Submitted:
16 March 2023
Posted:
17 March 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Spherical Crystals in the Prismatine Rock from Waldheim/Saxony
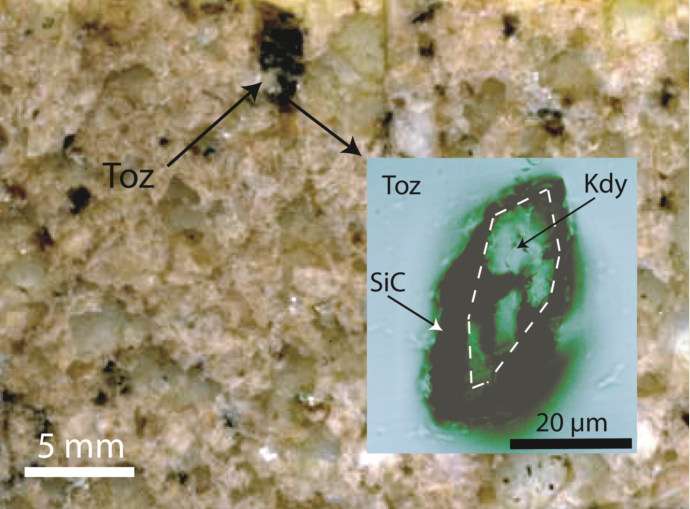
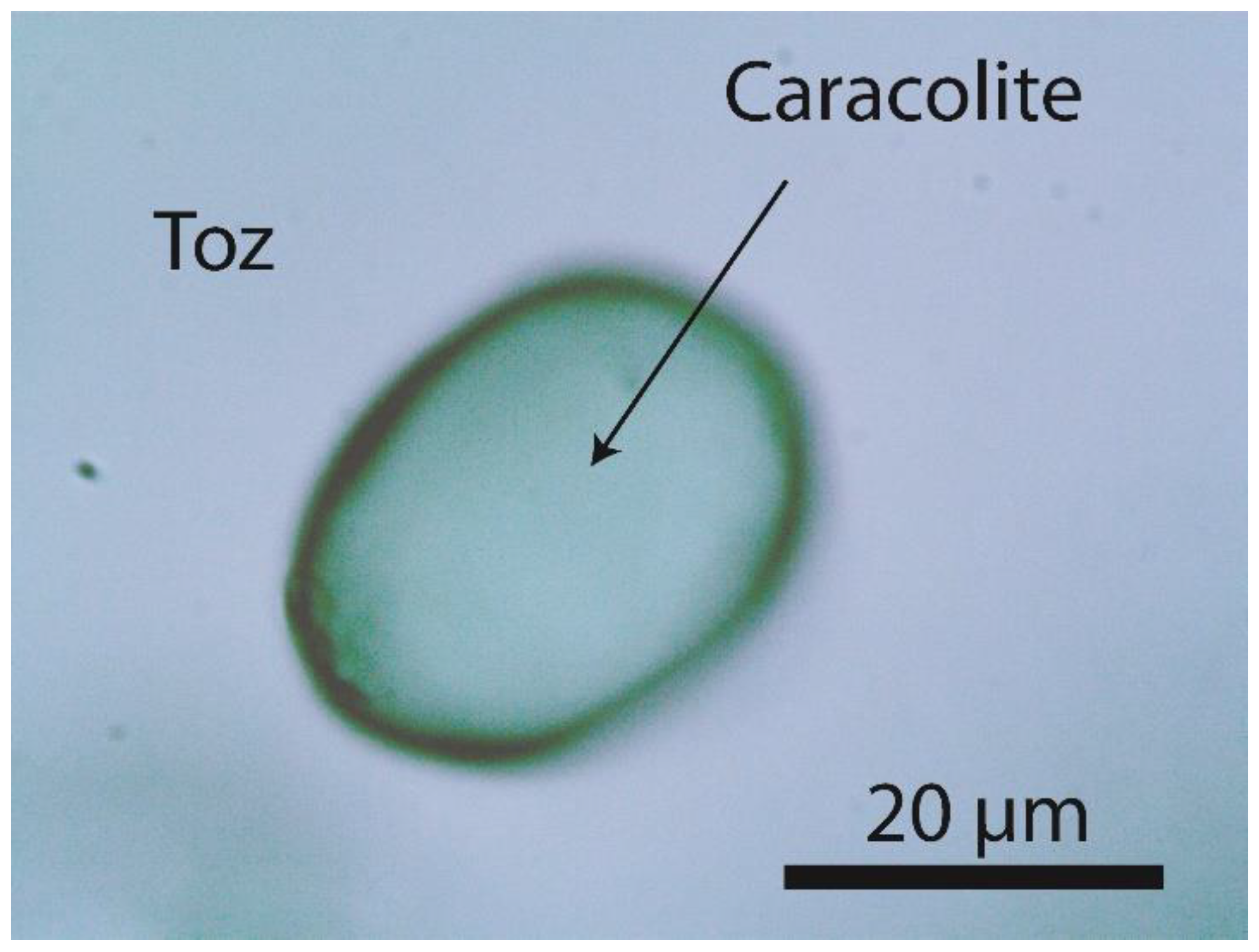
1.2. Spherical Crystals in topaz from the Greifenstein granite
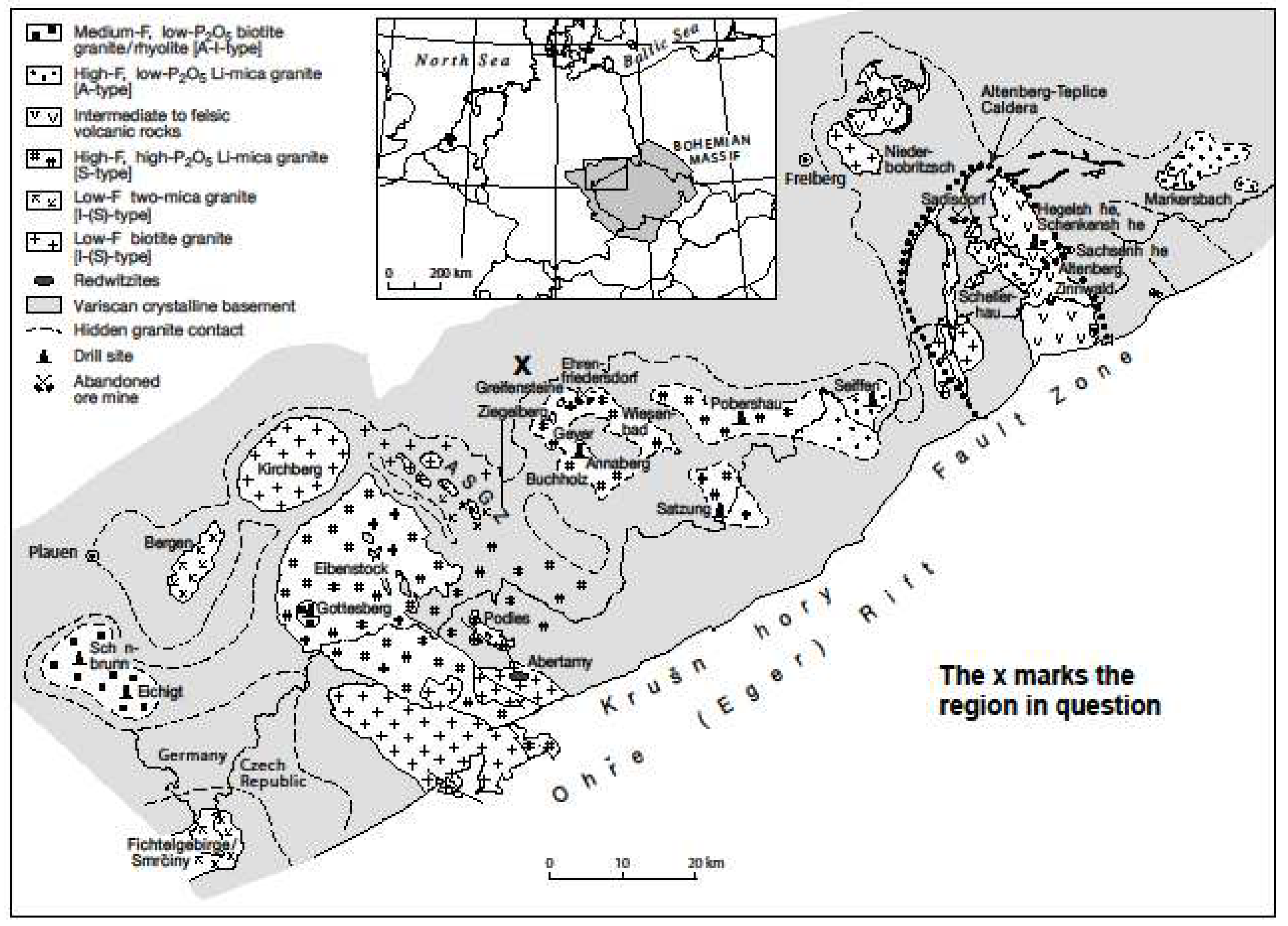
1.3. Sample
2. Results
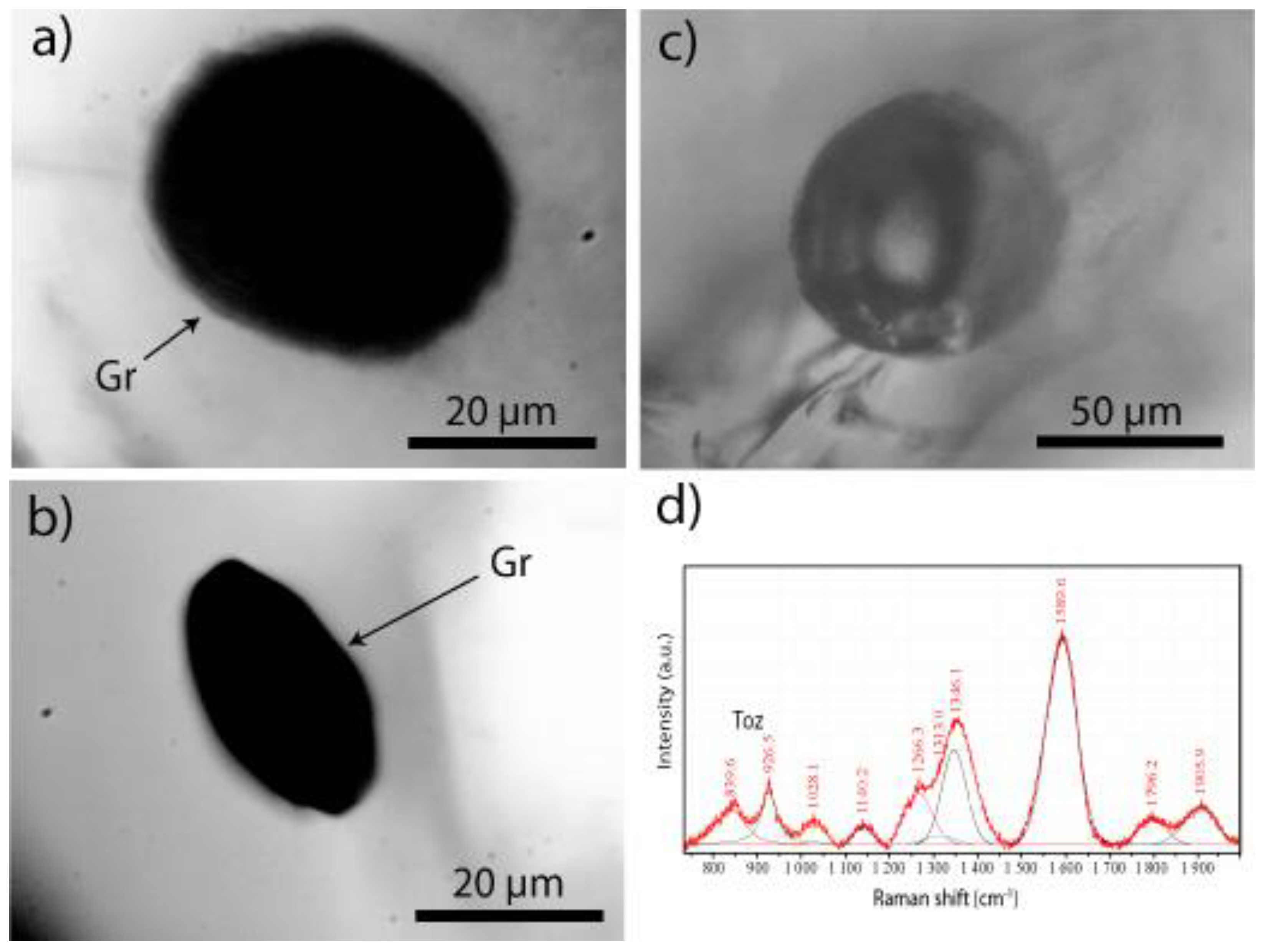
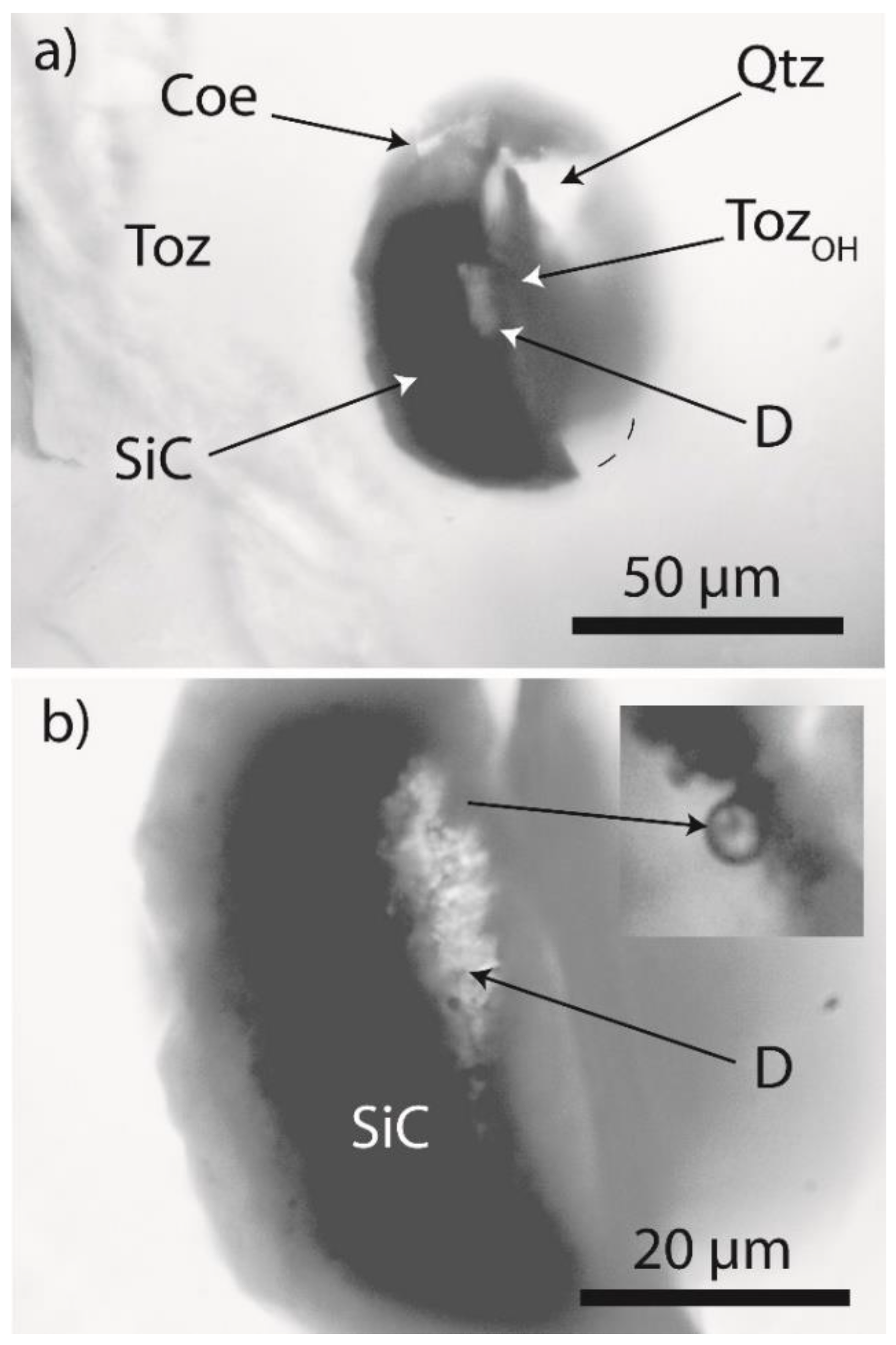
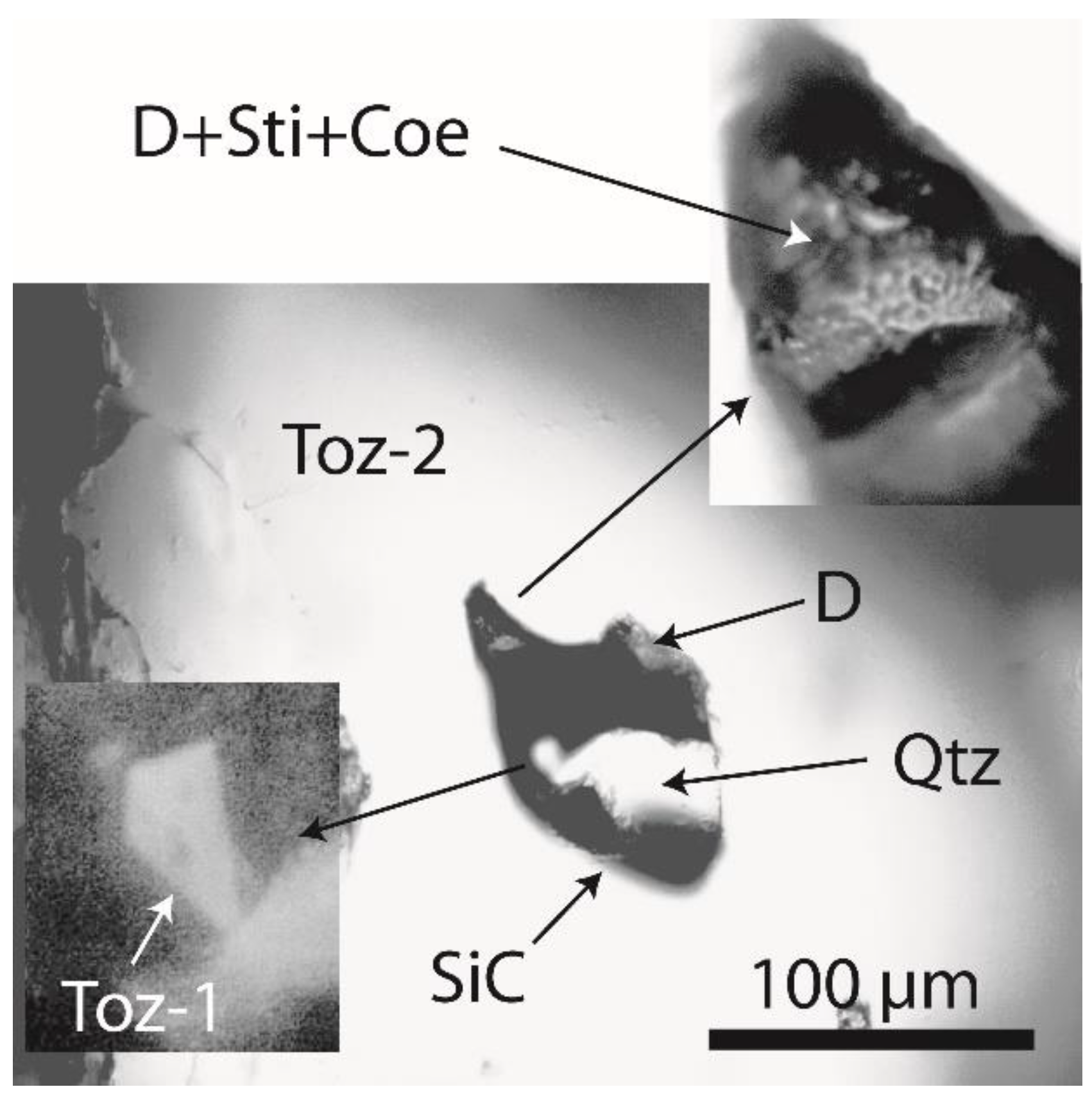
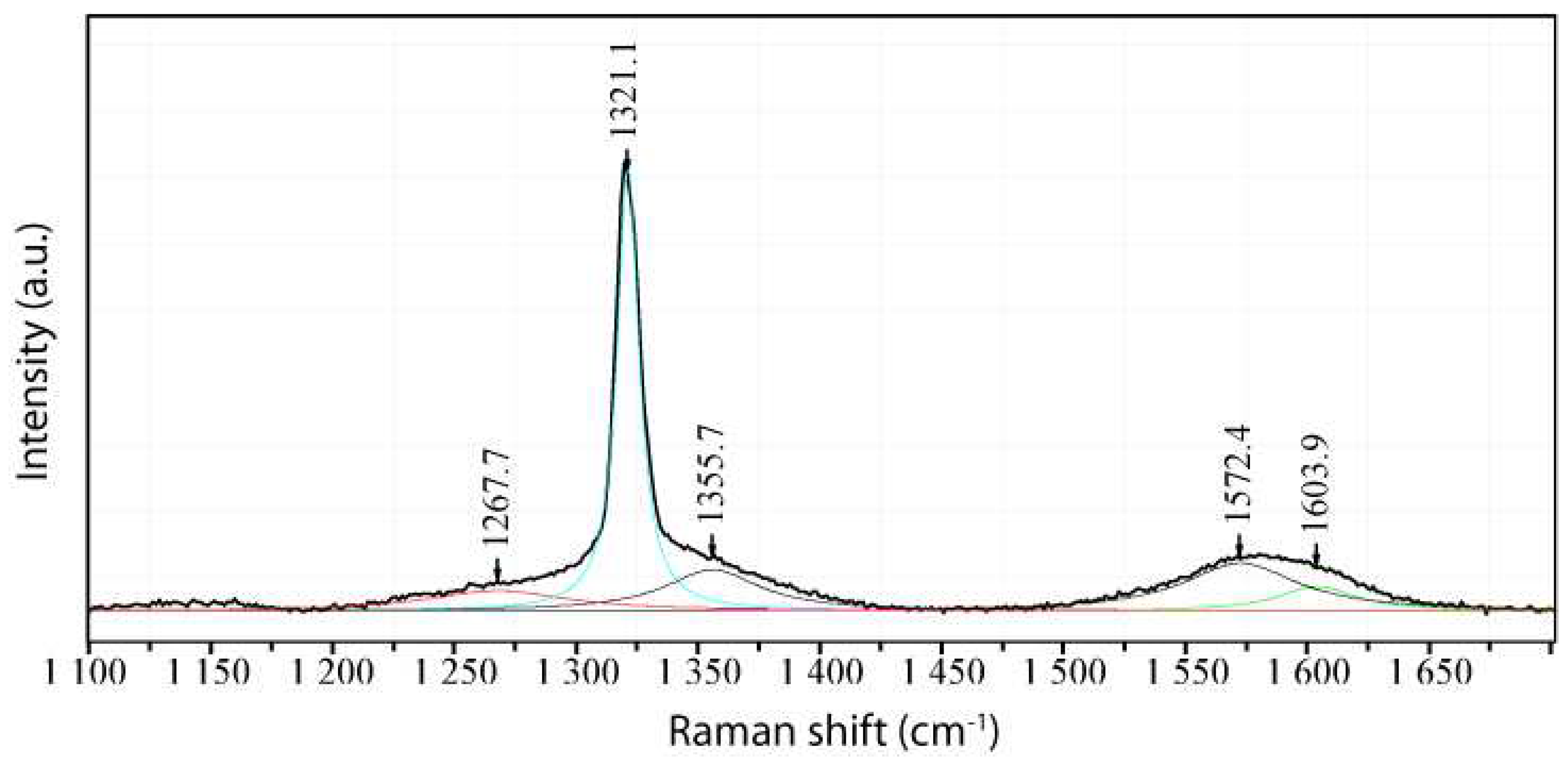
