Background
The cornea is a transparent, avascular, watch-glass like structure which forms anterior one-sixth of the outer fibrous coat of the eyeball and covers iris, pupil, and anterior chamber. Histologically, it has described 5 distinct layers: Corneal Endothelium, a monolayer of interdigitating hexagonal cells of 5 microns of thickness. The Descemet membrane, with a thickness of 10-12 microns, and can be observed two layers: anterior organized fetal banded layer, with 3 microns of thickness, and posterior unorganized non-banded layer with a thickness between 2-10 microns.
The greatest layer is corneal stroma or substantia propria, with 500-600 microns of thickness, with ≈80 % of water by weight. It is constituted by parallelly organized lamellae of collagen I, IV, and V in a mucopolysaccharide matrix. Their cellular content is scarce, and they are called keratocytes, Langerhans´ cells, pigmented melanocytes, macrophages, and histiocytes.
The next layer is Bowman´s membrane, with 8-14 microns of thickness, composed by unorganized type I collagen fibers in a glucose-amino-glycans (GAG) matrix and its acellular. The outermost layer is the corneal epithelium or anterior epithelium with a thickness of 50 microns. 3 cell types are described according to their location: superior or external and are 3 or 4 layers of squamous cells. Then the middle layer consisting of between one and three layers of flattened cells with polygonal form (wing cells); and a layer of deep basal cells, which secrete type IV collagen; which forms the basal membrane.
Introduction
The word metabolism means continuous change and in regards tissues can be defined as the series of chemical processes by which mass and energy is concatenated allowing the normal structure and function of a tissue.
It has been described two types of metabolism: Aerobic metabolism, this is: metabolism with O
2 and Anaerobic Metabolism or metabolism without O
2. However, both are theoretical in 95 % [
1]. The main functions of metabolism in cornea are to maintain the structural integrity so the cornea transparency is optimal to visual functions. Supposedly it is achieved through corneal dehydration.
The metabolic rate of the corneal epithelium is 10 folds than stroma. Theoretically, glucose has an almost incredible double function providing biomass precursors (carbon chains) and energy at the same time, and this to all the cells of the organism, without exception.
The prevalent dogma is as follows: in the eye, in this case the cornea, glucose mostly comes from aqueous humor, tear and limbal capillaries also contribute minimal amount of glucose and O2, glucose will also derive from corneal glycogen stored in corneal epithelium cells. Thereafter, 1 mol of glucose will be converted to the pyruvic acid and produce 2 mol lactic acid and 2 mol of ATP. In the Krebs cycle, 1 mol of glucose will utilize the pyruvic acid and O2 to produces 36 mol of ATP. Sadly, these concepts are controversial in 95 %, thereby are theoretical in its main part.
Tracking back, was Lavoisier in Paris, and Priestley in London; whose started the deeply rooted dogma that eukaryotic cell carefully combines oxygen with glucose to get energy, in other words, it is something like an improbable graduated combustion since oxygen combination occurs suddenly, like a flame. This chemical process cannot be fully explained and even less demonstrated so far, therefore the described reactions that are supposedly involved are theoretical in an astounding 95 %.
Although the cornea is avascular tissue except for a limited peripheral zone, the currently accepted metabolic requirements of living and respiration of the tissue suggest a negligible oxygen penetration from the cornea´s outer capillary network [
2].
Purportedly, the epithelium, stroma, and endothelium consume atmospheric oxygen without explanation of specific mechanisms. The necessary oxygen seems to comes from different structures such as: capillaries of the limbus, this is the limbal vasculature, and the precorneal tear film, with a PpO
2 of 155 mm Hg. By other hand, the endothelium obtains oxygen from aqueous humor with a PpO
2 pressure of 40 mm Hg. Therefore, the cornea allegedly respires primarily across its anterior and posterior surfaces [
3]. However, speed diffusion of oxygen in water (anterior chamber, aqueous tear layer) is extremely slowly. The diffusion coefficient of a gas is directly proportional to its solubility and inversely related to the square root of its molecular weight (MW):
But it is valid under ideal circumstances. We must keep in mind that atmospheric oxygen concentration is relatively low, and furthermore the cell must separate it from nitrogen, besides that tear film composition tends mainly to repeal more than attract atmospheric oxygen. Thereby, our finding about the unsuspected capacity of eukaryotic cell to dissociate the water molecules has an unusual importance because explains the presence of relatively high levels of molecular oxygen (O2) inside the cell, interstitial liquid, blood, aqueous humor, vitreous body, cephalospinal fluid, etc.
In the liquid phase, diffusion rates of gases are generally 10,000 to 600 000 times smaller than those in gaseous environments due to the much shorter mean free path between collisions with other molecules [
4].
Endothelium, epithelium, and stroma use 21, 40, and 39 % respectively of the total oxygen consumption of the cornea. Based on volumes of oxygen per unit volume tissue, epithelial oxygen utilization is about ten times that of the stroma and approximately 0.2 of the endothelia.
In regards the precorneal film, with a thickness ranged from 9 µm in frogs to 15 µm in gerbils (no tear film was detected in fish) measured with confocal microscopy that permit more accurate measurements of the separation between tear film and epithelial surfaces.
Figure 2.
This photographic computer-assisted effect gives an approximate idea of the thousands or millions of cells that normally conform the ocular surface, but cell respiration means that only expels CO2, but it cannot do something approximate to oxygen absorption, like the lung that does not absorbs atmospheric oxygen, and only expels CO2.
Figure 2.
This photographic computer-assisted effect gives an approximate idea of the thousands or millions of cells that normally conform the ocular surface, but cell respiration means that only expels CO2, but it cannot do something approximate to oxygen absorption, like the lung that does not absorbs atmospheric oxygen, and only expels CO2.
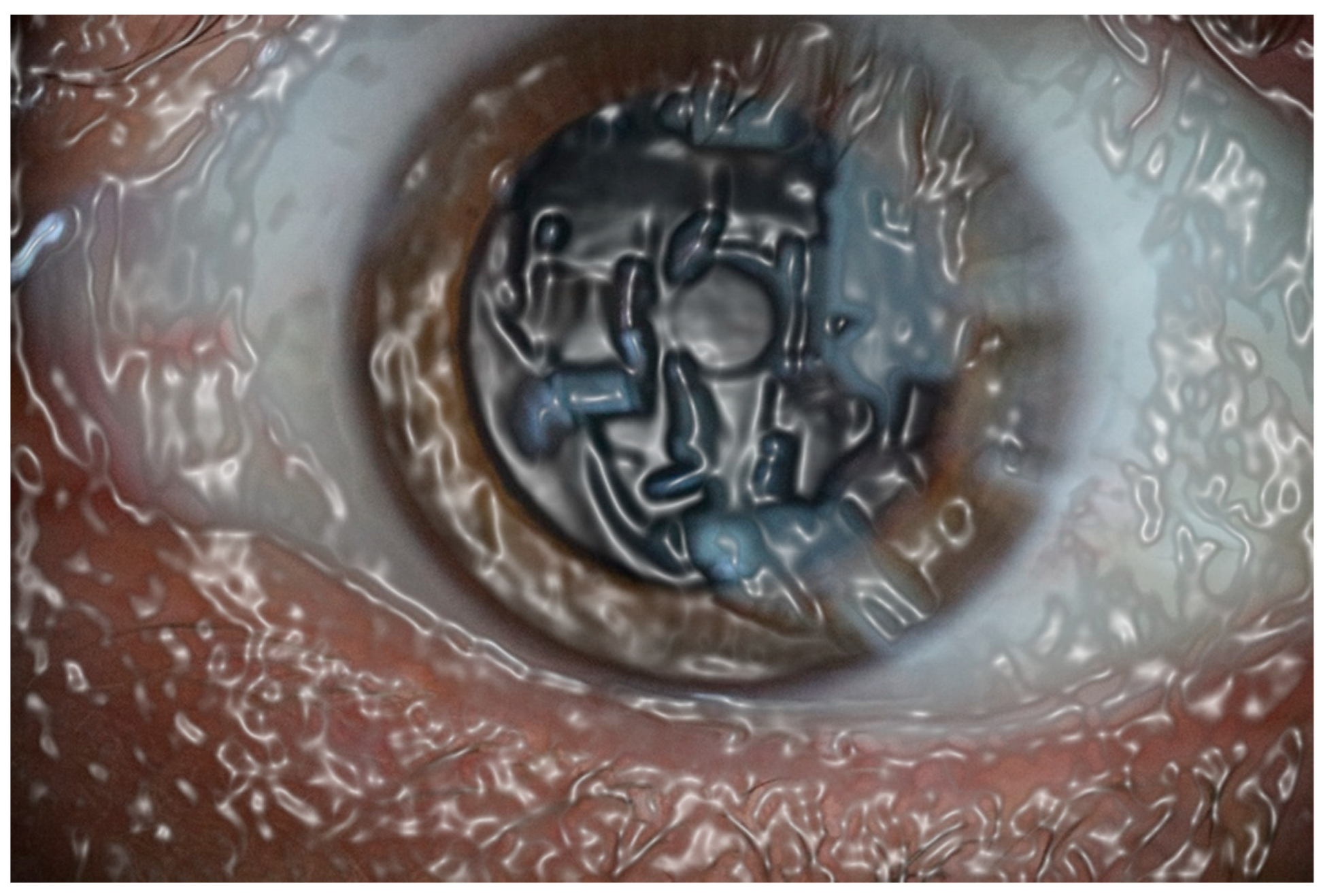
Figure 3.
