Introduction
The atypical thrombotic events associated with vaccination have been poorly described, but these, in turn, are very devastating when it comes to cerebral venous thrombosis. In fact, it has been shown to be the most fatal post-vaccination event against COVID-19.
Although it is true that thrombosis of the cerebral venous sinus is rare after vaccination with AstraZeneca or Johnson & Johnson COVID-19 (0.9-3.6 per million), this immunological phenomenon is much more frequent among hospitalized patients with COVID-19 (207 per million) (1). We present below a clinical case from our experience and professional practice where a venous thrombosis of both transverse sinuses occurred after vaccination with a ChAdOx1-S type agent against COVID-19.
Clinical Case Description
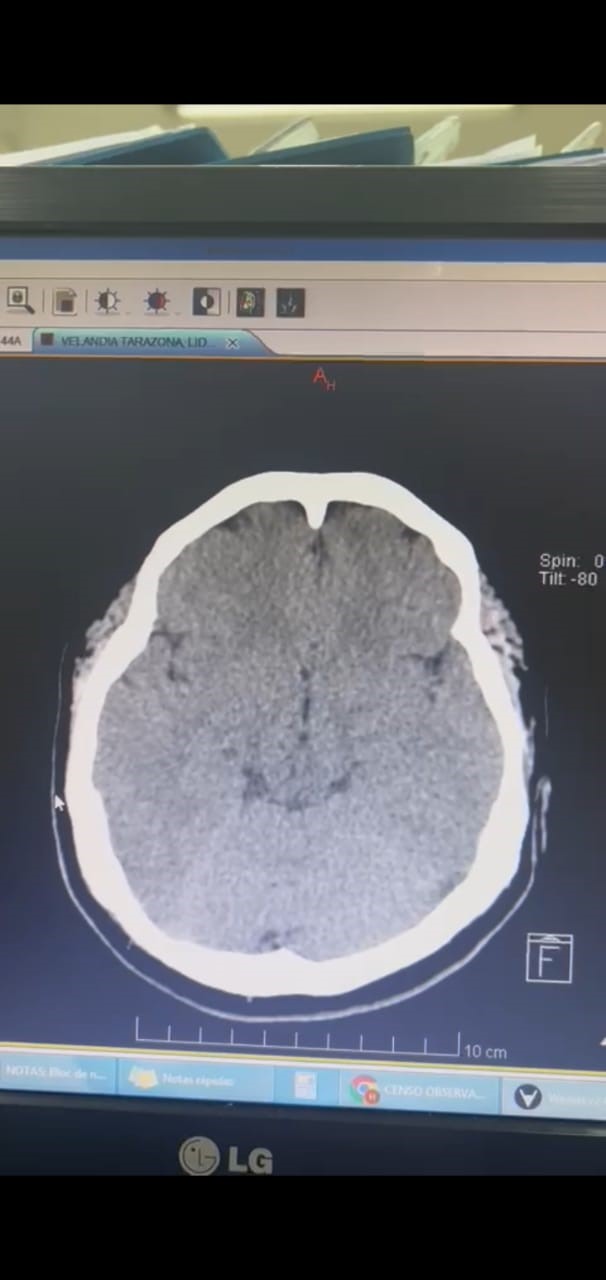
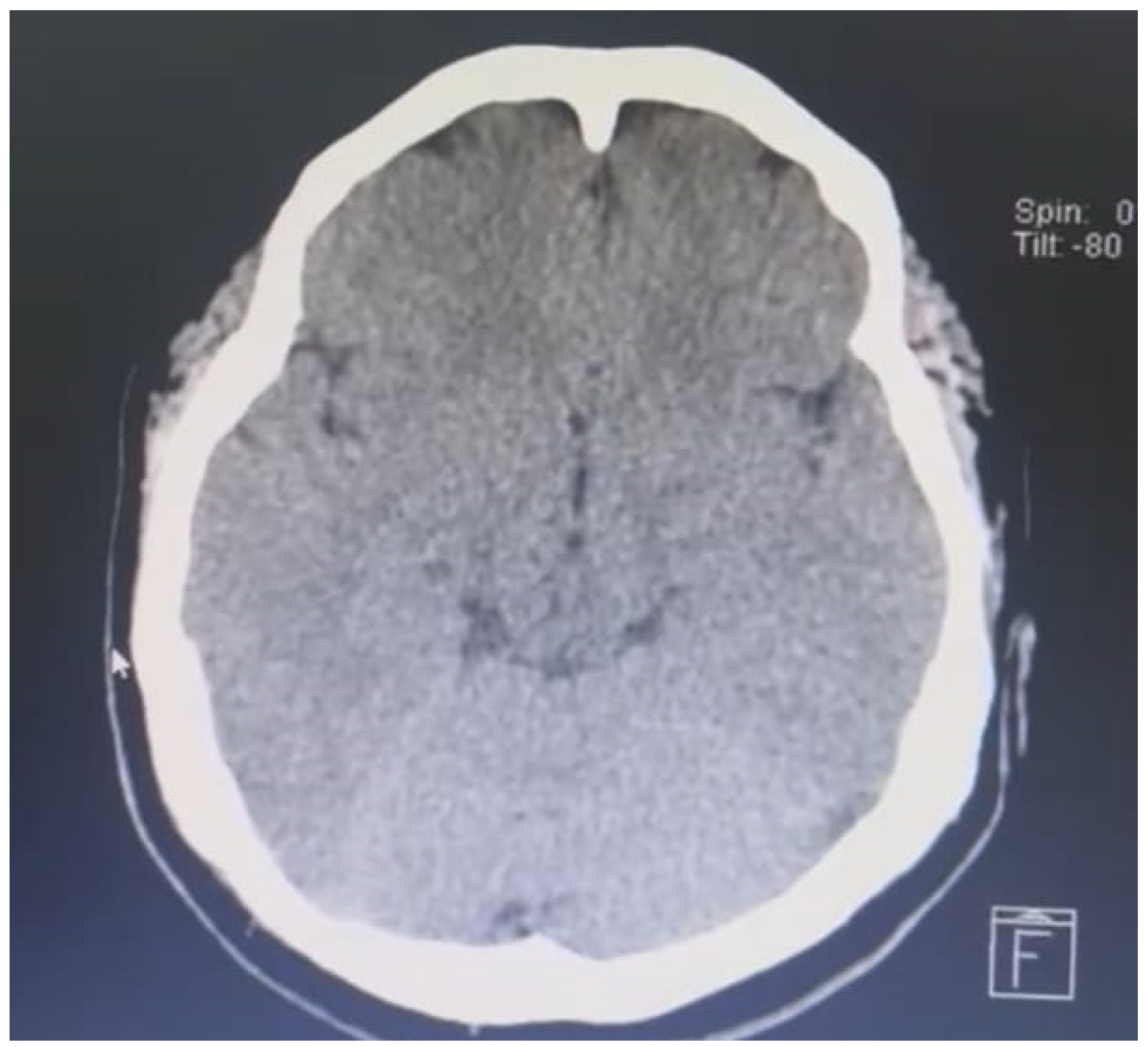
This is a 37-year-old female patient who presents after 11 days of vaccination with ChAdOx1-S agent against COVID-19 presents intense headache associated with symptoms of intracranial hypertension, after admission due to the aforementioned alarm symptoms and High blood pressure figures were initially taken to a simple cranial tomography showing the presence of cerebral edema and indirect signs of cerebral venous thrombosis.
Figure 1.
Simple brain tomography performed on admission.
Figure 1.
Simple brain tomography performed on admission.
