1. Introduction
The upwelling of hypoxic water masses that reach shallow water areas (called ‘Nigashio’ in Mikawa Bay and ‘Aoshio’ in Tokyo Bay, Japan) severely damage fishery and significantly influence the material cycle in the inner bay owing to the collapse of the tidal flat ecosystem (e.g., [
1,
2]). Rokujo tidal flat, located in the inner part of Mikawa Bay, central Japan (
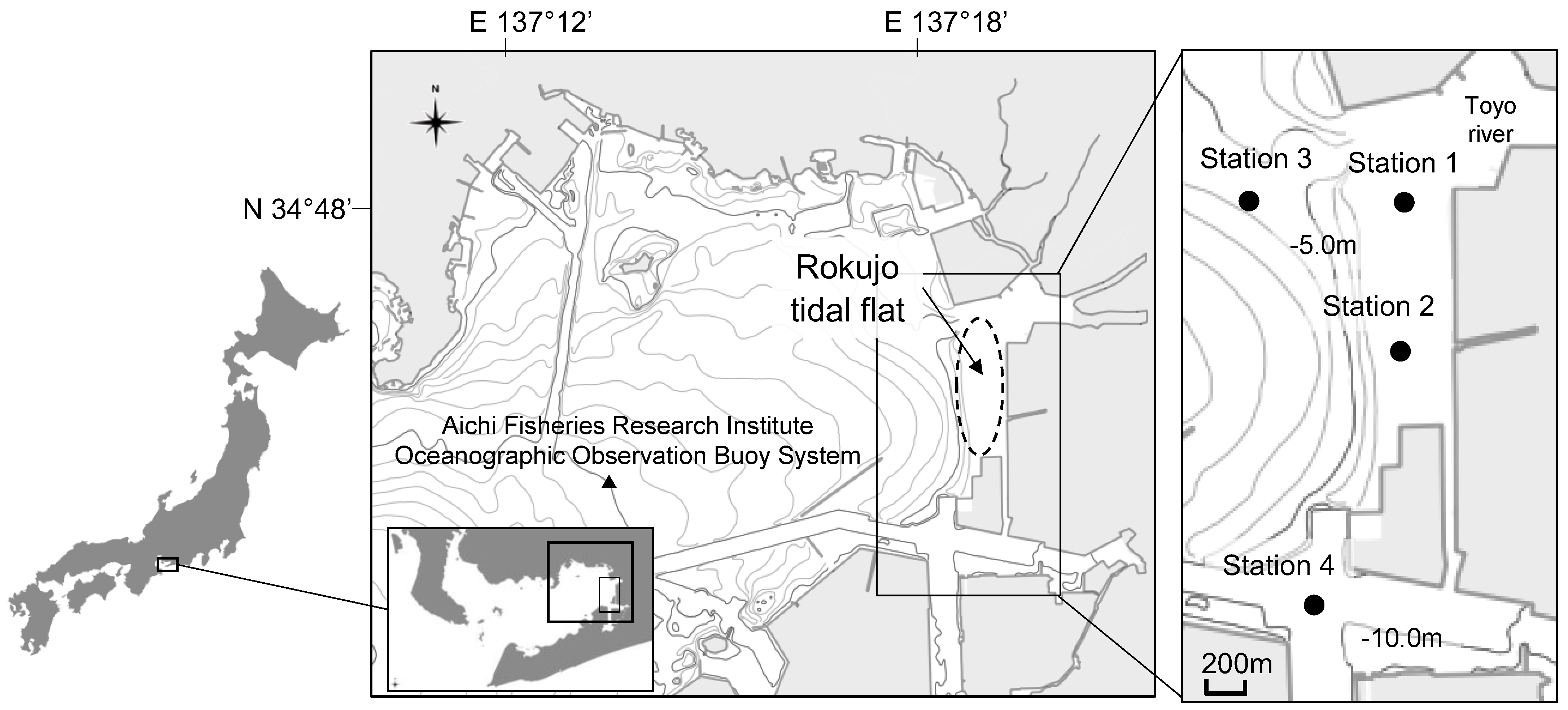
Figure 1), is a sea area with large numbers of juvenile asari clams,
Ruditapes philippinarum [
3]. Clam transplantation from this area contributes to fishery in the surrounding coastal areas. In addition, high-density juvenile asari clams can efficiently remove suspended matter by filter-feeding and play important roles in the material cycle in the bay, including maintaining water quality [
4]. During summer, hypoxic water masses accumulate in offshore areas and in the borrow pit, navigation channels, and anchorage areas [
5,
6,
7]. The upwelling of these masses have caused mass mortality of thousands of tons of asari clams (e.g., [
5]), which is extremely concerning for the fishery economy and the material cycle. The mass mortality of asari clams is thought to be a direct result of exposure to hypoxic water and hydrogen sulfide (H
2S) produced by sulfate reduction with hypoxic upwelling [
8]. However, in laboratory experiments, juvenile asari clams showed no decrease in survival rate in 48 h under anoxic conditions and approximately 50% survival rate in 48 h under 4.2 mg L
-1 hydrogen sulfide, indicating that they are extremely resistant to anoxia and hydrogen sulfide [
9]. Therefore, the upwelling of hypoxic water masses that leads to the death of organisms such as asari clams is not comprehensively understood. The following hypotheses present possible mechanisms underlying the upwelling of anoxic water masses and causing the mortality of asari clams, which are highly tolerant to hypoxia and hydrogen sulfide. The upwelling of hypoxic water masses may trigger the death of organisms with relatively low tolerance to hypoxia or hydrogen sulfide, and the organic matter loads from these organisms may in turn cause the formation of hydrogen sulfide and prolonged anoxia, leading to mass mortality of asari clams on the tidal flat.
Therefore, to elucidate the effects of the upwelling of hypoxic water masses on the tidal flat ecosystem, we conducted biological observations in the Rokujo tidal flat and water quality observations in the surrounding sea area, including the tidal flat. In addition, a simple laboratory experiment using sediments from a tidal flat containing benthic organisms was conducted to examine the possibility of hydrogen sulfide formation in tidal flats.
2. Materials and Methods
2.1. Study Area
The Rokujo tidal flat is a north-south extended estuarine tidal flat on the left bank of the Toyo River mouth, covering an area of 3.06 km
2 ([
10];
Figure 1). The tidal flat terrace extends approximately 1.0 km offshore westward, and the tidal flat edge is -0.7 m below the chart datum level (CDL), with the offshore side dropping to a depth of approximately 6–7 m. The surrounding water area, including the Rokujo tidal flat, is located at the port of Mikawa. The Rokujo tidal flat is a conservation area with high social value for fisheries and the environment; however, natural beaches, such as tidal flats, are scarce owing to coastal development [
11]. In addition, the navigation channels and anchorage areas for large vessels, where the water runs deeper than that of the surrounding area, are located south of the Rokujo tidal flat.
2.2. Macrobenthos Monitoring and Oceanographic Observation
To elucidate the effects of the upwelling of hypoxic water masses on the tidal flat ecosystem, water quality was continually observed along with macrobenthos monitoring at two stations on the tidal flat (Stations 1 (34°46.21′N, 137°18.86′E) and 2 (34°45.37′N, 137°18.88′E)). Macrobenthos in the bottom sediments were collected monthly using a quadrat (25×25 cm) from June to October 2016, and the samples sieved through a 1 mm mesh were used for species identification, followed by counting of individuals and measuring wet weight. The shell lengths of 30 asari clams were measured in each sample. Water temperature and salinity (Infinity-CTW, JFE Advantech Co., Ltd., Kobe, Japan), dissolved oxygen (DO; RINKO-W, JFE Advantech Co., Ltd., Kobe, Japan), and current velocity (Infinity-EM, JFE Advantech Co., Ltd., Kobe, Japan) were observed at 0.3 m above the sea bottom for 10-min intervals from June 15 to October 12, 2016. The wind direction and speed during the observation period were obtained from the oceanographic observation buoy system operated by the Aichi Fisheries Research Institute [
12], and the actual tidal level was obtained from the Mikawa Port Office as a factor affecting the flow environment in the study area. In addition, to monitor water quality around the tidal flat, water temperature, salinity, and DO in the water column were measured once a month from June to October 2016 at Stations 3 (34°46.20′N, 137°18.41′E) and 4 (34°43.82′N, 137°18.29′E) using a water quality sensor (AAQ1182, JFE Advantech Co., Ltd., Kobe, Japan). When DO was below 0.2 mg L
-1, water was collected using a Kitahara water sampler (Rigosha Co. Ltd., Tokyo, Japan), treated with zinc acetate, and taken to the laboratory to determine hydrogen sulfide concentration using the methylene blue method [
13].