This computer-assisted effect of a tear film with high viscosity means a significant distortion of retinal image. This would be the appearance of the mucin layer, the closest to the conjunctival and corneal cells, when removing the aqueous layer of the tear film.
Figure 3.
This computer-assisted effect of a tear film with high viscosity means a significant distortion of retinal image. This would be the appearance of the mucin layer, the closest to the conjunctival and corneal cells, when removing the aqueous layer of the tear film.
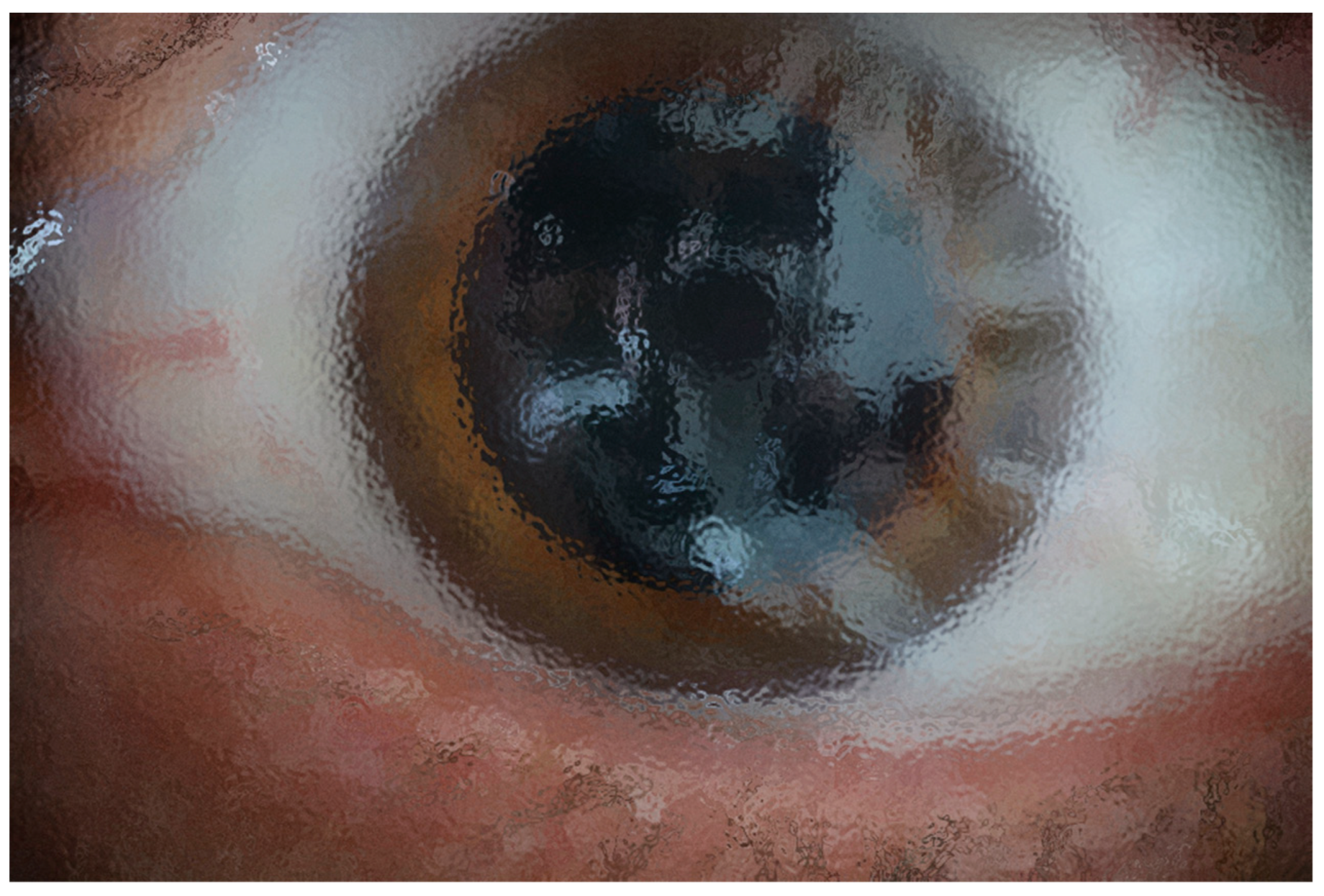
Figure 4.
In this computer-assisted effect, shows distortion of tear film with low viscosity but even higher than normal, therefore retinal image also was blurred.
Figure 4.
In this computer-assisted effect, shows distortion of tear film with low viscosity but even higher than normal, therefore retinal image also was blurred.
Figure 5.
In this computer-assisted effect of a photography taken only with natural light, the simulation of a high-viscosity tear film shows the significant image distortion that could be produced. The mucin layer, closest to conjunctival and corneal cells, functions as an interface between the aqueous layer of the tear film and cell membranes, decreasing water repulsion, providing support so that the tear film does not collapse and spread evenly across the entire width and length of the ocular surface.
Figure 5.
In this computer-assisted effect of a photography taken only with natural light, the simulation of a high-viscosity tear film shows the significant image distortion that could be produced. The mucin layer, closest to conjunctival and corneal cells, functions as an interface between the aqueous layer of the tear film and cell membranes, decreasing water repulsion, providing support so that the tear film does not collapse and spread evenly across the entire width and length of the ocular surface.
