Submitted:
27 March 2023
Posted:
28 March 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Artificial Intelligence Approaches for the Diagnosis of Obstructive Sleep Apnea
2. Materials and Methods
2.1. Definition of the System
2.1.1. Database Usage
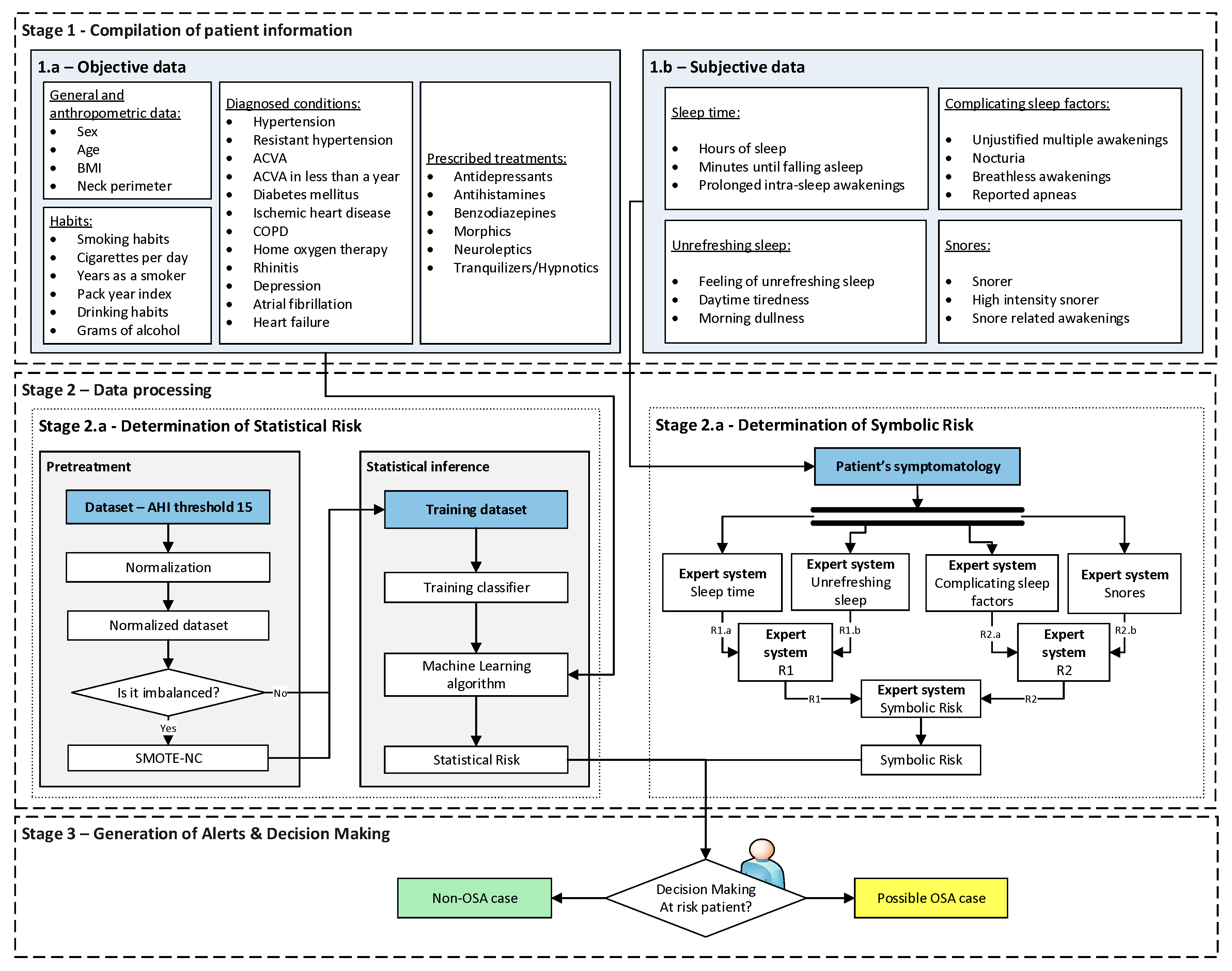
2.1.2. Conceptual Design and Description of the System
- Stage 1: Compilation of patient information
- Stage 1.a-Objective data: On the one hand, there is the more objective information, with a lower degree of subjectivity and interpretation, which is summarized in Table 1. This group has been divided into four subgroups for reasons of coherence and to facilitate the process of introducing the data into the forms. In the table, it is indicated whether each data type it is numerical or categorical.
| Subgroup | Data | Data type | Commentary |
|
General and anthropometric data |
Sex | Categorical | Male/Female |
| Age | Numerical | - | |
| Weight | Numerical | Not provided to the algorithm but used to determine the body mass index (BMI). | |
| Height | Numerical | Not provided to the algorithm but used to determine the body mass index (BMI). | |
| Body mass index (BMI) | Numerical | Data derived from height and weight. | |
| Neck perimeter | Numerical | - | |
| Subgroup | Data | Data type | Commentary |
|
Habits |
Smoker | Categorical | Yes/No/No longer |
| Cigarettes per day | Numerical | Not provided to the algorithm but used to determine the pack year index. | |
| Years as a smoker | Numerical | Not provided to the algorithm but used to determine the pack year index. | |
| Pack-year index | Numerical | Data derived from cigarettes per day and years smoking. | |
| Drinking habits | Categorical | No/Daily/Occasionally | |
| Grams of alcohol | Numerical | - | |
| Subgroup | Commentary | ||
| Diagnosed conditions |
All the comorbidities listed in Section 2.1.1 are included. Each of these fields is considered as categorical or binary, that is, either the pathology is suffered or not. | ||
| Subgroup | Commentary | ||
| Prescribed treatments |
All the drugs listed in Section 2.1.1 are included. Each of these fields is considered as categorical or binary, that is, prescribed treatments are or are not provided. |
||
- Stage 1.b-Subjective data: On the other hand, there is the subjective information, more interpretative, related to the symptoms reported by the patient and collected during a sleep interview. This information is summarized in Table 2. As in the previous table, it has been decided to divide this group into four subgroups for the sake of coherence and to simplify their subsequent treatment. In the table, together with each of the data a description of its nature is also presented, depending on whether it is numerical or categorical data.
- Stage 2: Data processing
- Stage 2.a–Determination of Statistical Risk: Once the most objective data has been collected, as presented in Stage 1.a, it is processed using a Machine Learning classification algorithm [29]. For the definition and configuration of the algorithm a clinical data set is used, which has already been introduced in Section 2.1.1. This data is preprocessed through normalizations and data augmentation, establishing an AHI threshold level equal to 15 for labeling the different patients according to the OSA and non-OSA classes. It is important to note that the medical team could modify this threshold if considered convenient. After this, once the model is adjusted and new patient data is available, a risk metric will be obtained as the output of the classifier, the Statistical Risk, which value will range from 0 to 100 and which can be understood as a percentage risk value of the patient actually suffering from OSA.
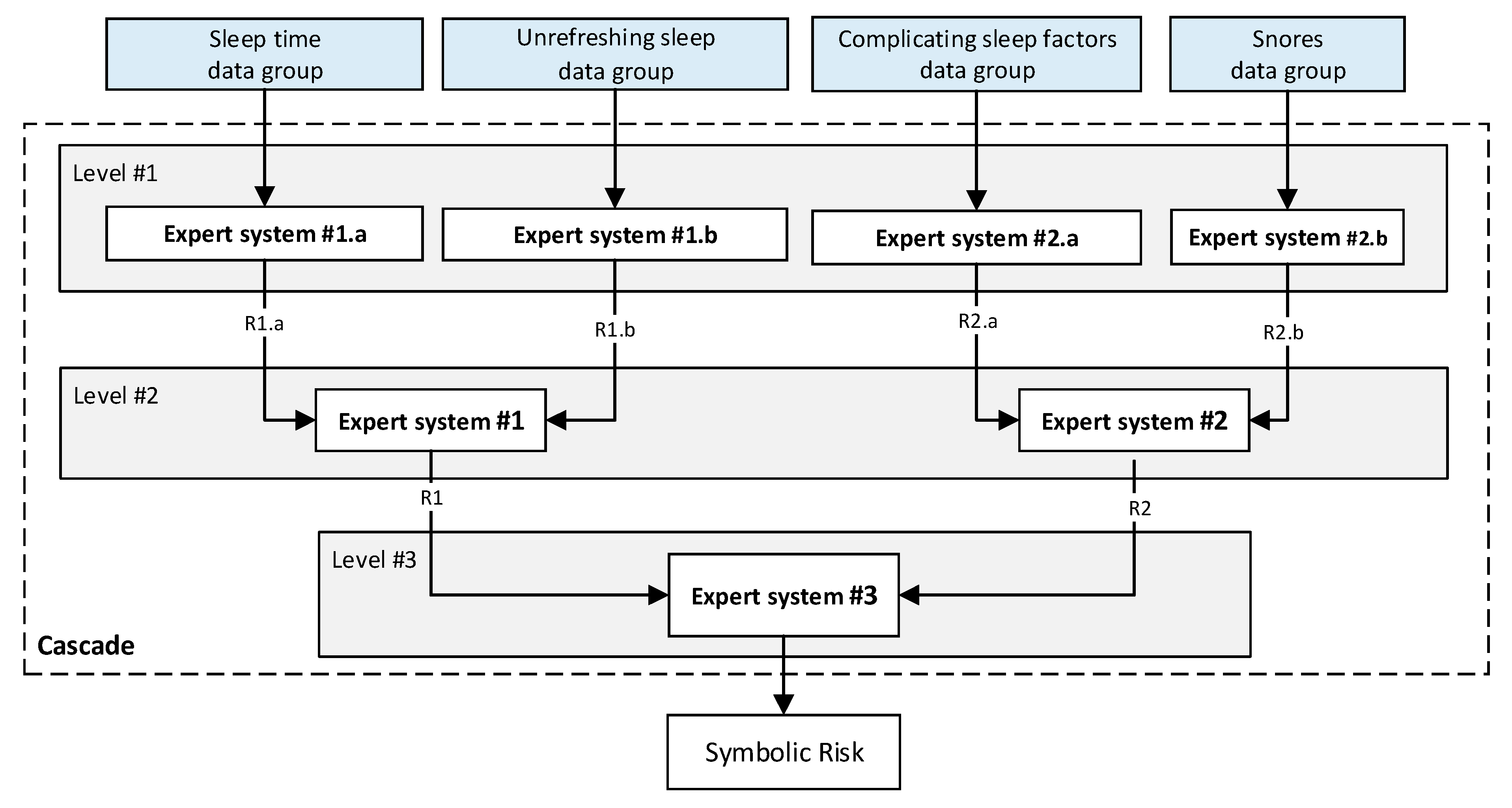
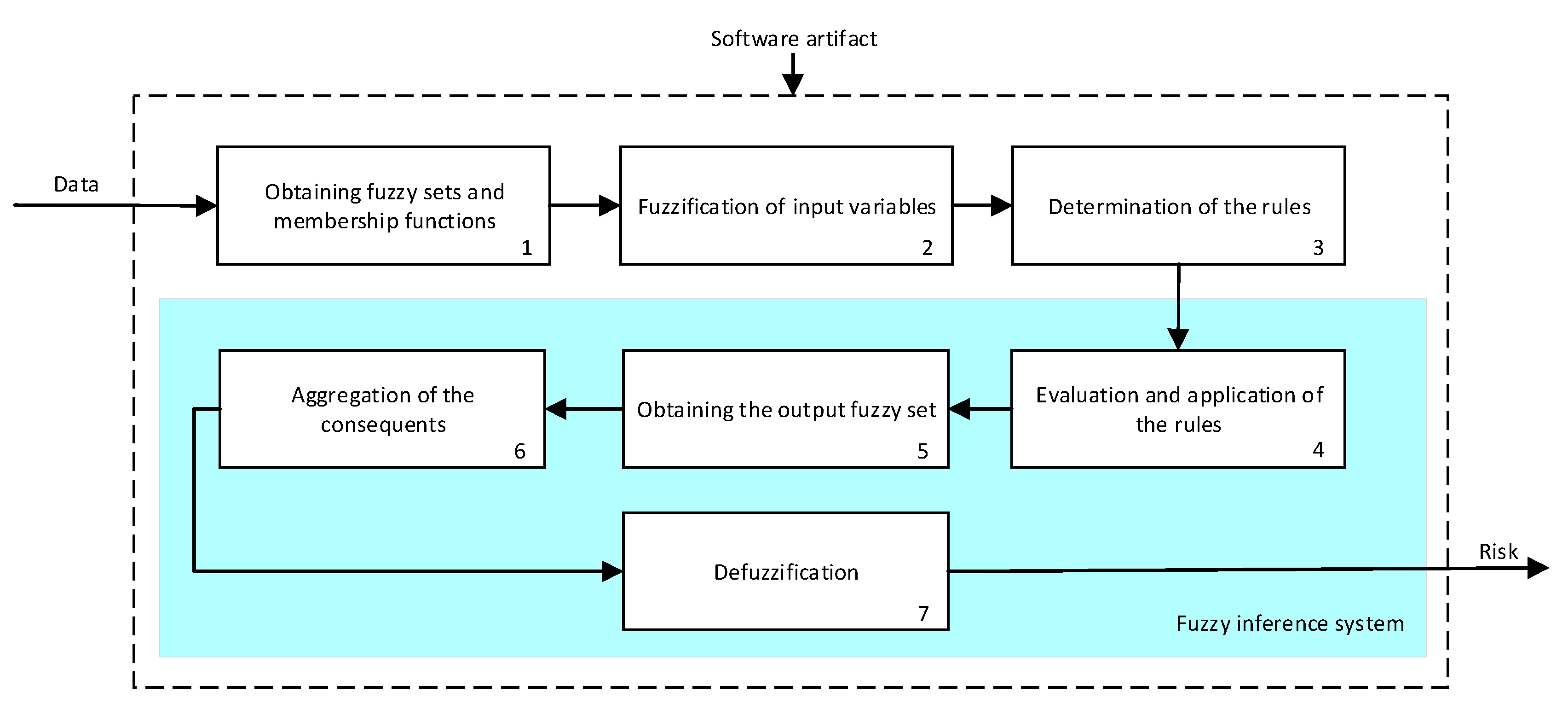
- Stage 2.b–Determination of the Symbolic Risk: Concurrently with Stage 2.a. [14,15,16,17], in Stage 2.b the more subjective data of the patient, collected in Stage 1.b, is processed. These have been split into groups, as mentioned in Stage 1. For their processing a series of expert systems are used, all of them based on Mamdani-type fuzzy inference systems [19,20,21,22], arranged in a three-level cascade as shown in Figure 2. This is because it is intended to perform a risk assessment based on different criteria, all of which are involved in the diagnosis of OSA, which allows reducing uncertainty and creating a more accurate and suitable knowledge base. Nevertheless, since this is a multicriteria approach and aiming to obtain a global risk indicator that groups and represents them, the risks obtained as an output of the expert systems in the first level of the cascade #1.a and #1.b, #2.a and #2.b, are simultaneously fuzzified as input to expert systems #1 and #2 in the second level of the cascade. The outputs of them are also fuzzified as inputs of the expert system #3, which consists on the last level of the cascade, determining as its output a general risk indicator that contemplates the risks of the previous levels. This indicator is named Symbolic Risk, which value will range from 0 to 100, representing the risk associated with the symptoms that a patient suffers when faced with a potential OSA case. It is important to point out that the management of uncertainty in the cascade is not related to probabilities but rather to the concept of membership, which is widely known and used in the field of fuzzy logic.
- Stage 3: Generation of Alerts & Decision Making
- Level 1: It refers to situations in which the level of risk is low, and it seems not to indicate an OSA condition. This status will be proposed when the percentage risk to be analyzed is lower than a Limit 1 value.
- Level 2: This refers to situations in which there is an intermediate level of risk, which does not apparently allow to distinguish whether or not it is an OSA case. This status will be proposed if the percentage risk value lies in the range [Limit 1 - Limit 2).
- Level 3: It refers to situations in which there is a high risk level that seems to indicate the presence of an OSA condition. This status will be proposed when the percentage risk to be analyzed is higher, or equal to, the Limit 2 value.
- Non-OSA case, do not perform diagnostic studies: This status will be proposed when the decision variable has a value lower than two.
- Doubtful case: This status will be proposed when the decision variable equals two. The medical team should assess whether it is necessary to perform further examinations, or suggest a new medical appointment after a period of time to reconsider the patient’s condition.
- Possible OSA case, perform diagnostic studies: This status will be proposed when the decision variable is larger than, or equal to, three.
