1. Introduction
With the rise of catheter-based procedures, the treatment of valvular heart disease has experienced profound changes during the past twenty years [
1]. In 2002 Alain Cribier reported the first successful transcatheter aortic valve implantation in a human and in 2008, Walther, Kempfert and colleagues described the principle of transcatheter valve-in-valve therapies [
2,
3,
4]. In 2009 Cheung and colleagues adopted the valve-in-valve concept to the mitral valve and in 2012 and 2013 the Dresden-group added early experiences with mitral valve-in-valve and valve-in-ring procedures [
5,
6,
7]. Despite that re-operative mitral valve surgery has been proven to be – at least in experienced hands - as safe as primary mitral surgery, catheter-based alternatives have some promising aspects, especially in high-risk or inoperable subpopulations [
5,
6,
8]. The early experiences confirmed short procedure times of a straightforward and less invasive catheter-based treatment strategy, but anyhow stated that nothing is known about durability or long-term outcomes [
5,
6]. Basically, this has not changed during the past decade. Despite the international and multicentric valve-in-valve registry (VIVID) which was set up by Danny Dvir confirming favorable immediate outcomes, still only little is known beyond one year of follow-up [
9,
10,
11,
12].
This gap in knowledge needs to be closed. Mitral valve disease is one of the most common valvular heart diseases and it is estimated that mitral regurgitation is the second most prevalent disease after aortic stenosis in adult patients with severe VHD [
13]. Of course, the current gold standard of treatment is surgical repair or replacement [
1]. But as life expectancy increases, we can expect a rise in the need for mitral valve reinterventions in failed repairs or degenerated prostheses.
For an adequate and evidence based decision-making process, it is unavoidable to gather more data – especially beyond the well described immediate and 1-year success. The present study sought to close the long-term data gap by adding a nearly 10-years follow-up of patients undergoing transcatheter mitral valve-in-valve or valve-in-ring procedures.
2. Patients and Methods
Study Design, Inclusion and Exclusion Criteria
The study is designed as a retrospective data analysis. The study population consisted of all consecutive patients who underwent a transcatheter mitral valve-in-valve or valve-in-ring procedure at our facility between September 2011 and December 2021 (n=54).
Accordingly, main inclusion criteria were presence of a failed biological mitral valve or recurrent mitral regurgitation after mitral repair as well as a Heart Team decision for a catheter-based procedure. This decision regularly was based on high surgical risk according to calculated, comorbidities or frailty in accordance to the past and present guidelines [
1].
Exclusion criteria were active endocarditis, emergent or salvage surgery. Concomitant procedures other than the mitral valve surgery at the index procedure as well as a history of endocarditis were not considered as exclusion criteria.
Data Collection, Ethic Statement and Study Endpoints
All patient data were anonymized before accessing the database. The institutional ethics committee approved the inclusion of the patients and waiver for the requirement for informed consent was granted (EK 53022010).
Study endpoints were specified in accordance with the Mitral Valve Academic Research Consortium criteria [
14]. Risk scores (EuroSCORE II and STS-PROM Score) were calculated for a redo surgical mitral valve procedure [
15,
16]. Follow-up data were obtained from all recorded patient contacts and available echocardiographic examinations.
Patients, Study Groups and Follow-up
A total of 54 patients according to the in- and exclusion criteria were included in the present study. The mean age was 76.5 ± 6.5 years, and 30 (55.6%) of the patients were male. Mean STS-PROM was 7.42 ± 4.24; the EuroSCORE II averaged 14.4 ± 8.72. The overall follow-up reached up to 9.9 years with a mean follow-up time of 4.8 ± 0.6 years. A total of 164.3 patient-years was analyzed.
According to the type of procedure, the study group was divided into two subpopulations: (a) Valve-in-Valve (ViV)-procedures including n=25 patients and (b) Valve-in-Ring (ViR)-procedures including n=29 patients.
Statistical analysis
Descriptive and analytic statistical analysis was performed. Continuous variables were expressed as means and standard deviations. Categorical variables were summarized as counts and percentages. A p-value under 0.05 was considered significant. Student’s t-test, Chi-squared test and Fisher’s exact test was used to compare demographic and postoperative data between the valve-in-valve (ViV) and the valve-in-ring (ViR) group. The Shapiro-Wilk test was used to assess the normality of the continues variables, and when abnormal distributions were detected, the Mann–Whitney non-parametric test was performed. Kaplan-Meier curves were used to demonstrate postoperative survival and time between the initial procedure and the reintervention. Univariable and multivariable Cox regression was conducted to evaluate possible risk factors for postoperative mortality. All the analyses were performed using R software, version 4.2.1 (R Foundation for Statistical Computing) [
17]. Results are presented in written and summarized in tabular and graphic form.
3. Results
Baseline Characteristics
Due to the differences in indications, both groups basically showed good comparability concerning their baseline characteristics risk profile. Nonetheless, some risk related items were not counterbalanced between ViR- and ViV-patients.
Age and sex did not differ significantly between the ViV- (77.4 ± 6.3 years; 52.0% male) and the ViR-patients (75.8 ± 6.7 years; 58.6% male) (p=0.35 and p=0.83, respectively). Cardiovascular risk factors such as arterial hypertension, diabetes mellitus, dyslipidemia and further pre-existing conditions in the medical history were equally distributed in both groups. Patients undergoing a ViV procedure had a significantly lower rate of preoperative history of permanent pacemaker implantation with 20.0% (n=5) compared to 69.0% (n=20) in the ViR-group (p<0.001).