2.3. Tidal Flat Sediment Incubation Experiment
Tidal flats with numerous organisms, such as the Rokujo tidal flat, may exhibit high concentrations of hydrogen sulfide with a chain of biological die-off triggered by the upwelling of hypoxic water masses. To investigate this possibility, we conducted a static incubation experiment in tidal flat sediments containing macrobenthos. The sediments were collected on August 18, 2016 at Station 1 using acrylic cores with an inner diameter of 44 mm and incubated at 28 °C, which is water temperature in tidal flats during summer, after sealing the acrylic cores. The DO of the water directly above the sediment was measured using an optical DO meter (ProODO, YSI Inc., Yellow Springs, Ohio, USA), and the hydrogen sulfide concentration was determined using the methylene blue method [
13] on days 3, 4, 6, and 20 of incubation.
3. Results
3.1. Macrobenthos Monitoring and Oceanographic Observation
3.1.1. Macrobenthos Monitoring
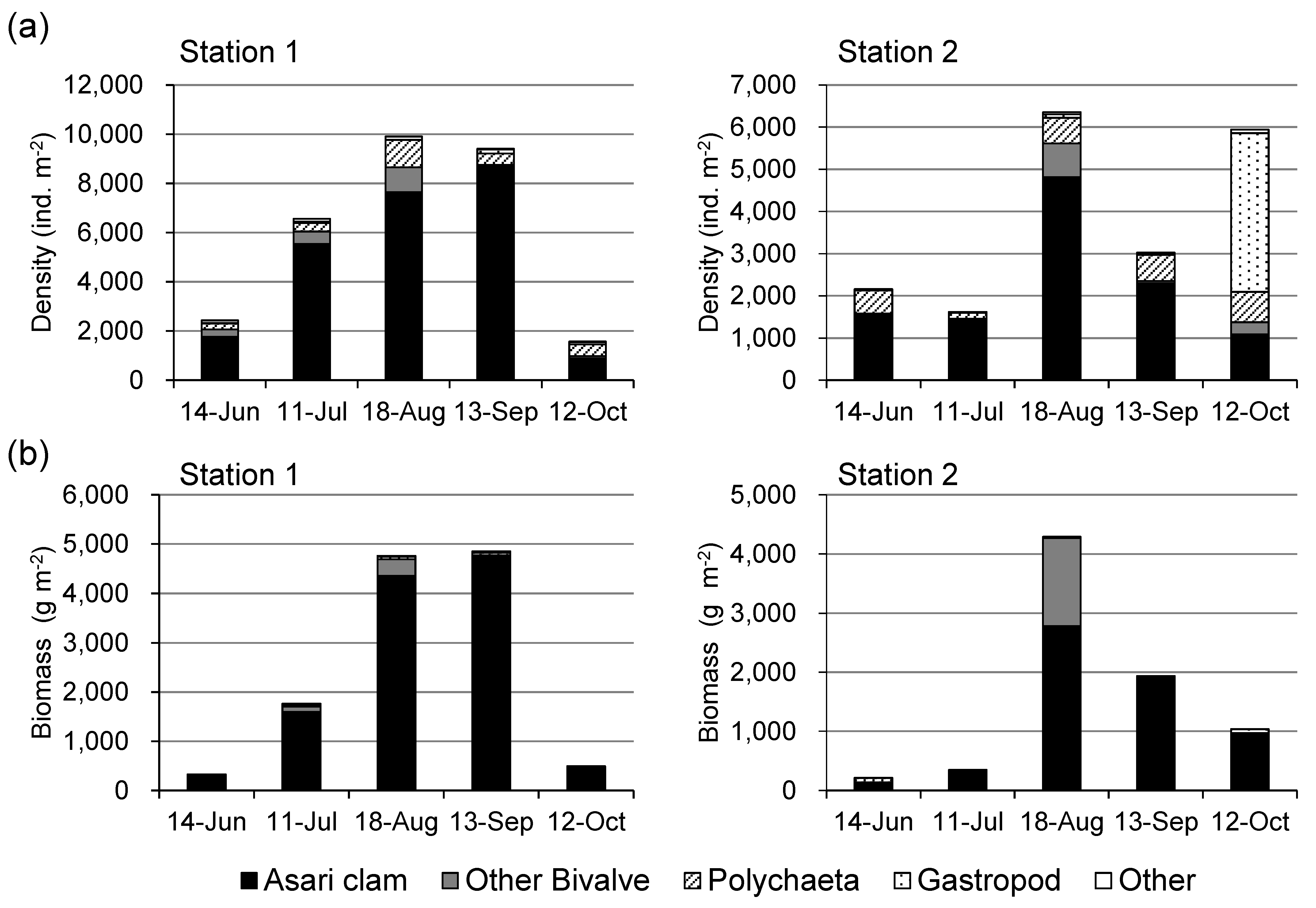
Figure 2 shows the monthly changes in the number and biomass of macrobenthos at Stations 1 and 2 in the tidal flat. The number and biomass of asari clams were predominant at both stations. At Station 1, both the number and biomass of macrobenthos increased until August, leveled off in September, and declined sharply in October. At Station 2, both the number and biomass of macrobenthos increased considerably in August, but declined in September. In October, both the number and biomass of asari clams decreased further, whereas the number of gastropods increased at Station 2.
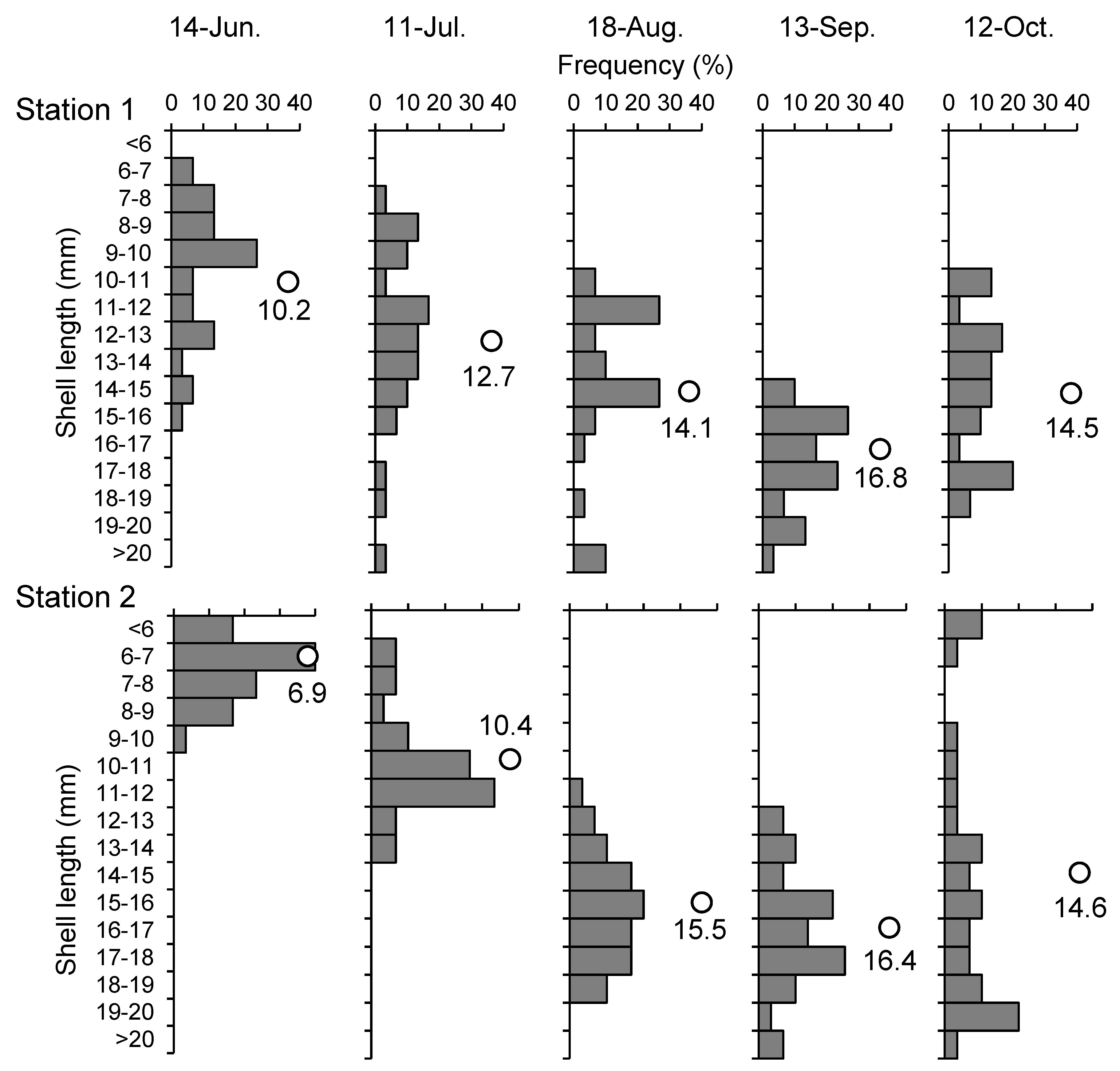
Figure 3 shows the composition and average length of the asari clam shells at the respective stations. Stations 1 and 2 comprised asari clams that were 6–16 mm and <10 mm long, respectively, and no large asari clams were observed in June. The average shell length was smaller at Station 2 than at Station 1 in June and July, but was the same in August and subsequent months.
