Introduction
Acute lymphoblastic leukemia (ALL) is the most common childhood cancer, but is less common in adolescents and young adults ( AYA, the age range varies from 15 to 39 years, depending on the study) and is rare in older adults. The 5-year survival rate of ALL is higher than 90% in children [
1], but falls significantly to 60-85% in adolescents and young adults [
2] and less than 30% in older adults [
3].
In terms of clinical and biological characteristics and response to treatment, AYA-ALL differs from the pediatric ALL population. Although survival of adolescents and young adults with acute lymphoblastic leukemia has improved with the use of pediatric inspired protocols [
4], the results of those who relapse remain poor.
The Philadelphia chromosome (Ph), resulting from the translocation of t(9;22)(q34;q11) and encoding rearrangement of BCR/ABL1 can be detected in approximately 20-30% of adult cases with ALL; its incidence increases with age and represents the most common form of ALL in the elderly population and is therefore a relatively rare event in AYA , less than 20% [
5]. The outcomes of patients with Ph-ALL have changed dramatically with the addition of tyrosine kinase inhibitors (TKI) to cytotoxic chemotherapy, increasing the rate of complete response to as high as 90% [
6].
The minimal residual disease (MRD) test is of extreme prognostic importance, as it is one of the most important risk factors for relapse, which has been confirmed by multivariate prognostic analyzes in many studies [
7].
Presentation
We discuss the case of a 21-year-old woman who came to the emergency department with altered general condition, fatigue, loss of appetite, cough, fever, and purpura. His medical history was irrelevant.
Laboratory findings on admission revealed life-threatening anemia (a hemoglobin of 2.3 g/dL ), severe thrombocytopenia (a platelet count of 24 x 109 / L) , and a normal leukocyte count of 6.8 x 109/L. In the peripheral blood smear (PBS), we found 6% medium-sized blasts with a morphological aspect suggestive of lymphoblasts (ALL-1, according to the FAB classification). Bone marrow examination revealed involvement of 38% blasts similar to those described in PBS.
Immunophenotyping by flow cytometry from bone marrow aspirate identified 33% cells with the following aspect: SSC low CD34+, cMPO-, cCD79a+, cCD3-, CD3-, CD7-, CD117-, CD13-, CD33-, CD10+ (CALLA) , CD19+, CD20-, sIgM kappa, lambda-, CD66c- (KOR-SA) , NG.2- , CD123-, cTdT-, CD58-. The described are based on the diagnosis of acute B-cell lymphoblastic leukemia, positive for CALLA, not otherwise specified, according to the 2016 World Health Organization criteria (WHO). Cytogenetic examination carried out on 10 metaphases revealed that ther were no numerical or structural alterations in chromosomes at this level of resolution (it should be noted that the cellularity of the bone marrow aspirate was low). In addition, a molecular study was performed that did not detect the Bcr-Abl transcript or the MLL fusion gene.
At the time of diagnosis, the patient had no neurologic signs or symptoms. Cerebrospinal fluid (CSF) studies and imaging also did not describe central nervous system (CNS) involvement.
Taking into account the available data : young age, normal white blood cells, precursor of the B lineage, positive for CALLA (CD10+), no evidence of CNS involvement, no Philadelphia chromosome, we classified the patient in a standard risk group.
Based on the preponderance of the data presented in the review, the best therapeutic approach for this patient would be intensive pediatric treatment. We decided to treat according to the Berlin-Frankfurt-Münster (BFM) protocol because we knew the following data from the literature. Patients with AYA-ALL, treated with the BFM protocol, had high overall survival (OS) and complete remission (CRD) rates and tolerated this pediatric regimen with acceptable toxicity. The OS and CRD rates were comparable to those achieved with hyper-CVAD, an adult leukemia therapy [
8].
