Submitted:
18 March 2023
Posted:
20 March 2023
You are already at the latest version
Abstract
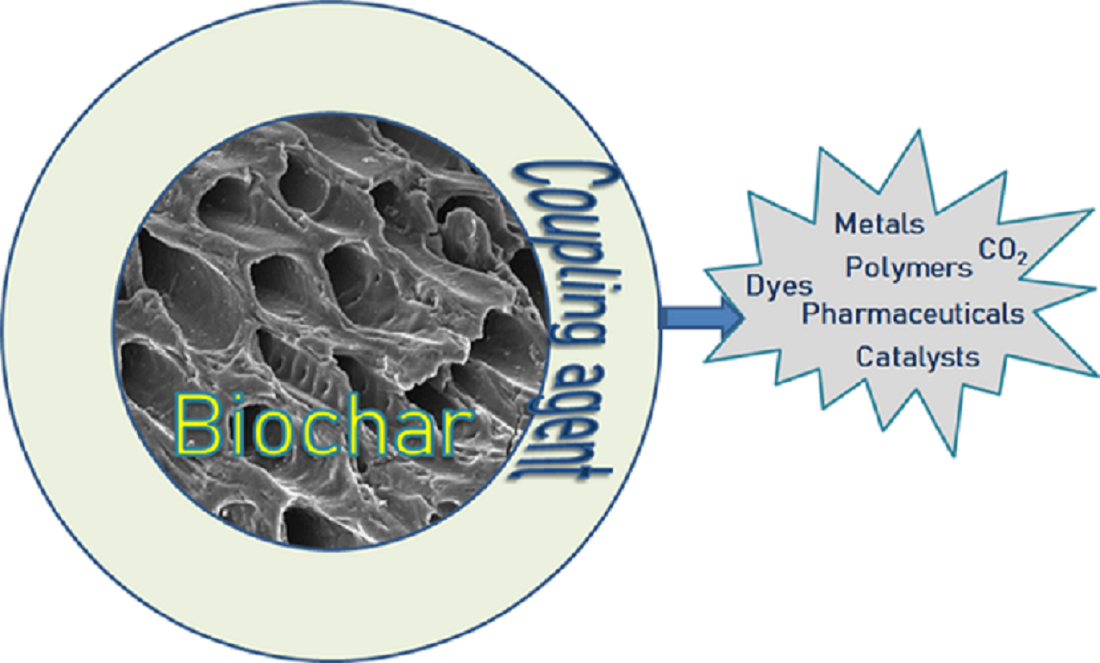
Keywords:
1. Introduction and Scope of the Review
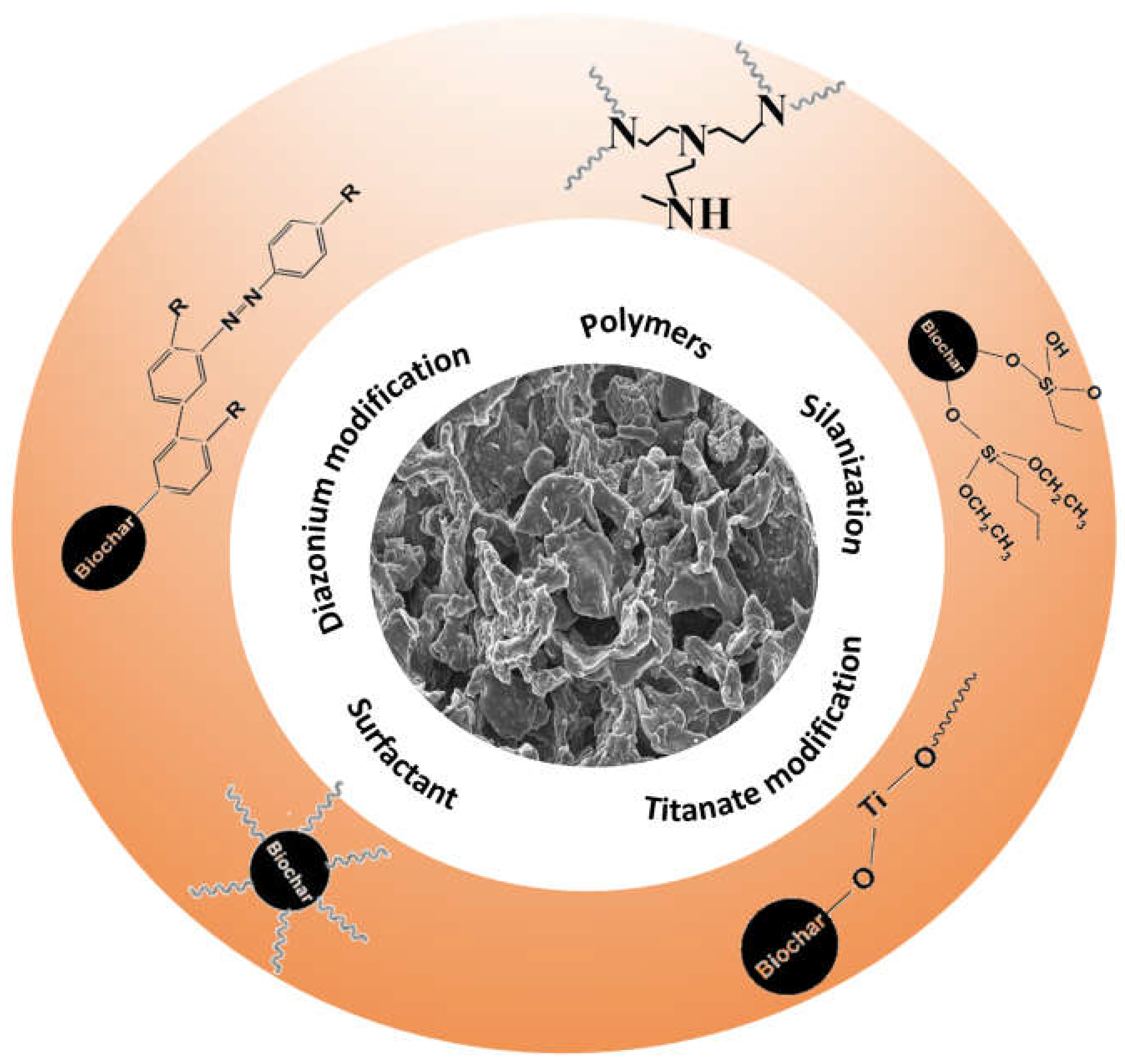
2. Biochar Surface Treatment Pathways
2.1. General Aspects
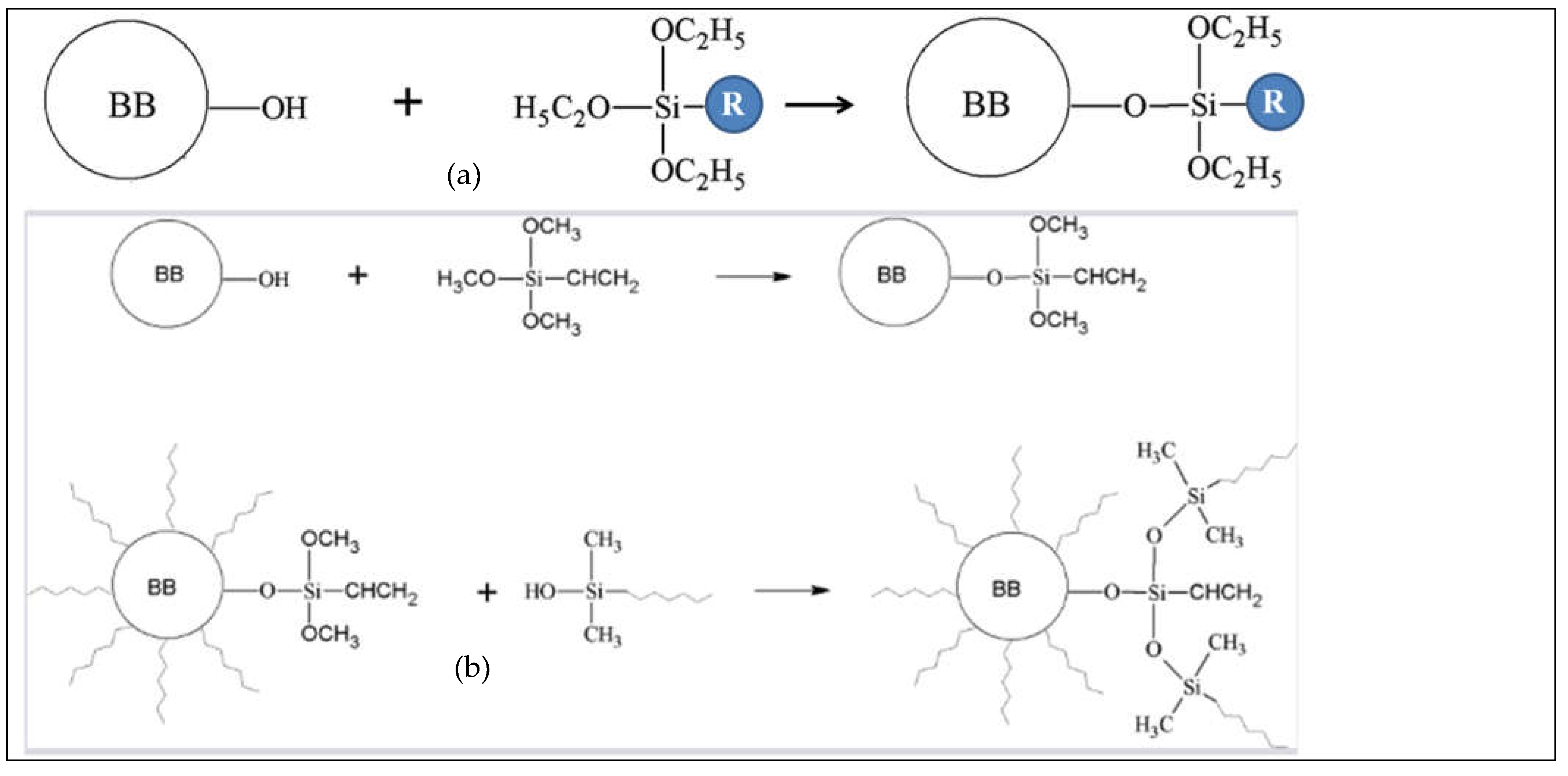
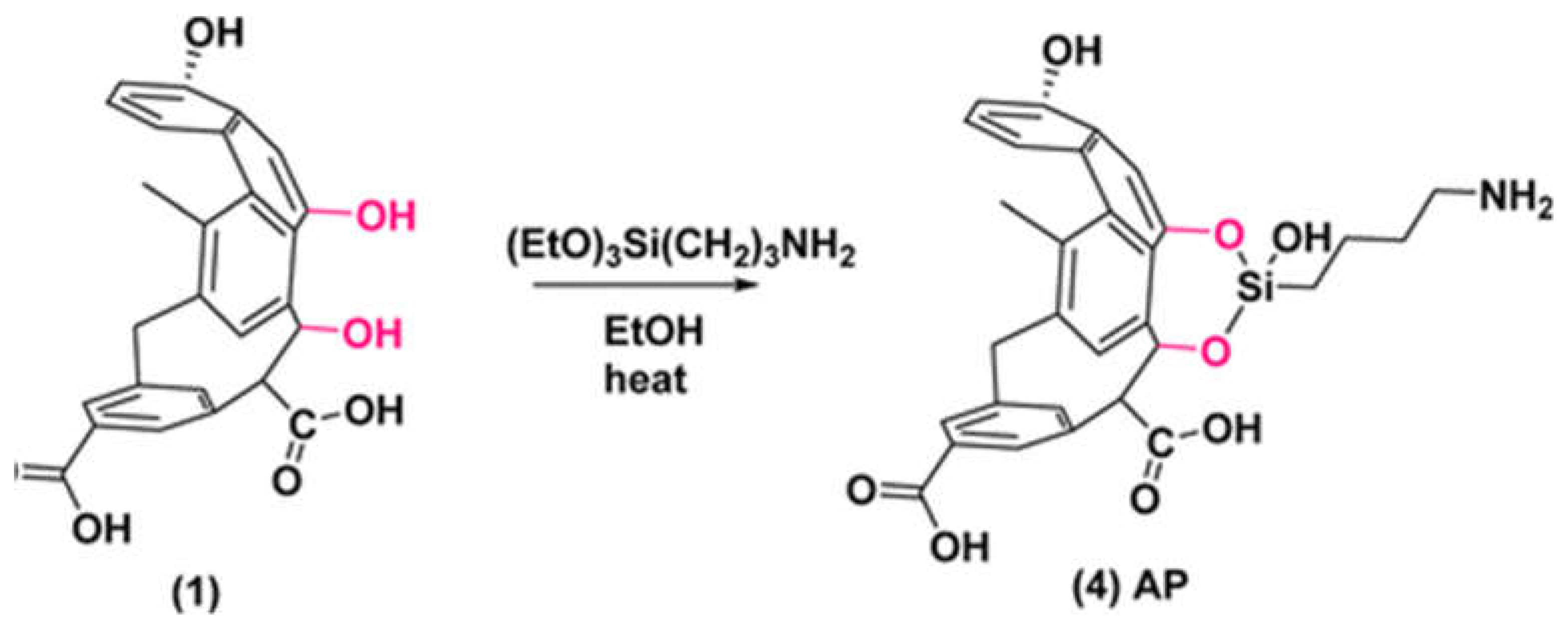
2.2. Surface Functionalization with Silane Coupling Agents
2.3. Titanate Surface Modifiers
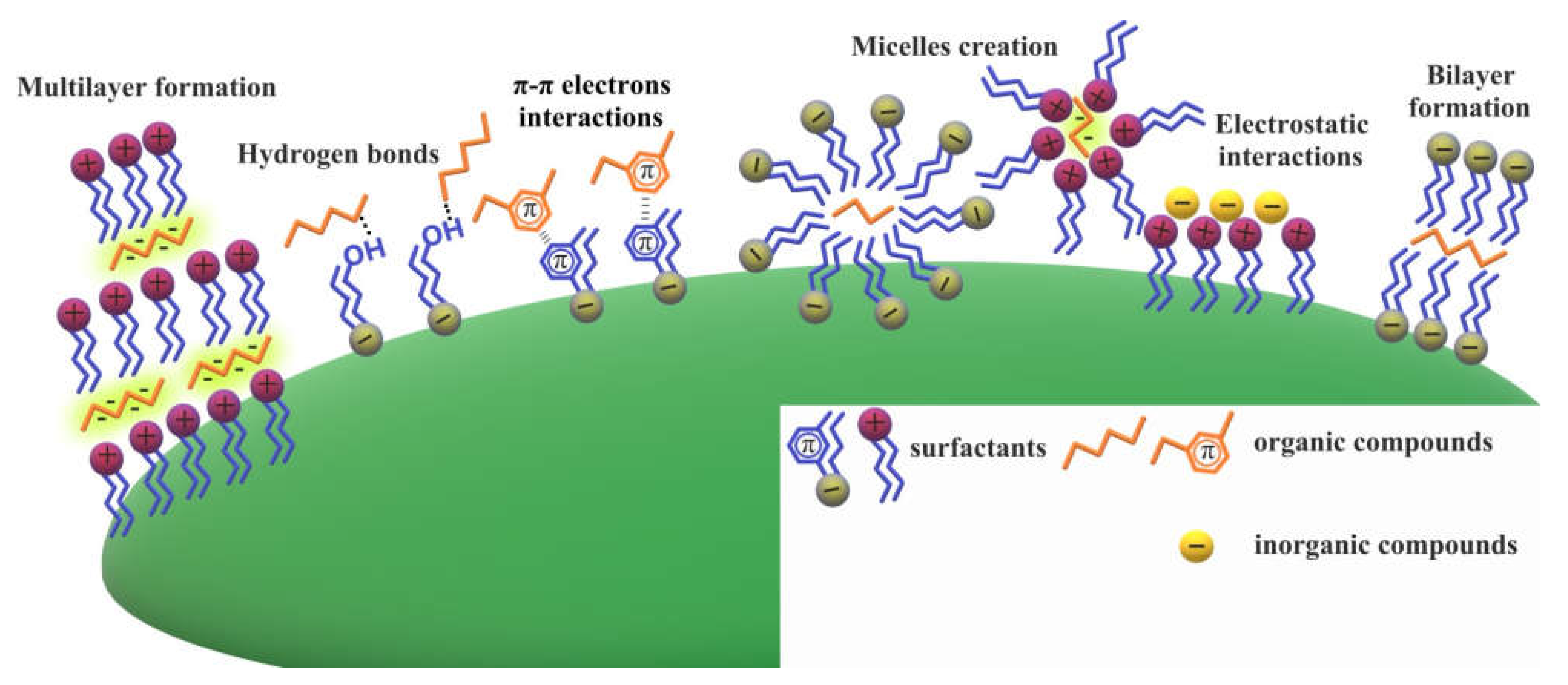
2.4. Modification of Biochar by Ionic and Nonionic Surfactants
