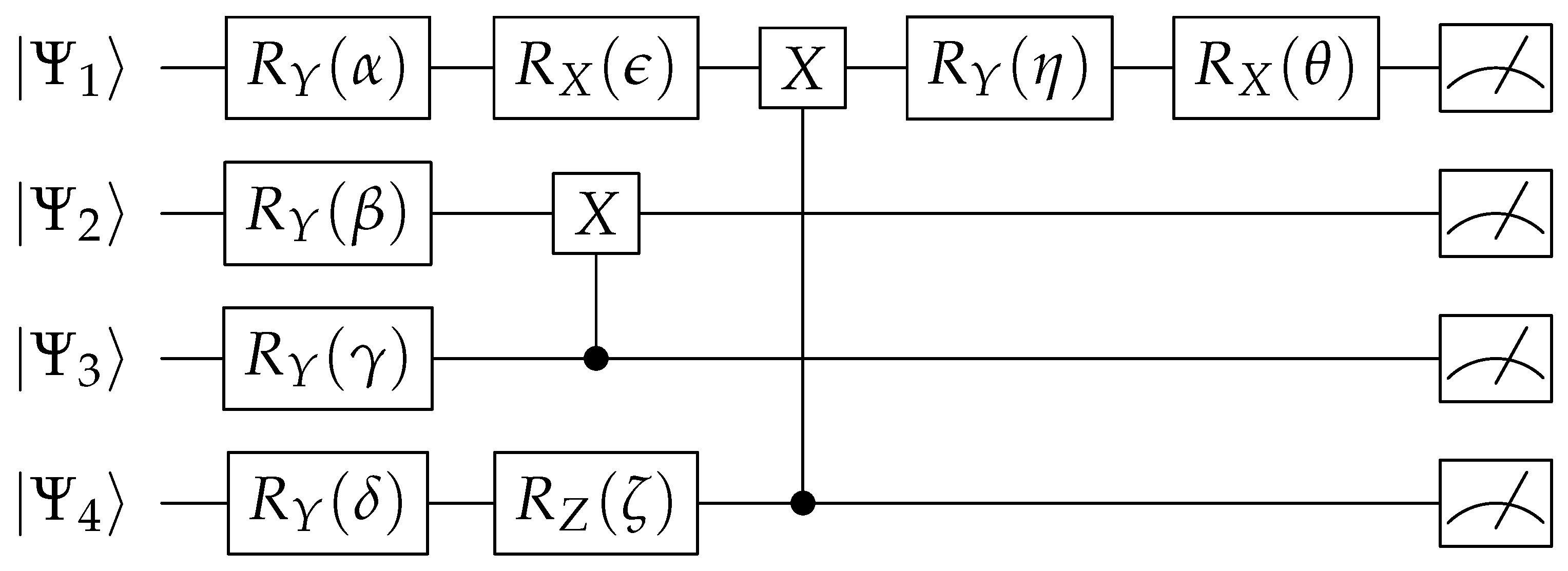1. Introduction
While a manual optical inspection of the eye can detect more obvious issues, fundus photography has proven its usefulness as being able to detect more subtle issue and being able to refer back over time for comparison and understanding of progression of a disease or risk thereof. Such subtle detection and comparison tasks can leverage the recent advancements in machine learning technology for accuracy as the data is available in digital format. Even though a well trained doctor may not require such methods, such tools can provide a standard for others and a frame of reference when there is doubt in diagnosis.
In the following sections, we introduce fundus photography, list its benefits, compare it with optical coherence tomography, give a quick overview of eye anatomy as it relates to fundus photography, provide more details on how to detect these diseases. After reviewing previous work and covering open problems in the field, we dive into developing a quantum machine learning method to classify ocular diseases automatically from fundus imagery.
1.1. Fundus Photography
Fundus photography is a specialized type of photography that captures high-resolution images of the retina. This can be used to detect diseases such as diabetic retinopathy at an early stage and monitor the progression of the disease.
During the fundus photography exam, the patient is seated in front of a camera and asked to look at a target in the center of the camera’s lens. The camera then takes several photographs of the retina. The images are usually analyzed by an eye care professional to look for signs of a disease such as diabetic retinopathy by identifying leaking blood vessels, blocked blood vessels, abnormal blood vessel growth, etc..
