Preprint
Hypothesis
IgG4 Antibodies Induced by mRNA Vaccines Generate Immune Tolerance to SARS-CoV-2’spike Protein by Suppressing the Immune System
Altmetrics
Downloads
3514
Views
19369
Comments
9
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Preprints on COVID-19 and SARS-CoV-2
Submitted:
25 March 2023
Posted:
27 March 2023
You are already at the latest version
Alerts
Abstract
Due to the health crisis caused by SARS-CoV-2, the creation of a new vaccine platform based on mRNA was implemented. Globally, around 13.32 billion COVID-19 vaccine doses of diverse platforms have been given, and up to this date, 69.7% of the total population received at least one injection of a COVID-19 vaccine. Although these vaccines prevent hospitalization and severe forms of the disease, increasing evidence has shown they do not produce sterilizing immunity, allowing people to suffer frequent re-infections. Recent research has also raised concerns that mRNA vaccines could induce immune tolerance, which, added to that caused by the virus itself, could complicate the clinical course of a COVID-19 infection. Furthermore, recent investigations have found high IgG4 levels in people who were administered two or more injections of mRNA vaccines. It has been suggested that an increase in IgG4 levels could have a protecting role by preventing immune over-activation, similar to that occurring during successful allergen-specific immunotherapy by inhibiting IgE-induced effects. Altogether, evidence suggests that the reported increase in the IgG4 levels detected after repeated vaccination with the mRNA vaccines is not a protective mechanism; rather, it may be a part of the immune tolerance mechanism to the spike protein that could promote unopposed SARS-CoV2 infection and replication by suppressing natural antiviral responses. IgG4-induced suppression of the immune system due to repeated vaccination can also cause autoimmune diseases, promotes cancer growth, and autoimmune myocarditis in susceptible individuals.
Keywords:
Subject: Medicine and Pharmacology - Pulmonary and Respiratory Medicine
1. Introduction
In a relatively short period after the beginning of the COVID-19 pandemic, two mRNA vaccines, BNT162b2 (Pfizer) and mRNA-1273 (Moderna) were granted the first-ever emergency use authorization. These mRNA vaccines represented a new type of vaccine that is comprised of synthetic mRNA molecules that contain the coding sequence necessary to build the SARS-CoV-2 Spike protein, which is encased in the lipid nanoparticles (LNPs) to allow for the delivery of mRNA to cells. The main characteristic of the mRNA vaccine platform is that the proteins are synthesized within the host cells, mimicking a natural infection with the SARS-CoV-2 [1].
Contemporary investigations have contrasted the seriousness of symptoms in COVID-19 individuals infected with the SARS-CoV-2 Alpha, Delta, and Omicron variants, as well as the effectiveness of mRNA immunizations versus each variant among individuals admitted to hospitals in the United States. COVID-19 vaccines were discovered to be quite efficient (90%) in avoiding intensive care unit (ICU) admissions caused by Alpha, Delta, and Omicron variants. However, three vaccine injections were needed to give protection against the Omicron variant, whereas two injections sufficiently safeguarded against the Alpha and Delta variants. When people were admitted to hospitals, the Omicron variant was linked to fewer clinical adverse outcomes than the Delta variant. However, despite that, the Omicron variant still produce considerable clinical symptoms and mortality [2,3,4,5,6].
Although being able to induce significant neutralizing anti-spike IgG and IgA responses, all three anti-COVID-19 vaccines (Pfizer, Moderna, and Astra Zeneca ChAdOx1) appeared to be only transiently protective against SARS-CoV-2 infection and transmission [7,8,9,10]. The high rate of breakthrough infections brought on by the Omicron variant suggests that the sterilizing protection offered by the existing immunization schedules is minimal [11,12]. The coronavirus has developed the ability to escape and deceive the immune system to favor its proliferation, making Omicron the most contagious variant to-date [13]. In previous work, we documented at least 7 evasion strategies that SARS-CoV-2 uses to elude immunological monitoring and attack, including the impairment of interferon synthesis, disruption in antigen presentation, evasion of humoral attack by constructing nanotubes, and induced lymphopenia through syncytia development, etc. (for review see [13]).
Severe SARS-CoV-2 infection has been linked to higher levels of IgG4 antibodies [14,15], and it has also been documented that mRNA vaccines trigger their synthesis [11]. It is, therefore, important to analyze this issue in depth. In this paper, we provide the scientific rationale showing that repeated vaccination with mRNA vaccines generates an immune tolerance mechanism, thereby favoring unopposed SARS-CoV-2 replication. The immediate consequence of this tolerance is the establishment of a permissive state of the host leading to chronic infection and other unintended consequences induced by mRNA vaccination.
2. Characteristics of the unusual IgG4 antibody
Several immunoglobulin classes and subclasses that constitute the antibody immune arsenal, including IgA, IgE, IgM, and IgG, are essentially identified by structure of their heavy chain constant region. Human immunoglobulins G (IgG) are divided into four subcategories based on the immunogenicity of their heavy chains (IgGl, IgG2, IgG3, and IgG4) [16,17,18]. Immunoglobulin subclasses differ in their basic physiologic regulation, localization throughout the organism, and engagement with receptors on immune system effector cells [19]. IgG4, the less prevalent subclass, is found in serum at mean values of 0.35-0.51 mg/ml [20], while the levels of IgG1, the most prevalent subclass, fluctuate between 5 and 12 mg/ml [21]. Due to its unusual biological characteristics and deficiency of effector functions, such as the ability to destroy infected cells through the activation of the complement system or using antibodies, IgG4 has been referred to as an unusual antibody by not adhering to the accepted theory of antibody structure and function [22,23].
The mechanism behind the reaction involving the replacement of one half of an antibody with another, also known as Fab arm exchange and specific to IgG4 antibodies, has been elucidated over the past twenty years [24]. The heavy chains can dissociate and then recombine arbitrarily due to the enhanced propensity of the natural IgG4 joint disulfide bonds to reduction, resulting in a heterogeneous group of IgG4 molecules with random heavy-chain and light-chain couples (Figure 1) [24].
The majority of IgG4 molecules will have two distinct Fab arms because of the half-antibody exchange, making them "bi-specific" and operationally univalent for a particular antigen. As a result, far from the other IgG subclasses, IgG4 antibodies in circulation are unable to form immunological complexes with antigens. IgG4 antibodies have a limited theoretical potential for immunological activation due to their weak affinity for C1q and Fc receptors. The production of immune complexes stimulates the complement system and the action of immune effector cells. Furthermore, IgG4 antibodies may be able to block the inflammatory effects of IgG1 or IgE antibodies by dislodging the binding of those with comparable specificities. The anti-inflammatory characteristic may offer insight into another important fact that IgG4 antibodies are typically formed after the prolonged contact with an allergen, hence reducing the level of chronic inflammation [24].
The designation "IgG4-related systemic disease" refers to several clinical manifestations that were formerly thought to be completely distinct diseases. The list of organs linked to this illness is continuously expanding. Regardless of the organ involved, tissue biopsies show significant histological similarities. However, there are slight variations between organs as well. The hallmark pathology findings include widespread fibrosis, numerous IgG4-positive plasma cells, and disperse lympho-plasmacytoid infiltrates [26].
2.1. IgG4: a protective or pathogenic antibody?
IgG4's reputation as a "blocking antibody" stems from its diminished capacity to elicit immune system effector reactions [27,28]. This implies that there will only be a minimal immune response when IgG4 interacts with molecules [29]. An IgG4 response can be either pathogenic or protective, depending on the situation. For instance, IgG4 is frequently referred to be a safeguarding blocking antibody because it can suppress or halt inflammation by competing with inflammatory IgE for antigen binding in the case of allergies and infections with helminth and filarial parasites. In contrast, IgG4 can lead to serious illness in several autoimmune disorders [30] as well as cancer [31,32]. Its bi-functionality will be thoroughly examined in the next subsections.
2.1.1. Protective role of IgG4 in allergy immunotherapy
IgG4's lack of effector action and the phenomena of half-antibody interchange create complicated considerations about whether these antibodies are harmful or whether they act as a counter-regulatory reaction to an enduring immunologic illness [24]. High concentrations of antigen-specific IgG4 are linked to the satisfactory results in the allergen-specific immunotherapy by inhibiting immunoglobulin E (IgE)-mediated effects (Figure 2), according to published studies [33,34]. In various aspects, developing a tolerance to allergens is an essential step in the development of a strong immune system. Hence, to develop prolonged desensitization against allergens, pathways involving modified allergen-specific memory T- and B-cell responses that lead to immunological tolerance are utilized [34,35,36].
2.1.2. IgG4-related disease and its pathogenesis
IgG4-related disease (IgG4-RD) is a fibro-inflammatory disorder named after the presence of numerous IgG4+ plasma cells in damaged tissues and of high serum IgG4 concentrations in most, but not all cases [37]. Several autoantibodies have been found in the serum of individuals with IgG4-RD, according to earlier reports [38,39,40,41,42,43]. Furthermore, it is well-known that steroid therapy is typically quite successful in treating IgG4-RD patients. These characteristics suggest that the illness is autoimmune in origin. Rituximab, an anti-CD20 antibody, produced remarkable clinical responses in IgG4-RD patients in recent investigations, accompanied by a sizable B cell and plasmablasts decrease [44].
These results imply that increased IgG and/or IgG4 concentrations in IgG4-RD individuals may play harmful roles [45]. Because of its particular biological traits, such as the capacity to interchange Fab arms [29], the incapacity to bind complement, and the weak affinity for Fc receptors [46], IgG4 is regarded as an anti-inflammatory immunoglobulin. IgG4 antibodies do, however, function as tissue-damaging autoantibodies in some disorders, as seen in myasthenia gravis [47], idiopathic membranous glomerulonephritis [48], and pemphigus vulgaris (PV) [49].
IgG4-RD includes a “wide variety of diseases, formerly diagnosed as Mikulicz’s disease (MD) [50], autoimmune pancreatitis (AIP) [51], Riedel thyroiditis [52], interstitial pneumonitis [53,54], interstitial nephritis [55,56], prostatitis, lymphadenopathy [57,58], retroperitoneal fibrosis (RPF) [59,60], and inflammatory aortic aneurysm [61]”. A significant part of the pathogenesis of at least 13 autoimmune disorders is also played by IgG4. It has been shown that laboratory animals passively infused with human total IgG or IgG4 develop signs in 5 of these 13 disorders, proving the pathogenicity of this antibody. IgG4-induced autoimmunity is suggested by the finding that the majority of antigen-specific autoantibodies are of the IgG4 class and that their concentrations correlate with the seriousness of the sickness for the eight remaining disorders [30]. For example, Myasthenia gravis (MG), which is characterized by the production of antibodies that attach to muscle-specific kinase (MuSK), is distinguished by sporadic muscular stiffness with significant involvement of the axial and bulbar muscles. At a certain stage during the illness, a significant portion of patients requires breathing support [62,63].
After the identification of MuSK antibodies in 2001, it quickly became evident that their IgG4 subclass predominance and correlation between titres and illness severity were key findings [64,65,66]. High purity IgG4 from MuSK MG patients was able to attach to neuromuscular connections in mouse muscle, but not IgG1-3 from the same patients or control IgG4. Injection with this antibody then caused a myasthenic phenotype in immune-compromised animals [67,68,69]. These tests conclusively demonstrated their pathogenicity [70].
2.1.2.1. IgG4 role in cancer
Immune checkpoint inhibitors, often known as cancer immunotherapy agents, prevent checkpoint proteins from attaching with their associated polypeptides., allowing cytotoxic CD8+ T lymphocytes (CTLs) to attack cancer cells. Immune checkpoint blocking (ICB) agents include anti-CTLA-4 (cytotoxic T-lymphocyte antigen 4) and anti-PD-1 (programmed cell death protein 1) monoclonal antibodies [71,72]. ICB has demonstrated therapeutic effectiveness in a wide range of cancer types, including advanced-stage cancer patients [73,74,75]. Regrettably, only 15–30% of cancer patients who have received treatment benefit from ICB's therapeutic efficacy [76]. Most crucially, new reports show that certain cancer patients receiving anti-PD-1 monoclonal antibody treatment have rapid disease advancement (also known as hyper progressive disease (HPD) instead of cancer remission [77,78,79]. Notably, the PD-1 antibody belongs to the IgG4 family. Furthermore, cancers, such as malignant melanoma [32], extrahepatic cholangiocarcinoma [80], and pancreatic cancer [81], have been linked to plasma B-cell infiltrates that are IgG4-positive. IgG4's contribution to cancer is poorly understood, but a groundbreaking study has added important new knowledge. Karagiannis et al. [32] studied malignant melanoma and found that IL-4 and IL-10 expression was elevated and that tumor-specific IgG4 was generated locally in the tumor tissues. It is common to think of IL-10 as an anti-inflammatory cytokine, however, this is only true in low quantities; at larger concentrations, it shows pro-inflammatory effects [82,83,84].