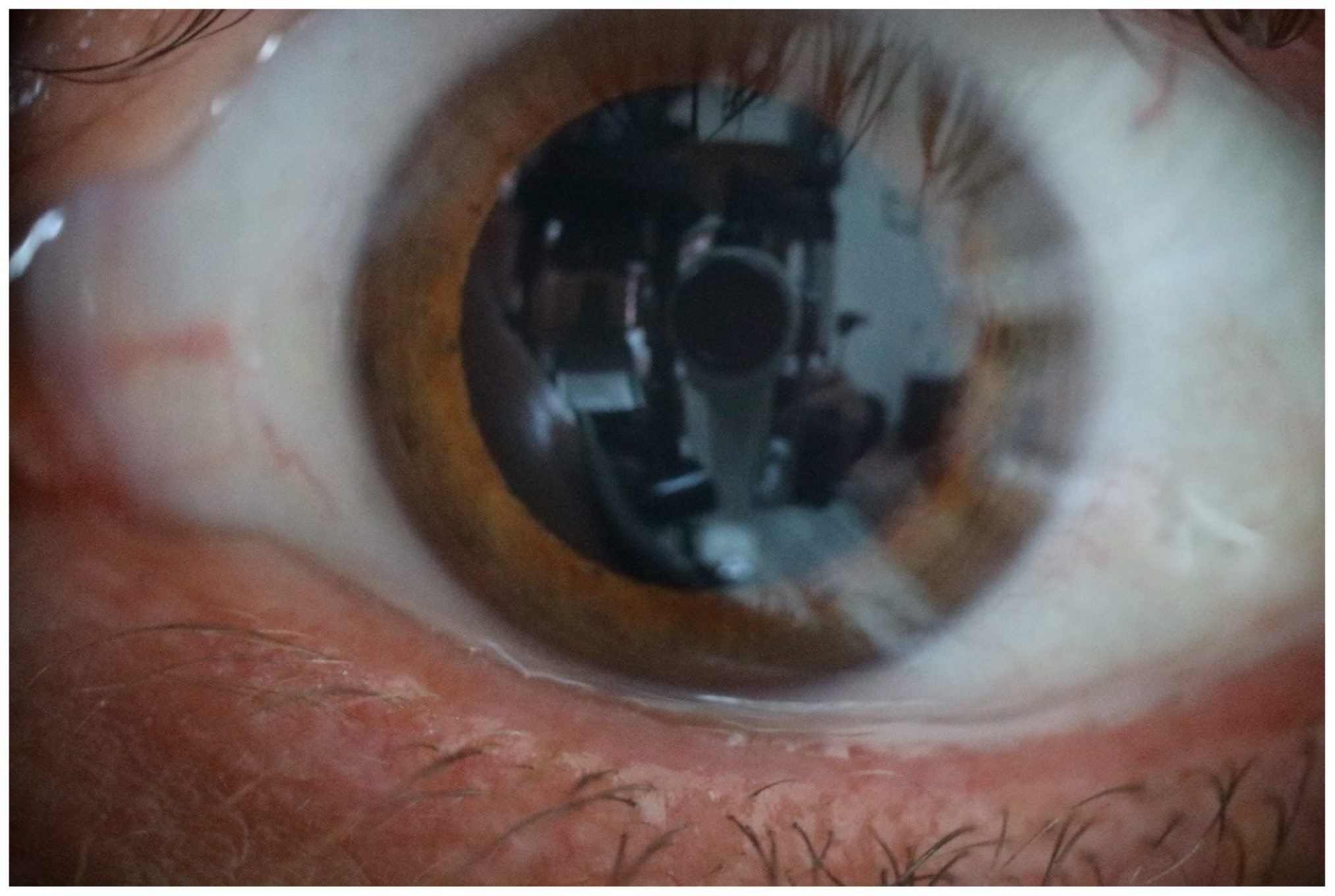
Figure 6.
This photograph taken with natural light only, shows the homogeneity of normal tear film and the clear image produced.
Figure 6.
This photograph taken with natural light only, shows the homogeneity of normal tear film and the clear image produced.
Figure 7.
Photography taken with natural light alone, where the image distortion is produced by simulated medium viscosity of tear film. The aqueous layer of the tear has an adequate thickness so that the surface of the tear film has a homogeneous surface allowing a sharp image.
Figure 7.
Photography taken with natural light alone, where the image distortion is produced by simulated medium viscosity of tear film. The aqueous layer of the tear has an adequate thickness so that the surface of the tear film has a homogeneous surface allowing a sharp image.
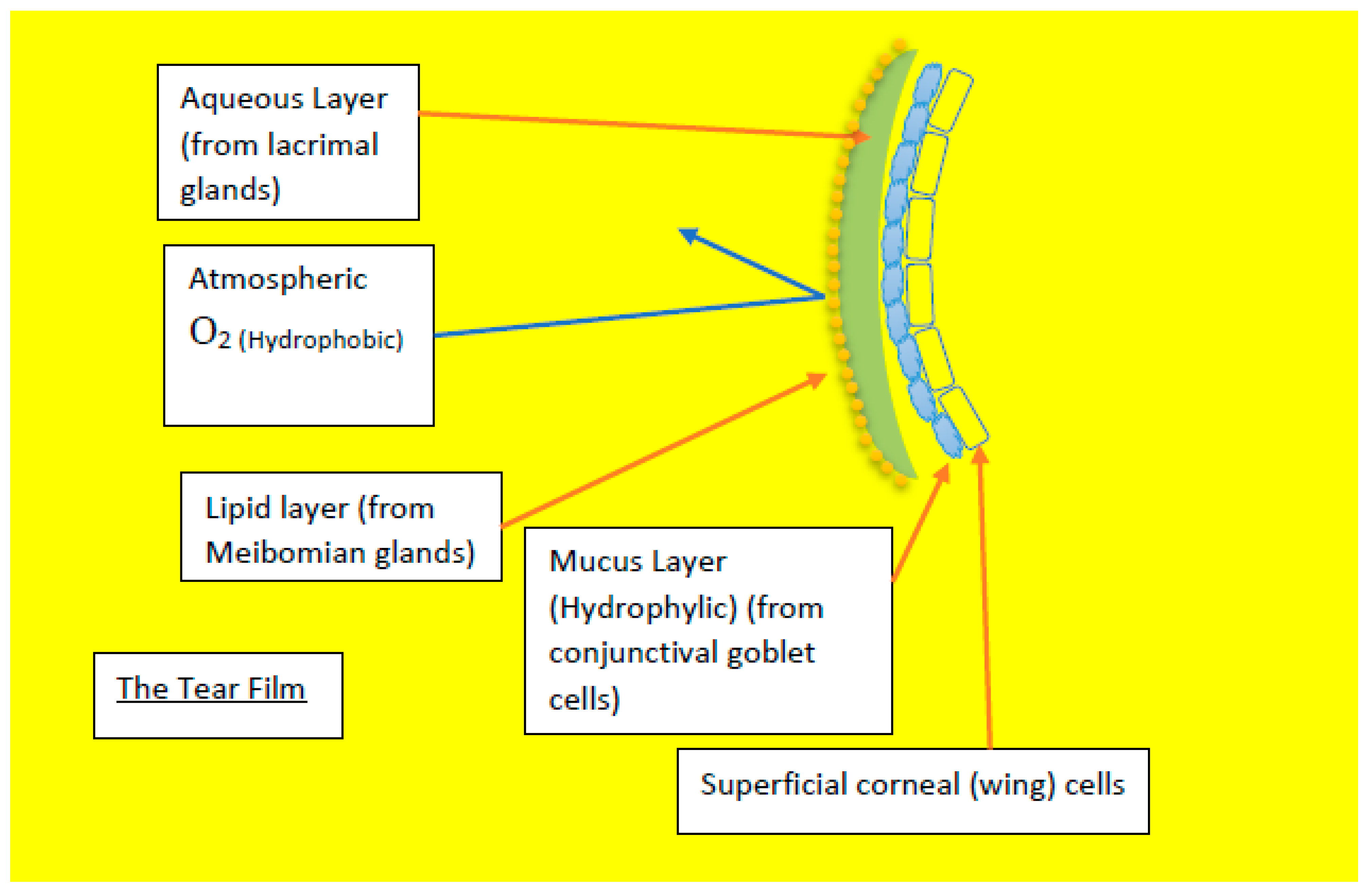
Figure 8.
Diagram showing the different components of the tear film that avoids the oxygen diffusion through tear film.
Figure 8.
Diagram showing the different components of the tear film that avoids the oxygen diffusion through tear film.
Thereby, when any gas is in contact with water, some gas will dissolve in the liquid (Henry´s Law) because water tends to repel oxygen and other gases, thereby, oxygen cannot pass through the water of precorneal film in any direction. The minimal amount that dissolves at a particular temperature depends on the pressure, or partial pressure of the gas. The dissolved gas and the undissolved gas are in equilibrium, with rapid forward and reverse reactions (under ideal conditions), there will be an equilibrium constant that give us information about the lowest energy state of the system.
Table 1.
Table of calculate values.
Table 1.
Table of calculate values.
| Gas |
KH (atm/M) |
| O2
|
769.23 |
| H2
|
1282.05 |
| CO2
|
29.41 |
| N2
|
1639.34 |
The data in the table are for equilibrium between the aqueous gas and the free gas.
To calculate the concentration of the molecule in solution:
Dissolved Oxygen and Henry’s Law
Oxygen dissolved in water can come from the atmosphere or mainly as a byproduct of the photosynthesis in aquatic plant. For water in balance with the atmosphere, the concentration is governed by Henry´s Law, and KH for O2 is 769.23. We can calculate the concentration of O2(aq) to be 0.00027 M.
