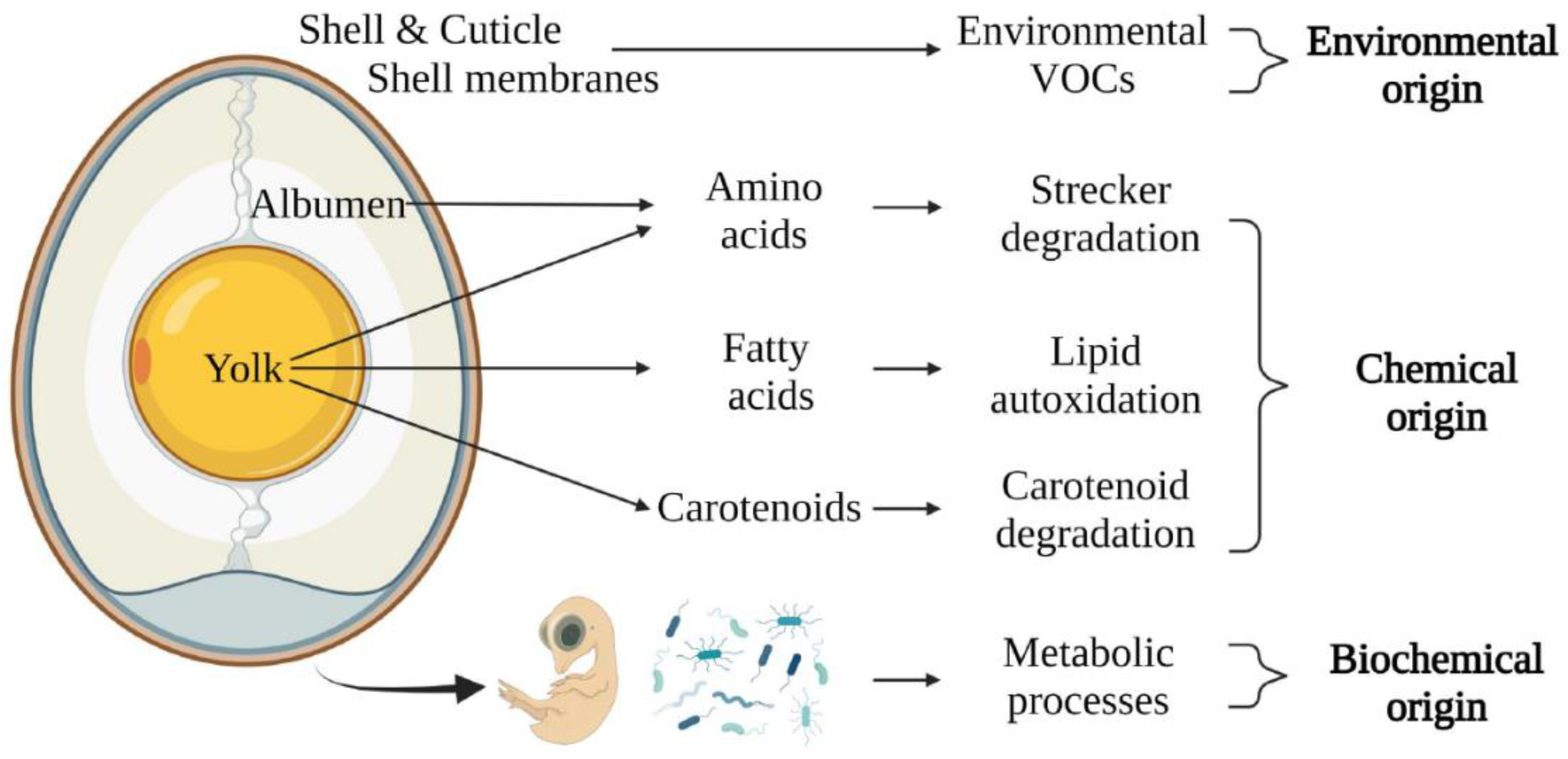
2.5. Volatile Organic Compounds
More recently, researchers also started working on detecting VOCs from avian eggs. These gases are released from the eggshell and can have different origins (e.g., environmental, chemical- or biochemical origins;
Figure 4). Furthermore, it has been demonstrated that the abundance of specific VOCs differs significantly between sexes during incubation [
43,
44].
Conventionally, the eggs are enclosed in a recipient to measure these VOCs. After a specific incubation time, extraction is performed by exposing the egg's equilibrated headspace to a sorbent (e.g., solid-phase micro-extraction fiber) or by performing a direct headspace analysis. Relative to the slower gas chromatography-mass spectrometer (
GC-MS), faster acquisition techniques are described in the patent literature for measuring egg volatiles, e.g., 1) chemical ionization techniques such as proton transfer reaction-mass spectrometry (
PTR-MS), 2) selected ion flow tube-mass spectrometry (
SIFT-MS) [45], or 3) optical techniques such as Terahertz (
THz) spectroscopy [
46,
47,
48]. The latter optical technique was included in this section since it is used explicitly for VOC sexing. Currently, no VOC
in ovo sexing system is commercially available.
VOC
in ovo sexing has the potential of non-destructively measuring emitted gases from the egg in an early stage or even on day 0 before the incubation starts (
Table 2) [
49]. Little is known about the models' robustness and if their accuracies are reproducible. This inherently depends on the limited dataset sizes due to the speed of conventional techniques that analyze at a rate of tens of minutes to one hour. Using faster acquisition techniques is expected to allow for more data and faster analysis times (in terms of seconds), which is also crucial for commercial implementation.
2.6. Other techniques
This section groups different
in ovo sexing approaches described in the literature with a unique methodology or without a defined category. Remarkably, the oldest patents were defined within this group, such as measuring magnetic polarity [
51,
52,
53,
54]s or using a galvanometer [
55]. Furthermore, no paper was reported on these pioneering
in ovo sexing technologies, and their patent protection has expired or is inactivated due to non-payment [
56].
The following two concepts are more recent but did not provide experimental data. The first approach utilized a plaster with a needle inserted through a hole in the eggshell. At the tip of the needle, a fluorescent marker signal could be read once in contact with a sex-specific molecule such as T or E2 [
57]. Although the inventors did not specify the marker, it is expected that it might be related to an immunosensing approach. Second, with an electrode close to the shell, a passive recording of the dynamic electromagnetic spectrum on the egg surface would allow sexual sorting
prior to incubation. According to the inventors, this electromagnetic field was intrinsically formed through the egg's biological, biochemical, and electrochemical processes [
58]. Whereas the patent with the plaster approach was granted, the second one is still under examination. These techniques have not been explored and might require more study. To conclude this section, an overview of all non-optical
in ovo sexing techniques is presented in
Table 2.
Table 2.
Overview of non-optical in ovo sexing techniques.
Table 2.
Overview of non-optical in ovo sexing techniques.
| Category |
Target |
Incubation day
Accuracy
Throughput
|
Technology (patents)/ research gaps (papers) |
Advantages (+)/
Drawbacks (-)
|
References |
| DNA analysis |
gDNA (e.g., CHD, XhoI, SWIM, HINTW) from different samples (e.g., AF, blood, feathers, brain) |
Days 8 to 13
100 %
Slow (slower in case of need for DNA extraction), PCR can take more than 60 minutes |
Technology > Research
Lack of papers using AF
Several possible female-specific sequences are not reported in the literature
|
+
High accuracy
Early in incubation (> day 2.5)
-
Lengthy processes and expensive
Can be highly invasive if not using AF |
Patents
⊗ Griffiths [14], ⊗ Clinton [19], ∅ Dalton et al. [59], ∅ Arie and Danielli [60], ∅ Molina and Espeut [61]
Papers
Li et al. [15], Haunshi et al. [17], Clinton et al. [18], He et al. [20] |
| Immunosensing |
Sexual hormones (e.g., E2 or estrone-3-sulfate) in AF or blood |
Minimum day 8
Reported as 99 % on day 9
Relatively slow using ELISA/RIA, which can take up to 90 minutes. |
Technology ≅ Research
Well defined in both papers and patents. |
+
Applicable in AF
-
Limited to after day 8 of incubation.
Lower accuracies than DNA analysis
Expensive and lengthy testing. |
Patents
∅ Tyczkowski et al. [26], ⊕ Butt and Tran [27], ∅ Einspanier [29], ⊕ Phelps [25]
Papers
Phelps et al. [11], Tanabe et al. [23], Weissmann et al. [30,62], Müller et al. [31], Aslam et al. [32], Wang et al. [33] |
| IMS and MS |
Metabolites in AF |
Days 9 to 10
90 % to higher than 95 %
Relatively fast |
Technology > Research
Only defined by two different groups of inventors in the patent literature
No work has been published in the scientific literature |
+
Quick sampling and relatively fast analysis
-
No scientific literature on physiological background |
Patents
∅ Daum and Atkinson [37], ∅ Bruins and Stutterheim [38,63], ∅ Stutterheim [64]
Papers
- |
| Genetic engineering |
gDNA |
Days 0 to 2.5
100 %
High if using labels |
Technology > Research
Only one short communication in papers, mainly present in patents. |
+
High accuracies at an early incubation stage.
-
Not accepted in several countries (see text)
Invasive injection of a labeled molecule (antibody/DNA sequence) |
Patents
∅ Decuypere and Fey [34], ∅ Offen [41], ⊗ Beisswanger [42]
Papers
Doran et al. [40] |
| VOC analysis |
VOCs released from the egg |
Day 0 or during incubation
Accuracy not defined
Seconds to minutes |
Technology > Research
Patents use real-time analysis systems such as THz spectroscopy, SIFT- or PTR-MS
Research papers use conventional SPME-GC-MS analysis and more research is required for generating robust sexing models |
+
Non-invasive
Potential for early-stage application
-
Contamination risk of egg odor by environment or other eggs |
Patents
∅ Rivers [45], ∅ Gabbai [48], ∅ Knepper et al. [65], ⊕ Tongli et al. [66]
Papers
Webster et al. [43], Costanzo et al. [44], Xiang et al. [49] |
| Other |
1) Electrical and magnetic polarity of the egg
2) Dynamic electromagnetic spectrum
3) Sex-specific compounds close to the eggshell |
Day 0
Accuracies unknown
No throughputs defined |
Technology > Research
Only defined in different patents and no scientific literature |
+
Before incubation
-
No scientific evidence |
Patents
⊗ Young [51], ⊗ Williams [52,53], ⊗ Chadfield [55], ⊗ Pacala et al. [56], ⊕ Visser [57], ∅ Rastoutsau et al. [58]
Papers
- |
| Abbreviations: d, day; gDNA, genomic DNA; AF, allantoic fluid; E2, estradiol; ELISA, enzyme-linked immunosorbent assay; RIA, radioimmunoassay; THz, terahertz; SPME-GC-MS, solid phase micro extraction–gas chromatography-mass spectrometry; SIFT-MS, selected ion flow tube-mass spectrometry; PTR-MS, proton transfer reaction-mass spectrometry; IMS and MS, ion mobility and mass spectrometry; ⊕, granted; ∅, pending; ⊗, ceased/rejected/discontinued. |
3. Optical in ovo sexing methods
This section describes the optical
in ovo sexing techniques. Optical techniques are considered to be both physical (i.e., the interaction between the sample and electromagnetic radiation) or geometrical (i.e., imaging) [
67]. These approaches are generally non-invasive and allow fast signal acquisition. Although in specific situations (e.g., Raman spectroscopy), a hole must be made through the shell or the membrane to access internal structures inside the egg. Similar to the previous section,
Table 3 summarizes the different approaches.
3.1. Raman and fluorescence spectroscopy
Generally, Raman spectroscopy is used after excitation with a monochromatic laser, having identical energy photons to analyze the frequencies of scattered radiation, obtain information about molecule energy levels, and study molecular vibrations and rotations. The broader fluorescence background intensity is directly re-emitted energy [
68].