Karagiannis et al. [32] also found that, in contrast to cancer-specific IgG1, cancer-specific IgG4 failed to activate two immunological processes that employ antibodies to identify and destroy cancer cells. Moreover, the IgG1 antibody was able to suppress cancer progression in an in vivo model while IgG4 failed to do so. IgG4 antibodies lack the ability to directly attack tumor cells and can interfere with the process of tumor cell death mediated by IgG1 antibodies. The inhibition of IgG1 binding and activation by Fc RI is the mechanism behind this obstructing activity. Such findings point to a previously un-researched feature of tumor-induced immune escape: IgG4 synthesis induced by tumors limits effector immune cell activities against tumors [32]. Another work [85] came to the same conclusion, that is, the IgG4 antibody is important and necessary for cancer immune evasion. In a cohort of individuals with esophageal cancer, B cells producing high IgG4 concentrations were markedly raised in malignant cells and also high in serum samples from patients. More IgG4 seems to be linked to more aggressive cancer growth, and both were strongly associated with higher cancer malignancy and poor prognosis. It was discovered that IgG4 can contend with IgG1 (as shown in Figure 3) in binding to Fc receptors present on some immune cells in vitro. This competition results in the inhibition of typical immune responses against cancer cells, such as cell and complement cytotoxicity, and cell phagocytosis, which are mediated by IgG1 antibodies.
Locally elevated levels of IgG4 in the cancer tissue hindered antibody-mediated anticancer responses, assist cancer in blocking the local immune response, and indirectly aided in cancer progression. Three separate immune-potent mice models supported this theory. It was discovered that local administration of IgG4 dramatically sped up the growth of implanted colorectal and breast tumors as well as skin papillomas caused by carcinogens. Researchers also examined the IgG4 antibody Nivolumab, which is used in cancer immunotherapy, and discovered that it dramatically accelerated the development of cancer in mice, when compared to phosphate buffer saline (PBS) and IgG1-treated groups [85].
Researchers used models of immunologically competent mice to evaluate their hypothesis and further explore the mechanism mediated by such antibodies. One model involved injecting non-cancer-specific IgG4 into the subcutaneous inoculation site for breast cancer cells. In comparison to other groups of mice (injected with PBS or IgG1 without IgG4), this group's cancer cell proliferation was dramatically accelerated, generating a significantly larger cancer mass by 21 days. Because IgG4 has no direct influence on cancer cell proliferation, these findings unambiguously indicate that cancer cells utilize the IgG4 antibody to block local immunological reactions and thus allow cancer growth in vivo via immune escape. This could explain the recently discovered hyper-progressive syndrome that is occasionally linked to cancer treatment with PD-1 inhibitors [85].
The immune system is able to detect cancers that might otherwise escape immune surveillance thanks to immune checkpoint inhibitory therapeutic antibodies that attach to the programmed cell death protein 1 (PD-1) receptor. Yet, IgG4 antibodies can also cause an autoimmune reaction by impeding the immune system's ability to be suppressed by regulatory T cells [86]. Intriguingly, the anti-PD-1 antibodies are class IgG4, raising the concern this therapy is a double edge sword. For instance, patients using immune checkpoint inhibitors alone or in combination have been linked to occurrences of acute myocarditis [87,88,89,90], sometimes with lethal consequences [91].
3. The role of IgG4 antibodies induced by mRNA vaccines
Researchers have reported that quickly upon the administration of the first two mRNA vaccine doses, the pro-inflammatory subclasses IgG1 and IgG3 dominated the IgG response. Nevertheless, a few months following the second Pfizer vaccine shot, spike-specific antibodies were further enhanced by a third mRNA injection and/or new infections caused by the SARS-CoV-2 variant [11]. Of all IgG antibodies generated against the spike protein, the IgG4 increased the most, steadily from 0.04% immediately after the second vaccination to 19.27% late after the third one. Such an increase in IgG4 levels was not observed in individuals who received either the same type or a different type of SARS-CoV-2 vaccine based on adenoviral vectors, proving that the mRNA Pfizer vaccine is the only one to cause this response. Surprisingly, 7 months after the second inoculation, the IgG4 levels in the serum of approximately half of the vaccinees surpassed the lower limit of detection [11]. To determine if the increase in IgG4 antibody concentration was exclusive to the homologous mRNA vaccination schedule utilized, researchers studied sera from an independent group that evaluated the immune system's capacity to react to immunization schedules that are similar and different, with the Pfizer and the adenoviral vector-based vaccine from AstraZeneca. Anti-spike IgG4 antibodies were again detected in 50% in the sera from the BNT-BNT group five to six months after the second vaccination, but just in one of the 51 serum samples from the other two vaccine groups. Significantly, following the third booster immunization, virtually in all vaccine recipients, a significant rise in IgG4 antibody levels was detected [11].
IgG4 responses have been infrequently reported with other vaccines, even after numerous inoculations, including that of the tetanus toxoid (TT) vaccine and the respiratory syncytial virus (RSV) [11]. These results provide support to the proposal that IgG4 class switching is not a common result of repeated antigen exposure from immunizations against other viruses or illnesses [11]. Even though natural infection with the measles virus can generate specific IgG4 antibodies [92], even persistent viral infections like the human cytomegalovirus (HCMV) do not produce a high amount of IgG4 antibodies [93].
A recently published study found that long-term IgG4 responses were produced by the mRNA vaccines but not by the vaccines using adenoviruses [94]. It's interesting to note that two mRNA vaccines, together with one AZD1222 (AstraZeneca) inoculation with an mRNA booster, and especially the mRNA-1273 vaccine, caused prolonged anti-S1 IgG4 responses in uninfected subjects. However, they were unable to detect this rise after two doses of the AZD1222 vaccine in uninfected individuals up to day 270, showing that only mRNA vaccines induce produce detectable and prolonged IgG4 responses until day 270. Importantly, in patients who had a previous COVID-19 infection (before vaccination), IgG4 did not rise, even after mRNA injections, implying that those with higher IgG4 levels are uninfected people who were immunized with mRNA vaccines before having their COVID-19 infection [94].
Compared to the BNT162b2 vaccine, the mRNA-1273 vaccination had a greater capability for inducing a prolonged IgG4 response. The amount and duration of the spike protein produced are presumably affected by the higher mRNA concentrations in the mRNA-1273 vaccine (100 µg) compared to the BNT162b2 vaccine (30 µg). Intriguingly, among the mRNA vaccines, the mRNA-1273 vaccine generated increased anti-S1 serum IgG4 concentrations in COVID-19 uninfected individuals with previously unknown repercussions on pathogen defense. Until day 270, uninfected people who received the adenovirus-based vaccine did not exhibit this long-lasting IgG4 response [94].
The problem associated with vaccines designed to be injected with low antigen concentration is a possible absence of immunological response, and traditionally there has been a strong connection to the "more is better" school of thought that persists, especially for the wide range of infectious diseases for whom there are not trustable immune predictors of vaccine-induced protection (human immunodeficiency virus (HIV), tuberculosis (TB), hepatitis C virus (HCV), etc.) [95]. The large amount (dose concentration) or repeated immunization with the same antigen (vaccine) tends to induce specific T cell tolerance (peripheral CD4) and subsequently inhibit immune responses [95,96]. However, a high antigen dose in primary immunization is seem recommended for lytic infections, which is required for both humoral and cellular immunity cooperation, while a low antigen dose is recommended for boosting [97,98]. A dose escalation technique is typically employed in clinical phase I vaccine investigations to find the dose that produces the best response. While this makes sense for diseases where there is no known immunological indicator of protection (thus, a robust response is probably superior to no response), the maximum dose that was tolerated and resulted in a positive response has often been adopted for following phase II/III investigations. Yet, significant arguments against this approach are supported by several major findings [95]:
1) When excessive quantities of antigen are injected, it can cause cell death, resulting in the loss of a specific group of T cells; this phenomenon is known as clonal deletion.
2) Immune tolerance may develop as a result of prolonged antigen exposure. T cells are an essential part of the immune system that detects and gets rid of infections and other foreign objects. Yet, these T cells may become desensitized and lose their capacity to react to repeated exposures when they are exposed to large concentrations of antigens, such as during repeated vaccination. Immune tolerance is a condition that can also result in the persistence of infections or the emergence of autoimmune diseases.
3) T cells can undergo a process known as "terminal differentiation" when vaccines are given in high concentrations, T cells undergo this process and become highly specialized, losing the capacity to divide and procreate. The immune system becomes exhausted as a result and is unable to mount a successful defense against subsequent illnesses. This is a problem since it might undermine the protective advantages of vaccinations. To balance the advantages of immunological protection and the potential disadvantages of immune exhaustion, it is crucial to carefully determine the ideal dose of vaccines.
4) Adverse outcomes are more likely to occur in groups getting greater doses.
5) The intensity of the reaction between an antigen and a T cell receptor or an antibody is referred to as avidity. The immune response is more effective in identifying and getting rid of the target antigen when avidity is high. High antigen dosages, however, can result in "immune exhaustion," a condition where the immune system's cells become desensitized and unable to mount a successful defense. Helper T cell and antibody avidity may decline as a consequence, impairing the immunological response to the target antigen. To establish a strong and effective immune response, it is crucial to thoroughly assess the ideal antigen dosages utilized in immunotherapy [95].
Billeskov et al. [95] provided proof of cases where lower vaccine antigen doses resulted in more positive responses from T cells, both for quality as judged by several effector capabilities and preventive efficiency in both animal and human experiments, and they presented arguments for the significance of reducing antigen dose for optimum protection in some models. They also encouraged experts in T-cell vaccination, in particular, to remember that sometimes, less certainly is more. In conclusion, is there a link between antigen dose concentration, repeated exposure, and the induction of IgG4 production? Or the elevated IgG4 concentration associated with COVID-19 vaccination is due to genetic predisposition? Because approximately half of the vaccinees showed a substantial increase in IgG4 concentration after the second mRNA inoculation [11], it is evident that such an increase is not caused by a genetic predisposition. Moreover, Moderna and Pfizer used the same antigen dose for their primary and booster vaccinations, which contradicts the vaccinology paradigm showing that a low antigen dose is recommended for boosting [97,98].
3.1. Lessons from the HIV vaccine trial
A study by Chung et al. contrasted repeated immunization with similar HIV vaccines in a scenario of an HIV vaccination trial. The protection (31.2%) afforded by one vaccine (RV144) was described by the authors as being linked with the production of IgG1 and IgG3 antibodies, whereas the protection of the other vaccine (VAX003) was negligible, and was associated with the production of IgG4 antibodies after multiple rounds of vaccinations [99]. Since the VAX003 vaccine increased levels of IgG4, which have historically been linked to reduced immunological efficiency, researchers wanted to know if the IgG4 production was merely triggered in connection with a disordered functional response or if it made a significant contribution to the improperly organized response. When IgG4 antibodies were eliminated from 16 similar samples from both trials, a significant increase in ADCP activity and a tendency toward greater ADCC for the VAX003 samples in comparison to bulk IgG was observed. These findings show that IgG4 antibodies may directly decrease antibody Fc-effector function rather than only being linked to the generation of an ad hoc reaction. Compared to VAX003, which produced uni-functional antibodies with significant amounts of IgG4 following seven protein vaccinations, RV144 produced highly functional IgG3 antibodies [99]. Therefore, several vaccinations and vaccine protocols may produce persistent antibody responses, but these IgG4 antibodies may not be as effective as the IgG1 and IgG3 subclasses. As a result, the IgG subclass change from fully efficient antibodies (IgG3) to IgG4 may constitute an important obstacle to the HIV vaccine’s success [100].
Such findings are similar to those recently reported after repeated mRNA vaccination; this IgG4 class shift was linked to a decreased ability of the spike-specific antibodies to promote complement deposition and antibody-dependent cellular phagocytosis. Additionally, vaccine-induced IgG3 antibodies improved immune functions such as ADCC and ADCP, whereas vaccine-induced IgG4 antibodies blocked these processes [99]. Similarly, in the HIV study, the removal of IgG4 antibodies from serum led to significant elevations in Fc-mediated effector activities, confirming a non-protective role for IgG4 antibodies. The unusually high production of IgG4 in the VAX003 group could be due to the repeated injection of seven vaccine doses containing high antigen concentration in the lack of appropriate adjuvant stimulation, which may have culminated in disproportionate B cell receptor activation [99].
From these data, it is clear that IgG4 production in the VAX003 group was associated with repeated boosting (seven rounds of immunization vs 4 rounds in the RV144 group), leading to reduced protection from HIV infection; moreover, this class switch to IgG4 may promote breakthrough infections due to the impairment in Fc-mediated antiviral responses [99]. This supports the notion that an increase in IgG4 subclasses could lead to extended viral persistence in case of infection, considering that Fc-mediated effector action is essential for viral elimination [11]. A study by Gazit et al. found that people who received vaccinations and booster shots had a 13-fold greater likelihood of contracting the COVID virus and getting sick. Also, this group saw the greatest number of hospitalizations. On the other hand, those who had COVID-19 infection before getting the vaccine were six times more likely to experience a breakthrough infection. Research showed that immunity acquired through natural disease provides better protection against infection and disease symptoms caused by the Delta variant of SARS-CoV-2 than the immunity provided by two injections with the BNT162b2 vaccine [101].