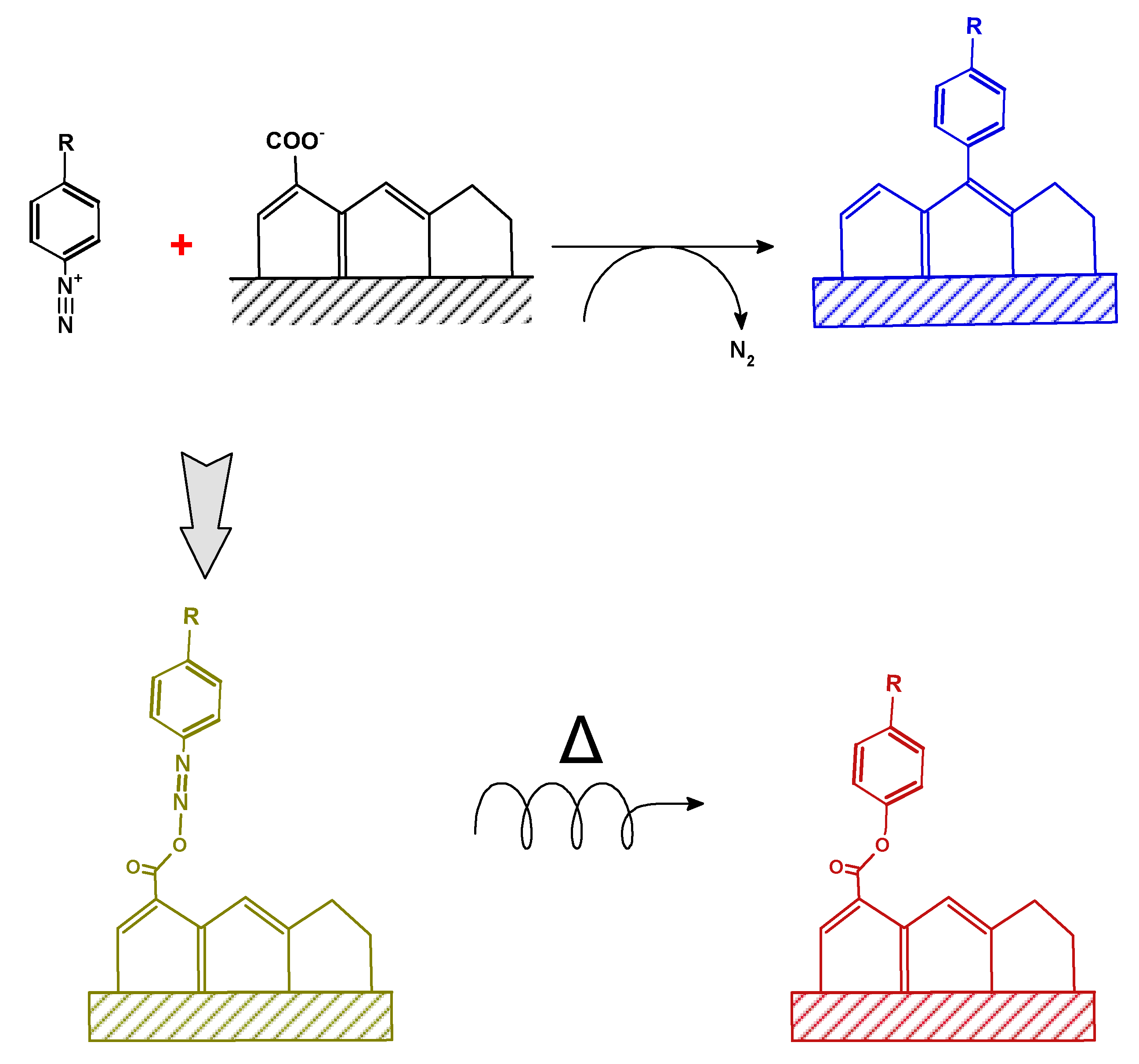
2.5. Surface Arylation with Diazonium Salts
2.6. Surface-Modified Biochar with Nitrogen-Based Compounds
3. Conclusions
- -
- in general, surface modification reduces the specific surface area and porous volume, but it is worth it because it imparts functionalities that unmodified biochar samples do not possess
- -
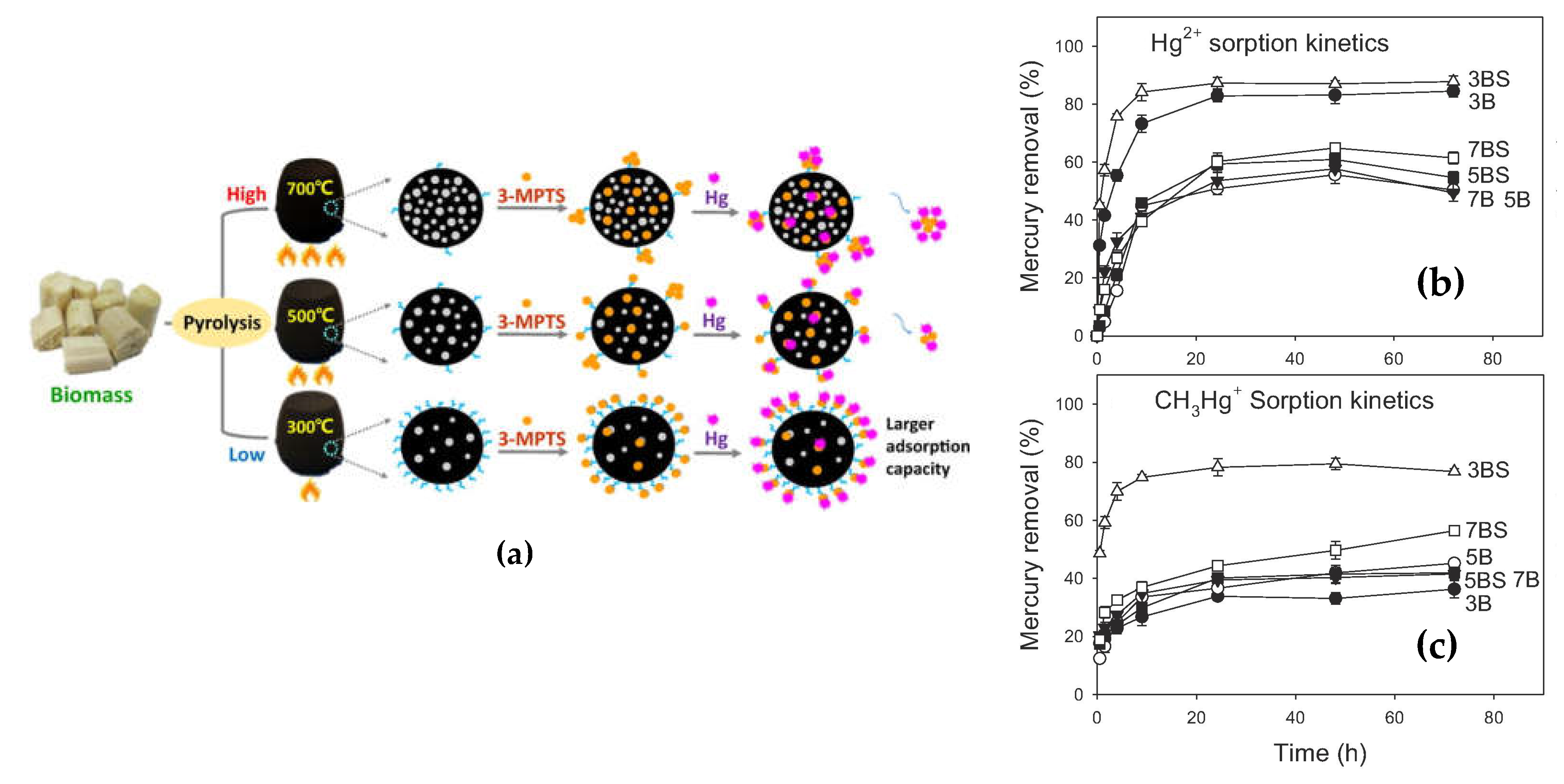
- silanization requires rich oxygen functionalities at the surface which could be obtained at moderate pyrolysis temperature, or after oxidization of the biochar.
