2. Theoretical justification
Suppose we want to calculate an arbitrary physical quantity L that characterizes some physical property of an arbitrary substance with the chemical formula AkBlCn... where the number of elements in the formula must be greater than one. The desired physical quantity L depends on the components of the substance and its structure. This quantity L can be the temperature of phase transition of any order and any other thermophysical quantity, mechanical, electrical, magnetic, optical and other characteristics of the substance. It can be assumed that L somehow depends on the similar physical quantities Li of all components of the substance under consideration: L = L(LA, LB, LC, ....). Since the amount of a component in a substance affects its properties, it should be concluded that chemical indexes should also be represented in the function: L = L(LA, LB, LC, ...., k, l, n, …). Since the atomic number of each component ultimately determines the electronic structure of a substance, we insist that the atomic numbers of all components also be represented in a functional relationship: L = L(LA, LB, LC, ...., k, l, n, …, A, B, C, …), where A is the atomic number of element A. The phase state of matter also determines L, and we assume that the state of matter should be represented by a structural parameter h: L = L(LA, LB, LC, ...., k, l, n, …, A, B, C, …, h). Since the properties of a substance can change due to changes in temperature T, pressure p, radiation dose D, static electromagnetic fields (E, B), etc., the value of L changes with such changes, but we will assume that L not depends on them directly, but only through Li and h: L(T,p,D,E,B,…) = L(LA(T,p,D,E,B,…), LB(T,p,D,E,B,…), LC(T,p,D,E,B,…), ...., k, l, n, …, A, B, C, …, h(T,p,D,E,B,…)). Since the basic physical properties of a chemical element depend on a set of integers that determine the position of the element in the periodic table, and given that the crystal structure is represented through a set of rational numbers representing the space group, we insist that the structural parameter h is a rational number, although it somehow depends on the lattice parameters: h = h(a, b, c, α, β, γ)Q, where Q is a set of rational numbers.
Now suppose that
L ≡
λABC… is the thermal conductivity of
AkBlCn... and we want to find its dependence on temperature only:
L(
T) =
L(
LA(
T),
LB(
T),
LC(
T), ....,
k,
l,
n, …,
A,
B,
C, …,
h(
T)). We found in [
4] that for oxides and silicates (
ZrO2,
ZrSiO4, (
U,
Zr)
SiO4,
UO2) the best agreement with experiment is given by the following formula:
where the parameter
h is associated with the most electronegative element, in our case with
C. It has been observed that
h is a constant over a certain range of temperatures. Thus, for example, for silicate (
U0.016Zr0.984)
SiO4 at temperature range
T (470, 1070]
K where
h = 1, the thermal conductivity is next:
where atomic numbers:
U = 92,
Zr = 40,
Si = 14,
O = 8. For zircon
ZrSiO4 in the temperature range
T[570, 1170]
K where
h = 2/3, the thermal conductivity is next:
where atomic numbers are
Zr = 40,
Si = 14,
O = 8.
We applied formula (1) to describe the thermal conductivity of carbides and nitrides and found (see the next section) that for some ceramics the structural parameter h is fixed in a certain temperature range, which makes it possible to represent the thermal conductivity of ceramics as the sum of the thermal conductivities of its components.
To assess the correctness of formula (1), relative errors were calculated in this work using the following formula:
where
λtheory is the result of using formula (1) and
λexp is taken from experimental data.
3. Discussion
For zirconium carbide
ZrC, formula (1) has the following form:
where atomic numbers are
Zr = 40,
C = 6. The result of calculations by formula (3) and comparison with the experiment [
1] is given in
Table 1. The thermal conductivity of carbon in the form of bulk diamond was taken from [
5], [
6], where theoretical data for bulk diamond are presented, which are in good agreement with the available experimental data [
7].
Table 1 shows that the parameter
h decreases with increasing
T. The step of changing
h between 400 and 600
K is Δ
h = 1 at 100
K. Therefore, we can express
h in terms of
T in this temperature range and obtain for
at
T [400, 600]
K:
where
. In order to fully express the thermal conductivity of a substance over the entire range of
T, it is necessary to know the dependence of
h on the lattice parameters as the temperature changes:
h = h(
a(
T)
, b(
T)
, c(
T)
, α(
T)
, β(
T)
, γ(
T))
Q.
Table 1.
Thermal conductivit
y of
ZrC calculated over formula (3) at different temperatures. Thermal conductivities for pure
Zr are taken from [
8], for bulk diamond – from [
5], [
6].
are the experimental values for
ZrC taken from [
1], where the data are corrected to 100% TD (total density).
are the values obtained from (3). Relative errors
δ are calculated according to (2).
