Introduction
By December 26
th, 2022, SARS-CoV-2 outbreak had infected around 657 million people and more than 6.5 million of COVID-19 related deaths have been reported [
1]. Several specific candidate treatments against have been tested in large randomized studies, but none has been reported and globally recognized as the optimal treatment [
2]. An early (< 5 days of symptoms) oral treatment to prevent complications before they occur and death (nirmatrelvir/ritonavir) has been validated by the WHO only in 2022, i.e. two years after the pandemics emergence [
3].
Starting March 2020, we decided in our hospital department upon a strategy including treatment with hydroxychloroquine (HCQ) and azithromycin (AZ) for COVID-19 patients. This choice was supported by early Chinese publications about the antiviral effects of chloroquine (CQ) and its derivatives against SARS-COV2, the demonstration of a synergistic effect in vitro of the HCQ-AZ combination on SARS-CoV-2, the HCQ and AZ immunomodulators effects, which may prevent the “cytokine storm” of COVID-19, the HCQ antithrombotic effects which might also be useful in the context of COVID-19 associated pulmonary embolism and coagulopathy, and the fact that HCQ-AZ had been associated with a reduction in viral shedding, with potential public health effects by reducing the duration of contagiousness [
2]. AZ has the added advantage of preventing superinfection [
2]. HCQ-AZM have been widely administered to patients and observational studies with thousands of cases have been published throughout the world [
4]. In our center in 2020, this combination was associated with lower mortality in 2,111 COVID-19 hospitalized patients [
5], and 10,429 COVID-19 outpatients [
6].
The possible toxicity of HCQ or HCQ-AZ has been highlighted in published or retracted studies [7-10]. More specifically, HCQ-AZ combination raised the question of a possible lengthening of the QT interval on the electrocardiogram (ECG), which could lead to an increased risk of torsades de pointes and sudden death [
11,
12]. While initial cardiac safety publications evaluating HCQ treatment in hospitalised patients with COVID-19 have shown significant QTc lengthening in some patients [
13,
14], another large study, evaluating HCQ safety in lower risk patients, showed only a modest QTc prolongation without clinical consequences [
15]. At this stage, monitoring of QTc had been suggested [
16,
17].
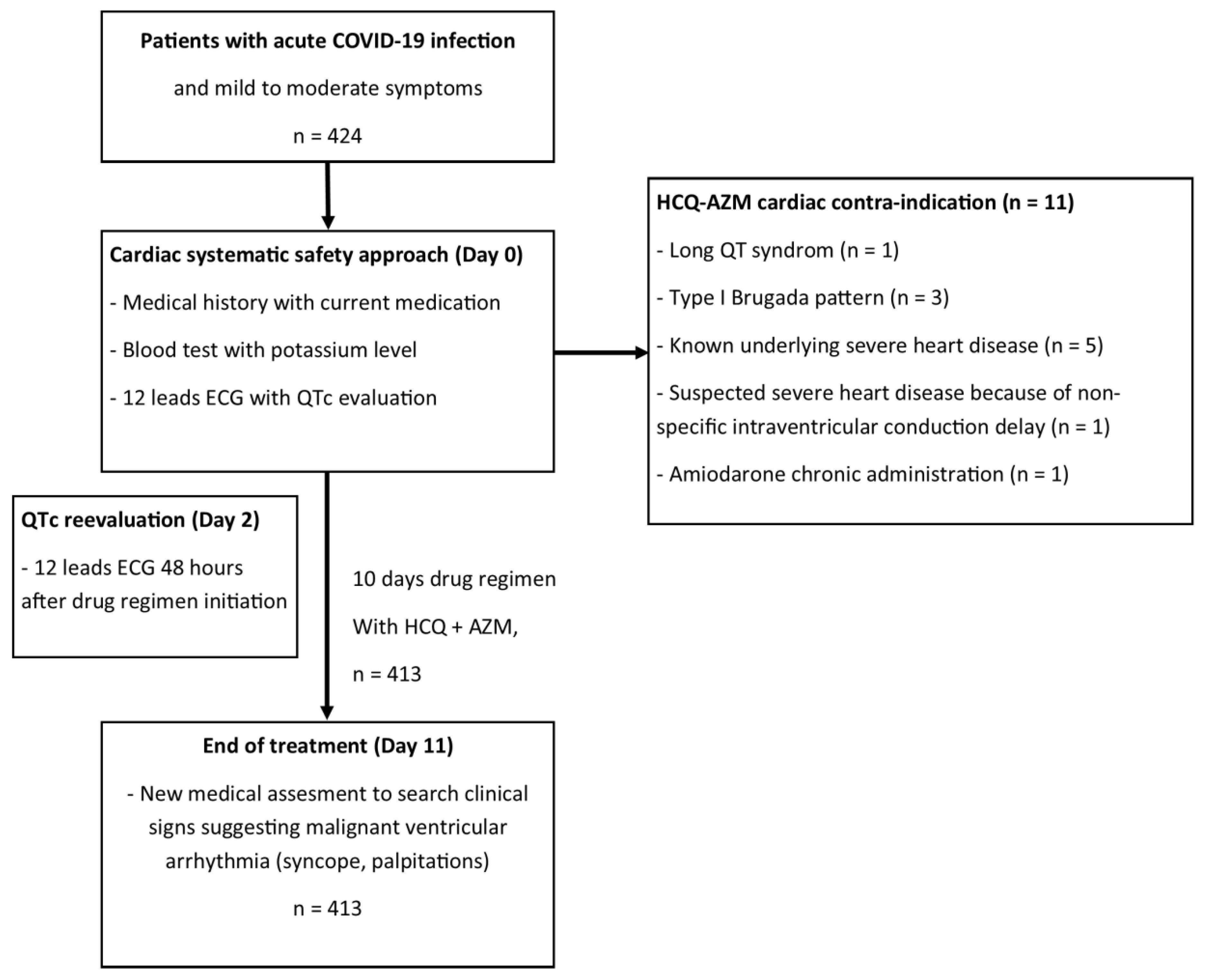
As the infectious diseases team at our academic hospital has been early involved in observing the role of HCQ-AZ in the treatment of COVID-19 infection, strict cardiac monitoring was immediately established, in the initial national context of lockdown and limited medical resources. Here, we report the details and results of our Cardiac Rhythm Safety Strategy, which was composed of an initial clinical evaluation followed by QTc monitoring in a cohort of 424 COVID-19 patients treated with HCQ-AZ.
Methods
The patients treated with HCQ-AZ were the within first adults seen for SARS-CoV-2 infection at the Institut Hospitalo-Universitaire (IHU) Méditerranée Infection, which is home to the infectious diseases department (ID) of the
