1. Introduction
In 1924, the Soviet biochemist Alexander I. Oparin (1894-1980) proposed a model according suggesting that the formation of liquid coacervates due to the liquid-liquid phase separations represented a crucial step in the origin of primitive life [
1]. The theory was based on the observation that droplets of organic molecules coalesce spontaneously in solution. Unfortunately, this model was ultimately not supported by the scientific community because it could not explain the existence of membrane-bound organelles and their contribution to cellular compartmentalization.
However, the so-called "LLPS-revolution" that took place in the 10s of the 21st century radically changed the idea of the spatial-temporal organization of the intracellular space in line with Oparin’s view. It became obvious that the liquid-liquid phase separation (LLPS) of biopolymers plays an important role in the formation, regulation, and functional activity of cellular compartments. An entire class of organelles being formed as a result of a tightly controlled LLPS and not enclosed by the membranes (membrane-less organelles MLOs) has been separated.
Membrane-less organelles (MLO) are present in eukaryotes, Achaea, and bacteria (and, likely in viruses as well). They can be formed in the nucleus,in the cytoplasm, as well as in the mitochondrial matrix and chloroplast stroma. To date, about a hundred different MLOs are known [
2]. Major nuclear MLOs are nucleoli, Cajal bodies, nuclear speckles, paraspeckles, nuclear stress bodies, amyloid bodies (A-bodies), and others. The cytoplasm also contains a number of MLOs, including processing bodies (P-bodies), stress granules, and germ granules [
3,
4]. While some MLOs are found in the cell under normal conditions (e.g. nucleoli, Cajal bodies, nuclear speckles, paraspeckles, histone loci bodies, P-bodies, etc.), others are formed under stress conditions.
One of the vital foundations for the existence and development of the cells is the ability of living organisms to adapt to changes in the environment. To survive, cells must constantly regulate the multitude of cascades of biochemical reactions in response to the intra- and extracellular signals. The ability of cells to form assemblages of proteins and nucleic acids in response to stress seems to be a conserved mechanism that allows them to withstand adverse conditions. Many cellular membrane-less organelles are condensates of proteins and nucleic acids. The formation and disassembly of such structures can be extremely fast [
5,
6], which ensures rapid metabolic and functional restructuring and response to stress [
7,
8]. Examples of such MLOs are nuclear stress bodies and A-bodies.
Nuclear stress bodies (nSBs) and Amyloid bodies (A-bodies or ABs) are two types of MLOs that are temporarily formed in the nucleus in response to stress and disintegrate during the period of post-stress recovery. Both of these stress-induced MLOs have a similar mechanisms of assembly and play a number of significant roles in the activation of survival programs and the normalization of cellular homeostasis after the cell exits unfavorable conditions. In this review, we will focus our attention on these MLOs and describe their functions, structure, and mechanism of formation.
2. Nucleolar stress bodies
Nuclear stress bodies (nSBs) were first discovered in the late 1980s when it became clear that these structures are associated with the cellular response to stress [
9]. nSBs are primate-specific MLOs that arise in response to various types of stress. The size and number of nSBs depend on the type and duration of stress, however, on average, one cell might contain several nSBs 1–2 μm in size [
6]. These organelles play an important role in the regulation of gene expression and the recovery of metabolic processes after stress exposure. However, specific functions, as well as the molecular mechanisms of the formation and decomposition of these compartments remain poorly understood to date.
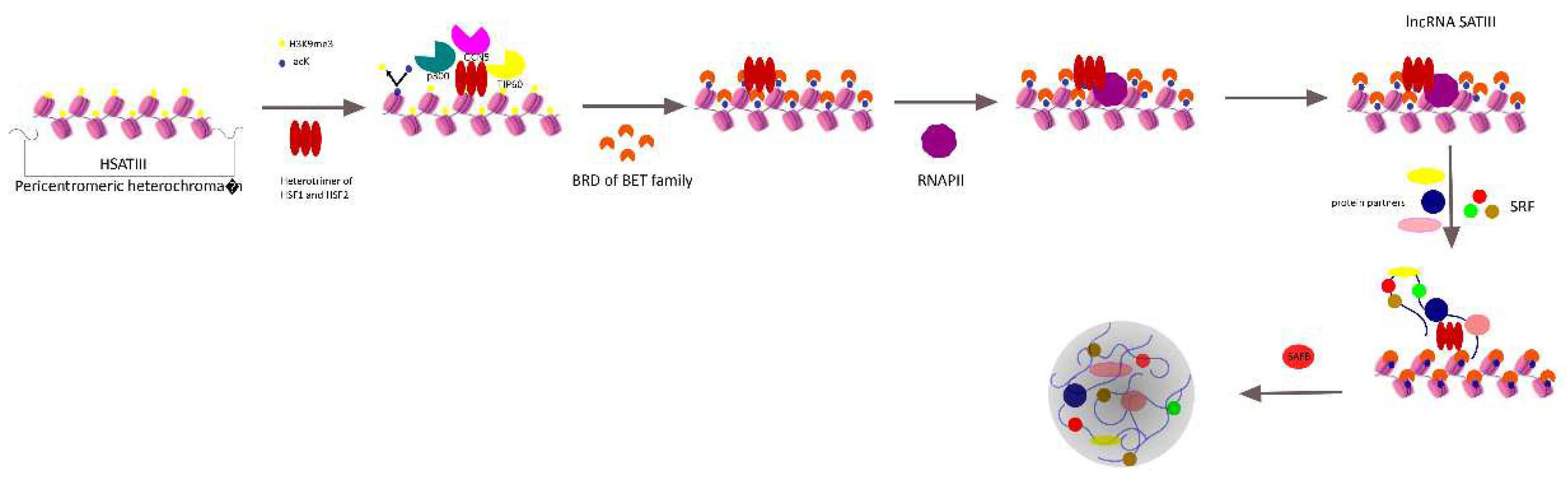
Structure and formation mechanism of nSBs
The assembly of nSBs is initiated by transcription of highly repetitive satellite III architectural noncoding RNAs, HSATIII arcRNAs, or simply HSATIII from the pericentromeric HSATIII repeat arrays of chromosomes 9, 12, and 15. However, nSBs are mainly associated with chromosome 9 (locus 9q12). HSATIII RNA is specific for primates; no formation of nuclear stress bodies or structures similar to them is observed in rodent cells in response to stress [
9]. However, it is known that in Drosophila melanogaster, so-called omega-speckles are formed in response to temperature stress. They are nuclear structures and contain a long non-coding RNA (lncRNA) Hsrω and many RNA-binding proteins, including various heterogeneous nuclear ribonucleoproteins (hnRNPs). Presumably, omega speckles are functional analogs of nSBs that arose independently in the course of evolution, which may emphasize the significance of such structures [
10].