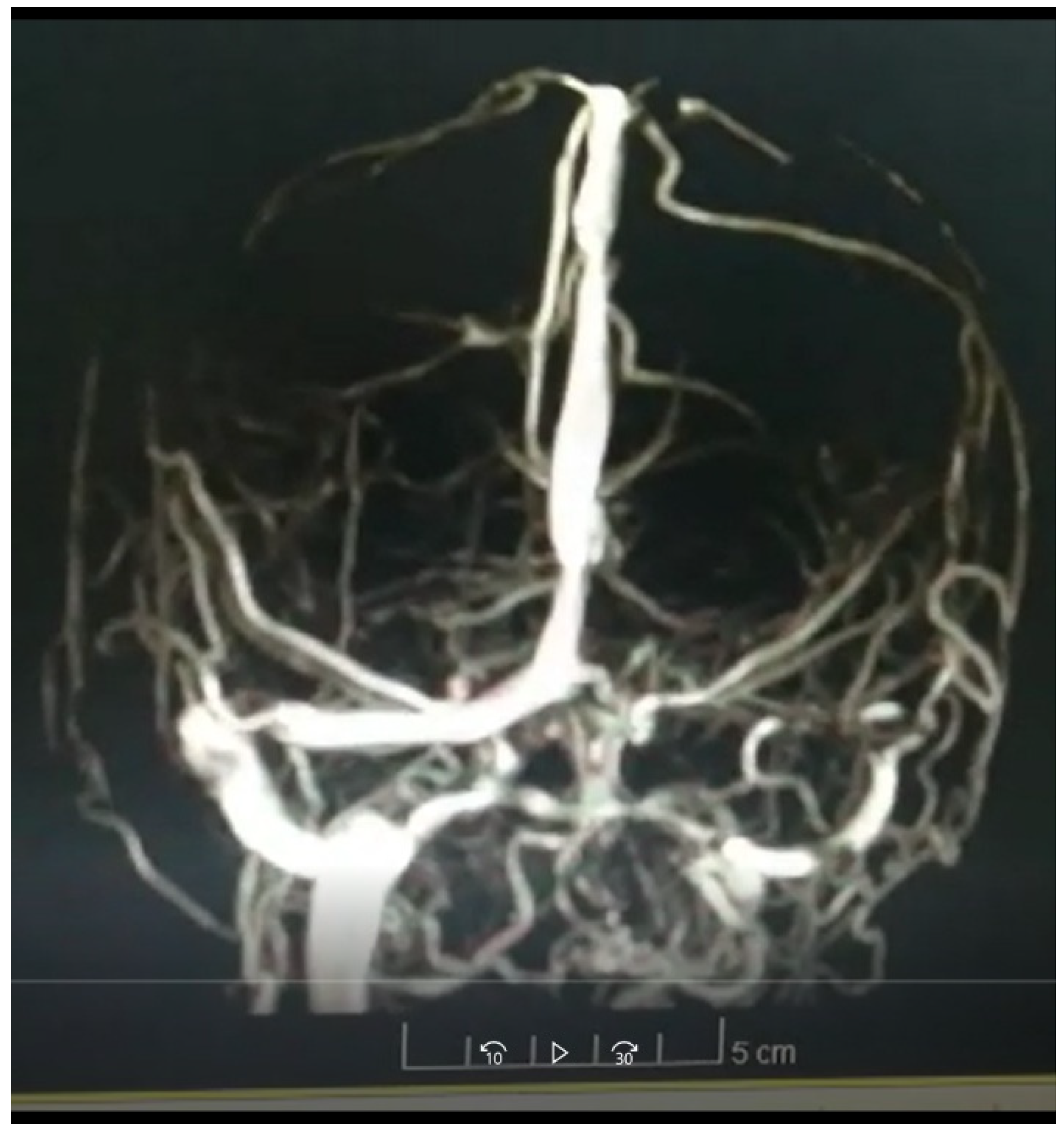
In the laboratories carried out on admission, only a thrombocytopenia of 135,000 was observed. It is then considered to carry out a brain angioresonance which showed the presence of thrombosis of both transverse venous sinuses in the right at the proximal level and in the left at the distal level.
Figure 2.
Angioresonance performed on the patient.
Figure 2.
Angioresonance performed on the patient.
No hypercoagulability states associated with deficit or overexpression of proteins involved in coagulation were found in the laboratories performed, Antithrombin III levels were normal, Protein C and S levels were normal, Homocysteine levels were normal, no found alterations in Factor V of Leyden. The levels of antinuclear antibodies were negative, no consumption of complement factors was found, and the antibodies against the neutrophil cytoplasm were negative.
No hypercoagulability states associated with deficit or overexpression of proteins involved in coagulation were found in the laboratories performed, Antithrombin III levels were normal, Protein C and S levels were normal, Homocysteine levels were normal, no found alterations in Factor V of Leyden.
The levels of antinuclear antibodies were negative, no consumption of complement factors was found, and the antibodies against the neutrophil cytoplasm were negative.