In ovo sexing Raman spectroscopy has been applied in an invasive and non-invasive way. The former analyzes the hemoglobin content in blood vessels [
69] or germinal disk cells [
70], and the latter focuses on sex hormones or other sex-related compounds in the eggshell [
71]. Both methods are further explained in this section.
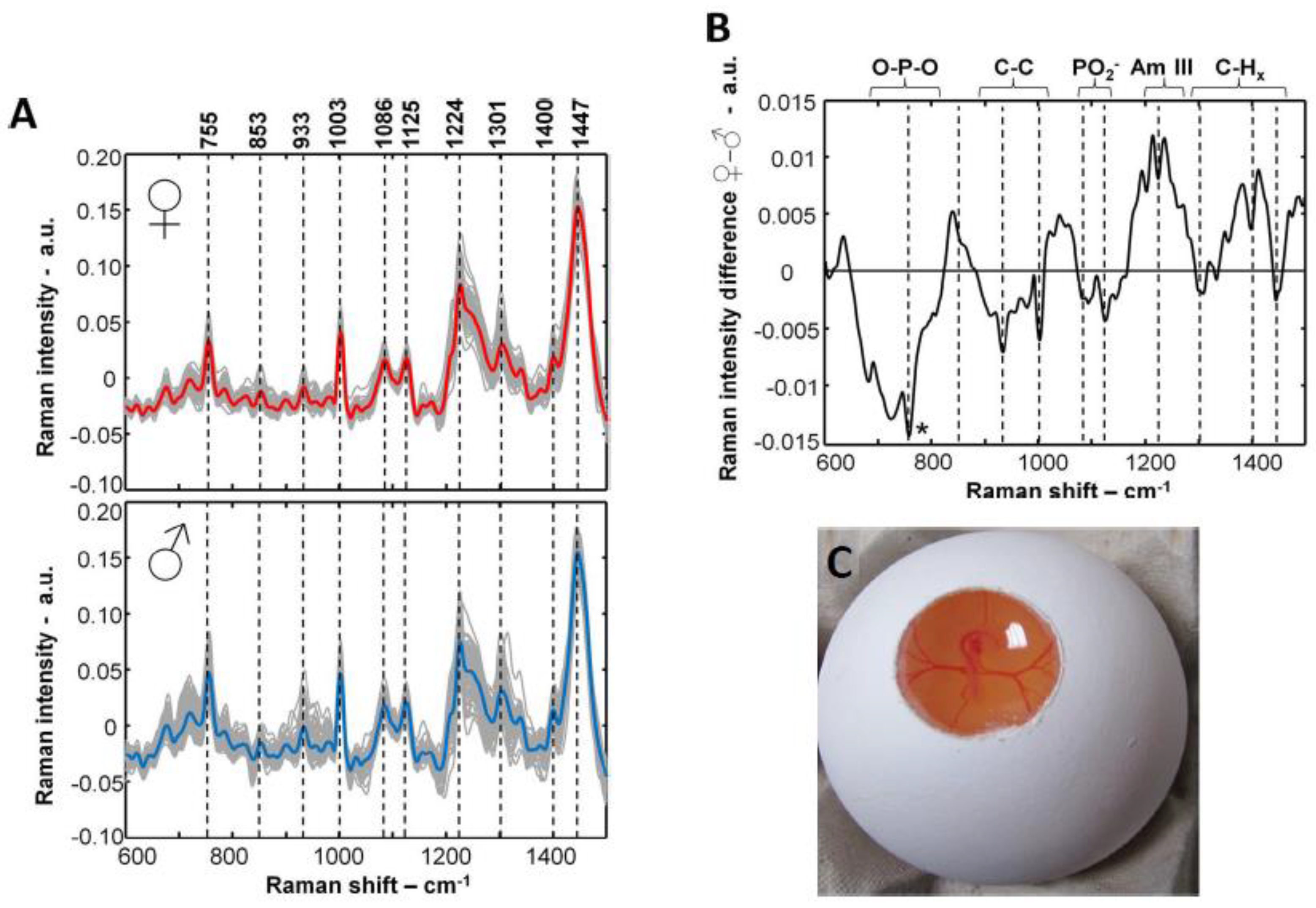
An invasive Raman and fluorescence spectroscopy
in ovo sexing method on day 3.5 was developed by Galli and co-authors and described in both patents and papers (
Figure 50) [
69,
72,
73,
74,
75,
76,
77]s. With this
in ovo sexing method, the laser excited a target (i.e., an extraembryonic blood vessel) and Raman bands with differences in blood chemistry and/or a higher fluorescence signal were observed in males due to a higher hemoglobin content (
Figure 5A and B).
In contrast to the previous technique, non-invasive Raman spectroscopy approaches are solely described in the patent literature [
71,
78]. The inventors claimed that sexually-discriminating substances such as sex-related genes, gene products, or sex hormones are spread throughout the egg and migrate to the eggshell. Subsequently, a focal point was set for a monochromatic laser to excite molecules inside or near the eggshell. Next, the reflected Raman signal was captured, and the spectral response would provide information on fertility and sex [
78]. However, no experimental data was provided on which substances were measured, on which incubation day it took place, or what the classification rate would be.
In another application, the detection of germinal disk cells' autofluorescence decay using a fluorescence spectrometer was described with a relatively low prediction accuracy (≈ 75 %) [
78]. Similarly, fluorescence excitation of blood vessels, germinal disk cells, or other embryonic structures was stated with accuracies ranging from 84.7 % to 92.3 % on day 3 to 6, respectively [
80]. Another application suggested using Raman spectroscopy to measure the germinal disk cells using, for example, feather pulp from which a model was built with a 95.4 % accuracy [
70,
81]. Finally, all these technologies required access through the eggshell to target the embryonic structures. This is considered an invasive approach that risks impacting embryonic development. Nevertheless, this technology can potentially be used early in the incubation process.
Despite the early detection, the intensive sampling procedure by windowing the eggshell (
Figure 5C), the sub-optimal analysis speed of 40 seconds per egg, and the 91 % accuracy of the invasive methods (see
Table 3) might explain why this technology has not been commercialized yet, even though it has been acquired and further developed by Agri Advanced Technologies (AAT GmbH, Visbek, Germany) [
69].
3.2. Infrared and Terahertz spectroscopy
Infrared spectroscopy (
IR) sexing is based on the difference in DNA content between sexes, whereby the males have approximately 2 % more DNA [
82]. These differences were detectable through an overall higher absorbance by male blastoderm cells extracted from the germinal disk, placed on an attenuated total reflection crystal [
83]. Like the Raman spectroscopic approach, IR can retrieve sex-specific information in a very early incubation stage (≤ day 0). This approach was applied even before incubation using Fourier-transform IR by Steiner et al. (83–85). Towards industrial application, the inventors integrated this crystal inside a probe with a cone-shaped tip that could be inserted through a hole in the eggshell [
85]. Due to the earlier-mentioned risk for the egg, the inventors stated in another paper that this method could not be further exploited [
69]. Nevertheless, the US patent is still active.
Similar to the IR spectroscopic technique, a THz spectroscopic approach was described, claiming that the waves would pass the intact shell and cause molecular vibrations of sex-specific DNA structures in germinal disk cells [
86]. THz wavebands are situated next to IR waves and can be absorbed by DNA molecules, thereby providing specific signatures [
87]. The patent for this THz approach has been granted, although no experimental data were reported.
In summary, although this early application holds commercialization potential, the process is relatively destructive, i.e., a small hole for probe insertion has to be made, increases contamination risks, and might affect hatchability, as shown in
Table 3.
3.3. Visible-Near-Infrared spectroscopy
Visible-near-infrared (
VIS-NIR) spectroscopy has the widest range of targets described for
in ovo sexing. This non-destructive technique measures vibrations caused by the stretching and bending of hydrogen bonds with carbon, oxygen, and nitrogen. Furthermore, it is widely used for assessing agricultural product quality and has the advantage of being fast, non-invasive, economical, and accurate [
88]. Classically, VIS-NIR spectroscopy consists of a single spectral dimension and can be extended by two spatial dimensions (i.e., X and Y), resulting in a three-dimensional hyperspectral image [
89], and enabling focusing on a specific region of interest within the egg.
The targets described in the state-of-the-art vary in 1) sexual-specific coloring [
90,
91,
92], 2) embryonic growth rate [
93], 3) sex-related blood absorption [
94,
95,
96], 4) heart rate or body movement [
97,
98,
99,
100], 5) egg yolk ratio [
101], 6) egg photoluminescence [
102], and 7) sex determining spectral features on the germinal disk or other regions in the egg [
103,
104,
105,
106,
107,
108,
109,
110,
111,
112,
113].The targets described in the state-of-the-art vary in 1) sexual-specific coloring [
90,
91,
92], 2) embryonic growth rate [
93], 3) sex-related blood absorption (94–96), 4) heart rate or body movement [
97,
98,
99,
100], 5) egg yolk ratio [
101], 6) egg photoluminescence [
102], and 7) sex determining spectral features on the germinal disk or other regions in the egg [
103,
104,
105,
106,
107,
108,
109,
110,
111,
112,
113]s.
In general, the techniques described in the patent literature for approaches 1 to 5 abovementioned did often not define accuracy or detection day. The egg opacity was used to measure embryonic growth, reporting an 84 % prediction accuracy from days 16 to 18 [
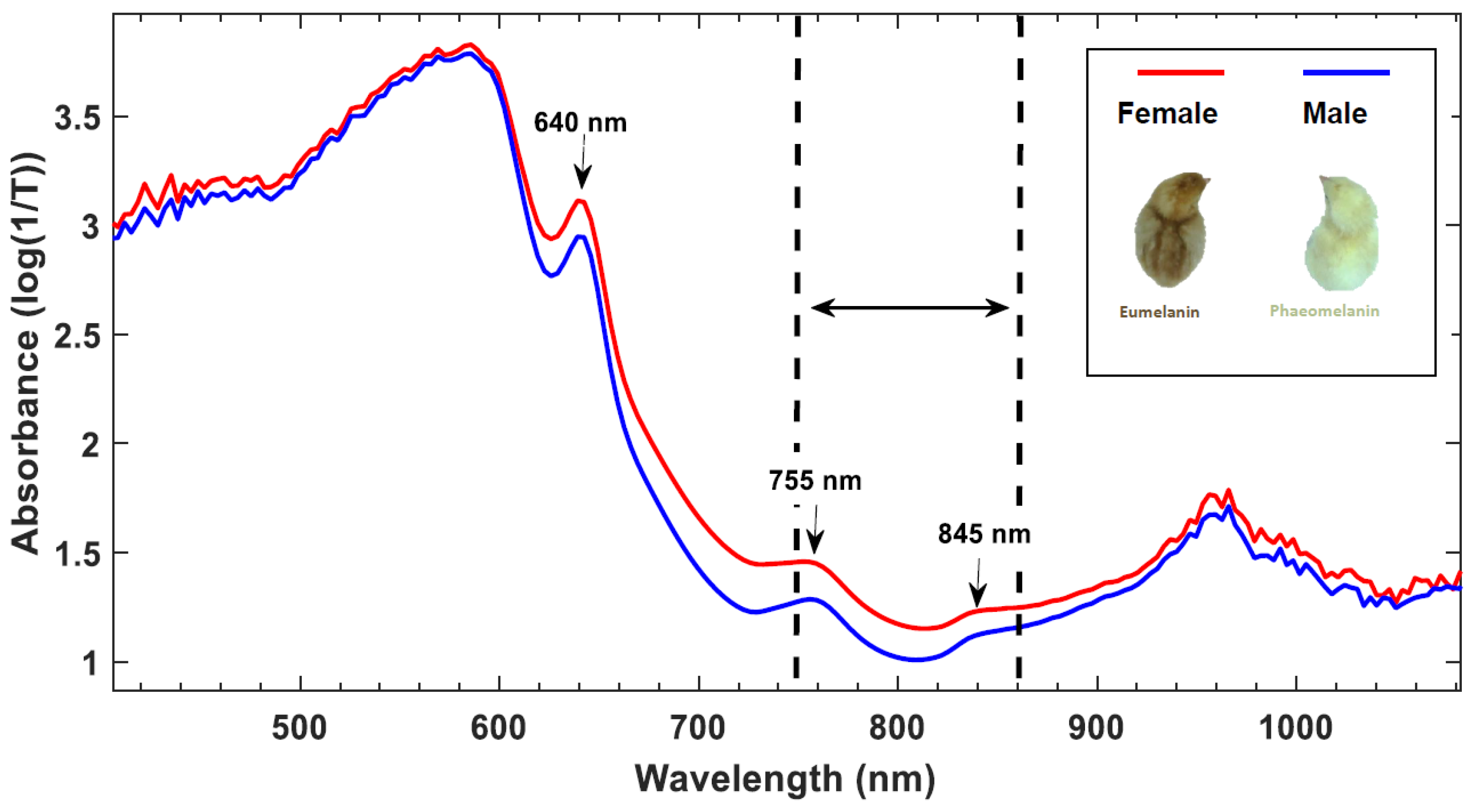
93]. Furthermore, VIS-NIR transmission measurements on eggs from chicken breeds with a sex-specific brown feather coloring is a well-established method being commercially applied by Agri Advanced Technologies (AAT GmbH, Visbek, Germany) on days 13 to 14 with an accuracy of up to 99 % in less than a second per egg (90–92). The more brown pigmentation in females (i.e., eumelanin) relative to the more yellow males (i.e., phaeomelanin) resulted in higher absorption of light in female embryos (
Figure 6).