Heat shock response (HSR) activates not only the transcription of genes encoding heat shock proteins (HSP) [
10] but also the transcription of pericentric heterochromatin (HC). The pericentric HC is formed by tandem repeats of satellite III elements, which have a modular structure consisting of the GGAAT pentamer sequences separated by the “terminator” sequence CAAC(C/A)CGAGT [
11]. Transcription activation is under the control of heat shock factor 1 (HSF1), a transcription factor present in the nucleus and cytoplasm of cells under normal conditions [
12,
13]. HSF2 is also involved in transcription activation [
14]. Although vertebrate cells express five additional members of the HSF family (HSF3, HSF4, HSF5, HSFX, and HSFY), the actual roles of these proteins are mostly unknown [
14].
Activation of pericentric HC is mainly observed at the 9q12 locus and leads to the accumulation of long non-coding (lnc) satellite III (sat III) RNA, which serves as a focus for the formation of nSBs. As a result of thermal exposure of the cell, HSF1 recruits HAT histone acetyltransferases, such as general control of amino acid synthesis protein 5 (GCN5, also known as lysine acetyltransferase 2 (KAT2), 60 kDa Tat-interactive protein (TIP60, also known as KAT5), and p300 to the region of pericentric heterochromatin, which leads to a redistribution of histone acetylation. The acetylated lysine residues of H3 and H4 histones then serve as docking sites for the bromodomain proteins of the BET family, triggering chromatin remodeling, RNAP II recruitment, and activation of the HSATIII transcription [
15]. Subsequently, the transcribed lncRNA HSATIII remains associated with its transcription sites (
Figure 1).
The peak of SatIII RNA expression is observed under cell stress (heat shock) at 42–43°C. Heat shock at 41°C or 44°C leads to ∼10% of maximal transcription, while lower temperatures (39–40°C) or high stress conditions (45°C) have almost no effect on SatIII DNA expression [
16]. Under the hyperosmotic stress, the transcriptional activation of SatIII DNA involves the transcription factor tonicity enhancer binding protein (TonEBP, also known as nuclear factor of activated T-cells 5, NFAT5) [
16]. TonEBP/NFAT5 is known to regulate gene expression in response to osmotic stress and is vital for kidney function and protection against elevated salt and urea levels in the renal medulla. Knockdown of TonEBP/NFAT5 with siRNA prevents nSB formation in response to stress, and the HSATIII sequence contains a putative TonEBP/NFAT5 binding site. Therefore, HSATIII RNA is a noncoding RNA whose transcription is controlled by different transcription factors under various stresses, and nSBs are an important part of the overall cellular response to stress, responding to various stress-sensitive pathways depending on the type of exposure [
16].
Cadmium sulfate and UV are also able to induce transcription of HSATIII RNA. Long-term exposure to cadmium sulfate is comparable to heat shock both in terms of the proportion of cells with nSBs (>90%), the number of nSBs per cell, and their sizes [
16]. A smaller proportion of cells with nSBs (30–40%) after UV irradiation, and the sizes of these structures are usually smaller. Interestingly, in all the cases described above, transcription mainly proceeds from the G-rich chain. C-rich transcripts are practically not found in cells not subjected to stress, and slightly increase after stress. Thus, the transcription of two DNA strands of HSATIII is asymmetric [
16].
In a study [
17], the authors induced knockdown of normal HSATIII expression using transient transfection with the antisense oligonucleotides and found that knockdown significantly reduced (~70%) the survival of HeLa cells after thermal exposure compared to the cells that were transiently transfected with control oligonucleotides and subjected to heat stress. They then transiently transfected the mammalian expression construct (pcDNA-Sat3) to allow constitutive transcription of HSATIII under normal (stress-free) conditions. Cells that constitutively expressed the cloned HSATIII repeats exhibited significantly lower survival after heat shock exposure compared to those transfected with a blank control plasmid. These data indicate not only the importance of HSATIII expression during thermal stress but also the toxic effect of HSATIII transcripts during ectopic expression [
17].
It is known that many proteins are involved in the biogenesis of nSBs. A recent proteomic analysis of isolated nSBs complexes has identified more than 140 proteins, mostly RNA-binding proteins (RBPs) that are involved in mRNA splicing, processing, and export [
18]. SATIII transcripts are known to colocalize with HSF1, HSF2, scaffold attachment factor B (SAFB), splicing factors CREBBP (cyclic adenosine monophosphate response element (CREB)-binding protein) [
17], serine/arginine-rich splicing factors 1 and 9 (SRSF1 and SRSF9) [
18,
19,
20], and many other proteins.
HSF1
HSF1 is the most studied heat shock transcription factor and is the classic HSF that responds to elevated temperatures and other forms of stress. Under normal physiological conditions, HSF1 exists in an inactive state associated with molecular chaperones. Under stress, it is rapidly released from the complex, trimerized, hyperphosphorylated, and acquires DNA-binding and transcriptional activity [
9,
14,
21]. Under constant moderate heat stress, the equilibrium shifts back to a monomeric, dephosphorylated, and Hsp-bound state. Interestingly, long-term stress leads to a decrease in HSF1 activity, which leads to a suppression of the ability to reactivate HSF1 during subsequent stress. A similar repression of HSF1 activity is observed during cell recovery after stress conditions. This acquired adaptation of cells to stressful influences is called stress thermotolerance [
21].
HSF2
Unlike HSF1, HSF2 undergoes a dimer-to-trimer transition upon activation and is not phosphorylated [
13]. The regulation of HSF1 and HSF2 expression differs sharply. Unlike stably and constitutively expressed HSF1, HSF2 is inducible. The study [
19] showed that HSF1 and HSF2 are functionally related. Heat shock produces HSF1 homotrimers and HSF1-HSF2-HSF1 heterotrimers, which activate and regulate HSATIII transcription in response to stress. The heterotrimerization mechanism of HSF1 and HSF2 likely provides transient regulation as heat shock lowers HSF2 levels, thereby limiting heterotrimerization due to limited HSF2 availability. Therefore, the HSF1-HSF2 heterotrimerization regulates stress-induced transcription [
19].
SAFB
SAFB is an hnRNP protein that binds to already formed stress granules via an RNA-binding domain [
20]. It is a protein of the nuclear matrix and is involved in such important cellular processes as Xist-regulated inactivation of the X chromosome [
20] and cell apoptosis [
22]. SAFB supports the organization of pericentromeric heterochromatin, interacts with repeat element RNAs, and drives phase separation. It is known that the depletion of SAFB leads to structural changes in the organization of the genome affecting expression of various genes [
23]. Therefore, the recruitment of SAFB into nSBs leads to the remodeling of genome compartmentalization, which probably helps the cell survival under stress [
24].
At the very beginning of the stress response, the main protein in nSBs is HSF1, but its amount rapidly decreases during the recovery period after the cessation of the stimulus. The binding of SAFB to nSBs begins an hour after exposure to mild thermal stress, reaching a maximum concentration 3 h after the end of the stress. In their 2019 article, T. Hirose
et al. suggested that in addition to the canonical HSF1/SAFB nSBs, additional stress nuclear bodies are also formed in the cell as a result of the interaction of the hnRNPM protein and HSATIII [
25]. Whether hnRNPM forms a specific distinct type of nSBs, or whether these formations represent one of the stages in the formation of nuclear stress bodies, remains to be seen.