3.1.2. Continuous Observation of Water Quality and Flow on the Tidal Flat
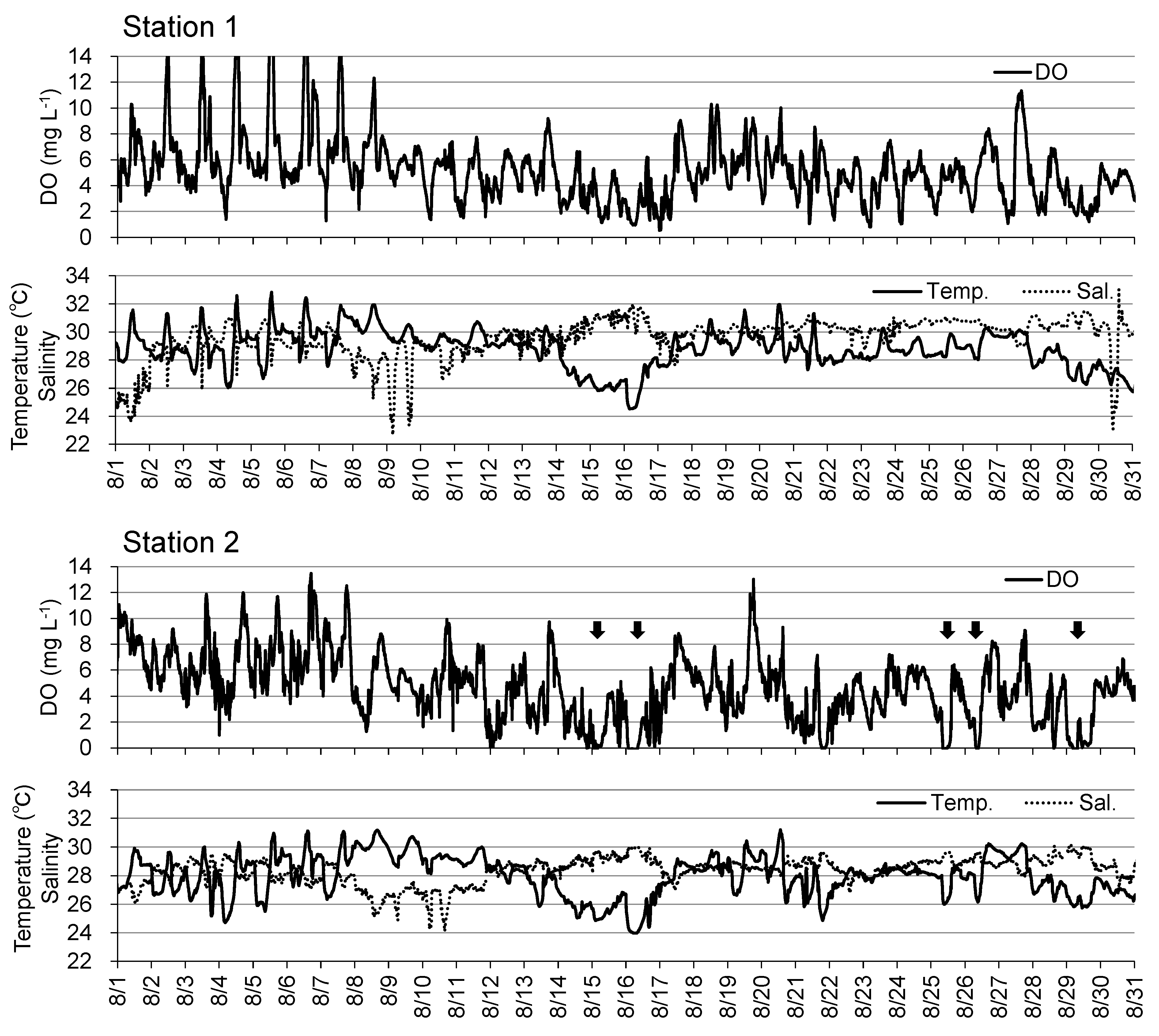
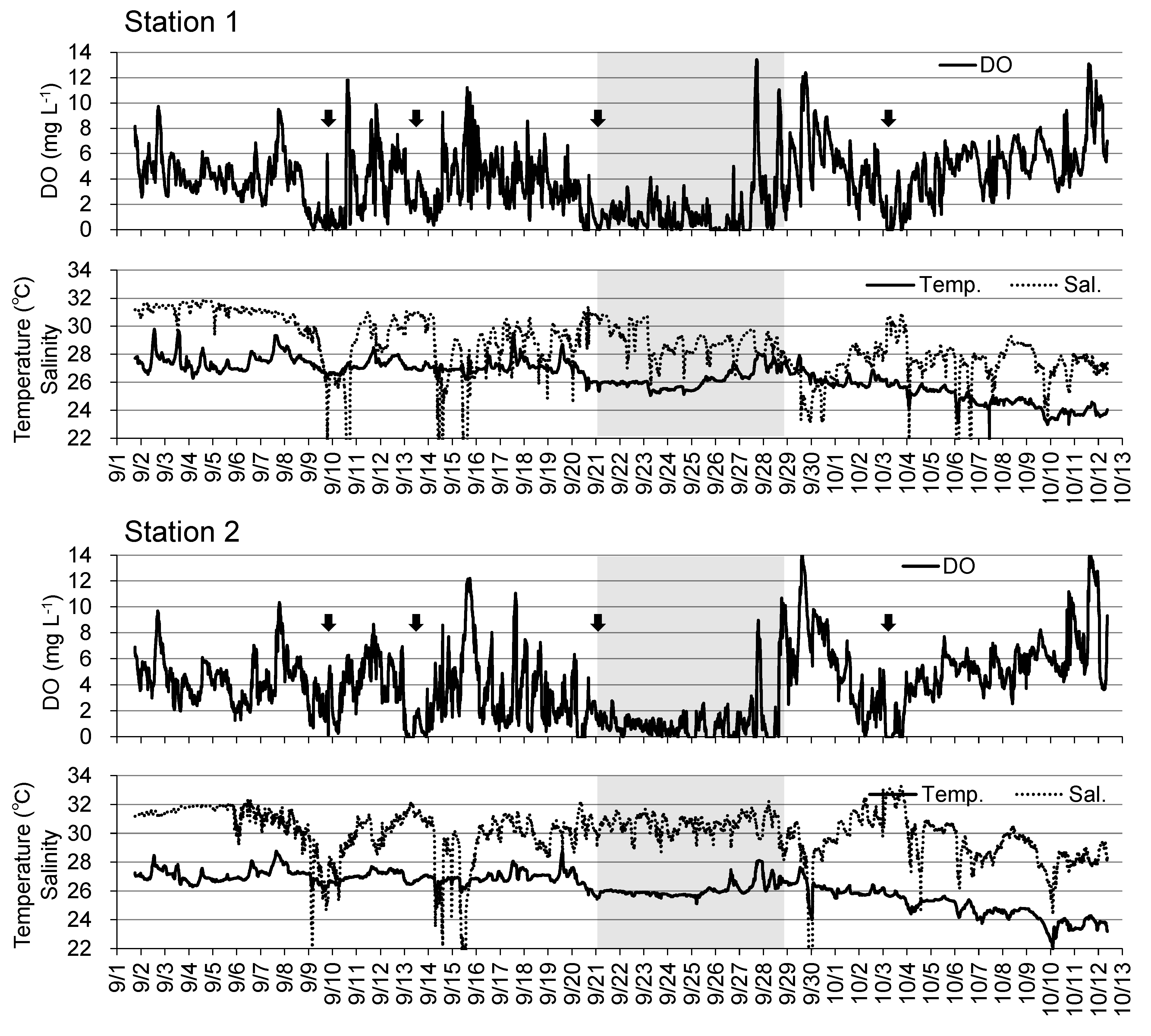
Figure 4 shows the results of continuous observations of DO, temperature, salinity, and flow at Stations 1 and 2 on the tidal flat since August, a period that includes macrobenthos reduction. At Station 1, DO below 3 mg L
-1 was observed several times in August, but there were no instances of 0 mg L
-1 DO. At Station 2, DO occasionally dropped to approximately 0 mg L
-1 during August (e.g., August 15, 16, 25, 26, and 29). Since September, instances of severe DO reduction were observed at both stations on September 9, 13, and 21, and October 3. In particular, the hypoxic period from September 21 lasted approximately one week. Low water temperature and high salinity were observed almost every time there was a considerable drop in DO at both stations throughout the observation period.
