1. Introduction
Toxoplasma gondii (
T. gondii) is an obligate intracellular parasite that infects approximately one- third of the human population. Members of the feline species are definitive hosts of
T. gondii while humans are intermediate hosts [
1]. Toxoplasmosis is more commonly presented as a latent infection whereas those infected are asymptomatic; however, severe symptomatic disease may precipitate in immunocompromised individuals and congenitally infected fetuses [
2]. Symptomatic
T. gondii infection may present as lymphadenopathy, chorioretinitis, hydrocephalus, and mental status changes [
2]. Toxoplasmosis of immunocompromised individuals, such as AIDS patients, is often a result of reactivation of latent infection ultimately presenting as neurological dysfunctions [
3]. For example, an increased frequency of Toxoplasma encephalitis in AIDS patients is recorded, specifically those with CD4+ T cell counts less than 200 cells/µL [
4]. Of the myriad clinical presentations in individuals infected with
T. gondii, the neuropsychiatric findings and behavioral changes associated with this parasite continue to be an area of research not well understood. Recent studies have suggested that latent
T. gondii infection is not entirely asymptomatic, with evidence of behavioral alterations in both rodents and humans infected with
T. gondii such as increased impulsivity, aggression, risky behavior, and suicidal behavior [
5,
6,
7]. Furthermore, many studies have found higher incidence of
T. gondii infection in schizophrenia patients as well as anxiety, depression, and other mental health illnesses [
8,
9]. However,
T. gondii infection in the context of drug dependence and the development of substance use disorder (SUD) remain to be elucidated. The mechanism for these neuropsychiatric changes is still unclear; however, these mechanisms are linked to
T. gondii’s dormant stages in the brain, subsequent neuroinflammation, and dopamine metabolism. Therefore, the purpose of the current review is to investigate various drugs of abuse and their associations with
T. gondii infection, and the role of inflammation and dopamine metabolism in the context of drug dependence and
T. gondii infection.
2. Methods
This review followed the Preferred Reporting Items for Systematic Reviews guidelines. The included articles were searched on the electronic databases, PubMed, Web of Science, and Scopus. Systematic review was included if published between the 1st of January 1970 and to the 30th of December 2022. Different key words and phrases were adopted: “T. gondii infection”, “Toxoplasmosis”, “T. gondii and drug use”, “T. gondii infection and dopamine”, “T. gondii infection and drugs of abuse”, “drugs of abuse”, “T. gondii infection and alcohol”, “SUD”, “Toxoplasmosis and SUD” and “Substance abuse and inflammation”. The keywords were matched through the Boolean operators AND or OR in the databases. Information related to the first author and year of publication, the objective of the study, the conclusion of the study, population screened, and main results were stored. A description and narrative synthesis was adopted to describe the results.
3. Results
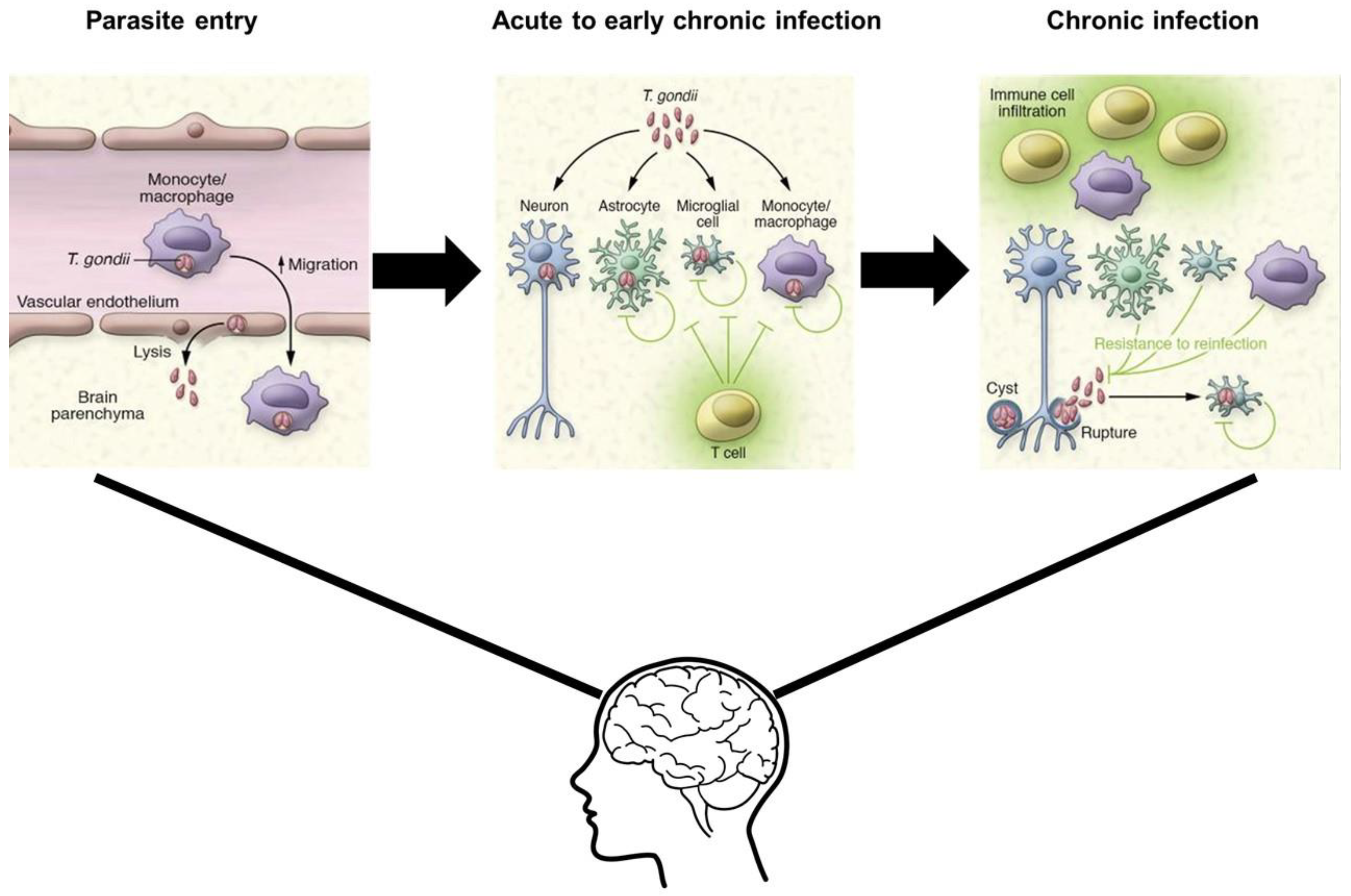
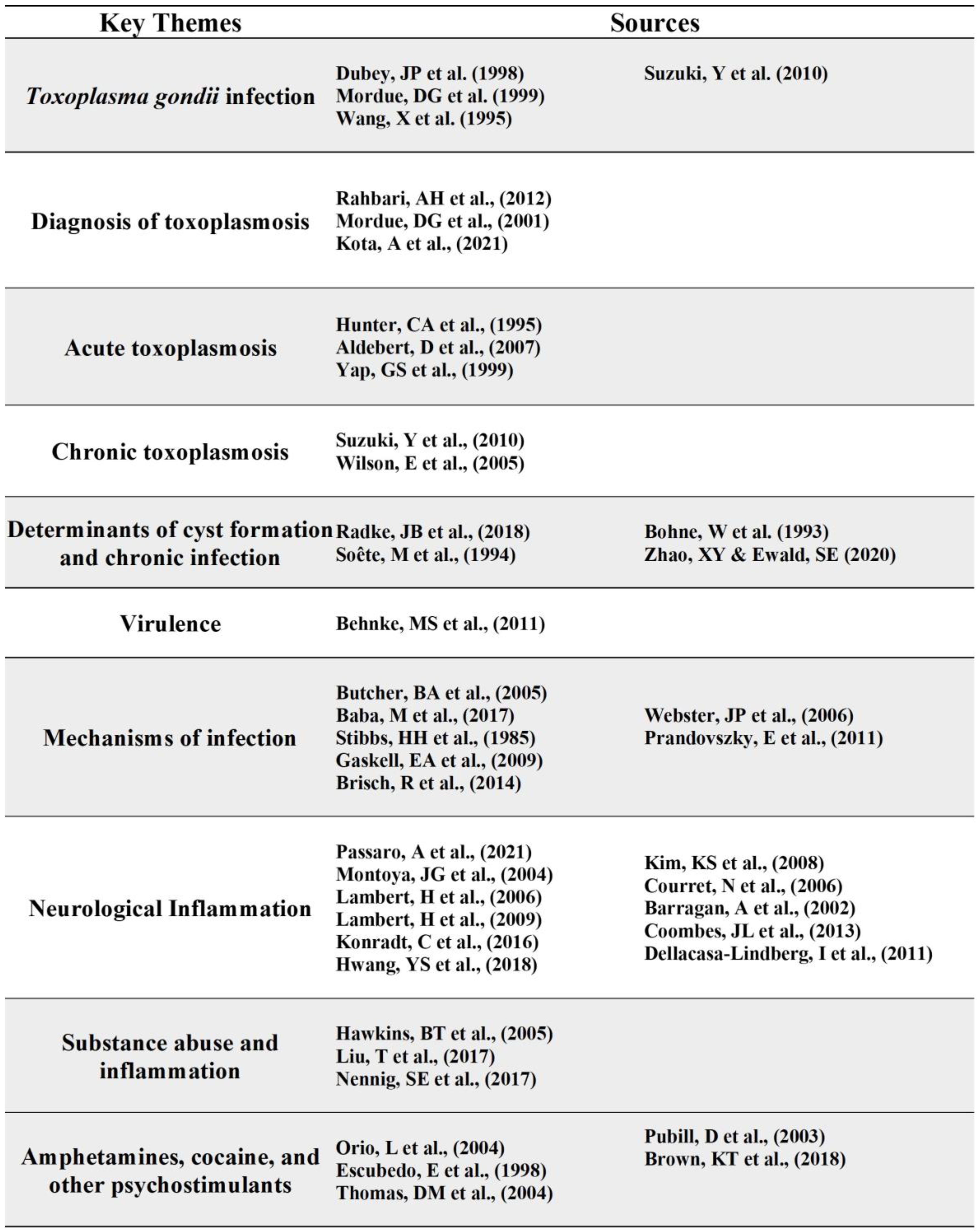
T. gondii Infection
Toxoplasma gondii (
T. gondii) is an obligate intracellular parasite transmitted primarily through the consumption of oocyst contaminated foods [
10]. Feline species are definitive hosts of
T. gondii and can shed highly infectious oocysts into the environment generating a wide variety of intermediate hosts [
10]. The three main stages in its life cycle are the following: sporozoites (oocysts), tachyzoites (replicative stage), and bradyzoites (tissue cysts) [
10]. Once the environmentally stable oocysts have been ingested by the intermediate host, the parasite transitions to the acute stage of infection [
10]. This stage involves rapidly replicating tachyzoites that disseminate throughout the body. Tachyzoites replicate every 6 to 8 hours within an intracellular parasitophorous vacuole which is essential to the growth of the parasite [
11].