SRSF1 and SRSF9
SRSF1 and SRSF9 are pre-mRNA splicing factors capable of interacting simultaneously with RNA and other protein components through the RNA recognition motif (RRM) and through the RS domain rich in arginine and serine residues [
18]. SRSF functions in pre-mRNA splicing are regulated by phosphorylation of the RS domain. Splicing factors SRSF are known to be rapidly dephosphorylated when exposed to thermal stress. During stress recovery, CDC-like kinase 1 (CLK1) is recruited to nSBs and re-phosphorylates SRSF9, which, by promoting intron retention, is involved in splicing inhibition [
26]. In other words, nSBs serve as a platform for rapid rephosphorylation of certain SRSFs and promote intron retention in hundreds of pre-mRNAs. There is another type of splicing regulation by nSBs that have been described. In a study [
27], it was shown that lncRNA HSATIII m
6 A-methylated in the GGAAU repeat, which mediates YTHDC1 (YTH domain-containing protein 1) sequestration to nSBs during recovery from the heat stress. This leads to the repression of m
6 A-dependent splicing of specific pre-mRNAs.
The two mechanisms described above, work in parallel to regulate splicing of target introns, with different features being recognized: a lower GC content and longer exon upstream for the YTHDC1 pathway and a relatively longer exon downstream for the SRSF9 pathway. However, at least some HSATIII target introns, such as RBM48 intron 3, are co-regulated by both mechanisms [
18,
27].
CREBBP
CREBBP is another important protein that is a part of nSBs. It is a transcriptional coactivator that forms a complex with various transcription factors and promotes the location of these complexes near the promoters of target genes. Recruitment of CREBBP to nSBs during heat shock occurs in an SRSF-1-dependent manner and contributes to the regulation of transcription under stress [
17].
In 2020, a study was published proving that nSBs are dynamic molecular condensates formed as a result of liquid-liquid phase separation [
28]. Furthermore, it has been shown that with an increase in the duration of stress exposure, the dynamics of HSF1 exchange with the nucleoplasm decreases sharply, which indicates a gradual hardening of HSF1-nSBs. Furthermore, after 16 h of incubation with the proteasome inhibitor MG132, HSF1 mobility decreased by 40% that was also confirmed by the addition of the liquid droplet condensate inhibitor 1,6-hexanediol, which showed a decrease in the mobile fraction by 54% after 8 h of proteotoxic exposure [
28]. The dynamism and reversibility of the nSB formation promote cell survival. The appearance of insoluble gel-like HSF1-nSBs leads to an increase in cell sensitivity to apoptosis [
28].
Biological functions of nSBs
At present, the biological function of nSBs is not fully understood. Initially, it was assumed that nSBs are the transcription centers of HSF1 target genes, but this assumption was refuted when it was unambiguously shown that the localization of nSBs does not coincide with the centers of expression of heat shock proteins [
27]. In addition, nSBs do not contain poly(A)-containing RNAs [
17]. However, it is clear that activation of HSATIII transcription in cells exposed to heat shock remodels gene expression on a genome-wide scale due to massive recruitment of HAT and transcription factors to nSBs [
15,
18,
19,
27].
In splicing control, nSBs serve a dual function: as "molecular sponges" for YTHDC1 sequestration, and as "reaction crucibles" for SRSF phosphorylation. Both mechanisms contribute to the repression of pre-mRNA splicing during recovery from heat stress [
19,
27].
Therefore, the formation of nSBs in response to stress ensures the occurrence of cytoprotective reactions and, probably, is of great importance in the cell life cycle.
A-Bodies
It is known that both the synthesis and biogenesis of ribosomes and the processing of ribosomal RNAs take place in the nucleolus [
29]. The nucleolus is a phase-separated biomolecular condensate in which a dynamic exchange of proteins with the surrounding nucleoplasm takes place [
30]. Each nucleolus is organized in layers that are clearly visible under electron microscopy (EM): fibrillar center (FC); dense fibrillar component (DFC) enriched in fibrillarin (FIB1); and a granular component (GC) containing nucleophosmin [
30]. It should be noted that such a multilayer structure is not unique to the nucleolus. For example, stress granules and other RNP fluid-like bodies exhibit a similar core-shell structure [
31,
32]. The transcription process occurs at the FC/DFC interface, the early stages of co-transcriptional processing and modification of pre-rRNA occur in the DFC, and the late stages of pre-rRNA processing and assembly with ribosomal proteins occur in peripheral GCs [
33,
34].
The nucleolus is the largest and most easily detectable MLO. They are assembled by phase separation of their molecular components. A study [
35] showed that subcompartments within the nucleolus are separate coexisting liquid phases. The layered organization of the droplets is caused by differences in the biophysical properties of the phases, especially in the surface tension of the droplets, which arise due to the peculiarities of their macromolecular components. Therefore, there is a molecular mechanism by which internally disordered regions (IDRs) facilitate the condensation of the protein into droplets, and RNA-binding domains confer subcompartment specificity. Phase separation contributes to the formation of a multilayer structure of the nucleolus, which determines its functions in ribosome biogenesis [
36].
Ribosome biogenesis is far from the only function of the nucleolus. In 1999, the nucleolar retention of proteins was first hypothesized, a phenomenon originally called “nucleolar sequestration”, which describes the ability of the nucleolus to sequester regulatory proteins in response to cellular signals [
37]. It was found that in the budding yeast Saccharomyces cerevisiae, the cell cycle regulator phosphatase CDC14, binding to the CFI1/NET1 protein, is sequestered in the nucleolus during the G1 and S phases. It is released during the anaphase, thereby facilitating the cell exit from mitosis [
35,
38,
39]. In mammalian cells, it was shown that E3 ubiquitin-protein ligase MDM2 (double minute 2 protein), a p53 inhibitor, can be temporarily localized in the nucleolus, thereby affecting the course of the cell cycle [
40]. Notably, many proteins sequestered in the nucleolus are immobile. This has been demonstrated for CDC14 [
41], MDM2, DNA (cytosine-5)-methyltransferase 1 (DNMT1) [
42], von Hippel-Lindau disease tumor suppressor (VHL), Piwi [
43], and E3 ubiquitin-protein ligase RNF8 (RING finger protein 8) [
44].