Even the protection that COVID-19 vaccines provide against severe symptoms and hospitalization, is now being questioned following an outbreak in an Israeli hospital that resulted in the deaths of five individuals (all with comorbidities) who were fully immunized [101]. This study casts some doubt on the notion that widespread immunization will produce herd immunity and stop COVID-19 outbreaks. This may have been true for the SARS-CoV-2 wild-type virus, but in the outbreak that is the subject of the cited study, 96.2% of those who were exposed received full vaccinations [102]. Similarly, Brosh-Nissimov et al. reported that among 17 Israeli hospitals, 34/152 (22%) fully immunized patients passed away from COVID-19. Noticeably, these individuals had a high prevalence of co-morbid disorders, such as congestive heart failure, chronic renal insufficiency, high blood pressure, diabetes, and lung disorders, that made them more vulnerable to developing severe COVID-19 [103].
Irrgang et al. [11] reported that it takes months for the IgG4 class switch to develop. Could this increase in IgG4 levels explain the reduced efficacy of mRNA vaccines detected after 6 months? [104]. Based on findings from the HIV trial [99], where decreased vaccine efficacy was linked to IgG4 production, we conclude that repeated mRNA vaccination is also correlated with reduced efficacy in protecting people from re-infection due to an increase in IgG4 levels.
3.2. Lessons from the Malaria vaccine trial
The merozoite surface protein 1 (MSP-1), the 175-kDa erythrocyte binding antigen (EBA-175), and the apical membrane antigen 1 (AMA-1) are the three major objectives of the natural immune response to the Plasmodium falciparum parasite, which causes Malaria. It was unclear, therefore, if antibodies to these antigens act as protective agents against clinical illness or only serve as exposure markers. In a group of 302 Mozambican children aging 5, 9, 12 and 24 months, highly specific tests were used to determine antibody responses to Plasmodium falciparum blood-stage antigens as part of a randomized, placebo-controlled trial between 2002 and 2004. The incidence of malaria throughout the follow-up period was found to be differently correlated with IgG subtype reactions to the EBA-175 antigen [105]. Since it is believed that the antibody isotype evoked by P. falciparum antigens is essential, the prophylactic effect of IgG has been attributed to the neutralizing (IgG1 and IgG3) rather than the non-neutralizing subtypes (IgG2) (IgG2 and IgG4) [106,107,108,109,110]. IgG1 reactivity to EBA-175 was consistent over the first year of life before rising in the following year.
While IgG4 reactivity was minimal in the first year but significantly rose by the age of 2 years, IgG3 reactivity remained moderate throughout the study period. IgG3 reactivity was stable throughout all time, while IgG4 was low during the first year but significantly rose by age 2 years. The study focused on the antibody responses of individuals at 5 and 12 months and investigated the incidence of malaria during two different time periods of risk, from 5 to 12 months and from 12 to 24 months. In their analysis, they noticed a distinct pattern for IgG subclasses to the EBA-175 antigen: higher concentrations of particular antibodies known as neutralizing IgG1 and IgG3 were linked to a reduced likelihood of contracting malaria in the second year. As the levels of IgG1 doubled, the risk of malaria reduced by about 50%, and when the levels of IgG3 doubled, the risk of malaria decreased by about 60% [105].
It is important to note that the probability of contracting malaria increased by around three times when non-neutralizing IgG4 levels doubled. Up to the age of 24 months, IgG1 and IgG3 demonstrated 51% and 56% protective effects respectively, however, IgG4 was linked to a higher risk of malaria infection throughout this age range [105]. It's interesting to note that a separate study also found a link between high IgG4 levels and a higher risk of infection and malaria exacerbations [111].
4. Discussion
Recent studies have raised concerns that inoculation with mRNA-based COVID-19 vaccines might result in the establishment of tolerance against the spike protein generated by host cells in response to vaccination. For example, a recent work by Irrgang et al. discovered that several months after the second immunization with the Pfizer vaccine, SARS-CoV-2-specific antibodies were mainly constituted of non-neutralizing IgG4 antibodies, which were enhanced even more by a third mRNA vaccination and/or SARS-CoV-2 variant breakthrough infections [11]. The authors commented that “independent of the underlying mechanism, the induction of antiviral IgG4 antibodies is a phenomenon infrequently described and raises important questions about its functional consequences” [11]. IgG4 antibodies are bi-functional: they can be protective but can also be directly pathogenic [112]. There has been a lot of research done on IgG4 in chronic allergen exposure models, where natural immunological tolerance is induced by giving an allergen in increasing doses [113]. The increase in IgG4 levels after the third immunization with the Pfizer vaccine could reflect a tolerance mechanism that could prevent immune over-reactivity (cytokine storm) and progression to a critical stage [11]. However, this exacerbated immune reaction does not occur in young and healthy people, it has been documented only in older patients with genetic susceptibility and those with comorbidities [114].
It has been suggested that an increase in IgG4 levels could have a protective role similar to that occurring during successful allergen-specific immunotherapy by inhibiting IgE-induced effects [11]. Allergen tolerance is an immune system adaptation characterized by a particular non-inflammatory response to an allergen that, under other conditions, would probably result in cell-mediated or humoral immunity, which would cause tissue inflammation and/or IgE synthesis [113]. In other words, the immune system “learns” to tolerate a foreign although innocuous antigen. However, a very different situation occurs when a virus invades our body. In this scenario, vaccine-induced tolerance can potentially have several negative, unintended consequences because tolerance to the spike protein could inhibit the immune system to detect and attack the pathogen (Figure 4), thus potentially exacerbating SARS-CoV2 pathology in individuals who suffer re-infection of COVID-19 in the setting of vaccine-induced immune suppression. For example, it was demonstrated that patients with severe COVID-19 who passed away had higher IgG4 levels than those who recovered [14]. More precisely, the death rate increased noticeably at 30 days when serum IgG4 concentrations were above 700 mg/dl and the ratio of IgG4 to IgG1 was above 0.05 [15]. Moreover, IgG4 levels were correlated with IL-6 levels [115], a known determinant of COVID-19-related mortality [115,116,117].
This leads us to conclude that it is incorrect to compare the increase in IgG4 levels between allergy treatments and the reported increase in IgG4 antibodies after repeated vaccination or infection with SARS-CoV-2. The induced tolerance against the spike protein could produce an impaired immune response against the virus when these patients suffer a re-infection. Although has a high rate of transmissibility. the severity of infections has fortunately been reduced as a result of a change in affinity towards the upper respiratory tract [118,119,120,121]. These findings may explain why Omicron infections caused fewer severe effects [122,123]. However, without an adequate protection level, even the new Omicron sub-variants (considered as mild) could cause severe multi-organ damage and death in immuno-compromised individuals. The result would be an immunodeficiency state in which any pathogen (in addition to SARS-CoV-2) could pose a significant risk for survival.
It is important to note that this virus causes severe immune suppression at different levels due to an evolved ability to evade our immune system [13]. There is now compelling evidence that only mRNA vaccines induce a remarkable increase in IgG4 levels, and such an increase was detected in COVID-19 uninfected individuals who received mRNA vaccinations before contracting COVID-19 infection, whereas for patients who had a previous COVID-19 infection before vaccination, IgG4 levels did not rise [94]. This is in contrast with findings from another study showing that the highest IgG4 levels were found in those individuals who developed a breakthrough infection after receiving three doses of mRNA vaccination, indicating that SARS-CoV-2 infections can also induce IgG4 production [11]. We suggest more research is needed to get a definitive conclusion about these different results.
In this regard, it was recently demonstrated that following the traditional vaccination scheme, the serum-neutralizing effectiveness in mice against the Delta and Omicron variants of the COVID-19 Pfizer vaccine was dramatically diminished after numerous booster doses [12]. Repeated antigen stimulation reportedly caused CD8+ T cells to become exhausted. These boosters also significantly diminished CD4+ and CD8+ T cell responses and enhanced programmed cell death protein 1 (PD-1) and lymphocyte activation gene-3 (LAG-3) production in these T cells [124]. The prolonged vaccination decreased the normal development of the germinal center and hindered the generation of memory B cells specific for RBD. This research additionally revealed that prolonged RBD vaccine booster immunization increased the concentration of the immunosuppressive cytokine IL-10 as well as the proportion of CD25+Foxp3+CD4+ Treg cells. The conventional SARS-CoV-2 vaccine's ability to provide immunological protection may be significantly impacted by over-vaccination. If this happens, either newly diagnosed COVID-19 cases or people who have already contracted the virus again may have a more severe case of the illness. This concept was proposed after seeing tolerance of both the humoral and cellular immune responses to prolonged booster immunization doses [124].
The HIV [99] and malaria trials [105] informed us that repeated vaccination was linked to reduced protection from infection, and this poor response was directly related to a higher IgG4 production. Moreover, it was suggested that this class switch might contribute to breakthrough infections due to impaired fc-mediated antiviral responses [99]. All in all, reviewed data indicates that IgG4 production induced by repeated vaccination does not in any way constitute a protective mechanism. There are also warning signs in recent literature that indicate the cellular immune response induced by the typical vaccination course may be severely compromised by repeated administration of the same booster shot or infection following vaccination, which, in combination with impaired antibody immune responses, may cause recipients' symptoms to worsen or their disease to last longer. Excessive vaccination is likely to create an immunosuppressive microenvironment that is crucial for promoting immunological tolerance. These findings show that repeated SARS-CoV-2 booster immunization in dense populations should be approached with caution [124].
In conclusion, the immune tolerance mechanism induced by mRNA vaccines could have at least 6 negative unintended consequences:
1) By ignoring the spike protein synthesized as a consequence of vaccination, the host immune system becomes vulnerable to re-infection with the new Omicron subvariants, allowing for free replication of the virus once a re-infection takes place. In this situation, we propose that even these less pathogenic Omicron subvariants could cause significant harm and even death in individuals with comorbidities and immuno-compromised.
2) mRNA and inactivated vaccines temporally impair interferon signaling [125,126], causing immune suppression and leaving the individual in a vulnerable situation against any other pathogen. In addition, this immune suppression could allow the re-activation of latent viral, bacterial, or fungal infections, and might also allow uncontrolled growth of cancer cells [127].
3) A tolerant immune system can allow SARS-CoV-2 persistence in the host and promote the establishment of a chronic infection, similarly to that generated by the hepatitis B virus (HBV), the human immune deficiency virus (HIV), and the hepatitis C virus (HCV) [128].
4) The combined immune suppression (produced by SARS-CoV-2 infection and further enhanced by vaccination) could explain a plethora of autoimmune conditions, cancers, re-infections, and deaths temporally associated with both. It is conceivable that the excess deaths reported in several highly COVID-19-vaccinated countries may be explained, in part, by this combined immunosuppressive effect.
5) Repeated vaccination could also lead to auto-immunity: In 2009, the results of an important study went largely unnoticed. Researchers discovered that in mice that are otherwise not susceptible to spontaneous autoimmune disorders, repeated administration of the antigen promotes systemic autoimmunity. The development of CD4+ T cells that can induce autoantibodies (autoantibody-inducing CD4+ T cells, or aiCD4+ T cells), which had their T cell receptors (TCR) modified, was triggered by excessive stimulation of CD4+ T cells. The aiCD4+ T cell was generated by new genetic TCR modification rather than a cross-reaction. The excessively stimulated CD8+ T cells induced them to develop into cytotoxic T lymphocytes (CTL) that are specific for an antigen. These CTLs were able to mature further by antigen cross-presentation, so in that situation, they induced autoimmune tissue damage resembling systemic lupus erythematosus (SLE) [129]. According to the self-organized criticality theory, when the immune system of the host is continually overstimulated by antigen exposure at concentrations that are higher (see page 11) than the immune system's self-organized criticality can tolerate, systemic autoimmunity inevitably occurs [130].
It has been proposed that the amount and duration of the spike protein produced are presumably affected by the higher mRNA concentrations in the mRNA-1273 vaccine (100 µg) compared to the BNT162b2 vaccine (30 µg) [94]. Thus, it is probable that the spike protein produced in response to mRNA vaccination is too high and last too much time in the body. That overwhelms the capacity of the immune system, thus leading to autoimmunity [129,130]. Indeed, several investigations have found that COVID-19 immunization is associated with the development of autoimmune responses [131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148].
6) Increased IgG4 levels induced by repeated vaccination can lead to autoimmune myocarditis: It has been discovered that IgG4 antibodies can also cause an autoimmune reaction by impeding the immune system`s ability to be suppressed by regulatory T cells [86]. Patients using immune checkpoint inhibitors alone or in combination have been linked to occurrences of acute myocarditis [87,88,89,90], sometimes with lethal consequences [91]. As anti-PD-1 antibodies are class IgG4, and these antibodies are also induced by repeated vaccination, it is plausible to suggest that excessive vaccination is associated with the occurrence of an increased number of myocarditis cases and sudden cardiac deaths.
Finally, these negative outcomes are not expected to affect all people who have received these mRNA vaccines. Individuals with genetic susceptibility, immune deficiencies, and co-morbidities probably would be the most likely to be affected. However, this gives rise to a disturbing paradox: if people who are the most affected by the COVID-19 disease (the elderly, diabetics, hypertensive, and immunocompromised people like those with HIV) are also more susceptible to suffering the negative effects of mRNA vaccines, is it then justified to booster them? As Omicron subvariants have been demonstrated to be less pathogenic, and mRNA vaccines do not protect against re-infection, clinicians should be aware of the possible detrimental effects on the immune system by administering boosters.