Earlier application of VIS-NIR was described for blood absorption on days 0 to 7 (94–96). Without explicitly mentioning accuracies, Rahman et al. [
94] found a significantly higher hemoglobin absorbance in males on day 3 (P < 0.001). In contrast, a higher hemoglobin absorbance was found in females on day 7 and was hypothesized to be a result of a temporary coupled effect of estrogen receptor synthesis and enzymes on erythropoiesis (P < 0.05).
Finally, multiple hyperspectral imaging approaches were described in the patent literature wherein spectral features of selected regions within the egg were used to determine the sex (e.g., the middle region or the germinal disc). The highest reported accuracies were 100 % on day 0 [
106], 82.86 % on day 10 [
108], and 80 % on day 12 [
107]. Especially for the day 0 models, the reproducibility should be investigated further since the inventors did not divide the dataset into a test set and a separate validation set for model robustness.Finally, multiple hyperspectral imaging approaches were described in the patent literature wherein spectral features of selected regions within the egg were used to determine the sex (e.g., the middle region or the germinal disc). The highest reported accuracies were 100 % on day 0 [
106], 82.86 % on day 10 [
108], and 80 % on day 12 [
107]. Especially for the day 0 models, the reproducibility should be investigated further since the inventors did not divide the dataset into a test set and a separate validation set for model robustness.
As summarized in
Table 3, the main advantages of VIS-NIR
in ovo sexing are the relatively fast signal acquisition time, potential for non-invasive application, and absence of consumables. Generally, it has a good balance between scientific and industrial reporting. However, sometimes there is a lack of physiological background on the spectral features to prove validation and guarantee the robustness of statistical models.
3.4. Nuclear Magnetic Resonance spectroscopy
Nuclear magnetic resonance (
NMR) is another suggested imaging technology that uses radiofrequency waves to induce an external magnetic field and observes the resonant absorption of energy by the excited nuclei [
115]. A first method was patented in which embryos were imaged using magnetic resonance imaging (
MRI) during the transfer from setter to hatcher [
116]. Here, the processed images revealed the sex by visualizing two testes in males and one ovarium in females. However, Davenel and co-researchers [
117] failed to identify gonads since these develop on top of the kidneys, and the MRI could not distinguish between both tissues. Furthermore, they questioned the applicability at industrial speed since gonad imaging using MRI was relatively slow.
Haase et al. [
118] described an MRI-NMR method in another patent. The inventors defined three NMR parameters in which they claimed to determine the sex starting from day 5. These NMR signals' specific origin was unknown and assumed to be a chemical shift of metabolites or hormones. This technology is under development for commercialization by Orbem (Orbem GmbH, Munich, Germany). Concerning the detection of embryonic metabolites or hormones, other non-imaging NMR solutions were reported in patent literature. One non-invasive method for metabolites was described on day 4, where the ratio between nucleobases from free-floating DNA relative to the abundance of aromatic amino acids in blood was significantly lower for males [
119]. Further, an invasive NMR method on freeze-dried AF from day 9 to 11 on metabolites (i.e., choline, valine, and glucose) was reported by Bruins and Stutterheim [
63]. Finally, a non-invasive approach in which differences in E2 and T would be measured through NMR was claimed [
120]. No scientific literature has reported metabolite or hormone detection for
in ovo sexing with NMR.
The main advantage of NMR is its potential for non-invasive analysis (see
Table 3). The moment of application depends on the target and is more in favor when early applicable. On the other hand, this approach has long acquisition times and requires more scientific reporting, wherein the sex-specific features are studied more deeply.
3.5. Morphometric studies
In the following category, egg and embryo morphometric studies were considered since both use images from industrial cameras. The first approach was patented by Taniguchi, who published different applications from 2001 to 2021 in which the egg shape was used to determine the sex (121–126). Although applicable before incubation, sex differences in egg dimensions are likely to be minimal, and ratios of these parameters will be required to improve the accuracy. The described concept relied on the idea that male eggs would generally be less spherical and more pointed than female eggs [
121]. Images were used to measure the shape by quantifying the egg length, width, and distance from the equator to the blunt end. The protection for this patent expired in 2019, and more recently, a follow-up invention included egg distortion [
126].
Concerning the concept of the more spherical-shaped female eggs and more pointed male eggs, similar indications have been published in a study on white-layer eggs [
127]. The researchers measured the width and length and calculated a shape index that would discriminate the sex from this. However, the measured overlap between the sexes was considerable and did not allow a highly accurate sexual separation.
The second approach used machine vision imaging of embryonic blood vessels around day 4 (128–132). This technique is assumed to be low-cost and requires a light source, an industrial camera, and a computer. After egg illumination, an asymmetry in the blood vessel branching was observed for the female embryos and was linked to the asymmetric gonadal development in which the right gonad regresses, and less blood is assigned (
Figure 7A). On the other hand, the blood vessel branching would be symmetric for the equally developing testes in the males (
Figure 7B). The authors obtained an accuracy of 89.74 % on the prediction set using white eggs [
128]. However, it is questioned whether this technique would also work for brown eggs, given that the brown shell might absorb more light and complicate the blood vessels' visualization through the shell.
Table 3.
Overview of optical in ovo sexing techniques.
Table 3.
Overview of optical in ovo sexing techniques.
| Category |
Target |
Incubation day
Accuracy
Throughput
|
Technology (patents)/ research gaps (papers) |
Advantages (+)/
Drawbacks (-)
|
References |
| Raman and fluorescence spectroscopy |
Blood, eggshell, germinal disk, or other embryonic structures. |
Day 3.5
91 % accuracy on blood
Day 6
92.3 % on embryo and blood
Low to high throughput depending on the invasiveness |
Technology ≅ Research
The applicability on blood vessels is well reported in academic literature and is protected by patents
Technology > Research
A scientific gap exists in the analysis of the eggshell, germinal disk, or other embryonic structures since these are only reported in patent literature |
+
Early applicable in incubation
No liquid or tissue sampling required
-
Intensive sampling procedure and relatively invasive process |
Patents
⊗ Baron et al. [71], ⊕ Steiner et al. [72], ⊕ Galli et al. [77], ⊕ Schortgen [78], ⊕ Opitz et al. [79], ⊕ Popp et al. [81], ⊕ Schart [133], ∅ Herzog and Hurlin [134], ∅ Steiner et al. [135], ⊕ Hurlin et al. [136]
Papers
Galli et al. [69,74,75], Gokdag et al. [137] |
| IR and THz spectroscopy |
Germinal disk |
Day 0
Accuracy not defined
Average throughput |
Technology ≅ Research
From the same group of authors, both patents and papers are reported for the IR approach
Technology > Research
Only a patent is reported for the non-invasive THz approach |
+
Applicable in an early stage
-
IR approach infringes hatchability |
Patents
∅ Steiner et al. [85], ∅ May et al. [86]
Papers
Steiner et al. [83,84] |
| VIS-NIR spectroscopy |
Heartbeat,
blood hemoglobin, movement, feather color, yolk ratio, or growth (opacity) |
Days 0 to 14 depending on the technology
High accuracies on color sexing 99 %
Blood sexing or other spectral features range from 80 to 90 %
High throughput |
Technology ≅ Research
Color sexing in the egg is commercially available and is described in both patent and paper literature
Blood detection through the eggshell is described in paper and patent literature
Technology > Research
Other VIS-NIR spectroscopy on specific egg regions on specific days is only described in patents and lacks scientific background |
+
Has the potential for non-invasive implementation
Equipment is affordable
Early application potential
-
Lack of physiological background for sex determining spectral features described in patents |
Patents
⊕ McKay [91], ∅ Preusse [96], ⊕ Fujitani [95,101], ⊗ Rams and Toellner [100], ∅ Hebrank [97], ∅ Wang et al. [98], ∅ Colvin [102], ⊕ Ngadi et al. [104,106], ⊗ Zhao et al. [105,113], ⊕ Rozenboim and Ben Dor [107], ⊗ Pan et al. [108], ∅ Green [109], ⊕ Zhu [110], ∅ Fischer and Meissner [111,112]
Papers
Gohler et al. [90], Corion et al. [92], Khaliduzzaman et al. [93], Rahman et al. [94], Li et al. [103], Pan et al. [138] |
| NMR spectroscopy |
Gonads, hormones, or metabolites |
Days 4 to 11 for hormones and metabolites, accuracy not defined and relatively fast
Day18 gonads, accuracy not defined and relatively slow |
Technology > Research
Almost only patent literature has been reported
A single research paper reports the difficulty of detecting gonads |
+
Non-invasive
Early incubation
-
No prediction accuracies are reported or known
Gonad sexing relatively late |
Patents
∅ Bruins and Stutterheim [63], ⊕ Reynells and Flegal [116], ∅ Haase et al. [118], ∅ Hergenroder et al. [119], ⊕ Sewiolo and Ziroff [120]
Papers
Davenel et al. [117] |
| Morphometric studies |
Eggshell morphology or blood morphology |
Day 0 for eggshell,
~93 to 100 %
Relatively fast (using imaging technique)
Day 4 for blood
89.74 %
Relatively high throughput expected, yet equilibrium time needed in a horizontal position |
Technology > Research
Numerous patents are filed and a few papers
One paper is using the formulas from a patent Not all papers did find sexual differences
Using the shape index, there was a trend for female eggs to be more spherical, although the overlap was big with male eggs
Blood vessel morphology sexing is described both in patent and paper literature, and it might require more research to verify the robustness |
+
Non-invasive
Before or early incubation
-
Not enough scientific evidence that proves this technique works robustly nor an explanation of why there are sexual differences in the egg shape
Blood vessel imaging requires a 2 minutes equilibration time in a horizontal position |
Patents
Taniguchi ⊕ [121], ∅ [122], ⊕ [123], ∅ [124], ∅ [125], ⊕ [126], ⊕ Xiaohui et al. [129], ⊗ Li et al. [130], ⊗ Li [131], ⊕ Chen et al. [132], ∅ [139]
Papers
Yilmaz-Dikmen et al. [127], Rutkowska et al. [140] |
|
Abbreviations: d, day; IR, infrared; THz, terahertz; VIS-NIR, visible-near-infrared; ⊕, granted; ∅, pending; ⊗, ceased/rejected/discontinued. |
This approach received increased interest due to the current higher performance of cameras and software for image analysis and has been explored to study eggshells non-invasively and blood vessel morphometry, enabling fast screening. Advantageous is that the first method was applicable before incubation, whereas the second method required blood vessel development in early incubation. However, whether the prediction accuracy is high enough to use morphometric differences between sexes is questioned. Nevertheless, the technique is described in both patents and papers and is expected to hold potential for early and non-invasive
in ovo sexing. An overview of all the optical
in ovo sexing techniques is presented in
Table 3.