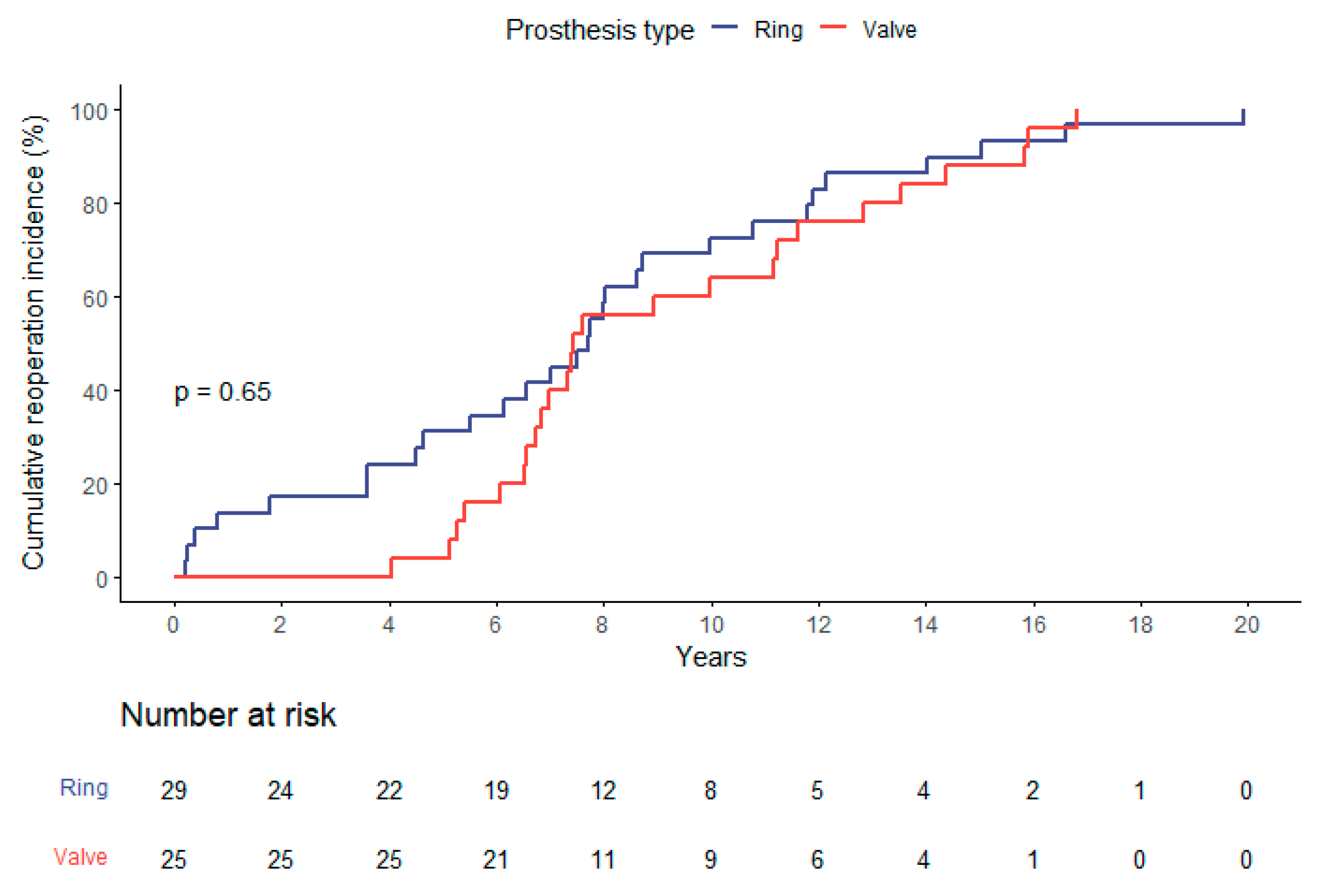
The calculated risk scores were consistently higher in the ViR group with an EuroSCORE II averaging 11.5 ± 7.5% and a mean STS-PROM Score of 5.9 ± 3.7% compared to 16.9 ± 9.0%, and 8.7 ± 4.3% in ViV-patients (both p=0.01). There was no significant difference concerning the time-intervall from the index-procedure to the present intervention. In ViV-patients this was in mean 9.3 ± 3.8 years and in ViR-patients 7.7 ± 5.0 years (p=0.20) (
Figure 1).
The indications for the index cardiac procedure differed significantly between both groups. In the ViV-cohort the index procedure was more frequently performed due to structural mitral disease (84.0%, n=21; p<0.01), whereas functional mitral disease was the most frequent indication for surgery amongst ViR-patients (72.4%, n=21; p<0.01).
Meanwhile the overall time to reintervention of the entire group is depicted in
Figure 1, the time to reintervention did not differ significantly comparing the indication for the initial procedure (functional compared to structural mitral valve disease; p=0.98).
Table 1 summarizes patient baseline characteristics.
Echocardiographic Baselines
The ViR-group showed a significantly worse left ventricular ejection fraction with 39.3 ± 15.1% compared to 53.6 ± 12.4% in the VIV-cohort (p<0.001). This finding was consistent with a higher LVEDD in ViR patients (49.1 ± 6.5 mm vs 58.6 ± 7.8 mm, p<0.001). Right ventricular function and distribution of the severity of mitral regurgitation were similar in both groups (
Table 2). There was a higher average peak (27.4 ± 6.4 mmHg vs. 18.4 ± 6.6mmHg; p<0.01) and mean gradients (10.7 ± 3.5 mmHg vs. 6.3 ± 2.7 mmHg; p<0.001) observed in ViV-patients compared to the ViR-cohort. This correlated with the higher incidence of moderate or severe stenosis (64.0% vs. 20.6%) in the ViV-cohort as primary type of failure. Preoperative echocardiographic data is summarized in
Table 2.