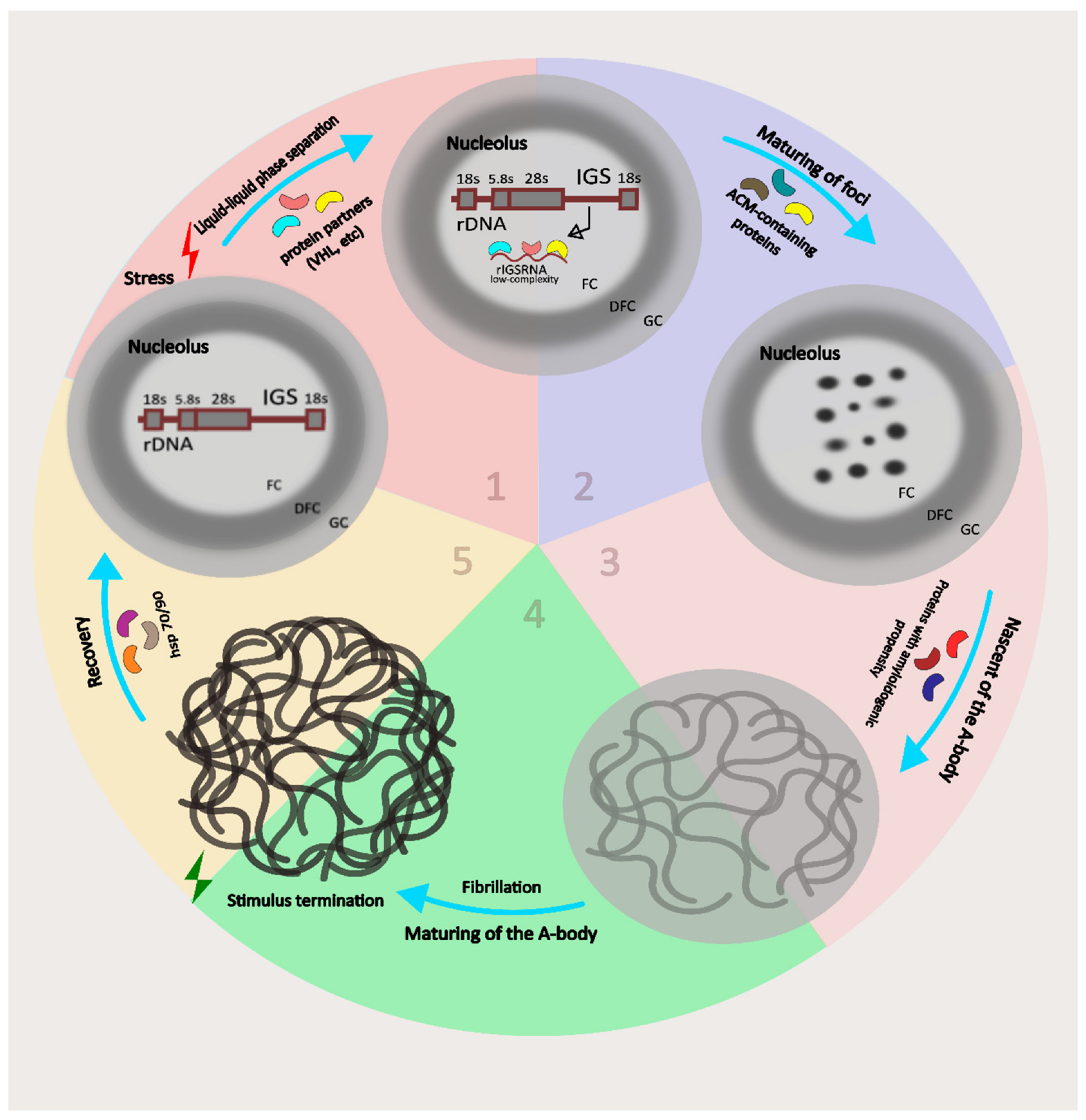
Mechanism of nucleolar sequestration, formation of A-bodies
Environmental stress factors such as heat shock, extracellular acidosis, and hypoxia trigger the expression of lncRNAs from the intergene spacer region of the rDNA cassette [
42]. There are approximately 200 copies of rDNA per haploid genome in mammals. As a rule, genes are distributed over several chromosomes and arranged in a tandem head-to-tail array, each of which contains a transcribed region that produces full-length pre-rRNA and an intergenic spacer (IGS) region [
45], which is a bundle of long non-coding transcripts [
46,
47].
Surprisingly, differences in the structure and length of IGS were found not only between species but also between individuals of the same species [
47,
48,
49]. IGS size ranges from ~2 kbp in yeast up to ~40 kbp in a human. This change in sequence, clearly observed in the increase in size, suggests that regulatory elements or even functional genes may have arisen in these spacer regions during evolution [
47]. The human IGS contains many different types of repeats, including simple repeats, microsatellites, repeats derived from retrotransposons, etc. [
47,
50]. In particular, numerous simple repeats [TC]n or [GA]n are found in human IGS. Three regions within IGS, enriched in simple repeats and predicted to form no secondary structure, are transcribed by Pol I RNA in response to certain cellular stresses and further processed into lncRNA ~300 bp in length [
42]. These regions are located at ~16 kb, ~22 kb, and ~28 kb downstream of the rRNA transcription start site. These lncRNAs have been named rIGS16, rIGS22, and rIGS28, respectively [
42,
51] and are induced by specific cellular stresses; rIGS16 and rIGS22 are induced by heat shock, while rIGS 28 is induced under conditions of extracellular acidosis.
These lncRNAs play a vital role in the reorganization of the nucleolus, in particular by redirecting certain proteins to this organelle. Such proteins have similar motifs called the amyloid converting motif (ACM). The ACMs of the studied proteins [
42,
51,
52] contain two distinct domains: a rich R/H sequence that borders a highly amyloidogenic region. The endogenous rIGSRNA interacts with the R/H residues, allowing the amyloidogenic domains to reach sufficient concentration, thus triggering the initial fibrillation event followed by protein polymerization. Probably due to the lack of a secondary structure, rIGSRNA has a larger surface area, which provides more binding sites for amyloidogenic proteins. As a result, the formation of an amyloidogenic riboprotein structure, the A-body, begins [
42]. As the A-body matures, more proteins aggregate in the nucleolus, which are immobilized through amyloidogenesis. Eventually, these ACM-containing proteins form a reversible mature A-body in the nucleolus (
Figure 2).
During the maturation of these organelles, there appears to be an intermediate liquid-like phase that gradually solidifies [
50,
53,
54]. The dismantling of A-bodies occurs within 1–2 h after the end of the stimulus; this process requires the presence of heat shock proteins HSP70 and HSP90 [
51,
52]. Therefore, A-bodies are gel-like solid-like entities formed as a result of a phase transition [
55]. Other solid-like structures are exemplified by the Balbiani bodies observed in
Xenopus [
56] and the pH-controlled transition of the cytoplasm from a liquid to a solid form in yeast [
57]. The fibrous, amyloid-like structure of A-bodies distinguishes them from other biomolecular condensates that have fluid-like properties, such as stress granules, nucleoli, and paraspeckles, because fluid-like condensates are dynamic, their constituents are mobile, they do not form fibers detectable by EM, and they usually do not stain with amyloidophilic dyes [
30,
40,
58,
59]. Thus, A-bodies represent a unique nucleolar structure based on their fibrous properties and rIGSRNA dependence.
In the article [
60], the protein composition of A-bodies was studied. In the course of the study, data were obtained that showed a high variability in the proteomic composition of A-bodies depending on stress exposure (heat shock, acidosis, or transcriptional/proteotoxic stress). Structures formed as a result of exposure to various stress factors can be different subtypes of A-bodies. Considering that transcription, translation, and metabolic factors are recruited into A-bodies induced by acidosis and heat shock, it can be assumed that gene expression is thus fine-tuned to help cope with specific damage.
At present, there is no unequivocal answer to the question of how proteins are specifically recruited under stress and converted into an amyloid form, although it is obvious that all proteins must have amyloidogenic properties. The model of A-bodies formation, in which organelle maturation is based on charge-based interactions between RNA and target proteins [
42,
52], does not take into account the stress-specificity of the proteomic composition. Probably, a second level of regulation of the formation of A-bodies is required. Interestingly, acidosis prior to or co-existing with heat shock prevented the sequestration of Flap Endonuclease 1 (FEN1, specific for heat shock) under conditions of heat shock [
60]. If heat alone was sufficient to refold FEN1, the protein would be recruited into A-bodies. Probably, acidotic conditions block the elements of the heat shock signaling pathway, i.e. the expression of heat shock-induced transcripts responsible for the sequestration of proteins in A-bodies is suppressed. It is possible that stress-specific targets can be modified in a certain way (for example, post-translationally), which leads to a phase transition and the formation of physiological amyloid aggregates. The resolution of this puzzle may, to some extent, provide insight into the cellular response to stress and potential sites of dysregulation for pathological aggregation.
It has been shown that only ~20% of heat shock A-bodies components can be found in acidosis-induced A-bodies [
60]. Proteins, such as VHL and CDC73 are characteristic of all types of A-bodies and can be used as A-body markers in research.
VHL
The von Hippel-Lindau tumor suppressor protein (VHL) is a vital component of the VBC-Cul2 E3 ubiquitin ligase complex as it acts as a substrate recognition protein and mediates the specificity of the degradation process [
61,
62,
63,
64]. VHL promotes recruitment, ubiquitination, and subsequent proteasomal degradation of alpha subunit of hypoxia-inducible factor (HIF) [
65,
66]. A decrease in extracellular pH (acidosis) triggers the translocation of VHL into the nucleolus, neutralizing its ability to degrade nuclear HIF even in the presence of oxygen. It has been shown that VHL passes from a highly dynamic nuclear-cytoplasmic distribution under standard growth conditions (21% O2, pH 7.4) to an immobilized state in the nucleolus when exposed to extracellular acidosis (1% O2, pH 6.0) [
67]. Only upon normalization of extracellular pH, VHL is released from the nucleolus and restores its mobility [
67]. VHL mapping revealed an amino acid fragment, originally called the nucleolar retention signal (NoDS), which is thought to be necessary and sufficient for protein immobilization in the nucleolus [
44].
A study [
51] analyzed the VHL fragment (residues 104–140; VHL104–140) and found that it interacts more effectively with endogenous rIGS 28 RNA during acidosis compared to other amyloidogenic or non-amyloidogenic regions. A more thorough study of VHL104–140 revealed the presence of an arginine/histidine cluster (residues 104–121), which is activated after a decrease in extracellular pH, mediating the retention of VHL in the nucleolus. This arginine/histidine rich sequence flanks a highly amyloidogenic motif (residues 122–140). It was found that proteins containing these motifs have all the properties of amyloids [
29]. Subsequently, the term "NoDS" was replaced by "amyloid-converting motif" (ACM) to emphasize its role in the transformation of the nucleolus into A-bodies. Notably, ACM is inactivated upon return to neutral pH conditions. Further research is needed to elucidate the mechanisms by which extracellular hydrogen ions activate ACM VHL, as well as what drives VHL sequestration during heat shock.
CDC73
Cell division control protein 73 (CDC73) is one of the key cellular regulators and is involved in transcriptional and post-transcriptional control pathways. It may influence cell cycle progression through the regulation of cyclin D1/PRAD1 expression. The protein is a component of the RNA polymerase II-associated protein 1 (PAF1) complex (PAF1C), which performs many functions during transcription by RNA polymerase II and is involved in the regulation of the development and maintenance of pluripotency of embryonic stem cells. Since CDC73 is an important marker protein present in all types of A-bodies from flies to humans [
68], its use as a marker in research provides a further opportunity to study the formation of these structures in various cell types.
A study [
68] showed that the formation of A-bodies can occur in cells derived from various species, such as monkeys, mice, rats, chickens, fish, and flies. Under body-specific conditions, A-bodies were detected in
D. melanogaster (flies) and
S. cerevisiae (yeast). It is important that the transcriptome sequences of scaffold lncRNAs are not conserved in humans, mice, and chickens [
68]. Nevertheless, there is a general pattern: stress leads to the expression of lncRNA of low complexity from the region immediately below the rRNA coding sequence in all three organisms [
68,
69]. These data suggest that the genomic arrangement and function of these scaffold transcripts are conserved across species, despite the lack of conservation in their primary sequence.
Functions of A-bodies
Although A-bodies have structural similarities to pathological amyloids, they are physiological and serve biological functions. Many proteins sequestered in A-bodies, for example, CDK1, POLD1, DNMT1, are involved in the regulation of the cell cycle [
51]. Capture and temporary retention of such proteins in A-bodies mediate the suppression of metabolic activity [
70] and arrest of DNA synthesis [
51]. This state is different from the resting state (G0) in the cell cycle when cells stop proliferating but remain metabolically active. Therefore, the biological role of A-body formation might be to provide cellular dormancy as an adaptive response to environmental stressors. That is, it is the “solid-like” properties of A-bodies that lead to a loss of metabolic activity and contribute to the transition of cells to a sleep mode, while the properties of “liquid-like” systems (dynamic, fluid) concentrate and facilitate biochemical processes (for example, nuclear bodies).
Another function of A-bodies is the local synthesis of nuclear proteins [
71]. A study [
49] identified translating ribosomes organized along filamentous assemblies that characterize solid condensate amyloid bodies. rIGS RNA silencing disrupted nuclear translation in acidosis and heat shock. Nuclear translation cessation correlated with A-bodies disassembly during stress recovery. Thus, it is likely that stressful conditions such as heat shock and acidosis stimulate nuclear fusion in A-bodies. Interestingly, lower eukaryotes (flies and yeasts) lacked local nuclear protein synthesis [
68]. This is most likely due to the fact that A-bodies mediated translation is a later evolutionary feature of this structure.
Therefore, A-bodies function not only as a preserving structure but also play an adaptive role in the fight against stress.
3. Conclusions
In this review, we attempted to summarize and analyze the literature data on two non-coding RNA-containing stress-induced nuclear MLOs that are formed by a similar principle, have an overlapping protein composition, but have significantly different physical properties, morphology, and functional activity. It carefully could be concluded that the more “rigid” A-bodies structure than nSBs correlates with more “passive” role of A-bodies in cellular stress-response, while dynamic properties of nSBs correspond to active stress-induced change of expression programs by these MLOs. In any case, a further coordinated study of these two similar and simultaneously different nuclear compartments will expand scientific knowledge about the processes of formation of stress-inducible membrane-less organelles, their participation in the regulation of cell signaling pathways, and will allow us to get closer to understanding the basic role of non-coding RNA in the regulation of the stress response.
Author Contributions
Writing—original draft preparation, A.A.G, A.V.F. V.N.U. A.S.F; writing—review and editing, V.N.U. A.A.G, A.V.F. A.S.F. K.K.T. I.M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Russian Science Foundation, grant number 21-75-10166 (A.V.F.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Oparin, A.I. The origine of Life; Moscow Worker: Moscow, 1924. [Google Scholar]
- Darling, A.L.; Uversky, V.N. Known types of membrane-less organelles and biomolecular condensates. In Droplets of Life: Membrane-Less Organelles, Biomolecular Condensates, and Biological Liquid-Liquid Phase Separation, 1st ed.; Uversky, V.N. Ed. Elsevier: Amsterdam, Netherlands, 2023. [Google Scholar]
- Mitrea, D.M.; Kriwacki, R.W. Phase separation in biology; functional organization of a higher order. Cell Commun. Signal. CCS 2016, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Decker, C.J.; Parker, R. P-bodies and stress granules: Possible roles in the control of translation and mRNA degradation. Cold Spring Harb. Perspect. Biol. 2012, 4, a012286. [Google Scholar] [CrossRef] [PubMed]
- Brangwynne, C.P.; Eckmann, C.R.; Courson, D.S.; Rybarska, A.; Hoege, C.; Gharakhani, J.; Jülicher, F.; Hyman, A.A. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 2009, 324, 1729–1732. [Google Scholar] [CrossRef] [PubMed]
- Chujo, T.; Hirose, T. Nuclear Bodies Built on Architectural Long Noncoding RNAs: Unifying Principles of Their Construction and Function. Mol. Cells 2017, 40, 889–896. [Google Scholar]
- Alberti, S.; Hyman, A.A. Biomolecular condensates at the nexus of cellular stress, protein aggregation disease and ageing. Nat. Rev. Mol. Cell Biol. 2021, 22, 196–213. [Google Scholar] [CrossRef] [PubMed]
- Deonarine, A.; Walker, M.W.G.; Westerheide, S.D. HSF-1 displays nuclear stress body formation in multiple tissues in Caenorhabditis elegans upon stress and following the transition to adulthood. Cell Stress Chaperones. 2021, 26, 417–431. [Google Scholar] [CrossRef]
- Biamonti, G.; Vourc'h, C. Nuclear stress bodies. Cold Spring Harb. Perspect. Biol. 2010, 2, a000695. [Google Scholar] [CrossRef]
- Jolly, C.; Lakhotia, S.C. Human sat III and Drosophila hsr omega transcripts: A common paradigm for regulation of nuclear RNA processing in stressed cells. Nucleic Acids Res. 2006, 34, 5508–5514. [Google Scholar] [CrossRef] [PubMed]
- Valgardsdottir, R.; Chiodi, I.; Giordano, M.; Cobianchi, F.; Riva, S.; Biamonti, G. Structural and functional characterization of noncoding repetitive RNAs transcribed in stressed human cells. Mol. Biol. Cell 2005, 16, 2597–2604. [Google Scholar] [CrossRef]
- Akerfelt, M.; Morimoto, R.I.; Sistonen, L. Heat shock factors: Integrators of cell stress, development and lifespan. Nat. Rev.. Mol. Cell Biol. 2010, 11, 545–555. [Google Scholar] [CrossRef]
- Alastalo, T.P.; Hellesuo, M.; Sandqvist, A.; Hietakangas, V.; Kallio, M.; Sistonen, L. Formation of nuclear stress granules involves HSF2 and coincides with the nucleolar localization of Hsp70. J. Cell Sci. 2003, 116, 3557–3570. [Google Scholar] [CrossRef]
- Kovacs, D.; Kovacs, M.; Ahmed, S.; Barna, J. Functional diversification of heat shock factors. Biol Futur 2022, 73, 427–439. [Google Scholar] [CrossRef]
- Col, E.; Hoghoughi, N.; Dufour, S.; Penin, J.; Koskas, S.; Faure, V.; Ouzounova, M.; Hernandez-Vargash, H.; Reynoird, N.; Daujat, S.; et al. Bromodomain factors of BET family are new essential actors of pericentric heterochromatin transcriptional activation in response to heat shock. Sci. Rep. 2017, 7, 5418. [Google Scholar] [CrossRef]
- Valgardsdottir, R.; Chiodi, I.; Giordano, M.; Rossi, A.; Bazzini, S.; Ghigna, C.; Riva, S.; Biamonti, G. Transcription of Satellite III non-coding RNAs is a general stress response in human cells. Nucleic Acids Res. 2008, 36, 423–434. [Google Scholar] [CrossRef]
- Goenka, A.; Sengupta, S.; Pandey, R.; Parihar, R.; Mohanta, G.C.; Mukerji, M.; Ganesh, S. Human satellite-III non-coding RNAs modulate heat-shock-induced transcriptional repression. J. Cell Sci. 2016, 129, 3541–3552. [Google Scholar] [CrossRef]
- Ninomiya, K.; Adachi, S. LncRNA-dependent nuclear stress bodies promote intron retention through SR protein phosphorylation. Embo J 2020, 39, e102729. [Google Scholar] [CrossRef]
- Sandqvist, A.; Björk, J.K.; Akerfelt, M.; Chitikova, Z.; Grichine, A.; Vourc'h, C.; Jolly, C.; Salminen, T.A.; Nymalm, Y.; Sistonen, L. Heterotrimerization of heat-shock factors 1 and 2 provides a transcriptional switch in response to distinct stimuli. Mol. Biol. Cell 2009, 20, 1340–1347. [Google Scholar] [CrossRef]
- Weighardt, F.; Cobianchi, F.; Cartegni, L.; Chiodi, I.; Villa, A.; Riva, S.; Biamonti, G. A novel hnRNP protein (HAP/SAF-B) enters a subset of hnRNP complexes and relocates in nuclear granules in response to heat shock. J. Cell Sci. 1999, 112, 1465–1476. [Google Scholar] [CrossRef]
- Morimoto, R.I. Dynamic remodeling of transcription complexes by molecular chaperones. Cell 2002, 110, 281–284. [Google Scholar] [CrossRef]
- Lee, Y.-B.; Colley, S.; Norman, M.; Biamonti, G.; Uney, J.B. SAFB re-distribution marks steps of the apoptotic process. Exp. Cell Res. 2007, 313, 3914–3923. [Google Scholar] [CrossRef]