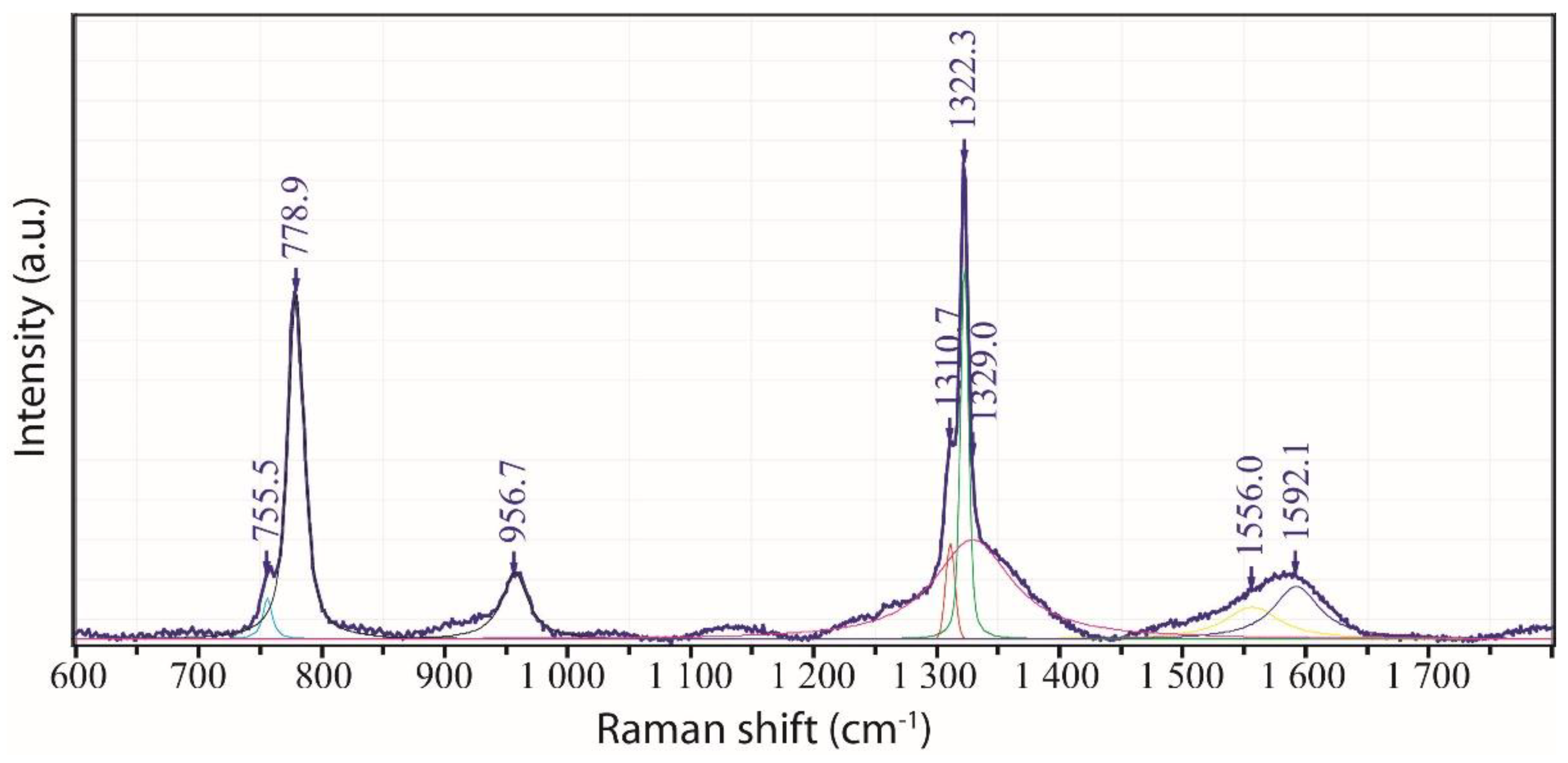
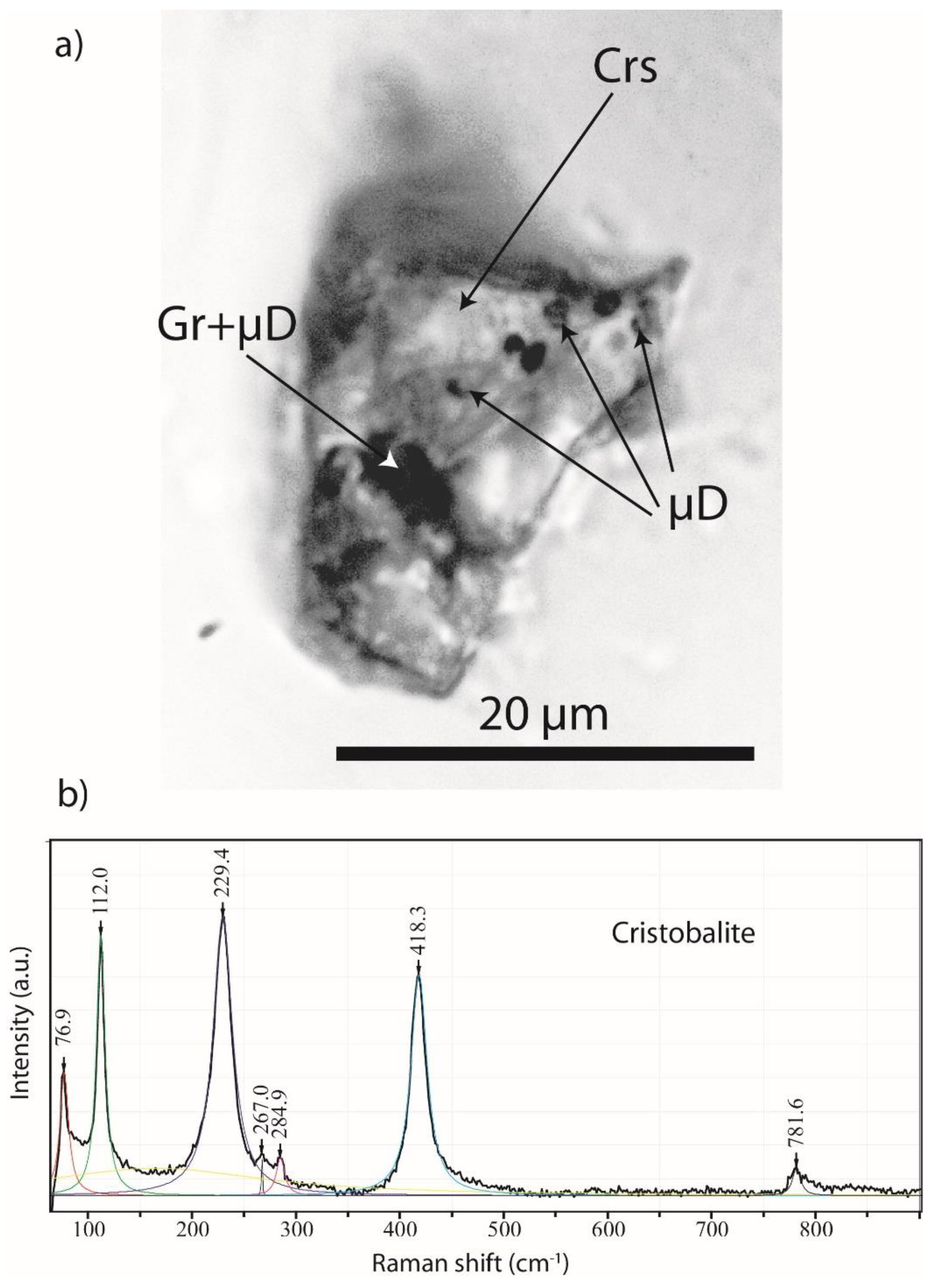
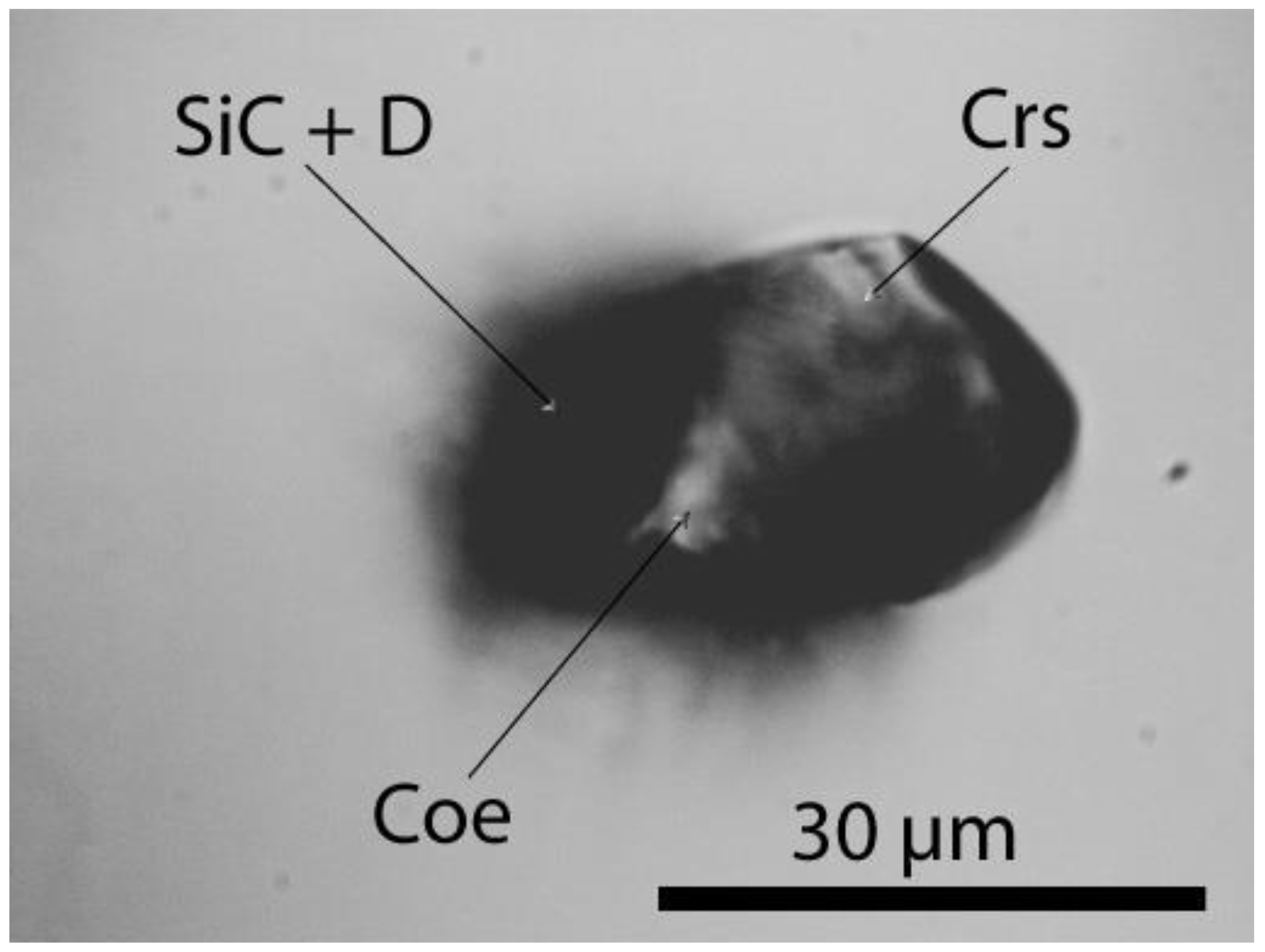
2.1. Diamond and Moissanite in Granite from Greifenstein
2.2. Natural Diamond and Moissanite in Inclusions in Granite
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Microscopy and Raman spectroscopy: Methodology
References
- Thomas, R.; Grew, E. Coesite inclusions in prismatine from Waldheim, Germany: New constrains on the pressure-temperature evolution of the Saxony Granulite Complex. Book of Abstracts, 3rd European Mineralogical Conference, EMC 2020, Cracow, Poland, 2021.