Author Contributions
Conceptualization, A.R.-C. and V.N.U.; validation, A.R.-C., E.M.R., W.M., and V.N.U.; formal analysis, A.R.-C., W.M., E.M.R. and V.N.U.; literature search, A.R.-C., E.M.R. and V.N.U.; data curation, A.R.-C., E.M.R. and V.N.U.; writing –original draft preparation, A.R.-C. and V.N.U.; writing – review and editing, A.R.-C., E.M.R., W.M., and V.N.U.; visualization, A.R.-C.; supervision, A.R.-C. and V.N.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Verbeke, R.; Lentacker, I.; De Smedt, S.C.; Dewitte, H. The dawn of mRNA vaccines: The COVID-19 case. Journal of Controlled Release 2021, 333, 511–520. [Google Scholar] [CrossRef]
- Lauring, A.S.; Tenforde, M.W.; Chappell, J.D.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D. Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. Bmj 2022, 376. [Google Scholar] [CrossRef]
- 3 Tenforde, M.W.; Self, W.H.; Gaglani, M.; Ginde, A.A.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; Mohr, N.M.; Zepeski, A.; McNeal, T. Effectiveness of mRNA vaccination in preventing COVID-19–associated invasive mechanical ventilation and death—United States, March 2021–January 2022. Morbidity and Mortality Weekly Report 2022, 71, 459. [Google Scholar] [CrossRef]
- Plumb, I.D.; Feldstein, L.R.; Barkley, E.; Posner, A.B.; Bregman, H.S.; Hagen, M.B.; Gerhart, J.L. Effectiveness of COVID-19 mRNA vaccination in preventing COVID-19–associated hospitalization among adults with previous SARS-CoV-2 infection—United States, June 2021–February 2022. Morbidity and Mortality Weekly Report 2022, 71, 549. [Google Scholar] [CrossRef]
- Rahmani, K.; Shavaleh, R.; Forouhi, M.; Disfani, H.F.; Kamandi, M.; Oskooi, R.K.; Foogerdi, M.; Soltani, M.; Rahchamani, M.; Mohaddespour, M. The effectiveness of COVID-19 vaccines in reducing the incidence, hospitalization, and mortality from COVID-19: A systematic review and meta-analysis. Frontiers in Public Health 2022, 10, 2738. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Self, W.H.; Adams, K.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D. Association between mRNA vaccination and COVID-19 hospitalization and disease severity. Jama 2021, 326, 2043–2054. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. New England journal of medicine 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Seow, J.; Graham, C.; Merrick, B.; Acors, S.; Pickering, S.; Steel, K.J.; Hemmings, O.; O’Byrne, A.; Kouphou, N.; Galao, R.P. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nature microbiology 2020, 5, 1598–1607. [Google Scholar] [CrossRef]
- Wang, Z.; Schmidt, F.; Weisblum, Y.; Muecksch, F.; Barnes, C.O.; Finkin, S.; Schaefer-Babajew, D.; Cipolla, M.; Gaebler, C.; Lieberman, J.A. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature 2021, 592, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, Y.C.; Tong, X.; Kang, J.; Avendaño, M.J.; Serrano, E.F.; García-Salum, T.; Pardo-Roa, C.; Riquelme, A.; Cai, Y.; Renzi, I. Omicron variant Spike-specific antibody binding and Fc activity are preserved in recipients of mRNA or inactivated COVID-19 vaccines. Science Translational Medicine 2022, 14, eabn9243. [Google Scholar] [CrossRef] [PubMed]
- Irrgang, P.; Gerling, J.; Kocher, K.; Lapuente, D.; Steininger, P.; Habenicht, K.; Wytopil, M.; Beileke, S.; Schäfer, S.; Zhong, J. Class switch towards non-inflammatory, spike-specific IgG4 antibodies after repeated SARS-CoV-2 mRNA vaccination. Science immunology 2022, eade2798. [Google Scholar]
- Subramanian, S.; Kumar, A. Increases in COVID-19 are unrelated to levels of vaccination across 68 countries and 2947 counties in the United States. European journal of epidemiology 2021, 36, 1237–1240. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Casillas, A.; Redwan, E.M.; Uversky, V.N. SARS-CoV-2: a master of immune evasion. Biomedicines 2022, 10, 1339. [Google Scholar] [CrossRef] [PubMed]
- Moura, A.D.; da Costa, H.H.; Correa, V.A.; de, S. Lima, A.K.; Lindoso, J.A.; De Gaspari, E.; Hong, M.A.; Cunha-Junior, J.P.; Prudencio, C.R. Assessment of avidity related to IgG subclasses in SARS-CoV-2 Brazilian infected patients. Scientific Reports 2021, 11, 17642. [Google Scholar] [CrossRef] [PubMed]
- Della-Torre, E.; Lanzillotta, M.; Strollo, M.; Ramirez, G.A.; Dagna, L.; Tresoldi, M. Serum IgG4 level predicts COVID-19 related mortality. European Journal of Internal Medicine 2021, 93, 107–109. [Google Scholar] [CrossRef]
- Grey, H.M.; Kunkel, H.G. H chain subgroups of myeloma proteins and normal 7S γ-globulin. The Journal of experimental medicine 1964, 120, 253. [Google Scholar] [CrossRef] [PubMed]
- Terry, W.D.; Fahey, J.L. Subclasses of human γ2-globulin based on differences in the heavy polypeptide chains. Science 1964, 146, 400–401. [Google Scholar] [CrossRef] [PubMed]
- Mayumi, M.; Kuritani, T.; Kubagawa, H.; Cooper, M. IgG subclass expression by human B lymphocytes and plasma cells: B lymphocytes precommitted to IgG subclass can be preferentially induced by polyclonal mitogens with T cell help. Journal of immunology (Baltimore, Md.: 1950) 1983, 130, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Lichtman, A.H.; Pober, J.S. Cellular and molecular immunology; Saunders: 1991.
- Meulenbroek, A. Human IgG subclasses: useful diagnostic markers for immunocompetence; CLB: 2002.
- Aucouturier, P.; Danon, F.; Daveau, M.; Guillou, B.; Sabbah, A.; Besson, J.; Preud'homme, J.L. Measurement of serum IgG4 levels by a competitive immunoenzymatic assay with monoclonal antibodies. Journal of immunological methods 1984, 74, 151–162. [Google Scholar] [CrossRef]
- Aalberse, R.C.; Schuurman, J. IgG4 breaking the rules. Immunology 2002, 105, 9–19. [Google Scholar] [CrossRef]
- Aalberse, R.; Stapel, S.; Schuurman, J.; Rispens, T. Immunoglobulin G4: an odd antibody. Clinical & Experimental Allergy 2009, 39, 469–477. [Google Scholar]
- Nirula, A.; Glaser, S.M.; Kalled, S.L.; Taylor, F.R. What is IgG4? A review of the biology of a unique immunoglobulin subtype. Current opinion in rheumatology 2011, 23, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, M.; Tanaka, A.; Maehara, T.; Furukawa, S.; Nakashima, H.; Nakamura, S. T helper subsets in Sjögren's syndrome and IgG4-related dacryoadenitis and sialoadenitis: a critical review. Journal of autoimmunity 2014, 51, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Khosroshahi, A.; Stone, J.H. A clinical overview of IgG4-related systemic disease. Current opinion in rheumatology 2011, 23, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, F.; Zhang, Y.; Wang, L.; Antonenko, S.; Zhang, S.; Zhang, Y.W.; Tabrizifard, M.; Ermakov, G.; Wiswell, D. Comprehensive analysis of the therapeutic IgG4 antibody pembrolizumab: hinge modification blocks half molecule exchange in vitro and in vivo. Journal of Pharmaceutical Sciences 2015, 104, 4002–4014. [Google Scholar] [CrossRef] [PubMed]
- Schuurman, J.; Van Ree, R.; Perdok, G.a.; Van Doorn, H.; Tan, K.; Aalberse, R. Normal human immunoglobulin G4 is bispecific: it has two different antigen-combining sites. Immunology 1999, 97, 693–698. [Google Scholar] [CrossRef]
- Van Der Neut Kolfschoten, M.; Schuurman, J.; Losen, M.; Bleeker, W.K.; Martínez-Martínez, P.; Vermeulen, E.; Den Bleker, T.H.; Wiegman, L.; Vink, T.; Aarden, L.A. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science 2007, 317, 1554–1557. [Google Scholar] [CrossRef] [PubMed]
- Huijbers, M.G.; Plomp, J.J.; van der Maarel, S.M.; Verschuuren, J.J. IgG4-mediated autoimmune diseases: a niche of antibody-mediated disorders. Annals of the New York Academy of Sciences 2018, 1413, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Daveau, M.; Fischer, J.P.; Rivat, L.; Rivat, C.; Ropartz, C.; Peter, H.H.; Cesarini, J.-P.; Kourilsky, F.M. IgG4 subclass in malignant melanoma. Journal of the National Cancer Institute 1977, 58, 189–192. [Google Scholar] [CrossRef]
- Karagiannis, P.; Gilbert, A.E.; Josephs, D.H.; Ali, N.; Dodev, T.; Saul, L.; Correa, I.; Roberts, L.; Beddowes, E.; Koers, A. IgG4 subclass antibodies impair antitumor immunity in melanoma. The Journal of clinical investigation 2013, 123, 1457–1474. [Google Scholar] [CrossRef]
- Akdis, C.; Blaser, K. Mechanisms of allergen-specific immunotherapy. Allergy 2000, 55, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Akdis, M. Healthy immune response to allergens: T regulatory cells and more. Current opinion in immunology 2006, 18, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Larché, M.; Akdis, C.A.; Valenta, R. Immunological mechanisms of allergen-specific immunotherapy. Nature Reviews Immunology 2006, 6, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Durham, S.R.; Walker, S.M.; Varga, E.-M.; Jacobson, M.R.; O'Brien, F.; Noble, W.; Till, S.J.; Hamid, Q.A.; Nouri-Aria, K.T. Long-term clinical efficacy of grass-pollen immunotherapy. New England Journal of Medicine 1999, 341, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Della-Torre, E.; Lanzillotta, M.a.; Doglioni, C. Immunology of IgG4-related disease. Clinical & Experimental Immunology 2015, 181, 191–206. [Google Scholar]
- Aparisi, L.; Farre, A.; Gomez-Cambronero, L.; Martinez, J.; De Las Heras, G.; Corts, J.; Navarro, S.; Mora, J.; Lopez-Hoyos, M.; Sabater, L. Antibodies to carbonic anhydrase and IgG4 levels in idiopathic chronic pancreatitis: relevance for diagnosis of autoimmune pancreatitis. Gut 2005, 54, 703–709. [Google Scholar] [CrossRef]
- Nishi, H.; Tojo, A.; Onozato, M.L.; Jimbo, R.; Nangaku, M.; Uozaki, H.; Hirano, K.; Isayama, H.; Omata, M.; Kaname, S. Anti-carbonic anhydrase II antibody in autoimmune pancreatitis and tubulointerstitial nephritis. Nephrology Dialysis Transplantation 2007, 22, 1273–1275. [Google Scholar] [CrossRef] [PubMed]
- Nishimori, I.; Miyaji, E.; Morimoto, K.; Nagao, K.; Kamada, M.; Onishi, S. Serum antibodies to carbonic anhydrase IV in patients with autoimmune pancreatitis. Gut 2005, 54, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Asada, M.; Nishio, A.; Uchida, K.; Kido, M.; Ueno, S.; Uza, N.; Kiriya, K.; Inoue, S.; Kitamura, H.; Ohashi, S. Identification of a novel autoantibody against pancreatic secretory trypsin inhibitor in patients with autoimmune pancreatitis. Pancreas 2006, 33, 20–26. [Google Scholar] [CrossRef]