4. Trends in in ovo sexing techniques
4.1. Publication trends of papers and patents over time
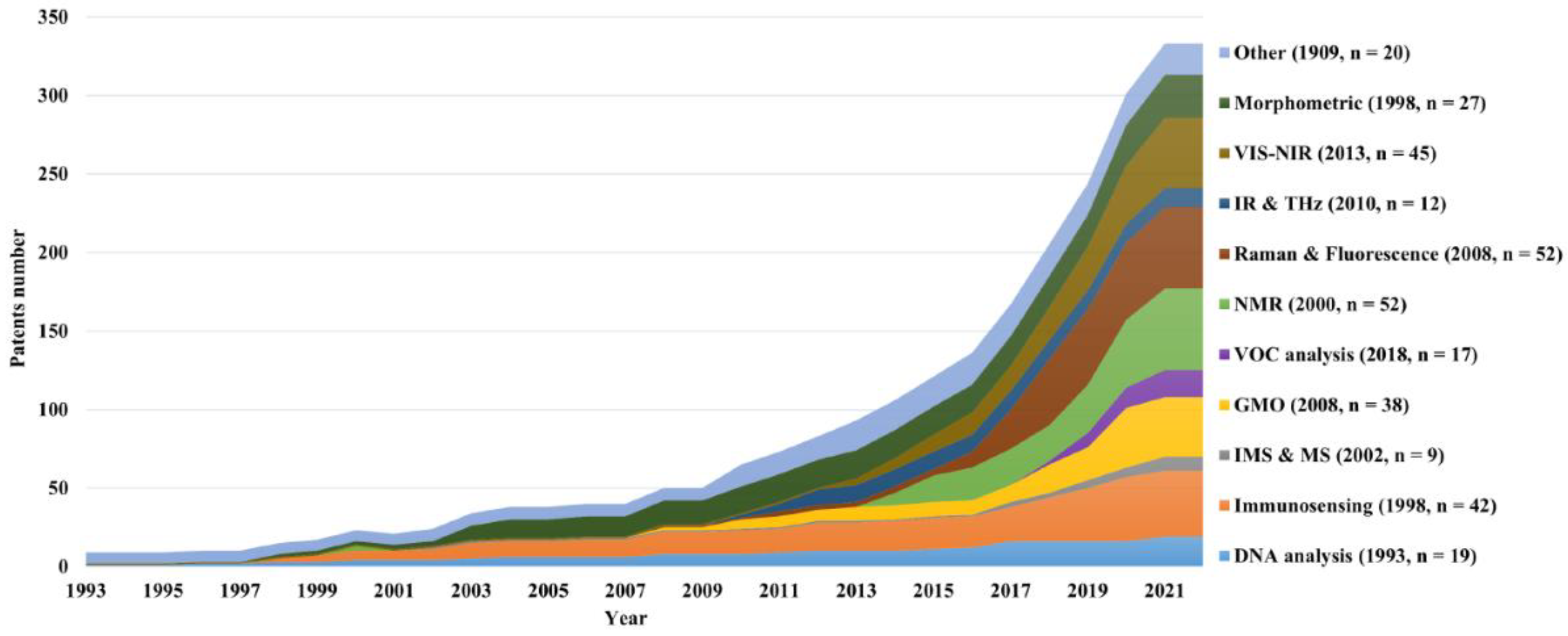
The quantitative information was organized in infographics to better understand the evolution of
in ovo sexing technologies.
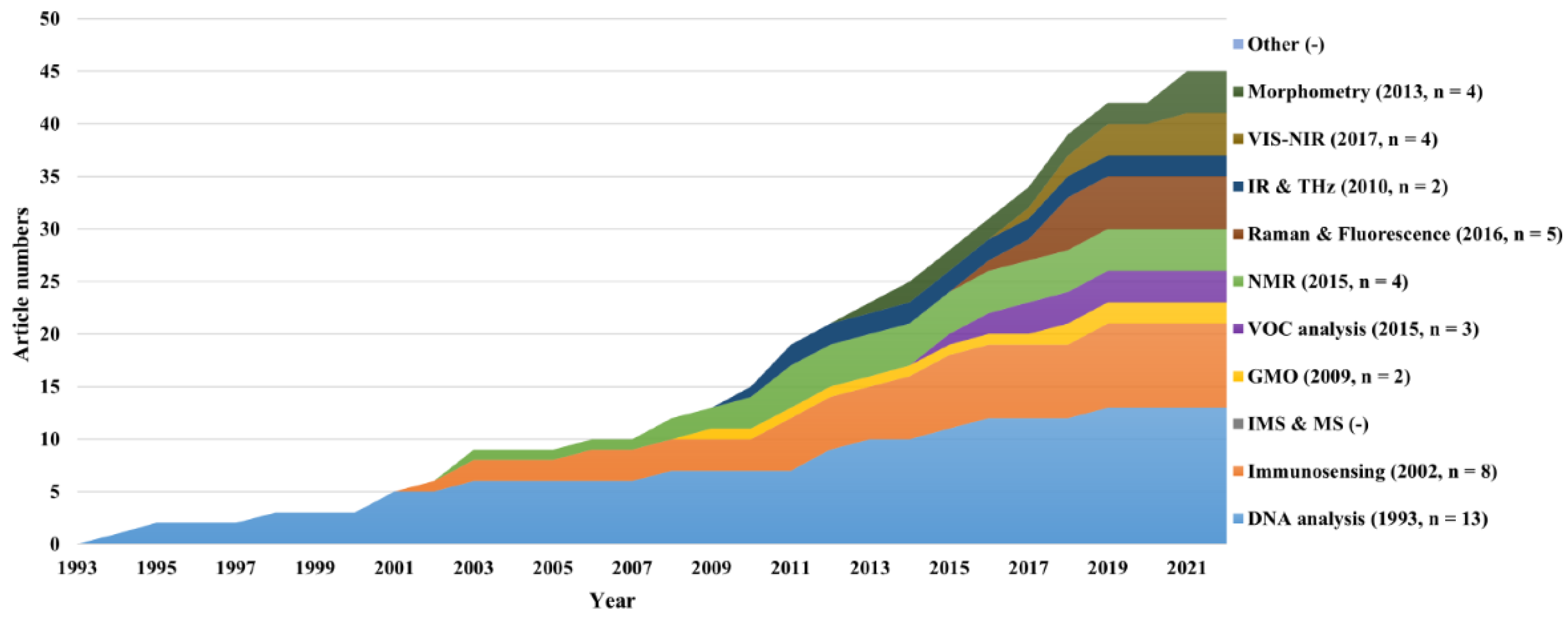
Figure 8 shows the scientific literature's growth over time for the different categories. Since the first scientific publication in 1994, constant growth has generally been observed for all techniques, with a total of 45 publications by 2021. No exponential growth was observed for any category, probably because no technology sufficed all the necessary market requirements. As of 2021, DNA analysis was the category having accumulated the most scientific publications (n = 13), followed by immunosensing (n = 8), perhaps due to the fact that these technologies were the first to be reported and usually applied as reference techniques.
In contrast, the IR & THz and GMO categories were the least reported techniques (each with two papers). The IMS and MS and the “other” category had no reported scientific literature. Notably, the curves seem to have reached a plateau more recently, which is hypothetically linked to the first official market entry of in ovo sexing techniques at the end of 2018: it is expected for innovative research activities to cease once a suitable technology is available to the market. Finally, it was assumed that the databases at the moment of the search in February 2022 might not have been completed yet for the publications of 2021.
Furthermore,
Figure 9 visualizes the growth of the patent application numbers through the years. The interest in a specific category over time was analyzed considering the publication number evolution throughout the years. For representation's sake, the timeline started in 1993, similar to
Figure 8. Previously to 1993, there had been seven patents from the "other" category (one for each of the following years: 1909, 1918, 1919, 1936 and 1955 as well as two from 1935), and one from VIS-NIR in 1935. As we can see from the data, the first-ever protected technology was covered in the "other" category for measuring magnetic polarity. From 1936 until 1993, there was a wide-year gap in which no patent was filed. From 1993 onwards, morphometric techniques have been concisely patented by the same inventors, with a considerable increase in 2004 (from 9 to 12). Steady growth with peaks in specific situations was observed concerning the remaining technologies, e.g., Raman and fluorescence technologies were first patented in 2008, reaching 52 patents in 2021. Between 2016 and 2018, Raman and fluorescence-related patents increased from 10 to 42. Some other examples of noteworthy increases were from 2018 to 2020: GMO (18 to 38), NMR (23 to 43), and VIS-NIR (21 to 37). Interestingly, these exponential growths were observed for some of the most recent techniques. The first GMO patent dated from 2008 (starting with two patents publications), Raman from 2008, and VOC analysis from 2018. In 2021, the category with the most patents was the category of Raman and fluorescence spectroscopy (n = 52). The category with the least patents was IMS and MS (n = 9). Considerable investment has been put into Raman and fluorescence spectroscopy since it can perform sexual detection on day 3.5 with high accuracy, which is an advantage for animal welfare (i.e., before the pain onset) and for the industry (i.e., the rapid replacement for female eggs). In total, 100 patent families have been found, resulting in 333 patent application numbers (multiple patent application numbers can be registered for one patent family if a patent has to be protected in multiple regions).
By comparing
Figure 8 and
Figure 9, it became apparent that most of the technologies were first protected with patents before being published in scientific papers, which can also be confirmed by looking at the references’ dates. Some examples of this were seen with DNA analysis, in which the first patent was filed in 1991, while the first paper dates to 1994, or even Raman which was patented in 2007, while only being reported in papers since 2016. In some cases, there were no published papers, only patent files, e.g., IMS and MS had their first patent filed in 1999, whilst no scientific publication has been reported so far. Exceptionally, the VOC analysis approach was first reported in a paper in 2015, whereas its first patent was published in 2018, being the most recently patented technology. Moreover, the growth trends between papers and patents tended to be different, with the latter looking similar to an exponential curve until 2017 when the growth stabilized until 2021. The different growth rates lead to seven times more patent applications than papers. This could be explained by the number of different patents (n = 333) filed under the same family number (n = 100).