T. gondii infection transitions to the bradyzoite stage, a slower replicating form, characterized by the presence of tissue cysts that lie dormant in muscle and brain tissue marking chronic infection with the parasite [
10]. Immunocompetent individuals with acquired toxoplasmosis are generally asymptomatic and are protected from symptomatic re-infection by host immune defenses. In contrast, immuno-compromised individuals with chronic infection, such as patients with AIDS, can experience reactivation of the latent infection in the brain, otherwise known as cerebral toxoplasmosis, and other organs where tissue cysts lie dormant [
10]. Reactivation of latent infection in the brain can be life threatening as bradyzoites convert to actively replicating tachyzoites resulting in Toxoplasma encephalitis (TE). Acute TE is a result of the parasite infecting microglia, astrocytes, and neurons and then later persisting in mainly neurons, marking chronic TE [
12]. During the transition from acute to chronic TE, the CD4+ and CD8+ T cell levels gradually decline, with CD8+ T cells at very low levels during chronic TE [
13]. Therefore, to understand the complexity of
T. gondii infection in both immunocompetent and immunocompromised individuals, the characteristics of acute versus chronic infection and the specific mechanisms
T. gondii utilizes to manipulate host immune responses must be identified (
Figure 1).
Diagnosis of Toxoplasmosis
Diagnosis of toxoplasmosis relies on the presence of specific immunoglobulin M and G (IgM and IgG) antibodies to Toxoplasma antigens. Acute toxoplasmosis can be identified by the presence of Toxoplasma-specific IgM antibodies; however, it has been reported that IgM may last longer in the serum following infection [
14]. Therefore, the IgG avidity test determines the timeline of acquired infection based on IgG affinity, with low avidity IgG antibodies indicating acute infection [
14]. Transmission can also occur congenitally during acute toxoplasmosis in a seronegative mother. Tachyzoites present in the blood may cross the placenta and infect the fetus leading to serious complications and birth defects [
10]. Conclusively,
T. gondii infection can be narrowed down to acute and chronic infection. Acute infection is characterized by actively replicating tachyzoites that, in immunocompetent individuals, can be cleared by host immune defenses. The parasites that do survive persist as slow-growing bradyzoite tissue cysts in tissues such as the brain, eye, cardiac, and skeletal muscle [
15]. Chronic infection is still poorly understood but can be characterized by the sustained immune response to
T. gondii. This is illustrated by the elevated Toxoplasma-specific IgG and IFN-γ in the sera [
16].
Acute Toxoplasmosis
Less well understood is the immunological response to infection. Nevertheless,
T. gondii infection relies on the manipulation of host immunity through the secretion of specific immunoregulatory cytokines, control of gene transcription, and modulation of cell adhesion and migration pathways. The Th1 type cell-mediated immune response is the primary resistance mechanism to the
T. gondii infection. This response involves the production of IL-12 by dendritic cells, macrophages, and neutrophils that then stimulates natural killer cells and T lymphocytes to produce IFN-γ [
17,
18]. IFN-γ plays a major role in controlling both acute and chronic stages of infection, by activating anti-parasitic effector cells such as macrophages, fibroblasts, and astrocytes [
19]. For immunocompetent individuals, the host adaptive immune response is imperative for the control of replication during an acute infection and the eventual formation of cysts in the brain and other organs that persist throughout the host’s life. Within a few weeks following acute infection, the host immune response eliminates tachyzoites. However, in immunocompromised individuals this process is hindered by the reduced CD4+ T cells population, leading to a decrease in IFN-γ levels and subsequent chronic infection in the brain [
20].
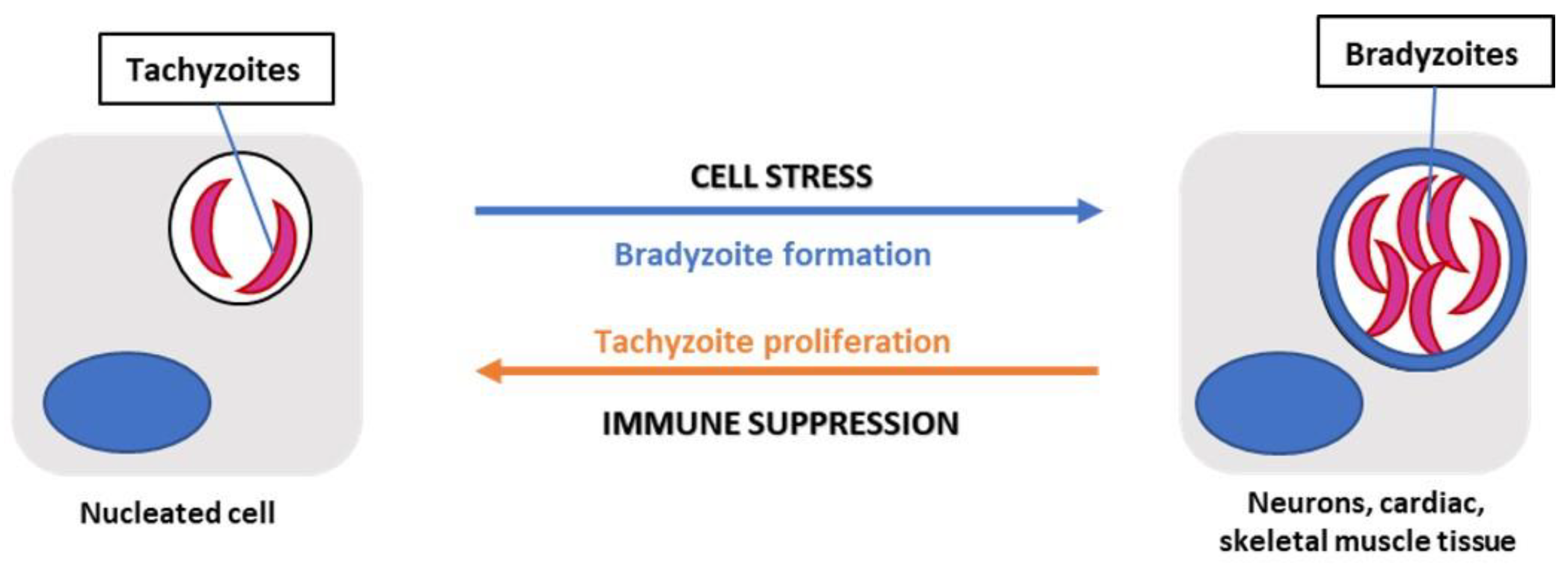
Chronic Toxoplasmosis
Chronic
T. gondii infection is characterized by the prevalence of bradyzoites and tissue cysts. These bradyzoites can be found in the brain as early as 3 days following infection and mature into tissue cysts primarily located in the grey matter and neurons of the brain [
10]. CD8+ T cells are key adaptive immune system mediators for the control of chronic infection and preventing reactivated infection in the brain. CD8+ T cells stimulate production of pro-inflammatory cytokines, predominantly IFN-γ, as well as anti-inflammatory cytokines, such as IL-17 and IL-27 [
20]. Activated CD8+ T cells have also been shown to lyse Toxoplasma-infected cells through perforin-mediated cytotoxic activity [
21]. A recent study performed by Suzuki et. al. suggests that this perforin-mediated cytotoxic activity by CD8+ T cells was able to remove Toxoplasma cysts from the brain [
21]. IL-10 inhibits the production of IL-12, IFN-y, TNF-a, and IL-6 from macrophages and microglia in the brain, thus limiting the lethal inflammatory response that causes TE [
22]. Ultimately, understanding the key players involved in host immune response is crucial for controlling
T. gondii infection and can provide new insights for potential therapeutic targets to reduce disease.
Virulence
Virulence is influenced by the genotype of the parasite. Type I strains are considered highly virulent in mice compared to Type II strains, which are more commonly associated with human toxoplasmosis [
27]. During infection, the control of toxoplasmosis is characterized by an elevated IFN-y dependent Th1 cytokine response. Type 1 (RH) and Type 2 (ME49/PTG)
T. gondii strains, elevate IFN-y, TNF-a, IL-12, and IL-18 expression during lethal infection [
11]. Ultimately, the expression of specific genes and virulence are important characteristics of this parasite’s life cycle and can be tracked via the associated immune response.
Mechanisms of Infection
Parasite egression is another key component of
T. gondii infection and dissemination into the brain. One mechanism is through infection of leukocytes as a vehicle for transport into target organs. For instance,
T. gondii has been shown to infect host macrophages thus causing a suppression of proinflammatory cytokines, such as IL-12 and TNF-a. This is accomplished by manipulation of the signaling cascade, Nuclear Factor- kappa B (NF-kB), specifically through the activation of STAT3 [
28]. The parasite can then cross capillary walls of the target organ by infecting endothelial cells. In a study conducted by Baba et. al., it was revealed that adhesion of
T. gondii tachyzoite infected leukocytes to endothelial cells triggered immediate egression of the parasite into the target organ, thus preventing the opportunity for an immune attack [
29]. The mechanism of parasite egress is a crucial aspect of
T. gondii infection and warrants further research into the specific cytokine expression during the transition from acute to chronic stage.