Table 1.
Thermal conductivit
y of
ZrC calculated over formula (3) at different temperatures. Thermal conductivities for pure
Zr are taken from [
8], for bulk diamond – from [
5], [
6].
are the experimental values for
ZrC taken from [
1], where the data are corrected to 100% TD (total density).
are the values obtained from (3). Relative errors
δ are calculated according to (2).
| T, ° K |
W/(m·K)
|
W/(m·K)
|
W/(m·K)
|
h |
W/(m·K)
|
δ, % |
| 300 |
19.5 |
22.7 |
1664 |
13/2 |
19.0 |
2.5 |
| 400 |
20.26 |
21.6 |
1441 |
26/5 |
20.50 |
1.2 |
| 500 |
20.42 |
21 |
1168 |
21/5 |
20.50 |
0.4 |
| 600 |
20.9 |
20.7 |
922 |
16/5 |
21.1 |
0.9 |
| 700 |
20.9 |
20.9 |
720 |
5/2 |
21.0 |
0.5 |
| 800 |
22.0 |
21.6 |
559 |
9/5 |
22.3 |
1.4 |
| 900 |
22.32 |
22.6 |
465 |
3/2 |
22.16 |
0.7 |
| 1000 |
23.31 |
23.7 |
425 |
13/10 |
23.20 |
0.5 |
For uranium carbide
UC, formula (1) has the following form:
where atomic numbers are
U = 92,
C = 6. The result of calculations by formula (4) and comparison with the experiment [
2], [
9] is given in
Table 2.
Table 2 shows that the parameter
h decreases with increasing
T. The negative value of
h = –1/666 at
T = 800
K requires studying the function
λUC =λUC(
h) at fixed
T, that is, at fixed
λU and
λC.
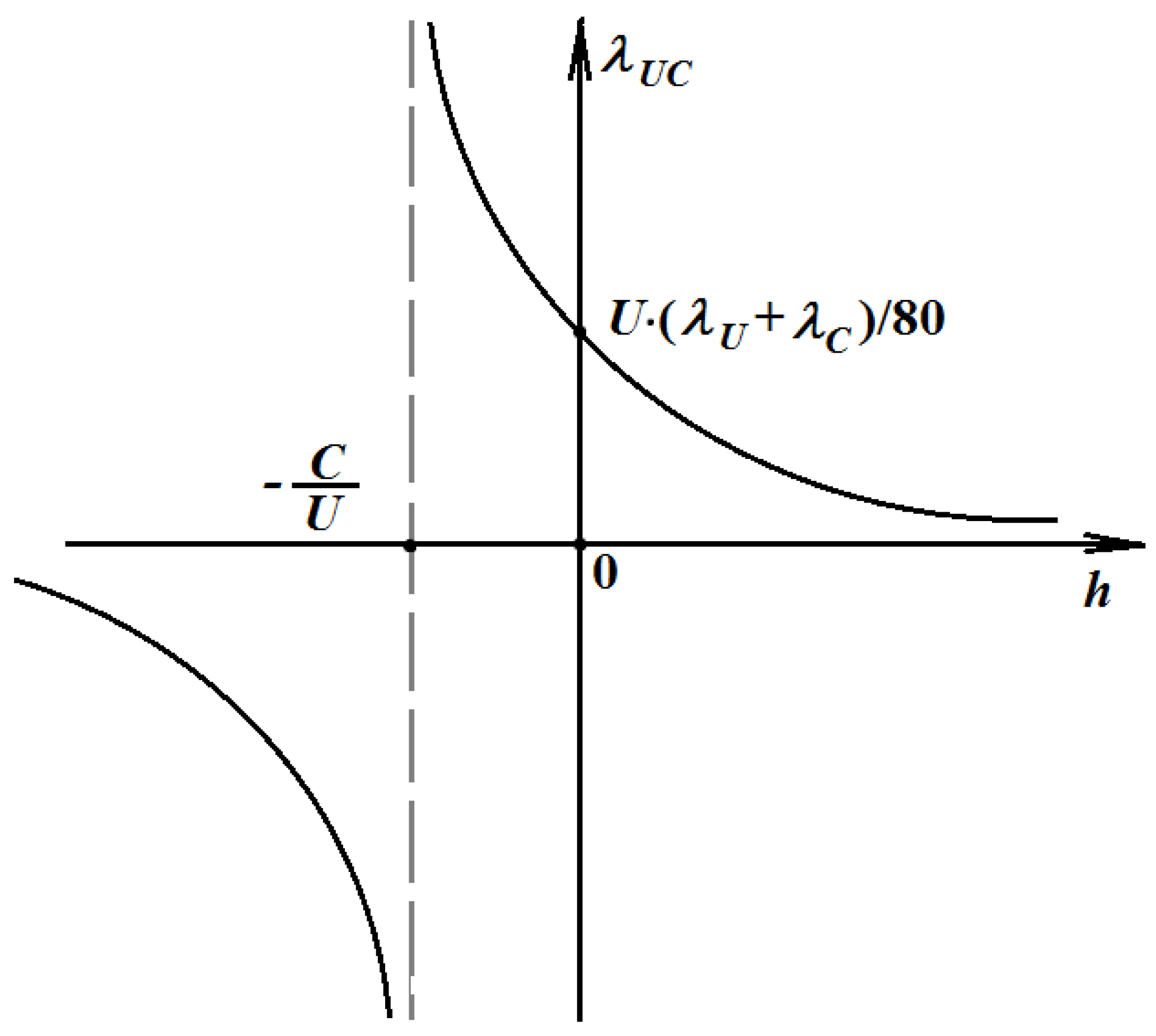
The function λUC =λUC(h) is shown in Fig.1. This is hyperbola with shifted center to the point . λUC(h) has an essential discontinuity at . At h = 0, The domain of positive λUC(h) is Q, where Q is a set of rational numbers. Only one value of h from this domain gives the physical value of λUC(h). The range of negative values of λUC(h) is not physical.