- -
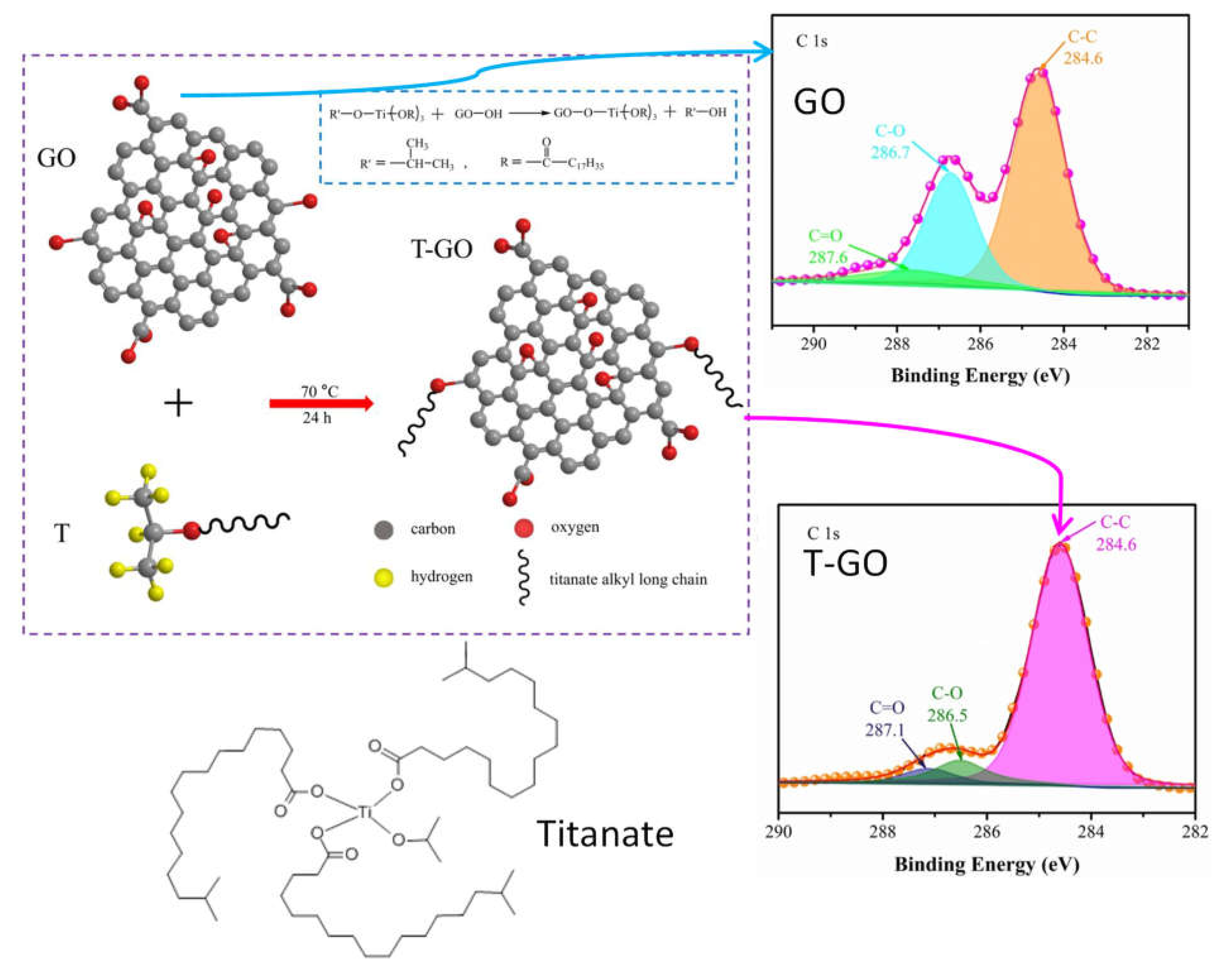
- titanate could be envisaged to create new biochar-polymer composites, if the primary intention is not to design biochar-titania photocatalysts
- -
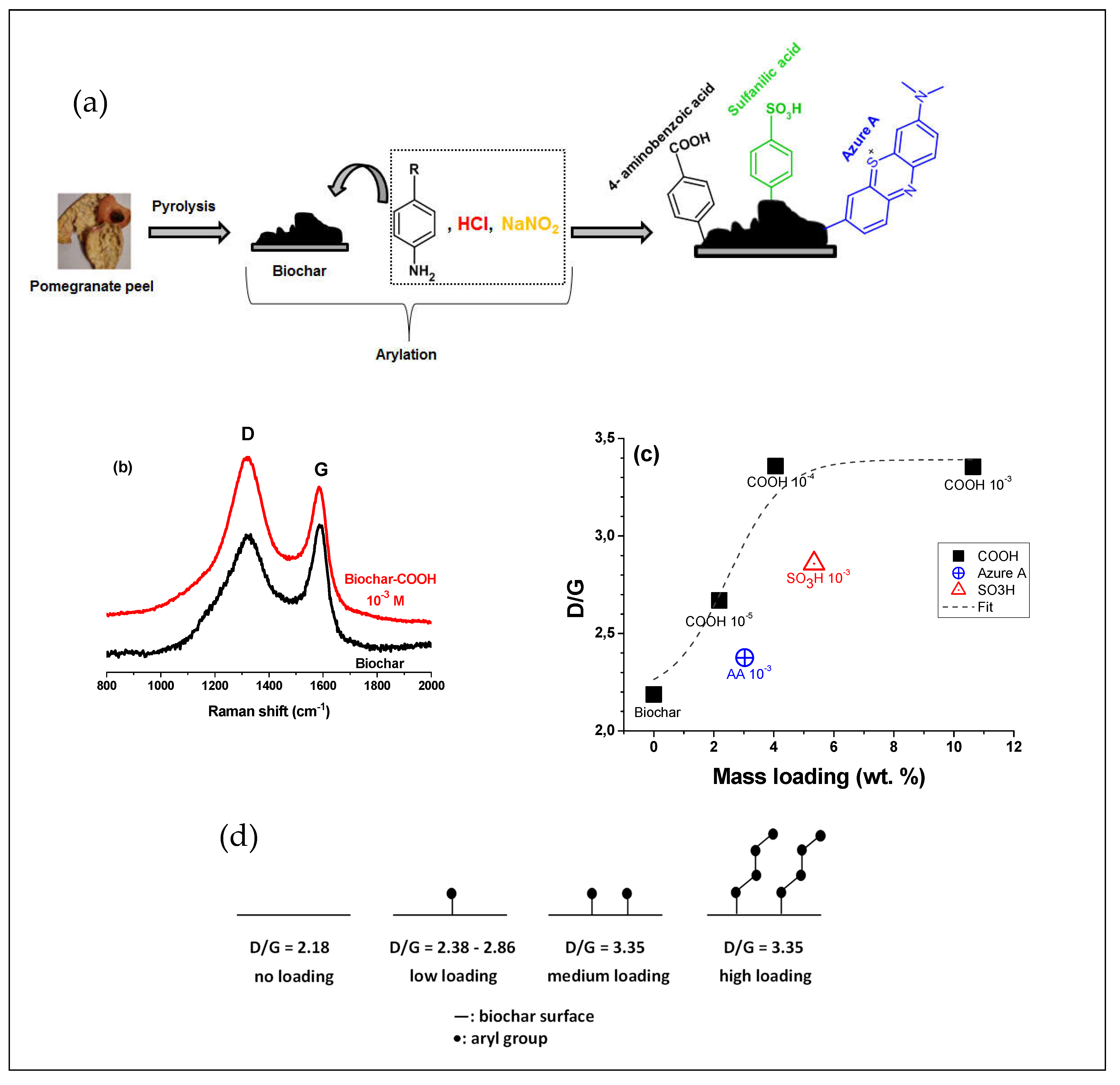
- aryl diazonium could be regarded as new coupling agents in materials surface chemistry as witnessed over the last three decades; their use to modify biochar particles is recent and very encouraging. Some of us have demonstrated that surface modification is limited by steric hindrance effects of the aryl group, and change in carbon structure of the biochar is significant at low initial diazonium concentration
- -
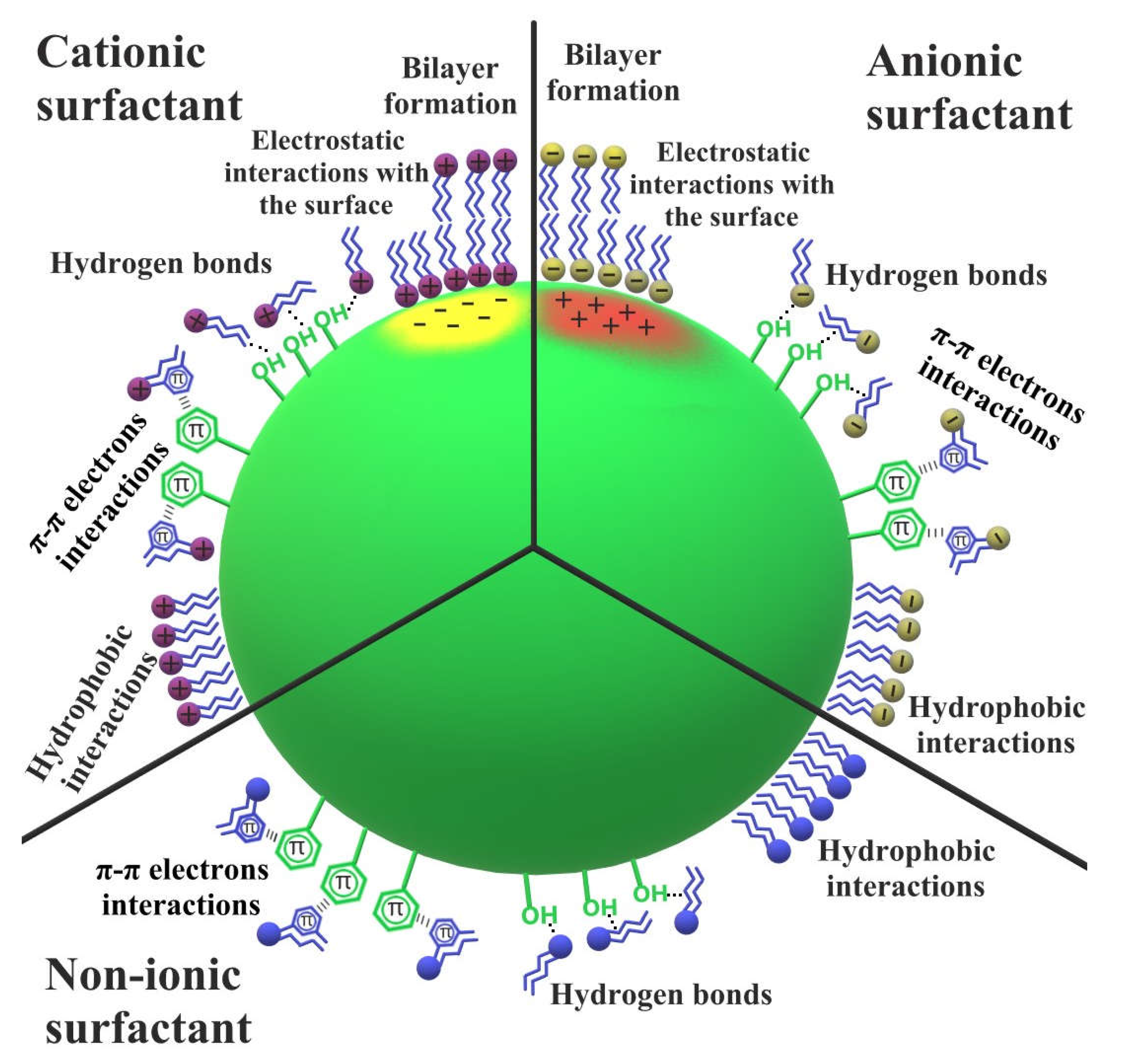
- modification with non-ionic and ionic surfactant adsorbed layers influences the textural characteristics of biochar, as well as acido-basic and hydrophilic-hydrophobic properties of its surface, changing the affinity for other substances present in the aqueous phase. The electrostatic, hydrogen bonds, π-π electrons and hydrophobic interactions are responsible for the surfactant molecules binding and can result in the formation of more complex surface structures, such as surfactant-adsorbate multilayers and micelles.