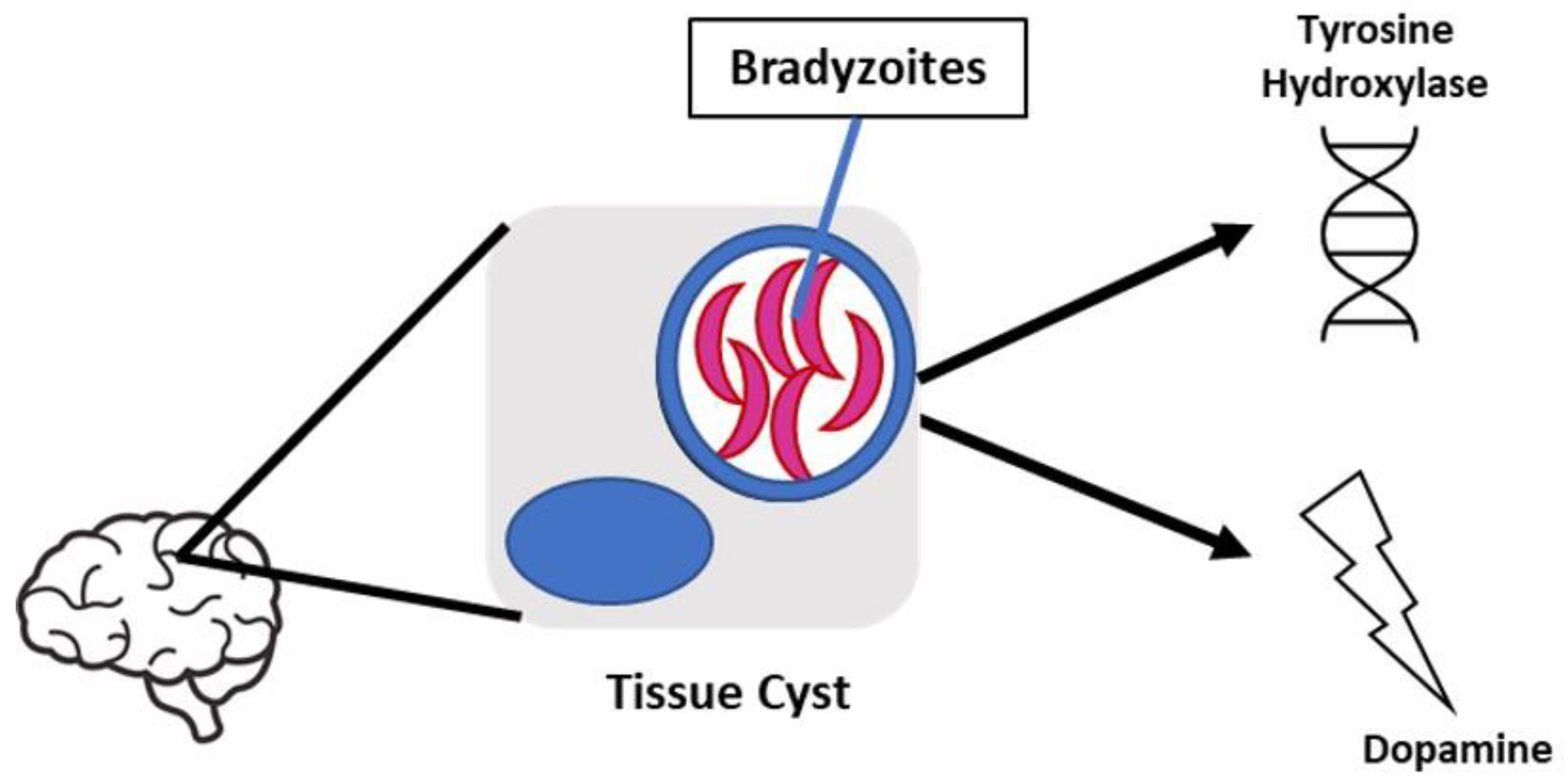
Behavioral manipulation is a key component of
T. gondii infection which may be attributed to alterations in dopamine metabolism; however, the mechanism for these changes is currently unknown. In a previous study conducted by Stibbs et al, it was observed that dopamine levels were 14% higher in mice with chronic
T. gondii infection compared to controls, while other neurotransmitters were unchanged [
30]. Subsequently, recent studies have identified two possible mechanisms that may play a role in behavior manipulation, 1) alteration of neurotransmitter signal transduction, indicated by the cessation of parasite-induced behavioral changes when administered medications for psychiatric diseases, and 2) the presence of tyrosine hydroxylase encoded in the parasite’s genome [
31]. Regarding psychiatric disease,
T. gondii seroprevalence has been increasingly associated with schizophrenia. Dopamine dysregulation has been shown to play a significant role in the development of schizophrenia which is why the principal antipsychotic medication used in its treatment is dopamine antagonist, haloperidol [
32]. Webster et al found that haloperidol as well as valproic acid, a mood stabilizing medication also used in the treatment of schizophrenia, prevented the development of behavior changes in latent toxoplasmosis- infected rodents [
33]. Furthermore, tyrosine hydroxylase is a rate-limiting enzyme involved in dopamine synthesis and induced during differentiation of the parasite’s tissue cyst stages. A study conducted by Prandovszky et al found tyrosine hydroxylase present in intracellular brain tissue cysts as well as a three-fold increase in dopamine release from
T. gondii infected dopaminergic cells compared to uninfected cells in vitro [
34]. Ultimately the presence of this enzyme may be responsible for the increased dopamine levels observed during infection. With the understanding that in brain tissue,
T. gondii cysts contain hundreds of bradyzoites, it is plausible that chronic
T. gondii infection could enhance dopamine release
in vivo. Taken together, dysregulation of dopamine metabolism can cause serious consequences on human behavior and potentially lead to neurological or psychiatric disorders, making it crucial to examine whether
T. gondii infection directly or indirectly alters dopamine levels in the brain (
Figure 3).
Neurological Inflammation
The innate immune response of the central nervous system consists of resident macrophages, known as microglia, as well as mast cells, astrocytes, and neurons. These microglia serve as primary immune cells in the brain and spinal cord with the functions of phagocytosis and activation of cytokines [
35]. Chronic
T. gondii infection is characterized by the presence of dormant bradyzoite cysts localized in muscle tissue and more dangerously, the brain. Toxoplasma encephalitis (TE) is caused by the reactivation of these bradyzoite cysts into fast-replicating tachyzoites resulting in serious brain damage, especially for immunocompromised individuals [
36]. However, it is still unclear how
T. gondii enters the brain and evades the host immune system. Recent reports suggest that tachyzoites disrupt the host leukocytes’ natural migration phenotype, such as dendritic cells and disseminate systemically through a “Trojan Horse” mechanism to rapidly reach the brain [
37,
38]. The “Trojan Horse” mechanism is characterized by
T. gondii tachyzoites that infect host leukocytes and cross the blood brain barrier without being detected [39-42]. Moreover, Konradt et al. observed that tachyzoites invade and lyse endothelial cells in brain vasculature prior to dissemination in the central nervous system [
43]. In addition to leukocytes, studies test the role of glial cells for parasite migration and dissemination. Dellacasa-Lindberg et al. showed that Toxoplasma-infected microglia exhibited minimal upregulation of major histocompatibility complex (MHC) II molecules and the costimulatory molecule, CD86, as well as a dramatic migratory phenotype when infected with live intracellular tachyzoites44. However, when studying IFN-y stimulated microglia, this migratory phenotype was minimal in comparison to the tachyzoite-infected microglia [
44]. Therefore, which cytokines are expressed that promote parasite migration and entry into the brain constitutes a noteworthy area of research. Hwang et al. showed an elevated and maintained expression of IL-12 and TGF-B for 12 weeks post infection as well as activation of the M1-type microglia, indicating a mixed anti-inflammatory and pro-inflammatory immune response for the control of infection [
45]. Taken together, these studies suggest that parasite migration and dissemination require tachyzoite infection of host leukocytes and glial cells; however, further research must be conducted to understand the specific cytokine profile involved which may elucidate how
T. gondii evades the host immune response upon entry to the brain, thus providing foundational knowledge for future therapies to combat the disease.
Substance abuse and Inflammation
Substance use has highly stimulating effects in the central nervous system that could lead to drug addiction and serious health complications, such as memory loss and cognitive decline. These conditions are primarily associated with neuroinflammation and neurotoxicity that cause neuronal cell damage. Recent research has shown that drugs of abuse disrupt the blood brain barrier (BBB) by altering tight junction formation making the brain susceptible to neuroinflammation [
46]. Alternatively, the neuroinflammation resulting from exposure to drugs of abuse can disrupt the BBB further increasing its permeability to foreign toxins. An important aspect of the innate immune response and regulation of inflammation is the transcription factor, nuclear factor kappa light chain enhancer of activated B cells (NFkB) [
47]. NFkB activation, upon exposure to drugs of abuse, causes innate immune gene induction leading to further inflammation and a progressive increase in addiction [
48]. In the following sections, drugs of abuse and their effects on inflammation will be reviewed.
Amphetamines, Cocaine, and other Psychostimulants
The neuropharmacology of amphetamines enhances the release of dopamine and serotonin as well as glutamate into the CNS. However, chronic amphetamine use can result in neurotoxicity marked by activated microglia [
49]. Microglial activation is also involved in methamphetamine-induced neurotoxicity, specifically in the striatum, cortex, and hippocampus [
50,
51,
52]. Subsequently, the activation of microglia results in secretion of proinflammatory cytokines and other reactive species that can cause damage to neuronal tissue. There is limited research on the specific inflammatory mediators involved, but Orio et al was able to show a significant increase in IL-1b after administration of MDMA [
49]. Therefore, neuroinflammation following amphetamine-related drugs of abuse can be noted by the increase in activation of microglia and subsequent secretion of proinflammatory cytokines as well as oxidative stress. Likewise, cocaine use activates astrocytes through signaling of innate immune receptors and subsequent upregulation of proinflammatory cytokines, such as IL-1b. For example, Brown et al observed that chronic cocaine use activated innate immune signaling pathways that ultimately contribute to the mechanisms of cocaine seeking [
53]. Furthermore, chronic cocaine abuse is implicated in HIV-1 associated neurological complications. This is illustrated by the enhancement of HIV-1 replication, increased permeability of the BBB, and the secretion of cytokines that decline CD4+ T cell counts. For example, Barr et al demonstrated that acute cocaine administration increased BBB permeability to the tracer, sodium fluorescein [
54]. Regarding cytokine expression, it was recently shown that active cocaine users expressed higher levels of IL-6 and downregulation of IL-10, an anti-inflammatory cytokine [
54]. Conversely, it was shown that active cocaine users have decreased IL-6 and TNF-a expression [
54]. Therefore, further research must be conducted to understand the inflammatory response upon psychostimulant exposure as it may provide context for the development of severe disease in immunocompromised individuals.
Alcohol
Alcohol use induces variable effects on neuroinflammation responses that ultimately causes neurodegeneration. For example, Crews et al observed that ethanol-induced NF-kB activation mediates the neurotoxicity caused by
in vivo models of binge ethanol administration [
55]. Specifically, alcohol treatment of brain slice cultures resulted in increased NF-kB binding to DNA markers in gene promoter regions and decreased protective transcription factor binding [
55]. These specific transcription factors promote neuronal survival and protection from excitotoxicity. The increase in NF-kB transcription results in upregulation of proinflammatory genes in the brain and less protection leaving the brain susceptible to damage [
55]. Chronic alcohol exposure increases proinflammatory gene expression through activation of toll-like receptor 4 (TLR4), however acute exposure has shown opposite results. For example, Pruett et al showed a weakened TNF-α, IL-1β, and IL-6 immune response to LPS upon acute ethanol exposure in mice [
56]. As for chronic administration, one study showed a marked increase in proinflammatory gene induction upon binge treatment with alcohol for 10 days in mice followed by LPS once the alcohol was cleared from system [
57]. In addition to cytokine expression, microglia activation is another marker of alcohol’s role in facilitating inflammation. It has been shown that alcohol can activate microglia directly through the stimulation of TLRs or indirectly from release of damage- associated molecular patterns [
58,
59]. Ultimately, the stimulation of these microglia is characterized by proinflammatory cytokine expression, oxidative stress, and further neuronal damage. Taken together, alcohol both acute and chronic administration influence neuroinflammation which increases neurodegeneration and the potential development of chronic illness.
Opioids
Opioid use is primarily associated with pain management; however, it is also implicated in the manipulation of inflammatory pathways. Like cocaine, opioid abusers have worse overall health and have an increased susceptibility to infectious disease, such as HIV infection. This is illustrated by immunosuppression upon opioid exposure such as decreased activation and chemotaxis of leukocytes and granulocytes upon morphine administration [
60]. Regarding cytokine expression, there is variation as to which cytokines are upregulated and downregulated. Piepenbrink et al has shown heroin users have higher levels of inflammatory cytokines such as TNF-a and IL-8 [
61]. However, Meijerink et al observed heroin abusers have lower levels of proinflammatory cytokines after the immune cells were in vitro stimulated by bacterial endotoxin lipopolysaccharide (LPS) [
62]. Furthermore, acute, and chronic administration of morphine produces mixed cytokine expression as shown in various rodent models. In both acute and chronic administration there was an increase in peripheral IL-1b expression as well as in CNS preparations, including the NAc [
63]. Additionally, there was an increase in IL-6 and IL-17 upon chronic administration and an increase in IL-10, TNF-a, IL-2, and IFN-c upon acute administration [
64,
65,
66]. This finding is indicative of the immunosuppressive properties of opioid use as well as an increased risk of developing chronic illness.