4.2. Geographical distribution of techniques
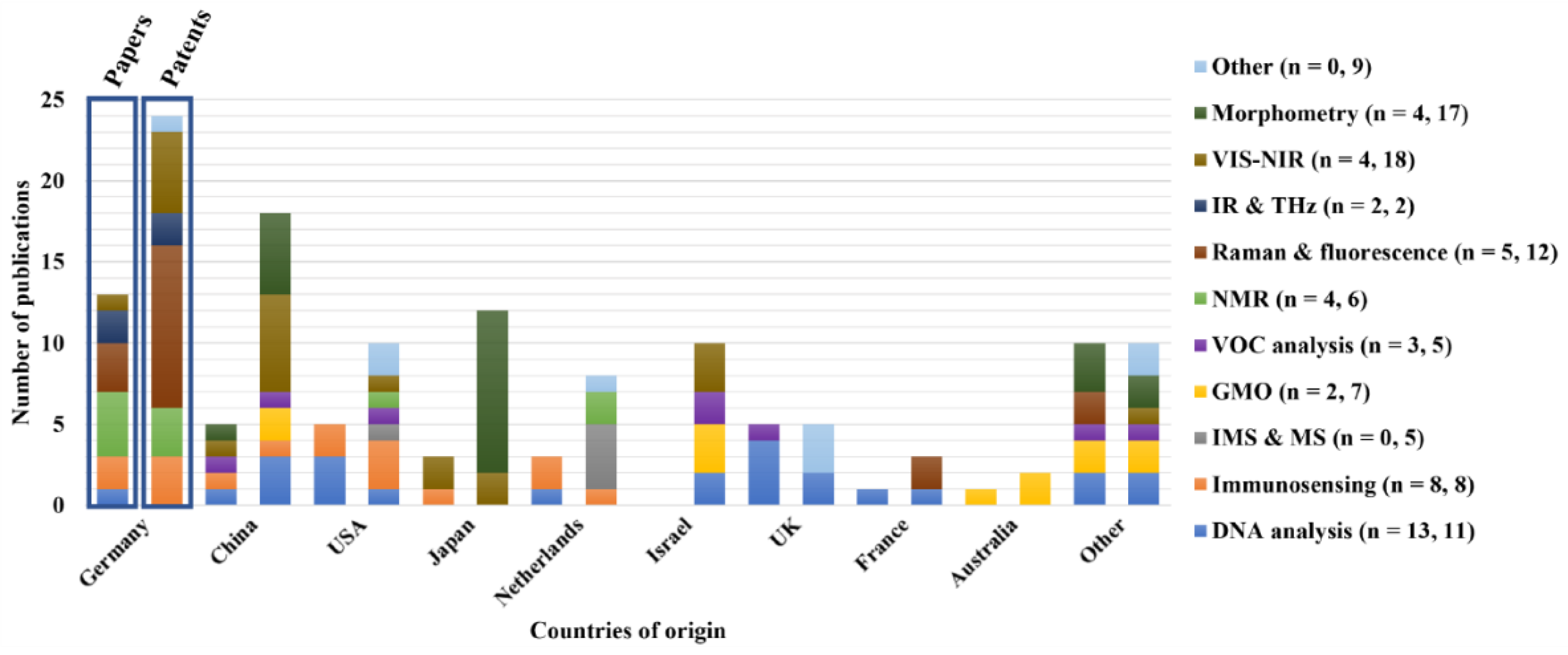
The geographical origin of the published papers and patent families are represented in
Figure 10. This representation shows the major world players and the balance between scientific and industrial materials. The origin of the patent families is different from the published patents and their protection in different countries. This distribution is presented in Section S3 of the supplementary materials. Concerning the patent origin, it was observed that Germany had the majority of patents (n = 24) whilst having the second largest number of papers (n = 13). Japan stood out with the largest number of publications (n = 14). Germany was the first to ban male chicken culling within the poultry industry [
5], potentially explaining the higher pressure to find new solutions and the high number of patents. Next to Germany, China (n = 18), Japan (n = 12), Israel (n = 10), and the USA (n = 10), were the countries with the highest number of patents (n = 55), accounting for 55 % of the overall patent number. These numbers were not necessarily at the same level as the number of papers. Overall, more patents were published in most countries than papers. Putatively, this was linked to the male culling problem requiring an industrial solution. Subsequently, researchers would prefer to protect their approach as soon as possible via a patent before disclosing their work through a paper.
An extreme example was Israel since this country had 12 patents and no papers. A more balanced example was the UK, with five papers and patents respectively, revealing that the scientific and industrial weight was similar. To further elaborate on the current poultry market shares between countries, China leads the list on egg production, followed by Europe, the US and India [
141]. The trend in egg production was positively correlated with the patent number for the US, China, and Germany (the latter being the biggest egg producer in the EU).
Furthermore, it was observed that the University of Dresden in Germany was the main publisher, with five papers and six patents on in ovo sexing. The Supplementary materials present these data in Section S4 and S5. Next to the immunosensing approach of the University of Leipzig and the company Seleggt, German researchers seem to predominantly work on optical in ovo sexing techniques.
Concerning the category landscape across countries, the most general category was DNA analysis (13 papers and 11 patents), present in Germany, the USA, China, the UK, The Netherlands, and France since nucleic acid techniques can be used as standards for other applications (an exception was seen in Japan). Observing the data, some of the most recent methods (e.g., VIS-NIR, IR and THz, or Raman and Fluorescence) were primarily present in Germany, representing the urge to comply with the industry's new governmental regulations (e.g., sex detection before day 7 of incubation). Some specific countries had a visible trend toward certain industrial applications, e.g., Japan had the highest number of morphometric patents (n = 17), China with VIS-NIR (n = 11 patents), and Australia with GMOs (n = 3). This same comparison was impossible to make for the papers since the number of publications was limited, and no preference towards any of the categories was observed.
4.3. Papers citations and legal status patents
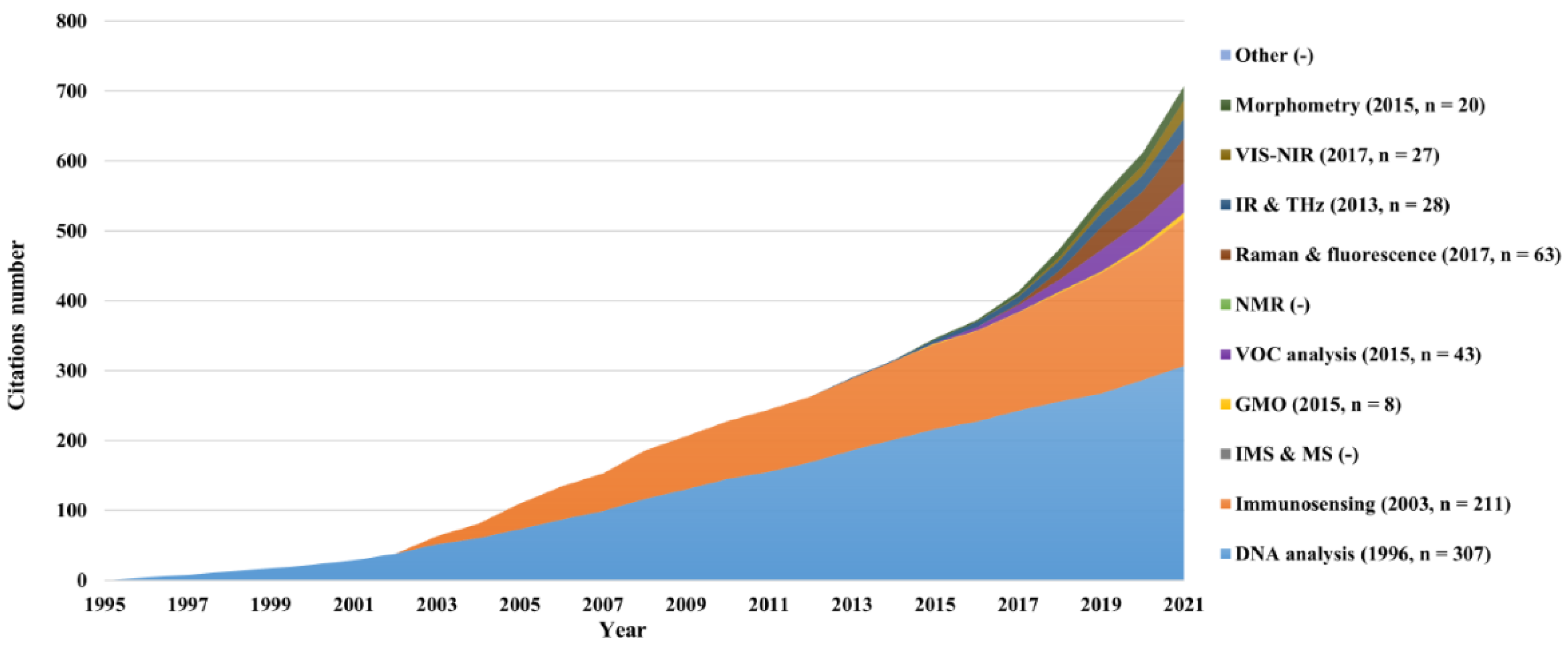
The cumulative number of citations per category was collated to quantify a method's influence in this field (
Figure 11). The analysis in this section was made at the level of categories rather than individual documents. The highest citation number was observed for the DNA analysis category (n = 307), followed by immunosensing (n = 211). These were also the first two categories to be published in papers and had the most papers overall (
Figure 8), while continuing to be the two most actively referred to and having the highest influence on the field. The category with the least citations was GMO (n = 8), mainly due to the presence of only two published papers. Furthermore, no citations were observed for the “other” and the IMS and MS categories due to the lack of papers. An evident increase over time was visible in the number of citations, closely related to the increasing number of papers through the years. An overview of the journals with two or more publications on
in ovo sexing is presented in Section S6 of the supplementary materials.
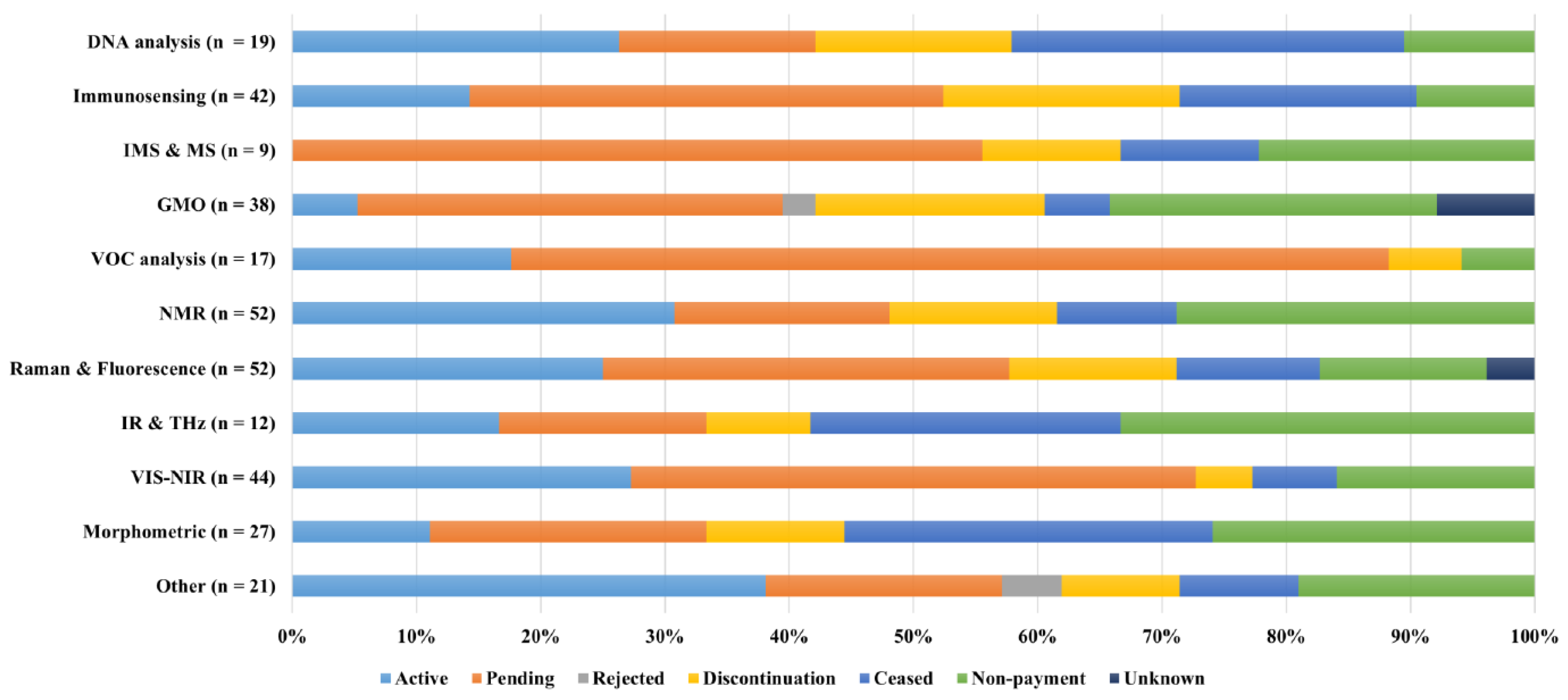
The patents’ legal statuses are presented in
Figure 12. This overview shows that many patents – regardless of their category – have been discontinued due to non-payment of the fees (13 % overall). This was the lowest (6 %) for VOC analysis patents potentially because this was the most recent sexing approach. Furthermore, the IR and THz approach had the highest ratio of 33 %, potentially because it was represented by the same group of inventors that regularly filed new patent applications.
