Submitted:
14 September 2023
Posted:
15 September 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Hypothesis
Postural Orthostatic Tachycardia Syndrome
Gut Microbiome
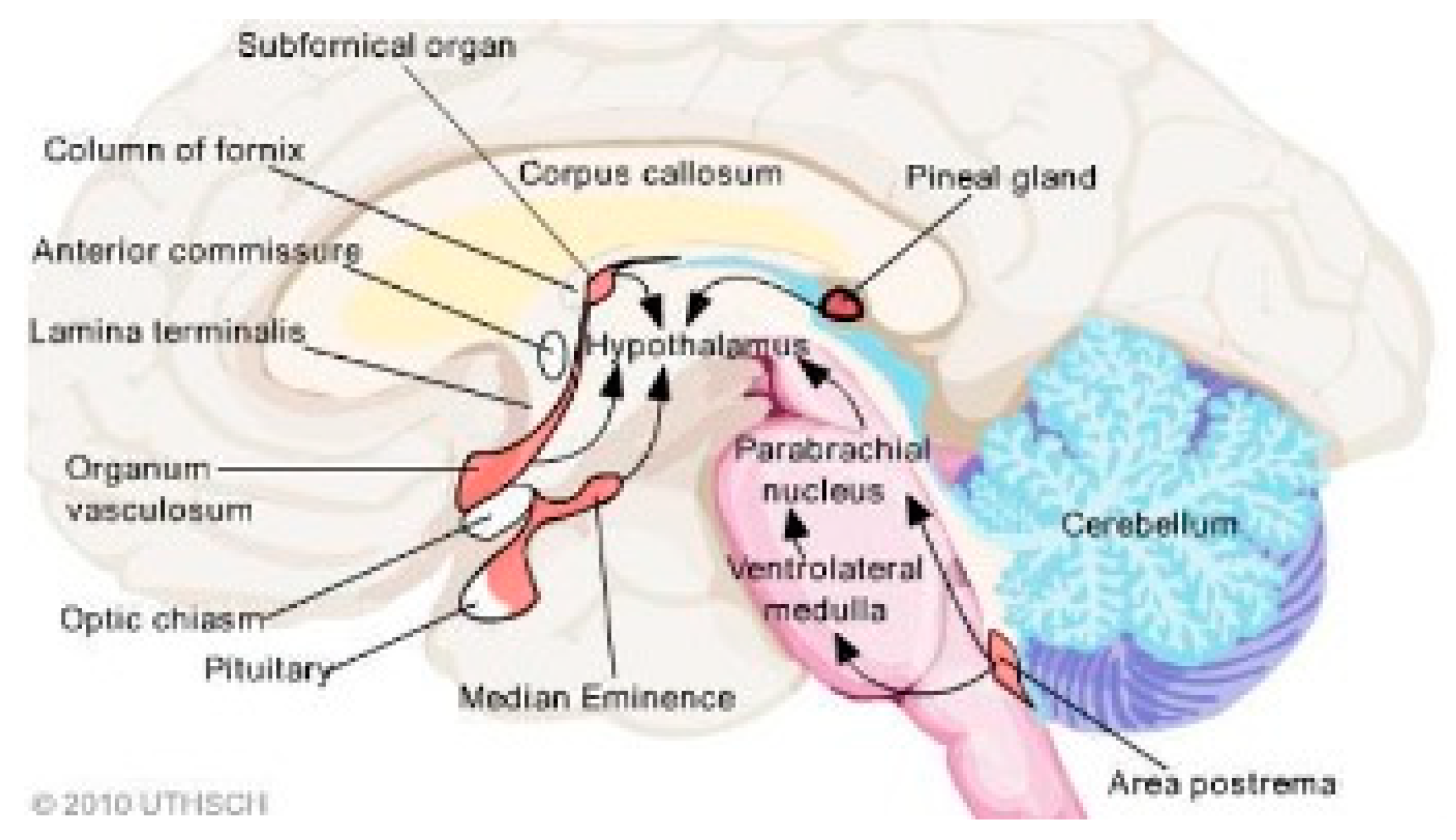
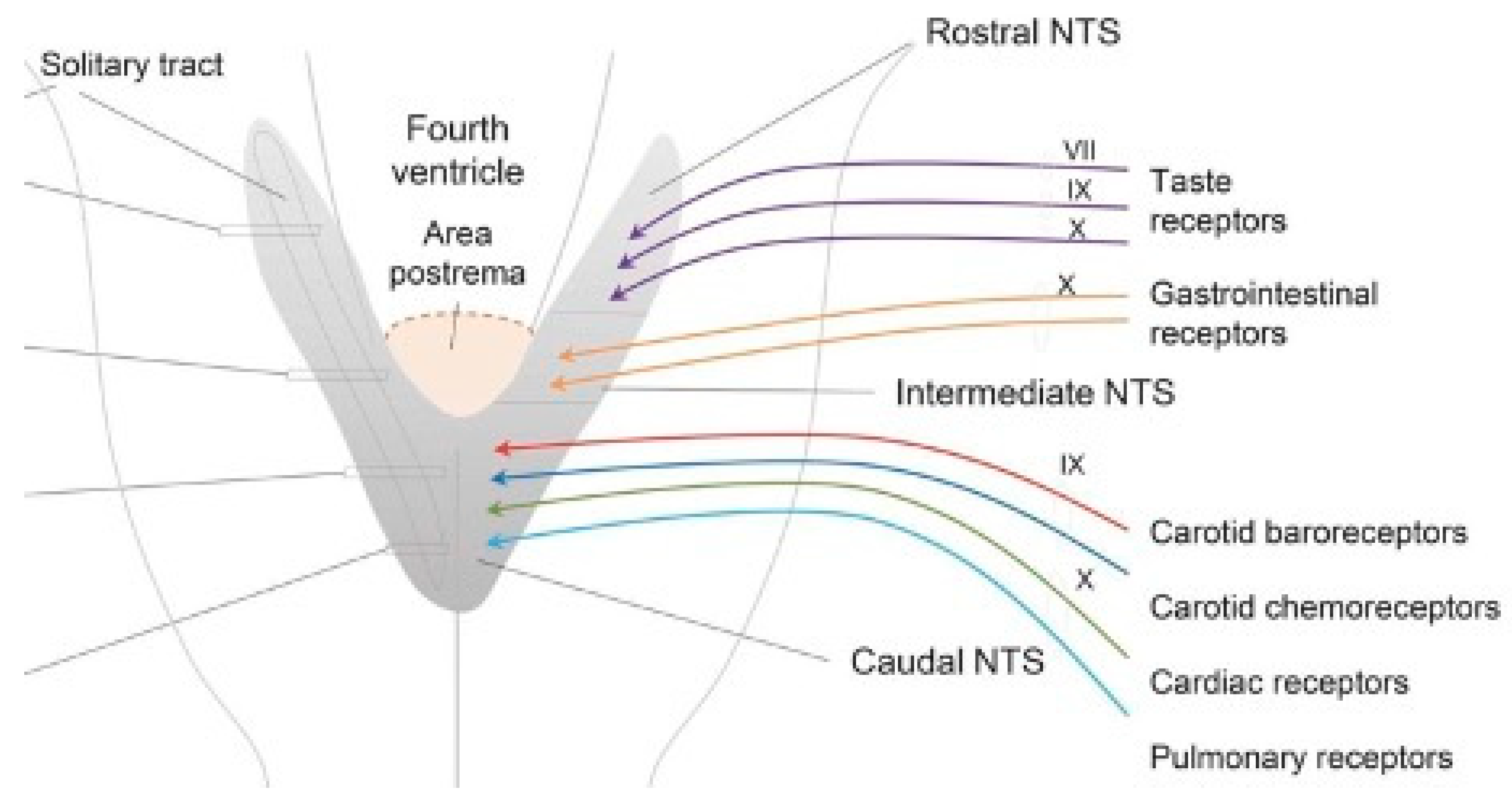
Circumventricular Organs
MTHFR and Homocysteine
Female Preponderance
Vitamin D and Oxidative Stress in Mitochondria
Antioxidants in Post Viral Fatigue Syndrome
Magnesium and the B Vitamins
Thoughts on Therapy
- 1. ARBs upregulate GABA, which displays anti-hypertension, anti-senescence, anti-diabetes, antioxidant, and anti-inflammatory properties (Sfera et al., 2022).
- 2. ARBs increase ACE, which degrades BKN, but block AT1Rs and hypertension induced dementia (Campbell et al., 1995)
- 3. ARBs alleviate POTS (Medow et al., 2005) and degrade BKN otherwise associated with AD (Singh et al., 2020).