- Thomas, R.; Davidson, P.; Rericha, A. Prismatine granulite from Waldheim/Saxony: Zircon-Reidite. J. Earth Envi. Sci. JEES-103 2022a, 1, 1–3.
- Thomas, R.; Davidson, P.; Rericha, A.; Recknagel, U. Discovery of stishovite in the prismatine-bearing granulite from Waldheim, Germany: A possible role of supercritical fluids of ultrahigh-pressure origin. Geosciences 2022, 12, 1–13. [Google Scholar] [CrossRef]
- Thomas, R.; Davidson, P.; Rericha, A.; Recknagel, U. Water-rich coesite in prismatine-granulite from Waldheim/Saxony. Veröffentlichungen Naturkunde Museum Chemnitz 2022c, 45, 67-80.
- Kalkowsky, E. (1907) Der Korundgranulit von Waldheim in Sachsen. Abh. Naturwissenschaftlichen Gesellschaft ISIS in Dresden. 47–65.
- Cesare, B.; Parisatto, M.; Mancini, L.; Peruzzo, L.; Franceschi, M.; Tacchetto, T.; Reddy, S.; Spiess, R.; Nestola, F.; Marone, F. Mineral inclusions are not immutable: Evidence of post-entrapment thermally-induced shape change of quartz in garnet. Earth Planet. Sci. Lett. 2021, 555, 116708. [Google Scholar] [CrossRef]