The images taken during the fundus photography exam can be used to detect a disease such as diabetic retinopathy in its early stages, even before the patient notices any symptoms. This is important because early detection and treatment can help prevent or slow the progression of the disease and prevent vision loss.
It is important to note that fundus photography is usually done after a dilated eye exam, and the images are interpreted by an eye care professional who is trained in interpreting the images. Additionally, fundus photography can also be used to monitor the progression of diabetic retinopathy over time. By comparing the current images with previous ones, the eye care professional can see if the disease is getting worse and make adjustments to the patient’s treatment plan accordingly.
1.2. Diagnostics Using Fundus Photography
Age-related macular degeneration (AMD) can be detected through fundus photography by looking for specific changes in the appearance of the macula. During a fundus photography exam, a special camera takes a high-resolution image of the inside of the eye, including the retina and macula. This image can be used to detect signs of AMD, including:
Drusen: small, yellow or white deposits that build up under the retina.
Pigment changes: dark areas or clumps of pigment in the macula that may indicate degeneration of the retinal pigment epithelium (RPE).
Atrophic changes: a thinning of the RPE, which can lead to vision loss in the central part of the visual field.
Neovascular changes: growth of new blood vessels in the retina, which can lead to vision loss if they bleed or cause scarring.
The presence and size of drusen, pigment changes, atrophic changes, and neovascular changes can provide important information about the severity of AMD and the risk of vision loss.
In addition to fundus photography, other diagnostic tools such as optical coherence tomography (OCT) and fluorescein angiography may also be used to evaluate patients with suspected AMD.
Cataracts can be detected through fundus photography by examining the appearance of the lens in the eye. During a fundus photography exam, a special camera takes a high-resolution image of the inside of the eye, including the lens. This image can be used to detect signs of cataracts, including:
Opacity: lens may appear cloudy or hazy, indicating the presence of a cataract.
Light scatter: light may scatter in the lens, leading to a decrease in visual acuity and the perception of glare.
Reduced clarity: lens may appear less transparent, reducing the clarity of the image captured by the camera.
In addition to fundus photography, other diagnostic tools such as slit lamp biomicroscopy and ultrasonography may also be used to evaluate patients with suspected cataracts. These exams provide detailed information about the size, shape, and location of the cataract and are used to guide treatment planning.
Through fundus photography, an eye care professional can detect and monitor several features of diabetic retinopathy such as:
Microaneurysms: small, dot-like changes in the blood vessels of the retina that occur in the early stages of diabetic retinopathy. They appear as small red or pink dots on the fundus photograph.
Retinal hemorrhages: bleeding in the retina appears as red or dark spots on the fundus photograph.
Cotton wool spots: small, white, fluffy areas of the retina that occur when the blood vessels in the retina are blocked. They appear as small white spots on the fundus photograph.
Hard exudates: yellow, lipid-rich deposits that form on the retina as a result of leakage from blood vessels. They appear as small yellow spots on the fundus photograph.
Venous beading: blood vessels in the retina may appear to be constricted or twisted, which is known as venous beading and can be seen on the fundus photograph.
New blood vessel formation: new blood vessels may grow on the surface of the retina and in the vitreous. They appear as delicate, fragile and abnormal vessels that can be seen on the fundus photograph.
Macular edema: this is a swelling of the macula, which is the central part of the retina responsible for sharp, clear vision. It can be seen on the fundus photograph as a blurry or distorted area in the center of vision.
Progressive retinal detachment: this could lead to blindness if left untreated.
Glaucoma can be detected through fundus photography by examining the appearance of the optic nerve head in the eye.
Optic nerve head cupping: change in the shape of the optic nerve head, indicating loss of retinal ganglion cells.
Nerve fiber layer loss: thinning of the nerve fiber layer surrounding the optic nerve head, indicating the loss of retinal ganglion cells.
Optic disc hemorrhage: presence of bleeding in the optic nerve head, which may indicate damage to the retinal ganglion cells.
Blocked veins: appearance of blocked veins near the optic nerve head, which may indicate increased pressure within the eye.