Water away the air/ water interface is not necessary in equilibrium with the air and can have even less oxygen than this quite small value. In fact, this is typical for the oxygen concentration in lakes and in the ocean to decrease with depth in the water.
Water in movement with shallow streams mixes better with air and tends to have a higher concentration than still water. Atmospheric pressure is lower at higher altitudes so water at higher elevations hold less dissolved oxygen than water at sea level.
The dissolved oxygen levels are higher in the summer and during daylight hours because this is when photosynthetic organisms produce more oxygen. At higher water temperature should be lower oxygen content.
Corneal tissues are most vulnerable to lowered dissolved oxygen levels in the early morning on hot summer days, and when precorneal film amount are low, water temperatures are high, and photosynthetic molecules have not produced enough oxygen through water dissociation.
AT 37 °C dissolved oxygen is 6.71 mg/L. At 33 and 34 °C there is 7.16 mg/L of dissolved oxygen in the precorneal film. (
Figure 1). However, this dissolved oxygen is coming from the water dissociation that happens unceasingly inside every cell, and not from the atmosphere [
5].
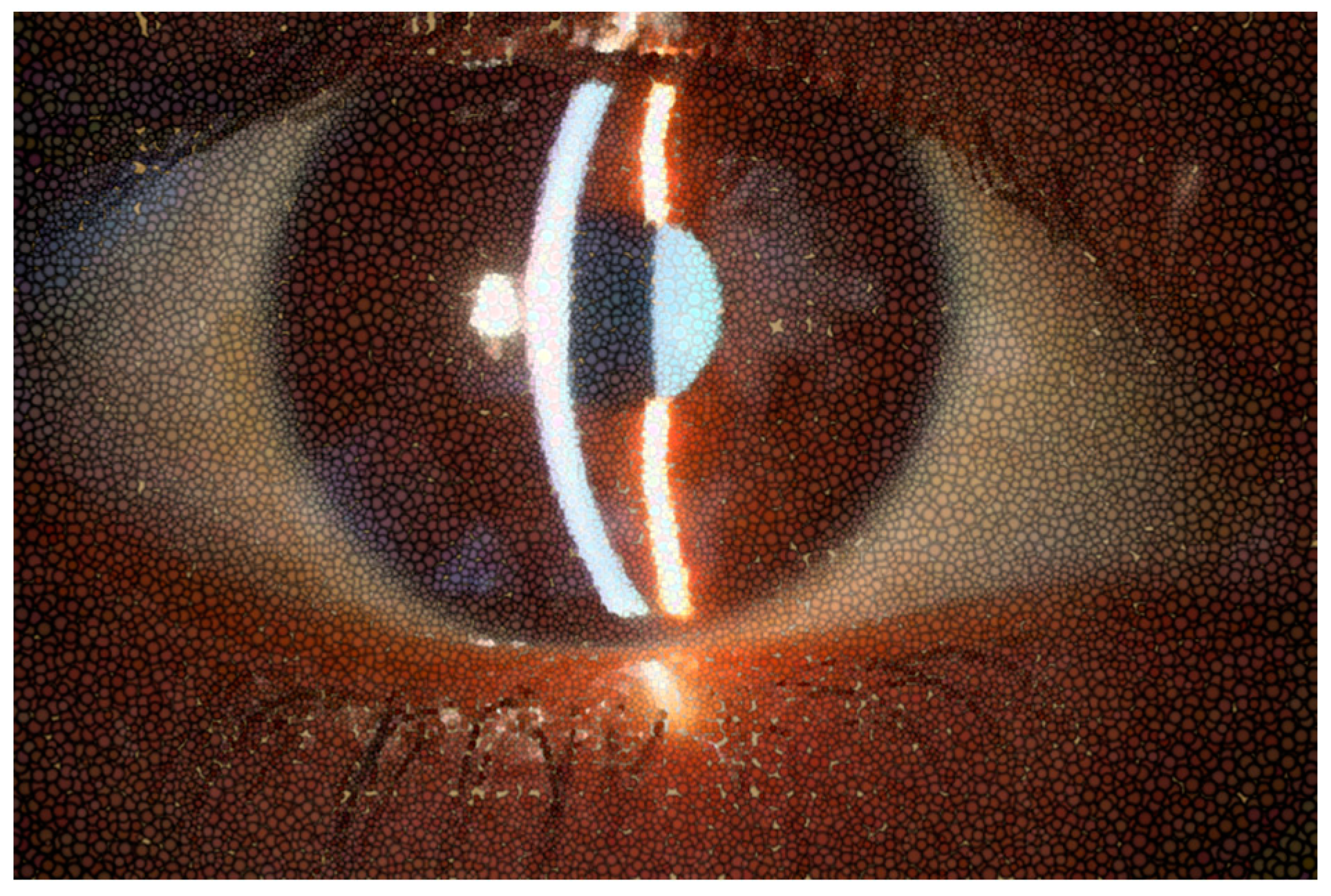
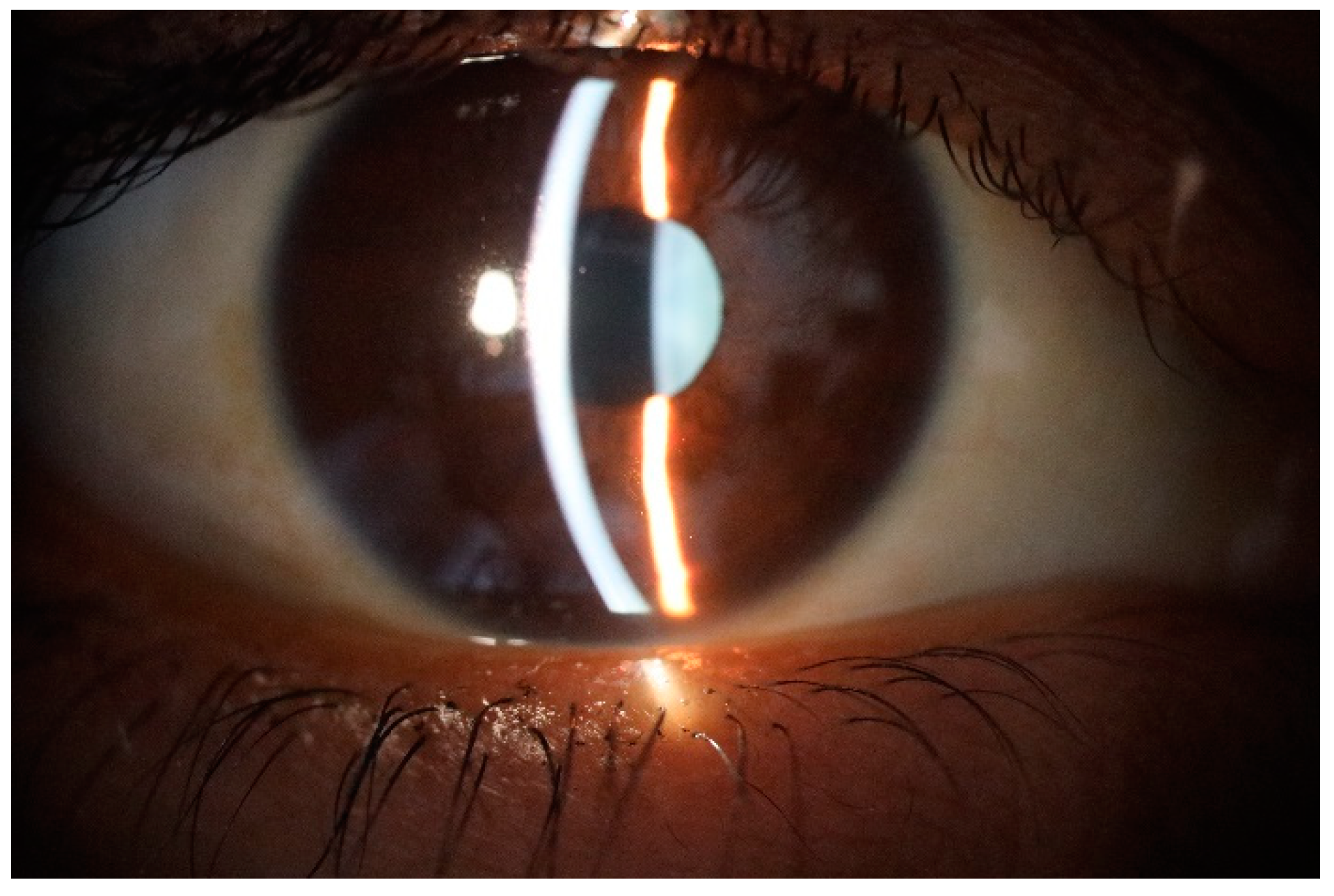
Figure 1.