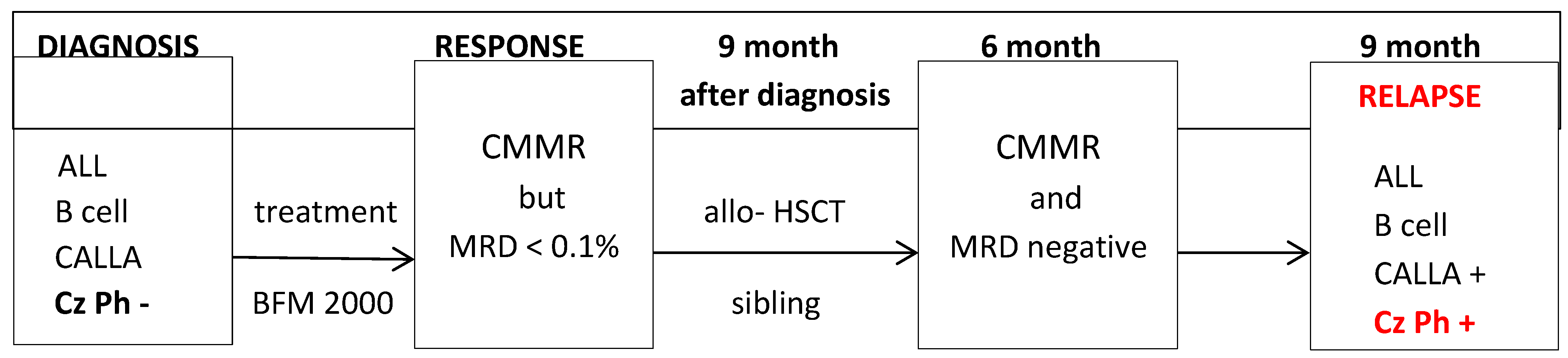
After 9 months of treatment, the patient is in complete medullary remission but with the presence < 0.1% of minimal residual disease (MRD), determined by multiparameter flow cytometry. MRD represents the most important prognostic information and decision support for the allocation to allogeneic hematopoietic stem cell transplantation (allo-HSCT) or other targeted therapy-a view uniformly supported by European and US experts [
9].
At this time, the patient underwent allo-HSCT from a matched related donor. The procedure was successful, with no major complications and no graft-versus-host disease (GVHD). Six months after allo-HSCT, the patient was in complete remission, with negative MRD and 100% chimerism. After another three months, the patient came to the scheduled follow-up in good general condition. However, PBS revealed a recurrence of blast cells, which raised suspicion of relapse of the disease.
We performed a reexamination to determine the current state of the disease. Morphologically, the blasts had a similar appearance to that at diagnosis (ALL -1). The bone marrow aspirate showed the presence of 85% lymphoblasts.
The immunophenotypic aspect of bone marrow blast cells showed some changes from the time of diagnosis, namely the positivity of the CD66c marker (KOR-SA), indicating the likelihood of the presence of the Philadelphia chromosome. This suspicion was confirmed by the result of the cytogenetic and molecular examination. The Philadelphia chromosome was detected in 34% of the analyzed 10 metaphases. In 45.1% of malignant cells, molecular analysis identified the BCR-ABL e1a2 transcript (m-BCR) corresponding to protein p190.
An examination of the central nervous system was also performed and relapse at this level was negated.
Figure 1.
Evolution of disease.
Figure 1.
Evolution of disease.
CMMR = complete medullary morphological remission ; MRD = minimum residual disease; Allo- HSCT = allogeneic hematopoietic stem cell transplantation; Cz Ph = Philadelphia Chromosome.
We started tyrosine kinase inhibitor (TKI) treatment with Dasatinib due its advantages in preventing CNS involvement [
10]. The selected dose of Dasatinib was 70 mg/day. Given the corticosteroid sensitivity the disease exhibited at the time of diagnosis, Dexamethasone therapy was also administered.
After only 3 days of TKI treatment, we observed the disappearance of blast precursors from peripheral blood. Note the occurrence of grade 2-3 liver toxicity, which required the interruption of TKI treatment. Complete remission of liver toxicity occurred after 7 days of Dasatinib treatment. Subsequently, TKI was administered again, but at a dose reduced to 50 mg/day.