Assistance Publique-Hôpitaux de Marseille (AP-HM), France, between 3 March 2020 and 5 April 2020. Our institute includes 75 hospital beds [
18]. COVID-19 patients could be hospitalized in five different ways at our institute: a) directly after screening in our day clinic, b) outpatients initially followed in our day clinic and then requiring hospitalization, c) from the emergency department, d) from other hospital wards or nursing homes, e) from intensive care units. During this early period of COVID-19 care, even mild patients could have been hospitalized as isolation request [
19]. Clinical severity was assessed using the National Early Warning Score adapted to COVID-19 patients (NEWS-2) upon hospital admission, with three categories of clinical deterioration: low score (NEWS-2 = 0–4), medium score (NEWS-2 = 5–6), and high score (NEWS-2 ≥7) [
20].
A systematic cardiac rhythm safety evaluation was performed before initiation of treatment using a simple assessment presented in
Table 1. Briefly, medical history and current medical status were thoroughly assessed for each patient. Once the decision to treat with HCQ-AZ combination had been taken by the ID clinicians, a 12-lead ECG was performed on each patient before treatment (baseline) and scheduled two days later after treatment had begun (Day 2). All the ECGs were reviewed by senior cardiologists. Heart rate, QRS duration and QT interval were measured, and the values automatically calculated by the recorder were collected. QTc was systematically calculated using the Bazett formula. No specific correction was made in cases of bundle branch block. Tracings were recorded at rest, with a paper speed of 25 mm/s and an amplitude calibration of 1 mm/mV (MAC® 3500 or MAC® 1600 recorder; GE Healthcare Europe, Freiburg, Germany). Treatment with HCQ and AZM was not started or was discontinued when the corrected QT interval (QTc; Bazett formula) was > 500 ms and the risk-benefit ratio of HCQ and AZM was estimated by the infectiologist and agreed with the cardiologist, at between 460 and 500 ms. Treatment was not started when the ECG showed patterns suggesting a channelopathy and the risk-benefit ratio was discussed when it showed other significant abnormalities (i.e., pathological Q waves, left ventricular hypertrophy, left bundle branch block). In addition, any drug with the potential to prolong the QT interval was discontinued or replaced for the duration of treatment. Standard blood chemistry was checked, especially potassium levels. Any hypokalaemia or hyperkalaemia was corrected before the initiation of treatment. The infectious diseases specialist was invited to contact a cardiologist whenever they felt the need, using a telephone “hot-line”, which was set-up in the emergency context of the pandemic. The drug regimen was as follows: HCQ, 200 mg three times a day for 10 days, plus AZ, 500 mg once a day on the first day, then 250 mg once a day for four days. All patients were physically seen or contacted by telephone on Day 11, i.e. one day after the end of the HCQ-AZM therapy.