Procedural Data
The stented tissue valve implanted during the index procedure of the ViV-patients had an average labeled size of ViV 29.7 ± 1.1 mm. In the ViR-cohort, the majority of annuloplasty rings implanted during the index procedure were semirigid (n=28; 96.6%). In one single case an incomplete flexible (n=1; 3.4%) ring had been used. The mean labeled size of the priorly implanted annuloplasty rings was ViR 28.4 ± 1.5 mm. During the actual procedure, all patients received a balloon-expandable transcatheter prosthesis. Mean labeled size was 27.9 ± 1.5 mm in the ViV- and 25.9 ± 1.7 mm in the ViR-cohort, which differed significantly (p<0.001). There was no significant difference between the groups in terms of access, prosthesis model, surgery time, rate of conversion, intraoperative complications, or procedure success. According the M-VARC criteria, the procedural success was low in both groups, which was mainly driven by increased transvalvular pressure gradients (
Table 3). When postprocedural mitral regurgitation was present, it predominantly consisted of paravalvular and less frequently transvalvular regurgitation. Left ventricular outflow tract obstruction was rare and occurred in 4.0% of the ViV- and 6.9% of the ViR-patients.
Table 3 summarizes procedural data.
Postoperative Course and Hospital Outcomes
Postoperative course and hospital outcomes were mainly comparable between both groups- The primary ICU-stay differed not significantly with 3.8 ± 6.8 days in the ViV-cohort and 4.3 ± 6.3 days in the ViR-cohort (p=0.96). The same was true for hospital stay (ViV: 9.9 ± 5.9 days vs. ViR 13.5 ± 8.0 days, p=0.13) and 30-day mortality with 4.0% (n=1) in the ViV- and 6.9% (n=2) in the ViR-group (p=1.00). The postoperative data are summarized in
Table 4.
Follow-Up Data
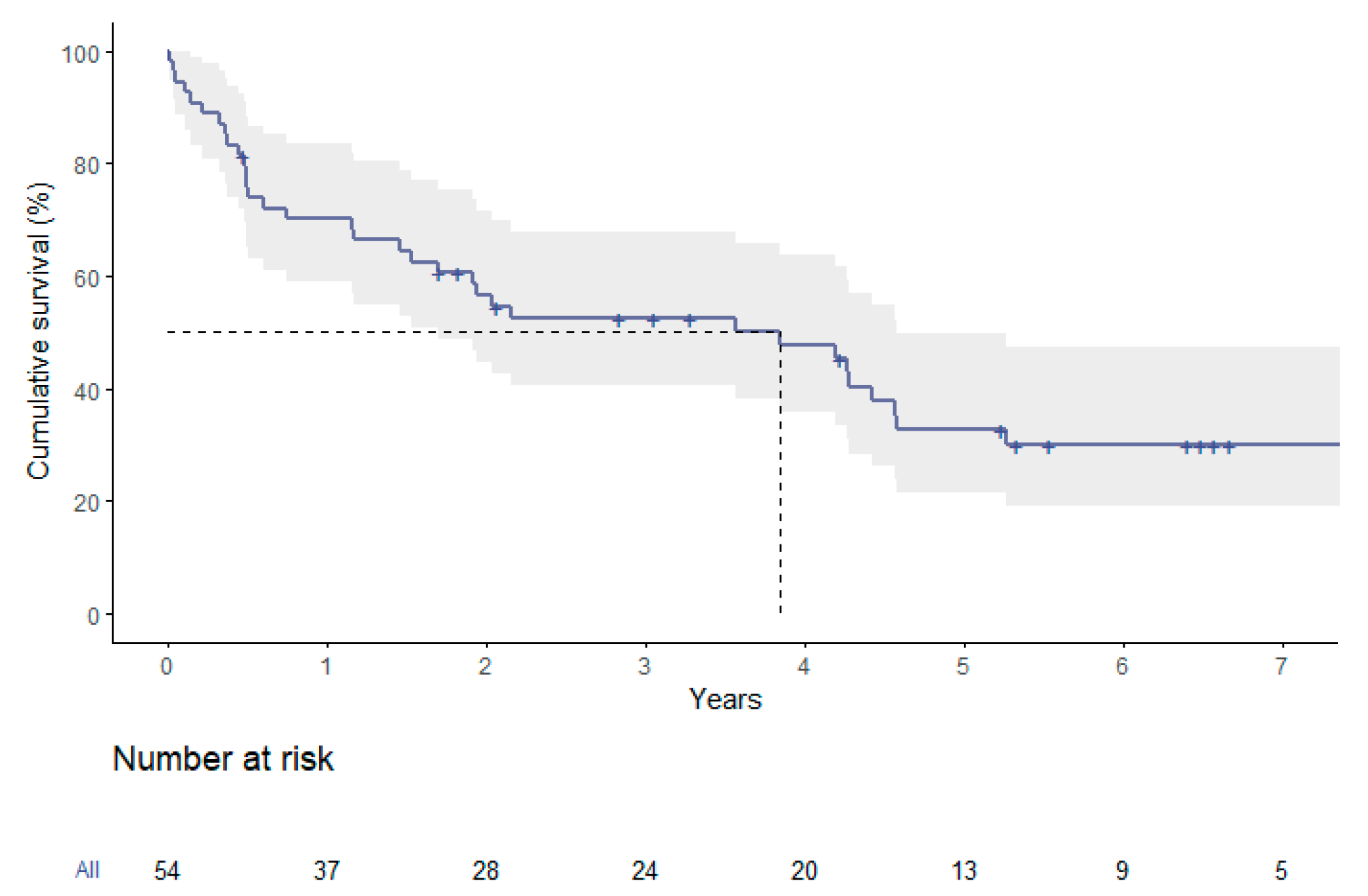
Survival analysis of the entire group showed a median overall survival of 3.9 years. The respective survival rates were 70.1 ± 6.3% at one year, 32.7 ± 7.0% at five years and 30.0 ± 6.9% at seven years (
Figure 2).