- Huo, X.; Ji, L.; Zhang, Y.; Lv, P.; Cao, X.; Wang, Q.; Yan, Z.; Dong, S.; Du, D.; Zhang, F.; et al. The Nuclear Matrix Protein SAFB Cooperates with Major Satellite RNAs to Stabilize Heterochromatin Architecture Partially through Phase Separation. Mol. Cell 2020, 77, 368–383. [Google Scholar] [CrossRef]
- Altmeyer, M.; Toledo, L.; Gudjonsson, T.; Grøfte, M.; Rask, M.B.; Lukas, C.; Akimov, V.; Blagoev, B.; Bartek, J.; Lukas, J. The chromatin scaffold protein SAFB1 renders chromatin permissive for DNA damage signaling. Mol. Cell 2013, 52, 206–220. [Google Scholar] [CrossRef]
- Aly, M.K.; Ninomiya, K.; Adachi, S.; Natsume, T.; Hirose, T. Two distinct nuclear stress bodies containing different sets of RNA-binding proteins are formed with HSATIII architectural noncoding RNAs upon thermal stress exposure. Biochem. Biophys. Res. Commun. 2019, 516, 419–423. [Google Scholar] [CrossRef]
- Simard, M.J.; Chabot, B. SRp30c is a repressor of 3' splice site utilization. Mol. Cell. Biol. 2002, 22, 4001–4010. [Google Scholar] [CrossRef]
- Ninomiya, K.; Iwakiri, J.; Aly, M.K.; Sakaguchi, Y.; Adachi, S.; Natsume, T.; Terai, G.; Asai, K.; Suzuki, T.; Hirose, T. m(6) A modification of HSATIII lncRNAs regulates temperature-dependent splicing. EMBO J. 2021, 40, e107976. [Google Scholar] [CrossRef]
- Gaglia, G.; Rashid, R.; Yapp, C.; Joshi, G.N.; Li, C.G.; Lindquist, S.L.; Sarosiek, K.A.; Whitesell, L.; Sorger, P.K.; Santagata, S. HSF1 phase transition mediates stress adaptation and cell fate decisions. Nat. Cell Biol. 2020, 22, 151–158. [Google Scholar] [CrossRef]
- Hernandez-Verdun, D. Nucleolus: From structure to dynamics. Histochem. Cell Biol. 2006, 125, 127–137. [Google Scholar] [CrossRef]
- Phair, R.D.; Misteli, T. High mobility of proteins in the mammalian cell nucleus. Nature 2000, 404, 604–609. [Google Scholar] [CrossRef]
- Hubstenberger, A.; Noble, S.L.; Cameron, C.; Evans, T.C. Translation repressors, an RNA helicase, and developmental cues control RNP phase transitions during early development. Dev. Cell 2013, 27, 161–173. [Google Scholar] [CrossRef]
- Jain, S.; Wheeler, J.R.; Walters, R.W.; Agrawal, A.; Barsic, A.; Parker, R. ATPase-Modulated Stress Granules Contain a Diverse Proteome and Substructure. Cell 2016, 164, 487–498. [Google Scholar] [CrossRef]
- Lafontaine, D.L.J.; Riback, J.A.; Bascetin, R.; Brangwynne, C.P. The nucleolus as a multiphase liquid condensate. Nat. Rev. Mol. Cell Biol. 2021, 22, 165–182. [Google Scholar] [CrossRef]
- Sawyer, I.A.; Sturgill, D.; Dundr, M. Membraneless nuclear organelles and the search for phases within phases. Wiley Interdiscip. Rev. RNA 2019, 10, e1514. [Google Scholar] [CrossRef]
- Visintin, R.; Hwang, E.S.; Amon, A. Cfi1 prevents premature exit from mitosis by anchoring Cdc14 phosphatase in the nucleolus. Nature 1999, 398, 818–823. [Google Scholar] [CrossRef]
- Feric, M.; Vaidya, N.; Harmon, T.S.; Mitrea, D.M.; Zhu, L.; Richardson, T.M.; Kriwacki, R.W.; Pappu, R.V.; Brangwynne, C.P. Coexisting Liquid Phases Underlie Nucleolar Subcompartments. Cell 2016, 165, 1686–1697. [Google Scholar] [CrossRef]
- Bachant, J.B.; Elledge, S.J. Mitotic treasures in the nucleolus. Nature 1999, 398, 757–758. [Google Scholar] [CrossRef]
- Shou, W.; Seol, J.H.; Shevchenko, A.; Baskerville, C.; Moazed, D.; Chen, Z.W.S.; Jang, J.; Shevchenko, A.; Charbonneau, H.; Deshaies, R.J. Exit from Mitosis Is Triggered by Tem1-Dependent Release of the Protein Phosphatase Cdc14 from Nucleolar RENT Complex. Cell 1999, 97, 233–244. [Google Scholar] [CrossRef]
- Mikhaleva, E.A.; Leinsoo, T.A.; Ishizu, H.; Gvozdev, V.A.; Klenov, M.S. The nucleolar transcriptome regulates Piwi shuttling between the nucleolus and the nucleoplasm. Chromosome Res. Int. J. Mol. Supramol. Evol. Asp. Chromosome Biol. 2019, 27, 141–152. [Google Scholar] [CrossRef]
- Weber, J.D.; Taylor, L.J.; Roussel, M.F.; Sherr, C.J.; Bar-Sagi, D. Nucleolar Arf sequesters Mdm2 and activates p53. Nat. Cell Biol. 1999, 1, 20–26. [Google Scholar] [CrossRef]
- Tomson, B.N.; Rahal, R.; Reiser, V.; Monje-Casas, F.; Mekhail, K.; Moazed, D.; Amon, A. Regulation of Spo12 phosphorylation and its essential role in the FEAR network. Curr. Biol. CB 2009, 19, 449–460. [Google Scholar] [CrossRef]
- Audas, T.E.; Jacob, M.D.; Lee, S. Immobilization of proteins in the nucleolus by ribosomal intergenic spacer noncoding RNA. Mol. Cell 2012, 45, 147–157. [Google Scholar] [CrossRef]
- Mikhaleva, E.A.; Leinsoo, T.A.; Ishizu, H.; Gvozdev, V.A.; Klenov, M.S. The nucleolar transcriptome regulates Piwi shuttling between the nucleolus and the nucleoplasm. Chromosome Res. 2019, 27, 141–152. [Google Scholar] [CrossRef]
- Mekhail, K.; Rivero-Lopez, L.; Al-Masri, A.; Brandon, C.; Khacho, M.; Lee, S. Identification of a common subnuclear localization signal. Mol. Biol. Cell 2007, 18, 3966–3977. [Google Scholar] [CrossRef]
- Paule, M.R.; White, R.J. Survey and summary: Transcription by RNA polymerases I and III. Nucleic Acids Res. 2000, 28, 1283–1298. [Google Scholar] [CrossRef]
- Bierhoff, H.; Dammert, M.A.; Brocks, D.; Dambacher, S.; Schotta, G.; Grummt, I. Quiescence-induced LncRNAs trigger H4K20 trimethylation and transcriptional silencing. Mol. Cell 2014, 54, 675–682. [Google Scholar] [CrossRef]