Outpatients were informed of the need to contact the centre in the event of unusual symptoms, including palpitations and syncope/dizziness, and were scheduled for an in-person follow-up appointment on Day 2 of treatment, and for remote follow-up afterwards via the COVID AP-HM® application or by telephone.
Quantitative variables are presented as means ± standard deviations and categorical variables as numbers (percentages). An analysis was conducted in patients for whom both the baseline ECG and the Day 2 ECG were available. Initial QTc and Day 2 QTc were compared by means of a paired T-test, in the overall population and according to subgroups (age, sex, cardiopathy, and hospitalisation). The predictive effect of various characteristics (age, heart rate, sex, and cardiopathy) on QTc prolongation ≥ 30 ms and ≥ 60 ms was assessed by estimating odds ratios (ORs) with 95% confidence intervals (CIs).
An analysis was conducted in a random subsample of the overall population, to compare the cardiologist’s interpretation and the automatic interpretation of the QTc. Correlation and agreement between measures were assessed by estimating the correlation and the intraclass correlation coefficient (ICC), respectively, with 95% CI. All analyses were performed using R software, version 3.6.3. All tests were two-sided, and P values < 0.05 were considered to be statistically significant.
This study is a retrospective analysis of medical data collected during systematic cardiac rhythm safety evaluation performed before initiation of a treatment that potentially prolonged the QTc interval to minimized cardiac consequences. Data were extracted from the patient medical files analysed and stored according to the European General Protection of Data Regulation as we declared onto the AP-HM register N° 2020-151 and 2020-152.
Results
A total of 424 patients are described in the present report (as illustrated in
Figure 1) and their epidemiological, clinical, and baseline ECG characteristics are presented in
Table 2. All patients were mild to moderate patients with a NEWs Score2 = < 5. The results showed that 11 (2.6%) of the patients were not given HCQ-AZ treatment for cardiac reasons: 1 (0.2%) patient had a QTc of 480 ms and a T-wave pattern suggestive of long QT syndrome; three (0.7%) patients showed type I Brugada pattern; five (1.2%) patients had a known severe heart disease (two ischemic cardiomyopathies, one hypertrophic cardiomyopathy, one idiopathic cardiomyopathy, and one valvular heart disease); one (0.2%) patient was suspected of having severe heart disease due to a severe non-specific intraventricular conduction delay; and one (0.2%) patient was receiving chronic treatment with amiodarone.
Within the total cohort (n=424), nine patients had an initial QTc over 460ms (2.1%), three of these were contra-indicated due to an underlying cardiopathy (n=2) or repolarisation pattern suggestive of long QT syndrome. Of the six who effectively received HCQ + AZM, four had a right bundle branch block. The last two patients presented neither a pattern of long QT syndrome, nor an underlying cardiomyopathy or family history of sudden cardiac death. Treatment was, therefore, approved in all six patients.
Table 2.
Patient characteristics.
Table 2.
Patient characteristics.
| Characteristic |
Value (N = 424) |
| Male sex – no. (%) |
208 (49.5) |
| Mean age ± SD – yr |
46.3 ± 16.1 |
| ≥ 65 years – no. (%) |
47 (11.1) |
| Clinical setting – no. (%) |
|
| Day care |
333 (78.5) |
| Inpatients |
91 (21.5) |
| Cardiovascular treatment – no. (%) |
|
| ACE inhibitors/ARBs |
34 (8.0) |
| Beta-blockers |
15 (3.5) |
| Diuretics |
17 (4.0) |
| Calcium channel blockers |
1 (0.2) |
| Digoxin |
1 (0.2) |
| Flecainide |
4 (0,9) |
| Amiodarone |
1(0.2) |
| Baseline ECG |
|
| Mean heart rate ± SD – beats/min |
74.6 ± 13.6 |
| Mean QRS duration ± SD – ms |
82.3 ± 1646 |
| Mean QTc duration ± SD – ms |
396.8 ± 29.4 |
| Initial ECG patterns suggesting: |
|
| Long QT interval – no. (%) |
1 (0.2) |
| Type I Brugada syndrome – no. (%) |
3 (0.7) |
| Bundle branch block – no. (%) |
40 (9.4) |
| Left ventricular hypertrophy – no. (%) |
4 (0.9) |
| Pathological Q waves – no. (%) |
9 (2.1) |
| Early repolarisation Pattern – no. (%) |
37 (8.7) |
| QTc risk score characteristics Tisdale score (points), median (IQR) |
7 (6–7) |
In consequence, 413 (97.4%) patients were prescribed HCQ-AZ for five days and HCQ for an additional five days. None of the patients reported palpitations suggestive of a malignant ventricular arrhythmia or syncope. Two patients died from non-sudden cardiac death at hospital (one Takotsubo syndrome and one acute respiratory distress). In both cases, their heart rhythm had been closely monitored by cardiac telemetry and did not show any signs of arrhythmia. None of the six patients with a basal QTc over 460ms had either QTc prolongation or symptoms suggestive of ventricular arrhythmia under HCQ-AZ.