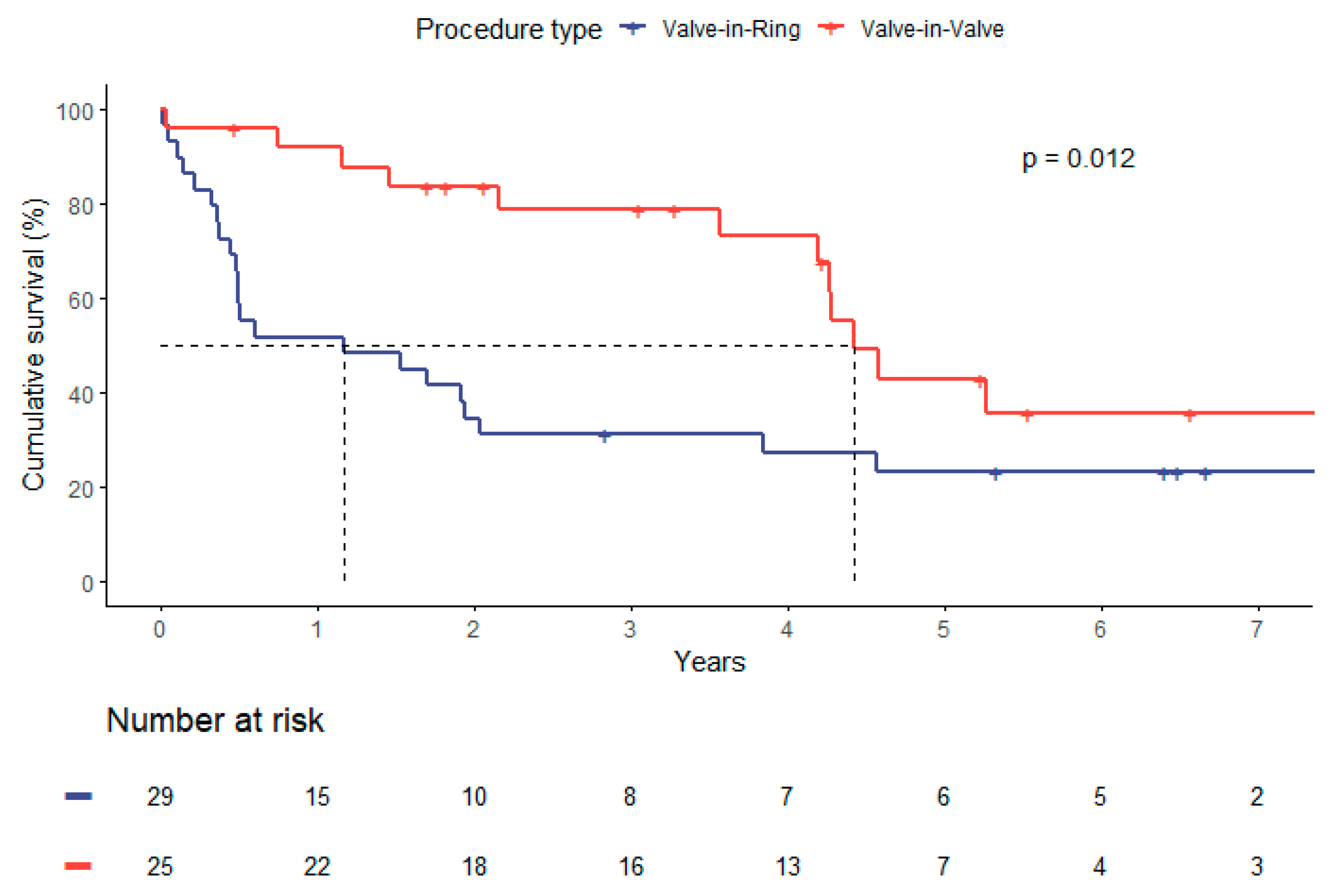
When comparing survival by type of procedure (ViV vs ViR), median survival was longer in the ViV-group with 4.4 versus 1.2 years, respectively (p=0.01). Higher survival rates in ViV-patients were also distinct at one year (91.8% vs 51.7%), five years (42.9% vs 23.3%) and seven years (35.7% vs 23.3%) as depicted in
Figure 3 (p = 0.012).