- 4. ARBs increase ACE2 (Zaheer et al., 2021) that degrades amyloid β-peptide (Aβ) in AD (Kehoe et al., 2016). The benefits of ACE2 in COVID-19 exceed its risks as a receptor for SARS-CoV-2 (Bastolla et al., 2022).
- 5. ARBs down regulate pro-inflammatory transforming growth factor-beta (TGF-β), increased in LC (Mahudpour et al., 2020), CFS (Yang et al., 2019), AD (Zhang et al., 2016), Marfan Syndrome, and EDS.
- 6. ARBs improve insulin sensitivity (Zhang et al., 2013) and are antidiabetic (Chang et al., 2014).
- 7. ARBs are neuroprotective (Villapol & Saavedra, 2015).
Conclusion
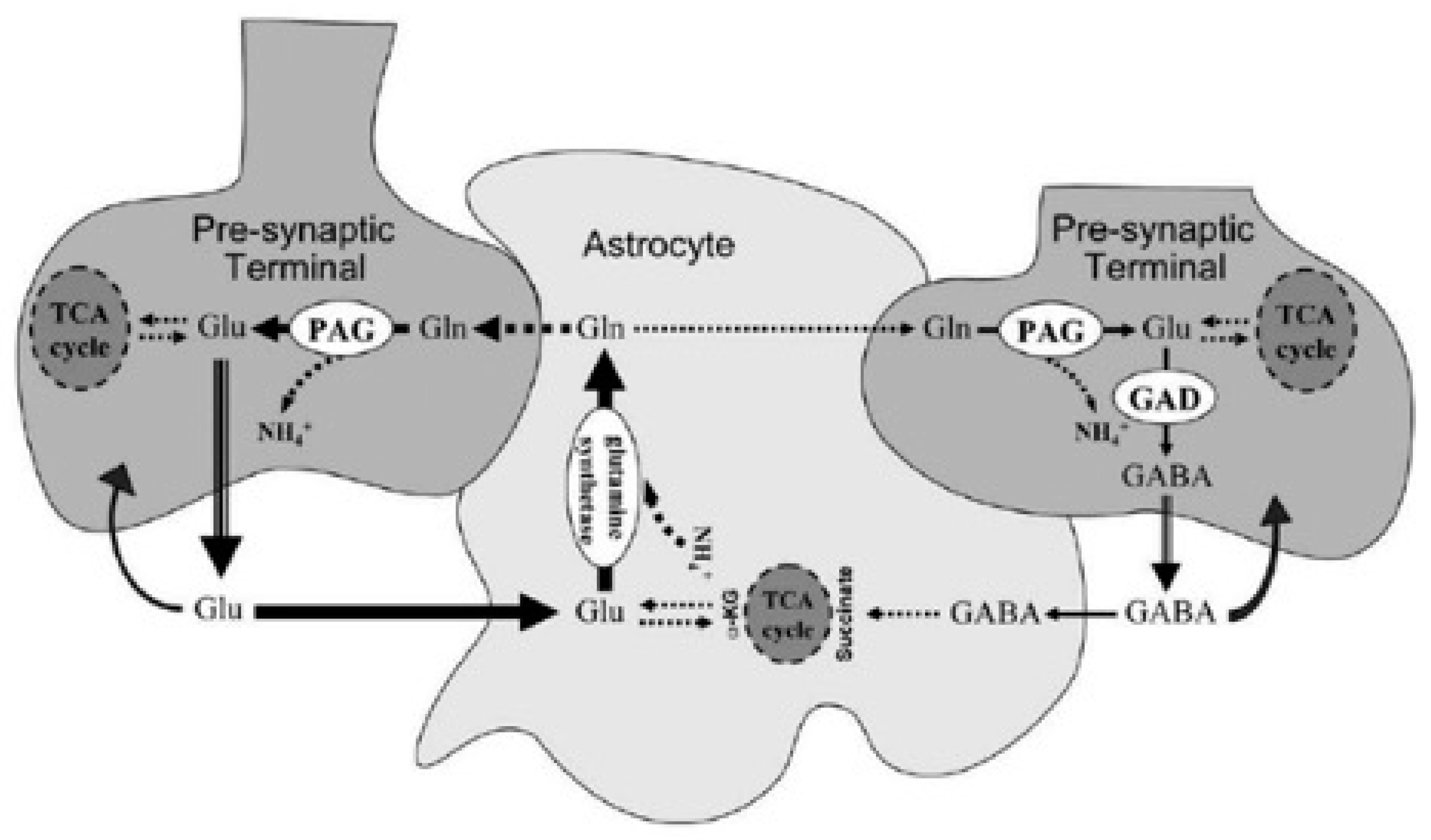
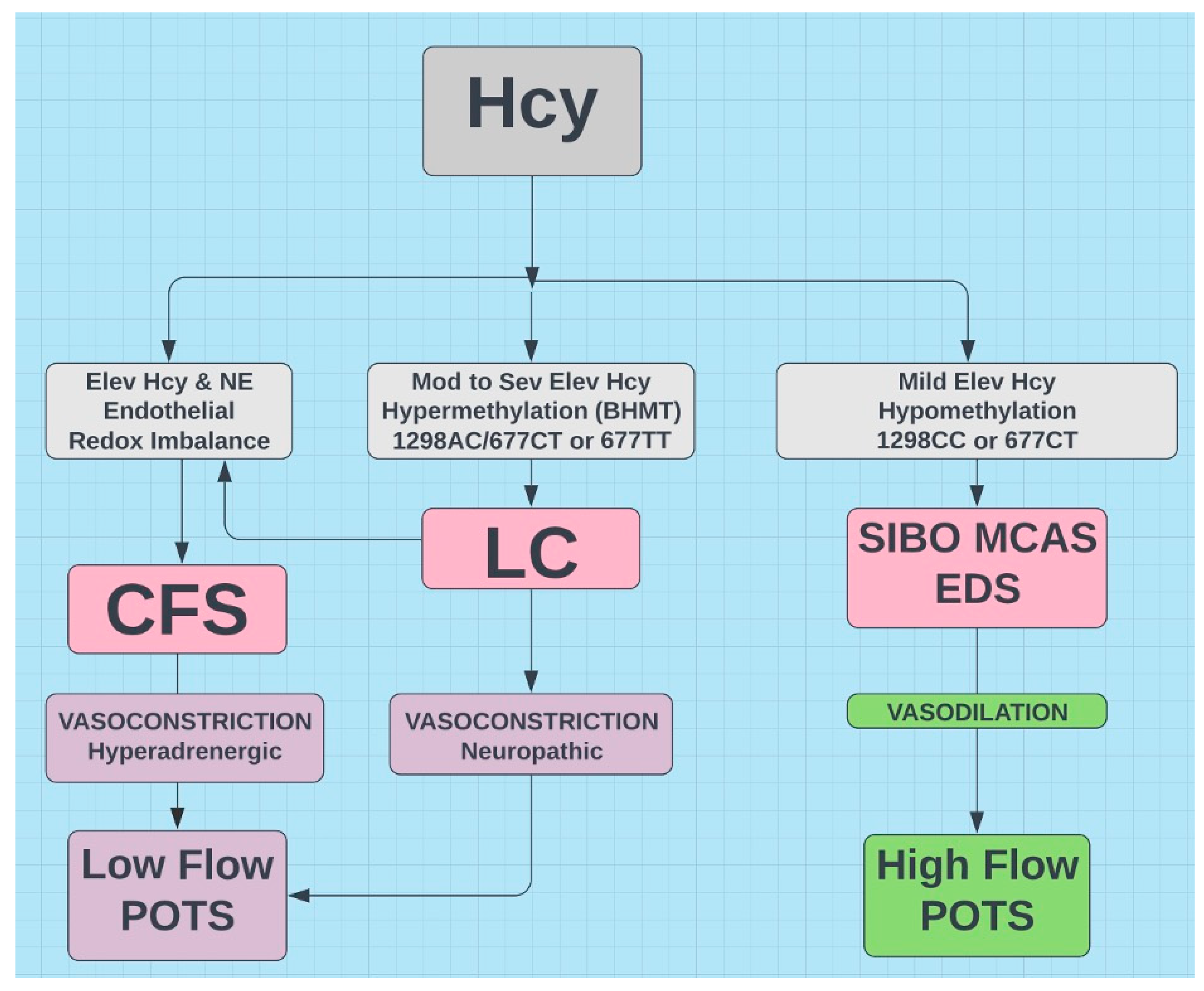
- Lack of gut microbiota diversity and elevated C-reactive protein, indicative of chronic information, are independent prognostic biomarkers for LC, CFS, and vitamin D deficiency (Moreira-Rosário et al., 2021). MTHFR mutations do not directly compromise gut microbiota diversity. The absence of the BBB in CVOs with nuclei that control baroreceptor function (area postrema and PVN) and neuroendocrine function (PVN), combined with increased Glu, histamine and decreased GABA producing bacteria, may in part explain pathogenesis of the myriad symptoms in these syndromes. Glu permeates CVOs and may overwhelm GABA neurotransmission. This suboptimal microbiome aggravates symptoms in those with the MTHFR 677T genotype (Regland et al., 2015; Ponti et al., 2021) and is seen in the vitamin D deficient (Thomas et al., 2020). Most with POTS have low 25(OH)D3 levels (Ashangari & Suoleman, 2015). Seasonal variation in the gut microbiome (Machado, 2023) may reflect the cold and flu season due to low vitamin D. These URIs cause and are caused by a sub optimal gut microbiome (Yildiz et al., 2018; Al-Khaldy et al., 2023; Waterhouse et al., 2019; Singh et al., 2020), increasing susceptibility to post viral fatigue syndrome, LC, CFS, POTS (Blitshteyn et alo., 2017), and some MTHFR variants (Okamoto et al., 2012). Unfortunately once chronic inflammation becomes entrenched and mitochondria oxidatively stressed (less CYP27B1) exogenous 1,25(OH)2D3 is needed (Warren et al., 2021). SIBO is an issue separate from gut microbiome diversity. Colon bacteria gain a foothold in the small bowel. This is associated with low volume high flow POTS seen in MCAS and EDS with histamine intolerance.