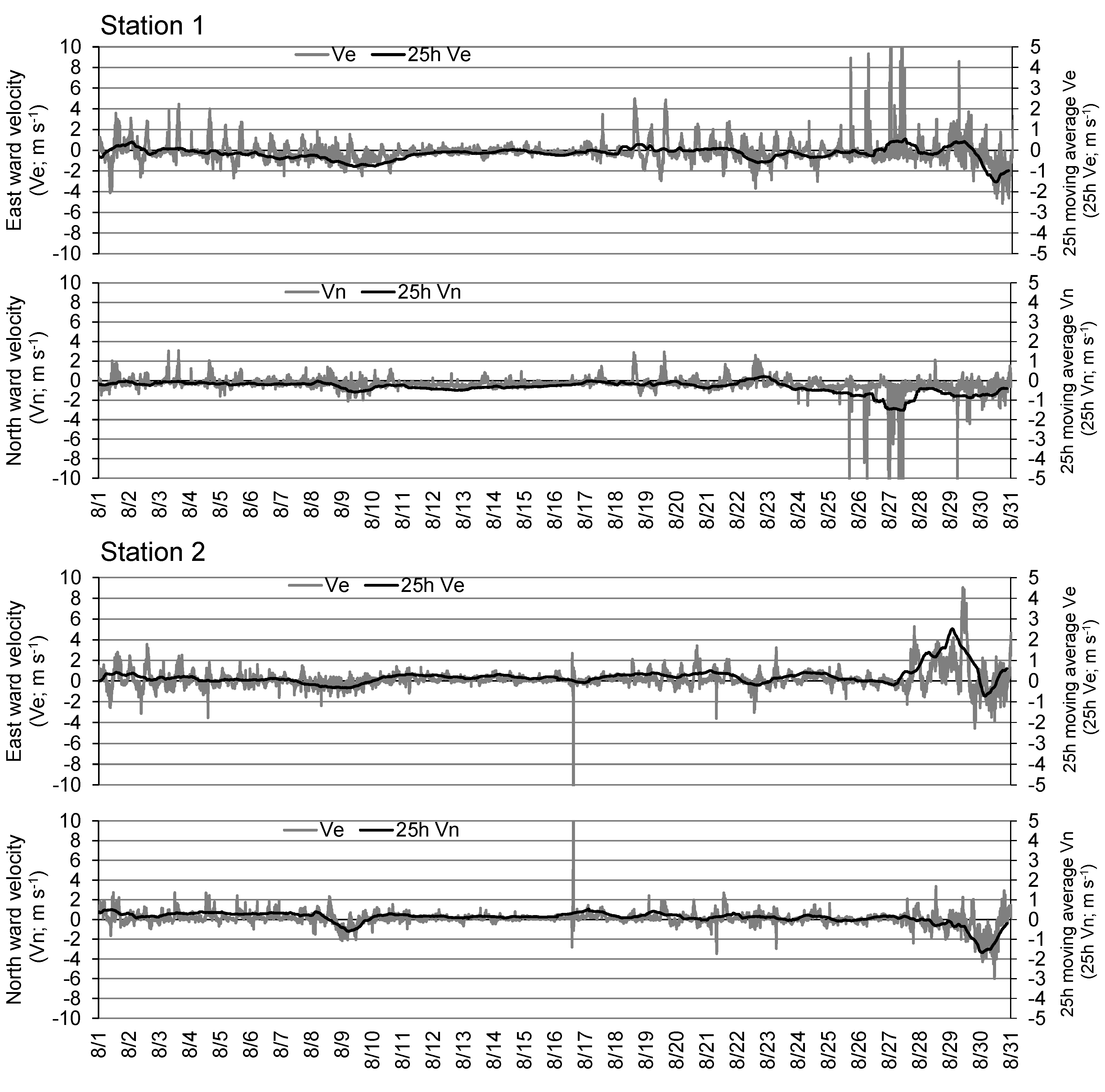
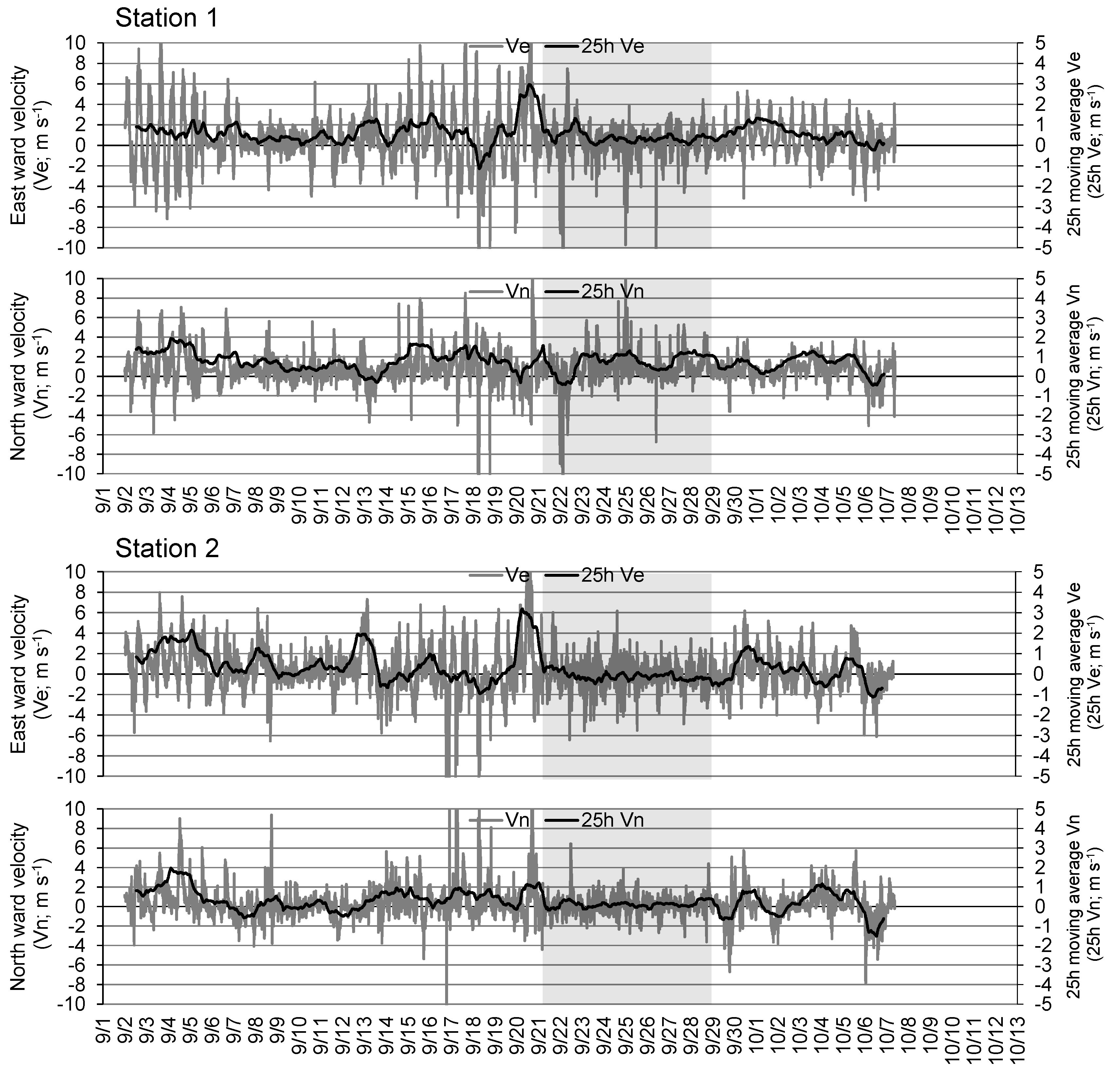
Figure 5 shows the east-west and north-south components of the current recorded at 10-min intervals, and their 25-h moving averages at the respective stations.