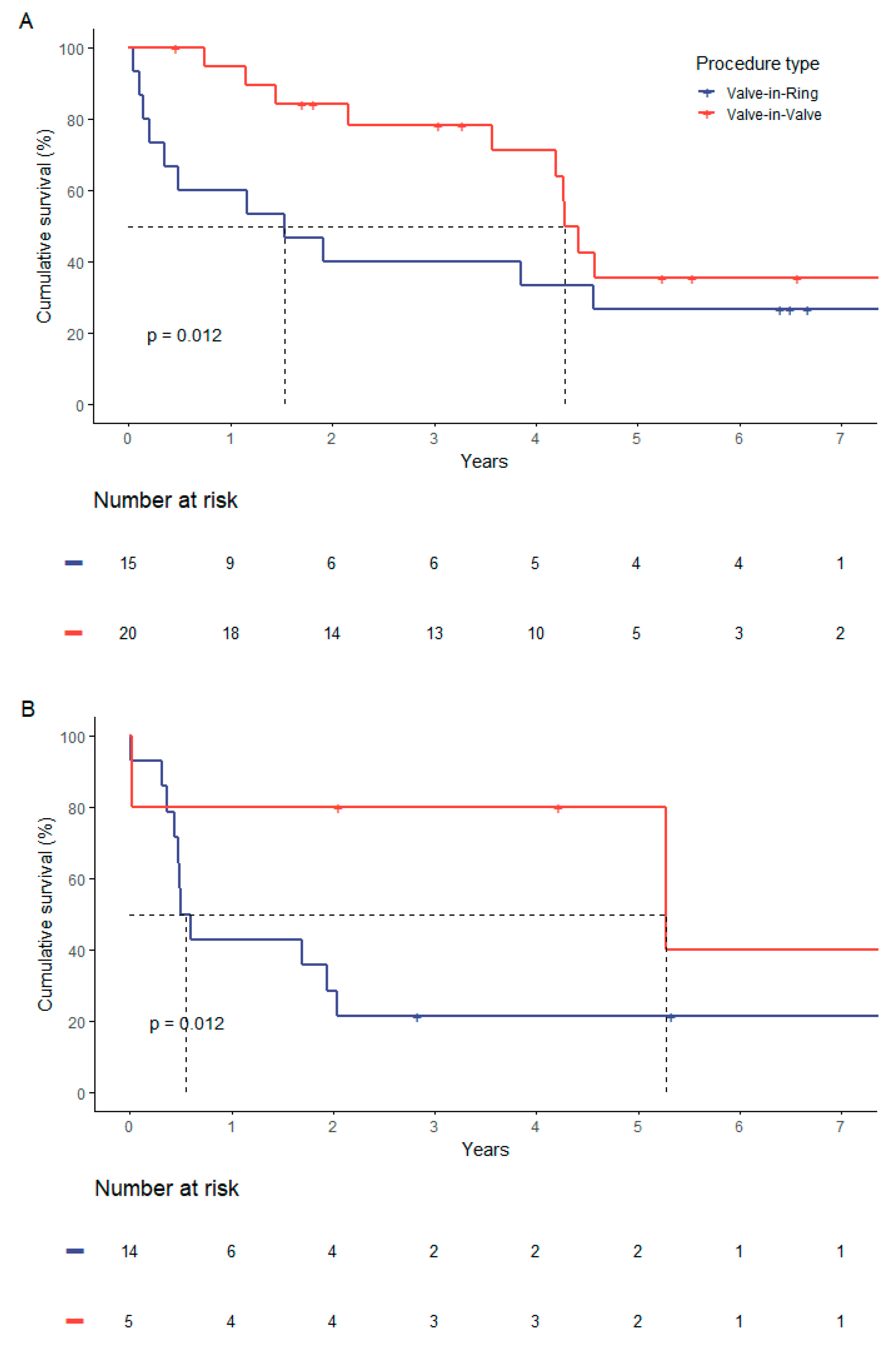
This coherence was independently from the risk stratification. If stratified after STS-PROM with separate consideration of a lower risk-strata (STS-Score < 8 %) and high risk -strata (STS-Score ≥ 8 %), the observed significantly higher mortality in the ViR-group persisted (
Figure 4).
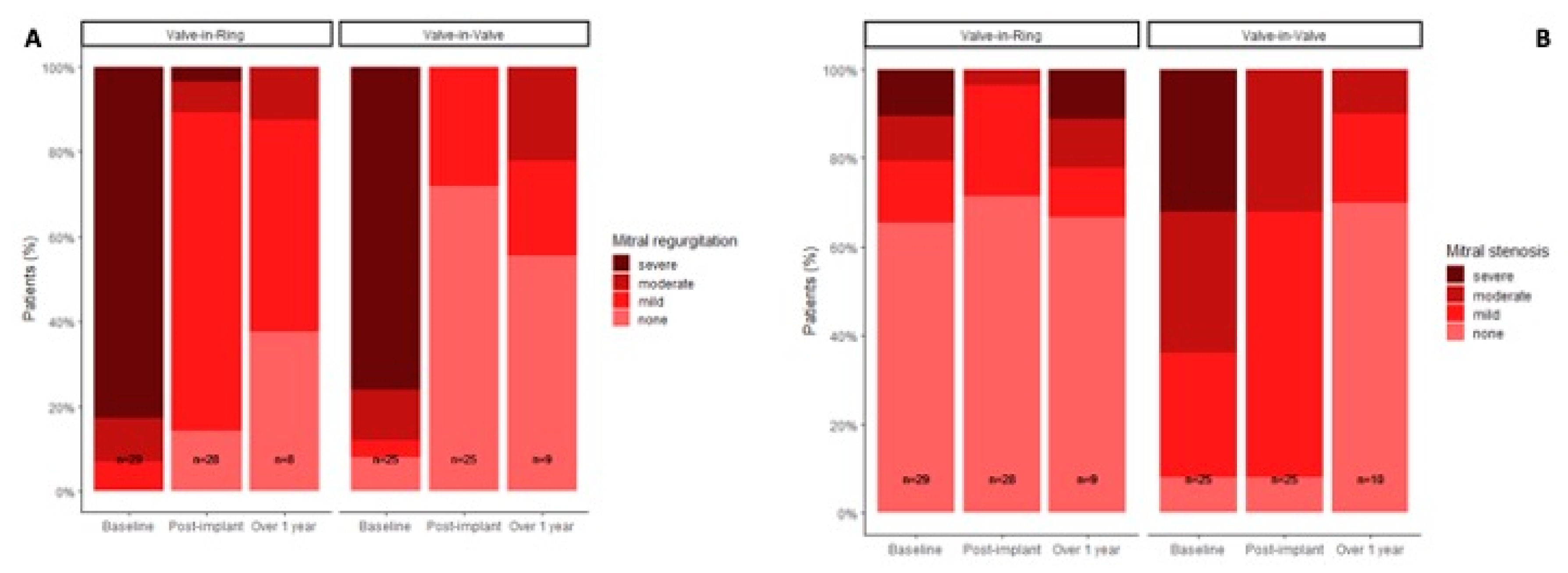
A significant reduction in mitral regurgitation in the postprocedural echocardiographic control was recorded. Echocardiographic follow-up after one year demonstrated that the initial results concerning regurgitation or stenosis were sustained independently from the performed type of procedure (
Figure 5).