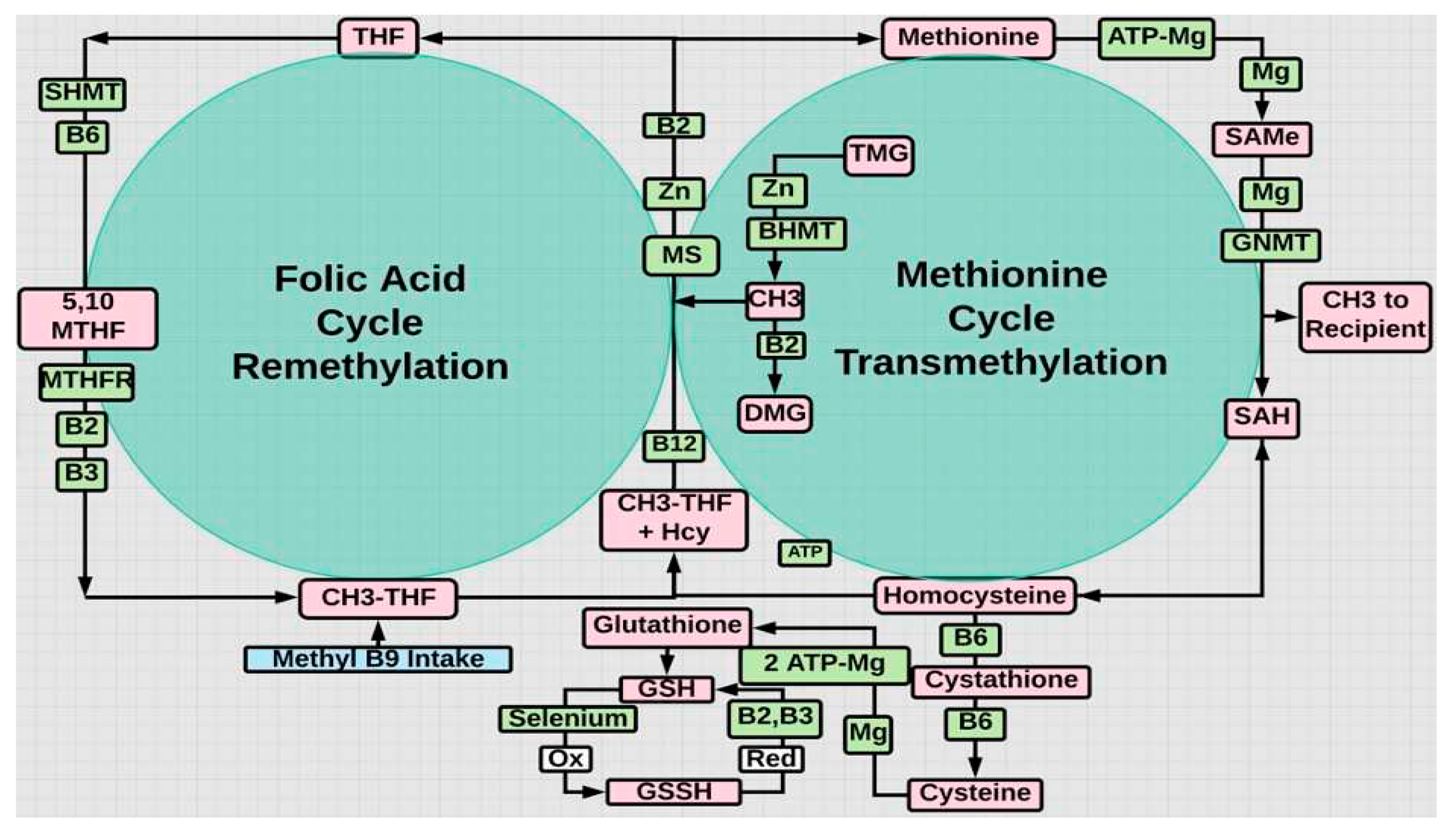
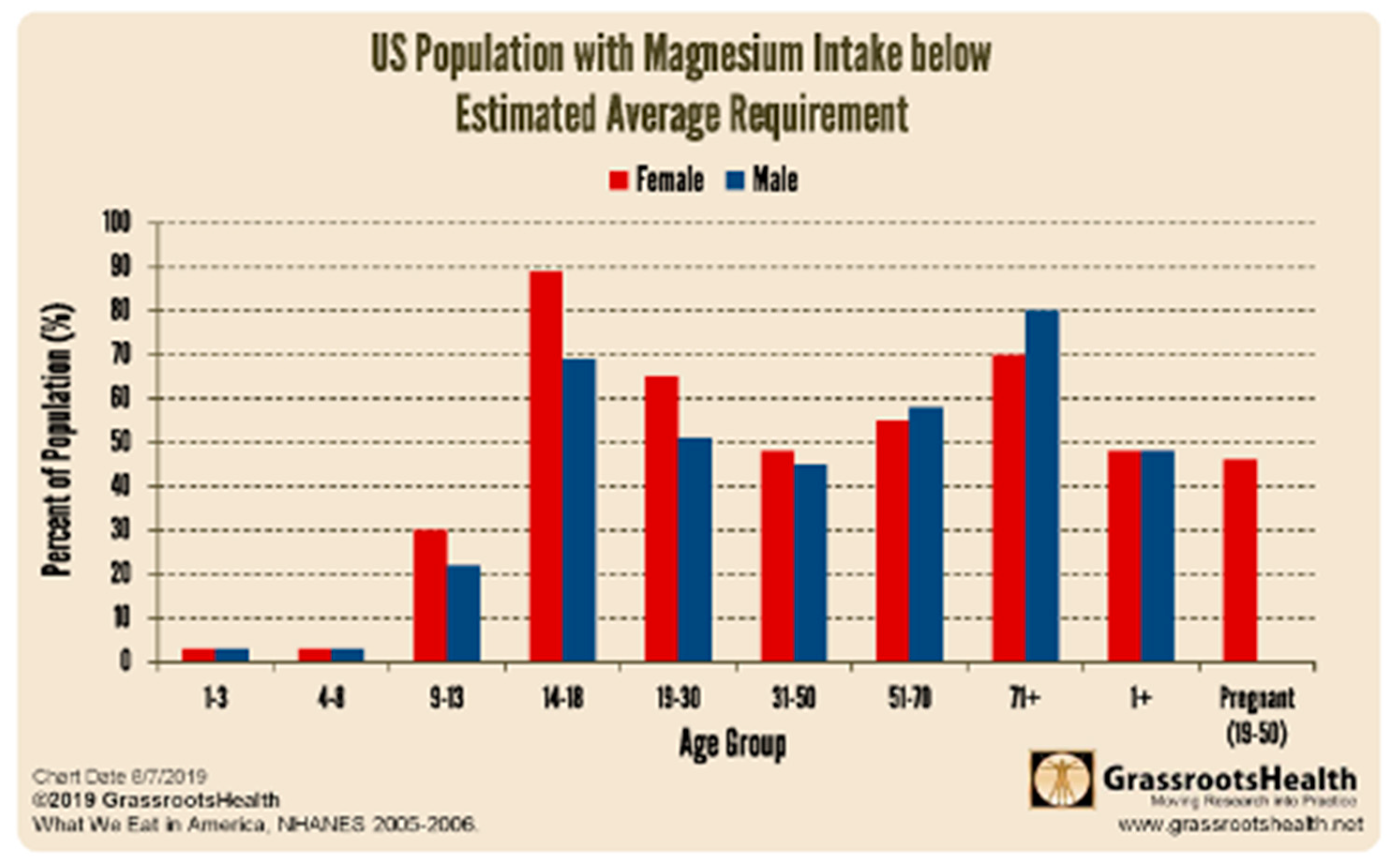
- Females in their reproductive years appear to be more susceptible to LC. This group is also more likely to be magnesium deficient (see Figure 7), probably due to the Western diet, high in calcium and low in magnesium. Estrogen, a mild ACE inhibitor, promotes BKN, linked to brain fog, cognitive decline, and AD. Estrogen also upregulates IFN-γ, produced by CD4+ and CD8+ T cells. Decreased IFN-γ translates to decreased C1INH and activation of the classic complement pathway and the KKS. Respiratory viral infections, increased in females, induce additional release of BKN (Stewart et al., 2008) 82. Estrogen induced upregulation of histamine (degradation requires transmethylation) enhances endothelial permeability and MCAS type low volume high flow POTS. The “post viral fatigue syndrome” in general may stem from a discriminating viral assault on T cells or from a URI induced depletion of gut microbiota. The loss of IFN-γ and the presence of estrogen potentiate chronic inflammation and endothelial permeability. Subsequent chronic inflammation induced oxidative stress from Hcy, residual spike protein S, and reactivated viruses conspire to overwhelm those with insufficient onboard antioxidants. In the absence of critical cofactors synthesis of the master antioxidant glutathione from Hcy cannot proceed (see Figure 5). This downregulates mitochondrial activity and upregulates fatigue (Wood et al., 2021). This also compromises the synthesis of 1,25(OH)2D3, the active form of vitamin D, and with it immune function.