In addition to fundus photography, other diagnostic tools such as visual field testing and optical coherence tomography (OCT) may also be used to evaluate patients with suspected glaucoma. These exams provide additional information about the extent and severity of vision loss and the health of the optic nerve.
Hypertension is not directly detected through fundus photography. However, the effects of high blood pressure on the blood vessels in the eye can be seen in fundus photography images.
Narrowing of blood vessels: blood vessels in the eye may appear narrower than normal, indicating increased pressure in the blood vessels.
Excess bleeding: presence of small hemorrhages in the retina, indicating increased pressure in the blood vessels.
Exudates: presence of yellow or white deposits near the blood vessels, indicating damage to the retina due to high blood pressure.
Pathological myopia, also known as high myopia, can be detected through fundus photography by examining the appearance of the back of the eye.
Elongation of the eye: longer than normal axial length of the eye, which can be seen in the fundus photography image.
Thinning of the retina: thinning of the retina, which can be seen in the fundus photography image as a decreased thickness.
Chorioretinal atrophy: reduced thickness of the choroid and retina, which can be seen in the fundus photography image as a lighter or more transparent area.
Degenerative changes in the retina: presence of degenerative changes in the retina, such as changes in the color and pattern of the retinal pigment epithelium or the presence of retinal tears, holes, or detachments.
In addition to fundus photography, other diagnostic tools such as ocular biometry, optical coherence tomography (OCT), and visual field testing may also be used to evaluate patients with suspected pathological myopia. These exams provide additional information about the extent and severity of vision loss and the health of the retina.
| AMD |
Cataract |
Diabetic |
Glaucoma |
Hypertension |
Patological myopia |
| Drusen |
Opacity |
Microaneurysms |
Optic nerve head cupping |
Narrowing of vessels |
Elongation of the eye |
| Pigment changes |
Light scatter |
Retinal hemorrhages |
Nerve fiber layer loss |
Excess bleeding |
Thinning of the retina |
| Atrophic changes |
Reduced clarity |
Cotton wool spots |
Optic disc hemorrhage |
Exudates |
Chorioretinal atrophy |
| Neovascular changes |
|
Hard exudates |
Blocked veins |
|
Retinal degeneration |
| |
|
Venous beading |
|
|
|
| |
|
Vessel formation |
|
|
|
| |
|
Macular edema |
|
|
|
| |
|
Retinal detachment |
|
|
|
1.3. Optical Coherence Tomography
Since many of these diseases can also be detected via Optical Coherence Tomography (OCT), let us differentiate why we focus on fundus photography in this work. Both are imaging techniques used to examine the retina, but they work in different ways and provide different types of information.
Fundus photography is a type of photography that captures high-resolution images of the retina. It is used to detect and monitor eye diseases by looking for changes in the blood vessels, such as leaking or blocked vessels, or abnormal blood vessel growth. Fundus photography can also be used to monitor the progression of such diseases over time.
On the other hand, Optical Coherence Tomography (OCT) is a non-invasive imaging test that uses light waves to take cross-sectional images of the retina. It provides detailed, high-resolution images of the different layers of the retina, including the nerve fiber layer, the ganglion cell layer, and the retinal pigment epithelium. These images can be used to detect and monitor diabetic retinopathy, as well as other eye diseases such as glaucoma and age-related macular degeneration.
OCT can also be used to detect and monitor macular edema, which is a swelling of the macula. OCT can also detect changes in the thickness of the retina, which can be an early sign of diabetic retinopathy and other eye diseases. The technology is also useful in detecting "hidden" diabetic retinopathy that may not be visible during a dilated eye exam or fundus photography.
In summary, fundus photography provides a detailed view of the overall retina, while OCT provides detailed, cross-sectional images of the different layers of the retina, which can detect subtle changes, it is used to evaluate the macula, macular edema and the retinal thickness. Both are useful diagnostic tools, and depending on the case, one or both may be used in the examination of diabetic retinopathy.
An eye care professional will typically decide when to use Optical Coherence Tomography (OCT) over fundus photography based on the specific needs of the patient and the type of eye condition being evaluated. OCT is often used to evaluate the macula, which is the central part of the retina responsible for sharp, clear vision. It can be used to detect and monitor macular edema, which is a swelling of the macula that can occur in diabetic retinopathy and other eye diseases. OCT is also useful in detecting "hidden" diabetic retinopathy that may not be visible during a dilated eye exam or fundus photography.