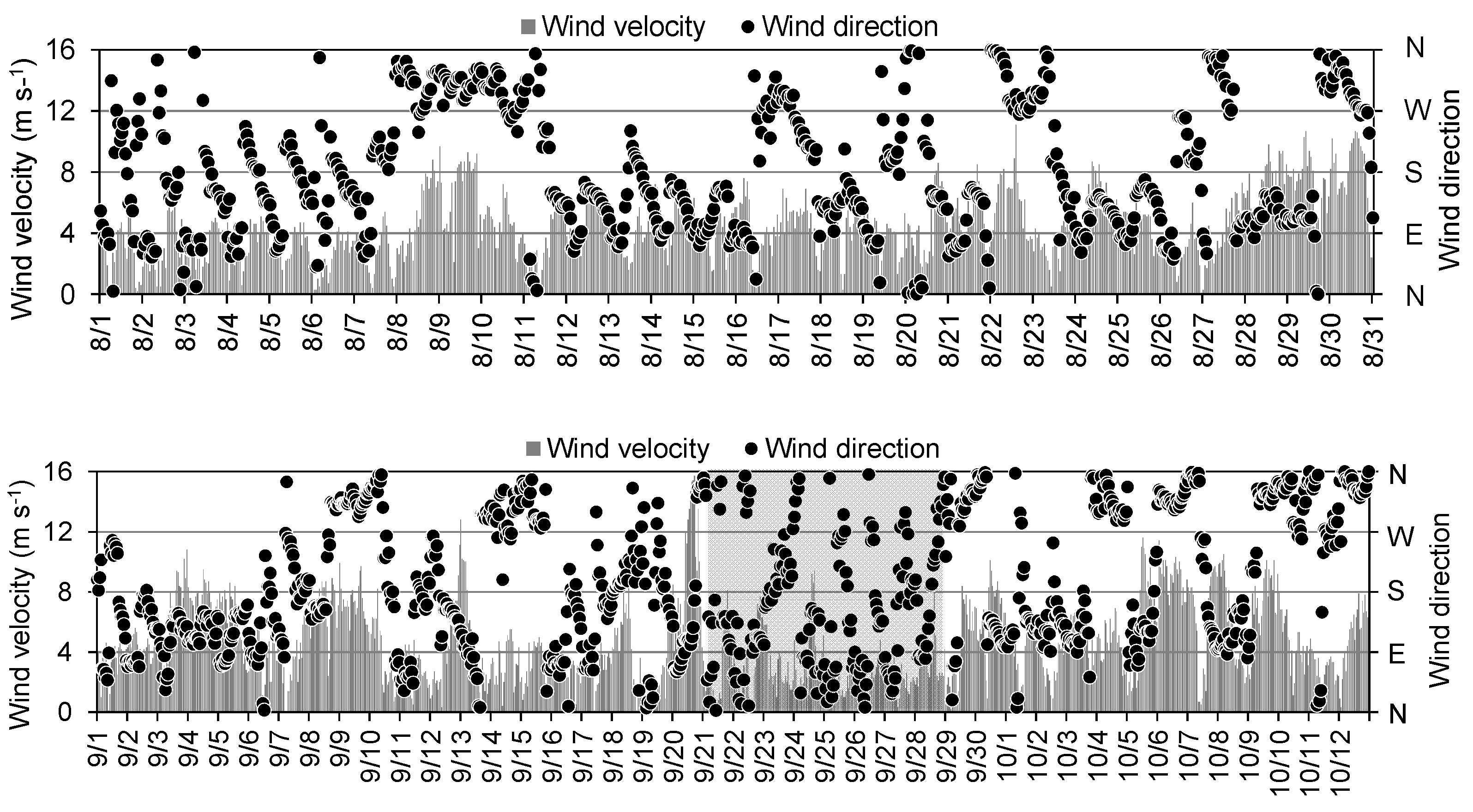
Figure 6 and
Figure 7 show the wind direction and speed at the oceanographic observation buoy system and the measured tide level in the Port of Mikawa, respectively. In the subsequent sections, we describe certain observation cases focusing on the flow environment before and after the reduction in DO. For example, on August 29, when DO decreased at Station 2 (
Figure 4), easterly winds of approximately 8 m s
-1 continued from the previous day (
Figure 6), and relatively strong eastward flow velocities were observed in the bottom layer of Station 2 (
Figure 5). On September 13, when DO decreased at both stations (
Figure 4), easterly winds of more than 10 m s
-1 blew from the previous day (
Figure 6), and an increase was observed in eastward flow velocity of the bottom layer at both stations (
Figure 5). From September 21 to 28, the hypoxic period continued at both stations (
Figure 4). Strong winds exceeding 15 m s
-1 were observed (
Figure 6), and the eastward flow velocity in the bottom layer increased temporarily at both stations on September 20 (
Figure 5), when the DO declined rapidly. Thereafter, a period of low wind velocity continued for approximately a week (
Figure 6), and at the beginning of the neap tide (
Figure 7), flow velocities remained relatively low until approximately September 28 (
Figure 5).
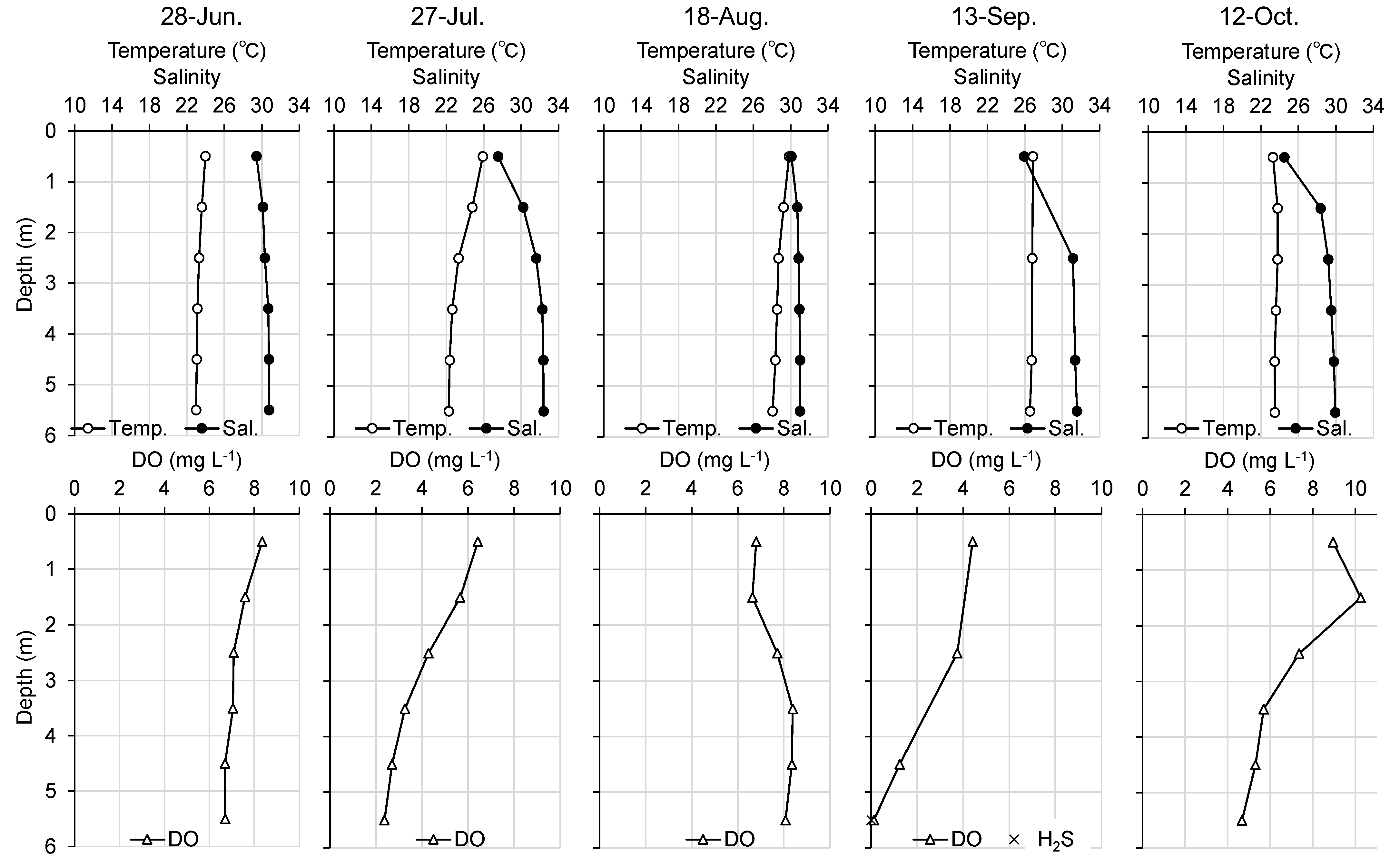
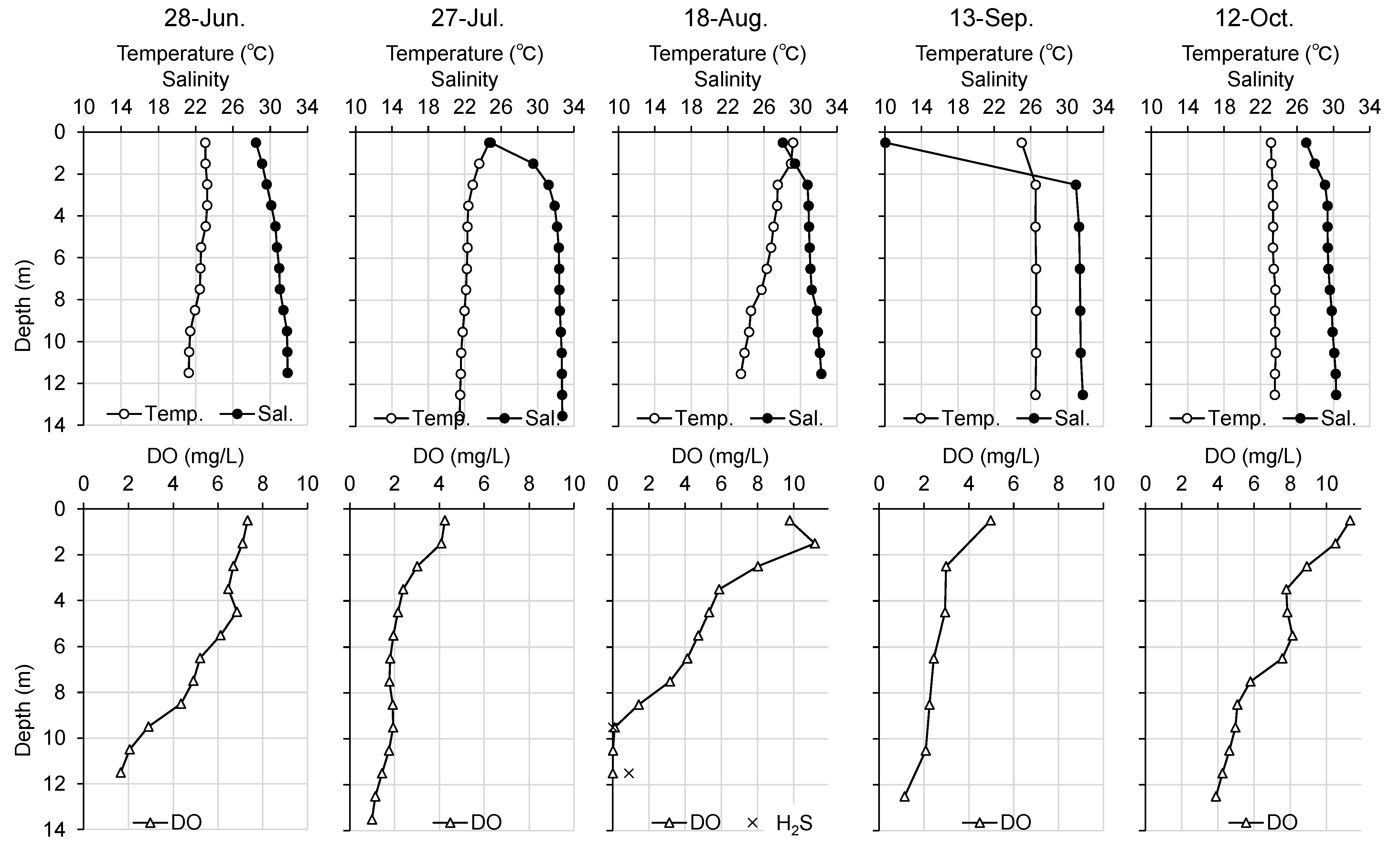
3.1.3. Water Quality Observation outside the Tidal Flat
Figure 8 and