Normal appearance of precorneal tear film photographed with slit lamp or biomicroscope.
Figure 1.
Normal appearance of precorneal tear film photographed with slit lamp or biomicroscope.
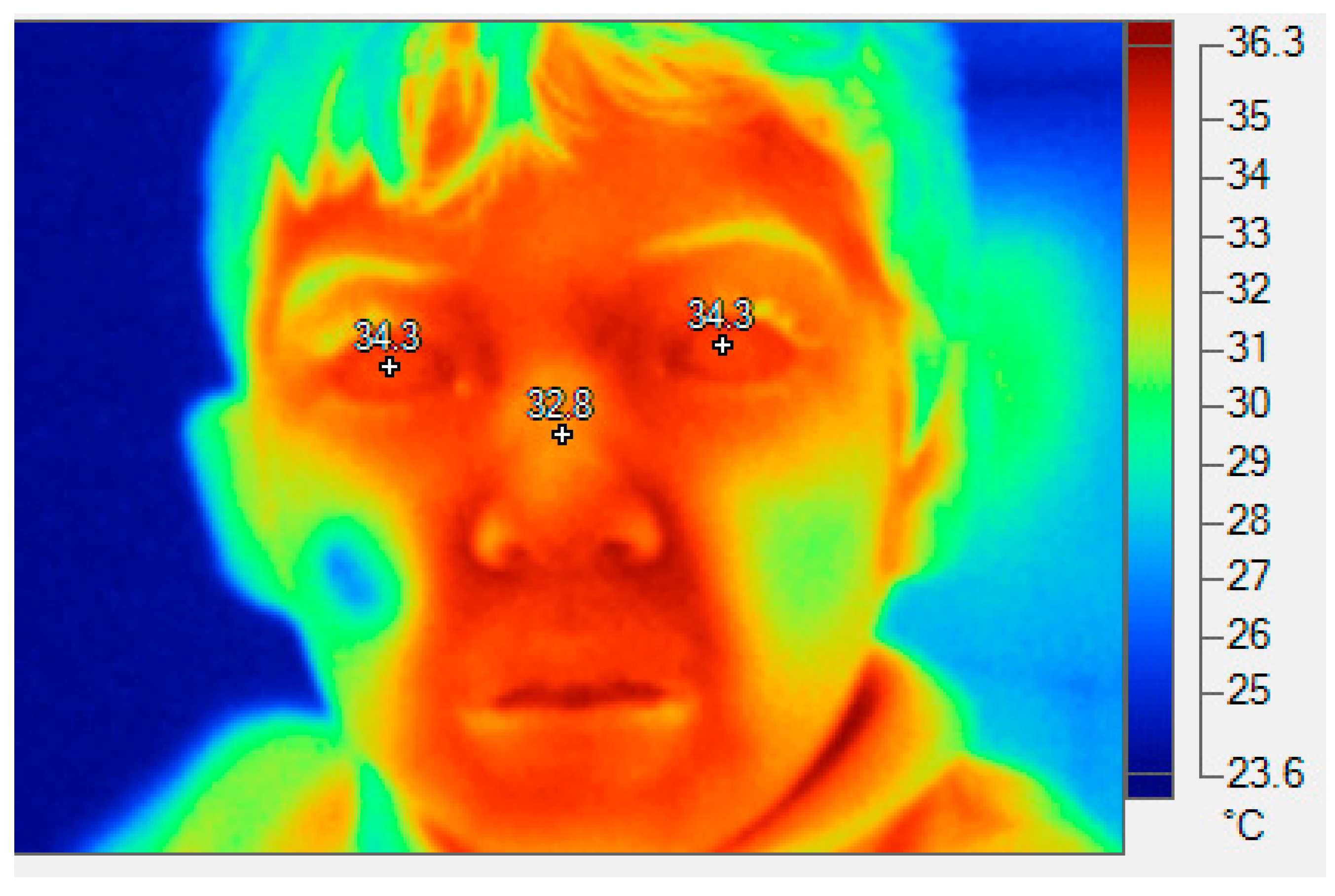
Figure 9.
A thermographic image of a child, showing the approximate temperature of the corneas. This is 34.3 ° C, in both eyes.
Figure 9.
A thermographic image of a child, showing the approximate temperature of the corneas. This is 34.3 ° C, in both eyes.
Traditionally, to corneal metabolism, the so far considered main factors are glucose, oxygen, and ATP. During aerobic metabolism, through Krebs cycle, 36 ATP are produced. Oppositely, during anaerobic metabolism, through glycolysis, 2 ATP are produced, and through Pentose shunt, a little bit more amount of ATP is generated, but not as much as in aerobic conditions.
Anaerobic metabolism is present normally in fetus and in cancer patients, but it is a phenomenon poorly understood. Supposedly, similar anaerobic condition in the cornea happens when a plastic contact lens is glued to the stroma. But this is a type of inefficient metabolism that can occur even in normoxia conditions.
Theoretically, eukaryotic cells like corneal epithelium cells obtain energy in form of ATP by the breakdown of glucose into lactic acid, CO2, and H2O.
The very structure of the mitochondrial membrane supposes a strong discrepancy in the biochemical/biomechanical model of the electron flow is, which is impermeable to the passage of the NADH reduced by the extra-mitochondrial glycolysis. Furthermore, one still cannot explain the fact that every NADH donates two electrons, for instance, while every O2 molecule needs four electrons to generate water, this is: 2H2O.
Experimental Background
In the first half of the past century, it was made studies trying to determine the respiration of the cornea [
6]. In England, researcher assumes that cornea possesses a respiratory mechanism, therefore gases are actively transpired through the agency of epithelium and endothelium [
7]. Some experimental results in rabbits, suggested the possibility that the endothelium uses the oxygen from the atmosphere.
But as the experimental circumstances were far from physiological (eyeballs enucleated, the epithelium scraped off, or there was not precorneal film), it was not possible to those researchers to determine whether also in the living animal the oxygen passes through the cornea from the air towards the anterior chamber.
Some research also was done in luxated eyes of rabbits, under a small glass bell, comparing the respiration of a normal cornea with that of a cornea deprived of its layers of epithelium and endothelium, through assessment of consumption of oxygen or the production of carbon dioxide. [
8]. They found that the CO
2 production and the O
2 consumption was similar after one hour between the normal and abnormal cornea. Only after four hours, the CO
2 production was the same for both corneas, but the O
2 consumption of the normal cornea was about 60 % greater than the consumption of abnormal cornea. This investigator assumes that the cornea forms a barrier to the permeation of O
2 and CO
2 only in one direction, being the oxygen able to travel only backwards through the cornea from the air towards the anterior chamber and carbon dioxide being able to pass through the cornea in opposite direction.
Interestingly, when the glass-bell was filled up with 100 % CO2, within one or two hours, the cornea became totally opaque, and second, absence of Oxygen in the surrounding atmosphere does not inhibit normal life of the cornea. This abnormally high CO2 concentration (100%), inhibit completely the highly accuracy of molecular mechanisms of water dissociation that are fundamental piece of life and functioning of the cell and therefore transparency of the cornea. The finding that in complete absence of oxygen in the atmosphere inside the glass-bell, the normal life of the cornea is unaffected, is compatible with the unraveling of the intrinsic property of several molecules inside the eukaryotic cell that can transform light power into chemical energy, through water dissociation, like happens in plants.
Bakker, some years later, replicate the experiments, but with albine rats, and with histological assessment, he dimensioned the effects of gases in situ, this is: on the endothelium cells. He places a few sutures in the eyelids to prevent closing.