Hallucinogens
The inflammatory effects of hallucinogens or psychedelics are still not well understood, but recent studies have suggested anti-inflammatory and immune-modulating properties of these substances. For example, LSD has shown the ability to suppress the proliferation of B cells and the production of pro-inflammatory cytokines IL-2, IL-4, and IL-6 in in vitro splenic lymphocytes derived from female rats [
67]. Additionally, a human study looking at ayahuasca effects in healthy volunteers observed a decrease in CD4 and CD3 cells and an increase in natural killer (NK) cells compared to placebo group and subjects treated with D-amphetamine [
68]. Furthermore, one study observed suppression of pro-inflammatory cytokine production upon administration of harmine, a component of ayahuasca, in mice treated with LPS simulating acute kidney injury [
69]. Ultimately, these studies suggest that these substances may have therapeutic effects for autoimmune diseases, but there is still a need for deeper investigation of these drugs’ effects on the immune system.
Marijuana
Marijuana, otherwise known as cannabis, contains cannabinoid compounds that act on various receptors involved in regulating the immune response. This can be exemplified by anti-inflammatory effects because of decreased release of pro-inflammatory mediators [
70]. The specific receptor proteins, CB1 and CB2, interact with cannabidiol (CBD) to provide anti-inflammatory and antioxidant properties [
70]. However, it is important to distinguish that CBD is the modulator of these properties as opposed to THC which does not affect the serum levels of pro-inflammatory cytokines [
71]. The role of the endocannabinoid system regarding neuroinflammation is exemplified by the increased expression of CB1 receptor and the potential upregulation of CB2 receptors upon neuroinflammatory conditions in the brain [
70]. A study observing the cytokine expression in rats’ brain upon treatment with LPS showed a decrease of TNF-α, IL-6, IL-1b, and IL-12 levels when cannabinoids were administered [
70]. The study also showed an increase in the production of TNF-α, IL-6, IL-1b, and IL-10 when cannabinoids were administered alone [
72]. This data shows that the endocannabinoid system has conflicting effects with cytokine expression, but there is a significant impact on their production and function. Additionally, the presence of TNF-α induces expression of CB1 and CB2 which is partially a result of activating NF-kB which triggers transcription of inflammatory proteins and cannabinoid receptors [
73] (
Figure 4).
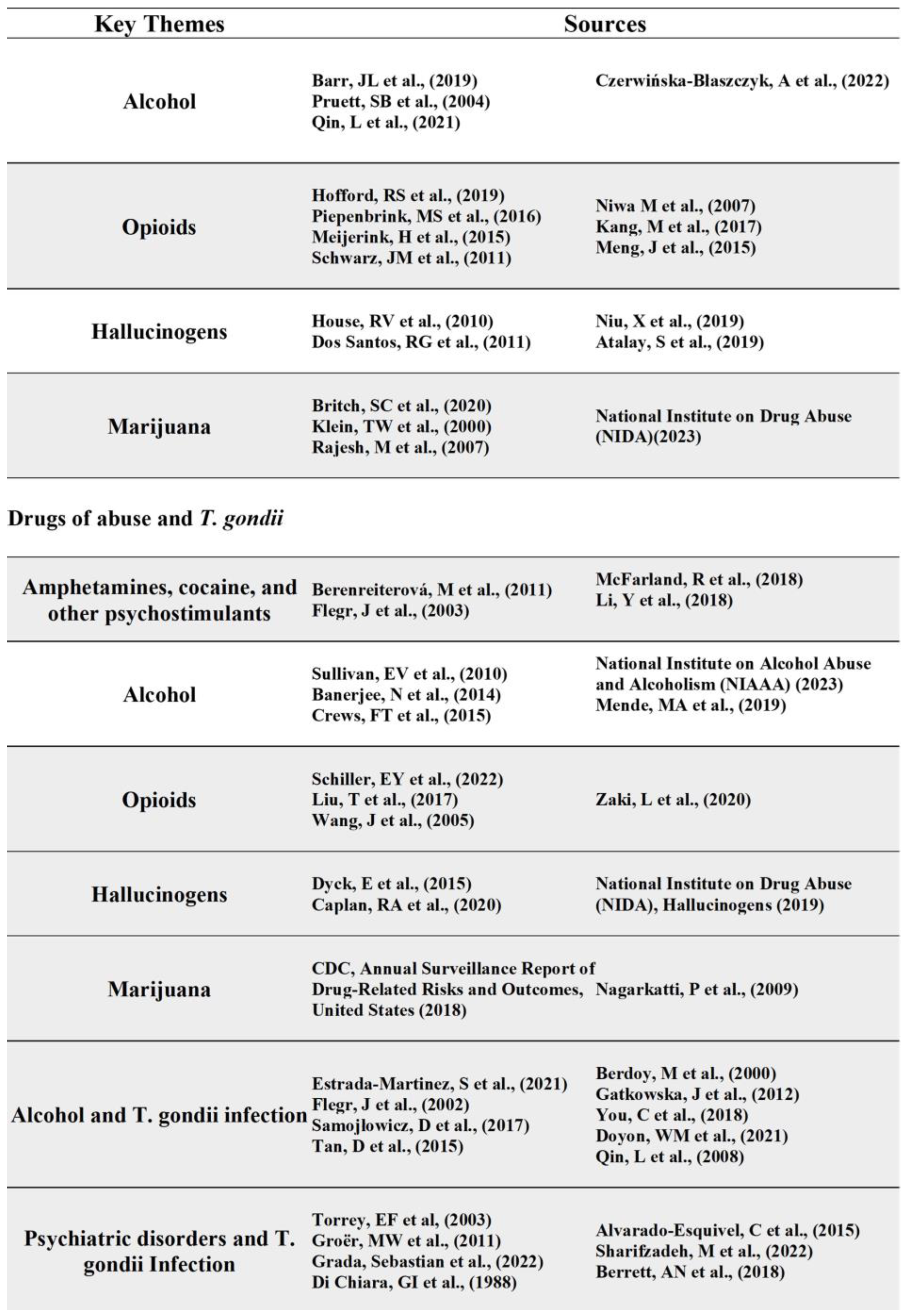
Drugs of Abuse and T. gondii
Drug abuse, a devastating and harmful neuropsychiatric disorder, continues to be a widely concerned public health issue. Drugs of abuse, such as alcohol, cocaine/psychostimulants, hallucinogens, opioids, and marijuana, all contribute to neurotoxicity and inflammation leading to further complications in the brain. In this section, we will review each drug of abuse to describe their mechanism of action and examine biological connections with T. gondii infection.
Amphetamines, Cocaine, and other Psychostimulants
Cocaine and psychostimulants, including amphetamines, methamphetamines, MDMA, etc. are sympathomimetics that inhibit catecholamine reuptake thus causing increases in blood pressure, heart rate, and body temperature. Abuse of these drugs can result in severity of these symptoms and possible death. According to the National Center for Health Statistics (NCHS) at the Centers for Disease Control and Prevention (CDC), the number of deaths since 2014 involving psychostimulants has significantly increased each year with 23,837 deaths in 2020 [
74]. Cocaine related death has also increased since 2014 with 19,447 deaths report in 2020 [
74]. These drugs act on the brain by increasing the availability of norepinephrine, dopamine, and serotonin at the synapse thus changing behavior and providing a sense of euphoria, alertness, agitation, and hyperactivity. However, more dangerous side effects of cocaine and psychostimulant abuse involve paranoia, psychosis, and hallucinations. Additionally, the inhibition of dopamine and monoamine reuptake can result in imbalanced free radical accumulation leading to oxidative stress and neuroinflammation. This neuroinflammation can be illustrated by activation of microglia and other inflammatory mediators that increase blood brain barrier permeability thus leading to loss of integrity and susceptibility to peripheral toxins in the brain. Likewise,
T. gondii infection has been shown to alter neurotransmitter transmission either directly or indirectly. The direct effect can be illustrated by the presence of cysts in specific areas of the brain such as the ventral tegmental area or amygdala resulting in behavioral modification [
75]. Furthermore, the immunological mechanisms elicited by the parasite may indirectly modulate monoamine pathways in the brain. Flegr et al suggested that dopamine (DA) is increased by activated cytokines, such as IL-2, upon infection [
76]. Further evaluation of DA transmission was studied by McFarland et al who observed that chronic
T. gondii infection in mice led to a blunted response to cocaine or amphetamines as well as a decreased the expression of dopamine transporter (DAT) and vesicular monoamine transporter 2 (VMAT2) [
77]. In addition to dopamine, Li et al showed chronic
T. gondii infection in mice induced anti-N-methyl-D-Aspartate (anti-NMDA) autoantibodies which was most likely triggered by tissue cysts [
78]. Ultimately, the relationship between cocaine/psychostimulants and
T. gondii infection regarding neurotransmitter pathways warrants further research on the effect of infection on abuse behavior and the potential development of psychiatric disease.
Alcohol
Alcohol is known for its depressant properties on the central nervous system but has dose-dependent side effects. Lower blood alcohol levels result in changes in personality, impaired judgement, and distractibility. As the levels increase, one may experience gait instability, visual disturbances, and more dangerously coma or even death due to respiratory depression [
79]. According to the 2019 National Survey on Drug Use and Health (NSDUH), 14.5 million people ages 12 years and older had alcohol use disorder (AUD). Additionally, an estimated 95,000 people die from alcohol related causes annually, making alcohol the third-leading preventable cause of death in the United States [
80]. Alcohol acts on the brain by exerting inhibitory or excitatory effects on dopaminergic, NMDA, and GABAergic systems, with chronic use increasing tolerance and addiction [
81]. Additionally, alcohol exposure induces the NF-kB pathway leading to persistent neuroimmune responses and glutamate excitotoxicity [
81]. Furthermore, alcohol abuse alters neuroplasticity and neural circuitry therefore accelerating cognitive decline [
82]. Like cocaine and psychostimulants, alcohol has been shown to cause brain injury due to neuroinflammation and cerebral atrophy [
83]. Likewise,
T. gondii can infect the same pathways and contributes to neurotoxicity indicative of an overlap with alcohol’s mechanism of action in the brain that may play a role in the development of further disease and modified behavior.