Figure 9 show the vertical profiles of water temperature, salinity, and DO at Station 3, a station offshore the tidal flat, and Station 4, a station in the navigation channel in the port, respectively. At Station 3, both the water temperature and salinity were vertically homogeneous in June, no obvious stratification was observed, and DO in the bottom layer was adequate. After July, stratification tended toward high water temperature and low salinity in the upper layer, and low water temperature and high salinity in the lower layer. The DO in the bottom layer was below 3 mg L
-1 in July, and the oxygen environment temporarily recovered in August. In September, however, the bottom layer DO decreased to 0.12 mg L
-1 and no hydrogen sulfides were detected. In October, the oxygen environment recovered. At Station 4, no obvious stratification was observed in June as was the case at Station 3; however, DO was below 3 mg L
-1 at depths below 10 m. In July, stratification occurred, and DO decreased throughout the water column. In August, DO was 0 mg L
-1 at depths below 9 m, and hydrogen sulfide was detected at 0.9 mg L
-1 in the deepest layer. The oxygen environment gradually improved from September onward.
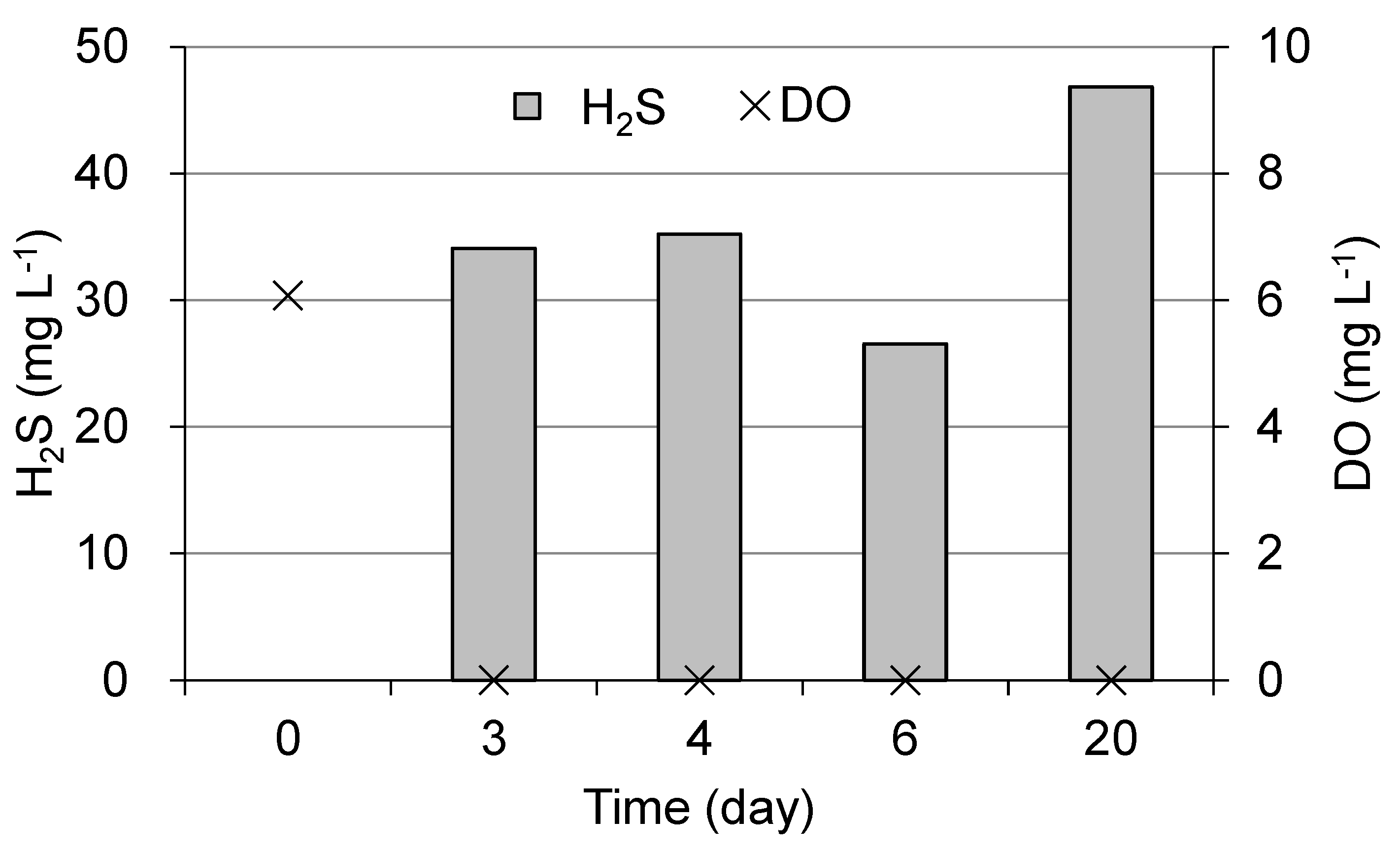
3.2. Tidal Flat Sediment Incubation Experiment
Figure 10 shows the DO and hydrogen sulfide concentrations in the water directly above the sediment during the incubation experiment. On the third day of incubation, the water directly above the sediment became anoxic, and the hydrogen sulfide concentration reached approximately 30 mg L
-1, after which it remained almost unchanged.