Fundus photography, on the other hand, provides a detailed view of the overall retina and is used to detect and monitor diabetic retinopathy and other eye diseases by looking for changes in the blood vessels, such as leaking or blocked vessels, or abnormal blood vessel growth. Fundus photography can also be used to monitor the progression of diabetic retinopathy over time.
OCT may also be used to detect changes in the thickness of the retina, which can be an early sign of diabetic retinopathy and other eye diseases. In cases where the doctor suspects macular edema or wants to evaluate the retinal thickness, OCT is the preferred method of examination. If the doctor is more interested in monitoring the progression of diabetic retinopathy over time, or wants to get an overall view of the retina, then fundus photography would be preferred.
In some cases, both fundus photography and OCT can be used together to provide a more comprehensive examination of the retina. The doctor may use the information from both tests to make a diagnosis and guide treatment decisions.
It is important to note that the choice of which test to use will depend on the clinical presentation of the patient, the doctor’s experience and expertise, and the availability of the equipment. For the rest of the paper, we focus our work on fundus photography only.
1.4. Eye Anatomy With Respect to Fundus Photography
The macula is a small, central area of the retina located in the back of the eye. It is responsible for sharp, clear vision, and it is the area of the retina that is most sensitive to light. The macula is about 5mm and is located in the center of the retina.
The macula is composed of special cells called cones, which are responsible for detecting color and fine details. This is why the macula is important for tasks such as reading, driving, and recognizing faces. Damage to the macula can cause a loss of central vision, which can make it difficult to perform these tasks.
The macula also contains a small depression called the fovea, which is the most sensitive part of the retina. The fovea is responsible for the sharpest vision, and it is the area of the retina that is used for reading, writing, and other tasks that require fine visual acuity.
Diseases that can affect the macula include age-related macular degeneration (AMD), diabetic retinopathy, and macular edema. These diseases can cause changes in the blood vessels, leading to swelling or bleeding in the macula, which can cause a loss of central vision.
The macula is located in the center of the retina, which is the light-sensitive tissue lining the back of the eye. The retina receives light from the lens and converts it into electrical signals that are sent to the brain via the optic nerve.
The macula is located about 5-6mm from the optic nerve head, which is the point where the optic nerve exits the eye and connects to the brain. The macula is responsible for sharp, clear central vision, and it is the area of the retina that is most sensitive to light. The fovea which is a small depression in the macula is the most sensitive part of the retina, it is responsible for the sharpest vision.
The macula is responsible for the central vision, while the peripheral retina (the area around the macula) is responsible for the side vision. The macula is surrounded by the peripheral retina, which is responsible for providing the brain with information about the location and movement of objects in the visual field.
Damage to the macula can cause a loss of central vision, which can make it difficult to perform tasks such as reading, writing, and recognizing faces.
2. Previous Work
[
1] provide a review of work on machine learning as it relates to fundus photography. [
2] also covers the literature of machine learning but for a more directed study of diabetic retinopathy severity diagnosis. [
3] implies that deep learning methods are quite reliable in this area with false negative rate reduction of 23% with only 2% false positive rate degradation against human graders. [
4] summarizes top solutions from a diabetic retinopathy diagnosis challenge.
The next set of work employ quantum machine learning and thus are most relevant to our work. [
5] implements a transfer learning based approach. The gist of such approaches is to start with an existing model, and then introduce modifications to the model so that it can target a different problem. For example a purely classical machine learning implementation can be a starting point and parts of the system can be converted to quantum. In this work, authors targeted APTOS 2019 Blindness Detection dataset using a pre-trained Inception V-3 neural network. The pre-trained network is quite deep with 48 layers and reduces into a 2048 feature vector. They use a four-qubit complex quantum circuit with 28 parameters and 18 CNOTs. Disadvantages of such system is complexity due to large number of layers, requirement to have a pre-trained network, and error-proneness of the quantum part due to high number of parameters and high count of CNOTs. Furthermore, the data available in the dataset are zoomed-in versions of fundus images, and hence are not how an original data will be available. In many cases, the curvature of the eye is not even visible.