- -
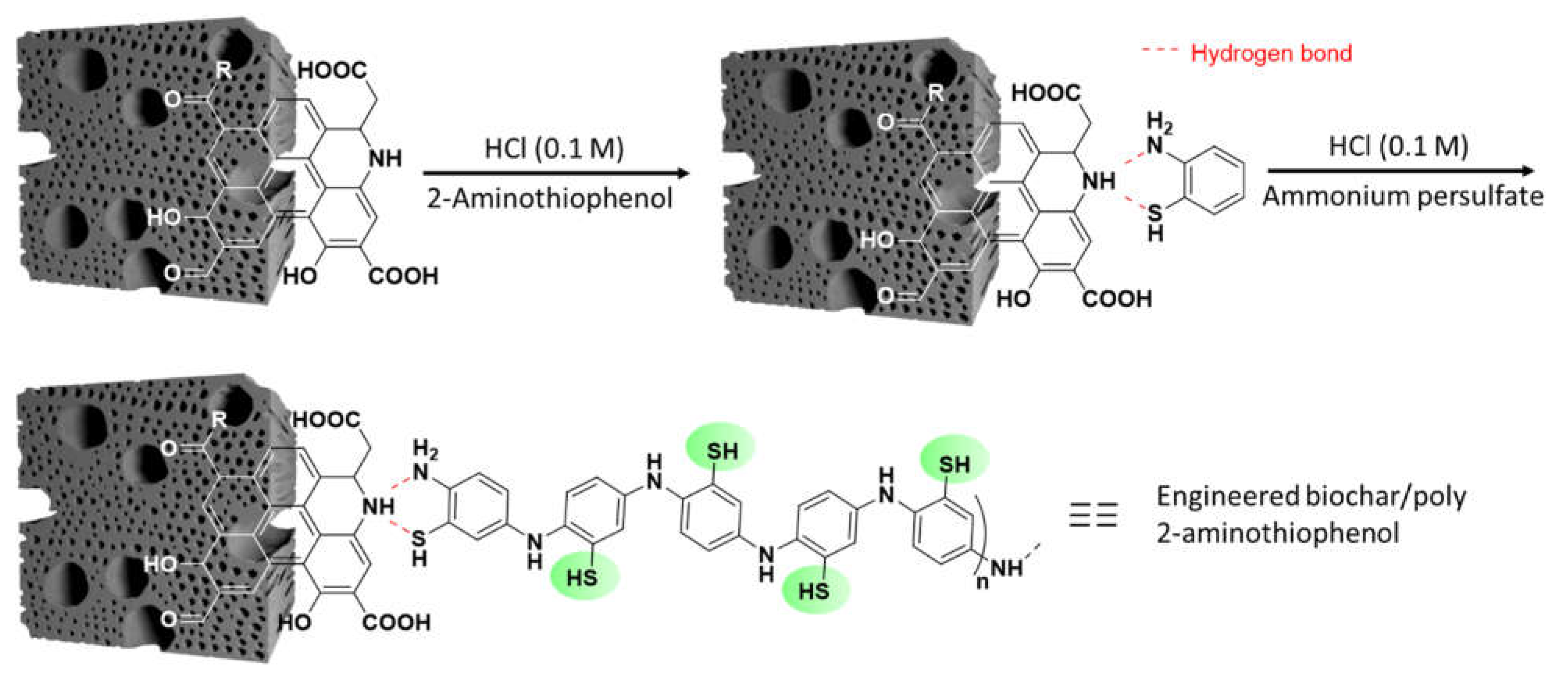
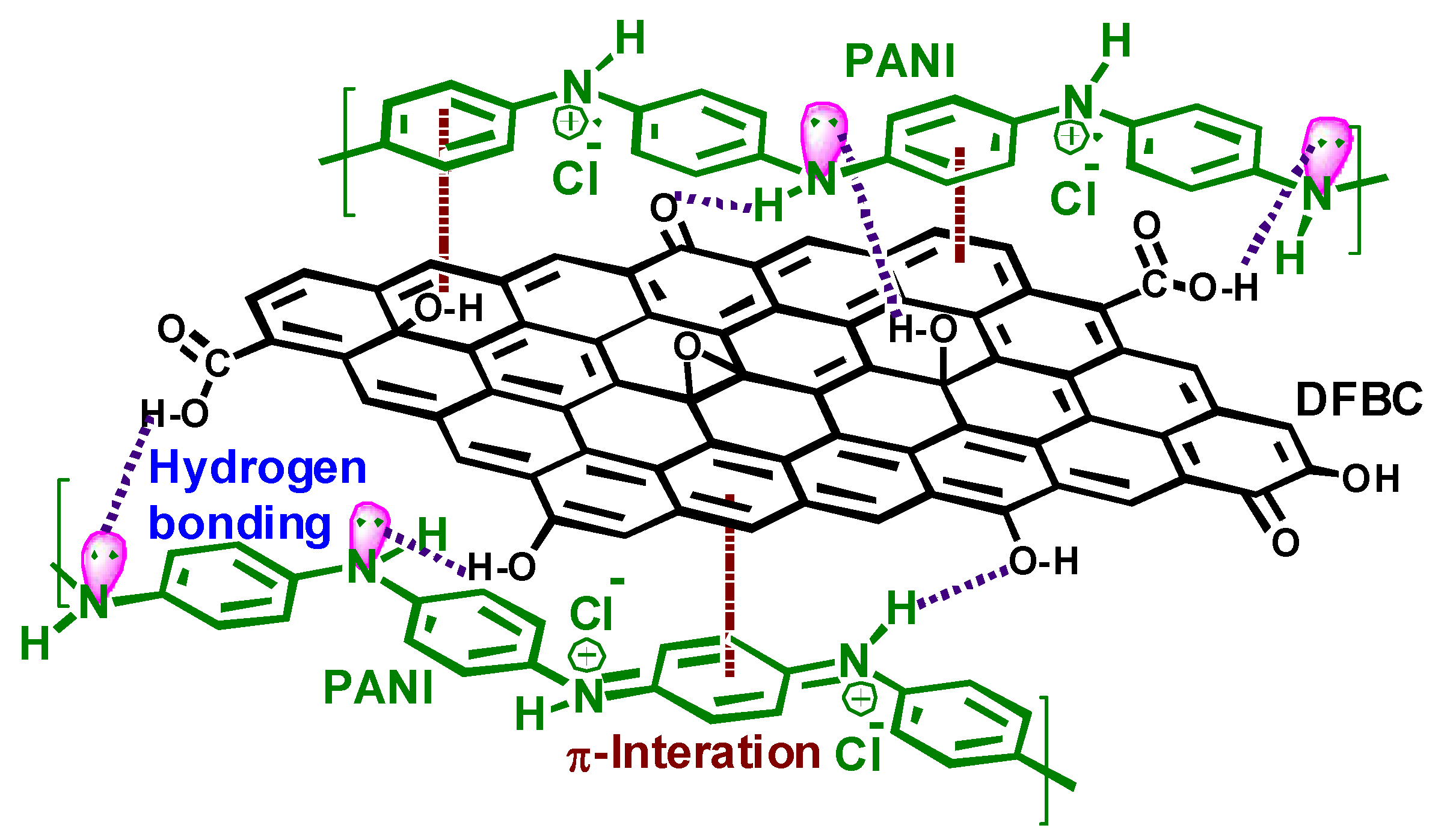
- the preparation of biochar-polyaniline composites are investigated by several researchers, particularly for the fabrication of supercapacitors, whereas poly(2-aminothiophenol) could be a remarkable alternative to provide surface-immobilized NH and SH groups able to chelate metal ions, for environmental remediation issues
- -
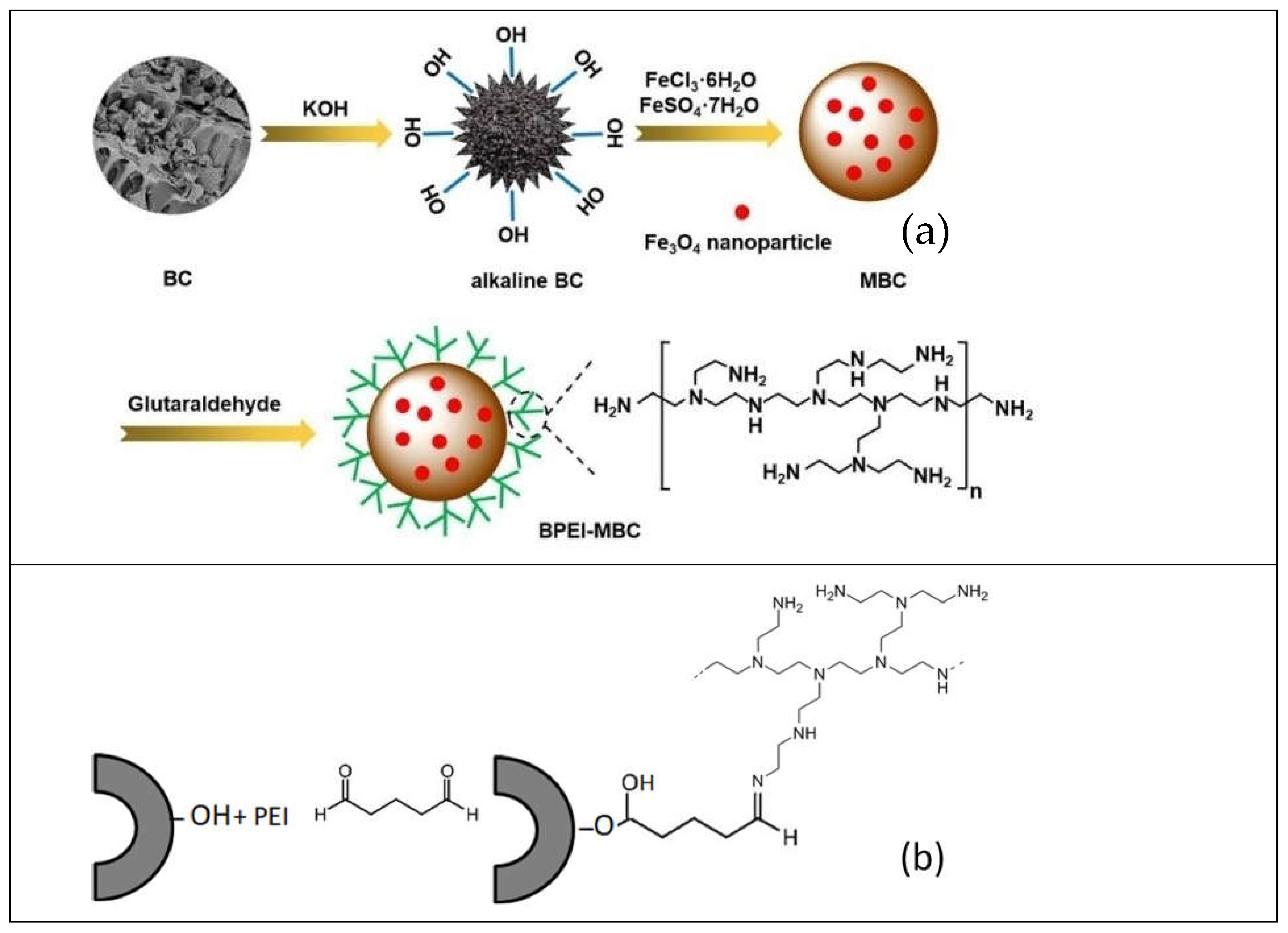
- polyethyleneimine is a nitrogen-rich polymer, and could be coated on biochar particles by adsorption from methanol or ethanol solution. Tight, covalent immobilization and crosslinking requires using organic coupling agents such as glutaraldehyde.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- S. Adhikari, W. Timms, M. A. P. Mahmud, Sci. Total Environ. 2022, 851, 158043. [CrossRef] [PubMed]
- S. Wijitkosum. Int. Soil Water Conserv. Res. 2022, 10, 335–341. [CrossRef]
- M. Harussani, S. Sapuan. Chem. Afr. 2021, 1–17.