- Endo, T.; Takizawa, S.; Tanaka, S.; Takahashi, M.; Fujii, H.; Kamisawa, T.; Kobayashi, T. Amylase α-2A autoantibodies: novel marker of autoimmune pancreatitis and fulminant type 1 diabetes. Diabetes 2009, 58, 732–737. [Google Scholar] [CrossRef]
- Frulloni, L.; Lunardi, C.; Simone, R.; Dolcino, M.; Scattolini, C.; Falconi, M.; Benini, L.; Vantini, I.; Corrocher, R.; Puccetti, A. Identification of a novel antibody associated with autoimmune pancreatitis. New England Journal of Medicine 2009, 361, 2135–2142. [Google Scholar] [CrossRef]
- Hart, P.A.; Topazian, M.D.; Witzig, T.E.; Clain, J.E.; Gleeson, F.C.; Klebig, R.R.; Levy, M.J.; Pearson, R.K.; Petersen, B.T.; Smyrk, T.C. Treatment of relapsing autoimmune pancreatitis with immunomodulators and rituximab: the Mayo Clinic experience. Gut 2013, 62, 1607–1615. [Google Scholar] [CrossRef]
- Shiokawa, M.; Kodama, Y.; Kuriyama, K.; Yoshimura, K.; Tomono, T.; Morita, T.; Kakiuchi, N.; Matsumori, T.; Mima, A.; Nishikawa, Y. Pathogenicity of IgG in patients with IgG4-related disease. Gut 2016, 65, 1322–1332. [Google Scholar] [CrossRef]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: from structure to effector functions. Frontiers in immunology 2014, 5, 520. [Google Scholar] [CrossRef]
- Plomp, J.J.; Huijbers, M.G.; van der Maarel, S.M.; Verschuuren, J.J. Pathogenic IgG4 subclass autoantibodies in MuSK myasthenia gravis. Annals of the New York Academy of Sciences 2012, 1275, 114–122. [Google Scholar] [CrossRef]
- Beck Jr, L.H.; Salant, D.J. Membranous nephropathy: recent travels and new roads ahead. Kidney international 2010, 77, 765–770. [Google Scholar] [CrossRef]
- Anhalt, G.J.; Labib, R.S.; Voorhees, J.J.; Beals, T.F.; Diaz, L.A. Induction of pemphigus in neonatal mice by passive transfer of IgG from patients with the disease. New England Journal of Medicine 1982, 306, 1189–1196. [Google Scholar] [CrossRef]
- Yamamoto, M.; Ohara, M.; Suzuki, C.; Naishiro, Y.; Yamamoto, H.; Takahashi, H.; Imai, K. Elevated IgG4 concentrations in serum of patients with Mikulicz's disease. Scandinavian journal of rheumatology 2004, 33, 432–433. [Google Scholar] [CrossRef]
- Okazaki, K.; Uchida, K.; Koyabu, M.; Miyoshi, H.; Takaoka, M. Recent advances in the concept and diagnosis of autoimmune pancreatitis and IgG4-related disease. Journal of gastroenterology 2011, 46, 277–288. [Google Scholar] [CrossRef]
- Dahlgren, M.; Khosroshahi, A.; Nielsen, G.P.; Deshpande, V.; Stone, J.H. Riedel's thyroiditis and multifocal fibrosclerosis are part of the IgG4-related systemic disease spectrum. Arthritis care & research 2010, 62, 1312–1318. [Google Scholar]
- Zen, Y.; Inoue, D.; Kitao, A.; Onodera, M.; Abo, H.; Miyayama, S.; Gabata, T.; Matsui, O.; Nakanuma, Y. IgG4-related lung and pleural disease: a clinicopathologic study of 21 cases. The American journal of surgical pathology 2009, 33, 1886–1893. [Google Scholar] [CrossRef] [PubMed]
- Inoue, D.; Zen, Y.; Abo, H.; Gabata, T.; Demachi, H.; Kobayashi, T.; Yoshikawa, J.; Miyayama, S.; Yasui, M.; Nakanuma, Y. Immunoglobulin G4–related lung disease: CT findings with pathologic correlations. Radiology 2009, 251, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Saeki, T.; Saito, A.; Yamazaki, H.; Emura, I.; Imai, N.; Ueno, M.; Nishi, S.; Miyamura, S.; Gejyo, F. Tubulointerstitial nephritis associated with IgG4-related systemic disease. Clinical and experimental nephrology 2007, 11, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Saeki, T.; Nishi, S.; Imai, N.; Ito, T.; Yamazaki, H.; Kawano, M.; Yamamoto, M.; Takahashi, H.; Matsui, S.; Nakada, S. Clinicopathological characteristics of patients with IgG4-related tubulointerstitial nephritis. Kidney international 2010, 78, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Kojima, M.; Takata, K.; Morito, T.; Asaoku, H.; Takeuchi, T.; Mizobuchi, K.; Fujihara, M.; Kuraoka, K.; Nakai, T. Systemic IgG4-related lymphadenopathy: a clinical and pathologic comparison to multicentric Castleman's disease. Modern Pathology 2009, 22, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Notohara, K.; Kojima, M.; Takata, K.; Masaki, Y.; Yoshino, T. IgG4-related disease: Historical overview and pathology of hematological disorders. Pathology international 2010, 60, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Hamanou, H.; Kawa, S.; Ochi, Y.; Unno, H.; Shiba, N.; Wajiki, M.; Nakazawa, K.; Shimojo, H.; Kiyosawa, K. Hydronephrosis associated with retroperitoneal fibrosis and sclerosing pancreatitis. The Lancet 2002, 359, 1403–1404. [Google Scholar] [CrossRef] [PubMed]
- Zen, Y.; Onodera, M.; Inoue, D.; Kitao, A.; Matsui, O.; Nohara, T.; Namiki, M.; Kasashima, S.; Kawashima, A.; Matsumoto, Y. Retroperitoneal fibrosis: a clinicopathologic study with respect to immunoglobulin G4. The American journal of surgical pathology 2009, 33, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.H.; Khosroshahi, A.; Hilgenberg, A.; Spooner, A.; Isselbacher, E.M.; Stone, J.R. IgG4-related systemic disease and lymphoplasmacytic aortitis. Arthritis and rheumatism 2009, 60, 3139–3145. [Google Scholar] [CrossRef]
- Evoli, A.; Tonali, P.A.; Padua, L.; Monaco, M.L.; Scuderi, F.; Batocchi, A.P.; Marino, M.; Bartoccioni, E. Clinical correlates with anti-MuSK antibodies in generalized seronegative myasthenia gravis. Brain 2003, 126, 2304–2311. [Google Scholar] [CrossRef]
- Farrugia, M.E.; Robson, M.D.; Clover, L.; Anslow, P.; Newsom-Davis, J.; Kennett, R.; Hilton-Jones, D.; Matthews, P.M.; Vincent, A. MRI and clinical studies of facial and bulbar muscle involvement in MuSK antibody-associated myasthenia gravis. Brain 2006, 129, 1481–1492. [Google Scholar] [CrossRef] [PubMed]
- McConville, J.; Farrugia, M.E.; Beeson, D.; Kishore, U.; Metcalfe, R.; Newsom-Davis, J.; Vincent, A. Detection and characterization of MuSK antibodies in seronegative myasthenia gravis. Annals of neurology 2004, 55, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Niks, E.; Van Leeuwen, Y.; Leite, M.; Dekker, F.; Wintzen, A.; Wirtz, P.; Vincent, A.; van Tol, M.; Jol-van der Zijde, C.; Verschuuren, J. Clinical fluctuations in MuSK myasthenia gravis are related to antigen-specific IgG4 instead of IgG1. Journal of neuroimmunology 2008, 195, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Ohta, K.; Shigemoto, K.; Fujinami, A.; Maruyama, N.; Konishi, T.; Ohta, M. Clinical and experimental features of MuSK antibody positive MG in Japan. European journal of neurology 2007, 14, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.N.; Reddel, S.W.; Gervásio, O.L.; Phillips, W.D. Anti-MuSK patient antibodies disrupt the mouse neuromuscular junction. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society 2008, 63, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.; Ghazanfari, N.; Ngo, S.; Gervasio, O.; Reddel, S.; Phillips, W. Patient autoantibodies deplete postsynaptic muscle-specific kinase leading to disassembly of the ACh receptor scaffold and myasthenia gravis in mice. The Journal of Physiology 2010, 588, 3217–3229. [Google Scholar] [CrossRef] [PubMed]
- Klooster, R.; Plomp, J.J.; Huijbers, M.G.; Niks, E.H.; Straasheijm, K.R.; Detmers, F.J.; Hermans, P.W.; Sleijpen, K.; Verrips, A.; Losen, M. Muscle-specific kinase myasthenia gravis IgG4 autoantibodies cause severe neuromuscular junction dysfunction in mice. Brain 2012, 135, 1081–1101. [Google Scholar] [CrossRef] [PubMed]
- Selcen, D.; Fukuda, T.; Shen, X.-M.; Engel, A.G. Are MuSK antibodies the primary cause of myasthenic symptoms? Neurology 2004, 62, 1945–1950. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Wolchok, J.D.; Chen, L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Science translational medicine 2016, 8, 328rv324–328rv324. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nature Reviews Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. New England Journal of Medicine 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.-J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. New England Journal of Medicine 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O'day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C. Improved survival with ipilimumab in patients with metastatic melanoma. New England Journal of Medicine 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Kamada, T. , Togashi, Y., Tay, C., Ha, D., Sasaki, A., Nakamura, Y.,... & Nishikawa, H. (2019). PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proceedings of the National Academy of Sciences, 116(20), 9999-10008.
- Champiat, S.; Dercle, L.; Ammari, S.; Massard, C.; Hollebecque, A.; Postel-Vinay, S.; Chaput, N.; Eggermont, A.; Marabelle, A.; Soria, J.-C. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1Hyperprogressive Disease with Anti-PD-1/PD-L1 Therapy. Clinical Cancer Research 2017, 23, 1920–1928. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Goodman, A.; Walavalkar, V.; Barkauskas, D.A.; Sharabi, A.; Kurzrock, R. Hyperprogressors after Immunotherapy: Analysis of Genomic Alterations Associated with Accelerated Growth RateGenomics of Immunotherapy-Associated Hyperprogressors. Clinical Cancer Research 2017, 23, 4242–4250. [Google Scholar] [CrossRef]
- Champiat, S.; Ferrara, R.; Massard, C.; Besse, B.; Marabelle, A.; Soria, J.-C.; Ferté, C. Hyperprogressive disease: recognizing a novel pattern to improve patient management. Nature Reviews Clinical Oncology 2018, 15, 748–762. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Shimoda, S.; Kimura, Y.; Sato, Y.; Ikeda, H.; Igarashi, S.; Ren, X.S.; Sato, H.; Nakanuma, Y. Significance of immunoglobulin G4 (IgG4)-positive cells in extrahepatic cholangiocarcinoma: molecular mechanism of IgG4 reaction in cancer tissue. Hepatology 2012, 56, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, M.; Inada, H.; Kanayama, K.; Shiraishi, T. A case of pancreatic ductal adenocarcinoma with marked infiltration with IgG4-positive cells. Journal of Cytology 2013, 30, 46. [Google Scholar] [CrossRef] [PubMed]
- Lauw, F.N.; Pajkrt, D.; Hack, C.E.; Kurimoto, M.; van Deventer, S.J.; van der Poll, T. Proinflammatory effects of IL-10 during human endotoxemia. The Journal of Immunology 2000, 165, 2783–2789. [Google Scholar] [CrossRef]
- Nagata, K.; Nishiyama, C. IL-10 in mast cell-mediated immune responses: anti-inflammatory and proinflammatory roles. International journal of molecular sciences 2021, 22, 4972. [Google Scholar] [CrossRef]