4. Discussion
4.1. Influence of Occasional Hypoxia on Macrobenthos in the Tidal Flat
The number and biomass of macrobenthos in tidal flats increased from June to August at Stations 1 and 2 (
Figure 2). These results were caused by the growth of juvenile asari clams, the dominant species in tidal flats, as shown in
Figure 2 and
Figure 3. From August to September, the macrobenthos number and biomass decreased at Station 2, but remained at Station 1 (
Figure 2). Hypoxia was observed from June, and the bottom layer became anoxic and hydrogen sulfides were detected in August at Station 4, relatively near Station 2, and located in the navigation channel (
Figure 9). The results of the continuous observation of the tidal flat showed that hypoxia was occasionally observed at Station 2, but no intensive hypoxia was observed at Station 1 in August (
Figure 4). Temporal hypoxia gradually decreases macrobenthos density, as in asari clams (e.g., [
1,
14]). The reduction in the number and biomass of macrobenthos at Station 2 from August to September was attributed to occasional hypoxia. Previous studies showed that the development of hypoxia in the bottom layer occurs earlier in the navigation channel than in the bay-wide area [
6,
7]. Therefore, the influence of hypoxia occurred earlier and was greater in the southern part of the tidal flat near the navigation channel than in the northern part of the tidal flat.
4.2. Influence of Continuous Hypoxia on Macrobenthos in the Tidal Flat
The number and biomass of asari clams decreased from September to October at Stations 1 and 2. Anoxia was observed in the bottom layer at Station 3, located offshore the tidal flat, and at Station 4 in September. Hypoxia was intermittently observed at both stations in September. In particular, hypoxia continued for approximately a week, from September 21. Wind and tidal current observations indicate that the bottom water flowed eastward when easterly winds blew hard on August 28, September 13, and September 20 (
Figure 5 and
Figure 6). These results suggest that the current direction in the bottom layer of the tidal flat was opposite that in the surface layer, and that hypoxic water masses with low temperature and high salinity in the offshore bottom layer were upwelling onto the tidal flat. Typhoon 16 caused strong winds on September 20 [
15]. We expected that the mass mortality of macrobenthos from September to October at both stations would be triggered by the extreme hypoxia observed in September (e.g., September 21). Moreover, the continuation of hypoxia after September 21 was considered a concern in the calm weather and stagnation of tidal currents owing to the neap tide.
4.3. Factors Affecting the Mass Mortality of Asari Clams
Juvenile asari clams are tolerant to hypoxia and hydrogen sulfide [
9]. Laboratory experiments at 24 °C, close to the in situ water temperature at the time of hypoxic water mass upwelling, showed that high concentrations of hydrogen sulfide (≥13.5 mg L
-1) resulted in reduced survival after 24 h but relatively low concentrations of hydrogen sulfide (2.8–6.4 mg L
-1) resulted in no decrease in survival after exposure for 24 h and a decrease in survival after exposure for 48 h [
9]. Therefore, the mortality of juvenile asari clams due to upwelling of hypoxic water masses may either require high concentrations of hydrogen sulfide or long-term exposure to ≥2 mg L
-1 hydrogen sulfide according to previous studies. However, explaining the mass mortality of juvenile asari clams caused by temporary upwelling of hypoxic water masses is difficult because no hydrogen sulfide was detected at Station 3, offshore of the tidal flat, and less than 1 mg L
-1 hydrogen sulfide was detected at Station 4, the navigation channel in the port.
The incubation experiments of tidal flat sediments containing macrobenthos resulted in hydrogen sulfides exceeding 30 mg L
-1 after 3 days of incubation (
Figure 10). The hydrogen sulfide release from the sediment was estimated to be at least 570 mg m
-2 day
-1 in this laboratory experiment, assuming that the sulfide concentration in the water was saturated on the third day.
Table 1 shows the results of sediment incubation in the present study compared with the previous study, the incubation experiment of sediment in the navigation channel near Station 4. The release rate of the previous experiment, using sediment severely polluted with organic matter, was approximately 390 mg m
-2 day
-1 [
16], which was lower than the result of the present experiment in tidal flat sediment. Because sediment organic matter (SOM: total organic matter + biological organic matter) at Station 1 on the tidal flat was higher than that at Station 4 in the navigation channel (
Table 1), tidal flat sediments are considered to have a potential release of highly concentrated hydrogen sulfide depending on environmental degradation.
These findings may indicate the following scenario for the mass mortality of asari clams in the Rokujo tidal flat triggered by upwelling hypoxia. First, upwelling hypoxia with or without hydrogen sulfide in tidal flats, caused by strong easterly winds, results in the death of species with relatively weak tolerance to hypoxia. For example,
Musculista senhousia and
Phacosoma japonicum are considered to have lower tolerance to hypoxia than asari clams [
17], and those living in the Rokujo tidal flat are quickly killed by upwelling hypoxia. In fact, we observed the death of
P. japonicum despite the presence of live asari clams associated with temporary hypoxia on the tidal flat in the past. Then, hydrogen sulfide is produced from these biogenic organic loads under anoxic conditions, mainly subsurface in the sediments, and under mild meteorological and hydrographic conditions, such as slight winds and neap tides. This results in a chain of biological die-offs in the tidal flat. Moreover, accumulated hydrogen sulfide in the tidal flat leads to the death of asari clams with relatively high tolerance to hypoxia and hydrogen sulfide and prolongs the period of hypoxic conditions. These chain reactions may cause mass mortality of asari clams. Sugahara et al. [
18] reported that a relatively high concentration of hydrogen sulfide was detected after the death of an individual, despite hypoxic conditions with no hydrogen sulfide, in an experiment using the bivalve
Corbicula japonica. In this study, sulfate-reducing bacteria produce hydrogen sulfide from organic acids in the bodies of
C. japonica and are released from the inside of the shell to the environment upon their death. This indicates that dead bivalves become the substrate for sulfate reduction, thus supporting our hypothesis for the mass mortality scenario of asari clams. The shallow area, including tidal flats, plays the role of purification around coastal areas via their ecosystem function (e.g., [
19]), but immediately turns from a sink to a source of nutrient load following the occurrence of hypoxia [
1,
20]. Moreover, the results of the present study suggest that the tidal flat ecosystem is a potential source of biogenic organic loads and hydrogen sulfide released by the chain of biological die-off triggered by upwelling hypoxia.
5. Conclusions
The study results suggest that the mass mortality of asari clams may be caused by the high concentration of hydrogen sulfide. This high concentration is attributed to sulfate reduction following the chain of biological die-off in the tidal flat caused by the upwelling of hypoxic water mass accompanied by meteorological events and calm conditions such as the neap tide period. However, to test this hypothesis, several issues have to be addressed. The sediment incubation experiment in this study did not reveal the detailed processes of hypoxia and hydrogen sulfide formation. In future, an experimental system that considers the biogenic organic load will be necessary. Another important issue is the in situ measurement of hydrogen sulfide concentrations in tidal flats during the upwelling of hypoxic water masses. The dynamics of hydrogen sulfide in the field during upwelling hypoxia need to be elucidated by observing the related enhancement. In addition, it is important to build a numerical model to simulate the chain of biological die-off, hydrogen sulfide release, and massive oxygen consumption in tidal flats triggered by upwelling hypoxia.
Author Contributions
R. S. designed the study, measured environmental factors, sampled macrobenthos, conducted experiments, and drafted the article. D. M. measured environmental factors and macrobenthic sampling T. I. measured environmental factors and sampled macrobenthos. S. K. designed the Study and gave final approval of the article. M. W. designed the study and drafted the manuscript. T. I. drafted the manuscript and provided final approval of the same. T. S. designed the study and provided final approval of the manuscript. All the authors have read and agreed to publish this version of the manuscript.
Funding
This study was supported by the Environment Research and Technology Development Fund of the Ministry of Environment, Japan (5-1404, principal investigator Prof. Yoshiyuki Nakamura).
Acknowledgments
We are thankful to Dr. Shogo Sugahara of Shimane University for his help and expertise with the analytical methods for hydrogen sulfide. We also appreciate the members of the Fisheries Environment Research Department at the Aichi Fisheries Research Institute.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Suzuki, T. Oxygen-deficient waters along the Japanese Coast and their effects upon the estuarine ecosystem. J. Environ. Qual 2001, 30, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Diaz, R.J.; Rosenberg, R. Spreading dead zones and consequences for marine ecosystems. Science 2008, 321, 926–929. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Agriculture, Forestry and Fisheries of Japan. White paper on fisheries 2014. Available online: https://www.jfa.maff.go.jp/j/kikaku/wpaper/h24/attach/pdf/09_3shou3setu.pdf (accessed on 4 February 2023).
- Sone, R.; Kamohara, S.; Yamada, S.; Suzuki, T.; Takabe, T. Water purification in the river mouth tidal flat with an abundant supply of the Manila clam Ruditapes philippinarum: A model study in June in Rokujo tidal flat, at the mouth of the Toyo River in Mikawa Bay, Japan. Bull. Jpn. Soc. Fish. Oceanogr 2015, 79, 117–129, (In Japanese, with English abstract). [Google Scholar]
- Takeda, K.; Ishida, M. Mass mortality of the short-necked clam, Ruditapes Philippinarum, caused by a niga-shio (bitter tide)in Mikawa Bay and dissolved oxygen deficiency in a borrow pit. J. Adv. Mar. Sci. Tech. Soc 2006, 12, 51–58. [Google Scholar]
- Waku, M.; Mukai, R.; Kamohara, S.; Honda, Y.; Tkabe, T. ; Mechanisms of environmental degradation in dead zones in Mikawa Bay, Japan. Bull. Aichi Fish. Res. Inst 2013, 18, 1–11. [Google Scholar]
- Waku, M.; Sone, R.; Inoue, T.; Ishida, T.; Suzuki, T.; Space-time distribution characteristics and interrelationship of sulfur and iron compounds in sediments: A model study in closed-section of Mikawa Bay, Japan. (submitted to Water).
- Kakino, J. Mortality of Manila clam caused by blue tide; effects of hypoxic water and sulfides. Bull. Chiba Pref. Fish. Exp 1982, 40, 1–6. [Google Scholar]
- Kodama, K.; Waku, M.; Sone, R.; Miyawaki, D.; Ishida, T.; Akatsuka, T.; Horiguchi, T. Ontogenetic and temperature-dependent changes in tolerance to hypoxia and hydrogen sulfide during the early life stages of the Manila clam Ruditapes philippinarum. Mar. Environ. Res 2018, 137, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Nature Conservation Bureau, Environment Agency. The Report of the Coastline Survey in the 4th National Survey on the Natural Environment. Nature Conservation Bureau, Environment Agency: Tokyo, Japan, 1994.
- Mikawa Port Office,Chubu Regional Development Bureau, Ministry of Land, Infrastructure, Transport and Tourism. Sea blue project. Available online: https://www.mikawa.pa.cbr.mlit.go.jp/gaiyou/jigyou/seablue/ (accessed on 4 February 2023).
- Aichi fisheries research institute oceanographic observation buoy systems. Available online: http://suisanshiken-buoy.jp/top/index.html (accessed on 4 February 2023).
- Cline, J.D. Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol. Oceanogr 1969, 14, 454–458. [Google Scholar] [CrossRef]
- Kamohara, S.; Yamada, S.; Waku, M.; Sone, R.; Iwata, Y. Movement of the early juvenile of Japanese littleneck clam Ruditapes philippinarum in Rokujo tidal flat of Mikawa Bay. Bull. Aichi Fish. Res. Inst. 2013, 18, 13–20. [Google Scholar]
- Japan meteorological agency. Tropical cyclone tracks. Available online: https://www.data.jma.go.jp/fcd/yoho/typhoon/route_map/bstv2016.html (accessed on 4 February 2023).
- Inoue, T.; Hagino, Y. Effects of three iron material treatments on hydrogen sulfide release from anoxic sediments. Water Sci. Technol 2022, 85, 305–318. [Google Scholar] [CrossRef]
- Hashiguchi, S.; Imao, K.; Kaneko, K.; Miyamukai, T.; Mori, A.; Toyohara, T.; Waku, M.; Ishida, M.; Suzuki, T. Quantification of a macrobenthic community’s response to hypoxia around a borrow pit in the inner part of Mikawa Bay, Japan. Fish. Eng 2012, 49, 1–12. [Google Scholar]
- Sugahara, S.; Suzuki, M.; Yamamuro, M.; Semura, H.; Kamiya, H.; Senga, Y.; Noda, K.; Egawa, M.; Seike, Y. Sulfide tolerance of Corbicula japonica by the difference in shell size. Aquacult. Sci 2017, 65, 83–87. [Google Scholar]
- Matsukawa, Y.; Sasaki, K. Budgets of nitrogen, phosphorus and suspended solid in an intertidal flat. Bull. Japan. Soc. Sci. Fish 1986, 52, 1791–1797. [Google Scholar] [CrossRef]
- Waku, M.; Hata, K.; Kaneko, K.; Suzuki, T.; Takabe, T. Adverse effects of dead zones in the coastal waters on the bay-wide material cycle and its remedial measures-An analyze with the ecosystem model in Mikawa Bay, Japan-. J. Adv. Mar. Sci. Tech. Soc 2013, 19, 15–27. [Google Scholar]
- Nakata, K.; Hata, K. Evaluation of the cleansing function of coastal tidal flats. J. Soc. Water Environ 1994, 17, 158–166. (in Japanese). [Google Scholar]
Figure 1.
Location of biological and oceanographic observation stations in Mikawa Bay, central Japan. Macrobenthos monitoring and continuous observations of water quality were conducted at Stations 1 and 2. Periodic observations of water quality were conducted at Stations 3 and 4.
Figure 1.
Location of biological and oceanographic observation stations in Mikawa Bay, central Japan. Macrobenthos monitoring and continuous observations of water quality were conducted at Stations 1 and 2. Periodic observations of water quality were conducted at Stations 3 and 4.
Figure 2.
Monthly changes in the number and biomass of macrobenthos from June to October, 2016 at Stations 1 and 2.
Figure 2.
Monthly changes in the number and biomass of macrobenthos from June to October, 2016 at Stations 1 and 2.
Figure 3.
Monthly changes of shell length composition (gray bars) and average shell length (open circles with numerical values) of asari clams from June to October, 2016 at Sts.1 and 2.
Figure 3.
Monthly changes of shell length composition (gray bars) and average shell length (open circles with numerical values) of asari clams from June to October, 2016 at Sts.1 and 2.
Figure 4.
Time–series of dissolved oxygen (DO), temperature and salinity at Stations 1 and 2 from August to October, 2016. Arrows indicate the large decline in DO. Gray hatch indicates the period of severe hypoxia.
Figure 4.
Time–series of dissolved oxygen (DO), temperature and salinity at Stations 1 and 2 from August to October, 2016. Arrows indicate the large decline in DO. Gray hatch indicates the period of severe hypoxia.
Figure 5.
Time-series of east-west and north-south component velocity of bottom current at Stations 1 and 2 from August to October, 2016. Gray hatch indicates the period of severe hypoxia in Stations 1 and 2.
Figure 5.
Time-series of east-west and north-south component velocity of bottom current at Stations 1 and 2 from August to October, 2016. Gray hatch indicates the period of severe hypoxia in Stations 1 and 2.
Figure 6.
Time-series of wind velocity and direction from the oceanographic observation buoy system operated by Aichi Fisheries Research Institute from August to October, 2016. Gray hatch indicates the period of severe hypoxia in Stations 1 and 2.
Figure 6.
Time-series of wind velocity and direction from the oceanographic observation buoy system operated by Aichi Fisheries Research Institute from August to October, 2016. Gray hatch indicates the period of severe hypoxia in Stations 1 and 2.
Figure 7.
Time-series of tidal level at Mikawa Port from August to October, 2016. Gray hatch indicates the period of severe hypoxia in Stations 1 and 2.
Figure 7.
Time-series of tidal level at Mikawa Port from August to October, 2016. Gray hatch indicates the period of severe hypoxia in Stations 1 and 2.
Figure 8.
Monthly changes of vertical profile of temperature, salinity, dissolved oxygen (DO), and hydrogen sulfide (H2S) in water column in Station 3 from June to October, 2016.
Figure 8.
Monthly changes of vertical profile of temperature, salinity, dissolved oxygen (DO), and hydrogen sulfide (H2S) in water column in Station 3 from June to October, 2016.
Figure 9.
Monthly changes in the vertical profile of temperature, salinity, dissolved oxygen (DO), and hydrogen sulfide (H2S) in water column at Station 4 from June to October, 2016.
Figure 9.
Monthly changes in the vertical profile of temperature, salinity, dissolved oxygen (DO), and hydrogen sulfide (H2S) in water column at Station 4 from June to October, 2016.
Figure 10.
Changes in dissolved oxygen (DO) and hydrogen sulfide (H2S) concentration of the water directly above the sediment incubated at 28 °C.
Figure 10.
Changes in dissolved oxygen (DO) and hydrogen sulfide (H2S) concentration of the water directly above the sediment incubated at 28 °C.
Table 1.
Flux of hydrogen sulfide, biological organic matter, and sedimentary organic matter in the sediments on the tidal flat and the navigation channel.
Table 1.
Flux of hydrogen sulfide, biological organic matter, and sedimentary organic matter in the sediments on the tidal flat and the navigation channel.
| Sediment collection site |
Hydrogen sulfide release
(mg m-2 day-1) |
Total organic matter
(TOC; g-C m-2) |
Biological organic matter
(Macrobenthos biomass; g-C m-2) |
Tidal flat
(Station 1) |
567.7 |
30.6 *2
|
93.2 *3
|
Navigation channel
(near Station 4) |
387.5 *1
|
76.8 *2
|
- |
| *1: Average value of reference core in Inoue and Hagino [16] |
| *2: Sone et al., unpublished data |
| *3: Converted from wet weight to carbon weight in reference to Sone et al. [4] and Nakata and Hata [21] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).