The immunological tests carried out for antiphospholipid syndrome were negative, based on all of the above, the thrombotic event was associated with the vaccine against COVID-19 agent ChAdOx1-S due to the time of evolution of symptoms after exposure to the vaccine agent 2 weeks prior to debut of the clinical picture.
Subsequently, the patient was taken to thrombectomy by Neuroradiology, achieving complete canalization of both transverse venous sinuses.
Discussion and Conclusions of the Case
This case is anecdotal given the low frequency of induced thrombocytopenia after vaccination for COVID-19. The German Society for Thrombosis and Haemostasis found that of approximately 2.2 million doses of AstraZeneca vaccine administered up to the time of publication of their article, a total of 31 cases of thrombosis had been reported, occurring 4 to 16 days later and thrombocytopenia was reported in 19 patients, with a fatal clinical course in nine (2).
A team from the University of Greifswald developed and evaluated the use of a rapid assay to diagnose thrombocytopenia induced after vaccination by COVID 19, the assay known as PIFPA (PF4-induced flow cytometry-based platelet activation) is a cytometric assay that is Based on a washed platelet assay, this test for platelet activation induced by platelet factor 4 after vaccination with ChAdOx1-S agent, has high specificity for vaccine-related antibodies but is currently only available in specialized laboratories (3 ).
The clinical presentation leading to the diagnosis of cerebral venous sinus thrombosis and thrombocytopenia after vaccination against COVID-19, according to reported cases included headache and lethargy in the first 14 days after vaccination (4).
The pathogenesis justifies the development of diagnostic tests. The US Centers for Disease Control (CDC) recommends treatment with alternatives to heparin in patients with headache or other symptoms that may suggest thrombotic events following COVID-19 vaccination.
It is essential that emergency physicians and general practitioners are aware of this condition, the findings of this type of event justify important clinical and public health implications.
CDC recommendations for these types of events are to avoid heparin and consider anticoagulation with alternatives to heparin. Cases with side effects related to COVID-19 vaccines are now at the forefront of regulatory bodies, as well as public opinion, and active surveillance programs are being developed in many countries, in which health workers report on the side effects that occur after the administration of the vaccine (5).
Based on the belief that increasing evidence supports an association between thrombosis and thrombocytopenia syndrome (TTS) and adenovirus vector-based vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a group of European and North American researchers has published a meta-analysis in Neurology (6) convinced that TTS and TTS-associated cerebral venous sinus thrombosis (CVOT) remain poorly characterized, so its essential objective has been to systematically evaluate the proportion of SVT among CTS cases and assess its characteristics and results.
The work included 69 studies in the qualitative analysis comprising 370 patients with CSVT out of a total of 4,182 patients with any thrombotic event associated with the administration of the SARS-CoV-2 vector-based vaccine. Twenty-three studies were also included in the quantitative meta-analysis. Among TSVC cases, the pooled proportion was 51%. Such an event was independently associated with a higher probability of developing it compared with patients without thrombocytopenia with thrombotic events after vaccination. The pooled CTS and CTS-associated CTS mortality rates were 28% and 38%, respectively. Thrombotic complications developed within two weeks of exposure to vector-based SARS-CoV-2 vaccines and predominantly affected women younger than 45 years, even in the absence of prothrombotic risk factors.
Therefore, they conclude that approximately half of the patients with CSVT have thrombocytopenia and that almost a third of the patients with CSVT do not survive, data that confirms that further research is necessary to identify the independent predictors of CSVT after vector-based vaccination. of adenovirus.
Regarding treatment Some experts recommend the administration of intravenous immunoglobulin or corticosteroids in patients with cerebral venous sinus thrombosis associated with thrombocytopenia and antibodies against platelet factor 4 (7).
In connection with anticoagulant management, anticoagulants other than heparin should be used, including direct factor Xa inhibitors, direct thrombin inhibitors, and fondaparinux. However, the effects of administering heparin to patients with such an adverse event remain unclear, with some evidence suggesting that heparin might inhibit antibody-mediated platelet activation specifically after COVID 19 vaccination. In patients receiving intravenous immunoglobulin and prednisolone, the platelet count increased despite continued heparin treatment (8). Similarly, serum samples from patients with thrombocytopenia after vaccination have been analyzed and platelet activation was found to be inhibited by heparins.