Table 2.
Thermal conductivit
y of
UC calculated over formula (4) at different temperatures. Thermal conductivities for pure
U are taken from [
8], for bulk diamond – from [
5], [
6].
are the experimental values for
UC taken from [
2], [
9].
are the values obtained from (4). Relative errors
δ are calculated according to (2).
Table 2.
Thermal conductivit
y of
UC calculated over formula (4) at different temperatures. Thermal conductivities for pure
U are taken from [
8], for bulk diamond – from [
5], [
6].
are the experimental values for
UC taken from [
2], [
9].
are the values obtained from (4). Relative errors
δ are calculated according to (2).
| T, ° K |
W/(m·K)
|
W/(m·K)
|
W/(m·K)
|
h |
W/(m·K)
|
δ, % |
| 300 |
751 |
27.6 |
1664 |
1/10 |
768 |
2.3 |
| 400 |
691 |
29.6 |
1441 |
1/11 |
706 |
2.2 |
| 500 |
703 |
31.7 |
1168 |
1/16 |
704.5 |
0.2 |
| 600 |
691 |
34 |
922 |
1/26 |
691.5 |
0.07 |
| 700 |
703 |
36.4 |
720 |
1/66 |
706 |
0.4 |
| 800 |
703 |
38.8 |
559 |
–1/666 |
703.7 |
0.1 |
Figure 1.
The graph of the function λUC(h) is represented by formula (4) at a fixed temperature, and hence constant thermal conductivities λU and λC. Only one value of hQ gives the physical value of .
Figure 1.
The graph of the function λUC(h) is represented by formula (4) at a fixed temperature, and hence constant thermal conductivities λU and λC. Only one value of hQ gives the physical value of .
For uranium nitride
UN, formula (1) has the following form:
where atomic numbers are
U = 92,
N = 7. The result of calculations by formula (5) and comparison with the experiment [
3] is given in
Table 3.
Table 3 shows that the parameter
h is a constant at
T [400, 900]
K and (900, 1200]
K. Therefore, we can describe
λUN as the sum of the thermal conductivities of its components:
Table 3.
Thermal conductivity of
UN calculated over formula (5) at different temperatures. Thermal conductivities for pure
U and
N are taken from [
8].
are the experimental values for
UN taken from [
3], where the data are corrected to 100% TD (total density).
are the values obtained from (5). Relative errors
δ are calculated according to (2).
Table 3.
Thermal conductivity of
UN calculated over formula (5) at different temperatures. Thermal conductivities for pure
U and
N are taken from [
8].
are the experimental values for
UN taken from [
3], where the data are corrected to 100% TD (total density).
are the values obtained from (5). Relative errors
δ are calculated according to (2).