As shown in
Table 3, in the treated patients, QTc was slightly but statistically significantly prolonged from baseline to 48 hours, mainly driven by a QTc prolongation in women.
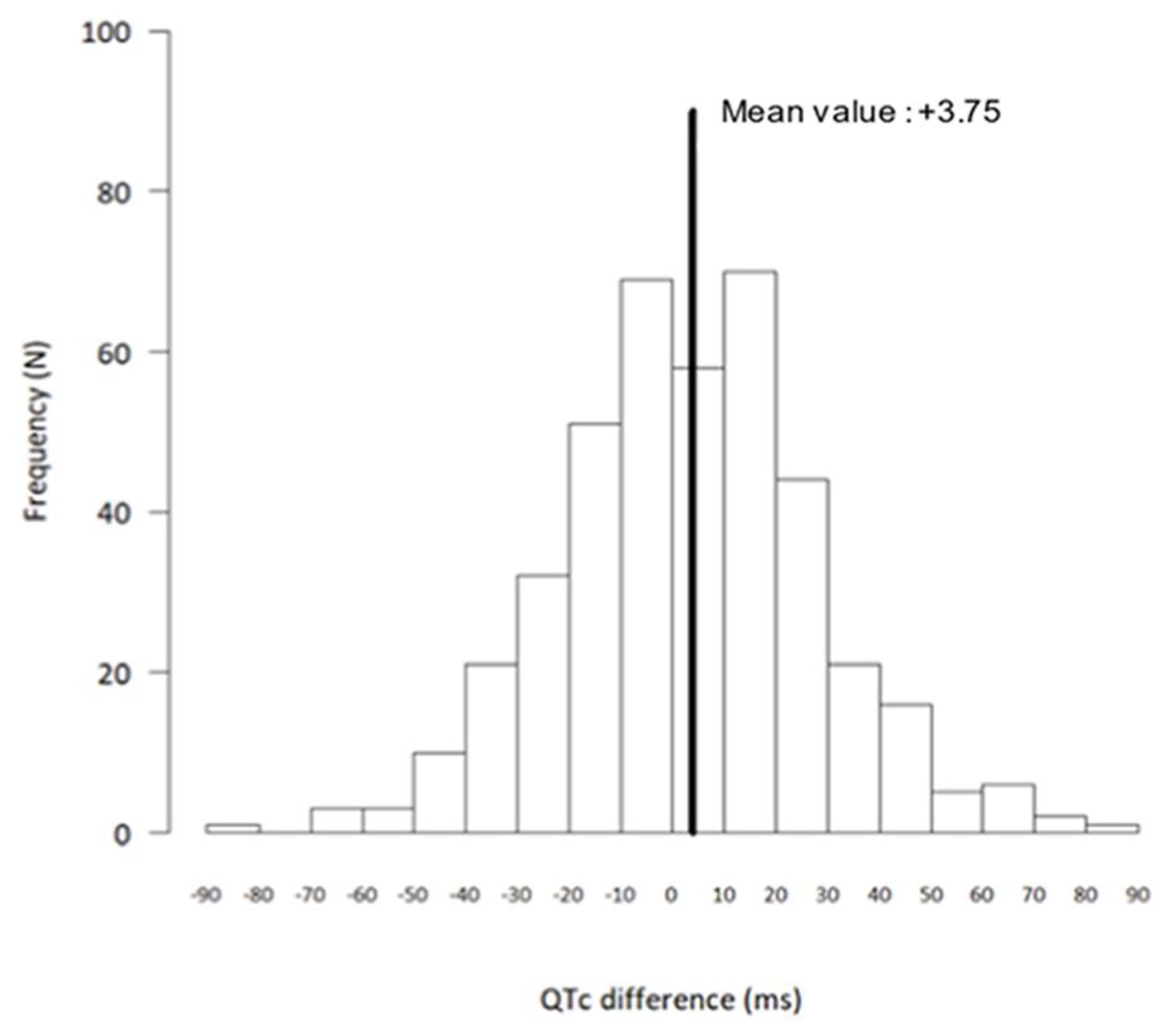
Figure 1 shows the distribution of the QTc changes at Day 2 as compared with baseline. A total of 53 patients (36 women) had a QTc prolongation of ≥ 30 ms. Gender was a risk factor as women had more frequently a QTc prolongation of ≥ 30 ms (OR, 2.17; 95% CI, 1.17 to 4.00; P=0.01). Ten patients (six women) had a QTc prolongation of ≥ 60 ms and none had a QTc > 500 ms. No clinical or electrocardiographic variables were statistically associated with QTc prolongation > 60 ms. At Day 2, heart rate was lower than at baseline (71.8 ± 12.5 beats/min vs. 74.6 ± 13.4 beats/min, respectively; P<0.0001), and the QRS duration was significantly prolonged (87.7 ± 13.3 ms vs. 81.5 ± 15.3 ms, respectively; P<0.0001).