[
6] utilized 2-qubit quantum machine learning for OCT images for a normal vs. choroidal neovascularization, diabetic macular edema, and drusen categories. Quantum portion consisted of angle embedding and entangling layers. The classical portion utilized a convolutional neural network. OCT images are quite different than fundus ones though and it is not clear if techniques developed for OCT could help fundus imagery.
[
7] use quantum machine learning to target RetinaMNIST dataset with 28x28 pixel images, i.e. a very small resolution. This dataset is for regression and not classification, and hence the methods proposed may not be directly applicable to our problem.
Readers may also refer to [
8] or other better known sources for an introduction to quantum computing in general.
2.1. Open Problems
There are several open problems in the field, some of which include:
Early detection: Despite advances in screening and imaging technologies, diabetic retinopathy can still be difficult to detect in its early stages. Developing more sensitive and specific methods for early detection is an ongoing area of research.
Understanding the underlying disease mechanisms: While much is known about the microvascular changes that occur in diabetic retinopathy, the underlying disease mechanisms are not fully understood. Further research is needed to identify the molecular and cellular pathways involved in the development of diabetic retinopathy.
Personalized treatment: The optimal treatment for diabetic retinopathy can vary depending on the individual patient and the stage of the disease. Developing personalized treatment plans that take into account a patient’s specific needs and characteristics is an ongoing area of research.
Telemedicine: Diabetic retinopathy is a leading cause of blindness in many parts of the world, especially in remote and rural areas. Telemedicine is a promising approach to addressing this problem by providing remote access to screening and diagnostic services. However, more research is needed to develop cost-effective and reliable telemedicine systems that can be used in low-resource settings.