Opioids
Prescription opioids are used primarily for the management of pain but have been at the center of misuse in the United States since the early 2000s. This public health crisis has been further exacerbated by the circulation of illegal opioids such as heroin and fentanyl [
84]. Opioids provide analgesic properties by acting as agonists primarily on the mu opioid receptors distributed throughout the central and peripheral nervous system. According to the NCHS report, opioid overdose deaths steadily increased from 21,088 in 2010 to 68,630 deaths in 2020 [
84]. The side effects presented with opioid intoxication vary from person to person but can include euphoria, agitation, delirium, and miotic pupils. Continued use of opioids can result in increased tolerance and dependence that may lead to withdrawal or overdose. Side effects of overdose result in respiratory depression, and can lead to altered mental state, and even death [
85]. Additionally, opioid abuse can also have long term complications such as increased susceptibility to infection and memory deficits. Regarding infection, opioids also alter blood brain barrier permeability through the upregulation of pro-inflammatory cytokines and tight junction protein disruption. A study by Wang et al observed that morphine impaired host innate immune response and increases the susceptibility to Streptococcus pneumoniae lung infection [
86].
T. gondii infection follows a similar mechanism of action to opioids by crossing the blood brain barrier and contributing to neuroinflammation. Furthermore, Zaki et al showed that mice receiving morphine before
T. gondii infection showed a significantly lower survival rate, increase in parasite load, and IFN-y level compared to mice treated with morphine after infection (p<0.01) [
87]. This study illustrates an alternative link between the response of
T. gondii infection to the therapeutic effects of opioids. Therefore, it is crucial to understand the overlap between opioid use and
T. gondii infection as it may provide context for the development of further disease or potential resistance to therapeutic opioid use.
Hallucinogens
Hallucinogens, such as d-lysergic acid diethylamide (LSD), psilocybin, and mescaline, are “mind altering” drugs derived from over 90 plant species or developed synthetically. Botanical forms have been used by humans for many years, often associated with sacred rituals and religious ceremonies. Synthetic forms, such as LSD, were developed in the early 1940s by Albert Hofmann, but were quickly banned by the federal government [
88]. However, recent studies have shown these compounds provide psychotherapeutic effects opening the door for further research. Hallucinogens primarily function through the serotonergic pathways by binding to and activating the 5-HT2 serotonin receptors. This activation in conjunction with other serotonin, dopamine, and adrenergic receptors contributes to the psychedelic effects of these compounds. Acute intoxication with hallucinogens may present with autonomic hyperactivity, such as fever, tachycardia, mydriasis, and hypertension. In more severe cases, serotonin syndrome might develop resulting in a triad of altered mental status, neuromuscular hyperactivity, and exacerbated autonomic dysfunction. The psychedelic effects include perceptual distortions such as depersonalization, derealization, and hallucinations as well as distortions of time and space and an altered state of consciousness. Long term use of hallucinogens is still not well understood; however, several reports of “flashbacks” have been made amongst 15% to 77% of users [
89]. Additionally, long term users can develop persistent psychosis or hallucinogen persisting perception disorder (HPPD) both of which are often seen in people who have a history of mental illness [
90]. Although there is not a concrete link between
T. gondii infection and hallucinogens, the manipulation of neurotransmitter transmission may suggest a potential relationship regarding behavior manipulation and potential development of disease.
Marijuana
Marijuana, also termed as cannabis or weed, has been used recreationally for its euphoric, “mind altering” effects attributed to the presence of tetrahydrocannabinol (THC). Marijuana- related disorders may become more prevalent with its increasing legalization amongst the United States. According to the CDC, approximately 4 million people in 2016 reported having a marijuana use disorder characterized by health problems, persistent or increasing use, and failure to meet daily life responsibilities [90, 91]. Conversely, marijuana has been used for medicinal and therapeutic purposes. For example, it has been used to combat nausea, pain, and provide other anti-inflammatory effects. The mechanism of action involves the activation of cannabinoid receptors located throughout the central and peripheral nervous system. Acute intoxication presents impaired short- term memory, incoordination, impaired judgment, and systemically as scleral injection, tachycardia, hypertension, and urinary retention. Other effects include initial anxiety, depersonalization, and euphoria which at higher doses could induce paranoia and psychosis. Chronic use can result in emotional lability, anxiety, insomnia, hyperreflexia, and diaphoresis. Additionally, marijuana use should be avoided during pregnancy and breastfeeding as it may increase the risk for complications. Regarding infection, cannabinoids obtain immunosuppressive properties exhibited by the decrease in TNF-α, IFN-γ, and GM-CSF levels [
92]. However, further research must be conducted to understand the effects on other immune mediators. Ultimately, the initial understanding of anti-inflammatory properties provides a potential link with the mechanism of
T. gondii infection in the brain (
Figure 5).
Further Evidence Linking T. gondii Infection and Alcohol Use Disorder
Alcohol exposure can cause dysregulation of the immune system; however, the association between
T. gondii infection and alcohol consumption is not well understood. In a study looking at the seroepidemiology of infection with
T. gondii in Durango, Mexico, there was a positive association between alcohol consumption and anti-
T. gondii IgG and IgM antibodies, specifically an 11.2% seroprevalence [
93]. Additionally, those infected with
T. gondii have displayed increased risky behavior due to the parasite’s ability to infect and persist in the central nervous system. Previous studies have attributed risky behavior to increased road traffic accidents and suicidal behaviors [
94]. Samojlowicz et al. studied this association between latent toxoplasmosis and excessive alcohol consumption by observing seroprevalence in three groups: risky behavior, control, and inconclusively risky behavior [
95]. The risky behavior group included data from deaths attributed to risky behavior such as: traffic accidents, suicide, substance overdose, and other risky behavior, such as disregard for reasonable safety precautions. Of the 535 individuals used in the study, anti IgG antibodies were found in 46.4% of all evaluated, with 51.1% of seropositive individuals being in the risky behavior group. When observing individual causes of death in the risky behavior group, a higher proportion of seropositive subjects were associated with substance overdose (53%, with 29.9% attributed to alcohol overdose) and other risky behavior (55%). Ultimately, the data indicated a strong positive correlation between anti-
T. gondii IgG antibodies and alcohol consumption when considering the relationship between
T. gondii infection and risky behavior [
95]. The underlying mechanism of parasite-induced behavior modification in humans is still unclear; however, evidence suggests that the presence of cysts diffusely localized throughout the brain plays a major role. One hypothesis for
T gondii’s ability to manipulate behavior involves the dysregulation of dopaminergic (DA) signaling in the brain, specifically in the nucleus accumbens (NAc) and ventral tegmental area (VTA). Moreover, infected rats display reduced DA content in the NAc and an increase in impulsivity compared to controls [
96]. In addition, Berdoy et al. demonstrated an increase in attraction rather than aversion when
T. gondii infected rodents were exposed to odors from the members of the family Felidae, suggesting that the parasite alters neurological activity of its intermediate hosts [
97]. Regarding other areas of the brain, Gatkowska et al. explained that the presence of cysts in the hippocampus and amygdala revealed diminished exploratory activity and less grooming behavior in infected mice compared to uninfected mice [
98].
Given that the NAc and VTA are areas of the brain important for regulating reward and reinforcement, there is a well described association between alcohol and dopamine transmission in the NAc and VTA [
99]. Doyon et al. observed DA neurons being stimulated by alcohol and an increase in the proportion of these neurons exhibiting pacemaker-like firing patterns [
100]. In addition to its role in the brain, alcohol has been shown to significantly alter immune function and inflammation in those affected. When administered daily doses of alcohol, mice exhibited an increase in proinflammatory cytokines, TNF-α, and MCP-I, as well as an enhanced cytokine response when pretreating lipopolysaccharide (LPS) induced mice with alcohol [
101]. More importantly, a prolonged proinflammatory response and subsequent decrease in IL-10 production in the brain occurs, as opposed to occurring in the liver and serum of alcohol exposed mice [
101]. Taken together, these studies suggest an increased risk of
T. gondii infection for those who consume or are exposed to alcohol. However, further research must be conducted to understand the specific cytokines expressed when simultaneously exposed to alcohol and infected with
T. gondii in both the acute and chronic stages.
Further Evidence Linking T. gondii Infection and Psychiatric Disorders
T. gondii infection has been linked to several behavioral and psychiatric disorders due to its presence in the brain and influence on dopamine transmission. For example,
T. gondii seropositivity has been associated with schizophrenia due to abnormal dopamine transmission [
102] as well as a risk factor for the development of depression and other psychiatric disorders [
103]. In addition to dopamine, there was a significantly higher prevalence of anti-
T. gondii IgG antibodies among patients in Western Romania with schizophrenia (69.77%), personality/behavior disorders (76.74%), and mental disorders concerning alcohol abuse (84.62%) compared to controls (p = 0.009, p = 0.005, p = 0.043, respectively) [
104]. Likewise, substance use is linked to the manipulation of dopamine transmission and the development of these diseases [
105]. But there is still a lack of information associated with
T. gondii infection and substance use. In a case control study performed in 2015 by Alvarado-Esquivel et al., it appeared that behaviors associated with substance abuse did not increase the risk
T. gondii infection, suggesting that latent toxoplasmosis may influence the degree of substance use rather than substance use increasing likelihood of infection [
106]. Additionally, in a case control study conducted by Sharifzadeh et al., drug dependent individuals in Iran were shown to have higher exposure to
T. gondii, through seroprevalence of anti-
T. gondii IgG antibody, compared to non-dependent individuals. The study further discussed the socioeconomic impact on drug dependent individuals as a contributing factor toward exposure to
T. gondii infection [
107]. Moreover, Berrett et al. observed that
T. gondii seropositivity was associated with a reduced likelihood of self-reported substance use, e.g tobacco, marijuana, heroin, and methamphetamines. The study discusses that this reduction in self-reporting could be due to
T. gondii’s influence on dopaminergic signaling in the brain [
108]. This data illustrates an association between
T. gondii and substance use, particularly with the overlap of dopaminergic signaling in both the parasite’s life cycle and drug dependence. Further research must be conducted to fully understand the biological effects of these associations.