The randomly selected subsample of ECGs used for comparison of manual QTc (cardiologist) and automatic QTc (recorder; Bazett formula) consisted of 200 ECGs from 109 women and 91 men. The automatic QTc was longer than the measured QTc (417.97 ± 24.83 ms automatic vs. 391.67 ± 24.69 ms manual; P<0.0001). Correlation between the two measures was strong (correlation coefficient, 0.87; 95% CI, 0.83 to 0.90), but agreement was moderate (intraclass correlation coefficient, 0.56; 95% CI, -0.08 to 0.84). The mean bias was 26.30 (95% CI, 24.54 to 28.06). Only one patient had an automatic QTc measurement that was shorter than the manual QTc measurement, with a difference of 1 ms.
Table 3.
Corrected QT Interval (QTc) Values Obtained at Baseline and at Day 2, and their Comparison.
Table 3.
Corrected QT Interval (QTc) Values Obtained at Baseline and at Day 2, and their Comparison.
| Variable |
Mean baseline QTc ± SD – ms |
Mean Day 2 QTc ± SD – ms |
Mean absolute difference in QTc (Day 2 vs baseline) ± SD – ms |
P value for comparison of QTc between baseline and Day 2 |
| General population (n = 413) |
396.0 ± 28.7 |
399.7 ± 28.7 |
+3.75 ± 25.4 |
0.003 |
| Sex |
|
|
|
|
| Female (n = 214) |
401.1 ± 27.4 |
407.0 ± 25.6 |
+5.61 ± 25.3 |
0.001 |
| Male (n = 199) |
390.2 ± 29 |
392.0 ± 29.8 |
+1.73 ± 25.5 |
0.31 |
| Age |
|
|
|
|
| < 65 years (n = 366) |
393.7 ± 27.4 |
397.2 ± 27.8 |
+3.56 ± 25.3 |
0.007 |
| ≥ 65 years (n = 47) |
413.9 ± 32.2 |
419.1 ± 27.8 |
+4.62 ± 26.6 |
0.19 |
| Cardiovascular disease |
|
|
|
|
| Absent (n = 350) |
392.2 ± 27.2 |
396.3 ± 28.0 |
+4.04 ± 25.9 |
0.004 |
| Present (n =63) |
416.8 ± 27.9 |
418.9 ± 24.5 |
+2.11 ± 22.7 |
0.47 |
| Patient setting |
|
|
|
|
| Day-care (n = 328) |
391.8 ± 27.8 |
395.9 ± 27.8 |
+4.11 ± 26.2 |
0.005 |
| Inpatient (n = 85) |
412.3 ± 26.3 |
414.6 ± 27.4 |
+2.33 ± 22.1 |
0.33 |
| |
Figure 2.
Distribution of differences in corrected QT interval (QTc): Day 2 versus baseline, showing absolute differences between Day 2 and baseline QTc (N = 413 patients).
Figure 2.
Distribution of differences in corrected QT interval (QTc): Day 2 versus baseline, showing absolute differences between Day 2 and baseline QTc (N = 413 patients).
Discussion
Our results were obtained in 424 SARS-CoV-2-infected patients with mild-to-moderate symptoms, who were candidates for a treatment combining HCQ-AZ for five days followed by HCQ alone for an additional five days, during the first weeks of covid patients’ care in our center. These results show that a pragmatic safety strategy can be implemented in the emergency setting, to ensure that this treatment has an acceptable cardiac rhythm safety. Based on the first medical assessment, including a 12-lead ECG, 2.6% of the patients did not receive the treatment for cardiac reasons. Among the patients who were eligible, treatment with HCQ-AZ did not lead to QTc prolongation necessitating the interruption of treatment and no sudden cardiac deaths were observed. Moreover, the automatic determination of the QTc by the ECG recorder appeared to be consistently longer than that measured by a cardiologist, suggesting that it may be safe to use this value. Initial cardiac safety publications evaluating HCQ treatment in hospitalised patients with COVID-19, with a mean age over 60, a longer basal QTc interval (respectively 396 ± 28.7 vs 435 ± 24 ms, p<0.001) and including severe and critically ill patients with COVID-19, have shown significant QTc lengthening in some patients [
13,
14] possibly leading to severe ventricular arrythmia. As shown in our cohort, a simple clinical evaluation with kalaemia and the use of a first ECG enabled us to initiate the treatment with acceptable safety in terms of potential arrhythmias, as confirmed by the second ECG and the clinical outcomes of our patients. The significant, but modest, QTc lengthening observed here is within the range of what has been reported when HCQ is combined with AZ [
15]. A slight but significant prolongation of the QRS duration was also observed and is consistent with the known effects of chloroquine on the electrocardiogram [
21]. In any case, our results in a higher risk population (i.e. initial QTc over 460 ms, severe and critically ill patients) have yet to be proven in a larger study.
HCQ is a derivative of chloroquine, which has similarities to quinine, and may therefore prolong the QT interval, although the effect is expected to be modest [
22]. AZ has low affinity for the hERG channel [
16], and no proarrhythmia potential of the two drugs in combination has been shown at therapeutic doses [
17].
We acknowledge that our monitoring strategy could have been stricter, but it should be borne in mind that these measures were implemented in the context of an ongoing and rapidly increasing pandemic, taking into account the balance between benefits and risks for patients. The strategy was consistent with safety guidelines issued by the American College of Cardiology [
23], which recommended that intensity of QT and arrhythmia monitoring should be considered in the context of risk level, resource availability and quarantine considerations. Our experience is in accordance with their proposal, which should reassure clinicians when using these drugs. Even in this context, relatively simple measures can be implemented, such as the ones we used: warning colleagues about the added risk of combining medications that could lengthen the QT interval or decrease kalaemia; reminding colleagues how to recognise, based on the patient’s history and medications, whether there is a risk of channelopathy or severe heart disease; being especially cautious with the elderly, especially women who, as we confirm here, may be most exposed to significant prolongation of the QTc interval. In addition, in this emergency situation we feel it is extremely important to put establish a range of means of facilitating “on-line” communication between the teams prescribing anti-infectious drugs and the cardiologists. Obviously, our results should not be extrapolated to situations in which these measures cannot be implemented.
In a smaller cohort [
24], we previously published the daily monitoring of QTc under HCQ-AZ using a smartwatch, in cases of early stage COVID-19 infections with mild to moderate symptoms. Although we did not show a high risk of QTc prolongation, we do not recommend using our strategy when more precise monitoring of the QT interval may be required. In such cases, monitoring must be implemented as recommended [
25], to allow patients to benefit from the treatment under the strictest safety conditions. This is particularly true in SARS-CoV-2-infected patients in intensive care, taking into account their increased risk of electrolyte disorders [
26].
Most of the patients (78.5%) in our cohort were outpatients, and only 11.1% were aged over 65, with mild to moderate symptoms. Given that treatment is not toxic and has a simple management protocol, that the at-risk population could not be perfectly identified at baseline, and that earliness is critical for efficacy, we have discussed elsewhere how this treatment should be initiated at the early stage of the disease [
6].
Our results show that automatic QTc measurement by an ECG recorder leads to a mean systematic error that overestimates the QTc compared with manual assessment. It could, therefore, be acceptable, given the pandemic circumstances, to generalise automatic assessment of QTc at the initiation of HCQ-AZ treatment. This strategy may reduce the number of patients in whom a second opinion from a cardiologist is needed e.g., when the automatic QTc exceeds 460 ms.
Also, it should be reminded that the Bazett formula was used to estimate the QTc, as this is the most common approach. This formula is suboptimal [
27] and overestimates the QTc, particularly when the heart rate is high, which might often be the case in febrile patients. Obviously, this will increase the safety process. These points are important when QTc monitoring may be performed using surrogates for conventional ECG recorders [
25].