- Saxton, R.A.; Tsutsumi, N.; Su, L.L.; Abhiraman, G.C.; Mohan, K.; Henneberg, L.T.; Aduri, N.G.; Gati, C.; Garcia, K.C. Structure-based decoupling of the pro-and anti-inflammatory functions of interleukin-10. Science 2021, 371, eabc8433. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, Q.; Zhao, C.; Zhu, Z.; Zhu, X.; Zhou, J.; Zhang, S.; Yang, T.; Zhang, B.; Li, J. An immune evasion mechanism with IgG4 playing an essential role in cancer and implication for immunotherapy. Journal for ImmunoTherapy of Cancer 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Hammers, H.; Lipson, E.J. Nivolumab: targeting PD-1 to bolster antitumor immunity. Future oncology 2015, 11, 1307–1326. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Marone, G.; Mercurio, V.; Galdiero, M.R.; Bonaduce, D.; Tocchetti, C.G. Immune checkpoint inhibitors and cardiac toxicity: an emerging issue. Current medicinal chemistry 2018, 25, 1327–1339. [Google Scholar] [CrossRef] [PubMed]
- Heinzerling, L.; Ott, P.A.; Hodi, F.S.; Husain, A.N.; Tajmir-Riahi, A.; Tawbi, H.; Pauschinger, M.; Gajewski, T.F.; Lipson, E.J.; Luke, J.J. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. Journal for immunotherapy of cancer 2016, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloomer, T.L. Fulminant myocarditis with combination immune checkpoint blockade. New England Journal of Medicine 2016, 375, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Läubli, H.; Balmelli, C.; Bossard, M.; Pfister, O.; Glatz, K.; Zippelius, A. Acute heart failure due to autoimmune myocarditis under pembrolizumab treatment for metastatic melanoma. Journal for immunotherapy of cancer 2015, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Matson, D.R.; Accola, M.A.; Rehrauer, W.M.; Corliss, R.F. Fatal myocarditis following treatment with the PD-1 inhibitor nivolumab. Journal of forensic sciences 2018, 63, 954–957. [Google Scholar] [CrossRef] [PubMed]
- Isa, M.B.; Martínez, L.; Giordano, M.; Passeggi, C.; de Wolff, M.C.; Nates, S. Comparison of immunoglobulin G subclass profiles induced by measles virus in vaccinated and naturally infected individuals. Clinical and Vaccine Immunology 2002, 9, 693–697. [Google Scholar] [CrossRef]
- Urban, M.; Winkler, T.; Landini, M.; Britt, W.; Mach, M. Epitope-specific distribution of IgG subclasses against antigenic domains on glycoproteins of human cytomegalovirus. Journal of Infectious Diseases 1994, 169, 83–90. [Google Scholar] [CrossRef]
- Buhre, J.S.; Pongracz, T.; Künsting, I.; Lixenfeld, A.S.; Wang, W.; Nouta, J.; Lehrian, S.; Schmelter, F.; Lunding, H.B.; Dühring, L. mRNA vaccines against SARS-CoV-2 induce comparably low long-term IgG Fc galactosylation and sialylation levels but increasing long-term IgG4 responses compared to an adenovirus-based vaccine. Frontiers in Immunology 2023, 13, 7835. [Google Scholar] [CrossRef] [PubMed]
- Billeskov, R.; Beikzadeh, B.; Berzofsky, J.A. The effect of antigen dose on T cell-targeting vaccine outcome. Human vaccines & immunotherapeutics 2019, 15, 407–411. [Google Scholar]
- Keck, S.; Schmaler, M.; Ganter, S.; Wyss, L.; Oberle, S.; Huseby, E.S.; Zehn, D.; King, C.G. Antigen affinity and antigen dose exert distinct influences on CD4 T-cell differentiation. Proceedings of the National Academy of Sciences 2014, 111, 14852–14857. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J.; McCormack, P.L. Reduced-Antigen, Combined Diphtheria, Tetanus, and Acellular Pertussis Vaccine, Adsorbed (Boostrix®) A Guide to Its Use as a Single-Dose Booster Immunization Against Pertussis. BioDrugs 2013, 27, 75–81. [Google Scholar] [CrossRef]
- McCormack, P.L. Reduced-Antigen, Combined Diphtheria, Tetanus and Acellular Pertussis Vaccine, Adsorbed (Boostrix®) A Review of its Properties and Use as a Single-Dose Booster Immunization. Drugs 2012, 72, 1765–1791. [Google Scholar] [CrossRef]
- Chung, A.W.; Ghebremichael, M.; Robinson, H.; Brown, E.; Choi, I.; Lane, S.; Dugast, A.-S.; Schoen, M.K.; Rolland, M.; Suscovich, T.J. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Science translational medicine 2014, 6, 228ra238–228ra238. [Google Scholar] [CrossRef] [PubMed]
- Karnasuta, C.; Akapirat, S.; Madnote, S.; Savadsuk, H.; Puangkaew, J.; Rittiroongrad, S.; Rerks-Ngarm, S.; Nitayaphan, S.; Pitisuttithum, P.; Kaewkungwal, J. Comparison of antibody responses induced by RV144, VAX003, and VAX004 vaccination regimens. AIDS research and human retroviruses 2017, 33, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Gazit, S.; Shlezinger, R.; Perez, G.; Lotan, R.; Peretz, A.; Ben-Tov, A.; Herzel, E.; Alapi, H.; Cohen, D.; Muhsen, K. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) naturally acquired immunity versus vaccine-induced immunity, reinfections versus breakthrough infections: a retrospective cohort study. Clinical Infectious Diseases 2022, 75, e545–e551. [Google Scholar] [CrossRef] [PubMed]
- Shitrit, P.; Zuckerman, N.S.; Mor, O.; Gottesman, B.-S.; Chowers, M. Nosocomial outbreak caused by the SARS-CoV-2 Delta variant in a highly vaccinated population, Israel, July 2021. Eurosurveillance 2021, 26, 2100822. [Google Scholar] [CrossRef]
- Brosh-Nissimov, T.; Orenbuch-Harroch, E.; Chowers, M.; Elbaz, M.; Nesher, L.; Stein, M.; Maor, Y.; Cohen, R.; Hussein, K.; Weinberger, M. BNT162b2 vaccine breakthrough: clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel. Clinical Microbiology and Infection 2021, 27, 1652–1657. [Google Scholar] [CrossRef]
- Nordström, P.; Ballin, M.; Nordström, A. Risk of infection, hospitalisation, and death up to 9 months after a second dose of COVID-19 vaccine: a retrospective, total population cohort study in Sweden. The Lancet 2022, 399, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Dobaño, C.; Quelhas, D.; Quintó, L.; Puyol, L.; Serra-Casas, E.; Mayor, A.; Nhampossa, T.; Macete, E.; Aide, P.; Mandomando, I. Age-dependent IgG subclass responses to Plasmodium falciparum EBA-175 are differentially associated with incidence of malaria in Mozambican children. Clinical and Vaccine Immunology 2012, 19, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Eisenhut, M. Immunity to blood stages of Plasmodium falciparum is dependent on a specific pattern of immunoglobulin subclass responses to multiple blood stage antigens. Medical hypotheses 2007, 69, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Mewono, L.; Maya, D.W.M.; Matsiegui, P.-B.; Agnandji, S.T.; Kendjo, E.; Barondi, F.; Issifou, S.; Kremsner, P.G.; Mavoungou, E. Interleukin-21 is associated with IgG1 and IgG3 antibodies to erythrocyte-binding antigen-175 peptide 4 of Plasmodium falciparum in Gabonese children with acute falciparum malaria. European cytokine network 2008, 19, 30–36. [Google Scholar]
- Oeuvray, C.; Bouharoun-Tayoun, H.; Gras-Masse, H.; Bottius, E.; Kaidoh, T.; Aikawa, M.; Filgueira, M.-C.; Tartar, A.; Druilhe, P. Merozoite surface protein-3: a malaria protein inducing antibodies that promote Plasmodium falciparum killing by cooperation with blood monocytes. 1994.
- Roussilhon, C.; Oeuvray, C.; Müller-Graf, C.; Tall, A.; Rogier, C.; Trape, J.-F.; Theisen, M.; Balde, A.; Pérignon, J.-L.; Druilhe, P. Long-term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein 3. PLoS medicine 2007, 4, e320. [Google Scholar] [CrossRef] [PubMed]
- Stanisic, D.I.; Richards, J.S.; McCallum, F.J.; Michon, P.; King, C.L.; Schoepflin, S.; Gilson, P.R.; Murphy, V.J.; Anders, R.F.; Mueller, I. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infection and immunity 2009, 77, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Aucan, C.; Traoré, Y.; Tall, F.; Nacro, B.; Traoré-Leroux, T.; Fumoux, F.; Rihet, P. High immunoglobulin G2 (IgG2) and low IgG4 levels are associated with human resistance to Plasmodium falciparum malaria. Infection and immunity 2000, 68, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Trampert, D.C.; Hubers, L.M.; van de Graaf, S.F.; Beuers, U. On the role of IgG4 in inflammatory conditions: lessons for IgG4-related disease. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 2018, 1864, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Pajno, G.; Barberio, G.; De Luca, F.; Morabito, L.; Parmiani, S. Prevention of new sensitizations in asthmatic children monosensitized to house dust mite by specific immunotherapy. A six-year follow-up study. Clinical & Experimental Allergy 2001, 31, 1392–1397. [Google Scholar]
- Angioni, R.; Sanchez-Rodriguez, R.; Munari, F.; Bertoldi, N.; Arcidiacono, D.; Cavinato, S.; Marturano, D.; Zaramella, A.; Realdon, S.; Cattelan, A. Age-severity matched cytokine profiling reveals specific signatures in Covid-19 patients. Cell death & disease 2020, 11, 1–12. [Google Scholar]
- Della-Torre, E.; Campochiaro, C.; Cavalli, G.; De Luca, G.; Napolitano, A.; La Marca, S.; Boffini, N.; Da Prat, V.; Di Terlizzi, G.; Lanzillotta, M. Interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hyperinflammation: an open-label cohort study. Annals of the rheumatic diseases 2020, 79, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Della-Torre, E.; Della-Torre, F.; Kusanovic, M.; Scotti, R.; Ramirez, G.A.; Dagna, L.; Tresoldi, M. Treating COVID-19 with colchicine in community healthcare setting. Clinical Immunology (Orlando, Fla.) 2020, 217, 108490. [Google Scholar] [CrossRef] [PubMed]
- Della-Torre, E.; Lanzillotta, M.; Campochiaro, C.; Cavalli, G.; De Luca, G.; Tomelleri, A.; Boffini, N.; De Lorenzo, R.; Ruggeri, A.; Rovere-Querini, P. Respiratory impairment predicts response to IL-1 and IL-6 blockade in COVID-19 patients with severe pneumonia and hyper-inflammation. Frontiers in Immunology 2021, 12, 675678. [Google Scholar] [CrossRef] [PubMed]
- Peacock, T.P.; Brown, J.C.; Zhou, J.; Thakur, N.; Sukhova, K.; Newman, J.; Kugathasan, R.; Yan, A.W.; Furnon, W.; De Lorenzo, G. The altered entry pathway and antigenic distance of the SARS-CoV-2 Omicron variant map to separate domains of spike protein. biorxiv 2022, 15, e0241955. [Google Scholar]
- McMahan, K.; Giffin, V.; Tostanoski, L.H.; Chung, B.; Siamatu, M.; Suthar, M.S.; Halfmann, P.; Kawaoka, Y.; Piedra-Mora, C.; Jain, N. Reduced pathogenicity of the SARS-CoV-2 Omicron variant in hamsters. Med 2022, 3, 262–268.e264. [Google Scholar] [CrossRef] [PubMed]