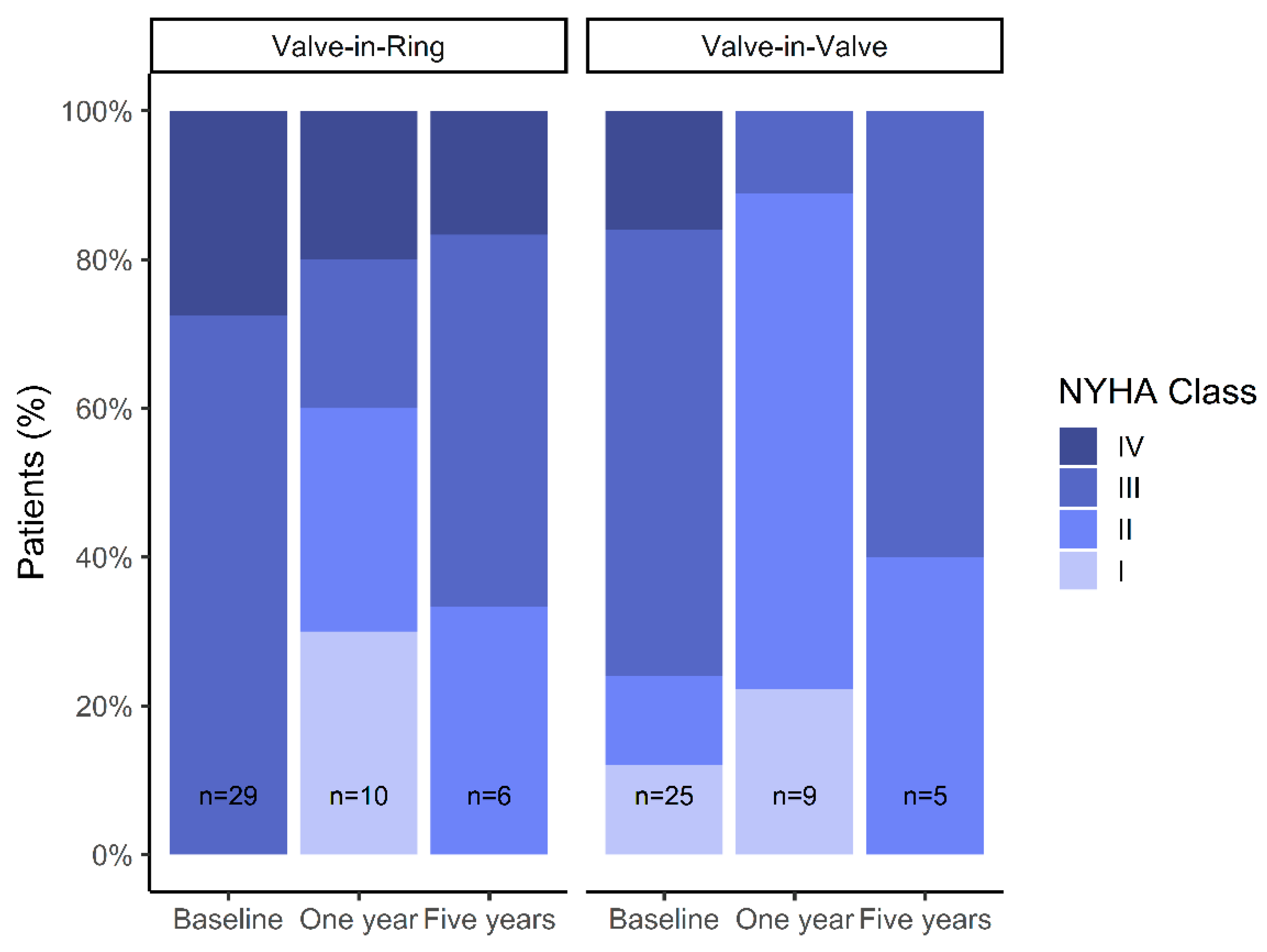
We did not observe significant changes in LVEF in follow-up measurements. Significant improvements in NYHA class at 1-year follow-up were observed in both groups, but NYHA functional class appeared to deteriorate again after 5-years of follow-up (
Figure 6).
Survival Analysis
Several possible predictors of long-term survival were assessed using univariable Cox regression. A Valve-in-Ring procedure was found to be a significant predictor of mortality with a hazard ratio of 2.36 (95% CI 1.19 - 4.67; p = 0.01). The further evaluated parameters failed to show any significance: prosthesis size (HR 0.93, 95% CI 0.77 – 1.1, p=0.4), ejection fraction (HR 0.98, 95% CI 0.96 – 1.00, p=0.1), EuroSCORE II (HR 1.04, 95% CI 0.99 – 1.08, p=0.06) and STS-Score (HR 1.07, 95% CI 0.99 – 1.15, p=0.08), initial surgery for primary versus secondary valve disease (HR 1.55, 95% CI 0.80 – 2.99, p=0.19), or postoperative mitral stenosis or regurgitation grade (p=0.82 and 0.48). Multivariable regression analysis did not identify any further risk factors.
4. Discussion
Concerning the immediate outcomes, transcatheter mitral valve implantation is a viable alternative in high-risk patients presenting with a degenerated bioprosthesis or recurrent regurgitation after mitral repair. The observed 30-day mortality proved to be lower or at least to be comparable to the predicted mortality by scores [
11,
12,
18]. These findings could be confirmed by our current study. Here, the observed 30-day mortality likewise was lower than predicted mortality, which was true for the type of procedure (ViV or ViR) as well as for both used scoring systems (STS-PROM and EuroSCORE II). Nonetheless, the 30-days mortality remains substantial. This has to be interpreted with regard to a real high risk population, consisting of patients whom rejected for a redo surgical procedure. With that background, the comparatively low 30-day mortality rates are acceptable.
Despite certain risk factors being more pronounced in ViR patients (higher EuroSCORE II and STS-PROM score, a lower ejection fraction), we did not identify any of these factors to be an independent predictor of inferior outcome. Intraprocedural and postprocedural outcomes did not differ significantly between groups. Device success was similarly low to previously reported success rates in both groups [
12,
18]. As reported by Simonato et al., the low device success and subsequently procedure success rate was mainly driven by mean transvalvular gradients, being mostly ≥5mmHg in postinterventional measurements. When modified criteria were used (mean gradient ≤10mmHg), the procedural success rates were notably higher, but still not comparable to what is common after a surgical redo-procedure.
However, as previously observed in similar studies, these initially encouraging results worsen in the long-term. This was particularly true for patients undergoing a ViR-procedure. Our observations with a significantly shorter median survival in patients who had previously undergone a mitral annuloplasty confirmed these findings. Hu et al., in a systematic review of literature, reported six-month mortality rates of 18.5% for ViV and 38.5% for ViR patients, despite the mean overall in-hospital mortality was satisfying with 5.8% [
19]. The inferior outcome of ViR persisted when subgroups were evaluated separately – patients with reduced ejection fraction, patient with preserved ejection fraction, patients with high-risk scores, patients with low-risk scores. This indicates that, independent of other risk factors, the implantation of a transcatheter aortic valve prosthesis in a mitral annuloplasty ring is associated with higher long-term mortality of any cause compared to the implantation of the same prosthesis in a previously implanted biological valve prosthesis. This finding confirms the results from other studies [
12,
19].
The key aspect distinguishing our study from previous ones is that we present a single-center high-volume experience with long-term follow-up. By reporting on all consecutive patients who underwent this transcatheter procedure we can avoid the selection bias that can occur in registries [
12,
18]. Furthermore, despite the overall lower number of cases in the present series compared to multicenter studies, we have a much higher single center case load. For example, Guerrero et al. reported 4.22 ViV and 2.12 ViR cases per hospital in 172 hospitals. The low center case load in registry-patients could mitigate to some extent the inherent effects of a learning curve [
12,
18].
It has been previously suggested that the inferior long-term survival in ViR patients may be attributable to one or multiple factors – preoperative comorbidities and cardiac dysfunction, higher risk of potential LVOT obstruction or mismatch in the shape of the annuloplasty ring and the transcatheter aortic valve prosthesis. Cardiac dysfunction may be more pronounced in the annuloplasty group, due to the hesitancy to operate a previously reconstructed mitral valve. However, there is no way to measure the interval from the time at which an intervention would have been indicated to the time it was performed and compare it in both groups. Therefore, a longer delay in surgery and subsequently more severe deterioration in cardiac function in the ViR group can neither be confirmed nor ruled out. An LVOT-obstruction is theoretically more likely in ViR patients as the anterior mitral leaflet is still present in all patients and comes in direct contact with the newly implanted prosthesis, whereas in ViV patients it has already been either resected or is being retracted by the initial valve prosthesis. Our data does not support these thesis by an overall low rate of LVOT-obstruction.
It remains, that the inability of the circular valve to adapt the irregular D-shaped form of the annuloplasty ring probably causes hemodynamic and functional deficits is less effective in removing the predominant cause of failure, which in the long-term leads to a diminished prognosis [
18,
20]. This assumption is supported by our finding, that postprocedural mitral regurgitation as well as paravalvular mitral regurgitation were significantly more frequent in the ViR-group. However, whether this is causative for the long-term outcomes remains speculative and warrants further investigation.
5. Conclusions
ViV and ViR procedures are safe and effective on the short-term, but fail to prove durability in the long-term. Particularly ViR seems to be less effective in removing the primary mode of failure, which on the long-run seems to be causative for inferior clinical outcomes.
Nonetheless, the observations made, have carefully to be interpreted in the context of an old and high-risk population of treated patients.
But anyhow, the results of the present series do not support the indication extend towards younger or lower-risk patients.
Institutional Review Board Statement
The institutional ethics committee approved the inclusion of the patients and waiver for the requirement for informed consent was granted (EK 53022010).
Informed Consent Statement
The institutional ethics committee approved the inclusion of the patients and waiver for the requirement for informed consent was granted (EK 53022010).
Data Availability Statement
Data available on request due to restrictions. The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical restrictions
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; Bonis, M. de; Paulis, R. de; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632, . [CrossRef]
- Kempfert, J.; van Linden, A.; Linke, A.; Borger, M.A.; Rastan, A.; Mukherjee, C.; Ender, J.; Schuler, G.; Mohr, F.W.; Walther, T. Transapical off-pump valve-in-valve implantation in patients with degenerated aortic xenografts. The Annals of thoracic surgery 2010, 89, 1934–1941, . [CrossRef]
- Walther, T.; Falk, V.; Dewey, T.; Kempfert, J.; Emrich, F.; Pfannmüller, B.; Bröske, P.; Borger, M.A.; Schuler, G.; Mack, M.; et al. Valve-in-a-valve concept for transcatheter minimally invasive repeat xenograft implantation. J. Am. Coll. Cardiol. 2007, 50, 56–60, . [CrossRef]
- Cribier, A.; Eltchaninoff, H.; Bash, A.; Borenstein, N.; Tron, C.; Bauer, F.; Derumeaux, G.; Anselme, F.; Laborde, F.; Leon, M.B. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002, 106, 3006–3008, . [CrossRef]
- Wilbring, M.; Alexiou, K.; Tugtekin, S.M.; Arzt, S.; Ibrahim, K.; Matschke, K.; Kappert, U. Pushing the limits-further evolutions of transcatheter valve procedures in the mitral position, including valve-in-valve, valve-in-ring, and valve-in-native-ring. J. Thorac. Cardiovasc. Surg. 2014, 147, 210–219, . [CrossRef]
- Wilbring, M.; Alexiou, K.; Tugtekin, S.M.; Sill, B.; Hammer, P.; Schmidt, T.; Simonis, G.; Matschke, K.; Kappert, U. Transapical transcatheter valve-in-valve implantation for deteriorated mitral valve bioprostheses. The Annals of thoracic surgery 2013, 95, 111–117, . [CrossRef]
- Cheung, A.; Webb, J.G.; Wong, D.R.; Ye, J.; Masson, J.-B.; Carere, R.G.; Lichtenstein, S.V. Transapical transcatheter mitral valve-in-valve implantation in a human. The Annals of thoracic surgery 2009, 87, e18-20, . [CrossRef]
- Speiser, U.; Pohling, D.; Tugtekin, S.-M.; Charitos, E.; Matschke, K.; Wilbring, M. Redo surgery for noninfective isolated mitral valve disease: Initial outcome and further follow-up compared to primary surgery. Journal of cardiac surgery 2022, 37, 1990–1997, . [CrossRef]
- Yoon, S.-H.; Whisenant, B.K.; Bleiziffer, S.; Delgado, V.; Schofer, N.; Eschenbach, L.; Fujita, B.; Sharma, R.; Ancona, M.; Yzeiraj, E.; et al. Transcatheter Mitral Valve Replacement for Degenerated Bioprosthetic Valves and Failed Annuloplasty Rings. J. Am. Coll. Cardiol. 2017, 70, 1121–1131, . [CrossRef]
- Dvir, D.; Webb, J.G.; Bleiziffer, S.; Pasic, M.; Waksman, R.; Kodali, S.; Barbanti, M.; Latib, A.; Schaefer, U.; Rodés-Cabau, J.; et al. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA 2014, 312, 162–170, . [CrossRef]
- Yoon, S.-H.; Whisenant, B.K.; Bleiziffer, S.; Delgado, V.; Dhoble, A.; Schofer, N.; Eschenbach, L.; Bansal, E.; Murdoch, D.J.; Ancona, M.; et al. Outcomes of transcatheter mitral valve replacement for degenerated bioprostheses, failed annuloplasty rings, and mitral annular calcification. Eur. Heart J. 2019, 40, 441–451, . [CrossRef]
- Simonato, M.; Whisenant, B.; Ribeiro, H.B.; Webb, J.G.; Kornowski, R.; Guerrero, M.; Wijeysundera, H.; Søndergaard, L.; Backer, O. de; Villablanca, P.; et al. Transcatheter Mitral Valve Replacement After Surgical Repair or Replacement: Comprehensive Midterm Evaluation of Valve-in-Valve and Valve-in-Ring Implantation From the VIVID Registry. Circulation 2021, 143, 104–116, . [CrossRef]
- Iung, B.; Delgado, V.; Rosenhek, R.; Price, S.; Prendergast, B.; Wendler, O.; Bonis, M. de; Tribouilloy, C.; Evangelista, A.; Bogachev-Prokophiev, A.; et al. Contemporary Presentation and Management of Valvular Heart Disease: The EURObservational Research Programme Valvular Heart Disease II Survey. Circulation 2019, 140, 1156–1169, . [CrossRef]
- Stone, G.W.; Adams, D.H.; Abraham, W.T.; Kappetein, A.P.; Généreux, P.; Vranckx, P.; Mehran, R.; Kuck, K.-H.; Leon, M.B.; Piazza, N.; et al. Clinical trial design principles and endpoint definitions for transcatheter mitral valve repair and replacement: part 2: endpoint definitions: A consensus document from the Mitral Valve Academic Research Consortium. Eur. Heart J. 2015, 36, 1878–1891, . [CrossRef]
- O’Brien, S.M.; Shahian, D.M.; Filardo, G.; Ferraris, V.A.; Haan, C.K.; Rich, J.B.; Normand, S.-L.T.; DeLong, E.R.; Shewan, C.M.; Dokholyan, R.S.; et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2--isolated valve surgery. The Annals of thoracic surgery 2009, 88, S23-42, . [CrossRef]
- Nashef, S.A.M.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. EuroSCORE II. Eur. J. Cardiothorac. Surg. 2012, 41, 734-44; discussion 744-5, . [CrossRef]
- R Development Core Team. R: A language and environment for statistical computing; R Foundation for Statistical Computing: Vienna, Austria, 2020.
- Guerrero, M.; Vemulapalli, S.; Xiang, Q.; Wang, D.D.; Eleid, M.; Cabalka, A.K.; Sandhu, G.; Salinger, M.; Russell, H.; Greenbaum, A.; et al. Thirty-Day Outcomes of Transcatheter Mitral Valve Replacement for Degenerated Mitral Bioprostheses (Valve-in-Valve), Failed Surgical Rings (Valve-in-Ring), and Native Valve With Severe Mitral Annular Calcification (Valve-in-Mitral Annular Calcification) in the United States: Data From the Society of Thoracic Surgeons/American College of Cardiology/Transcatheter Valve Therapy Registry. Circ. Cardiovasc. Interv. 2020, 13, e008425, . [CrossRef]
- Hu, J.; Chen, Y.; Cheng, S.; Zhang, S.; Wu, K.; Wang, W.; Zhou, Y. Transcatheter mitral valve implantation for degenerated mitral bioprostheses or failed surgical annuloplasty rings: A systematic review and meta-analysis. Journal of cardiac surgery 2018, 33, 508–519, . [CrossRef]
- Wan, S.; Lee, A.P.W.; Jin, C.-N.; Wong, R.H.L.; Chan, H.H.M.; Ng, C.S.H.; Wan, I.Y.P.; Underwood, M.J. The choice of mitral annuloplastic ring-beyond “surgeon’s preference”. Ann. Cardiothorac. Surg. 2015, 4, 261–265, . [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).