- H. Liu, Y. Liu, X. Li, X. Zheng, X. Feng, A. Yu, in Book Adsorption and Fenton-like Degradation of Ciprofloxacin Using Corncob Biochar-Based Magnetic Iron–Copper Bimetallic Nanomaterial in Aqueous Solutions, ed., ed. by Editor, City, 2022, Volume 12, Chap. Chapter.
- Mukherjee, N. Goswami, D. Dhak. Dhak, Chem. Afr. 2022. [CrossRef]
- M. Bartoli, R. Arrigo, G. Malucelli, A. Tagliaferro, D. Duraccio. Polymers 2022, 14, 2506.
- N. Bélanger, S. Prasher, M.-J. Dumont. Polym. -Plast. Technol. Mater. 2023, 62, 54–75.
- M. M. Harussani, S. M. Sapuan, G. Nadeem, T. Rafin, W. Kirubaanand. Def. Technol. 2022, 18, 1281–1300. [CrossRef]
- T. Huggins, H. Wang, J. Kearns, P. Jenkins, Z. J. Ren. Bioresour. Technol. 2014, 157, 114–119.
- S. Tabac, D. Eisenberg. Curr. Opin. Electrochem. 2021, 25, 100638. [CrossRef]
- Y. Snoussi, I. Sifaoui, A. M. Khalil, A. K. Bhakta, O. Semyonov, P. S. Postnikov, L. Michely, R. Pires, S. Bastide, J. E.-P. Barroso, J. L. Morales, M. M. Chehimi. Mater. Today Commun. 2022, 32, 104126. [CrossRef]
- Y. Snoussi, I. Sifaoui, M. El Garah, A. M. Khalil, J. E. Piñero, M. Jouini, S. Ammar, J. Lorenzo-Morales, M. M. Chehimi. Waste Manag. 2023, 155, 179–191. [CrossRef] [PubMed]
- H. W. Lee, H. Lee, Y.-M. Kim, R.-s. Park, Y.-K. Park. Chin. Chem. Lett. 2019, 30, 2147–2150. [CrossRef]
- U. Kamran, S.-J. Park. J. Clean. Prod. 2021, 290, 125776. [CrossRef]
- R. P. Lopes, D. Astruc. Coord. Chem. Rev. 2021, 426, 213585.
- D. Luo, L. Wang, H. Nan, Y. Cao, H. Wang, T. V. Kumar, C. Wang. Environ. Chem. Lett. 2022. [CrossRef]
- L. Goswami, A. Kushwaha, S. R. Kafle, B.-S. Kim. Kim, Catalysts 2022, 12, 817.
- D. C. C. d. S. Medeiros, C. Nzediegwu, C. Benally, S. A. Messele, J.-H. Kwak, M. A. Naeth, Y. S. Ok, S. X. Chang, M. Gamal El-Din. Sci. Total Environ. 2022, 809, 151120. [CrossRef]
- X. Pan, Z. Gu, W. Chen, Q. Li. Sci. Total Environ. 2021, 754, 142104.
- M. M. Chehimi, J. Pinson, F. Mousli, Aryl Diazonium Salts and Related Compounds: Surface Chemistry and Applications. Editor, Springer Nature, 2022.
- C. Li, Y. Gao, A. Li, L. Zhang, G. Ji, K. Zhu, X. Wang, Y. Zhang. Environ. Pollut. 2019, 254, 113005. [CrossRef]
- F. Asgharzadeh, R. R. Kalantary, M. Gholami, A. J. Jafari, M. Kermani, H. Asgharnia. Biomass Convers. Biorefinery 2021. [CrossRef]
- X. Wang, J. Feng, Y. Cai, M. Fang, M. Kong, A. Alsaedi, T. Hayat, X. Tan. Sci. Total Environ. 2020, 708, 134575. [CrossRef]
- S. Detriche, A. K. Bhakta, P. N’Twali, J. Delhalle, Z. Mekhalif, in Book Assessment of Catalyst Selectivity in Carbon-Nanotube Silylesterification, ed., ed. by Editor, City, 2020, Vol. 10, Chap. Chapter.
- P. Walker, Handbook of adhesive technology 2003, 2, 205–221.
- Y. Lan, W. Wang. Adv. Polym. Technol. 2018, 37, 1979–1986. [CrossRef]
- M. Zhang, H. Zhu, B. Xi, Y. Tian, X. Sun, H. Zhang, B. Wu, in Book Surface Hydrophobic Modification of Biochar by Silane Coupling Agent KH-570, ed., ed. by Editor, City, 2022, Vol. 10, Chap. Chapter.
- H. Bamdad, K. Hawboldt, S. MacQuarrie. Energy Fuels 2018, 32, 11742–11748. [CrossRef]
- P. Moradi, M. Hajjami. RSC Adv. 2022, 12, 13523–13534. [CrossRef] [PubMed]
- P. Moradi, M. Hajjami. New J. Chem. 2021, 45, 2981–2994.
- Y. Huang, S. Xia, J. Lyu, J. Tang. Chem. Eng. J. 2019, 360, 1646–1655. [CrossRef]
- W. Ran, H. Zhu, X. Shen, Y. Zhang. Constr. Build. Mater. 2022, 329, 127057. [CrossRef]
- B. Wu, B. Xi, X. He, X. Sun, Q. Li, Q. Ouche, H. Zhang, C. Xue, in Book Methane Emission Reduction Enhanced by Hydrophobic Biochar-Modified Soil Cover, ed., ed. by Editor, City, 2020, Vol. 8, Chap. Chapter.
- K. Sheng, S. Zhang, S. Qian, C. A. Fontanillo Lopez. Compos. Part B: Eng. 2019, 165, 174–182. [CrossRef]
- Y. Qin, B. Xi, X. Sun, H. Zhang, C. Xue, B. Wu. Front. Bioeng. Biotechnol. 2022, 10. [CrossRef]
- G. Devi, N. Nagabhooshanam, M. Chokkalingam, S. K. Sahu. Polym. Compos. 2022, 43, 5996–6003. [CrossRef]
- P. Moradi, M. Hajjami, F. Valizadeh-Kakhki. Appl. Organomet. Chem. 2019, 33, e5205. [CrossRef]
- H. Li, L. Sun, W. Li. J. Mater. Sci. 2022, 57, 13845–13870.
- Y. Lu, X. Li, C. Wu, S. Xu. J. Alloys Compd. 2018, 750, 197–205. [CrossRef]
- N. W. Elshereksi, M. Ghazali, A. Muchtar, C. H. Azhari. Dent. Mater. J. 2017, 2016–014.
- B. Ali Sabri, S. Meenaloshini, N. M. Abreeza, A. N. Abed. Mater. Today: Proc. 2021. [CrossRef]
- Y. Zeng, Y. Xue, L. Long, J. Yan. Water Air Soil Pollut. 2019, 230, 50–10.
- K. D. Cai, W. F. Mu, in Book Activated carbon modified by titanate coupling agent for supercapacitor, ed., ed. by Editor, Trans Tech Publ, City, 2012, Vol. 347, Chap. Chapter, pp. 3649–3652.
- X. Li, C. Gan, Z. Han, H. Yan, D. Chen, W. Li, H. Li, X. Fan, D. Li, M. Zhu. Carbon 2020, 165, 238–250. [CrossRef]
- M. Shahverdi, E. Kouhgardi, B. Ramavandi. Data Brief 2016, 9, 163–168. [CrossRef] [PubMed]
- L. Mathurasa, S. Damrongsiri. Appl. Environ. Res. 2017, 39, 11–22.
- M. Wiśniewska, P. Nowicki, T. Urban. J. Mol. Liq. 2021, 332, 115872. [CrossRef]
- F. Wang, Q. Zeng, W. Su, M. Zhang, L. Hou, Z.-L. Wang. J. Chem. 2019, 2019, 2428505. [CrossRef]
- C. Kosaiyakanon, S. Kungsanant. Environ. Nat. Resour. J. 2019, 18, 21–32.
- X. Mi, G. Li, W. Zhu, L. Liu. J. Chem. 2016, 2016.
- M. Wiśniewska, P. Nowicki. Colloids Surf. A: Physicochem. Eng. Asp. 2020, 585, 124179. [CrossRef]
- K. Anas, S. Y. Pratama, A. Izzah, M. A. Kurniawan. 2021, 2021, 8. [CrossRef]
- K. Anas, A. Izzah, S. Y. Pratama, F. I. Fajarwati. AIP Conf. Proc. 2020, 2229, 030024. [CrossRef]
- W. Que, L. Jiang, C. Wang, Y. Liu, Z. Zeng, X. Wang, Q. Ning, S. Liu, P. Zhang, S. Liu. J. Environ. Sci. 2018, 70, 166–174. [CrossRef]
- H. He, W. Li, H. Deng, L. Kang, R. Qiu, X. Li, W. Liu, Z. Meng. Desalination Water Treat. 2019, 148, 195–201.
- M. Wiśniewska, P. Nowicki, K. Szewczuk-Karpisz, M. Gęca, K. Jędruchniewicz, P. Oleszczuk. Sep. Purif. Technol. 2021, 276, 119297. [CrossRef]
- Z. Hua, S. Wan, L. Sun, Z. Yu, X. Bai. J. Chem. Technol. Biotechnol. 2018, 93, 3044–3055. [CrossRef]
- P. Griess. Annalen 1858, 106, 123–125.
- S. V. Heines. J. Chem. Educ. 1958, 35, 187. [CrossRef]
- F. Mo, G. Dong, Y. Zhang, J. Wang. Org. Biomol. Chem. 2013, 11, 1582–1593. [CrossRef] [PubMed]
- A. Mohamed, Z. Salmi, S. A. Dahoumane, A. Mekki, B. Carbonnier, M. M. Chehimi. Adv. Colloid Interface Sci. 2015, 225, 16–36.
- P. Mirzaei, S. Bastide, A. Aghajani, J. Bourgon, E. Leroy, J. Zhang, Y. Snoussi, A. Bensghaier, O. Hamouma, M. M. Chehimi. Chehimi, Langmuir 2019, 35, 14428–14436.
- S. A. Ali H. Jawad, Lee D. Wilson, Syed Shatir A. Syed-Hassan, Zeid A. ALOthman, Mohammad Rizwan Khan. Chin. J. Chem. Eng. 2021, 32, 281–290. [CrossRef]
- M. H. Abdel-Aziz, E. Z. El-Ashtoukhy, M. Bassyouni, A. F. Al-Hossainy, E. M. Fawzy, S. M. S. Abdel-Hamid, M. S. Zoromba. Carbon Lett. 2021, 31, 863–878. [CrossRef]
- M. Khalil, R. Msaadi, W. Sassi, I. Ghanmi, R. Pires, L. Michely, Y. Snoussi, A. Chevillot-Biraud, S. Lau-Truong, M. M. Chehimi. Carbon Lett. 2022, 32, 1519–1529. [CrossRef]
- L. Rodd, M. A. Creighton, C. A. Vaslet, J. R. Rangel-Mendez, R. H. Hurt, A. B. Kane. Environ. Sci. Technol. 2014, 48, 6419–6427. [CrossRef]
- Bensghaïer, Z. Bensghaïer, Z. Salmi, B. Le Droumaguet, A. Mekki, A. A. Mohamed, M. Beji, M. M. Chehimi. Surf. Interface Anal. 2016, 48, 509–513. [Google Scholar] [CrossRef]
- Y. Snoussi, M. El Garah, A. M. Khalil, S. Ammar, M. M. Chehimi. Appl. Organomet. Chem. 2022, 36, e6885. [CrossRef]
- K. Malins, V. Kampars, J. Brinks, I. Neibolte, R. Murnieks. Appl. Catal. B: Environ. 2015, 176-177, 553–558. [CrossRef]
- S. Niu, Y. Ning, C. Lu, K. Han, H. Yu, Y. Zhou. Energy Convers. Manag. 2018, 163, 59–65. [CrossRef]
- T. Zhang, H. Wei, J. Gao, S. Chen, Y. Jin, C. Deng, S. Wu, H. Xiao, W. Li. Mol. Catal. 2022, 517, 112034. [CrossRef]
- Malaika, K. Ptaszyńska, M. Kozłowski. Fuel 2021, 288, 119609. [CrossRef]
- L. J. Konwar, P. Mäki-Arvela, J.-P. Mikkola. Chem. Rev. 2019, 119, 11576–11630. [CrossRef] [PubMed]
- Y. Zhong, Q. Deng, P. Zhang, J. Wang, R. Wang, Z. Zeng, S. Deng. Fuel 2019, 240, 270–277. [CrossRef]
- Z. Wan, Y. Sun, D. C. W. Tsang, E. Khan, A. C. K. Yip, Y. H. Ng, J. Rinklebe, Y. S. Ok. Chem. Eng. J. 2020, 401, 126136. [CrossRef]
- N. Kasera, P. Kolar, S. G. Hall. Biochar 2022, 4, 17. [CrossRef]
- Z. Lin, R. Wang, S. Tan, K. Zhang, Q. Yin, Z. Zhao, P. Gao. J. Environ. Manag. 2023, 334, 117503. [CrossRef]
- R. Chatterjee, B. Sajjadi, W.-Y. Chen, D. L. Mattern, N. O. Egiebor, N. Hammer, V. Raman. Energy Fuels 2019, 33, 2366–2380. [CrossRef]
- M. Shahabi Nejad, H. Sheibani. J. Environ. Chem. Eng. 2022, 10, 107363. [CrossRef]
- R. C. Thomas, J. E. Houston, R. M. Crooks, T. Kim, T. A. Michalske. J. Am. Chem. Soc. 1995, 117, 3830–3834. [CrossRef]
- Herath, C. Reid, F. Perez, C. U. Pittman, T. E. Mlsna. J. Environ. Manag. 2021, 296, 113186. [CrossRef] [PubMed]
- K. R. Thines, E. C. Abdullah, M. Ruthiraan, N. M. Mubarak, M. Tripathi. J. Anal. Appl. Pyrolysis 2016, 121, 240–257. [CrossRef]
- D. Thomas, N. B. Fernandez, M. D. Mullassery, R. Surya. Inorg. Chem. Commun. 2020, 119, 108097. [CrossRef]
- Y. Ma, T. Zhang, P. Zhu, H. Cai, Y. Jin, K. Gao, J. Li. Sci. Total Environ. 2022, 821, 153453. [CrossRef] [PubMed]
- Y. Ma, W.-J. Liu, N. Zhang, Y.-S. Li, H. Jiang, G.-P. Sheng. Bioresour. Technol. 2014, 169, 403–408. [CrossRef]
- J. Qu, X. Zhang, F. Bi, S. Wang, X. Zhang, Y. Tao, Y. Wang, Z. Jiang, Y. Zhang. Environ. Pollut. 2022, 313, 120103. [CrossRef]
- M. Gęca, M. Wiśniewska, P. Nowicki, in Book Simultaneous Removal of Polymers with Different Ionic Character from Their Mixed Solutions Using Herb-Based Biochars and Activated Carbons, ed., ed. by Editor, City, 2022, Vol. 27, Chap. Chapter.
- E. Parameswari, R. Kalaiarasi, V. Davamani, T. Ilakiya, P. Kalaiselvi, S. P. Sebastian, in Biochar and its Application in Bioremediation, ed. by R. Thapar Kapoor, H. Treichel, M. P. Shah, Springer Nature Singapore, Singapore, 2021, pp. 27–48.
- H. Tian, C. Huang, P. Wang, J. Wei, X. Li, R. Zhang, D. Ling, C. Feng, H. Liu, M. Wang, Z. Liu. Bioresour. Technol. 2023, 369, 128452. [CrossRef]
- Q. Jiang, W. Xie, S. Han, Y. Wang, Y. Zhang. Colloids Surf. A: Physicochem. Eng. Asp. 2019, 583, 123962. [CrossRef]
- Z. Xiao, Y. Xie, H. Militz, C. Mai. 2010, 64, 483–488. [CrossRef]
- D. Pandey, A. Daverey, K. Dutta, K. Arunachalam. Environ. Monit. Assess. 2022, 194, 880. [CrossRef] [PubMed]
- J. Fan, C. Cai, H. Chi, B. J. Reid, F. Coulon, Y. Zhang, Y. Hou, J. Hazard. Mater. 2020, 388, 122037. [CrossRef]
- F. Saremi, M. R. Miroliaei, M. Shahabi Nejad, H. Sheibani, J. Mol. Liq. 2020, 318, 114126. [CrossRef]
- L. Boubkr, A. K. Bhakta, Y. Snoussi, C. Moreira Da Silva, L. Michely, M. Jouini, S. Ammar, M. M. Chehimi, in Book Highly Active Ag-Cu Nanocrystal Catalyst-Coated Brewer’s Spent Grain Biochar for the Mineralization of Methyl Orange and Methylene Blue Dye Mixture, ed., ed. by Editor, City, 2022, Vol. 12, Chap. Chapter.
- J. Omiri, Y. Snoussi, A. K. Bhakta, S. Truong, S. Ammar, A. M. Khalil, M. Jouini, M. M. Chehimi. Colloids Interfaces 2022, 6. [CrossRef]
- N. Ayedi, B. Rzig, N. Bellakhal. Chem. Afr. 2023. [CrossRef]
- H. A. Alharbi, B. H. Hameed, K. D. Alotaibi, S. S. Al-Oud, A. S. Al-Modaihsh. Front. Environ. Sci. 2022, 10, 996953.
- K. Jlassi, S. Mallick, A. Eribi, M. M. Chehimi, Z. Ahmad, F. Touati, I. Krupa. Sens. Actuators B: Chem. 2021, 328, 129058.
| Running title | Review main topic | Year review published | References |
|---|---|---|---|
| Biochar for catalytic biorefinery and environmental processes | The article summarizes the knowledge on the production of biochar, its classical activation with aggressive compounds and its use to expediate biorefinery operations and degradation of environmental pollutants | 2019 | [13] |
| Chemically modified carbonaceous adsorbents for CO2 capture | The review discusses activation, surface amination or carboxylation, and doping of various carbon allotropes, including biochar, for extensive capture of CO2. | 2021 | [14] |
| Biochar as a support for nanocatalysts and other reagents | The review paper summarizes the knowledge on the use of biochar-supported nanocatalyst, and discusses the mode of coordination of the catalyst nanoparticles by the biochar. | 2021 | [15] |
| Phosphorus adsorption by functionalized biochar | Inefficient crop fertilization induces water pollution by phosphorus. The review discusses biomass pre-treatment as well as post-modification of biochar to obtain efficient phosphorus porous adsorbents | 2022 | [16] |
| Surface modification of biochar for dye removal | The review discusses several strategies of obtaining functional biochar for the removal of dyes from water. | 2022 | [17] |
| Biochar for the removal of contaminants from industrial wastewaters | The paper reviews methods for obtaining pristine and engineered biochar for the efficient removal of numerous contaminants from industrial wastewaters. | 2022 | [18] |
| Biochar as a reinforcing bio-based filler in rubber composites. | This article is focussed on the key properties of highly reinforcing fillers such as particle size, structure, and surface activity are discussed. The review essentially covers silane modification of biochar. | 2023 | [7] |
| Coupling agent | Biomass | Surface treatment conditions | Properties of engineered biochar | Potential application | References |
|---|---|---|---|---|---|
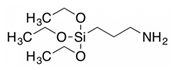 |
Sawmill residues | Char/water=10/1; 2 wt.% APTES; condensation at pH 3-4 for 1h followed by reaction at 70 °C for 6h. Post-activation in air at 560 °C. |
SSA=394 m²/g; 0.24 wt.% N; | CO2 adsorption capacity of 3.7 mmol/g | [28] |
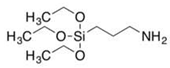 KH-550 KH-550 |
Oil sludge | C2H5OH: H2O: KH-550 ratio of 70: 25: 5, left for 60 min. Then, oil sludge pyrolysis residue added and stirred for 1 h, followed by drying at 60 °C. | Rheological properties (rutting resistance factor, creep stiffness, and high temperature performance) improvement of the asphalt mortar | Road engineering | [32] |
KH-570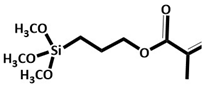
|
Rice straw | Mixture of pre-treated biochar + 20% (v/v) KH-570, 72% (v/v) absolute C2H5OH + 8% (v/v) H2O, magnetically stirred at 30°C, mixed with modifier for 12h, washed with ethanol, filtered and dried at 50°C. | Enhanced CH4 oxidation | Soil cover for landfill gas control. | [33] |
YDH-171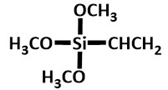
|
lodgepole pine bark | Synthesized biochar at 600°C and modified by YDH-171. Further, 3 wt.% modified were taken for further experiment | PV performance: separation factor (11.3) and flux (227.25 g m−2 h−1). |
PV membranes (Separating C2H5OH from H2O. |
[26] |
A-189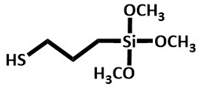
|
Bamboo | Pre-treated ultrafine bamboo char was mixed with A-189 (16 w/w%). Following silane treatment, oven-dried for 24 at 105 °C. | Tensile strength (18.87 MPa) and tensile modulus (272 MPa) increased by 99.3 % and 104.9%). | Polymer reinforcement | [34] |
KH-570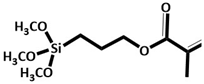
|
Rice straw | - | H2O absorption =1.27 g (g biochar) −1 Water proofing, MOB growth promotion, ventilation and efficient CH4 reduction. |
Land fill cover soil | [35] |
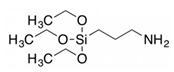 APTES APTES |
Onion peels | Silane (3 wt.%) was mixed with C2H5OH- H2O solution (95 wt % C2H5OH + water + acid 5%), stirred for 10 at room temp. Then, ball milled Co-biochar was incorporated, rinsed for 10 min and filtered, and finally dried at 110°C. | High tensile strength and EMI: −44.37 dB and − 49.62 dB for X and Ku band | PVA composite For EMI application |
[36] |
CPTMS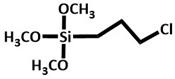
|
Chicken manure | Pyrolyzed biomass (400-800 °C, 1 to 2h) was treated with CPTMS followed by dispersion in 1.5 mmol TBA and toluene and stirred for 48 h at 90°C. Residue was isolated, washed with C2H5OH and dried (50°C). Then, Pd (OAc) and NaBH4 treatment at optimum condition. | In the synthesis of bipheneyl derivatives (yield = 97%, TON = 135, and TOF = 405) | C-C coupling reaction (Suzuki–Miyaura and Heck–Mizoroki cross-coupling reactions.) | [37] |
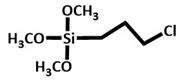 |
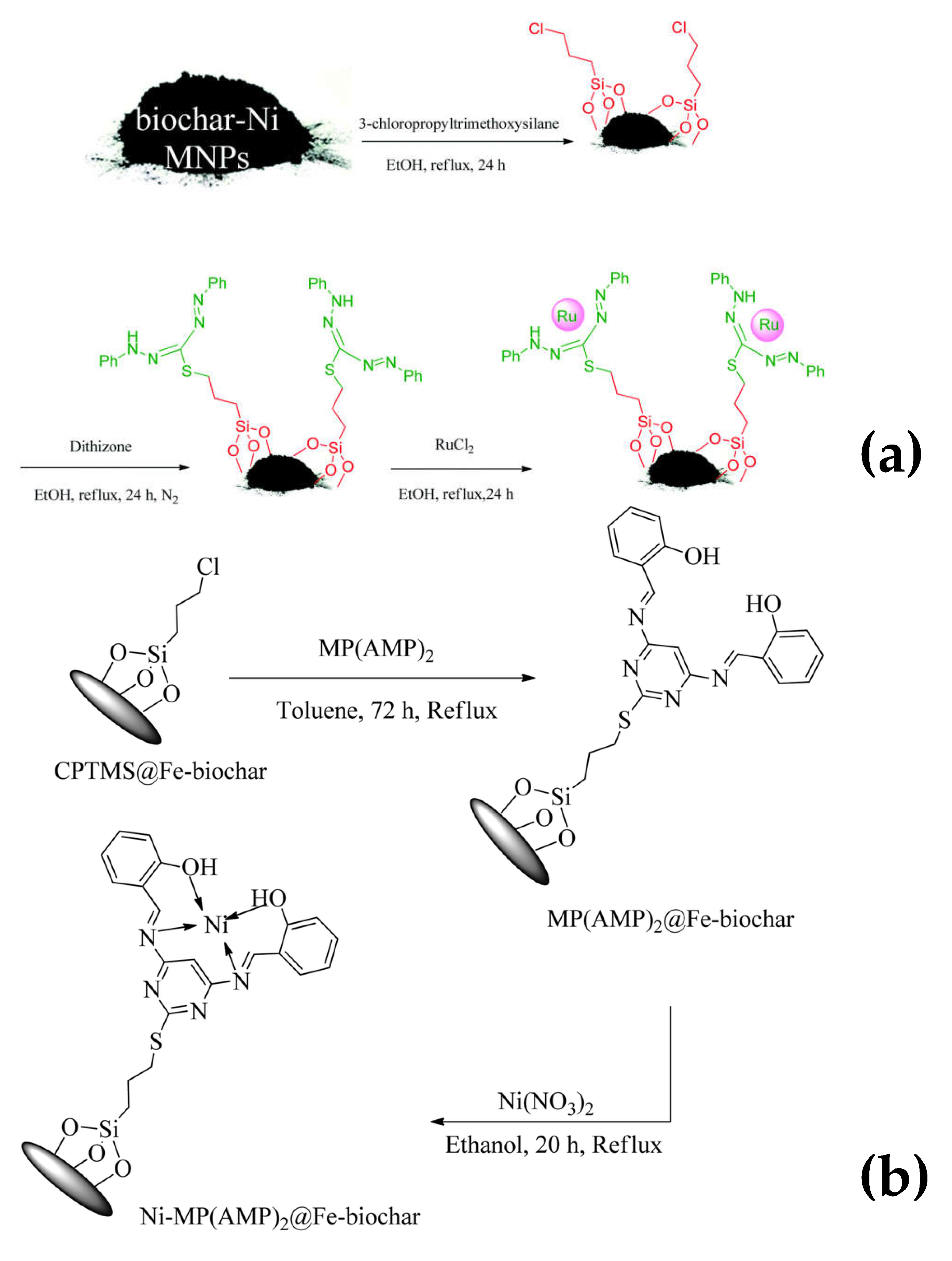
Chicken manure | Biochar-Ni magnetic composite were refluxed with 1.5 silane in the presence ot EToH for 24 h. Several other steps followed by treatment with dithizone and Rucl2 leads to formation of Ru-dithizone@biochar-Ni MNPs | High catalytic activity for C-C coupling reaction of iodobenzene or chlorobenzene with 96 % yield. | Suzuki C-C coupling reaction | [29] |
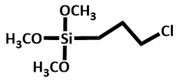 |
Chicken manure | CPTMS@Fe-biochar treated with MP(AMP)2 and refluxed for 72 h in the presence of toluene and then the product is refluxed with ethanol and nickel nitrate for 20h. | Enhanced catalytic efficiency. Catalyst recovery through magnet. Homoselectivity was observed. %yield = 98%. Recyclable upto 9 times. | Catalysis ( synthesis of tetrazole derivatives) |
[30] |
| Surfactant | Biomass used for biochar preparation | Surfactant immobilization conditions | Properties of modified biochar | Application | Reference |
|---|---|---|---|---|---|
Cationic CTAB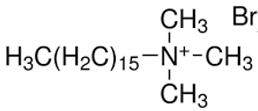
|
Populus alba | C0: 22 mg/L Dried at 105 ℃ |
- | Cr(VI) | [45] |
| Rice husk | C0:1457 mg/L Dried at 60 ℃ |