Artificial intelligence and Machine learning: With the recent success of deep learning in medical imaging, there is a growing interest in using these techniques to improve the diagnosis and treatment of diabetic retinopathy. However, more research is needed to develop robust and accurate AI-based methods that can be used in a clinical setting.
Cost-effectiveness: Diabetic retinopathy treatment can be expensive and not accessible for many people, especially in low-income countries. More research is needed to identify cost-effective screening and treatment options that can be implemented in low-resource settings.
These are some of the open problems in diabetic retinopathy, and new advances are being made in these areas through ongoing research.
3. Experimental Results
From these open problems, in this paper we target the artificial intelligence and machine learning aspect. We use a pre-categorized dataset for our imagery [
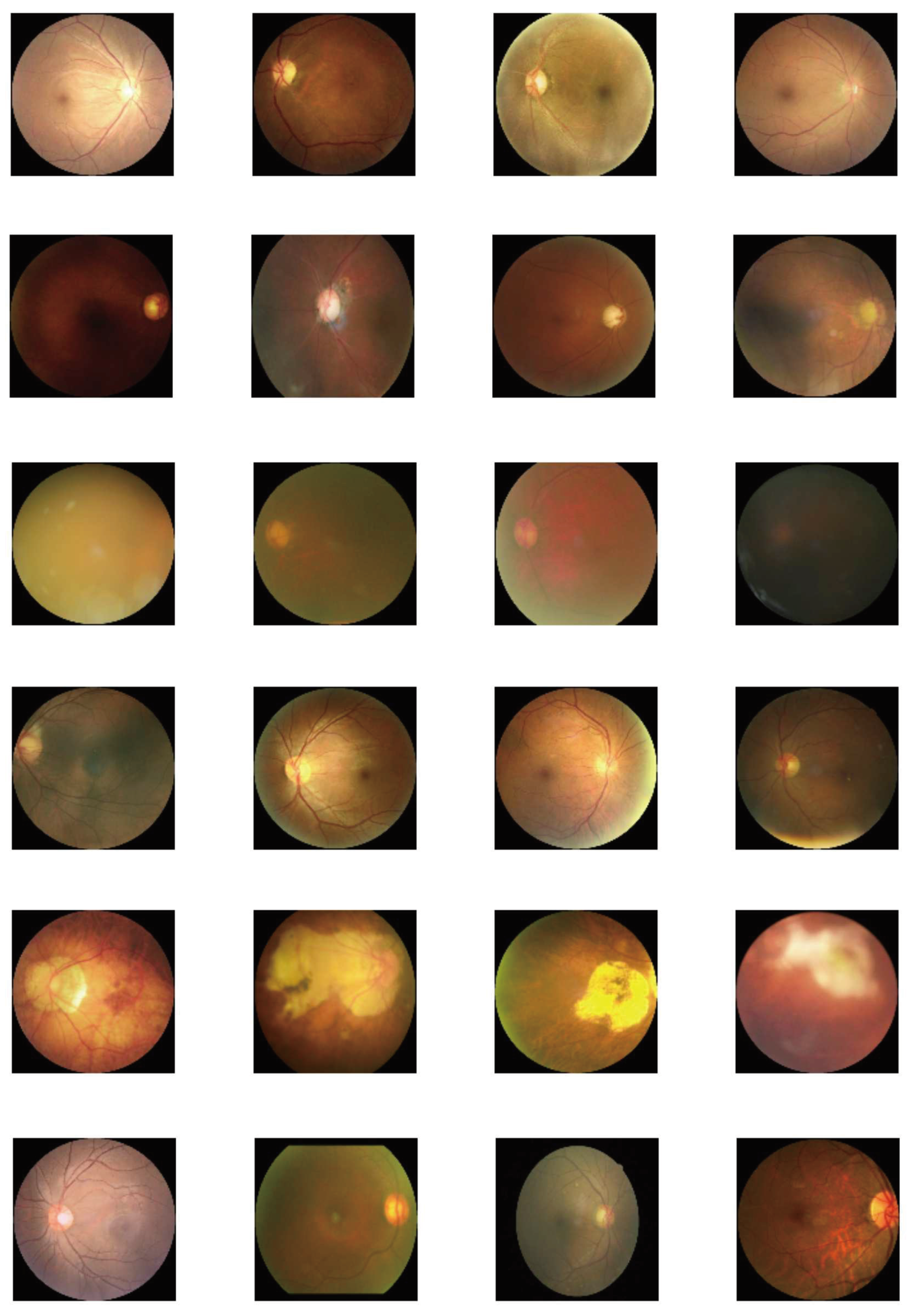
9]. We train our models using 800 patients and test them on 200 patients and report results on accuracy and loss on the latter. Sample data we have used are shown in
Figure 1 with multiple examples of a disease on each row. Not all fundus images are symmetric as we notice that some have been skewed. We use Intel i-7 CPU with 2.2GHz in single-threading mode. We use Pennylane to simulate the quantum implementation. We use Adam optimizer with sparse categorical crossentropy as the loss function.
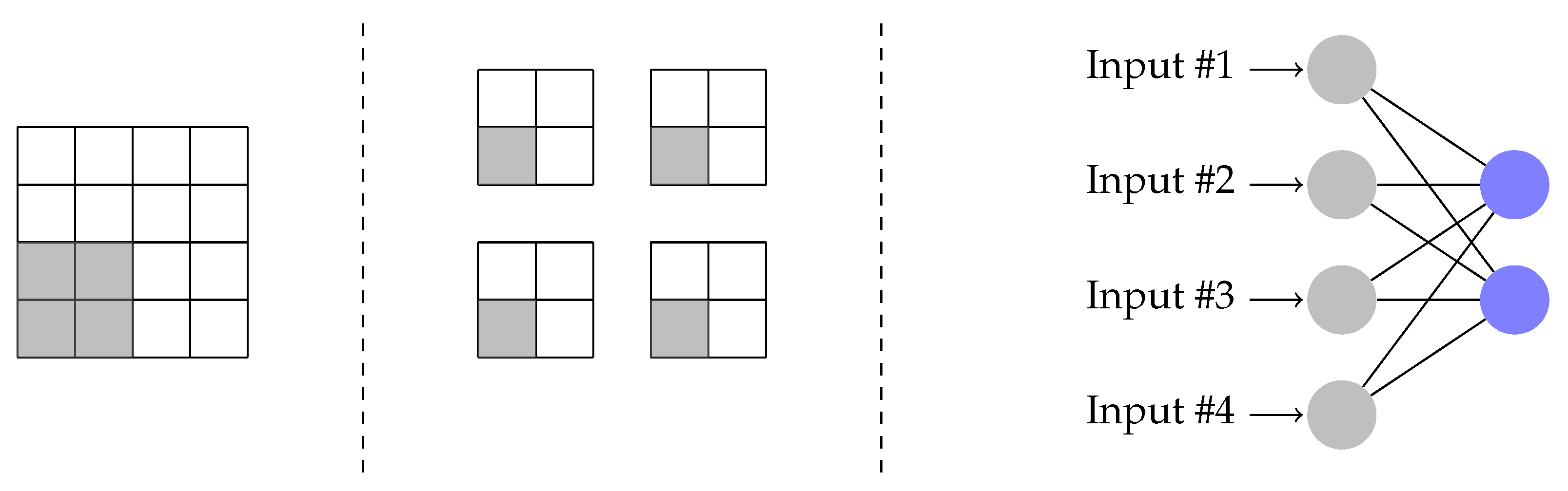
Input images are all 512 by 512 pixels. This resolution provides sufficient details that one may be able to diagnose a disease using these images directly. We first downsample them to 128x128 pixels to improve processing speed. We implement a quanvolutional network similar to [
10].