- In the presented hypothesis protein methylation plays a prominent role. It is at the center of the MTHFR gene variants. It manifests in LC (Nikesjö et al., 2022) and POTS (Li et al., 2018) high flow type. MTHFR status is easily available via 23andme or a blood test for Hcy. However, given the prevalence of the MTHFR variants in the general population and from which the laboratory reference values for Hcy are determined, any value near the upper limit of normal may be cause for concern. Elevated Hcy carries many short term (thrombosis) and long-term (neurodegenerative disease) problems, best addressed in a timely manner. Mild elevation of Hcy is associated with hypomethylation. Significantly elevated Hcy associated with the 677TT variant appears to trigger hypermethylation via an alternative B2 dependent “rescue” pathway (Fryar-Williams, 2016). Another protein impacted by hypomethylation is histamine linked to SIBO. DNA can also be differentially methylated (hypo and hyper methylated regions) and is associated with chronic inflammation, cancer, and increasing age. Given the high frequency of this polymorphism in the general population and its potential devastating effects, everyone should be privy to their risks and those of their offspring, especially in the post Covid era.
Abbreviation
| ADH | Antidiuretic hormone (vasopressin) |
| ARBs | Angiotensin receptor blockers |
| BBB | Blood brain barrier |
| BKN | Bradykinin |
| CFS | Chronic Fatigue Syndrome |
| COMT | Catechol-O-methyltransferase |
| CRH | Corticotropin-releasing hormone |
| CVOs | Circumventricular organs |
| DAO | Diamine oxidase |
| EDS | Ehlers Danlos syndrome |
| FB | Fibromyalgia |
| Glu | Glutamate |
| GnRH | Gonadotropin releasing hormone |
| Hcy | Homocysteine |
| HNMT | Histamine N-methyltransferase |
| HPA | Hypothalamus-pituitary-adrenal |
| LC | Long COVID |
| MCAS | Mast cell activation syndrome |
| MTHFR | Methylenetetrahydrofolate reductase |
| P5P | Pyridoxal-5-phosphate |
| POTS | Postural orthostatic tachycardia syndrome |
| PVN | Paraventricular nucleus |
| RLS | Restless legs syndrome |
| SAMe | S-adenosylmethionine |
| SIBO | Small intestine bacterial overgrowth |
Acknowledgements
Disclosures
References
- Regland, B.; Forsmark, S.; Halaouate, L.; Matousek, M.; Peilot, B.; Zachrisson, O.; Gottfries, C.-G. Response to Vitamin B12 and Folic Acid in Myalgic Encephalomyelitis and Fibromyalgia. PLOS ONE 2015, 10, e0124648. [Google Scholar] [CrossRef]
- Ponti, G.; Pastorino, L.; Manfredini, M.; Ozben, T.; Oliva, G.; Kaleci, S.; Iannella, R.; Tomasi, A. COVID-19 spreading across world correlates with C677T allele of the methylenetetrahydrofolate reductase (MTHFR) gene prevalence. J. Clin. Lab. Anal. 2021, 35, e23798. [Google Scholar] [CrossRef]
- Fedorowski, A. Postural orthostatic tachycardia syndrome: clinical presentation, aetiology and management. J. Intern. Med. 2018, 285, 352–366. [Google Scholar] [CrossRef]
- Chadda, K.R.; Blakey, E.E.; Huang, C.L.-. .-H.; Jeevaratnam, K. Long COVID-19 and Postural Orthostatic Tachycardia Syndrome- Is Dysautonomia to Be Blamed? Front. Cardiovasc. Med. 2022, 9, 860198. [Google Scholar] [CrossRef]
- Goldstein, J. Betrayal by the Brain: The Neurologic Basis of Chronic Fatigue Syndrome, Fibromyalgia Syndrome, and Related Neural Network. 1st edition Routledge 1996 USA.
- Stewart, J.M.; Glover, J.L.; Medow, M.S. Increased plasma angiotensin II in postural tachycardia syndrome (POTS) is related to reduced blood flow and blood volume. 110. [CrossRef]
- Okamoto, L.E.; Raj, S.R.; Peltier, A.; Gamboa, A.; Shibao, C.; Diedrich, A.; Black, B.K.; Robertson, D.; Biaggioni, I. Neurohumoral and haemodynamic profile in postural tachycardia and chronic fatigue syndromes. 122. [CrossRef]
- Li, Y.; He, B.; Li, H.; Zhang, Q.; Tang, C.; Du, J.; Jin, H. Plasma Homocysteine Level in Children With Postural Tachycardia Syndrome. Front. Pediatr. 2018, 6, 375. [Google Scholar] [CrossRef]
- Yu, X.; Li, H.; Murphy, T.A.; Nuss, Z.; Liles, J.; Liles, C.; Aston, C.E.; Raj, S.R.; Fedorowski, A.; Kem, D.C. Angiotensin II Type 1 Receptor Autoantibodies in Postural Tachycardia Syndrome. J. Am. Hear. Assoc. 2018, 7. [Google Scholar] [CrossRef]
- Fedorowski, A.; Li, H.; Yu, X.; Koelsch, K.A.; Harris, V.M.; Liles, C.; Murphy, T.A.; Quadri, S.M.S.; Scofield, R.H.; Sutton, R.; et al. Antiadrenergic autoimmunity in postural tachycardia syndrome. Eur. 2016, 19, 1211–1219. [Google Scholar] [CrossRef]
- Badiudeen, T.; Forsythe, E.A.; Bennett, G.; Li, H.; Yu, X.; Beel, M.; Nuss, Z.; Blick, K.E.; Okamoto, L.E.; Arnold, A.C.; et al. A functional cell-based bioassay for assessing adrenergic autoantibody activity in postural tachycardia syndrome. J. Transl. Autoimmun. 2019, 2, 100006. [Google Scholar] [CrossRef]
- Wirth, K.; Scheibenbogen, C. A Unifying Hypothesis of the Pathophysiology of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): Recognitions from the finding of autoantibodies against ß2-adrenergic receptors. Autoimmun. Rev. 2020, 19, 102527. [Google Scholar] [CrossRef]
- Briquez, P.S.; Rouhani, S.J.; Yu, J.; Pyzer, A.R.; Trujillo, J.; Dugan, H.L.; Stamper, C.T.; Changrob, S.; Sperling, A.I.; Wilson, P.C.; et al. Severe COVID-19 induces autoantibodies against angiotensin II that correlate with blood pressure dysregulation and disease severity. Sci. Adv. 2022, 8, eabn3777. [Google Scholar] [CrossRef]
- Barki-Harrington, L.; Luttrell, L.M.; Rockman, H.A. Dual Inhibition of β-Adrenergic and Angiotensin II Receptors by a Single Antagonist. Circulation 2003, 108, 1611–1618. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yu, B.; Liu, Z.; Li, J.; Ma, M.; Wang, Y.; Zhu, M.; Yin, H.; Wang, X.; Fu, Y.; et al. Homocysteine directly interacts and activates the angiotensin II type I receptor to aggravate vascular injury. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Häggström, M.; Richfield, D. Diagram of the pathways of human steroidogenesis. WikiJournal Med. 2014, 1. [Google Scholar] [CrossRef]
- Hall, K.T.; Battinelli, E.; Chasman, D.I.; Ridker, P.M.; Psaty, B.M.; Rotter, J.I.; Kaptchuk, T.J.; Tracy, R.P.; Wassel, C.L.; Mukamal, K.J. Catechol-O-Methyltransferase and Cardiovascular Disease: MESA. J. Am. Hear. Assoc. 2019, 8, e014986. [Google Scholar] [CrossRef] [PubMed]
- Małecki, J.M.; Davydova, E.; Falnes, P. . Protein methylation in mitochondria. J. Biol. Chem. 2022, 298, 101791. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, YK, et al “Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19”. Gut 70 (2021):698-706 https://gut.bmj.
- König, R.S.; Albrich, W.C.; Kahlert, C.R.; Bahr, L.S.; Löber, U.; Vernazza, P.; Scheibenbogen, C.; Forslund, S.K. The Gut Microbiome in Myalgic Encephalomyelitis (ME)/Chronic Fatigue Syndrome (CFS). Front. Immunol. 2022, 12, 628741. [Google Scholar] [CrossRef]
- Strandwitz, P.; Kim, K.H.; Terekhova, D.; Liu, J.K.; Sharma, A.; Levering, J.; McDonald, D.; Dietrich, D.; Ramadhar, T.R.; Lekbua, A.; et al. GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol. 2019, 4, 396–403. [Google Scholar] [CrossRef]
- Pokusaeva, K.; Johnson, C.; Luk, B.; Uribe, G.; Fu, Y.; Oezguen, N.; Matsunami, R.K.; Lugo, M.; Major, A.; Mori-Akiyama, Y.; et al. GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol. Motil. 2016, 29. [Google Scholar] [CrossRef]
- Ko, C.Y.; Lin, H.-T.V.; Tsai, G.J. Gamma-aminobutyric acid production in black soybean milk by Lactobacillus brevis FPA 3709 and the antidepressant effect of the fermented product on a forced swimming rat model. Process. Biochem. 2013, 48, 559–568. [Google Scholar] [CrossRef]
- Mayengbam, S.; Chleilat, F.; Reimer, R.A. Dietary Vitamin B6 Deficiency Impairs Gut Microbiota and Host and Microbial Metabolites in Rats. Biomedicines 2020, 8, 469. [Google Scholar] [CrossRef]
- Dev, S.; Mizuguchi, H.; Das, A.K.; Matsushita, C.; Maeyama, K.; Umehara, H.; Ohtoshi, T.; Kojima, J.; Nishida, K.; Takahashi, K.; et al. Suppression of Histamine Signaling by Probiotic Lac-B: a Possible Mechanism of Its Anti-allergic Effect. J. Pharmacol. Sci. 2008, 107, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ye, Z.; Houston, C.M.; Zecharia, A.Y.; Ma, Y.; Zhang, Z.; Uygun, D.S.; Parker, S.; Vyssotski, A.L.; Yustos, R.; et al. Wakefulness Is Governed by GABA and Histamine Cotransmission. Neuron 2015, 87, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Jiménez, F.J.; Esguevillas, G.; Alonso-Navarro, H.; Zurdo, M.; Turpín-Fenoll, L.; Millán-Pascual, J.; Adeva-Bartolomé, T.; Cubo, E.; Navacerrada, F.; Amo, G.; et al. Gamma-aminobutyric acid (GABA) receptors genes polymorphisms and risk for restless legs syndrome. Pharmacogenomics J. 2018, 18, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, L.B.; Brook, J.B.; Walters, A.S.; Goris, A.; Afrin, L.B.; Molderings, G.J. Restless legs syndrome is associated with long-COVID in women. Sleep Med. 2022, 18, 1413–1418. [Google Scholar] [CrossRef]
- Civelek, G.M.; Ciftkaya, P.O.; Karatas, M. Evaluation of restless legs syndrome in fibromyalgia syndrome: An analysis of quality of sleep and life. J. Back Musculoskelet. Rehabilitation 2014, 27, 537–544. [Google Scholar] [CrossRef]
- Dodson, C.; Bagai, K.; Weinstock, L.B.; Thompson, E.; Okamoto, L.E.; Peltier, A.; Raj, S.R.; Walters, A.S. Restless legs syndrome is increased in postural orthostatic tachycardia syndrome. Sleep Med. 2021, 17, 791–795. [Google Scholar] [CrossRef]
- Blum, D.J.; During, E.; Barwick, F.; Davidenko, P.; Zeitzer, J.M. 0009 Restless Leg Syndrome: Does It Start With A Gut Feeling? Sleep 2019, 42, A4–A4. [Google Scholar] [CrossRef]
- Riera, J.J.; Schousboe, A.; Waagepetersen, H.S.; Howarth, C.; Hyder, F. The micro-architecture of the cerebral cortex: Functional neuroimaging models and metabolism. NeuroImage 2008, 40, 1436–1459. [Google Scholar] [CrossRef]
- Jammoul, M.; Naddour, J.; Madi, A.; Reslan, M.A.; Hatoum, F.; Zeineddine, J.; Abou-Kheir, W.; Lawand, N. Investigating the possible mechanisms of autonomic dysfunction post-COVID-19. Auton. Neurosci. 2022, 245, 103071–103071. [Google Scholar] [CrossRef]
- Kaur, C.; Ling, E.-A. The circumventricular organs. 32. [CrossRef]
- Cytokines Driving Sympathetic Nervous System Activation In The Subfornical Organ: Implications For Heart Failure And Hypertension (2015) Gabriel Bassi https://brainimmune.
- Cutsforth-Gregory, J.K.; Benarroch, E.E. Nucleus of the solitary tract, medullary reflexes, and clinical implications. Neurology 2017, 88, 1187–1196. [Google Scholar] [CrossRef]
- Page, M.C.; Cassaglia, P.A.; Brooks, V.L.; Erdos, B.; Clifton, R.R.; Liu, M.; Li, H.; McCowan, M.L.; Sumners, C.; Scheuer, D.A.; et al. GABA in the paraventricular nucleus tonically suppresses baroreflex function: alterations during pregnancy. Am. J. Physiol. Integr. Comp. Physiol. 2011, 300, R1452–R1458. [Google Scholar] [CrossRef]
- Haam, J.; Popescu, I.R.; Morton, L.A.; Halmos, K.C.; Teruyama, R.; Ueta, Y.; Tasker, J.G. GABA Is Excitatory in Adult Vasopressinergic Neuroendocrine Cells. J. Neurosci. 2012, 32, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Kim, Y.-B.; Kim, J.S.; Bin Kim, W.; Kim, Y.S.; Han, H.C.; Colwell, C.S.; Cho, Y.-W.; Kim, Y.I. GABAergic inhibition is weakened or converted into excitation in the oxytocin and vasopressin neurons of the lactating rat. Mol. Brain 2015, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kakizawa, K.; Watanabe, M.; Mutoh, H.; Okawa, Y.; Yamashita, M.; Yanagawa, Y.; Itoi, K.; Suda, T.; Oki, Y.; Fukuda, A. A novel GABA-mediated corticotropin-releasing hormone secretory mechanism in the median eminence. Sci. Adv. 2016, 2, e1501723–1501723. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.M.; Abbott, G.; Mair, J.; Prescott, M.; Campbell, R.E. Mapping GABA and glutamate inputs to gonadotrophin-releasing hormone neurones in male and female mice. J. Neuroendocr. 2018, 30, e12657. [Google Scholar] [CrossRef]
- Wiens, S.C.; Trudeau, V.L. Thyroid hormone and γ-aminobutyric acid (GABA) interactions in neuroendocrine systems. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 2006, 144, 332–344. [Google Scholar] [CrossRef]
- Unnikrishnan P, Diabetes Insipidus, Dept of Neuroanesthesia SCTIMST, Trivandrum, Kerala, India https://www.uzhnu.edu. 2398.
- Miwa, K. Down-regulation of renin–aldosterone and antidiuretic hormone systems in patients with myalgic encephalomyelitis/chronic fatigue syndrome. J. Cardiol. 2017, 69, 684–688. [Google Scholar] [CrossRef]
- Pereira, G.; Gillies, H.; Chanda, S.; Corbett, M.; Vernon, S.D.; Milani, T.; Bateman, L. Acute Corticotropin-Releasing Factor Receptor Type 2 Agonism Results in Sustained Symptom Improvement in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Front. Syst. Neurosci. 2021, 15. [Google Scholar] [CrossRef]
- Coffin, S.T.; Black, B.K.; Biaggioni, I.; Paranjape, S.Y.; Orozco, C.; Black, P.W.; Dupont, W.D.; Robertson, D.; Raj, S.R. Desmopressin acutely decreases tachycardia and improves symptoms in the postural tachycardia syndrome. Hear. Rhythm. 2012, 9, 1484–1490. [Google Scholar] [CrossRef]
- Diep, P.-T.; Chaudry, M.; Dixon, A.; Chaudry, F.; Kasabri, V. Oxytocin, the panacea for long-COVID? a review. Horm. Mol. Biol. Clin. Investig. 2022, 43, 363–371. [Google Scholar] [CrossRef]
- Goldstein, JA. , Betrayal by the Brain: The Neurologic Basis of Chronic Fatigue Syndrome, Fibromyalgia Syndrome, and Related Neural Network 2012 Haworth Medical Press.
- Amir, S. Stimulation of the paraventricular nucleus with glutamate activates interscapular brown adipose tissue thermogenesis in rats. Brain Res. 1990, 508, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Frosst, P.; Blom, H.J.; Milos, R.; Goyette, P.; Sheppard, C.A.; Matthews, R.G.; Boers, G.J.H.; den Heijer, M.; Kluijtmans, L.A.J.; van den Heuve, L.P.; et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat. Genet. 1995, 10, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadeq, D.W.; Nasrallah, G.K. The Spectrum of Mutations of Homocystinuria in the MENA Region. Genes 2020, 11, 330. [Google Scholar] [CrossRef] [PubMed]
- Nefic, H, et al. “The Frequency of the 677C>T and 1298A>C Polymorphisms in the Methylenetetrahydrofolate Reductase (MTHFR) Gene in the Population” Med Arch. 2018 Jun;72(3):164-169.
- Carpenè, G.; Negrini, D.; Henry, B.M.; Montagnana, M.; Lippi, G. Homocysteine in coronavirus disease (COVID-19): a systematic literature review. Diagnosis 2022, 9, 306–310. [Google Scholar] [CrossRef]
- Regland, B.; Andersson, M.; Abrahamsson, L.; Bagby, J.; Dyrehag, L.E.; Gottfries, C.G. Increased Concentrations of Homocysteine in the Cerebrospinal Fluid in Patients with Fibromyalgia and Chronic Fatigue Syndrome. Scand. J. Rheumatol. 1997, 26, 301–307. [Google Scholar] [CrossRef]
- Bottiglieri, T.; Laundy, M.; Crellin, R.; Toone, B.K.; Carney, M.W.P.; Reynolds, E.H. Homocysteine, folate, methylation, and monoamine metabolism in depression. J. Neurol. Neurosurg. Psychiatry 2000, 69, 228–232. [Google Scholar] [CrossRef]
- Oner, P.; Yilmaz, S.; Doğan, S. High Homocysteine Levels Are Associated with Cognitive Impairment in Patients Who Recovered from COVID-19 in the Long Term. J. Pers. Med. 2023, 13, 503. [Google Scholar] [CrossRef]
- Ren, J.-C.; Wu, Y.-X.; Wu, Z.B.; Zhang, G.-H.; Wang, H.B.; Liu, H.B.; Cui, J.-P.B.; Chen, Q.; Liu, J.; Frank, A.; et al. MTHFR Gene Polymorphism Is Associated With DNA Hypomethylation and Genetic Damage Among Benzene-Exposed Workers in Southeast China. J. Occup. Environ. Med. 2018, 60, e188–e192. [Google Scholar] [CrossRef]
- Nikesjö, F.; Sayyab, S.; Karlsson, L.; Apostolou, E.; Rosén, A.; Hedman, K.; Lerm, M. Defining post-acute COVID-19 syndrome (PACS) by an epigenetic biosignature in peripheral blood mononuclear cells. Clin. Epigenetics 2022, 14, 1–6. [Google Scholar] [CrossRef]
- Fryar-Williams, S. Fundamental Role of Methylenetetrahydrofolate Reductase 677 C → T Genotype and Flavin Compounds in Biochemical Phenotypes for Schizophrenia and Schizoaffective Psychosis. Front. Psychiatry 2016, 7, 172. [Google Scholar] [CrossRef]
- Shaker, MM, et al. “Correlation of methylation status in MTHFR promoter region with recurrent pregnancy loss” J Genet Eng Biotechnol. 2021 Mar 22;19(1):44.
- Walsh, WJ. “Nutrient Power: Heal Your Biochemistry and Heal Your Brain”. 2012. [Google Scholar]
- Bonds, R.S.; Midoro-Horiuti, T. Estrogen effects in allergy and asthma. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Balnis, J.; Madrid, A.; Hogan, K.J.; Drake, L.A.; Adhikari, A.; Vancavage, R.; Singer, H.A.; Alisch, R.S.; Jaitovich, A. Persistent blood DNA methylation changes one year after SARS-CoV-2 infection. Clin. Epigenetics 2022, 14, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sarı, I.K.; Keskin, O.; Keskin, A.S.; Elli̇dağ, H.Y.; Harmandar, O. Is Homocysteine Associated with the Prognosis of Covid-19 Pneumonia. Int. J. Clin. Pr. 2023, 2023, 1–8. [Google Scholar] [CrossRef]
- Rhein, V.F.; Carroll, J.; He, J.; Ding, S.; Fearnley, I.M.; Walker, J.E. Human METTL20 Methylates Lysine Residues Adjacent to the Recognition Loop of the Electron Transfer Flavoprotein in Mitochondria. J. Biol. Chem. 2014, 289, 24640–24651. [Google Scholar] [CrossRef] [PubMed]
- de Vega, W.C.; Vernon, S.D.; McGowan, P.O. DNA Methylation Modifications Associated with Chronic Fatigue Syndrome. PLOS ONE 2014, 9, e104757. [Google Scholar] [CrossRef] [PubMed]
- Benelli, M.; Franceschini, G.M.; Magi, A.; Romagnoli, D.; Biagioni, C.; Migliaccio, I.; Malorni, L.; Demichelis, F. Charting differentially methylated regions in cancer with Rocker-meth. Commun. Biol. 2021, 4, 1–15. [Google Scholar] [CrossRef]
- McKinney, B.C.; Lin, C.-W.; Rahman, T.; Oh, H.; Lewis, D.A.; Tseng, G.; Sibille, E. DNA methylation in the human frontal cortex reveals a putative mechanism for age-by-disease interactions. Transl. Psychiatry 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Takahashi, T.; Ellingson, M.K.; Wong, P.; Israelow, B.; Lucas, C.; Klein, J.; Silva, J.; Mao, T.; Oh, J.E.; Tokuyama, M.; et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 2020, 588, 315–320. [Google Scholar] [CrossRef]
- Pujantell, M.; Altfeld, M. Consequences of sex differences in Type I IFN responses for the regulation of antiviral immunity. Front. Immunol. 2022, 13, 986840. [Google Scholar] [CrossRef]
- Chambers, P.W. (2022) Long Covid, Short Magnesium. Open Access Library Journal, 9, e8736. https://www.scirp.org/journal/paperinformation.aspx? 1174. [Google Scholar]
- Goetzl, EJ, et al. “Gender specificity of altered human immune cytokine profiles in aging” FASEB J. 2010 Sep;24(9):3580-9.
- De Benedetti, F.; Prencipe, G.; Bracaglia, C.; Marasco, E.; Grom, A.A. Targeting interferon-γ in hyperinflammation: opportunities and challenges. Nat. Rev. Rheumatol. 2021, 17, 678–691. [Google Scholar] [CrossRef]
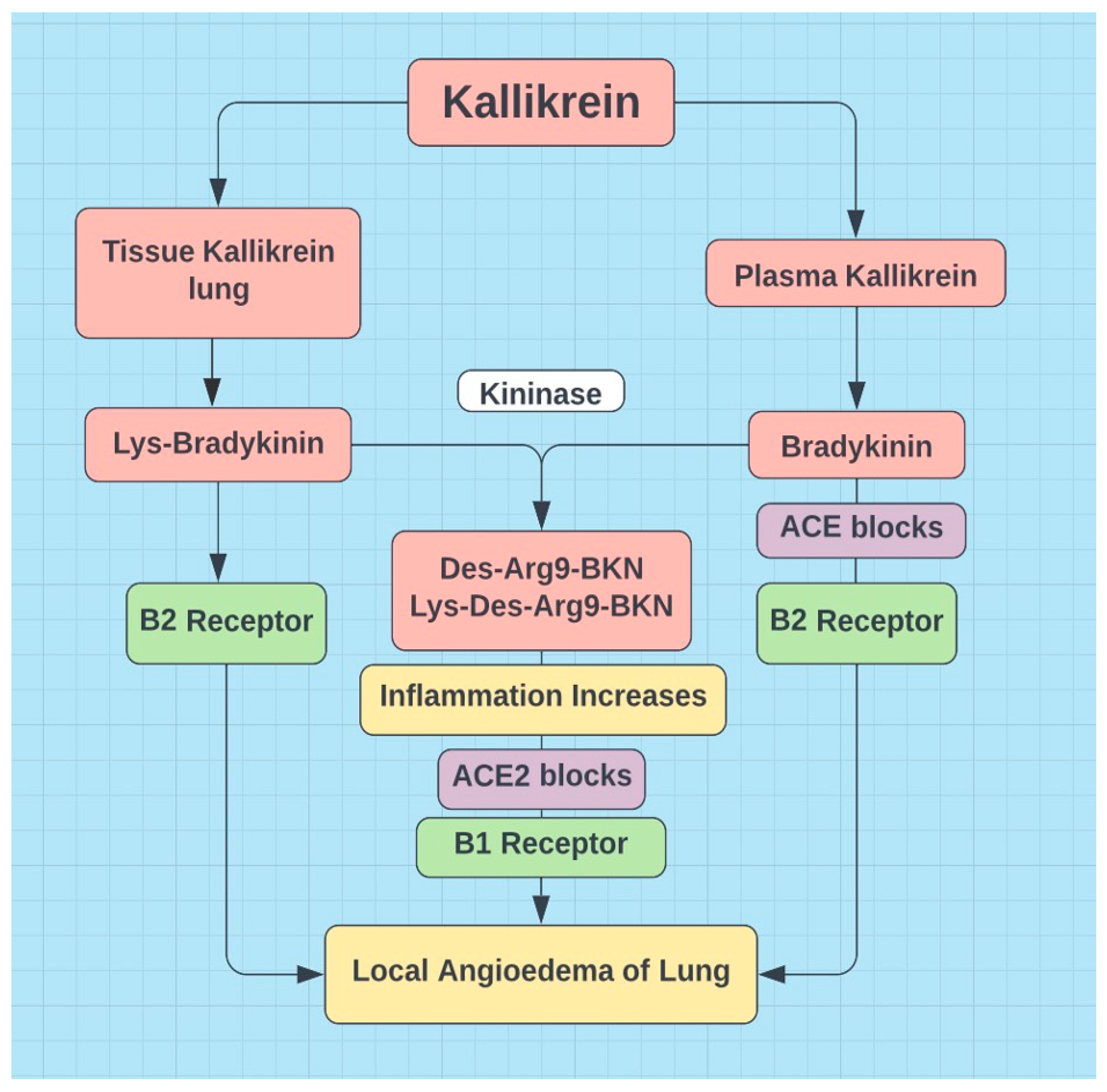
- Bossi, F.; Peerschke, E.I.; Ghebrehiwet, B.; Tedesco, F. Cross-talk between the complement and the kinin system in vascular permeability. Immunol. Lett. 2011, 140, 7–13. [Google Scholar] [CrossRef]
- Kuklina, E.M. T Lymphocytes as Targets for SARS-CoV-2. Biochem. (Moscow) 2022, 87, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Chambers, P.W. Basigin Binds Spike S on SARS-CoV2. OALib 2021, 08, 1–7. [Google Scholar] [CrossRef]
- Ma, Q.; Hao, Z.-W.; Wang, Y.-F. The effect of estrogen in coronavirus disease 2019. Am. J. Physiol. Cell. Mol. Physiol. 2021, 321, L219–L227. [Google Scholar] [CrossRef] [PubMed]
- Herrera, A.Y.; Hodis, H.N.; Mack, W.J.; Mather, M. Estradiol Therapy After Menopause Mitigates Effects of Stress on Cortisol and Working Memory. J. Clin. Endocrinol. Metab. 2017, 102, 4457–4466. [Google Scholar] [CrossRef] [PubMed]
- Stelzig, K.E.; Canepa-Escaro, F.; Schiliro, M.; Berdnikovs, S.; Prakash, Y.S.; Chiarella, S.E. Estrogen regulates the expression of SARS-CoV-2 receptor ACE2 in differentiated airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L1280–L1281. [Google Scholar] [CrossRef] [PubMed]
- Mendes, G.M.d.M.; Nascimento, I.J.B.D.; Marazzi-Diniz, P.H.; Da Silveira, I.B.; Itaborahy, M.F.; Viana, L.E.; Silva, F.A.; Santana, M.F.; Pinto, R.A.; Dutra, B.G.; et al. The des-Arg9-bradykinin/B1R axis: Hepatic damage in COVID-19. Front. Physiol. 2022, 13, 1080837. [Google Scholar] [CrossRef]
- van de Veerdonk, F.L.; Netea, M.G.; van Deuren, M.; van der Meer, J.W.; de Mast, Q.; Brüggemann, R.J.; van der Hoeven, H. Kallikrein-kinin blockade in patients with COVID-19 to prevent acute respiratory distress syndrome. eLife 2020, 9. [Google Scholar] [CrossRef]
- Stewart, J.M.; Taneja, I.; Glover, J.; Medow, M.S.; Lang, J.A.; Krajek, A.C.; McNeely, B.D.; Meade, R.D.; Fujii, N.; Seely, A.J.E.; et al. Angiotensin II type 1 receptor blockade corrects cutaneous nitric oxide deficit in postural tachycardia syndrome. Am. J. Physiol. Circ. Physiol. 2008, 294, H466–H473. [Google Scholar] [CrossRef]
- Kiowski, W.; Linder, L.; Kleinbloesem, C.; van Brummelen, P.; Bühler, F.R. Blood pressure control by the renin-angiotensin system in normotensive subjects. Assessment by angiotensin converting enzyme and renin inhibition. Circulation 1992, 85, 1–8. [Google Scholar] [CrossRef]
- Persson, P.B. Renin: origin, secretion and synthesis. J. Physiol. 2003, 552, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.L.; Jiang, L.; Adams, J.S.; Xu, Z.Z.; Shen, J.; Janssen, S.; Ackermann, G.; Vanderschueren, D.; Pauwels, S.; Knight, R.; et al. Vitamin D metabolites and the gut microbiome in older men. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.C.; Davis, C.T.; Zhu, W.; Bowman-Kirigin, J.A.; Walker, A.E.; Tai, Z.; Thomas, K.R.; Donato, A.J.; Lesniewski, L.A.; Li, D.Y. Dietary Vitamin D and Its Metabolites Non-Genomically Stabilize the Endothelium. PLOS ONE 2015, 10, e0140370–e0140370. [Google Scholar] [CrossRef]
- Moreira-Rosário, A.; Marques, C.; Pinheiro, H.; Araújo, J.R.; Ribeiro, P.; Rocha, R.; Mota, I.; Pestana, D.; Ribeiro, R.; Pereira, A.; et al. Gut Microbiota Diversity and C-Reactive Protein Are Predictors of Disease Severity in COVID-19 Patients. Front. Microbiol. 2021, 12, 705020. [Google Scholar] [CrossRef] [PubMed]
- Bikle, DD. “Vitamin D metabolism, mechanism of action, and clinical applications”. Chem Biol. 21.3 (2014): 319-29.
- Warren, T.; McAllister, R.; Morgan, A.; Rai, T.S.; McGilligan, V.; Ennis, M.; Page, C.; Kelly, C.; Peace, A.; Corfe, B.M.; et al. The Interdependency and Co-Regulation of the Vitamin D and Cholesterol Metabolism. Cells 2021, 10, 2007. [Google Scholar] [CrossRef]
- Ashangari, C.; Suleman, A. Abstract 121: Vitamin D Deficiency Study in Postural Orthostatic Tachycardia Syndrome. Circ. Cardiovasc. Qual. Outcomes 2015, 8. [Google Scholar] [CrossRef]
- Machado, CD. “The human gut microbiome displays diurnal and seasonal rhythmic patterns,” abstract 395, on Sunday, , at 11:20 am, Digestive Disease Week (2023) in Chicago, IL https://news.ddw. 7 May.