| T, K |
,W/(m·K)
|
,W/(m·K)
|
,W/(m·K)
|
h |
,W/(m·K)
|
δ, % |
| 300 |
13.0 |
27.6 |
0.026 |
1/9 |
12.9 |
0.8 |
| 400 |
14.5 |
29.6 |
0.03252 |
1/10 |
14.7 |
1.4 |
| 500 |
15.9 |
31.7 |
0.03864 |
1/10 |
15.8 |
0.6 |
| 600 |
17.15 |
34.0 |
0.044 |
1/10 |
16.92 |
1.3 |
| 700 |
18.25 |
36.4 |
0.0493 |
1/10 |
18.11 |
0.8 |
| 800 |
19.0 |
38.8 |
0.054 |
1/10 |
19.3 |
1.6 |
| 900 |
20.0 |
41.3 |
0.0587 |
1/10 |
20.5 |
2.5 |
| 1000 |
20.9 |
43.9 |
0.063 |
1/9 |
20.5 |
1.9 |
| 1100 |
21.7 |
46.3 |
0.0672 |
1/9 |
21.7 |
0 |
| 1200 |
22.3 |
49.0 |
0.0713 |
1/9 |
22.9 |
2.7 |
where
h = 1/10 at
T [400, 900]
K and
h = 1/9 at
T (900, 1200]
K.
For thorium nitride
ThN, formula (1) has the following form:
where atomic numbers are
Th = 90,
N = 7. The result of calculations by formula (6) and comparison with the experiment [
3] is given in
Table 4.
Table 4 shows that the parameter
h gradually increases with increasing
T.
For mixed ceramics
ThxU1-xN, where
x = 0.2 and 0.5, formula (1) has the following form:
where atomic numbers are
Th = 90,
U = 92,
N = 7. The result of calculations by formula (7) and comparison with the experiment [
3] is given in
Table 5. Since
Table 4.
Thermal conductivit
y of
ThN calculated over formula (6) at different temperatures. Thermal conductivities for pure
Th and
N are taken from [
8].
are the experimental values for
ThN taken from [
3], where the data are corrected to 100% TD (total density).
are the values obtained from (6). Relative errors
δ are calculated according to (2).
Table 4.
Thermal conductivit
y of
ThN calculated over formula (6) at different temperatures. Thermal conductivities for pure
Th and
N are taken from [
8].
are the experimental values for
ThN taken from [
3], where the data are corrected to 100% TD (total density).
are the values obtained from (6). Relative errors
δ are calculated according to (2).
| T, K |
,W/(m·K)
|
,W/(m·K)
|
,W/(m·K)
|
h |
,W/(m·K)
|
δ,% |
| 300 |
46.6 |
54 |
0.026 |
1/42 |
46.5 |
0.2 |
| 400 |
45.24 |
54.5 |
0.03252 |
1/37 |
45.53 |
0.6 |
| 500 |
43.9 |
55.1 |
0.03864 |
1/30 |
43.4 |
1.1 |
| 600 |
42.7 |
55.8 |
0.044 |
1/27 |
42.5 |
0.5 |
| 700 |
41.5 |
56.4 |
0.0493 |
1/24 |
41.3 |
0.5 |
| 800 |
40.26 |
56.9 |
0.054 |
1/22 |
40.44 |
0.4 |
| 900 |
39.16 |
57.3 |
0.0587 |
1/20 |
39.28 |
0.3 |
| 1000 |
38.05 |
57.8 |
0.063 |
1/18 |
37.97 |
0.2 |
| 1100 |
37.06 |
58.3 |
0.0672 |
1/17 |
37.39 |
0.9 |
| 1200 |
36.06 |
58.7 |
0.0713 |
1/16 |
36.66 |
1.7 |
Table 5.
Thermal conductivities of (
Th0.2,
U0.8)
N and (
Th0.5,
U0.5)
N calculated over formula (7) at different temperatures.
λexp are the experimental values for (U
x,Zr
1-x)SiO
4 taken in [
3], red left column is for
(Th0.2,U0.8)N and right blue is for
(Th0.5,U0.5)N. Thermal conductivities for pure
Th and
U are taken from [
8].
λtheory are the values obtained from (7), red left column is for
(Th0.2,U0.8)N and right blue is for
(Th0.5,U0.5)N. For
h is the same coloring as for
λexp and
λtheory. Relative errors
δ are calculated according to (2) and are given in brackets.
Table 5.
Thermal conductivities of (
Th0.2,
U0.8)
N and (
Th0.5,
U0.5)
N calculated over formula (7) at different temperatures.
λexp are the experimental values for (U
x,Zr
1-x)SiO
4 taken in [
3], red left column is for
(Th0.2,U0.8)N and right blue is for
(Th0.5,U0.5)N. Thermal conductivities for pure
Th and
U are taken from [
8].