After an additional 7 days of Dasatinib treatment, the bone marrow evaluation already showed remission and the presence of the bcr-abl transcript was no longer detected on the molecular evaluation.
Discussion
This case of acute lymphoblastic leukemia with a precursor of the B lineage, Ph-negative, in a patient with AYA with early relapse, less than one year, after bone marrow allograft transplantation, raises several questions.
The biological, morphological, immunophenotypic, cytogenetic and molecular characteristics at the time of diagnosis were not predictive of relapse. Certain characteristics of blast cells might allow predictions of the presence of certain genetic or molecular alterations and of the prognosis. It is well known that the expression of the markers CD66c [
11], CD33 [
12] or CD13 [
13] can be correlated with the presence of the Philadelphia chromosome.
Ng.2 (neuronal glial antigen-2) also has predictive value for MLL rearrangements [
14]. In our case, the absence of the Philadelphia chromosome, the BCR-ABL1 fusion gene, and the absence of the MLL gene was later confirmed by cytogenetic and molecular studies. The presence of CALLA provides a good prognosis for the disease.
The choice of treatment with a protocol oriented to the pediatric population was made taking into account the fact that it improves the response rate and the duration of remission. The persistence of minimal residual disease at the end of chemotherapy led to the indication of bone marrow allotransplantation. When feasible, allogeneic HSCT is preferable to standard intensive pediatric chemotherapy in MRD positive patients to reduce the risk of relapse and increase survival from ⩽25% without HSCT to approximately 45-55% (GMALL, NILG, GRAALL studies, reviewed by Bassan et al.) [
15]. However, 9 months after bone marrow transplantation, the patient relapsed with a new chromosomal change compared to the time of diagnosis, Philadelphia chromosome.
Several hypotheses can be considered that underlie the relapse of the disease. One is that there was a dormant clone that could not be identified at diagnosis that escaped immune system control? Is this mutation acquired during the development of the disease or is it secondary to treatment? Could possible contamination by the donor also be considered? The expression of BCR-ABL1 (p210 transcript) has been detected at very low levels in peripheral blood cells of some healthy individuals but not in cord blood cells [
16]. The biological explanation for this observation remains largely unexplained [
17].
After complete remission with TKI, we consider therapy with T cells expressing the chimeric antigen receptor (CAR-T cells). CAR-T cells have shown promise as a treatment option for patients with relapsed ALL, with impressive remission rates of 70-90% [
18,
19]. The question of whether CAR -T is a stand-alone therapy or a bridge to transplantation cannot be answered in general terms given the current state of research [
20]. There is a lack of randomised trials comparing approaches with consolidating HSCT to approaches in which patients do not progress to HSCT but are strictly followed for T cell persistence and MRD remission after infusion [
21]. We are also looking for an unrelated donor for a possible second allo-HSCT.
In view of all this, the question arises whether it would not be more effective to include TKIs in treatment in prophylactic doses and after certain clinical trials, starting from the diagnosis of ALL?
Conclusions
The Philadelphia chromosome is the most common cytogenetic alteration associated with acute lymphoblastic leukemia. It leads to an unfavorable prognosis for the disease. However, the introduction and development of TKIs have improved response rate and survival in this disease. The Philadelphia chromosome may be present at diagnosis or may occur later in the course of the disease and contribute to relapse of the disease.