Once again, no torsade de pointe was identified in the 424 included patients. Furthermore, in order to identify the covid patients most at risk based on the present study, we found that HCQ significantly prolongs QTc in women less than 65 years of age managed on an outpatient basis who do not have cardiovascular disease (
Table 3). This is a population with an extremely low risk of torsade de pointes. In contrast, we did not find any significant QTc prolongation in male patients, older than 65 years, with cardiovascular disease or hospitalized (
Table 3). Hence, in the population with the more severe cardiac vulnerability, we found that the risk of torsade de pointe, based on QTc prolongation, was lower. Based on cardiologic expertise, selected patients should benefit from smartwatch electrocardiogram and artificial intelligence, demonstrated by our team as a relevant and modern approach [
24]. Finally, in the literature, studies with careful and expert cardiac monitoring did not find sudden death and cardiac mortality related to HCQ-AZ treatment [
28].
Conclusions
Staring April 2020, the indications for the Day 2 control ECG were restricted after an initial workup showing that all contraindicative repolarization abnormalities had been detected on the first ECG, and that HCQ-Az could be used safely. This ECG monitoring was conducted in an emergency situation as an early response to the ongoing COVID-19 pandemic. This report does not aim to contribute to knowledge of the efficacy of treating COVID-19 with HCQ-AZ. Indeed, we have reported our 2020 data elsewhere [
5,
6]. More results will soon be discussed in the SARS-CoV-2 variants, vaccination and post-vaccination era. Here we wanted to focus in details about the so-called cardiac toxicity of HCQ-AZ combination. Our results indicate that the risks of severe arrhythmia induced by combined HCQ-AZ therapy COVID-19, if any, can be minimised by the institution of simple clinical management, including careful assessment of contraindications (mainly cardiological history, co-medications, kalaemia, initial ECG), interruption of other drugs prolonging QTc if possible and correction of hypo- or hyperkalaemia. This has been confirmed by when treating more than 30,000 in our center in 2020 and 2021 including moderate to severe 4,000 hospitalized patients. If this simple and systematic cardiac rhythm safety evaluation is performed, and contraindications are respected, treatment with HCQ-AZ in early-stage COVID-19 is safe and is not associated with clinically relevant cardiac rhythm side effects. This work is evidence to the fact that QT-prolonging anti-infective drugs (Supplementary
Table 4) can be used safely in acute life-threatening infections, provided that a strict protocol and close collaboration between infectious disease specialists and rhythmologists are followed.
Author Contributions
M.M.: Investigation, resources, writing-original draft; J.-C.L.: Investigation, resources, writing- review and editing; J.H.: Formal analysis; F.F.: Formal analysis; J.C.D.: Conceptualization, methodology, Supervision; P.P.: Conceptualization, supervision; P.B.: Conceptualization, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
No specific source of funding.
Conflicts of Interest
None of the authors declare have any conflicts of interest to declare.
References
- Coronavirus Resource Center. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Accessed at https://coronavirus.jhu.edu/map.html on December 26th, 2022.
- Gautret P, Million M, Jarrot PA, Camoin-Jau L, Colson P, Fenollar F, et al. Natural history of COVID-19 and therapeutic options. Expert Rev Clin Immunol 2020, 16, 1159-1184. [CrossRef]
- World Health Organization. (2022). Nirmatrelvir-ritonavir for COVID-19. World Health Organization. https://apps.who.int/iris/handle/10665/359751.
- Global HCQ/CQ studies. https://c19study.com.