Bakker found that after 12 hours in an atmosphere of pure nitrogen, the cornea remained quite clear and microscopically no injurious influence was found. Furthermore, Bakker concludes that in normal circumstances the cornea does not use the oxygen from the surrounding atmosphere. In regards CO2, he found that under 100 % CO2 atmosphere, within half an hour, the cornea became totally opaqued.
If carbon dioxide normally diffuses away through the cornea from the anterior chamber towards the surrounding atmosphere, it is true that a concentration of 8% of carbon dioxide outside the eye is high enough to impede he eventual permeation of CO
2 through the cornea. Histologically, it was found an abnormally high number of cell divisions, possibly as consequence of an irritating action of CO
2 [
9]. One manifestation of low levels of water dissociation and thereby insufficient levels of oxygen (and hydrogen) inside the cell is, precisely, decontrolled mitosis.
Thereby the knowledge concerning respiration of the cornea is ambiguous, however, the question whether the cornea uses the oxygen of the surrounding air now is close from settled.
The Precorneal Film
The behavior of precorneal tear film is like a fluid shell with minimal effect of blinking and saccades on tear film distribution and dynamics [
10]. Clinical studies using laser interferometry and confocal microscopy, have been shown that the tear film structure was found to be different from earlier descriptions. Mucus, and not aqueous fluid, formed a substantial portion of the film. Thickness studies of precorneal tear film gave values of about 40 µm, more than four times greater than previous estimates [
11].
During a blink, the upper lid reaches speeds of up to 30 cm/sec [
12] and the eye rotates several degrees in the opposite direction [
13] and moves into the orbit by up to 1.6 mm. Pressures of 10-51 mmHg have been measured between lid and eye during lid closure [
14].
The etiology of many tear film disorders is not understood. Sometimes there is inflammation of the lacrimal gland with less production of aqueous fluid [
15].
Water: Properties and Behavior
Despite its scarcity across the universe, water is so abundant on Earth that we aren’t always aware of how special it is. For starters, water is the only substance that exists naturally on our planet as a solid (ice and snow), liquid (rivers, lakes, and oceans), and a gas (water in the atmosphere as humidity). As you might recall (or can read about in our module on States of Matter), water molecules are in a different energy state in each phase. The amount of energy required to go from solid to liquid and liquid to gas is related to how water molecules interact with each other. Those interactions are, in turn, related to how the atoms within a water molecule interact with each other.
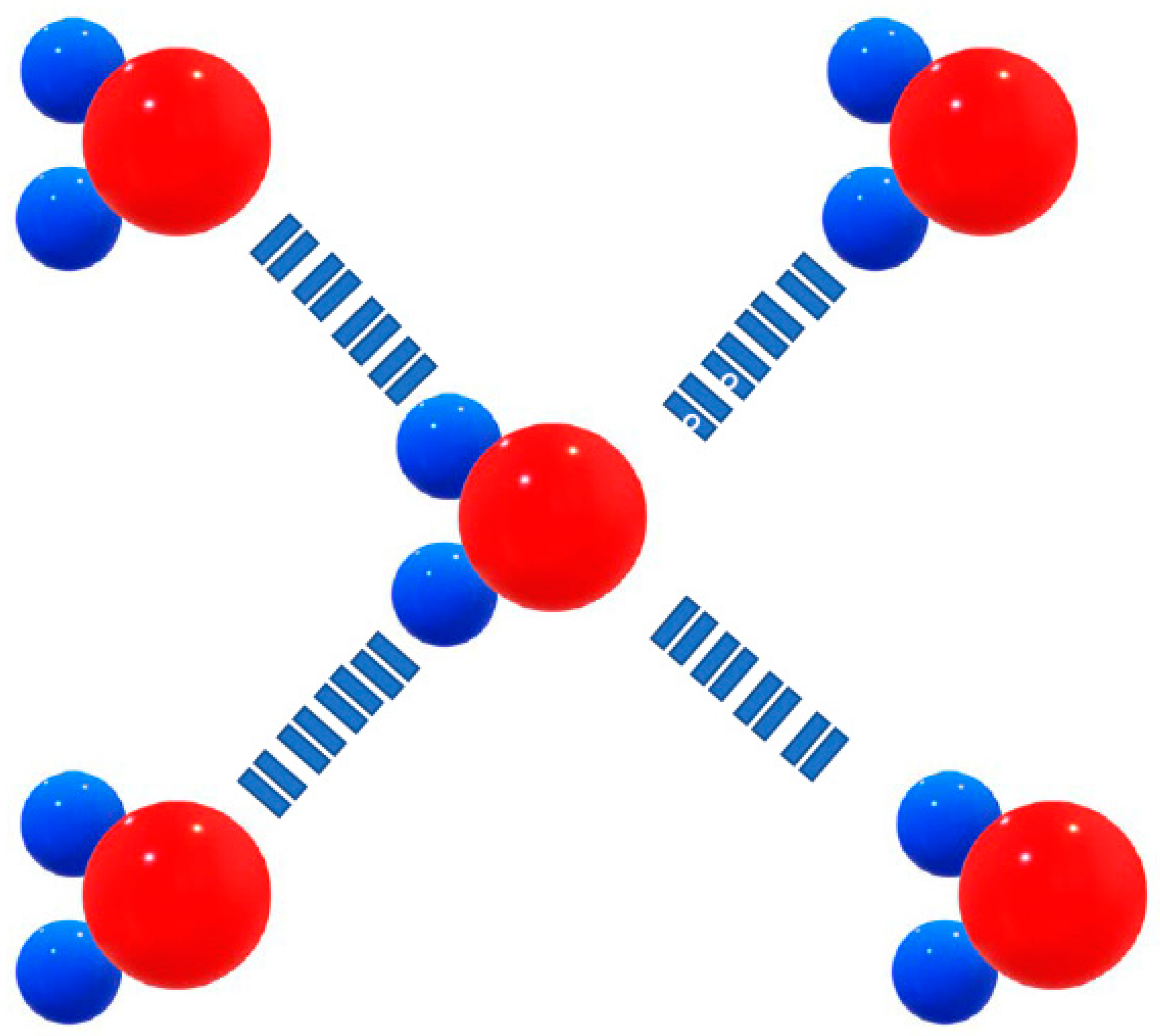
In the bond between oxygen and hydrogen, the electrons are shared unequally, drawn a bit more to the oxygen. As a result, a partial negative charge (ð-) forms at the oxygen end of the molecule, and a partial positive charge (ð+) forms at each of the hydrogen atom ends.