Figure 2 illustrates our flow. We pick four non-overlapping image pixels at a time. We use each pixel value to alter a quantum gate parameter. We convert pixel values in RGB color format to grayscale, normalize to [0,1] range, multiply with
, and use as input parameter to the gates in first layer of the quantum circuit (
,
,
,
) shown in
Figure 3. Remaining gate values in this circuit are fixed as shown in
Table 1, the values are in radians. The input to the quantum circuit indicated by
to
can be kept as all
or
.
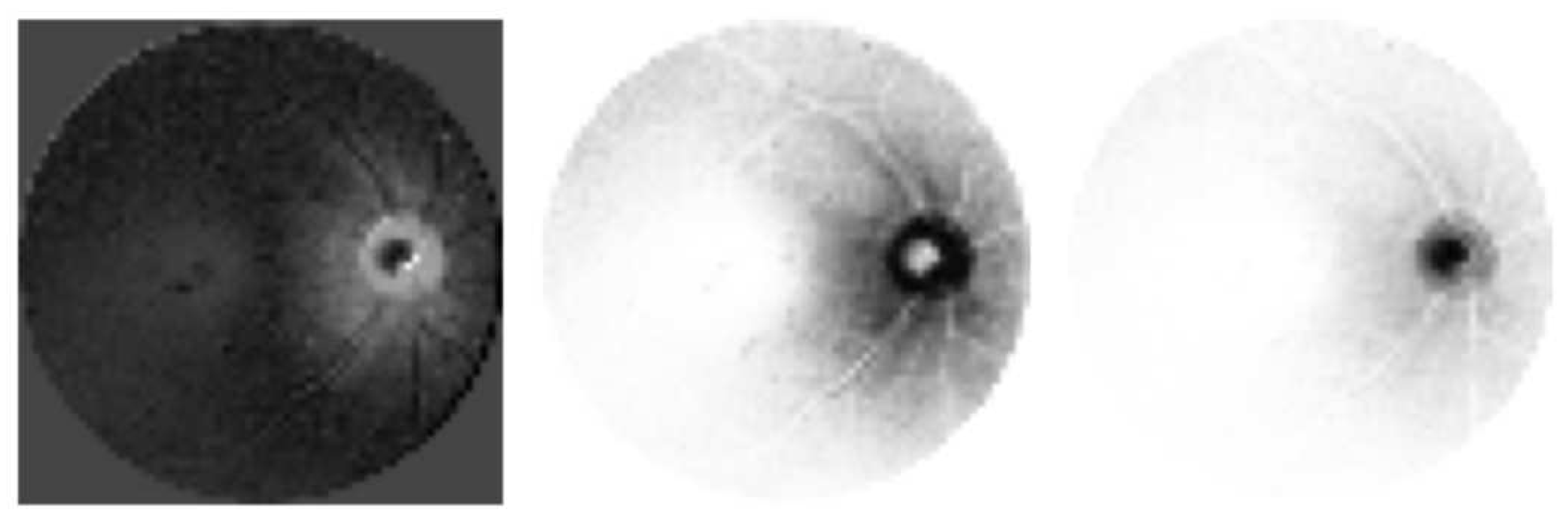
Each qubit is measured to convert values back to analog domain. If one is to observe this step,
Figure 4 provides examples of types of data that could be acquired from the same input image. Essentially different parts and features of the images may be highlighted. Next, we flatten outputs into a first layer of a neural network. The output of this neural network is fully connected to 8 classes. The learning process targets optimizing the values of the neural network.