- Lagier JC, Million M, Cortaredona S, Delorme L, Colson P, Fournier PE, et al. Outcomes of 2111 COVID-19 Hospitalized Patients Treated with Hydroxychloroquine/Azithromycin and Other Regimens in Marseille, France, 2020: A Monocentric Retrospective Analysis. Ther Clin Risk Manag 2022, 18, 603-617. [CrossRef]
- Million M, Lagier JC, Tissot-Dupont H, Ravaux I, Dhiver C, Tomei C, et al. Early combination therapy with hydroxychloroquine and azithromycin reduces mortality in 10,429 COVID-19 outpatients. Rev Cardiovasc Med 2021, 22, 1063-1072. [CrossRef]
- Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ 2020, 369, m1849. [CrossRef]
- Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A, et al. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N Engl J Med 2020, 383, 2041. [CrossRef]
- Self WH, Semler MW, Leither LM, Casey JD, Angus DC, Brower RG, et al. Effect of Hydroxychloroquine on Clinical Status at 14 Days in Hospitalized Patients With COVID-19: A Randomized Clinical Trial. JAMA 2020, 324, 2165. [CrossRef]
- Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G, et al. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med 2020, 382, 2411-2418. [CrossRef]
- Chatre C, Roubille F, Vernhet H, Jorgensen C, Pers Y-M. Cardiac Complications Attributed to Chloroquine and Hydroxychloroquine: A Systematic Review of the Literature. Drug Saf 2018, 41, 919-31. [CrossRef]
- Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med 2012, 366, 1881-90. [CrossRef]
- Chorin E, Dai M, Shulman E, Wadhwani L, Bar-Cohen R, Barbhaiya C, et al. The QT interval in patients with COVID-19 treated with hydroxychloroquine and azithromycin. Nat Med 2020, 26, 808-809. [CrossRef]
- Bessière F, Roccia H, Delinière A, Charrière R, Chevalier P, Argaud L, et al. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit. JAMA Cardiol 2020, 5, 1067-1069. [CrossRef]
- Gasperetti A, Biffi M, Duru F, Schiavone M, Ziacchi M, Mitacchione G, et al. Arrhythmic safety of hydroxychloroquine in COVID-19 patients from different clinical settings. Europace 2020, 22, 1855-1863. [CrossRef]
- Hancox JC, Hasnain M, Vieweg WVR, Crouse ELB, Baranchuk A. Azithromycin, cardiovascular risks, QTc interval prolongation, torsade de pointes, and regulatory issues: A narrative review based on the study of case reports. Ther Adv Infect Dis 2013, 1, 155-65. [CrossRef]
- Fossa AA, Wisialowski T, Duncan JN, Deng S, Dunne M. Azithromycin/Chloroquine combination does not increase cardiac instability despite an increase in monophasic action potential duration in the anesthetized guinea pig. Am J Trop Med Hyg 2007, 77, 929-38. [CrossRef]
- Brouqui P, Drancourt M, Raoult D, On behalf of the IHU Task Force. COVID-19 Management at IHU Méditerranée Infection: A One-Year Experience. J Clin Med 2021, 10, 2881. [CrossRef]
- Million M, Lagier JC, Gautret P, Colson P, Fournier PE, Amrane S, et al. Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: A retrospective analysis of 1061 cases in Marseille, France. Travel Med Infect Dis 2020, 35, 101738. [CrossRef]
- Liao X, Wang B, Kang Y. Novel coronavirus infection during the 2019-2020 epidemic: preparing intensive care units-The experience in Sichuan Province, China. Intensive Care Med 2020, 46, 357–360. [CrossRef]
- Haeusler IL, Chan XHS, Guérin PJ, White NJ. The arrhythmogenic cardiotoxicity of the quinoline and structurally related antimalarial drugs: a systematic review. BMC Medicine 7 nov 2018, 16, 200. [CrossRef]
- Costedoat-Chalumeau N, Hulot J-S, Amoura Z, Leroux G, Lechat P, Funck-Brentano C, et al. Heart conduction disorders related to antimalarials toxicity: an analysis of electrocardiograms in 85 patients treated with hydroxychloroquine for connective tissue diseases. Rheumatology (Oxford) 2007, 46, 808-10. [CrossRef]
- American College of Cardiology. Ventricular Arrhythmia Risk Due to Hydroxychloroquine-Azithromycin Treatment For COVID-19. Available at: https://www.acc.org/latest-in-cardiology/articles/2020/03/27/14/00/ventricular-arrhythmia-risk-due-to-hydroxychloroquine-azithromycin-treatment-for-covid-19 (accessed December, 26, 2022).
- Maille B, Wilkin M, Million M, Rességuier N, Franceschi F, Koutbi-Franceschi L, et al. Smartwatch electrocardiogram and artificial intelligence for assessing cardiac-rhythm safety of drug therapy in the COVID-19 pandemic. The QT-logs study. Int J Cardiol 2021, 331, 333-339. [CrossRef]
- Roden Dan M., Harrington Robert A., Poppas Athena, Russo Andrea M. Considerations for drug interactions on QTc in exploratory COVID-19 treatment. Circulation 2020, 141, e906-7. [CrossRef]
- Chen D, Li X, Song Q, Hu C, Su F, Dai J. Hypokalemia and clinical implications in patients with coronavirus disease 2019 (COVID-19). MedRxiv 2020, 2020.02.27.20028530. [CrossRef]
- Vandenberk B, Vandael E, Robyns T, Vandenberghe J, Garweg C, Foulon V, et al. Which QT correction formulae to use for QT monitoring? J Am Heart Assoc 2016, 5, e003264. [CrossRef]
- O'Connell TF, Bradley CJ, Abbas AE, Williamson BD, Rusia A, Tawney AM et al. Hydroxychloroquine/Azithromycin therapy and QT prolongation in hospitalized patients with COVID-19. JACC Clin Electrophysiol. 2021, 7(1), 16-25. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).