- Meng, B.; Abdullahi, A.; Ferreira, I.A.; Goonawardane, N.; Saito, A.; Kimura, I.; Yamasoba, D.; Gerber, P.P.; Fatihi, S.; Rathore, S. Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity. Nature 2022, 603, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Willett, B.J.; Grove, J.; MacLean, O.A.; Wilkie, C.; De Lorenzo, G.; Furnon, W.; Cantoni, D.; Scott, S.; Logan, N.; Ashraf, S. SARS-CoV-2 Omicron is an immune escape variant with an altered cell entry pathway. Nature microbiology 2022, 7, 1161–1179. [Google Scholar] [CrossRef] [PubMed]
- Shuai, H.; Chan, J.F.-W.; Hu, B.; Chai, Y.; Yuen, T.T.-T.; Yin, F.; Huang, X.; Yoon, C.; Hu, J.-C.; Liu, H. Attenuated replication and pathogenicity of SARS-CoV-2 B. 1.1. 529 Omicron. Nature 2022, 603, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Wolter, N.; Jassat, W.; Walaza, S.; Welch, R.; Moultrie, H.; Groome, M.; Amoako, D.G.; Everatt, J.; Bhiman, J.N.; Scheepers, C. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. The Lancet 2022, 399, 437–446. [Google Scholar] [CrossRef]
- Gao, F.-X.; Wu, R.-X.; Shen, M.-Y.; Huang, J.-J.; Li, T.-T.; Hu, C.; Luo, F.-Y.; Song, S.-Y.; Mu, S.; Hao, Y.-N. Extended SARS-CoV-2 RBD booster vaccination induces humoral and cellular immune tolerance in mice. Iscience 2022, 25, 105479. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Xu, J.; Xia, H.; Wang, Y.; Zhang, C.; Chen, W.; Zhang, H.; Liu, Q.; Zhu, R. Comprehensive investigations revealed consistent pathophysiological alterations after vaccination with COVID-19 vaccines. Cell discovery 2021, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.N.; Devlin, J.C.; Buus, T.B.; Koide, A.; Shwetar, J.; Cornelius, A.; Samanovic, M.I.; Herrera, A.; Mimitou, E.P.; Zhang, C. SARS-CoV-2 mRNA vaccine elicits a potent adaptive immune response in the absence of IFN-mediated inflammation observed in COVID-19. MedRxiv 2021. [Google Scholar]
- Seneff, S.; Nigh, G.; Kyriakopoulos, A.M.; McCullough, P.A. Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs. Food and Chemical Toxicology 2022, 164, 113008. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, Y.; Moorman, J.P.; Yao, Z.Q.; Jia, Z.S. Viral (hepatitis C virus, hepatitis B virus, HIV) persistence and immune homeostasis. Immunology 2014, 143, 319–330. [Google Scholar] [CrossRef]
- Tsumiyama, K.; Miyazaki, Y.; Shiozawa, S. Self-organized criticality theory of autoimmunity. PLoS One 2009, 4, e8382. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, S. Cause of systemic lupus erythematosus: a novel self-organized criticality theory of autoimmunity. Expert Review of Clinical Immunology 2011, 7, 715–717. [Google Scholar] [CrossRef] [PubMed]
- Gadi, S.R.; Brunker, P.A.; Al-Samkari, H.; Sykes, D.B.; Saff, R.R.; Lo, J.; Bendapudi, P.; Leaf, D.E.; Leaf, R.K. Severe autoimmune hemolytic anemia following receipt of SARS-CoV-2 mRNA vaccine. Transfusion 2021, 61, 3267–3271. [Google Scholar] [CrossRef] [PubMed]
- Bril F, Al Diffalha S, Dean M, Fettig DM. 2021. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: Causality or casualty? Journal of Hepatology 75:222–224. [CrossRef]
- Watad, A.; De Marco, G.; Mahajna, H.; Druyan, A.; Eltity, M.; Hijazi, N.; Haddad, A.; Elias, M.; Zisman, D.; Naffaa, M.E. Immune-mediated disease flares or new-onset disease in 27 subjects following mRNA/DNA SARS-CoV-2 vaccination. Vaccines 2021, 9, 435. [Google Scholar] [CrossRef] [PubMed]
- Portuguese, A.J.; Sunga, C.; Kruse-Jarres, R.; Gernsheimer, T.; Abkowitz, J. Autoimmune-and complement-mediated hematologic condition recrudescence following SARS-CoV-2 vaccination. Blood Advances 2021, 5, 2794–2798. [Google Scholar] [CrossRef]
- Ghielmetti, M.; Schaufelberger, H.D.; Mieli-Vergani, G.; Cerny, A.; Dayer, E.; Vergani, D.; Beretta-Piccoli, B.T. Acute autoimmune-like hepatitis with atypical anti-mitochondrial antibody after mRNA COVID-19 vaccination: a novel clinical entity? Journal of autoimmunity 2021, 123, 102706. [Google Scholar] [CrossRef]
- Vuille-Lessard, É.; Montani, M.; Bosch, J.; Semmo, N. Autoimmune hepatitis triggered by SARS-CoV-2 vaccination. Journal of autoimmunity 2021, 123, 102710. [Google Scholar] [CrossRef] [PubMed]
- Chamling, B.; Vehof, V.; Drakos, S.; Weil, M.; Stalling, P.; Vahlhaus, C.; Mueller, P.; Bietenbeck, M.; Reinecke, H.; Meier, C. Occurrence of acute infarct-like myocarditis following COVID-19 vaccination: just an accidental co-incidence or rather vaccination-associated autoimmune myocarditis? Clinical Research in Cardiology 2021, 110, 1850–1854. [Google Scholar] [CrossRef] [PubMed]
- Clayton-Chubb, D.; Schneider, D.; Freeman, E.; Kemp, W.; Roberts, S. Comment to the letter of Bril F et al.“Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: causality or casualty?”. J Hepatol 2021, 75, 1249–1250. [Google Scholar] [CrossRef] [PubMed]
- Minocha, P.K.; Better, D.; Singh, R.K.; Hoque, T. Recurrence of acute myocarditis temporally associated with receipt of the mRNA coronavirus disease 2019 (COVID-19) vaccine in a male adolescent. The Journal of pediatrics 2021, 238, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Elrashdy, F.; Tambuwala, M.M.; Hassan, S.S.; Adadi, P.; Seyran, M.; Abd El-Aziz, T.M.; Rezaei, N.; Lal, A.; Aljabali, A.A.; Kandimalla, R. Autoimmunity roots of the thrombotic events after COVID-19 vaccination. Autoimmunity reviews 2021, 20, 102941. [Google Scholar] [CrossRef] [PubMed]
- Garrido, I.; Lopes, S.; Simões, M.S.; Liberal, R.; Lopes, J.; Carneiro, F.; Macedo, G. Autoimmune hepatitis after COVID-19 vaccine–more than a coincidence. Journal of autoimmunity 2021, 125, 102741. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Wang, P.; Li, X.M.; Shuai, Z.W.; Ye, D.Q.; Pan, H.F. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology 2022, 165, 386–401. [Google Scholar] [CrossRef] [PubMed]
- Fatima, Z.; Reece, B.R.; Moore, J.S.; Means Jr, R.T. Autoimmune hemolytic anemia after mRNA COVID vaccine. Journal of Investigative Medicine High Impact Case Reports 2022, 10, 23247096211073258. [Google Scholar] [CrossRef] [PubMed]
- Mahroum, N.; Lavine, N.; Ohayon, A.; Seida, R.; Alrais, M.; Zoubi, M.; Bragazzi, N.L. COVID-19 vaccines and the rate of autoimmune adverse events: insights from a literature review. Frontiers in immunology 2022, 3221. [Google Scholar]
- Finsterer, J. Neurological side effects of SARS-CoV-2 vaccinations. Acta Neurologica Scandinavica 2022, 145, 5–9. [Google Scholar] [CrossRef]
- Garg, R.K.; Paliwal, V.K. Spectrum of neurological complications following COVID-19 vaccination. Neurological Sciences 2022, 43, 3–40. [Google Scholar] [CrossRef] [PubMed]
- Kaulen, L.D.; Doubrovinskaia, S.; Mooshage, C.; Jordan, B.; Purrucker, J.; Haubner, C.; Seliger, C.; Lorenz, H.M.; Nagel, S.; Wildemann, B. Neurological autoimmune diseases following vaccinations against SARS-CoV-2: a case series. European journal of neurology 2022, 29, 555–563. [Google Scholar] [CrossRef]
- Kwon, H.; Kim, T. Autoimmune encephalitis following ChAdOx1-S SARS-CoV-2 vaccination. Neurological Sciences 2022, 43, 1487–1489. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, R.; Giovanellla, L.; Campennì, A. SARS-CoV-2 vaccine may trigger thyroid autoimmunity: Real-life experience and review of the literature. Journal of Endocrinological Investigation 2022, 45, 2283–2289. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
IgG4 antibody has a distinctive structure. A. Two heavy chains and two light chains make up the IgG4 antibody. B. The Fc fragment of one IgG4 molecule can react with the Fc fragment of another. C. When half-molecules are exchanged (called a Fab-arm interchange), IgG4 combines two distinct specificities into a unique molecule (bispecific antibody). Reproduced from Moriyama, M., Tanaka, A., Maehara, T., Furukawa, S., Nakashima, H., Nakamura, S. (2014). T helper subsets in Sjögren's syndrome and IgG4-related dacryoadenitis and sialoadenitis: a critical review. Journal of Autoimmunity, 51, 81-88. [25]: “This is an open access article distributed under the terms of the Creative Commons CC-BY license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited”.
Figure 1.
IgG4 antibody has a distinctive structure. A. Two heavy chains and two light chains make up the IgG4 antibody. B. The Fc fragment of one IgG4 molecule can react with the Fc fragment of another. C. When half-molecules are exchanged (called a Fab-arm interchange), IgG4 combines two distinct specificities into a unique molecule (bispecific antibody). Reproduced from Moriyama, M., Tanaka, A., Maehara, T., Furukawa, S., Nakashima, H., Nakamura, S. (2014). T helper subsets in Sjögren's syndrome and IgG4-related dacryoadenitis and sialoadenitis: a critical review. Journal of Autoimmunity, 51, 81-88. [25]: “This is an open access article distributed under the terms of the Creative Commons CC-BY license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited”.
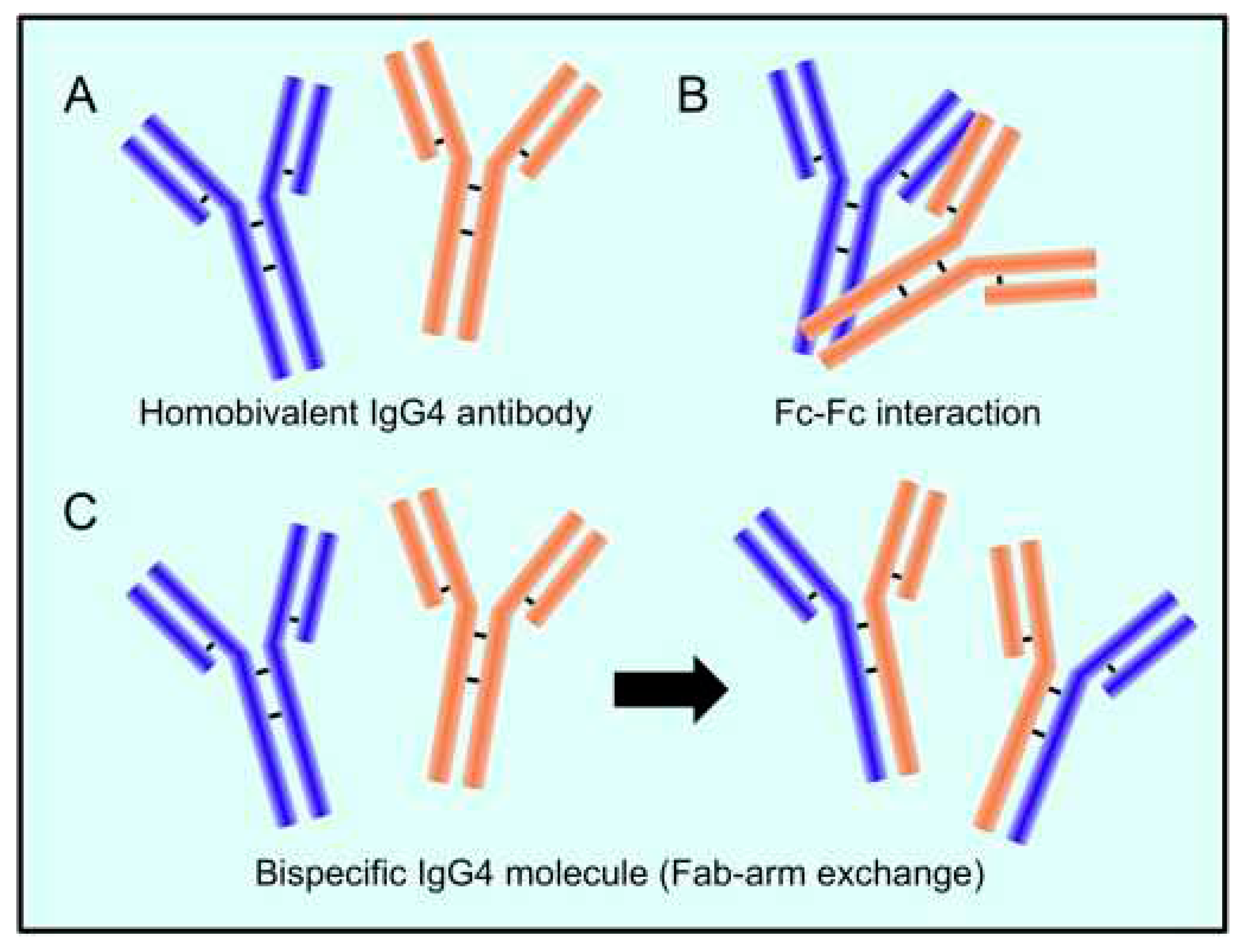
Figure 2.
In A, a pollen grain is recognized through the fragment antigen-binding region (Fab) of an IgE antibody. After that, the IgE attaches to its receptor, called Fc epsilon RI (FcεRI), located on eosinophil leukocytes, and induces histamine release from cytoplasmic granules. Histamine is a vasoactive peptide that causes symptoms such as itching, sneezing, runny nose, itchy throat, eyes, and ears, and trouble breathing during a pollen-induced allergic reaction. In B, the Fc region of an IgG4 antibody binds to the fragment crystallizable (Fc) region of an IgE antibody, inhibiting its binding to the FcεRI receptor and thus blocking IgE-mediated effects. Created with Biorender.
Figure 2.
In A, a pollen grain is recognized through the fragment antigen-binding region (Fab) of an IgE antibody. After that, the IgE attaches to its receptor, called Fc epsilon RI (FcεRI), located on eosinophil leukocytes, and induces histamine release from cytoplasmic granules. Histamine is a vasoactive peptide that causes symptoms such as itching, sneezing, runny nose, itchy throat, eyes, and ears, and trouble breathing during a pollen-induced allergic reaction. In B, the Fc region of an IgG4 antibody binds to the fragment crystallizable (Fc) region of an IgE antibody, inhibiting its binding to the FcεRI receptor and thus blocking IgE-mediated effects. Created with Biorender.
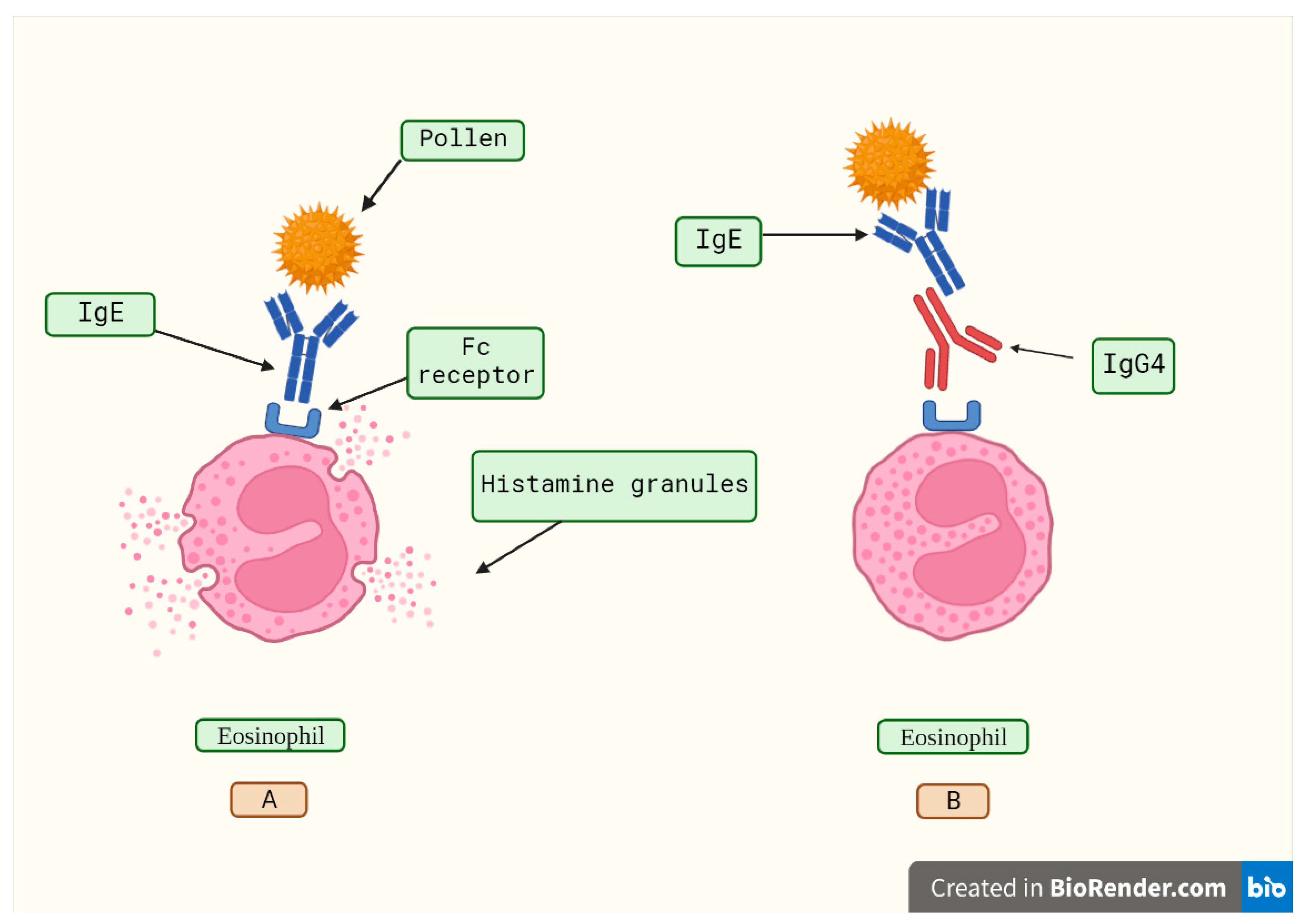
Figure 3.