- Lin,Y. ;Hu,Q.;Meng,Y.;Walter,M.; Mao,H. Evidence for the stability of ultrahydrous stishovite in Earth's lower mantle. PNAS USA 2020, 117, 184–189.
- Rötzler, J.; Hagen, B.; Hoernes, S. Geothermometry of the ultrahigh-temperature Saxon granulites revised. Part I: New evidence from key mineral assemblages and reaction textures. Eur. J. Mineral. 2008, 20, 1097–1115. [Google Scholar] [CrossRef]
- Hagen, B.; Hoernes, S.; Rötzler, J. Geothermometry of the ultrahigh-temperature Saxon granulites revised. Part II. Thermal peak conditions and cooling rates inferred from oxygen-isotope fractionation. Eur. J. Mineral. 2008, 20, 1117–1133. [Google Scholar] [CrossRef]
- Thomas, R.; Webster, J.D. Strong tin enrichment in a pegmatite-forming melt. Mineral. Deposita 2000, 35, 570–582. [Google Scholar] [CrossRef]
- Hösel, G. (1994) Das Zinnerz-Lagerstättengebiet Ehrenfriedersdorf/Erzgebirge. Bergbaumonographie, Bd. 1, 195 p.
- Förster, H.-J.; Romer, R.L. (2010) Carboniferous magmatism, 287-308. In: Linnemann, U.; Romer, R.L. (eds.) Pre-Mesozoic Geology of Saxo-Thuringia. Schweizerbart Sci. Pub., 499 p. + DVD.
- Thomas, R.; Klemm, W. (1997) Microthermometric study of silicate melt inclusions in Variscan Granites from SE Germany: Volatile contents and entrapment conditions. J. Petrol. 1997, 38, 1753–1765. [Google Scholar] [CrossRef]
- Acosta-Vigil, A.; Stöckert, B.; Hermann, J.; Yaxley, G.; Cesare, B.; Bartoli, O. Remelting of nanogranitoids in UHP felsic granulites from Erzgebirge (Bohemian Massif, Germany. Am. Geophysical Union, Fall Meeting 2017, abstract #V24B-06.
- Xue, X.; Kanzaki, M.; Fukui, H. Unique crystal chemistry of two polymorphs of topaz-OH: A multi-nuclear NMR and Raman study. Am. Mineral. 2010, 95, 1276–1293. [Google Scholar] [CrossRef]
- Keller, D.S.; Ague, J.J. Possibilities for misidentification of natural diamond and coesite in metamorphic rocks. Neues Jb. Miner. Abh 2022, 197, 253–261. [Google Scholar] [CrossRef]
- Mendili, Y.E.; Orberger, B.; Charteigner, D.; Bardeau, J.-F.; Gascoin, S.; Petit, S.; Perez, O. Occurrence of SiC and diamond polytypes, chromite and uranophane in breccia from nickel lateries (Ne Caledonia): Combined analyses. Minerals 2022, 12, 196. [Google Scholar] [CrossRef]
- Shumiliva, T.G.; Mayer, E.; Isaenko, S.I. Natural monocrystalline lonsdaleite. Dokl. Earth Sc. 2011, 441, 1552–1554. [Google Scholar] [CrossRef]
- Hemley, R.J. (1987) Pressure dependence of Raman spectra of SiO2 polymorphs: -quartz, coesite, and stishovite. High-Pressure Research in Mineral Physics, ed. By M.H. Manghnani and Y. Syono, pp. 347-359.
- Misra, A.; Tyagi, P.K.; Yadav, B.S.; Rai, P. , Misra, D.S. Hexagonal diamond synthesis on h-GaN strained films. Appl. Phys. Lett. 2006, 89, 071911-1–071911-3. [Google Scholar] [CrossRef]
- Černok, A. (2015) Diversity of compressional mechanisms among SiO2 polymorphs: case of coesite and cristobalite. Dissertation, Bayreuth, Germany, 121 p.
- Soufi, M. Origin and physical-chemical control of topaz crystallization in felsic igneous rocks: Contrasted effect of temperature on its OH–F substitution. Earth-Sci. Rev. 2021, 213, 103467. [Google Scholar] [CrossRef]
- Bureau, H.; Keppler, H. Complete miscibility between silicate melts and hydrous fluids in the upper mantle: experimental evidence and geochemical implications. Earth Planet. Sci. Lett. 1999, 165, 187–196. [Google Scholar] [CrossRef]
- Ni, H.; Zhang, L.; Xiong, X.; Mao, Z.; Wang, J. Supercritical fluids at subduction zones: Evidence, formation condition, and physicochemical properties. Earth-Sci. Rev. 2017, 167, 62–71. [Google Scholar] [CrossRef]
- Thomas, R.; Davidson, P.; Rericha, A.; Voznyak, D.K. Water-rich melt inclusions as "frozen" samples of the supercritical state in granites and pegmatites reveal extreme element enrichment resulting under non-equilibrium conditions. Mineral. J. (Ukraine). 2022, 44, 3–15. [Google Scholar] [CrossRef]
- Thomas, R.; Davidson, P.; Appel, K. The enhanced element enrichment in the supercritical states of granite-pegmatite systems. Acta Geochim. 2019, 38, 335–349. [Google Scholar] [CrossRef]
- Zagorsky, V.Y. Deep fluid flow–melt interaction and problems of granite–pegmatite system petrogenesis. Abstracts in Granitic pegmatites: the state of the art. Memórias Porto 2007, 8, 106–107. [Google Scholar]
- Russell, J.K.; Porritt, L.A.; Lavallee, Y.; Dingwell, D.B. Kimberlite ascent by assimilation-fuelled buoyancy. Nature 2012, 481, 352–356. [Google Scholar] [CrossRef]
- Jakubová, P.; Kotková, J.; Wirth, R.; Škoda, R.; Haifler, J. Morphology and Raman spectral parameters of Bohemian microdiamonds: implications to elastic geothermobarometry. J. Geosci. 2022, 67, 239–257. [Google Scholar] [CrossRef]
- Massonne, H.-J.; Nasdala, L. Characterization of an early metamorphic stage through inclusions in zircon of the diamondiferous quartzofeldsathic rock from the Erzgebirge, Germany. Am. Mineral. 2003, 88, 883–889. [Google Scholar] [CrossRef]
- Stöckhert, B.; Duyster, J.; Trepmann, C.; Massonne, H.-J. Microdiamonds daughter crystals precipitated from supercritical COH + silicate fluids included in garnet, Erzgebirge, Germany. Geology 2001, 29, 391–394. [Google Scholar] [CrossRef]
- Schröcke, H. Zur Paragenese erzgebirgischer Zinnlagerstätten. Neues Jb. Mineral. Abh. 1954, 87/1, 33-109 (1954).
| Sample | Band ~1332 (cm-1) | ± 1σ kenna(cm-1) | FWHMkenna(cm-1) | ± 1σ kenna(cm-1) | Number of measurements |
| Natural diamond: | |||||
| Diamond in SiC-I | 1325.4 | 4.40 | 10.8 | 4.1 | 28 |
| Diamond in SiC-II | 1324.5 | 4.30 | 7.7 | 3.6 | 50 |
| Diamond in SiC-II xxx-1 | 1320.9 | 0.40 | 7.3 | 0.4 | 10 |
| Diamond in SiC-II xxx-2 | 1322.4 | 2.40 | 8.9 | 2.9 | 13 |
| Diamond in SiC-III | 1322.8 | 2.80 | 9.4 | 5.0 | 20 |
| Σ Diamond in SiC | 1323.9 | 3.55 | 8.8 | 3.6 | 121 |
| Diamond in cristobalite | 1331.1 | 1.10 | 6.7 | 1.3 | 10 |
| Diamond in MI in zircon* | 1330.5 | 0.55 | 5.8 | 1.2 | 41 |
| Technical diamond for sample preparation: | |||||
| Diamond cutting disk 1) | 1332.5 | 0.42 | 5.5 | 0.3 | 28 |
| Diamond cutting disk 2)4) | 1332.5 | 0.53 | 4.9 | 0.14 | 28 |
| Polishing wheel 3)4) | 1330.5 | 5.40 | 31.0 | 16.6 | 28 |
| Diamond spray D1 5) | 1336.4 | 6.90 | 67.8 | 17.0 | 28 |
| Diamond spray D0.25 6) | 1334.6 | 5.80 | 100.0 | 6.5 | 28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