Furthermore, some patents were pending before being active due to a requirement for an examination. This ranged from 16 % for DNA analysis (i.e., one of the oldest approaches) to 70 % for VOC analysis (the most recent approach). The active patents could be found in all categories except for IMS and MS. Overall, 21 % of the patents were still active as of January 2022. The collected data showed that 19 % of the patent fees were not paid in time, hereby losing protection. At last, there were also ceased patents (granted at some point but expired; 13 %). Thus, the applicants lost their rights over their technology. The authors can renew these patents by updating their inventions and filing them for examination.
In conclusion, actively granted patents were observed in all categories demonstrating that investments continue to be made regardless of the approach. There was no outstanding category for which a noticeable ratio of granted patents was observed. It was observed that both VOC analysis techniques and IMS and MS approaches have more than 50 % pending, indicating their potential growth.
5. Conclusion
Since 1907, in ovo sexing technologies have been developed to tackle the issue of male chick culling in the poultry industry. This review shows the effort that has been put into developing in ovo sexing technologies, enabling sex determination before hatching. Until now, all the reported technologies (both in patents and papers) do not satisfy the market requirements or create other problems, such as environmental or new ethical issues. Based on this review, no clear consensus can yet be reached on which technology is best suited for in ovo sexing, which is mirrored by the newly emerging techniques. Among all the presented technologies, we considered the optical techniques promising since they offer the potential for non-invasive and high throughput screening. However, most optical techniques fail to meet the accuracy or incubation time requirements. An exception is Raman spectroscopy, an invasive optical technique with the desired early detection (day 3.5). However, more improvements are needed to be made less invasive, and guarantee unaffected hatchability.
Furthermore, the technology that most recently emerged in patents was the VOC analysis approach. The potential of being non-invasive and early applicable has been demonstrated in papers. However, it requires more research to robustly define the relevant biomarkers and detect them with fast equipment. Other standard technologies, such as DNA analysis, immunosensing, or even mass spectrometry, are well-known analyses used across different applications and will most likely continue to be used for in ovo sexing.
However, to finally succeed in placing a technology in the industrial setting, it is first necessary to obtain harmony between industry, academia, and governments to decide on the best approach and requirements to solve male day-old chick culling.
Author Contributions
MC and SS are first shared co-authors of this work and have equally analyzed the literature and written the manuscript. DS, BDK, MH and JL contributed to the structure of the manuscript and critically reviewed the content. All authors read and approved the final manuscript.
Funding
This work has received funding from the Flemish environment department, the Foundation for Food and Agricultural Research [EggTech-0000000028], and the Research Foundation – Flanders [SB project 1SC7219N and SB project 1S54823N].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Gratitude is expressed to Julie Callaert and Mariëtte Du Plessis from ECOOM, the center for research & development monitoring for their help in building the search key and performing the patent search in the PATSTAT database. Finally, the authors are also grateful for the support of Cameron Dierendonck in setting up the concept of this review paper.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Authors’ information (optional)
Not applicable.
Abbreviations
| IMS |
ion mobility spectrometry |
| MS |
mass spectrometry |
| GMO |
genetic modified organisms |
| VOC |
volatile organic compounds |
| PCR |
polymerase chain reaction |
| DMRT-1 |
doublesex and mab-3 related transcription factor 1 |
| CHD |
chromodomain helicase DNA |
| WPKCI |
W-protein kinase C iota |
| gDNA |
genomic DNA |
| AF |
allantoic fluid |
| FRET |
fluorescence resonance energy transfer |
| qPCR |
quantitative PCR |
| ELISA |
enzyme-linked immunosorbent assay |
| RIA |
radioimmunoassay |
| DHT |
dihydrotestosterone |
| A4 |
androstenedione |
| E1 |
estrone |
| E2 |
estradiol |
| E1S |
estrone sulfate |
| P4 |
progesterone |
| T |
testosterone |
| PGCs |
primordial germ cells |
| GC-MS |
gas chromatography-mass spectrometer |
| PTR-MS |
proton transfer reaction-mass spectrometry |
| SIFT-MS |
selected ion flow tube-mass spectrometry |
| THz |
Terahertz |
| SPME-GS-MS |
solid phase micro extraction-gas chromatography-mass spectrometry |
| VIS-NIR |
visible-near-infrared |
| NMR |
nuclear magnetic resonance |
| MRI |
magnetic resonance imaging |
| IR |
infrared |
References
- Bruijnis MRN, Blok V, Stassen EN, Gremmen HGJ. Moral “lock-in” in responsible innovation: The ethical and social aspects of killing day-old chicks and its alternatives. J Agric Environ Ethics. 2015;28(5):939–60. [CrossRef]
- Reithmayer C, Mußhoff O, Danne M. Alternatives to culling male chicks – the consumer perspective. Br Food J. 2020;122(3):753–65. [CrossRef]
- Gautron J, Réhault-Godbert S, Van de Braak TGH, Dunn IC. Review: What are the challenges facing the table egg industry in the next decades and what can be done to address them? Animal [Internet]. 2021;(xxxx):100282. Available from. [CrossRef]
- Reithmayer C, Mußhoff O. Consumer preferences for alternatives to chick culling in Germany. Poult Sci. 2019;98(10):4539–48. [CrossRef]
- Der Bundesregierung. Entwurf eines gesetzes zur änderung des tierschutzgesetzes – verbot des kükentötens. Der Bundesregierung; 2019 p. 176.
- Française, R. Journal Officiel - Lois et Décrets [Internet]. Paris; 2022 p. 56–7. Available from: https://www.legifrance.gouv.fr/jorf/jo/2022/02/06/0031.
- Consiglio DEL, Sono E. REGOLAMENTO (CE) N. 1099/2009 DEL CONSIGLIO del 24 settembre 2009 relativo alla protezione degli animali durante l’abbattimento [Internet]. Brussels: Unione europea; 2009 p. 1–30. Available from: https://eur-lex.europa.eu/legal-content/IT/TXT/?uri=celex%3A32009R1099.
- Busse M, Kernecker ML, Zscheischler J, Zoll F, Siebert R. Ethical Concerns in Poultry Production: A German Consumer Survey About Dual Purpose Chickens. J Agric Environ Ethics [Internet]. 2019;32(5–6):905–25. Available from. [CrossRef]
- Rosenbruch, M. The sensitivity of chicken embryos in incubated eggs. ALTEX. 1997;14(3):111–3.
- Mellor DJ, Diesch TJ. Birth and hatching: Key events in the onset of awareness in the lamb and chick. N Z Vet J [Internet]. 2007;55(2):51–60. Available from. [CrossRef]
- Phelps P, Bhutada A, Bryan S, Chalker A, Ferrell B, Neuman S, et al. Automated identification of male layer chicks prior to hatch. Worlds Poult Sci J. 2003;59(1):33–8.
- Krautwald-Junghanns ME, Cramer K, Fischer B, Förster A, Galli R, Kremer F, et al. Current approaches to avoid the culling of day-old male chicks in the layer industry, with special reference to spectroscopic methods. Poult Sci. 2018;97(3):749–57. [CrossRef]
- Sinclair A, Smith C. Sex-determination and methods of specifying same [Internet]. 2010. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DWO2010088742A1.
- Griffiths, R. Avian CHD genes and their use in methods for sex identification in birds [Internet]. Vol. 1. WO9639505A1, 1996. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DWO9639505A1.
- Li H, Hu Y, Song C, Ji G, Liu H, Xu W, et al. A new primer for sex identification of ducks and a minimally invasive technique for sampling of allantoic fluid to detect sex during bird embryo development. Sex Dev. 2015;9(3):173–81. [CrossRef]
- Petitte JN, Kegelmever AE. Rapid sex determination of chick embryos using the polymerase chain reaction1. Anim Biotechnol. 1995;6(2):119–30.
- Haunshi S, Pattanayak A, Bandyopadhaya S, Saxena SC, Bujarbaruah KM. A simple and quick DNA extraction procedure for rapid diagnosis of sex of chicken and chicken embryos. J Poult Sci. 2008;45:75–81. [CrossRef]
- Clinton M, Nandi S, Zhao D, Olson S, Peterson P, Burdon T, et al. Real-Time Sexing of Chicken Embryos and Compatibility with in ovo Protocols. Sex Dev. 2016;10(4):210–6. [CrossRef]
- Clinton, M. Avian sex determination method [Internet]. 2004. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DWO2004016812A1.
- He L, Martins P, Huguenin J, Van TNN, Manso T, Galindo T, et al. Simple, sensitive and robust chicken specific sexing assays, compliant with large scale analysis. PLoS One. 2019;14(3):1–17. [CrossRef]
- Smith CA, Roeszler KN, Hudson QJ, Sinclair AH. Avian sex determination: What, when and where? Cytogenet Genome Res. 2007;117(1–4):165–73.
- Smith CA, Sinclair AH. Sex determination: Insights from the chicken. BioEssays. 2004;26(2):120–32. [CrossRef]
- Tanabe Y, Nakamura T, Fujioka K, Doi O. Production and secretion of sex steroid hormones by the testes, the ovary, and the adrenal glands of embryonic and young chickens (Gallus domesticus). Gen Comp Endocrinol. 1979;39(1):26–33. [CrossRef]
- Gill D, V. , Robertson HA, Betz TW. In vivo estrogen synthesis by the developing chicken (Gallus gallus) embryo. Gen Comp Endocrinol. 1983;49(2):176–86.
- Phelps, P. Method of sorting birds in ovo [Internet]. 1998. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DWO9814781A1.
- Tyczkowski J, Mahato D, Chalker A. Competitive particle immunoassay methods utilizing fluorescence microscopy [Internet]. 2006. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DWO2006124456A3.
- Butt T, Tran HT. Compositions and methods for gender sorting [Internet]. 2002. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DCA2445162A1.
- Tran HT, Ferrell W, Butt TR. An estrogen sensor for poultry sex sorting. J Anim Sci. 2010;88(4):1358–64. [CrossRef]
- Einspanier, A. Method for the in-ovo sex identification of chicks [Internet]. 2017. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DWO2017109133A1.