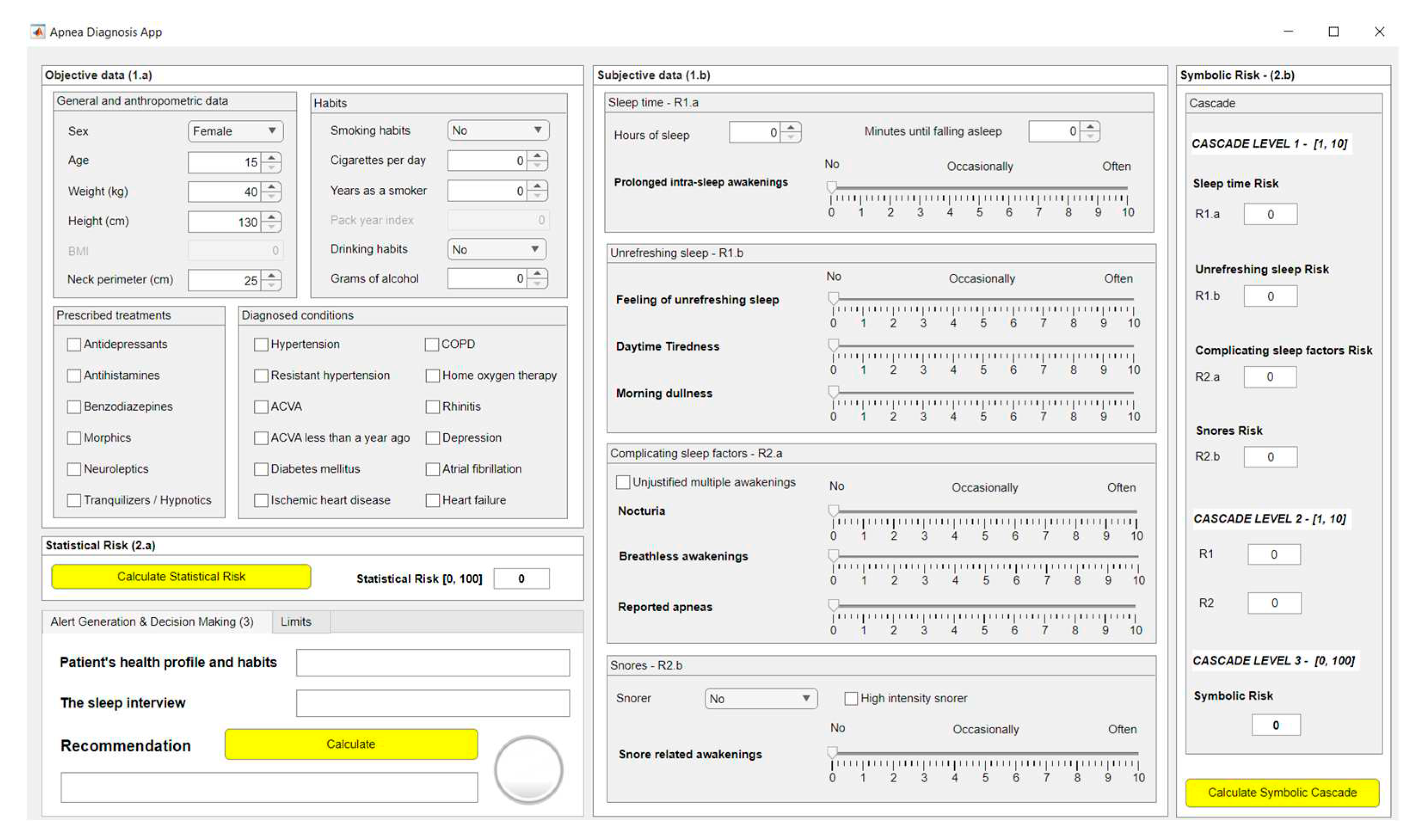
2.2. Implementation of the System
2.2.1. Data Acquisition
2.2.2. Data Processing
- Classification Algorithm Based on Machine Learning
- Cascade of Expert Systems
- First level: at the upper level of the cascade, the processing of the four groups of information previously introduced in Stage 2.b of Section 2.1.2 is carried out (‘sleep time’ group, ‘unrefreshing sleep’ group, ‘complicating sleep factors’ group and ‘snores’ group). For this purpose, four expert systems are used to obtain a risk indicator (R1.a, R1.b, R2.a and R2.b respectively) at the output of each of them after the defuzzification process. These indicators determine the hazard level associated to suffering from OSA related to each group of data.
- Second level: at the second level of the cascade, the data from the first level is processed using two expert systems with the aim of aggregating their outputs. This is so because of the decision to group the risks obtained in the first level of the cascade in couples (R1.a and R1.b, R2.a and R2.b) according to the degree of affinity between the starting data. As a result, two risk indicators (R1 and R2 respectively) which show the hazard associated with suffering from OSA, related to the groups of data linked to each indicator, are determined at the output of the expert systems after the defuzzification process.