λtheory are the values obtained from (7), red left column is for
(Th0.2,U0.8)N and right blue is for
(Th0.5,U0.5)N. For
h is the same coloring as for
λexp and
λtheory. Relative errors
δ are calculated according to (2) and are given in brackets.
| T, K |
,W/(m·K)
|
,W/(m·K)
|
,W/(m·K)
|
,W/(m·K)
|
|
|
(δ,%) |
(δ,%) |
| 300 |
17.4±1.8
|
20.1±1.9
|
54 |
27.6 |
-20/63 |
-10/77 |
17.3(0.6) |
20.1(0) |
| 400 |
18.1±1.8
|
21.5±1.9
|
54.5 |
29.6 |
-20/63 |
-10/73 |
18.2(0.5) |
21.5(0) |
| 500 |
19.8±1.8
|
22.5±2
|
55.1 |
31.7 |
-20/63 |
-10/73 |
19.1(3.5) |
22.2(1.3) |
| 600 |
20.6±1.9
|
23.5±2.1
|
55.8 |
34 |
-20/63 |
-10/73 |
20.1(2.4) |
23.0(2.1) |
| 700 |
21.3±2.1
|
24.4±2.2
|
56.4 |
36.4 |
-20/63 |
-10/71 |
21.2(0.5) |
24.3(0.4) |
| 800 |
21.9±2.2
|
25.1±2.2
|
56.9 |
38.8 |
-20/63 |
-10/71 |
22.3(1.8) |
25.1(0) |
| 900 |
22.5±2.2
|
25.7±2.5
|
57.3 |
41.3 |
-20/64 |
-10/71 |
22.7(0.9) |
25.8(0.4) |
| 1000 |
23.2±2.2
|
26.4±2.3
|
57.8 |
43.9 |
-20/65 |
-10/71 |
23.2(0) |
26.7(1.1) |
| 1100 |
23.6±2.5
|
27.0±2.5
|
58.3 |
46.3 |
-20/66 |
-10/71 |
23.5(0.4) |
27.4(1.5) |
| 1200 |
24.2±2.2
|
27.5±2.6
|
58.7 |
49 |
-20/67 |
-10/73 |
24.0(0.8) |
27.6(0.4) |
the influence of λN is negligible, since λN << x·λTh, (1–x)·λU over the entire temperature range, we excluded it from the calculations.
Table 5 shows that the parameter
h is a constant for some temperature intervals. Therefore, we can describe, for example,
as the sum of the thermal conductivities of its components:
where
h = –10/73 at
T [400, 600]
K and
h = –10/71 at
T (600, 1100]
K.
For zirconium nitride
ZrN, formula (1) has the following form:
where atomic numbers are
Zr = 40,
N = 7. The result of calculations by formula (8) and comparison with the experiment [
1] is given in
Table 6. Since the influence of
λN is negligible, since
λN <<
λZr over the entire temperature range, we excluded it from the calculations.
Table 6 shows that the parameter
h is a constant for some temperature intervals. Therefore, we can describe
λZrN as the sum of the thermal conductivities of its components:
Table 6.
Thermal conductivit
y of
ZrN calculated over formula (8) at different temperatures. Thermal conductivities for pure
Zr are taken from [
8].
are the experimental values for
ZrN taken from [
1], where the data are corrected to 100% TD (total density).
are the values obtained from (8). Relative errors
δ are calculated according to (2). .
Table 6.
Thermal conductivit
y of
ZrN calculated over formula (8) at different temperatures. Thermal conductivities for pure
Zr are taken from [
8].
are the experimental values for
ZrN taken from [
1], where the data are corrected to 100% TD (total density).
are the values obtained from (8). Relative errors
δ are calculated according to (2). .
| T, K |
,W/(m·K)
|
,W/(m·K)
|
h |
,W/(m·K)
|
δ,% |
| 300 |
38.2 |
22.7 |
-5/41 |
37.4 |
2.0 |
| 370 |
41.0 |
21.9 |
-5/39 |
40.9 |
0.2 |
| 470 |
43.0 |
21.2 |
-5/38 |
42.7 |
0.7 |
| 570 |
44.0 |
20.8 |
-5/37 |
45.6 |
3.6 |
| 670 |
46.0 |
20.84 |
-5/37 |
45.7 |
0.6 |
| 770 |
46.0 |
21.4 |
-5/37 |
47.0 |
2.2 |
| 870 |
46.4 |
22.3 |
-4/30 |
46.8 |
0.9 |
| 970 |
49.3 |
23.4 |
-4/30 |
49.1 |
0.4 |
| 1070 |
49.2 |
24.54 |
-3/23 |
48.2 |
2 |
| 1170 |
50.3 |
25.7 |
-3/23 |
50.4 |
0.2 |
| 1270 |
52.5 |
26.7 |
-3/23 |
52.4 |
0.2 |
| 1370 |
52.5 |
27.6 |
-3/23 |
54.2 |
3.2 |
at
T [1070, 1370]
K.