- Weissmann A, Reitemeier S, Hahn A, Gottschalk J, Einspanier A. Sexing domestic chicken before hatch: A new method for in ovo gender identification. Theriogenology [Internet]. 2013;80(3):199–205. Available from. [CrossRef]
- Müller W, Eising CM, Dijkstra C, Groothuis TGG. Sex differences in yolk hormones depend on maternal social status in leghorn chickens (Gallus gallus domesticus). Proc R Soc B Biol Sci. 2002;269(1506):2249–55. [CrossRef]
- Aslam MA, Hulst M, Hoving-Bolink RAH, Smits MA, de Vries B, Weites I, et al. Yolk concentrations of hormones and glucose and egg weight and egg dimensions in unincubated chicken eggs, in relation to egg sex and hen body weight. Gen Comp Endocrinol [Internet]. 2013;187:15–22. Available from. [CrossRef]
- Wang Y, Jin G, Ma M, Xiang X. Sex differences in serum steroid hormone levels during embryonic development in hen eggs. Poult Sci. 2019;98(11):6053–62. [CrossRef]
- Decuypere E, Fey F. Method for avian sex determination. 2011;1(19):33. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DUS2014099265A1.
- Güneş E, Movassaghi H, Unsal F, Güneş NT. GMO Policies and Practices: A Global Overview with Special Focus on Turkey. In: Policy Issues in Genetically Modified Crops: A Global Perspective. 2020. p. 29–56.
- Arce L, Gallegos J, Garrido-Delgado R, Medina LM, Sielemann S, Wortelmann T. Ion mobility spectrometry a versatile analytical tool for metabolomics applications in food science. Curr Metabolomics. 2014;2(4):264–71. [CrossRef]
- Daum KA, Atkinson DA. Gender determination of avian embryo [Internet]. US63365339B1, 2002. Available from: https://worldwide.espacenet.com/patent/search/family/026795695/publication/US6365339B1?q=pn%3DUS6365339B1.
- Bruins W, Stutterheim W. Method and system for the non-destructive in ovo determination of fowl gender [Internet]. WO2017204636A2, 2017. Available from: https://worldwide.espacenet.com/patent/search/family/059366469/publication/WO2017204636A2?q=pn%3DWO2017204636A2&queryLang=en%3Ade%3Afr.
- Bruetschy, C. The EU regulatory framework on genetically modified organisms (GMOs). Transgenic Res [Internet]. 2019;28(s2):169–74. Available from. [CrossRef]
- Doran TJ, Morris KR, Wise TG, O’Neil TE, Cooper CA, Jenkins KA, et al. Sex selection in layer chickens. Anim Prod Sci. 2018;58(3):476–80. [CrossRef]
- Offen, D. Methods for gender determination of avian embryos in unhatched eggs and means thereof [Internet]. 2018. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DWO2017094015A1.
- Beisswanger, R. Method for suppressing the male sex in birds, specifically in laying poultry, by inserting a lethal DNA sequence into sex chromosomes that is active only in male embryos Roland 2002.pdf [Internet]. 2002. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DDE10248361A1.
- Webster B, Hayes W, Pike TW. Avian egg odour encodes information on embryo sex, fertility and development. PLoS One. 2015;10(1). [CrossRef]
- Costanzo A, Panseri S, Giorgi A, Romano A, Caprioli M, Saino N. The odour of sex: Sex-related differences in volatile compound composition among barn swallow eggs carrying embryos of either sex. PLoS One. 2016;11(11):1–17.
- Rivers, AR. System and method for determining the sex and viability of poultry eggs prior to hatching [Internet]. US20210181174A1, 2021. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DUS2021181174A1.
- Knepper P, O’Hayer M, Hoopes J. System and method for in ovo sexing of avian embryos [Internet]. WO2018023105A1, 2018. p. 33. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DWO2018023105A1.
- Tongli S, Lin X, Xiaohai Y. The invention discloses a terahertz poultry embryo sex detection device [Internet]. CN208891459U, 2019. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DCN208891459U.
- Gabbai, E. A system and method for non-invasively determining egg properties [Internet]. WO2019021275A1, 2019. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DWO2019021275A1.
- Xiang X, Hu G, Jin Y, Jin G, Ma M. Nondestructive characterization gender of chicken eggs by odor using SPME/GC-MS coupled with chemometrics. Poult Sci. 2022;101(3):101619. [CrossRef]
- Corion M, Ketelaere B De, Hertog M, Lammertyn J. Profiling the emission of volatile organic compounds from chicken hatching eggs in the first half of incubation. In: IFRG eMeeting 2021. [Online]: IFRG; 2021.
- Young, RD. Electrical and magnetic polarity and sex indicator [Internet]. GB176672A, 1922. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DGB176672A.
- Williams, Henry A. Improvements in and connected with appliances adapted to inidcate the sex of any living thing and for other analogous purposes [Internet]. 1920. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DGB150655A.
- Williams, HA. A new apparatus to indicate the sex of birds or animals and to tell whether an egg is fertile and what sex it will hatch [Internet]. GB190900909A, 1909. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DGB190900909A.
- Pacala N, Bencsik I, Bencsik I, Corin Ni, Stanculet JJ, Anna BA. Apparatus and method for determining the genetic sex [Internet]. 2007. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DRO121316B1.
- Chadfield, C. Improvements in or relating to methods of and means for determining the presence of living organisms and or the sex of living organisms [Internet]. GB462237A, 1935. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DGB462237A.
- Pacala N, Bencsik I, Bencsik I, Corin Ni, Stanculet JJ, Anna BA. Apparatus and method for determining the genetic sex [Internet]. RO121316B1, 2007. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DRO121316B1.
- Visser CFT. Method for preparing an invasive test of an egg and for determining a gender of an embryo in an egg [Internet]. US2012231487A1, 2012. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DEP2473030B1.
- Rastoutsau U, Lukyanau A, Vinogradov S, Kosolobov A, Galitsky N, Gromov R. A method of identifying the state of eggs in the process of incubation and a system for implementing the same [Internet]. WO2018042225A1, 2018. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DWO2018042225A1.
- Dalton M, Daly J, Mccabe M, Walsh P. Genomic analysis device and methods of use [Internet]. 2016. Available from: https://worldwide.espacenet.com/patent/search/family/053887156/publication/WO2016020838A1?q=pn%3DWO2016020838A1.
- Arie A, Danielli A. Method and system for detecting a target within a population of molecules [Internet]. US20190137504A1, 2019. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DUS2019137504A1.
- Molina F, Espeut J. Method for determining a specific characteristic of an embryo in an unhatched egg [Internet]. Vol. 1. EP3599465A1, 2020. p. 1–34. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DEP3599465A1.
- Weissmann A, Förster A, Gottschalk J, Reitemeier S, Krautwald-Junghanns ME, Preisinger R, et al. In ovo-gender identification in laying hen hybrids: effects on hatching and production performance. Eur Poult Sci. 2014;78. [CrossRef]
- Bruins W, Stutterheim W. Gender, viability and/or developmental stage determination of avian embryos in ovo [Internet]. WO2014021715A3, 2014. Available from: https://worldwide.espacenet.com/patent/search/family/050028615/publication/WO2014021715A2?q=WO 2014%2F021715.
- Stutterheim WM, Van Bommel L, Bruins WS, Dijksterhuis E, Van De Wel J, Leemker M. Egg characteristic determining device [Internet]. WO2021145771A1, 2021. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DNL2016818B1.
- Knepper P, O’Hayer M, Hoopes J. System and method for in ovo sexing of avian embryos [Internet]. WO2018023105A1, 2018. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DWO2018023105A1.
- Shi T, Xu L, Yu X. The invention discloses a terahertz poultry embryo sex detection device [Internet]. CN208891459U, 2019. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DCN208891459U.
- Kingslake R, Thompson BJ. Optics. In: Encyclopedia Britannica [Internet]. 2020. Available from: https://www.britannica.
- Atkins P, de Paula J, Keeler J. Physical chemistry. 11th ed. Biophysical Chemistry. Oxford University Press; 2018.
- Galli R, Preusse G, Schnabel C, Bartels T, Cramer K, Krautwald-Junghanns ME, et al. Sexing of chicken eggs by fluorescence and Raman spectroscopy through the shell membrane. PLoS One [Internet]. 2018;13(2). Available from. [CrossRef]
- Harz M, Krause M, Bartels T, Cramer K, Rösch P, Popp J. Minimal invasive gender determination of birds by means of UV-resonance Raman spectroscopy. Anal Chem. 2008;80(4):1080–6. [CrossRef]
- Baron S, Catherinot L, Sarr Y, Schortgen M, Thouand G, Assaf AA. Procede non invasif de determination de la fertilite et ou du sexe d un oeuf [Internet]. FR3075965A1, 2019. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DFR3075965A1.
- Steiner G, Preusse G, Galli R, Koch E. Method and device for optical in ovo sex determination of fertilized and incubated birds’ eggs [Internet]. US20190383782A1, 2019. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DUS2019383782A1.
- Galli R, Koch E, Preusse G, Schnabel C, Bartels T, Krautwald-Junghanns M-E, et al. Contactless in ovo sex determination of chicken eggs. Curr Dir Biomed Eng [Internet]. 2017;3(2):131–4. Available from: http://www.degruyter.com/view/j/cdbme.2017.3.issue-2/cdbme-2017-0027/cdbme-2017-0027. [CrossRef]
- Galli R, Preusse G, Uckermann O, Bartels T, Krautwald-Junghanns ME, Koch E, et al. In ovo sexing of chicken eggs by fluorescence spectroscopy. Anal Bioanal Chem [Internet]. 2017;409(5):1185–94. Available from. [CrossRef]
- Galli R, Preusse G, Uckermann O, Bartels T, Krautwald-Junghanns M-E, Koch E, et al. In ovo sexing of domestic chicken eggs by raman spectroscopy. Anal Chem. 2016;88:8657–63. [CrossRef]
- Steiner G, Preusse G, Koch E, Galli R, Schnabel C, Preusse J. Method for classifying spectra of objects having complex information content [Internet]. CA3047337A1, 2018. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DCA3047337A1.
- Galli R, Preusse G, Koch E, Steiner G, Krautwald-Junghanns M-EE, Bartels T. Method and device for the Raman spectroscopic, in ovo sex determination of fertilised and incubated birds’ eggs [Internet]. AU2015283366B2, 2016. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DAU2015283366B2.
- Schortgen, M. Non-invasive device for determining the fertility and/or the sex of an egg, and corresponding method [Internet]. US20170160202A1, 2017. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DUS10060854B2.
- Opitz J, Fischer B, Morgenstern P, Schreiber J, Gerich C. Methods for determining the sex of bird’s eggs [Internet]. US008364247B2, 2013. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DUS8364247B2.
- Dörksen H, Krahl J, Staufenbiel J. Device and method for in-ovo determination of the sex of a fertilised bird egg [Internet]. WO2021144420A1, 2021. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DWO2021144420A1.
- Popp J, Roesch P, Bartels T, Krause M, Harz M. Bird sex determination involves testing DNA relevant cell material of bird with light, and measuring molecule oscillations, where spectrum of molecule oscillations resulting from light is detected, and is compared [Internet]. DE102007013107A1, 2008. p. 1–5. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DDE102007013107A1.
- Tiersch, TR. Identification of sex in chickens by flow cytometry. Worlds Poult Sci J. 2003;59(1):25–32.
- Steiner G, Bartels T, Stelling A, Krautwald-Junghanns M-E, Fuhrmann H, Sablinskas V, et al. Gender determination of fertilized unincubated chicken eggs by infrared spectroscopic imaging. Anal Bioanal Chem [Internet]. 2011 Jul 9 [cited 2017 Oct 17];400(9):2775–82. Available from: http://link.springer.com/10.1007/s00216-011-4941-3. [CrossRef]
- Steiner G, Bartels T, Krautwald-Junghanns M-E, Koch E. Bird sexing by Fourier transform infrared spectroscopy. In: Mahadevan-Jansen A, Petrich W, editors. Biomedical Vibrational Spectroscopy IV: Advances in Research and Industry [Internet]. 2010. p. 75600D. Available from: https://proceedings.spiedigitallibrary.org/proceeding.aspx?doi=10.1117/12.841627.