It is extremely important that, in the case of acute leukemia relapse but also throughout the evolution of the disease, a complete and thorough reevaluation, both morphological and immunophenotypic, as well as cytogenetic and molecular, is carried out, because there may be acquisitions of new changes compared to the time of diagnosis, which require other targeted lines of treatment.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Inaba, H.; Mullighan, C.G. Pediatric acute lymphoblastic leukemia. Haematologica 2020, 105, 2524–2539. [Google Scholar] [CrossRef] [PubMed]
- Rytting, M.E.; Thomas, D.A.; O'Brien, S.M.; Ravandi-Kashani, F.; Jabbour, E.J.; Franklin, A.R.; Kadia, T.M.; Pemmaraju, N.; Daver, N.G.; Ferrajoli, A.; et al. Augmented Berlin-Frankfurt-Münster therapy in adolescents and young adults (AYAs) with acute lymphoblastic leukemia (ALL). Cancer 2014, 120, 3660–3668. [Google Scholar] [CrossRef]
- Sive, J.I.; Buck, G.; Fielding, A.; Lazarus, H.M.; Litzow, M.R.; Luger, S.; Marks, D.I.; McMillan, A.; Moorman, A.V.; Richards, S.M.; et al. Outcomes in older adults with acute lymphoblastic leukaemia (ALL): results from the international MRC UKALL XII/ECOG2993 trial. Br. J. Haematol. 2012, 157, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Stock, W.; Luger, S.M.; Advani, A.S.; Yin, J.; Harvey, R.C.; Mullighan, C.G.; Willman, C.L.; Fulton, N.; Laumann, K.M.; Malnassy, G.; et al. A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10403. Blood 2019, 133, 1548–1559. [Google Scholar] [CrossRef] [PubMed]
- Moorman, A.V. New and emerging prognostic and predictive genetic biomarkers in B-cell precursor acute lymphoblastic leukemia. Haematologica 2016, 101, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Kantarjian, H.M.; Short, N.J.; Samra, B.; Khoury, J.D.; Shamanna, R.K.; Konopleva, M.; Jain, N.; DiNardo, C.D.; Khouri, R.; et al. Prognostic Factors for Progression in Patients With Philadelphia Chromosome- Positive Acute Lymphoblastic Leukemia in Complete Molecular Response Within 3 Months of Therapy With Tyrosine Kinase Inhibitors. Cancer 2021, 127, 2595–2814. [Google Scholar] [CrossRef] [PubMed]
- Bassan, R.; Intermesoli, T.; Scattolin, A.; Viero, P.; Maino, E.; Sancetta, R.; Carobolante, F.; Gianni, F.; Stefanoni, P.; Tosi, M.; et al. Minimal residual disease assessment and riskbased therapy in acute lymphoblastic leukemia. Clin Lymphoma Myeloma Leuk 2017, 17, S2–S9. [Google Scholar] [CrossRef]
- Rytting, M.E.; Thomas, D.A.; O'Brien, S.M.; Ravandi-Kashani, F.; Jabbour, E.J.; Franklin, A.R.; Kadia, T.M.; Pemmaraju, N.; Daver, N.G.; Ferrajoli, A.; et al. Augmented Berlin-Frankfurt-Münster therapy in adolescents and young adults (AYAs) with acute lymphoblastic leukemia (ALL). Cancer 2014, 120, 3660–3668. [Google Scholar] [CrossRef]
- Short, N.J.; Jabbour, E.; Albitar, M.; de Lima, M.; Gore, L.; Jorgensen, J.; Logan, A.C.; Park, J.; Ravandi, F.; Shah, B.; et al. Recommendations for the assessment and management of measurable residual disease in adults with acute lymphoblastic leukemia: a consensus of North American experts. Am. J. Hematol. 2019, 94, 257–265. [Google Scholar] [CrossRef]
- Porkka, K.; Koskenvesa, P.; Lundan, T.; Rimpiläinen, J.; Mustjoki, S.; Smykla, R.; Wild, R.; Luo, R.; Arnan, M.; Brethon, B.; et al. Dasatinib crosses the blood-brain barrier and is an efficient therapy for central nervous system Philadelphia chromosome -positive leukemia. Blood 2008, 112, 1005–1012. [Google Scholar] [CrossRef]