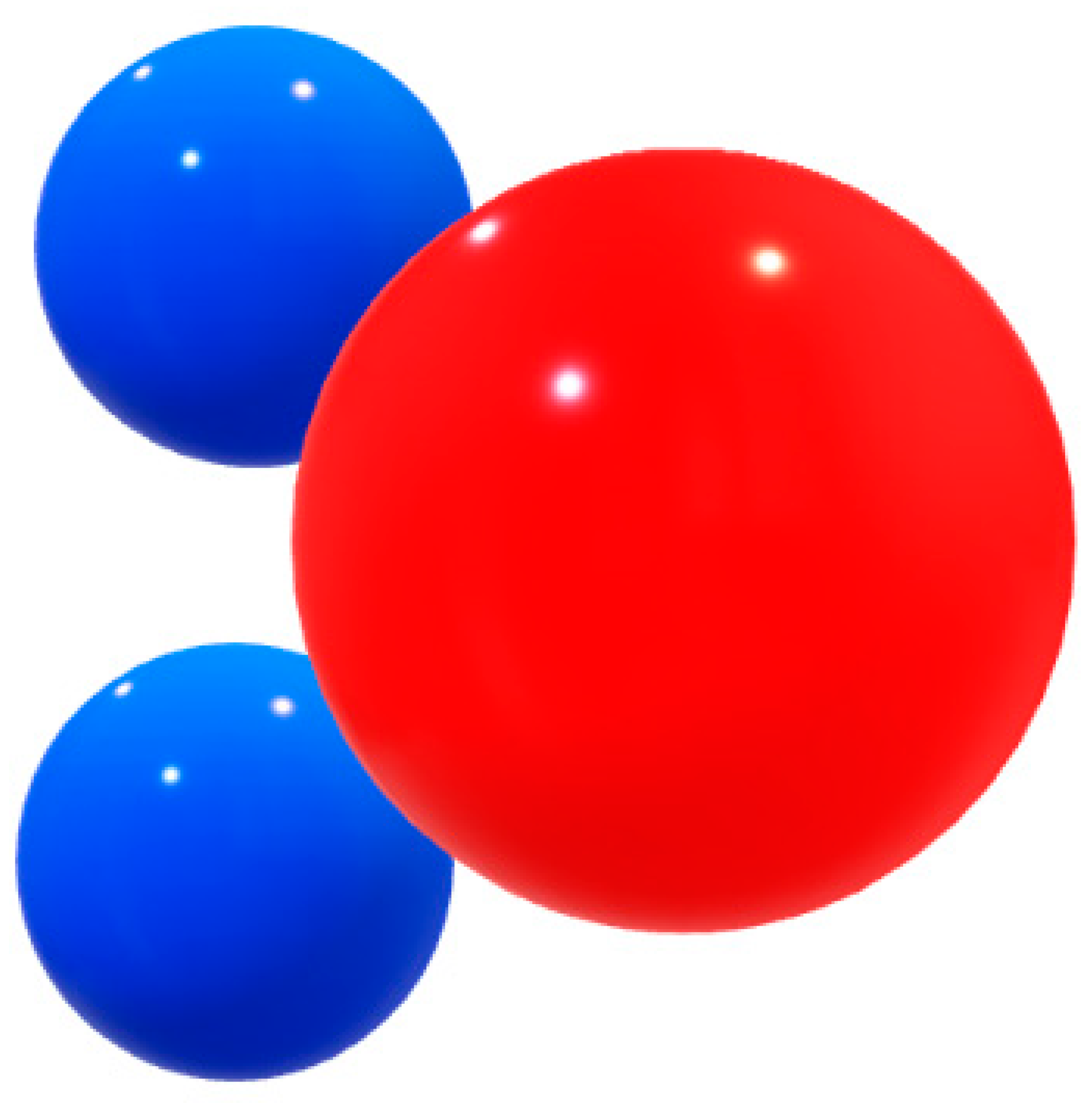
Figure 10.
Blue spheres: Hydrogen, with a partial positive charge (δ+), red ball represents Oxygen, with a partial negative charge – (δ-).
Figure 10.
Blue spheres: Hydrogen, with a partial positive charge (δ+), red ball represents Oxygen, with a partial negative charge – (δ-).
Since the hydrogen and oxygen atoms in the molecule carry opposite (though partial) charges, nearby water molecules are attracted to each other like tiny little magnets. The electrostatic attraction between the ð+ hydrogen (ð stands for partial charge, a value less than the charge of an electron) and the ð- oxygen in adjacent molecules is called hydrogen bonding (
Figure 11).
Figure 11.
Hydrogen bonds between water molecules. The slight negative charge on the oxygen atom is attracted to the slight positive charge on a hydrogen atom.
Figure 11.
Hydrogen bonds between water molecules. The slight negative charge on the oxygen atom is attracted to the slight positive charge on a hydrogen atom.
In general terms, properties of water arise from hydrogen bonding. Thus, the liquid flows because intermolecular attractions can be broken and reformed. A liquid freezes when the kinetic energy (movement) is reduced (i.e., the temperature is reduced) enough that the attractive forces between molecules can no longer be broken, and the molecules become locked in a static lattice.
In the case of water, though, the shape of the molecule and the strength of the hydrogen bonds affect the arrangement of the molecules. In liquid water, hydrogen bonding pulls molecules closely together. As water freezes, the dipole ends when charges repel each other, forcing the molecules into a fixed lattice in which they are farther from each other than they are in liquid water. More space between molecules makes the ice less dense than liquid water, and thus it floats.
Water is sometimes referred to as the “universal solvent,” because it dissolves more compounds than any other liquid known. The polarity of the water molecule allows it to readily dissolve other polar molecules, as well as ions. Most biological molecules, such as DNA, proteins, and vitamins are polar, and important ions such as sodium and potassium are also charged. In order for any of these compounds to carry out functions in the body, they have to be able to circulate in the blood and the fluid within and between cells, all of which are mostly water. Because of its polarity, water can dissolve these and other substances, allowing their free movement around the body. A few biomolecules, such as fats and cholesterol, aren’t polar, and don’t dissolve in water – however, the body has developed unique ways to circulate and store these substances.
O2 is not a polar molecule; it dissolves because the polar charges in the water molecule induce a temporary and relatively weak dipole in the molecular oxygen. Cohesion occurs when molecules of the same kind are attracted to each other. In the case of water, the molecules form strong hydrogen bonds, which hold the substance together. Water is the highest cohesive non-metallic liquid.
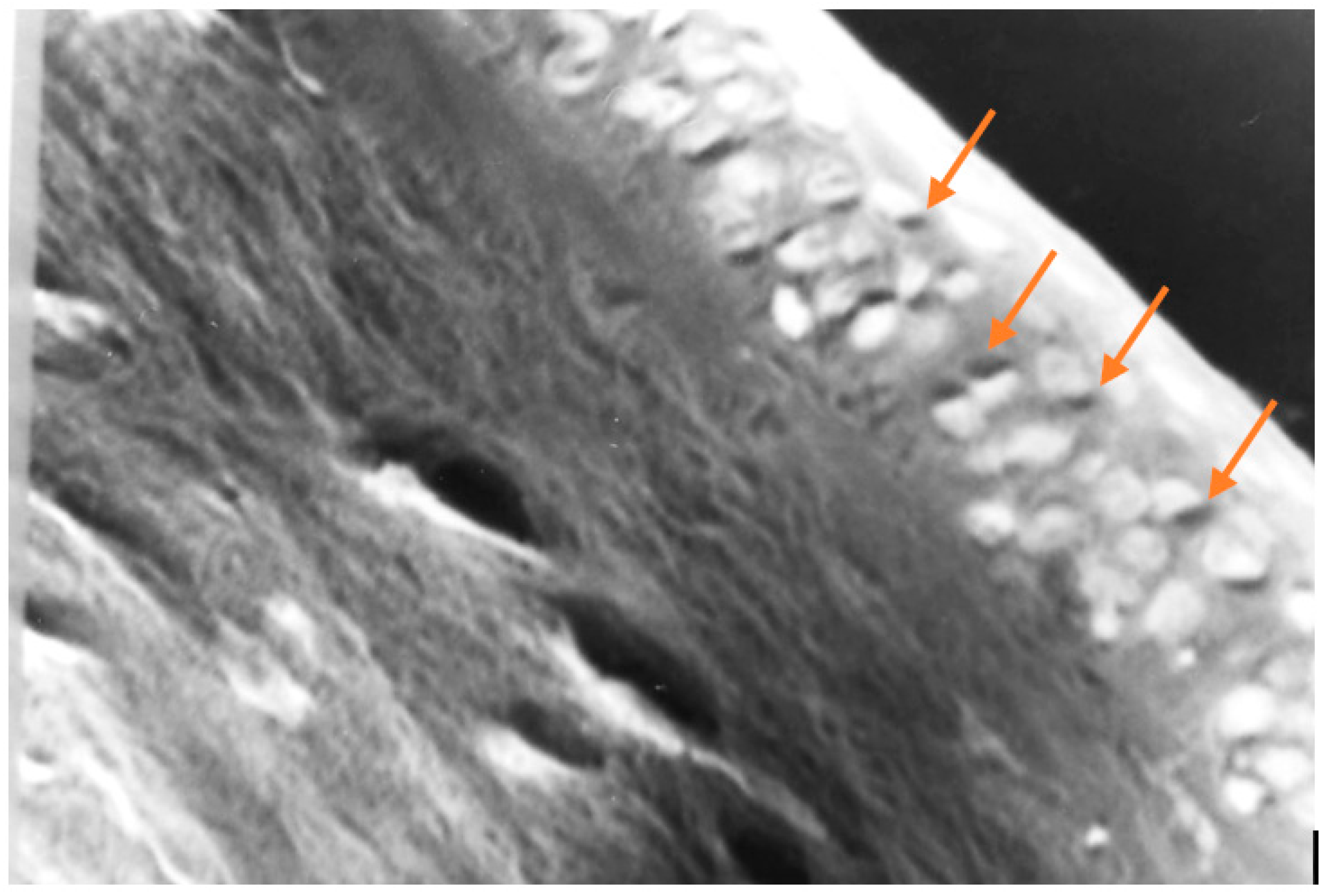
Figure 12.