- Smirnov, E.; Cmarko, D.; Mazel, T.; Hornáček, M.; Raška, I. Nucleolar DNA: The host and the guests. Histochem. Cell Biol. 2016, 145, 359–372. [Google Scholar] [CrossRef]
- Arnheim, N.; Krystal, M.; Schmickel, R.; Wilson, G.; Ryder, O.; Zimmer, E. Molecular evidence for genetic exchanges among ribosomal genes on nonhomologous chromosomes in man and apes. Proc. Natl. Acad. Sci. USA 1980, 77, 7323–7327. [Google Scholar] [CrossRef]
- Reeder, R.H.; Brown, D.D.; Wellauer, P.K.; Dawid, I.B. Patterns of ribosomal DNA spacer lengths are inherited. J. Mol. Biol. 1976, 105, 507–516. [Google Scholar] [CrossRef]
- Gonzalez, I.L.; Sylvester, J.E. Complete sequence of the 43-kb human ribosomal DNA repeat: Analysis of the intergenic spacer. Genomics 1995, 27, 320–328. [Google Scholar] [CrossRef]
- Audas, T.E.; Audas, D.E.; Jacob, M.D.; Ho, J.J.D.; Khacho, M.; Wang, M.; Perera, J.K.; Gardiner, C.; Bennett, C.A.; Head, T.; et al. Adaptation to Stressors by Systemic Protein Amyloidogenesis. Dev. Cell 2016, 39, 155–168. [Google Scholar] [CrossRef]
- Wang, M.; Tao, X.; Jacob, M.D.; Bennett, C.A.; Ho, J.J.D.; Gonzalgo, M.L.; Audas, T.E.; Lee, S. Stress-Induced Low Complexity RNA Activates Physiological Amyloidogenesis. Cell Rep 2018, 24, 1713–1721. [Google Scholar] [CrossRef]
- Kellermayer, M.S.; Karsai, A.; Benke, M.; Soós, K.; Penke, B. Stepwise dynamics of epitaxially growing single amyloid fibrils. Proc. Natl. Acad. Sci. USA 2008, 105, 141–144. [Google Scholar] [CrossRef]
- Rambaran, R.N.; Serpell, L.C. Amyloid fibrils: Abnormal protein assembly. Prion 2008, 2, 112–117. [Google Scholar] [CrossRef]
- Wang, M.; Bokros, M.; Theodoridis, P.R.; Lee, S. Nucleolar Sequestration: Remodeling Nucleoli Into Amyloid Bodies. Front Genet 2019, 10, 1179. [Google Scholar] [CrossRef]
- Boke, E.; Ruer, M.; Wühr, M.; Coughlin, M.; Lemaitre, R.; Gygi, S.P.; Alberti, S.; Drechsel, D.; Hyman, A.A.; Mitchison, T.J. Amyloid-like Self-Assembly of a Cellular Compartment. Cell 2016, 166, 637–650. [Google Scholar] [CrossRef]
- Munder, M.C.; Midtvedt, D.; Franzmann, T.; Nüske, E.; Otto, O.; Herbig, M.; Ulbricht, E.; Müller, P.; Taubenberger, A.; Maharana, S.; et al. A pH-driven transition of the cytoplasm from a fluid- to a solid-like state promotes entry into dormancy. eLife 2016, 5. [Google Scholar] [CrossRef]
- Shin, Y.; Brangwynne, C.P. Liquid phase condensation in cell physiology and disease. Science 2017, 357. [Google Scholar] [CrossRef]
- Shin, Y.; Brangwynne, C.P. Liquid phase condensation in cell physiology and disease. Science 2017, 357. [Google Scholar] [CrossRef]
- Marijan, D.; Tse, R.; Elliott, K.; Chandhok, S.; Luo, M.; Lacroix, E.; Audas, T.E. Stress-specific aggregation of proteins in the amyloid bodies. FEBS Lett 2019, 593, 3162–3172. [Google Scholar] [CrossRef]
- Iwai, K.; Yamanaka, K.; Kamura, T.; Minato, N.; Conaway, R.C.; Conaway, J.W.; Klausner, R.D.; Pause, A. Identification of the von Hippel-lindau tumor-suppressor protein as part of an active E3 ubiquitin ligase complex. Proc. Natl. Acad. Sci. USA 1999, 96, 12436–12441. [Google Scholar] [CrossRef]
- Kaelin, W.G. Jr. Molecular basis of the VHL hereditary cancer syndrome. Nat. Rev. Cancer 2002, 2, 673–682. [Google Scholar] [CrossRef]
- Kibel, A.; Iliopoulos, O.; DeCaprio, J.A.; Kaelin, W.G. Jr. Binding of the von Hippel-Lindau tumor suppressor protein to Elongin B and C. Science 1995, 269, 1444–1446. [Google Scholar] [CrossRef]
- Lisztwan, J.; Imbert, G.; Wirbelauer, C.; Gstaiger, M.; Krek, W. The von Hippel-Lindau tumor suppressor protein is a component of an E3 ubiquitin-protein ligase activity. Genes Dev. 1999, 13, 1822–1833. [Google Scholar] [CrossRef]
- Jaakkola, P.; Mole, D.R.; Tian, Y.M.; Wilson, M.I.; Gielbert, J.; Gaskell, S.J.; von Kriegsheim, A.; Hebestreit, H.F.; Mukherji, M.; Schofield, C.J.; et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 2001, 292, 468–472. [Google Scholar] [CrossRef]
- Maxwell, P.H.; Wiesener, M.S.; Chang, G.-W.; Clifford, S.C.; Vaux, E.C.; Cockman, M.E.; Wykoff, C.C.; Pugh, C.W.; Maher, E.R.; Ratcliffe, P.J. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 1999, 399, 271–275. [Google Scholar] [CrossRef]
- Mekhail, K.; Khacho, M.; Carrigan, A.; Hache, R.R.; Gunaratnam, L.; Lee, S. Regulation of ubiquitin ligase dynamics by the nucleolus. J. Cell Biol. 2005, 170, 733–744. [Google Scholar] [CrossRef]
- Lacroix, E.; Pereira, L.; Yoo, B.; Coyle, K.M.; Chandhok, S.; Zapf, R.; Marijan, D.; Morin, R.D.; Vlachos, S.; Harden, N.; et al. Evolutionary conservation of systemic and reversible amyloid aggregation. J. Cell Sci. 2021, 134. [Google Scholar] [CrossRef]
- Lacroix, E.; Audas, T.E. Keeping up with the condensates: The retention, gain, and loss of nuclear membrane-less organelles. Front. Mol. Biosci. 2022, 9, 998363. [Google Scholar] [CrossRef]
- Mekhail, K.; Rivero-Lopez, L.; Khacho, M.; Lee, S. Restriction of rRNA synthesis by VHL maintains energy equilibrium under hypoxia. Cell Cycle 2006, 5, 2401–2413. [Google Scholar] [CrossRef]
- Theodoridis, P.R.; Bokros, M.; Marijan, D.; Balukoff, N.C.; Wang, D.; Kirk, C.C.; Budine, T.D.; Goldsmith, H.D.; Wang, M.; Audas, T.E.; et al. Local translation in nuclear condensate amyloid bodies. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).