The suggested pathway for immune evasion evolved by cancer cells through IgG4 produced from B lymphocytes is depicted diagrammatically. Prolonged exposure to cancer antigens causes B cells to change their class and generate IgG4. With its Fc-Fc binding characteristic, such enhanced IgG4 can interact with cancer-bound IgG as well as Fc receptors on immune effector cells. Increased IgG4 in the cancer microenvironment promotes an efficient immune evasion mechanism for cancer due to its special structural and biological properties. The acronyms ADCC, ADCP, CDC, and NK stand for antibody-dependent cell-mediated cytotoxicity, antibody-dependent cell phagocytosis, complement-dependent cytotoxicity, and natural killer cells, respectively. Reproduced from Wang H, Xu Q, Zhao C, Zhu Z, Zhu X, Zhou J, Zhang S, Yang T, Zhang B, Li J, Yan M, Liu R, Ma C, Quan Y, Zhang Y, Zhang W, Geng Y, Chen C, Chen S, Liu D, Chen Y, Tian D, Su M, Chen X, Gu J. (2020). An immune evasion mechanism with IgG4 playing an essential role in cancer and implication for immunotherapy. Journal for Immuno Therapy of Cancer, 8 (2), e000661. [85]: “This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial”.
Figure 3.
The suggested pathway for immune evasion evolved by cancer cells through IgG4 produced from B lymphocytes is depicted diagrammatically. Prolonged exposure to cancer antigens causes B cells to change their class and generate IgG4. With its Fc-Fc binding characteristic, such enhanced IgG4 can interact with cancer-bound IgG as well as Fc receptors on immune effector cells. Increased IgG4 in the cancer microenvironment promotes an efficient immune evasion mechanism for cancer due to its special structural and biological properties. The acronyms ADCC, ADCP, CDC, and NK stand for antibody-dependent cell-mediated cytotoxicity, antibody-dependent cell phagocytosis, complement-dependent cytotoxicity, and natural killer cells, respectively. Reproduced from Wang H, Xu Q, Zhao C, Zhu Z, Zhu X, Zhou J, Zhang S, Yang T, Zhang B, Li J, Yan M, Liu R, Ma C, Quan Y, Zhang Y, Zhang W, Geng Y, Chen C, Chen S, Liu D, Chen Y, Tian D, Su M, Chen X, Gu J. (2020). An immune evasion mechanism with IgG4 playing an essential role in cancer and implication for immunotherapy. Journal for Immuno Therapy of Cancer, 8 (2), e000661. [85]: “This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial”.
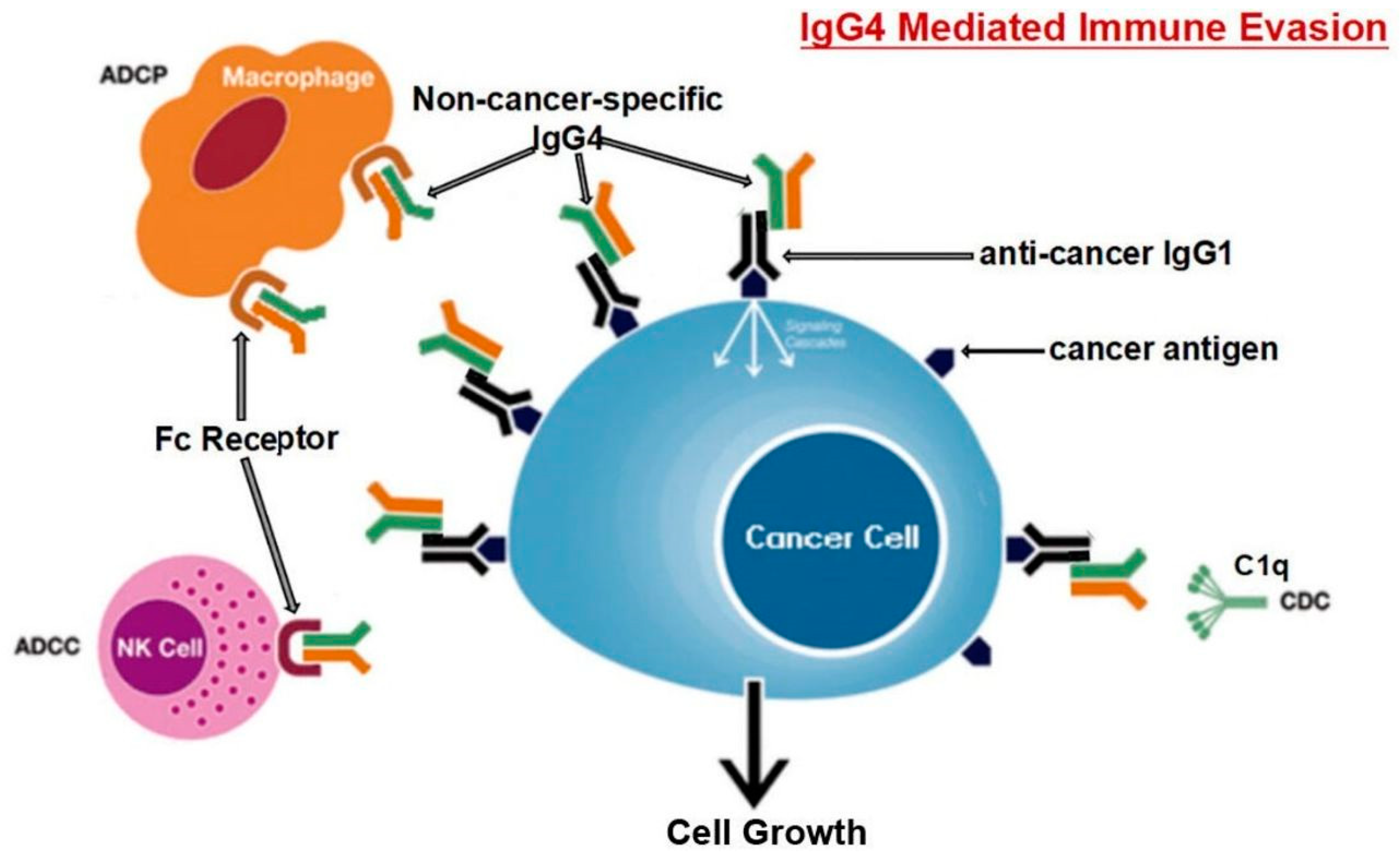
Figure 4.
An effective humoral response induced by vaccination consists of the synthesis of high IgG3 concentrations. A) IgG3 antibodies attach to viral antigens exposed on infected cells' membranes through its variable region. This antibody has a constant region (Fc) that is recognized by the corresponding receptor found on cytotoxic T cells and other immune cells. The cytotoxic T cell becomes activated and releases chemical agents that destroy the infected cell. B) Repeated vaccination induces high IgG4 levels (depicted in red). This antibody inhibits the attachment of the Fc region from the IgG3 antibody to its receptor located on cytotoxic T cells, thus blocking its activation, and in consequence, the infected cell is not destroyed. In this sense, repeated boosting causes the switch to the production of high IgG4 levels, which impair immune responses. Created with Biorender.
Figure 4.
An effective humoral response induced by vaccination consists of the synthesis of high IgG3 concentrations. A) IgG3 antibodies attach to viral antigens exposed on infected cells' membranes through its variable region. This antibody has a constant region (Fc) that is recognized by the corresponding receptor found on cytotoxic T cells and other immune cells. The cytotoxic T cell becomes activated and releases chemical agents that destroy the infected cell. B) Repeated vaccination induces high IgG4 levels (depicted in red). This antibody inhibits the attachment of the Fc region from the IgG3 antibody to its receptor located on cytotoxic T cells, thus blocking its activation, and in consequence, the infected cell is not destroyed. In this sense, repeated boosting causes the switch to the production of high IgG4 levels, which impair immune responses. Created with Biorender.
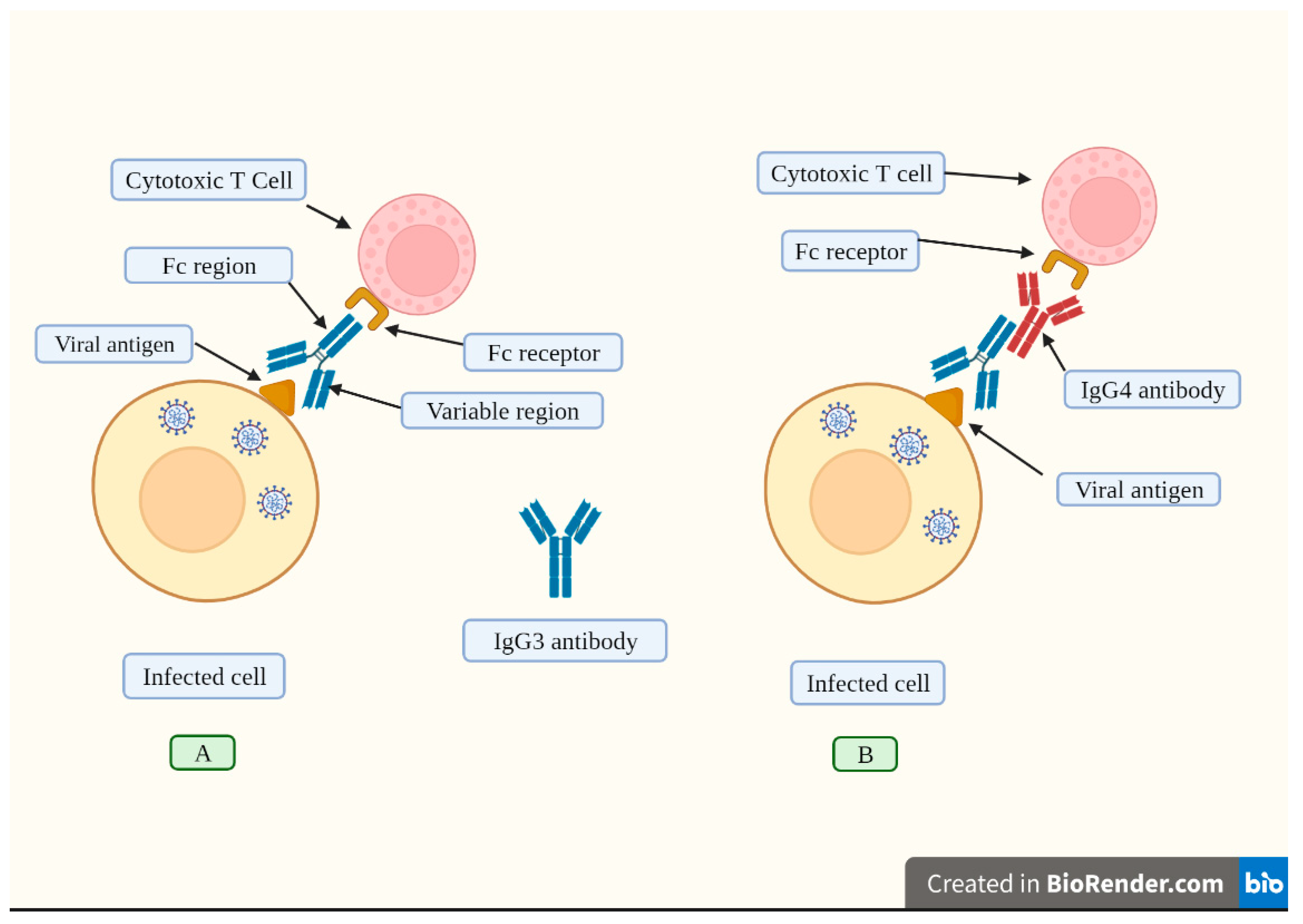
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Cellular and humoral immunogenicity of a candidate DNA Vaccine Expressing SARS-CoV-2 Spike Subunit 1
Khalid A. Alluhaybi
et al.
,
2021
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated