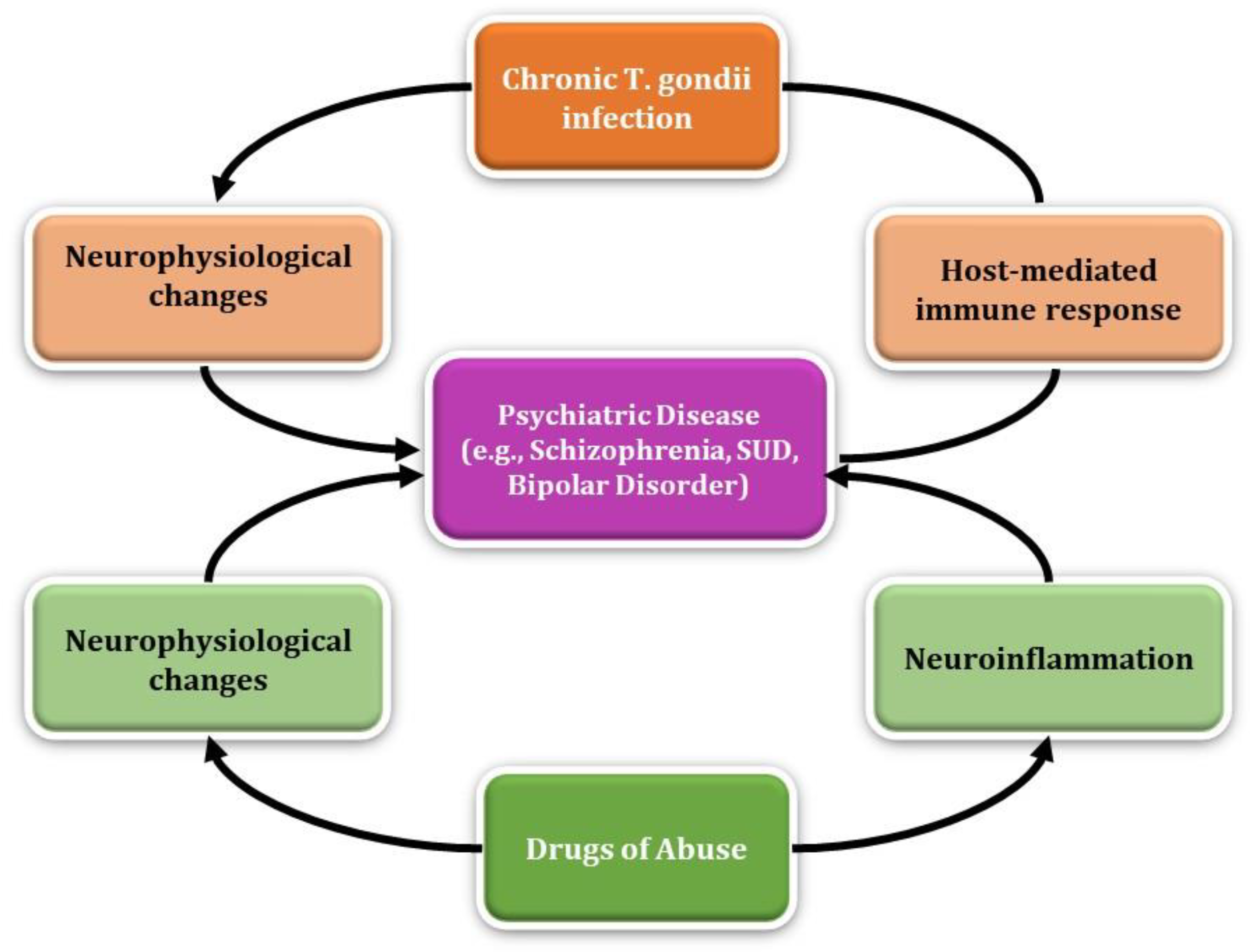
4. Discussion
T. gondii is a complex, intracellular parasite responsible for the manipulation of host immune pathways and subsequent neuropsychiatric consequences that are especially detrimental in immunocompromised individuals. Likewise, drugs of abuse continue to be a major public health crisis potentially leading to the development of substance use disorder (SUD). This systematic review provides potential connections between the two topics, setting the foundation for further research, however
T. gondii infection in the context of drug dependence and the development of SUD remains to be elucidated. It is important to characterize the mechanisms associated with dopamine metabolism of drug dependence and withdrawal in the context of
T. gondii infection, evaluate the role of inflammation in the brain, and identify potential drug-and-sex specific underpinnings of these associations. It is understood that
T. gondii infection has both acute and chronic stages that can be potentially detrimental in immunocompromised individuals. Chronic toxoplasmosis is characterized by bradyzoites and tissue cysts leading to stimulation of CD8+ T cells and production of pro-inflammatory cytokines. Additionally, control of this parasite is characterized by the production of anti-inflammatory cytokines, such as IL-10, limiting a severe inflammatory response. However, these protective mechanisms are impaired in immunocompromised individuals making that population more susceptible to developing Toxoplasma encephalitis (TE) and other tissue damage. There is still ongoing research into the inflammatory effects of specific drugs of abuse, with preliminary data showing mixed results. As stated previously, some drugs of abuse have immunosuppressive effects, such as alcohol, posing the question of whether those with SUD may protect
T. gondii from host immunity during chronic infection. The highly stimulating effects of these substances in the brain and their ability to disrupt the BBB permeability pose a risk for further neurological damage associated with
T. gondii infection. Currently, research on the immunological effects of alcohol is more prevalent compared to other drugs of abuse, making alcohol abuse a useful model for understanding
T. gondii infection in the context of drug dependence. In the Durango, Mexico seroepidemiology study, the positive association between alcohol consumption and anti-
T. gondii antibodies provides preliminary evidence of the link between the two. However, it may be worth simplifying the research to understand the key biological markers and cytokine expression involved in
T. gondii infection under alcohol exposure. This may elucidate the mechanisms of parasite entry and the potential protective effects employed by alcohol for
T. gondii to evade host immunity. Understanding these mechanisms may provide foundational knowledge of this complex parasite’s effects in the brain and the additional risks of SUD in the context of opportunistic infections (
Table 1).
Aside from the immunological perspective, chronic
T. gondii infection can lead to behavioral changes such as impulsivity, aggression, and self-directed violence. Additionally,
T. gondii infection has been linked to the development of schizophrenia and other psychiatric diseases due to its increase in dopamine metabolism, neuromodulating effects, and the presence of tyrosine hydroxylase in its genome. The studies evaluating dopamine antagonist medication, such as haloperidol, on
T. gondii associated behavior changes could be expanded into therapeutic use for
T. gondii infected individuals, given the evidence of reduced predator-risk behavior in infected rats. Therefore, evaluating other anti-psychotic medications and the anti-
T. gondii medications, such as pyrimethamine and sulfadiazine, will improve understanding as to their inhibitory effects regarding behavior changes associated with the parasite. Dopamine is associated with human behavior including pleasure, aggression, memory, and substance use disorder; however, the result of the studies on the association of
T. gondii infection and drugs of abuse are not well understood. The frequency of substance abuse may be associated with substance-induced modification of dopamine-receptor densities and basal dopaminergic activity. Likewise,
T. gondii can directly or indirectly influence dopaminergic activity in infected cells making one more susceptible to neuropsychiatric complications. For example, the studies evaluating the effects of psychostimulants, like cocaine and amphetamines, and the reduced expression of key signaling receptors in chronic
T. gondii infected mice implicates the parasite in blunting of host response to dopaminergic drugs. Ultimately, this review illustrates the many parallels in the mechanisms elicited by drugs of abuse and
T. gondii infection, such as immunomodulating effects and neurophysiological changes, that may pose a risk for developing psychiatric disease. The parallels between these entities poses two questions: 1) whether
T. gondii infection can predispose those with an SUD to developing further psychiatric disease and 2) whether SUD is a risk factor for developing behavioral and psychiatric complications associated with
T. gondii infection (
Table 2).
5. Conclusions
The current state of research on T. gondii infection is expanding, but there remains several unanswered questions regarding its inflammatory response and neuropsychiatric consequences. Likewise, research on drugs of abuse have long been untouched, but with the recent support, attempts to understand their effects have increased. The purpose of this review was to provide a comprehensive summary of the existing research on both topics and the potential connections between the two. The current limitations in research include a gap in knowledge on T. gondii’s cytokine expression profile upon parasite entry to the brain, its effects on the inflammatory response, and dopamine metabolism of T. gondii infection upon administration of drugs of abuse. Additionally, further research is necessary to understand the immunomodulating effects of various drugs of abuse in the context of T. gondii infection, thus providing foundational knowledge on potential protective mechanisms. Limitations of this review include excluded literature due to narrowed analysis of studies found under specific keywords in each database. Based on the results, this review provides foundational knowledge on the hypothesis that SUD is a potential risk factor for behavioral and psychiatric complications associated with T. gondii infection. However, further research is necessary to understand T. gondii infection in the context of drug dependence, withdrawal, and the complex development of SUD.
Author Contributions
AS provided substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; AS and VJ drafted the article or revised it critically for important intellectual content; VJ gave final approval of the version of the article to be published; all authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Dr. Scott Steffensen and Ben for their contributions and guidance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dubey JP, Miller NL, Frenkel JK. The Toxoplasma gondii oocyst from cat feces. J Exp Med. 1970;132(4):636-662. [CrossRef]
- Halonen SK, Weiss LM. Toxoplasmosis. Handb Clin Neurol. 2013;114:125-145. [CrossRef]
- Robert-Gangneux, F., & Dardé, M. L. (2012). Epidemiology of and diagnostic strategies for toxoplasmosis. Clinical microbiology reviews, 25(2), 264–296. [CrossRef]
- Luft, B. J., & Remington, J. S. (1992). Toxoplasmic encephalitis in AIDS. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 15(2), 211–222. [CrossRef]
- Flegr, J., Havlícek, J., Kodym, P., Malý, M., & Smahel, Z. (2002). Increased risk of traffic accidents in subjects with latent toxoplasmosis: a retrospective case-control study. BMC infectious diseases, 2, 11. [CrossRef]
- Ling V.J., Lester D., Mortensen P.B., Langenberg P.W., Postolache T.T.: Toxoplasma gondii seropositivity and suicide rates in women. J. Nerv. Ment. Dis. 2011; 199: pp. 440-444.
- Kocazeybek B., Oner Y.A., Turksoy R., et. al.: Higher prevalence of toxoplasmosis in victims of traffic accidents suggest increased risk of traffic accident in Toxoplasma -infected inhabitants of Istanbul and its suburbs. Forensic Sci. Int. 2009; 187: pp. 103-108.
- Sugden K., Moffitt T.E., Pinto L., Poulton R., Williams B.S., Caspi A.: Is Toxoplasma gondii infection related to brain and behavior impairments in humans? Evidence from a population-representative birth cohort. PLoS One 2016; 11:.
- Flegr J. Effects of toxoplasma on human behavior. Schizophr Bull. 2007;33(3):757-760. [CrossRef]
- Dubey JP, Lindsay DS, Speer CA. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin Microbiol Rev. 1998;11(2):267-299. [CrossRef]
- Mordue DG, Hakansson S, et al. Toxoplasma gondii resides in a vacuole that avoids fusion with host cell endocytic and exocytic vesicular trafficking pathways. Experimental parasitology. 1999;92(2):87–99.
- Wang, Xisheng et al. “Importance of CD8(+)Vbeta8(+) T cells in IFN-gamma-mediated prevention of toxoplasmic encephalitis in genetically resistant BALB/c mice.” Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research vol. 25,6 (2005): 338-44. [CrossRef]
- Suzuki, Yasuhiro et al. “Removal of Toxoplasma gondii cysts from the brain by perforin-mediated activity of CD8+ T cells.” The American journal of pathology vol. 176,4 (2010): 1607-13. [CrossRef]
- Rahbari, A. H., Keshavarz, H., Shojaee, S., Mohebali, M., & Rezaeian, M. (2012). IgG avidity ELISA test for diagnosis of acute toxoplasmosis in humans. The Korean journal of parasitology, 50(2), 99–102. [CrossRef]
- Mordue DG, Monroy F, et al. Acute Toxoplasmosis Leads to Lethal Overproduction of Th1 Cytokines. The Journal of Immunology, 2001, 167: 4574-4584.
- Sturge, Carolyn R, and Felix Yarovinsky. “Complex immune cell interplay in the gamma interferon response during Toxoplasma gondii infection.” Infection and immunity vol. 82,8 (2014): 3090-7. [CrossRef]
- Kota, Archana S. and Nadeem Shabbir. “Congenital Toxoplasmosis.” StatPearls, StatPearls Publishing, 25 July 2021.
- Hunter, C A et al. “Studies on the role of interleukin-12 in acute murine toxoplasmosis.” Immunology vol. 84,1 (1995): 16-20.
- Aldebert D, Durand F, Mercier C, Brenier-Pinchart MP, Cesbron-Delauw MF, Pelloux H. Toxoplasma gondii triggers secretion of interleukin-12 but low level of interleukin-10 from the THP-1 human monocytic cell line. Cytokine. 2007;37(3):206-211. [CrossRef]
- Yap, G S, and A Sher. “Effector cells of both nonhemopoietic and hemopoietic origin are required for interferon (IFN)-gamma- and tumor necrosis factor (TNF)-alpha-dependent host resistance to the intracellular pathogen, Toxoplasma gondii.” The Journal of experimental medicine vol. 189,7 (1999): 1083-92. [CrossRef]
- Suzuki, Yasuhiro et al. “Removal of Toxoplasma gondii cysts from the brain by perforin-mediated activity of CD8+ T cells.” The American journal of pathology vol. 176,4 (2010): 1607-13. [CrossRef]
- Wilson, Emma H et al. “A critical role for IL-10 in limiting inflammation during toxoplasmic encephalitis.” Journal of neuroimmunology vol. 165,1-2 (2005): 63-74. [CrossRef]
- Radke JB, Worth D, Hong D, et al.. Transcriptional repression by ApiAP2 factors is central to chronic toxoplasmosis. PLOS Pathogens. 2018;14(5):e1007035. [CrossRef]
- Soête, M et al. “Experimental induction of bradyzoite-specific antigen expression and cyst formation by the RH strain of Toxoplasma gondii in vitro.” Experimental parasitology vol. 78,4 (1994): 361-70. [CrossRef]
- Bohne, W et al. “Induction of bradyzoite-specific Toxoplasma gondii antigens in gamma interferon-treated mouse macrophages.” Infection and immunity vol. 61,3 (1993): 1141-5. [CrossRef]
- Zhao, X. Y., & Ewald, S. E. (2020). The molecular biology and immune control of chronic Toxoplasma gondii infection. The Journal of clinical investigation, 130(7), 3370–3380. [CrossRef]
- Behnke, M. S., Khan, A., Wootton, J. C., Dubey, J. P., Tang, K., & Sibley, L. D. (2011). Virulence differences in Toxoplasma mediated by amplification of a family of polymorphic pseudokinases. Proceedings of the National Academy of Sciences of the United States of America, 108(23), 9631–9636. [CrossRef]
- Butcher BA, Kim L, Panopoulos AD, Watowich SS, Murray PJ, Denkers EY. IL-10-independent STAT3 activation by Toxoplasma gondii mediates suppression of IL-12 and TNF-alpha in host macrophages. J Immunol. 2005;174(6):3148-3152. [CrossRef]
- Baba, Minami et al. “Adhesion of Toxoplasma gondii tachyzoite-infected vehicle leukocytes to capillary endothelial cells triggers timely parasite egression.” Scientific reports vol. 7,1 5675. 18 Jul. 2017. [CrossRef]
- Stibbs H. H. (1985). Changes in brain concentrations of catecholamines and indoleamines in Toxoplasma gondii infected mice. Annals of tropical medicine and parasitology, 79(2), 153–157. [CrossRef]
- Gaskell, E. A., Smith, J. E., Pinney, J. W., Westhead, D. R., & McConkey, G. A. (2009). A unique dual activity amino acid hydroxylase in Toxoplasma gondii. PloS one, 4(3), e4801. [CrossRef]
- Brisch, R., Saniotis, A., Wolf, R., Bielau, H., Bernstein, H. G., Steiner, J., Bogerts, B., Braun, K., Jankowski, Z., Kumaratilake, J., Henneberg, M., & Gos, T. (2014). The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: old fashioned, but still in vogue. Frontiers in psychiatry, 5, 47. [CrossRef]
- Webster, J. P., Lamberton, P. H., Donnelly, C. A., & Torrey, E. F. (2006). Parasites as causative agents of human affective disorders? The impact of anti-psychotic, mood-stabilizer and anti-parasite medication on Toxoplasma gondii's ability to alter host behaviour. Proceedings. Biological sciences, 273(1589), 1023–1030. [CrossRef]
- Prandovszky, E., Gaskell, E., Martin, H., Dubey, J. P., Webster, J. P., & McConkey, G. A. (2011). The neurotropic parasite Toxoplasma gondii increases dopamine metabolism. PloS one, 6(9), e23866. [CrossRef]
- Passaro, Austin P et al. “Immune Response in Neurological Pathology: Emerging Role of Central and Peripheral Immune Crosstalk.” Frontiers in immunology vol. 12 676621. 10 Jun. 2021. [CrossRef]
- Montoya, J G, and O Liesenfeld. “Toxoplasmosis.” Lancet (London, England) vol. 363,9425 (2004): 1965-76. [CrossRef]
- Lambert, Henrik et al. “Induction of dendritic cell migration upon Toxoplasma gondii infection potentiates parasite dissemination.” Cellular microbiology vol. 8,10 (2006): 1611-23. [CrossRef]
- Lambert, Henrik et al. “The Toxoplasma gondii-shuttling function of dendritic cells is linked to the parasite genotype.” Infection and immunity vol. 77,4 (2009): 1679-88. [CrossRef]
- Kim KS. Mechanisms of microbial traversal of the blood–brain barrier. Nature Rev Microbiol. 2008; 6:625–634. [PubMed: 18604221].
- Courret N, et al. CD11c- and CD11b-expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood. 2006; 107:309–316. [PubMed: 16051744].
- Barragan A. Transepithelial migration of Toxoplasma gondii is linked to parasite motility and virulence. J Exp Med. 2002; 195:1625–1633. [PubMed: 12070289].
- Coombes JL, et al. Motile invaded neutrophils in the small intestine of Toxoplasma gondii-infected mice reveal a potential mechanism for parasite spread. Proc Natl Acad Sci USA. 2013; 110:E1913–E1922. [PubMed: 23650399].
- Konradt, Christoph et al. Endothelial cells are a replicative niche for entry of Toxoplasma gondii to the central nervous system. Nature microbiology vol. 1 16001. 15 Feb. 2016. [CrossRef]
- Dellacasa-Lindberg, Isabel et al. “Migratory activation of primary cortical microglia upon infection with Toxoplasma gondii.” Infection and immunity vol. 79,8 (2011): 3046-52. [CrossRef]
- Hwang, Young Sang et al. “Characteristics of Infection Immunity Regulated by Toxoplasma gondii to Maintain Chronic Infection in the Brain.” Frontiers in immunology vol. 9 158. 5 Feb. 2018. [CrossRef]
- Hawkins, B. T., & Davis, T. P. (2005). The blood-brain barrier/neurovascular unit in health and disease. Pharmacological reviews, 57(2), 173–185. [CrossRef]
- Liu, T., Zhang, L., Joo, D., & Sun, S. C. (2017). NF-κB signaling in inflammation. Signal transduction and targeted therapy, 2, 17023–. [CrossRef]
- Nennig, S. E., & Schank, J. R. (2017). The Role of NFkB in Drug Addiction: Beyond Inflammation. Alcohol and alcoholism (Oxford, Oxfordshire), 52(2), 172–179. [CrossRef]
- Orio, Laura et al. “3,4-Methylenedioxymethamphetamine increases interleukin-1beta levels and activates microglia in rat brain: studies on the relationship with acute hyperthermia and 5-HT depletion.” Journal of neurochemistry vol. 89,6 (2004): 1445-53. [CrossRef]
- Escubedo, E et al. “Microgliosis and down-regulation of adenosine transporter induced by methamphetamine in rats.” Brain research vol. 814,1-2 (1998): 120-6. [CrossRef]
- Thomas, David M et al. “Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation.” The Journal of pharmacology and experimental therapeutics vol. 311,1 (2004): 1-7. [CrossRef]
- Pubill D, Canudas AM, Pallàs M, Camins A, Camarasa J, Escubedo E. Different glial response to methamphetamine- and methylenedioxymethamphetamine-induced neurotoxicity. Naunyn-Schmiedeberg's Archives of Pharmacology. 2003;367(5):490-499. [CrossRef]
- Brown, Kyle T et al. “Innate immune signaling in the ventral tegmental area contributes to drug-primed reinstatement of cocaine seeking.” Brain, behavior, and immunity vol. 67 (2018): 130-138. [CrossRef]
- Barr, J. L., Brailoiu, G. C., Abood, M. E., Rawls, S. M., Unterwald, E. M., & Brailoiu, E. (2020). Acute cocaine administration alters permeability of blood-brain barrier in freely-moving rats- Evidence using miniaturized fluorescence microscopy. Drug and alcohol dependence, 206, 107637. [CrossRef]
- Crews, F. T., Sarkar, D. K., Qin, L., Zou, J., Boyadjieva, N., & Vetreno, R. P. (2015). Neuroimmune Function and the Consequences of Alcohol Exposure. Alcohol research : current reviews, 37(2), 331–351.
- Pruett, S. B., Zheng, Q., Fan, R., Matthews, K., & Schwab, C. (2004). Acute exposure to ethanol affects Toll-like receptor signaling and subsequent responses: an overview of recent studies. Alcohol (Fayetteville, N.Y.), 33(3), 235–239. [CrossRef]
- Crews, F. T., Sarkar, D. K., Qin, L., Zou, J., Boyadjieva, N., & Vetreno, R. P. (2015). Neuroimmune Function and the Consequences of Alcohol Exposure. Alcohol research : current reviews, 37(2), 331–351.
- Qin, Liya, and Fulton T Crews. “NADPH oxidase and reactive oxygen species contribute to alcohol-induced microglial activation and neurodegeneration.” Journal of neuroinflammation vol. 9 5. 12 Jan. 2012. [CrossRef]
- Czerwińska-Błaszczyk, A., Pawlak, E., & Pawłowski, T. (2022). The Significance of Toll-Like Receptors in the Neuroimmunologic Background of Alcohol Dependence. Frontiers in psychiatry, 12, 797123. [CrossRef]
- Hofford, R. S., Russo, S. J., & Kiraly, D. D. (2019). Neuroimmune mechanisms of psychostimulant and opioid use disorders. The European journal of neuroscience, 50(3), 2562–2573. [CrossRef]
- Piepenbrink MS, Samuel M, Zheng B, Carter B, Fucile C, Bunce C, Kiebala M, Khan AA et al. (2016) Humoral dysregulation associated with increased systemic inflammation among injection heroin users. PLoS ONE, 11, e0158641.
- Meijerink H, Indrati A, Utami F, Soedarmo S, Alisjahbana B, Netea MG, van Crevel R, Wisaksana R et al. (2015) Heroin use is associated with suppressed pro-inflammatory cytokine response after LPS exposure in HIV-infected individuals. PLoS ONE, 10, e0122822.
- Niwa M, Nitta A, Yamada Y, Nakajima A, Saito K, Seishima M, Noda Y & Nabeshima T (2007) Tumor necrosis factora and its inducer inhibit morphine-induced rewarding effects and sensitization. Biol. Psychiatry, 62, 658–668.
- Kang, M., Mischel, R. A., Bhave, S., Komla, E., Cho, A., Huang, C., Dewey, W. L., & Akbarali, H. I. (2017). The effect of gut microbiome on tolerance to morphine mediated antinociception in mice. Scientific reports, 7, 42658. [CrossRef]
- Meng, J., Banerjee, S., Li, D., Sindberg, G. M., Wang, F., Ma, J., & Roy, S. (2015). Opioid Exacerbation of Gram-positive sepsis, induced by Gut Microbial Modulation, is Rescued by IL-17A Neutralization. Scientific reports, 5, 10918. [CrossRef]
- Schwarz, J. M., Hutchinson, M. R., & Bilbo, S. D. (2011). Early-life experience decreases drug-induced reinstatement of morphine CPP in adulthood via microglial-specific epigenetic programming of anti-inflammatory IL-10 expression. The Journal of neuroscience : the official journal of the Society for Neuroscience, 31(49), 17835–17847. [CrossRef]
- Immunological consequences of in vitro exposure to lysergic acid diethylamide (LSD) R.V. House, P.T. Thomas and H.N. Bhargava Immunopharmacol. Immunotoxicol., 16 (1994), pp. 23-40. [CrossRef]
- Dos Santos, R. G., Valle, M., Bouso, J. C., Nomdedéu, J. F., Rodríguez-Espinosa, J., McIlhenny, E. H., Barker, S. A., Barbanoj, M. J., & Riba, J. (2011). Autonomic, neuroendocrine, and immunological effects of ayahuasca: a comparative study with d-amphetamine. Journal of clinical psychopharmacology, 31(6), 717–726. [CrossRef]
- Niu, X., Yao, Q., Li, W., Zang, L., Li, W., Zhao, J., Liu, F., & Zhi, W. (2019). Harmine mitigates LPS-induced acute kidney injury through inhibition of the TLR4-NF-κB/NLRP3 inflammasome signalling pathway in mice. European journal of pharmacology, 849, 160–169. [CrossRef]
- Atalay, S., Jarocka-Karpowicz, I., & Skrzydlewska, E. (2019). Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants (Basel, Switzerland), 9(1), 21. [CrossRef]
- Britch, S. C., Goodman, A. G., Wiley, J. L., Pondelick, A. M., & Craft, R. M. (2020). Antinociceptive and Immune Effects of Delta-9-Tetrahydrocannabinol or Cannabidiol in Male Versus Female Rats with Persistent Inflammatory Pain. The Journal of pharmacology and experimental therapeutics, 373(3), 416–428. [CrossRef]
- Klein, T. W., Lane, B., Newton, C. A., & Friedman, H. (2000). The cannabinoid system and cytokine network. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine (New York, N.Y.), 225(1), 1–8. [CrossRef]
- Rajesh, M., Mukhopadhyay, P., Bátkai, S., Haskó, G., Liaudet, L., Huffman, J. W., Csiszar, A., Ungvari, Z., Mackie, K., Chatterjee, S., & Pacher, P. (2007). CB2-receptor stimulation attenuates TNF-alpha-induced human endothelial cell activation, transendothelial migration of monocytes, and monocyte-endothelial adhesion. American journal of physiology. Heart and circulatory physiology, 293(4), H2210–H2218. [CrossRef]
- https://nida.nih.gov/research-topics/trends-statistics/overdose-death-rates.
- Berenreiterová, M., Flegr, J., Kuběna, A. A., & Němec, P. (2011). The distribution of Toxoplasma gondii cysts in the brain of a mouse with latent toxoplasmosis: implications for the behavioral manipulation hypothesis. PloS one, 6(12), e28925. [CrossRef]
- Flegr J, Preiss M, Klose J, et al. Decreased level of psychobiological factor novelty seeking and lower intelligence in men latently infected with the protozoan parasite Toxoplasma gondii : dopamine, a missing link between schizophrenia and toxoplasmosis? Biol Psychol. 2003;63:253–268;
- McFarland, Ross et al. “AAH2 gene is not required for dopamine-dependent neurochemical and behavioral abnormalities produced by Toxoplasma infection in mouse.” Behavioural brain research vol. 347 (2018): 193-200. [CrossRef]
- Li, Y., Viscidi, R. P., Kannan, G., McFarland, R., Pletnikov, M. V., Severance, E. G., Yolken, R. H., & Xiao, J. (2018). Chronic Toxoplasma gondii Infection Induces Anti-N-Methyl-d-Aspartate Receptor Autoantibodies and Associated Behavioral Changes and Neuropathology. Infection and immunity, 86(10), e00398-18. [CrossRef]
- Sullivan, E. V., Harris, R. A., & Pfefferbaum, A. (2010). Alcohol's effects on brain and behavior. Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism, 33(1-2), 127–143.
- https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/alcohol-facts-and-statistics.
- Banerjee N. (2014). Neurotransmitters in alcoholism: A review of neurobiological and genetic studies. Indian journal of human genetics, 20(1), 20–31. [CrossRef]
- Crews, F. T., Sarkar, D. K., Qin, L., Zou, J., Boyadjieva, N., & Vetreno, R. P. (2015). Neuroimmune Function and the Consequences of Alcohol Exposure. Alcohol research : current reviews, 37(2), 331–351.
- Mende M. A. (2019). Alcohol in the Aging Brain - The Interplay Between Alcohol Consumption, Cognitive Decline and the Cardiovascular System. Frontiers in neuroscience, 13, 713. [CrossRef]
- https://nida.nih.gov/research-topics/trends-statistics/overdose-death-rates.
- Schiller EY, Goyal A, Mechanic OJ. Opioid Overdose. [Updated 2022 Sep 19]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470415/.
- Wang, Jinghua et al. “Morphine impairs host innate immune response and increases susceptibility to Streptococcus pneumoniae lung infection.” Journal of immunology (Baltimore, Md. : 1950) vol. 174,1 (2005): 426-34. [CrossRef]
- Zaki, Leila et al. “Toxoplasma gondii: Preventive and therapeutic effects of morphine and evaluation of treatment parameters of tachyzoites and infected macrophages in vitro and in a murine model.” EXCLI journal vol. 19 514-527. 20 Apr. 2020. [CrossRef]
- Dyck E. (2015). LSD: a new treatment emerging from the past. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne, 187(14), 1079–1080. Advance online publication. [CrossRef]
- Caplan, R. A., Zuflacht, J. P., Barash, J. A., & Fehnel, C. R. (2020). Neurotoxicology Syndromes Associated with Drugs of Abuse. Neurologic clinics, 38(4), 983–996. [CrossRef]
- NIDA. Hallucinogens DrugFacts. National Institute on Drug Abuse website. https://nida.nih.gov/publications/drugfacts/hallucinogens. April 22, 2019 Accessed September 4, 2022.
- Centers for Disease Control and Prevention. 2018 Annual Surveillance Report of Drug-Related Risks and Outcomes — United States. Surveillance Special Report 2pdf icon. Centers for Disease Control and Prevention, U.S. Department of Health and Human Services. Published August 31, 2018.
- Nagarkatti, Prakash et al. “Cannabinoids as novel anti-inflammatory drugs.” Future medicinal chemistry vol. 1,7 (2009): 1333-49. [CrossRef]
- Estrada-Martinez S, Pe´rez-A´lamos AR, Ibarra-Segovia M, Beristaı´n-Garcia I, RamosNeva´rez A, Saenz-Soto L, et al. (2021) Seroepidemiology of Toxoplasma gondii infection in people with alcohol consumption in Durango, Mexico. PLoS ONE 16(1): e0245701. [CrossRef]
- Flegr, J., Havlícek, J., Kodym, P., Malý, M., & Smahel, Z. (2002). Increased risk of traffic accidents in subjects with latent toxoplasmosis: a retrospective case-control study. BMC infectious diseases, 2, 11. [CrossRef]
- Samojłowicz D, Borowska-Solonynko A, Kruczyk M. New, previously unreported correlations between latent Toxoplasma gondii infection and excessive ethanol consumption. Forensic Sci Int. 2017;280:49-54. [CrossRef]
- Tan, D., Soh, L.J.T., Lim, L.W., Daniel, T.C.W., Zhang, X., Vyas, A., 2015. Infection of male rats with Toxoplasma gondii results in enhanced delay aversion and neural changes in the nucleus accumbens core. Proceedings of the Royal Society B: Biological Sciences 282, 20150042.. [CrossRef]
- Berdoy M, Webster JP, Macdonald DW. Fatal attraction in rats infected with Toxoplasma gondii. Proc Biol Sci. 2000;267(1452):1591-1594. [CrossRef]
- Gatkowska, J., Wieczorek, M., Dziadek, B., Dzitko, K., & Dlugonska, H. (2012). Behavioral changes in mice caused by Toxoplasma gondii invasion of brain. Parasitology research, 111(1), 53–58. [CrossRef]
- You, C., Vandegrift, B., Brodie, M.S., 2018. Ethanol actions on the ventral tegmental area: novel potential targets on reward pathway neurons. Psychopharmacology 235, 1711–1726.. [CrossRef]
- Doyon, W. M., Ostroumov, A., Ontiveros, T., Gonzales, R. A., & Dani, J. A. (2021). Ethanol produces multiple electrophysiological effects on ventral tegmental area neurons in freely moving rats. Addiction biology, 26(2), e12899. [CrossRef]
- Qin L, He J, Hanes RN, Pluzarev O, Hong J-S, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. Journal of Neuroinflammation. 2008;5(1):10. [CrossRef]
- Torrey, E Fuller, and Robert H Yolken. “Toxoplasma gondii and schizophrenia.” Emerging infectious diseases vol. 9,11 (2003): 1375-80. [CrossRef]
- Groër, Maureen W et al. “Prenatal depression and anxiety in Toxoplasma gondii-positive women.” American journal of obstetrics and gynecology vol. 204,5 (2011): 433.e1-7. [CrossRef]
- Grada, Sebastian et al. “Toxoplasma gondii Infection in Patients with Psychiatric Disorders from Western Romania.” Medicina (Kaunas, Lithuania) vol. 58,2 208. 30 Jan. 2022. [CrossRef]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85(14):5274-5278. [CrossRef]
- Alvarado-Esquivel, Cosme et al. “Toxoplasma gondii exposure in patients suffering from mental and behavioral disorders due to psychoactive substance use.” BMC infectious diseases vol. 15 172. 3 Apr. 2015. [CrossRef]
- Sharifzadeh, M., Rezanezhad, H., Solhjoo, K. et al. Sero-molecular survey on Toxoplasma gondii infection among drug addicted and non-addicted individuals: a case–control study. BMC Infect Dis 22, 19 (2022). [CrossRef]
- Berrett AN, Gale SD, Erickson LD, et al. Toxoplasma gondii seropositivity and substance use in US adults. Folia Parasitologica. 2018 Sep;65:2018.011. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).