- Owaidah, T.M.; Rawas, F.I.; Al khayatt, M.F.; Elkum, N.B. Expression of CD66c and CD25 in Acute Lymphoblastic Leukemia as a Predictor of the Presence of BCR/ABL Rearrangement. Hematol. Oncol. Stem Cell Ther. 2008, 1, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Corrente, F.; Bellesi, S.; Metafuni, E.; Puggioni, P.L.; Marietti, S.; Ciminello, A.M.; Za, T.; Sorà, F.; Fianchi, L.; Sica, S.; et al. Role of Flow-Cytometric Immunophenotyping in Prediction of BCR/ABL1 Gene Rearrangement in Adult B-Cell Acute Lymphoblastic Leukemia: FLOW-CYTOMETRY IN BCR/ABL1 ADULT B-ALL. Cytom. B Clin. Cytom. 2018, 94, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Hrušák, O.; Porwit-MacDonald, A. Antigen Expression Patterns Reflecting Genotype of Acute Leukemias. Leukemia 2002, 16, 1233–1258. [Google Scholar] [CrossRef] [PubMed]
- BeLopez-Millan, B.; Sanchéz-Martínez, D.; Roca-Ho, H.; Gutiérrez-Agüera, F.; Molina, O.; de la Guardia, R.D.; Torres-Ruiz, R.; Fuster, J.L.; Ballerini, P.; Suessbier, U.; et al. NG2 antigen is a therapeutic target for MLL-rearranged B-cell acute lymphoblastic leukemia. Leukemia 2019, 33, 1557–1569. [Google Scholar] [CrossRef]
- Bassan, R.; Intermesoli, T.; Scattolin, A.; Viero, P.; Maino, E.; Sancetta, R.; Carobolante, F.; Gianni, F.; Stefanoni, P.; Tosi, M.; et al. Minimal residual disease assessment and risk based therapy in acute lymphoblastic leukemia. Clin Lymphoma Myeloma Leuk 2017, 17, S2–S9. [Google Scholar] [CrossRef]
- Biernaux, C.; Loos, M.; Sels, A.; Huez, G.; Stryckmans, P. Detection of major bcr-abl gene expression at a very low level in blood cells of some healthy individuals. Blood 1995, 86, 3118–3122. [Google Scholar] [CrossRef]
- Janz, S.; Potter, M.; Rabkin, C.S. Lymphoma- and leukemia-associated chromosomal translocations in healthy individuals. Genes Chromosomes Cancer 2003, 36, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Davila, M.L.; Riviere, I.; Wang, X.; Bartido, S.; Park, J.; Curran, K.; Chung, S.S.; Stefanski, J.; Borquez-Ojeda, O.; Olszewska, M.; et al. Efficacy and toxicity management of 19-28z CAR T cell therapy B cell acute lymphoblastic leukemia. Sci. Transl. Med. 2014, 6, 224ra25. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Kochenderfer, J.N.; Stetler-Stevenson, M.; Cui, Y.K.; Delbrook, C.; Feldman, S.A.; Fry, T.J.; Orentas, R.; Sabatino, M.; Shah, N.N.; et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia childre and young adults: A phase 1 dose-escalatio trial. Lancet 2015, 385, 517–528. [Google Scholar] [CrossRef]
- Candoni, A.; Rambaldi, A.; Fanin, R.; Velardi, A.; Arcese, W.; Ciceri, F.; Lazzarotto, D.; Lussana, F.; Olivieri, J.; Grillo, G.; et al. Outcome of Allogeneic Hematopoietic Stem Cell Transplantation in Adult Patients with Philadelphia ChromosomePositive Acute Lymphoblastic Leukemia in the Era of Tyrosine Kinase Inhibitors: A RegistryBased Study of the Italian Blood and Marrow Transplantation Society (GITMO). Biol Blood Marrow Transplant 2019, 25, 2388–2397. [Google Scholar] [CrossRef]
- Buechner, J.; Caruana, I.; Künkele, A.; Rives, S.; Vettenranta, K.; Bader, P.; Peters, C.; Baruchel, A.; Calkoen, F.G. Chimeric Antigen Receptor T-Cell Therapy in Paediatric B-Cell Precursor Acute Lymphoblastic Leukaemia: Curative Treatment Option or Bridge to Transplant? Front. Pediatr. 2022, 9, 784024. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).