At the time the patient is under anticoagulant management with Warfarin due to normalization of platelet counts, there is no evidence that she can justify the use of direct oral anticoagulants (9).
The presence of cerebral venous thrombosis after vaccination for COVID-19 is an area under constant investigation. At the time of writing this manuscript, we must rely on the guidelines of international scientific societies on the most appropriate diagnosis and treatment, for the moment the tests diagnostic techniques by flow cytometry are limited in underdeveloped countries.
The limitations of this clinical case were those derived from not having flow cytometric tests, the questions to be resolved are; determine, based on the scientific evidence that is in development, which would be the therapeutic strategies with the best results, since there are no studies with large samples of patients and the scientific evidence available to date comes from case reports due to the infrequent nature of said complication.
Finally, we highlight the role of surveillance programs against mass vaccination against COVID-19 developed by government and academic entities, since these will allow us to obtain the best evidence in this regard (10), the evidence is scarce at the moment, but in the In the future, it will be possible to obtain the most appropriate conclusions derived from the highest quality scientific evidence.
References
- Bikdeli B, Chatterjee S, Arora S, Monreal M, Jimenez D, Krumholz HM, Goldhaber SZ, Elkind MSV, Piazza G. Cerebral Venous Sinus Thrombosis in the U.S. Population, After Adenovirus-Based SARS-CoV-2 Vaccination, and After COVID-19. J Am Coll Cardiol. 2021 Jul 27;78(4):408-411. [CrossRef]
- Oldenburg, J, Klamroth, R, Langer, F, et al. Diagnosis and management of vaccine-related thrombosis following AstraZeneca COVID-19 vaccination: guidance statement from the GTH. Hamostaseology. Epub ahead of print 1 April 2021. [CrossRef]
- Handtke, S, Wolff, M, Zaninetti, C, et al. A Flow cytometric assay to detect platelet activating antibodies in VITT after ChAdOx1 nCov-19 vaccination. Blood. Epub ahead of print 4 May 2021. [CrossRef]
- CDC Health Alert Network. Cases of cerebral venous sinus thrombosis with thrombocytopenia after receipt of the Johnson & Johnson COVID-19 vaccine. https://emergency.cdc.gov/han/2021/han00442.asp.
- Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med 2021. [CrossRef]
- Lina Palaiodimou, Maria-Ioanna Stefanou, Aristeidis H. Katsanos, Diana Aguiar de Sousa, Jonathan M. Coutinho, Pagona Lagiou, Ioannis Michopoulos, Androniki Naska, Sotirios Giannopoulos, Konstantinos Vadikolias, Konstantinos I. Voumvourakis, Vasiliki Papaevangelou, Theodoros I Vassilakopoulos, Sotirios Tsiodras, Georgios Tsivgoulis. Cerebral Venous Sinus Thrombosis and Thrombotic Events After Vector-Based COVID-19 Vaccines. A Systematic Review and Meta-analysis. Neurology Nov 2021, 97(21) e2136-e2147. [CrossRef]
- Furie KL, Cushman M, Elkind MSV, Lyden PD, Saposnik G; American Heart Association/American Stroke Association Stroke Council Leadership. Diagnosis and Management of Cerebral Venous Sinus Thrombosis with Vaccine-Induced Immune Thrombotic Thrombocytopenia. Stroke. 2021 Jul;52(7):2478-2482. [CrossRef]
- Jaax, ME, Krauel, K, Marschall, T, et al. Complex formation with nucleic acids and aptamers alters the antigenic properties of platelet factor 4. Blood 2013; 122(2): 272–281.
- Sheikh, S. Thrombotic Thrombocytopenia and Central Venous Sinus Thrombosis Post - COVID-19 Vaccination and its Treatment with Heparin Alternatives. J Coll Physicians Surg Pak. 2021 Jul;31(7):149. [CrossRef]
- Klein NP, Lewis N, Goddard K, Fireman B, Zerbo O, Hanson KE, Donahue JG, Kharbanda EO, Naleway A, Nelson JC, Xu S, Yih WK, Glanz JM, Williams JTB, Hambidge SJ, Lewin BJ, Shimabukuro TT, DeStefano F, Weintraub ES. Surveillance for Adverse Events After COVID-19 mRNA Vaccination. NEVER. 2021 Oct 12;326(14):1390-1399. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).