- Yildiz, S.; Mazel-Sanchez, B.; Kandasamy, M.; Manicassamy, B.; Schmolke, M. Influenza A virus infection impacts systemic microbiota dynamics and causes quantitative enteric dysbiosis. Microbiome 2018, 6, 1–17. [Google Scholar] [CrossRef]
- Groeneveld, J.M.; Ballering, A.V.; van Boven, K.; Akkermans, R.P.; Hartman, T.C.O.; A Uijen, A. Sex differences in incidence of respiratory symptoms and management by general practitioners. Fam. Pr. 2020, 37, 631–636. [Google Scholar] [CrossRef]
- Al-Khaldy, N.S.; Al-Musharaf, S.; Aljazairy, E.A.; Hussain, S.D.; Alnaami, A.M.; Al-Daghri, N.; Aljuraiban, G. Serum Vitamin D Level and Gut Microbiota in Women. Healthcare 2023, 11, 351. [Google Scholar] [CrossRef]
- Waterhouse, M.; Hope, B.; Krause, L.; Morrison, M.; Protani, M.M.; Zakrzewski, M.; Neale, R.E. Vitamin D and the gut microbiome: a systematic review of in vivo studies. Eur. J. Nutr. 2018, 58, 2895–2910. [Google Scholar] [CrossRef]
- Singh, P.; Rawat, A.; Alwakeel, M.; Sharif, E.; Al Khodor, S. The potential role of vitamin D supplementation as a gut microbiota modifier in healthy individuals. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Blitshteyn, S. Vitamin B1 deficiency in patients with postural tachycardia syndrome (POTS). Neurol. Res. 2017, 39, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Know, Lee. T: Mitochondria and the Future of Medicine, 2018.
- Atanassova, N. and Koeva, Y. (2012) Hydrohysteroid Dehydrogenases—Biological Role and Clinical Importance—Review. In: Canuto, R.A., Ed., Dehydrogenases, IntechOpen, London. [CrossRef]
- Depeint, F.; Bruce, W.R.; Shangari, N.; Mehta, R.; O’Brien, P.J. Mitochondrial function and toxicity: Role of the B vitamin family on mitochondrial energy metabolism. Chem. Biol. Interact. 2006, 163, 94–112. [Google Scholar] [CrossRef] [PubMed]
- Azzini, E.; Ruggeri, S.; Polito, A. Homocysteine: Its Possible Emerging Role in At-Risk Population Groups. Int. J. Mol. Sci. 2020, 21, 1421. [Google Scholar] [CrossRef]
- Plasma Methylation Enzyme and Nutrition Guide, Doctor’s Data, Inc., St. Charles, Illinois https://site-akiajqrf22xmaqzsiz6q.s3.amazonaws.com/DDI+Website/Resource+Guides/78338+DDI+PlasmaMethylationEnzyme+WP_R4.
- Weinstock, L.B.; Brook, J.B.; Myers, T.L.; Goodman, B. Successful treatment of postural orthostatic tachycardia and mast cell activation syndromes using naltrexone, immunoglobulin and antibiotic treatment. BMJ Case Rep. 2018, 2018. [Google Scholar] [CrossRef]
- Uy, P.; Jehangir, A.; Yap, J.E.; Rao, S. S493 SIBO and SIFO Prevalence in Patients With Ehlers-Danlos Syndrome Based on Duodenal Aspirates/Culture. Am. J. Gastroenterol. 2021, 116, S218–S219. [Google Scholar] [CrossRef]
- Rao, S.S.C.; Bhagatwala, J. Small Intestinal Bacterial Overgrowth. Clin. Transl. Gastroenterol. 2019, 10, e00078. [Google Scholar] [CrossRef]
- Sánchez-Pérez, S.; Comas-Basté, O.; Duelo, A.; Veciana-Nogués, M.T.; Berlanga, M.; Latorre-Moratalla, M.L.; Vidal-Carou, M.C. Intestinal Dysbiosis in Patients with Histamine Intolerance. Nutrients 2022, 14, 1774. [Google Scholar] [CrossRef]
- Courseault, J.; Kingry, C.; Morrison, V.; Edstrom, C.; Morrell, K.; Jaubert, L.; Elia, V.; Bix, G. Folate-dependent hypermobility syndrome: A proposed mechanism and diagnosis. Heliyon 2023, 9, e15387. [Google Scholar] [CrossRef]
- Hadtstein, F.; Vrolijk, M. Vitamin B-6-Induced Neuropathy: Exploring the Mechanisms of Pyridoxine Toxicity. Adv. Nutr. Int. Rev. J. 2021, 12, 1911–1929. [Google Scholar] [CrossRef]
- Chambers P, Antioxidants and Long Covid. Open Access Library Journal (2022) 9:e9414. https://www.scirp.org/journal/paperinformation.aspx? 1208.
- Sfera, A.; Thomas, K.G.; Sasannia, S.; Anton, J.J.; Andronescu, C.V.; Garcia, M.; Sfera, D.O.; Cummings, M.A.; Kozlakidis, Z. Neuronal and Non-Neuronal GABA in COVID-19: Relevance for Psychiatry. Reports 2022, 5, 22. [Google Scholar] [CrossRef]
- Campbell, D.J.; Kladis, A.; Valentijn, A.J. Effects of Losartan on Angiotensin and Bradykinin Peptides and Angiotensin-Converting Enzyme. J. Cardiovasc. Pharmacol. 1995, 26, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Chen, Z.-L.; Ghosh, D.; Strickland, S.; Norris, E.H. Increased plasma bradykinin level is associated with cognitive impairment in Alzheimer's patients. Neurobiol. Dis. 2020, 139, 104833–104833. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, J.; Kim, H.; Kim, J.S. Correlation of ACE2 with RAS components after Losartan treatment in light of COVID-19. Sci. Rep. 2021, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, P.G.; Wong, S.; AL Mulhim, N.; Palmer, L.E.; Miners, J.S. Angiotensin-converting enzyme 2 is reduced in Alzheimer’s disease in association with increasing amyloid-β and tau pathology. Alzheimer's Res. Ther. 2016, 8, 1–10. [Google Scholar] [CrossRef]
- Bastolla, U.; Chambers, P.; Abia, D.; Garcia-Bermejo, M.-L.; Fresno, M. Is Covid-19 Severity Associated With ACE2 Degradation? Front. Drug Discov. 2022, 1. [Google Scholar] [CrossRef]
- Mahmudpour, M.; Roozbeh, J.; Keshavarz, M.; Farrokhi, S.; Nabipour, I. COVID-19 cytokine storm: The anger of inflammation. Cytokine 2020, 133, 155151–155151. [Google Scholar] [CrossRef]
- Yang, T.; Yang, Y.; Wang, D.; Li, C.; Qu, Y.; Guo, J.; Shi, T.; Bo, W.; Sun, Z.; Asakawa, T. The clinical value of cytokines in chronic fatigue syndrome. J. Transl. Med. 2019, 17, 1–12. [Google Scholar] [CrossRef]
- Zhang, X, et al. “TGF-β1 factor in the cerebrovascular diseases of Alzheimer’s disease”. Eur Rev Med Pharmacol Sci. 20.24 (2016): 5178-5185. https://pubmed.ncbi.nlm.nih. 2805.
- Zhang, Z.; Liu, C.; Gan, Z.; Wang, X.; Yi, Q.; Liu, Y.; Wang, Y.; Lu, B.; Du, H.; Shao, J.; et al. Improved Glucose-Stimulated Insulin Secretion by Selective Intraislet Inhibition of Angiotensin II Type 1 Receptor Expression in Isolated Islets of db/db Mice. Int. J. Endocrinol. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Chang, C.-H.; Chang, Y.-C.; Wu, L.-C.; Lin, J.-W.; Chuang, L.-M.; Lai, M.-S. Different angiotensin receptor blockers and incidence of diabetes: a nationwide population-based cohort study. Cardiovasc. Diabetol. 2014, 13, 91–91. [Google Scholar] [CrossRef]
- Villapol, S.; Saavedra, J.M. Neuroprotective Effects of Angiotensin Receptor Blockers. Am. J. Hypertens. 2014, 28, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, L.B.; Schmeidler, J.; Lesser, G.T.; Beeri, M.S.; Purohit, D.P.; Grossman, H.T.; Haroutunian, V. Less Alzheimer disease neuropathology in medicated hypertensive than nonhypertensive persons. Neurology 2009, 72, 1720–1726. [Google Scholar] [CrossRef] [PubMed]
- van Dalen, J.W.; Marcum, Z.A.; Gray, S.L.; Barthold, D.; van Charante, E.P.M.; van Gool, W.A.; Crane, P.K.; Larson, E.B.; Richard, E. Association of Angiotensin II–Stimulating Antihypertensive Use and Dementia Risk. Neurology 2020, 96, e67–e80. [Google Scholar] [CrossRef] [PubMed]
- Wood, E, et al. “Role of mitochondria, oxidative stress and the response to antioxidants in myalgic encephalomyelitis/chronic fatigue syndrome: A possible approach to SARS-CoV-2 ‘long-haulers’?” Chronic Dis Transl Med. 7.1 (2021): 14-26.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