- Steiner G, Koch E, Krautwald-Junghanns M-E, Bartels T. Method and device for determining the sex of fertilized, non-incubated bird eggs [Internet]. US8624190B2, 2014. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DUS8624190B2.
- May T, Weber K, Popp J. Method and arrangement for non-invasive non-destructive identification of molecule-specific and or biological properties of an internal structure of a biological examination item through an optically impenetrable barrier [Internet]. WO2014086335A1, 2014. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DWO2014086335A1.
- Zhang W, Brown ER, Rahman M, Norton ML. Observation of terahertz absorption signatures in microliter DNA solutions. Appl Phys Lett. 2013;102(2):2–6. [CrossRef]
- De Ketelaere B, Bamelis F, Kemps B, Decuypere E, De Baerdemaeker J. Non-destructive measurements of the egg quality. Worlds Poult Sci J. 2004;60(3):289–302. [CrossRef]
- Amigo JM, Babamoradi H, Elcoroaristizabal S. Hyperspectral image analysis. A tutorial. Anal Chim Acta. 2015;896:34–51.
- Göhler D, Fischer B, Meissner S. In-ovo sexing of 14-day-old chicken embryos by pattern analysis in hyperspectral images (VIS/NIR spectra): A non-destructive method for layer lines with gender-specific down feather color. Poult Sci [Internet]. 2016;96(1):1–4. Available from: https://academic.oup.com/ps/article-lookup/doi/10.3382/ps/pew282. [CrossRef]
- McKay, JC. Spectrophotometric analysis of embryonic chick feather color [Internet]. US20140069336A1, 2014. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DUS2014069336A1.
- Corion M, Keresztes J, De Ketelaere B, Saeys W. In ovo sexing of eggs from brown breeds with a gender-specific color using visible-near-infrared spectroscopy: effect of incubation day and measurement configuration. Poult Sci [Internet]. 2022;101(5):101782. Available from. [CrossRef]
- Khaliduzzaman A, Shinichi F, Ayuko K, Tetsuhito S, Yuichi O, Naoshi K. Non-invasive broiler chick embryo sexing based on opacity value of incubated eggs. Comput Electron Agric [Internet]. 2019;158(March):30–5. Available from. [CrossRef]
- Rahman A, Syduzzaman M, Khaliduzzaman A, Fujitani S, Kashimori A, Suzuki T, et al. Nondestructive sex-specific monitoring of early embryonic development rate in white layer chicken eggs using visible light transmission. Br Poult Sci [Internet]. 2020;61:209–16. Available from. [CrossRef]
- Fujitani, S. Nondestructive inspection device of hatching egg, and hatching egg inspection program used for the same [Internet]. JP2017227471A, 2017. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DJP2017227471A.
- Preusse G, Steiner G, Galli R, Koch E, Schnabel C. Method for in-ovo fertilisation determination and gender determination on a closed egg [Internet]. 2020. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DUS2020400640A1.
- Hebrank, JH. Methods and apparatus for detecting the presence of eggs in an eggs in an egg flat [Internet]. WO2006078499A2, 2006. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DWO2006078499A2.
- Wang M, Luo J, Wu L, Han D, Zeng Y. Device and method for gender identification of egg embryo based on heart rate measurement [Internet]. CN109431484A, 2019. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DCN109431484A.
- Wu L, Luo J, Wang M, Han D, Zeng Y. Egg embryo sex determination device based on heart rate measurement [Internet]. CN209611122U, 2019. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DCN209611122U.
- Rams P, Toellner A. Improvements in and relating to the determination of the sex of eggs of fowls [Internet]. GB457543A, 1935. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DGB457543A.
- Fujitani, S. Sexual orientation selection device of hatching egg before incubation [Internet]. JP2019017336A, 2019. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DJP2019017336A.
- Colvin, A. Devices and methods for determining analytes [Internet]. WO2018154389A1, 2018. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DWO2018154389A1.
- Li Q-X, Wang QH, Ma M, Xiao S-J, Shi H. Non-destructive detection of male and female information of early duck embryos based on visible/near infrared spectroscopy and deep learning. Spectrosc Spectr Anal. 2021;41(6):2021.
- Ngadi M, Liu L, Zheng C. Systems, devices, and methods for detecting fertility and gender of unhatched eggs [Internet]. WO2016131124A1, 2016. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DWO2016131124A1.
- Zhao X, Song Z, Guan Y, Tan F, Shang T, Di G, et al. Gender identification method for chick embryo in near-infrared hatching egg at earlier stage incubation [Internet]. CN103472008A, 2013. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DCN103472008A.
- Ngadi M, Liu L, Zheng C. Systems, devices, and methods for detecting fertility and gender of unhatched eggs [Internet]. US20200302604A1, 2020. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DUS2020302604A1.
- Rozenboim I, Ben Dor E. Hyperspectral Identification of Egg Fertility and Gender [Internet]. US20130044210A1, 2013. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DUS2013044210A1.
- Pan L, Hu P, Tu K, Zhang W, Sun Y, Li Z. Gender determination method for chicken hatching egg incubation early embryo based on hyperspectral image [Internet]. CN104316473A, 2015. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DCN104316473A.
- Green, Y. Method and system for spectral determination of egg gender and fertility [Internet]. WO2018235070A1, 2018. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DWO2018235070A1.
- Zhu Z, Hong Q, Wu L, Wang Q, Ma M. Early-stage chick embryo gender identification method [Internet]. CN109142248A, 2019. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DCN109142248A.
- Fischer B, Meissner S. Device for examining hatching eggs [Internet]. WO2019174661A1, 2019. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DWO2019174661A1.
- Fischer B, Meissner S. Apparatus for inspecting bird eggs [Internet]. JP2021099334A, 2021. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DJP2021099334A.
- Zhao X, Cai L, Song Z, Guan Y, Tan F, Tian F, et al. Near-infrared gender recognition device for chicken embryo in hatching egg during early stage of incubation [Internet]. CN103461218A, 2013. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DCN103461218A.
- Corion M, Keresztes J, De Ketelaere B, Saeys W. Innovative, non-invasive, fast and highly accurate visible- near-infrared in ovo sexing method on brown chicken eggs. In: 26th World’s Poultry Congress. [Online]: World’s Poultry Congress; 2020.
- Nageswara Rao, BD. Nuclear magnetic resonance: An early history. Resonance. 2015;20(11):969–85.
- Reynells RD, Flegal CJ. Method and apparatus for avian pre-hatch sex determination [Internet]. US6029080A, 2000. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DUS6029080A.
- Davenel A, Eliat PA, Quellec S, Nys Y. Attempts for early gender determination of chick embryos in ovo using Magnetic Resonance Imaging. XXII Eur Symp Qual Poult Meat XVI Eur Symp Qual Eggs Egg Prod. 2015;2–5.
- Haase A, Schusser BM, Molina-Romero M, Goméz PA, Aigner M, Huber S, et al. Automated noninvasive determining the sex of an embryo and the fertility of a brid’s egg [Internet]. WO2019092265A1, 2019. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DWO2019092265A1.
- Hergenröder R, Lambert J, Telfah A. Method for determining the sex of bird eggs in ovo [Internet]. WO2020126253A1, 2020. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DWO2020126253A1.
- Sewiolo B, Ziroff A. Method for determining the sex of an embryo in an egg [Internet]. US20160057977A1, 2016. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DUS2016057977A1.
- Taniguchi, R. Method for sexing fowl egg and apparatus therefor [Internet]. JP2001238559A, 2001. Available from: https://worldwide.espacenet.com/patent/search/family/026582006/publication/JP2001238559A?q=pn%3DJP2001238559A.
- Taniguchi, R. Method and apparatus for determining the sex of a fertilized egg [Internet]. US20040040515A1, 2004. Available from: https://worldwide.espacenet.com/patent/search/family/031977095/publication/US2004040515A1?q=pn%3DUS2004040515A1.
- Taniguchi, R. Method and apparatus for determining the sex of a fertilized egg [Internet]. US007167579B2, 2007. Available from: https://worldwide.espacenet.com/patent/search/family/027800473/publication/US7167579B2?q=US7167579B2.
- Taniguchi, R. Steric strain measuring method of egg [Internet]. JP2010038897A, 2010. Available from: https://worldwide.espacenet.com/patent/search/family/042011587/publication/JP2010038897A?q=pn%3DJP2010038897A.
- Taniguchi, R. Method for identifying sex of fertilized egg based on outline thereof [Internet]. JP2011142866A, 2011. p. 1–4. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DJP2011142866A.
- Taniguchi, R. Sex identification device for fertilized eggs, sex identification method for fertilized eggs, and program [Internet]. EP3878274A1, 2021. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DEP3878274A1.
- Yilmaz-Dikmen B, Dikmen S. A morphometric method of sexing white layer eggs. Rev Bras Ciência Avícola [Internet]. 2013;15(3):203–10. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1516-635X2013000300006&lng=en&nrm=iso&tlng=en.
- Zhu ZH, Ye ZF, Tang Y. Nondestructive identification for gender of chicken eggs based on GA-BPNN with double hidden layers. J Appl Poult Res. 2021;30(4). [CrossRef]
- Xiaohui D, Ziqiaojing Y, Zhengting L. Method forlossless determination of genders of poultry embryo eggs [Internet]. CN107041325A, 2017. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DCN107041325B.
- Li Z, Li R, Wang T. Method and monitoring device for early discriminating embryonic male and female of birds [Internet]. CN1189616A, 1998.Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DCN1189616A.
- Li, Z. Method and apparatus for judging sex of domestic chicken in embryo egg early incubation period [Internet]. CN1742569A, 2006. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DCN1742569A.
- Chen L, Zhang C, Xue X, Fan S, Huang W, Li J, et al. Sex detection system and method for chicken embryonated eggs [Internet]. CN110771533A, 2020. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DCN110771533A.
- Schart, C. Method and device for adjusting the laser focus of an excitation laser for vessels through which blood flows for optical measurements - Schart 2017.pdf [Internet]. EP3244197A1, 2017. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DEP3244197A1.
- Herzog S, Hurlin J. Method and system for determining the sex in-ovo or determining the sex of living birds [Internet]. WO2018115280A1, 2018. Available from: https://worldwide.espacenet.com/patent/search? 2018.
- Steiner G, Preusse G, Koch E, Galli R, Schnabel C. Producing a hole in a bird egg for determining the sex of the bird egg [Internet]. 2018. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DUS2019339244A1.
- Hurlin J, Meissner S, Fischer B. Method and apparatus for creating an opening in the calcified shell [Internet]. US10458967B2, 2019. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DUS10458967B2.
- Gokdag U, Cinar E. Application of genetic algorithm for egg gender determination. In 26th IEEE Signal Processing and Communications Applications Conference (SIU); 2018 [cited 2022 May 3]. Available from: https://www.webofscience.com/wos/woscc/full-record/WOS:000511448500190.
- Pan L, Zhang W, Yu M, Sun Y, Gu X, Ma L, et al. Gender determination of early chicken hatching eggs embryos by hyperspectral imaging. Nongye Gongcheng Xuebao/Transactions Chinese Soc Agric Eng. 2016;32(1):181–6.
- Taniguchi, R. Sex determination method of sperm egg and device thereof [Internet]. JP2018186779A, 2018. Available from: https://worldwide.espacenet.com/patent/search?q=pn%3DJP2018186779A.
- Rutkowska J, Dubiec A, Nakagawa S. All eggs are made equal: Meta-analysis of egg sexual size dimorphism in birds. J Evol Biol. 2014;27(1):153–60. [CrossRef]
- United Nations Statistics Division. UNData - A world of information [Internet]. 2022. Available from: https://data.un.org/Data.aspx?q=Number+of+R&d=FAO&f=itemCode%3A1067.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).