Surface charge change to positive | Inorganic nitrogen fertilizers removal | [46] | |
| Peanut shells Corncobs |
C0: 100 mg/L pH=3, 6, 9 |
- | Poly(acrylic acid) adsorption | [47] | |
| Peanut shells | C0: 17-272 mg/L T: 25 ℃ pH: 6 |
- | Bisphenol A adsorption | [48] | |
| Coffee husk | C0: 2550 mg/L T: 30 ℃ Dried at 60 ℃ |
Specific surface area decrease (from 750.1 to 557.4 m2/g) Pore volume decrease (from 0.3541 to 0.3192 cm3/g) Pore mean diameter increase (from 18.9 to 22.9 Å) |
Reactive dyes removal | [49] | |
| Cornstalks | C0: 10 mg/L Dried at 60 ℃ Neutral pH |
Surface charge change to positive | Orange II and methylene blue adsorption | [50] | |
Anionic SDBS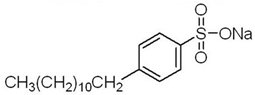
|
Rice husk | C0: 437.5 mg/L Dried at 60 ℃ |
Surface charge change to negative | Inorganic nitrogen fertilizers removal | [46] |
Anionic SDS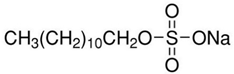
|
Peanut shells Corncobs |
C0: 100 mg/L pH: 3, 6, 9 |
- | Poly(acrylic acid) adsorption | [47] |
| Peat | C0: 100 mg/L pH: 3, 4.5, 6, 9 |
- | Poly(acrylic acid) adsorption | [51] | |
Anionic SDBS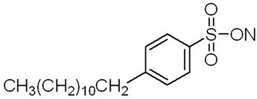
|
Peanut shells | C0: 24.5-392 mg/L T: 25 ℃pH: 6 |
- | Bisphenol A adsorption | [48] |
| Cassava peels | C0: 30-150 mg Dried at 110 ℃ |
More numerous surface active sites availability | Methylene blue adsorption | [52] | |
Anionic SDS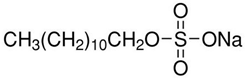
|
Cassava peels | C0: 30-150 mg/L Dried at 110 ℃ |
- | Methylene blue adsorption | [53] |
| Peanut shells | C0: 6-30 mg/L Dried at 65 ℃ |
Specific surface area increase (from 51.37 to 85.62 m2/g) Pore volume increase (from 0.232 to 0.283 cm3/g) Pore mean diameter decrease (from 3.794 to 1.543 nm) |
Methylene blue adsorption | [54] | |
Anionic SDBS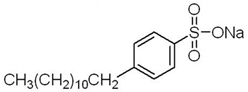
|
Corncob Furfural residue |
C0: 61.25-3062.5 mg/L pH: 2-10 |
Norfloxacin adsorption | [21] | |
Anionic SDS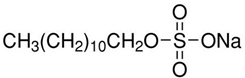
|
Corncob Furfural residue |
C0: 61.25-3062.5 mg/L pH: 2-10 |
Norfloxacin adsorption | [21] | |
Amphoteric BS-12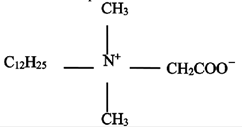
|
- | Modification 25-200 % | - | Phenanthrene adsorption | [55] |
Non-ionic Triton X-100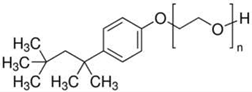
|
Horsetail herb | C0: 100 mg/L pH: 3 |
- | Poly(acrylic acid) and Pb(II) adsorption | [56] |
| Peanut shells Corncobs |
C0: 100 mg/L pH: 3, 6, 9 |
- | Poly(acrylic acid) adsorption | [47] | |
Non-ionic Tween 20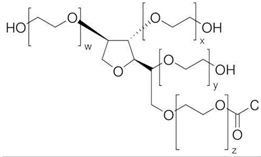
|
Peanut shells | C0: 3.5-56 mg/L T: 25 ℃ pH: 6 |
- | Bisphenol A adsorption | [48] |
PEG2000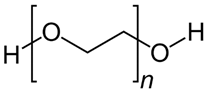
|
Eucalyptus sawdust | Surfactant dissolved in ethanol T: 60 ℃ Neutral pH |
Specific surface area decrease (from 462 to 448 m2/g) Pore volume increase (from 0.309 to 0.392 cm3/g) Pore mean diameter increase (from 2.679 to 3.497 nm) |
Metronidazole adsorption | [57] |
Pluronic P-123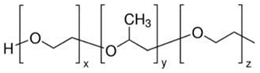
|
Eucalyptus sawdust | Surfactant dissolved in ethanol T: 60 ℃ Neutral pH |
Specific surface area decrease (from 462 to 436 m2/g) Pore volume increase (from 0.309 to 0.3586 cm3/g) Pore mean diameter increase (from 2.679 to 3.289 nm) |
Metronidazole adsorption | [57] |
Pluronic F-127
|
Eucalyptus sawdust | Surfactant dissolved in ethanol T: 60 ℃ Neutral pH |
Specific surface area decrease (from 462 to 221 m2/g) Pore volume decrease (from 0.309 to 0.2465 cm3/g) Pore mean diameter increase (from 2.679 to 4.466 nm) |
Metronidazole adsorption | [57] |
| Coupling agent | Biomass | Surface treatment conditions | Properties of engineered biochar | Potential application | References |
|---|---|---|---|---|---|
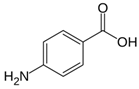 |
Pomegranate peel | Reaction with in situ generated diazonium salt, in 37% HCl, amine:NaNO2 = 1:1; final diazonium concentration=10-5-10-3 M | Controlled arylation, mass loading = 2.2-10.6 wt/wt%, D/G=2.7-3.4 (peak area ratio). | NA, potential heavy metal removal, | [65] |
| Sugarcane bagasse | Reaction of 300 mg biochar with in situ generated diazonium salt, in 37% HCl, amine:NaNO2 = 1:1 (6 mmol:6 mmol); | Arylation yields the grafting of 2 SO3 per 100 biochar carbon atoms. D/G=1.39 for biochar and 1.45 for biochar-SO3H. Arylation with in situ generated diazonium in concentrated HCl induces channel formation within the biochar. | In situ deposition of Ag(I) and Cu(II) ions, followed by reduction using sugarcane bagasse extract. | [68] | |
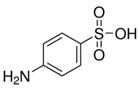 |
Pomegranate peel | Reaction with in situ generated diazonium salt, in 37% HCl, amine:NaNO2 = 1:1; final diazonium concentration=10-3 M | Mass loading = 5.3%, D/G=2.9 (peak area ratio) | NA, potential heavy metal removal | [65] |
| Bamboo | Sulfanilic acid/Biochar molar ratio= 0.1-1.5, in 25% HCl, at 30-80 °C, for 2-60 min. | Total acid density=1.69 mmol/g. SSA decreased from 919 to 225 m²/g upon arylation. |
Acid catalyst. Up to 96% catalyzed esterification of oleic acid with ethanol. | [70] | |
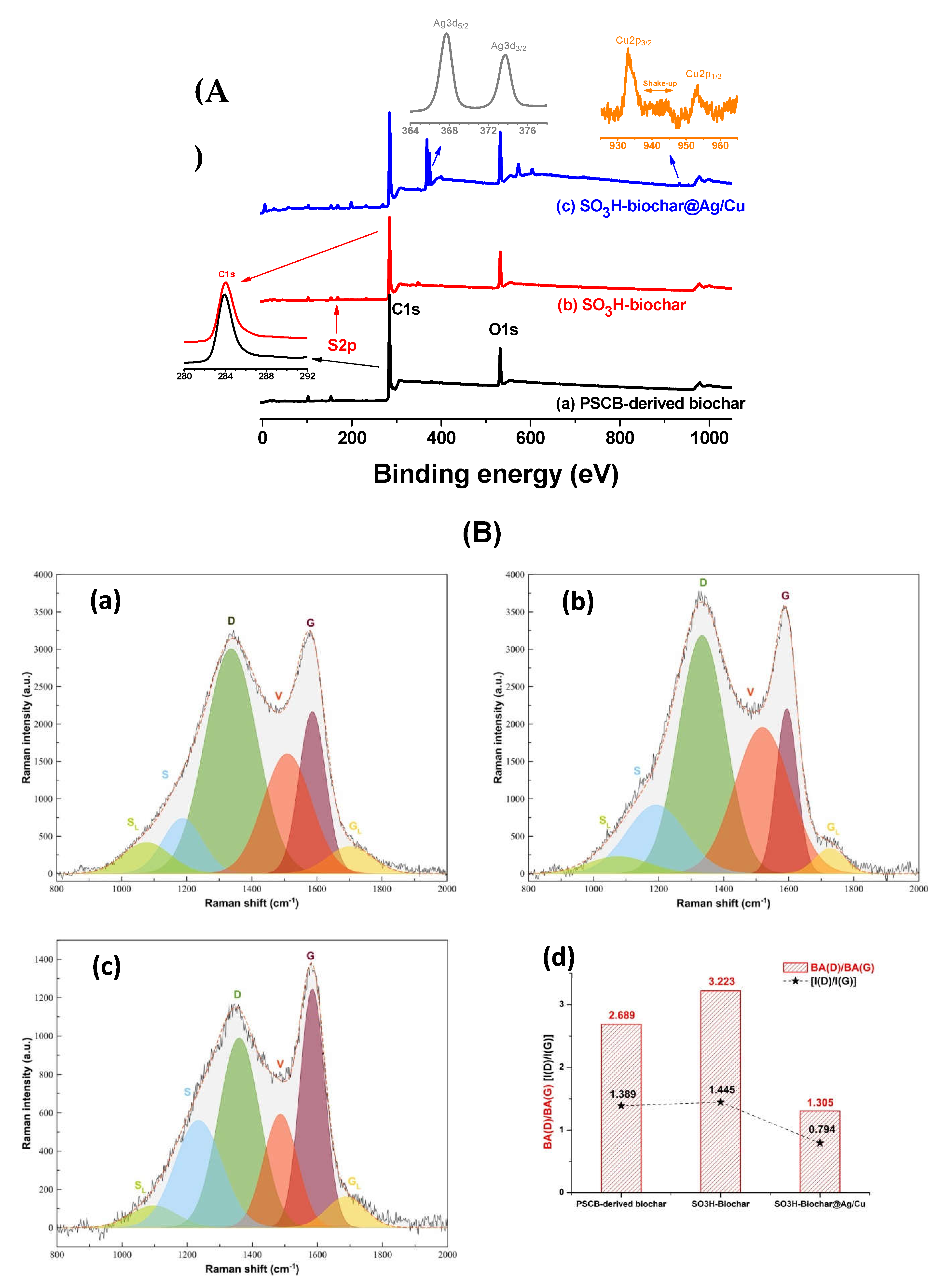
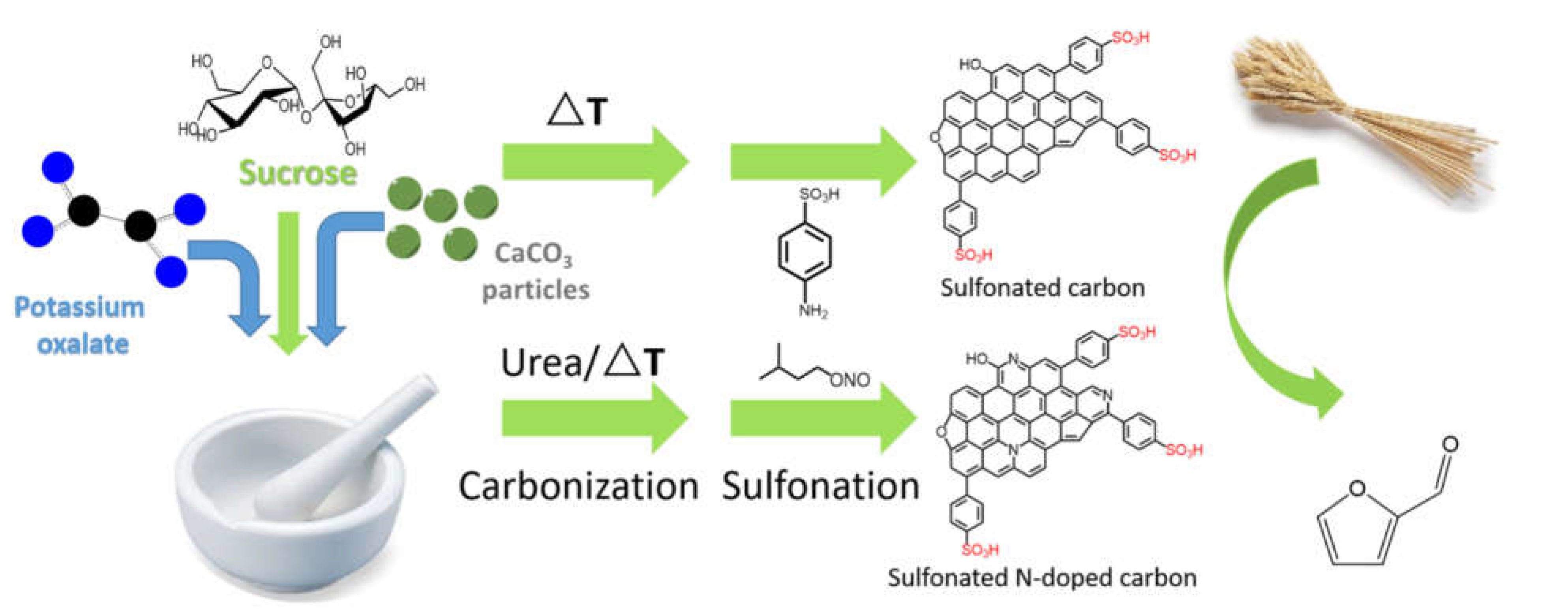
| Sucrose-K2C2O4•H2O- CaCO3-Urea (1-1-1-0 or 1-1-1-0.5 wt.fractions) | Biochar preparaed at 600-800 °C without urea, and at 750 °C in the presence of urea. 1g biochar mixed with 4 g sulfanilic acid and 2 g isoamyl nitrite, in 150 mL DIW. Arylation at 80 °C overnight. | Drastic reduction of SSA from 1422 to 190 m²/g for biochar prepared at 800 °C, and from 1658 to 30 m² for biochar-urea and sulfonated biochar-urea. Sulfur content: 0.08 to 2.29 mmol/g. With urea, very high arylationD/G increased with arylation. | Catalytic furfural production from wheat straw. | [71] | |
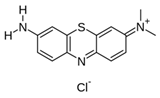 |
Pomegranate peel | Reaction with in situ generated diazonium salt, in 37% HCl, amine:NaNO2 = 1:1; final diazonium concentration=10-3 M | Mass loading = 5.3%, D/G=2.9 (peak area ratio) | NA, potential radical polymerization photoinitiation | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