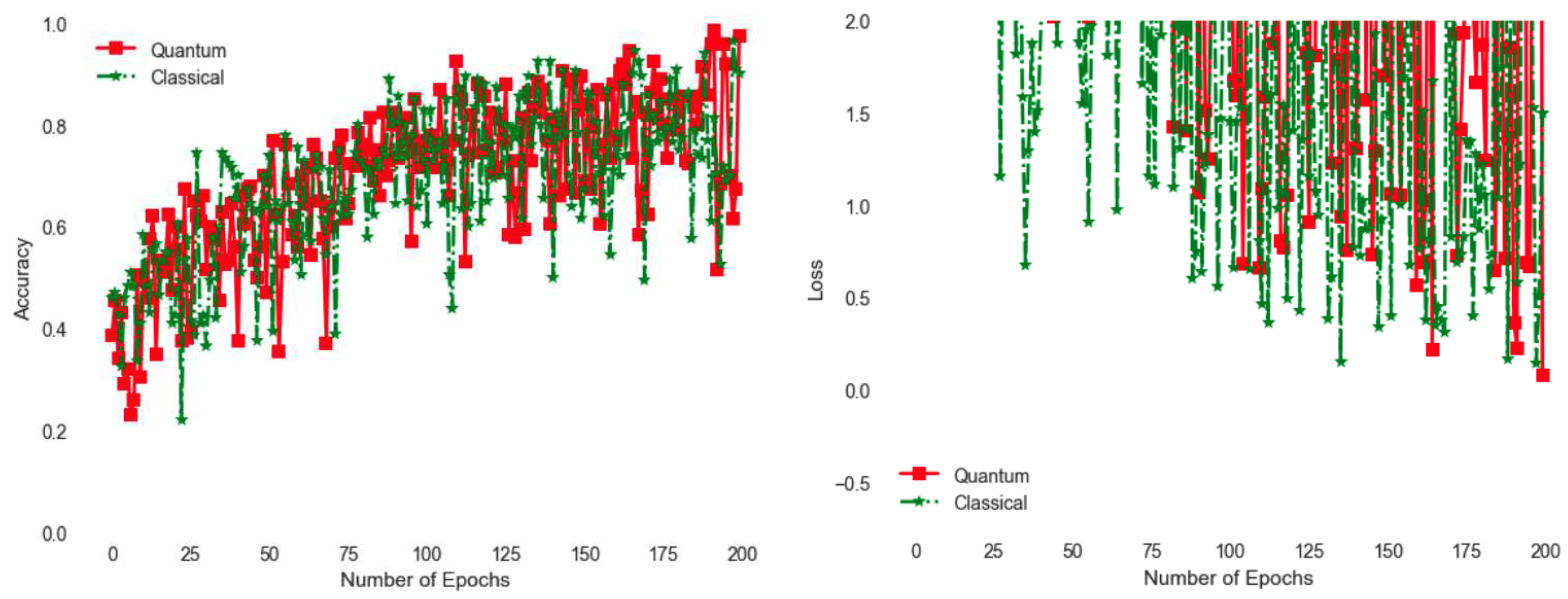
Figure 5 compares quantum implementation with a classical one. The classical one is rather simple, with the quanvolutional circuit replaced with a classical logic. For example if third pixel is larger than 0.5, we use it to invert the second pixel by subtracting it from 1. Similarly the fourth pixel controls the first one. With the current implementation, the quantum version shows better accuracy as shown with larger accuracy towards the end of the 200 epoch training cycle. Similarly observing
Figure 5, loss is even better for the quantum version as measured by smaller values of loss function. Loss is a measure of how well the model performs across different samples, i.e. squared error with respect to an expected value.