In this photomicrograph taken with a light microscope, and then digitally processed, the collagen fibers that make up the corneal stroma can be seen on the left, and on the right the cells that make up the epithelial layer of the cornea. The yellow arrows point to dark spots, within some epithelial cells, which could correspond to melanin. Melanin is not easy to identify under the microscope, not even by histochemistry. (100 X, H & E).
Figure 12.
In this photomicrograph taken with a light microscope, and then digitally processed, the collagen fibers that make up the corneal stroma can be seen on the left, and on the right the cells that make up the epithelial layer of the cornea. The yellow arrows point to dark spots, within some epithelial cells, which could correspond to melanin. Melanin is not easy to identify under the microscope, not even by histochemistry. (100 X, H & E).
Figure 13.
The photomicrograph of
Figure 12, with a computer-assisted effect to give an idea of how the tissues would look in the presence of water. Let us remember that they are normally dehydrated during staining on purpose to study the histological sections under a microscope examination making visible intracellular structures that otherwise appear invisible under a light microscope.
Figure 13.
The photomicrograph of
Figure 12, with a computer-assisted effect to give an idea of how the tissues would look in the presence of water. Let us remember that they are normally dehydrated during staining on purpose to study the histological sections under a microscope examination making visible intracellular structures that otherwise appear invisible under a light microscope.
Conclusion
Water is the only substance that exists naturally on our planet as a solid (ice and snow), liquid (rivers, lakes, and oceans), and a gas (water in the atmosphere as humidity). And now we can add a fourth state in which water unfolds inside the eukaryotic cell, and provides microbubbles of hydrogen and oxygen, which come from the unsuspected ability of the human body to dissociate the water molecule through various molecules, like the plants.
At this time, the most important molecule in this regard is melanin, since it is the only one capable of dissociating water and reassociating hydrogen and oxygen, as melanin seems to tolerate oxygen toxicity quite well. The rest of the described molecules that can dissociate water but irreversibly (they do not re-form it) are hemoglobin, myoglobin, cytochrome P 450, and bilirubin [
16].
The formation of microbubbles in eukaryotic cells involves reorganizing cell biology, since by dissociating water, the cell obtains oxygen and hydrogen at the same time. Until now it was only thought that plants could transform the power of light into chemical energy, but our observation completely changes the dogma and reduces the role of glucose to the universal precursor of biomass, destroying the belief that it also provided energy [
17].
The mechanism by which the cell extracts oxygen from the water it contains is astonishingly exact and has not changed since the dawn of time. But said accuracy is disturbed by polluted water, polluted air, pesticides, herbicides, fertilizers, phosphates, solvents, industrial waste, alcohol, external temperatures, etc., and by unbalancing this fundamental process for life, cells simply disorganize and manifest some type of disease or disorder. It seems that all diseases start at this level, for as long as the levels of oxygen and hydrogen that the cell obtains from the dissociation of water are within the narrow range of life's parameters, cell functions well, as it has done ever since the beginning of time.
The etiology of many tear film disorders is not understood, but it was thought that the high levels of oxygen that we have inside our cells and fluids came from the atmosphere, but when the oxygen in the atmosphere was characterized in 1750, it was also found to be relatively low. 100 years later, in 1850, when the study of biochemistry was deepened, it was found that inside the body we have almost 5 times more oxygen than the atmosphere; and it has been a matter of debate ever since. To date, the explanation of oxygen levels inside the body is based on Krogh's mathematical model, which is so intricate that it cannot even be verified experimentally [
18].
Sometimes there is inflammation of the lacrimal gland with less production of aqueous fluid [
19]. Reduced conjunctival goblet cell density also is associated with tear film disorder [
20]. Quantity of aqueous fluid has not proved to be a reliable guide to diagnosis or treatment of clinical conditions. Changes in quantity, viscous-elastic properties, or concentration of mucus may be important.
Therefore, to date, ocular surface diseases have been managed by managing the symptoms with some compounds, but without going to the bottom of the matter. And therefore, the results are very modest. So, we are at the beginning of a new era in ophthalmology and related areas, which will allow us to understand, prevent, and treat in a more realistic way, the different diseases that afflict our current society.
Acknowledgments
This work was supported by an unrestricted grant from Human Photosynthesis™ Research Center. Aguascalientes 20000, México.
References
- Stobbe, M. D. The road to knowledge: from biology to databases and back again. Dissertation at Faculty of Medicine in the University of Amsterdam. 2012. Retrieved from http://dare.uva.nl/record/428591 in Sept 2014.
- Freeman, Ralph D. Oxygen Consumption by the component layers of the cornea. J. Physiol. (1972), 225, pp. 15-32. [CrossRef]
- Kaura R and Tiffany JM: The role of mucous glycoproteins in the tear film. In The Precorneal Tear Film, Holly FJ, editor. Lubbock, TX, Dry Eye Institute, 1986, pp. 728-732.
- Krogh A. “The rate of diffusion of gases through animal tissues with some remarks on the coefficient of invasion”. J Physiol 52 (1919): 391-408.
- Herrera AS (2017) Towards a New Ophthalmic Biology and Physiology: The Unsuspected Intrinsic Property of Melanin to Dissociate the Water Molecule. MOJ Cell Sci Rep 4(2): 00084. [CrossRef]
- Bakker A. SOME RESEARCH ON THE RESPIRATION OF THE CORNEA IN ALBINO RATS. Br J Ophthalmol. 1947 Feb;31(2):100-8. [CrossRef]
- Duke-Elder. Text-book of Ophtalmology. 1946 Editorial Henry Kimpton London.
- Bullot, G. Jl. Of Physiology., Vol. XXXI, p. 359. 1904.
- Bakker, A. Ned- Tydschr. Geneeesk., Vol. LXXXV, p. 1121. 1941.
- Yokoi N, Bron AJ, Georgiev GA. The precorneal tear film as a fluid shell: the effect of blinking and saccades on tear film distribution and dynamics. Ocul Surf. 2014 Oct;12(4):252-66. [CrossRef]
- Prydal JI, Artal P, Woon H, Campbell FW. Study of human precorneal tear film thickness and structure using laser interferometry. Invest Ophthalmol Vis Sci. 1992 May;33(6):2006-11.
- Doane MG: Interaction of eyelids and tears in corneal wetting and the dynamics of the normal human eyeblink. Am J Ophthalmol 89:507, 1980.
- Kennard DW and Smythe GL: Interaction of mechanisms causing eye and eyelid movement. Nature 197:50, 1963.
- Miller D: Pressure of the lid on the eye. Arch Ophthalmol 78:328, 1967.
- Bron AJ: Prospects for the dry eye. Trans Ophthalmol Soc UK 104:801, 1985.
- Herrera, A.S. (2015) The Biological Pigments in Plants Physiology. Agricultural Sciences, 6, 1262- 1271. [CrossRef]
- Herrera AS, Del C A Esparza M, Md Ashraf G, Zamyatnin AA, Aliev G. Beyond mitochondria, what would be the energy source of the cell? Cent Nerv Syst Agents Med Chem. 2015;15(1):32-41. [CrossRef]
- Gjedde A. Christian Bohr og De syv små Djaevle: Et laerestykke i 4 akter om iltdiffusionsstriden mellem Christian Bohr og August Krogh [Christian Bohr and the Seven Little Devils]. Dan Medicinhist Arbog. 2004:13-39. Danish.
- Creeth JM: Constituents of mucus and their separation. Br Med Bull 34(1): 17, 1978.
- Carl Marfurt; Miracle C. Anokwute; Kaleigh Fetcko; Erin Mahony-Perez; Hassan Farooq; Emily Ross; Maraya M. Baumanis; Rachel L. Weinberg; Megan E. McCarron; Joseph L. Mankowski. Comparative Anatomy of the Mammalian Corneal Subbasal Nerve Plexus. Investigative Ophthalmology & Visual Science December 2019, Vol.60, 4972-4984. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).