- Third level: at the third level of the cascade, the data from the second level of the cascade (R1 and R2) are processed using a single expert system. At its output, after the defuzzification process a risk indicator is obtained, the Symbolic Risk, which indicates the hazard level associated to the patient suffering from OSA according to the subjective input data.
2.2.3. Generation of Alerts and Decision Making
3. Results
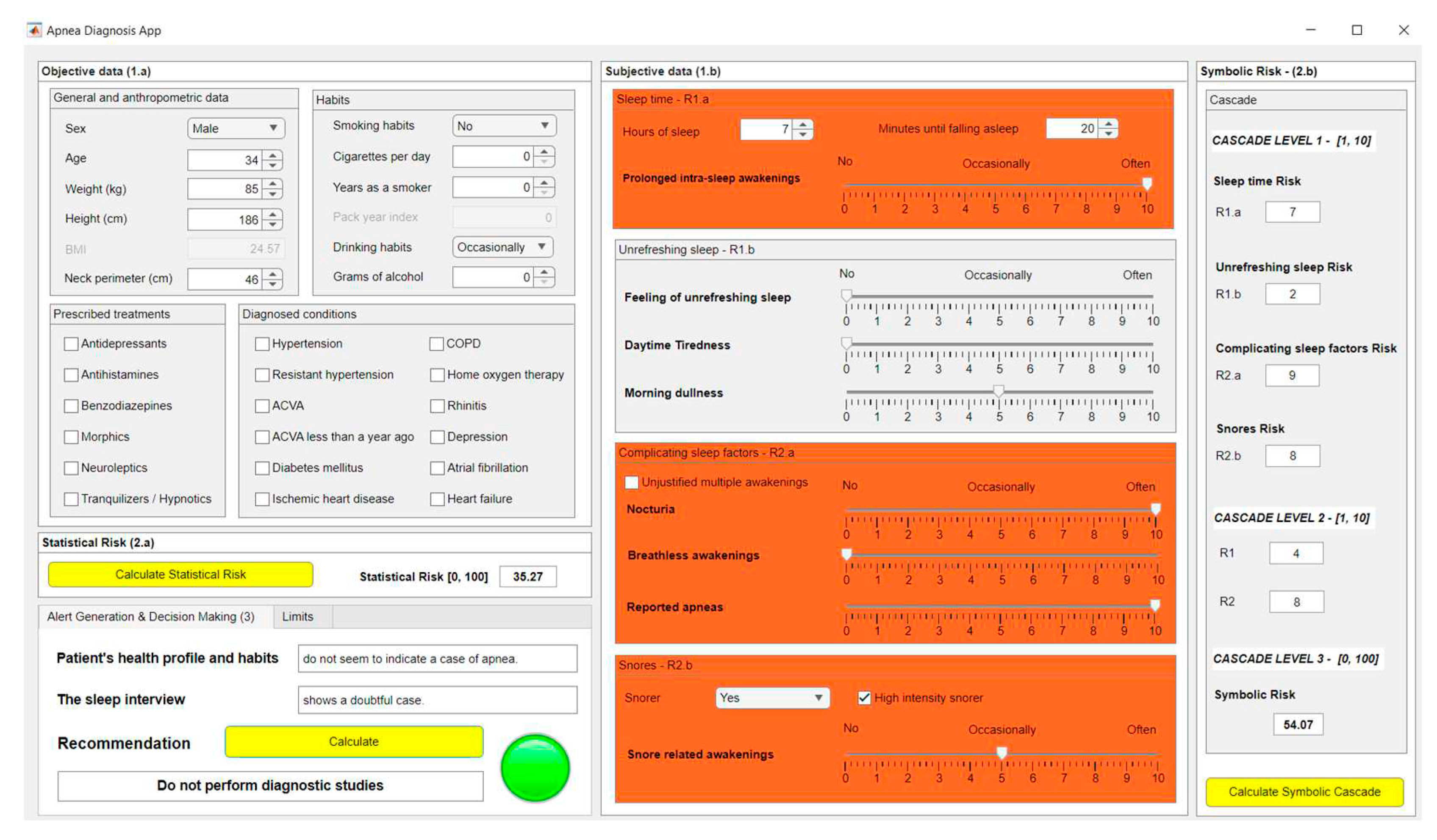
3.1. Compilation of the Patient’s Information
3.2. Data Processing
3.3. Generation of Alerts and Decision Making
3.3. Interpretation of the Results
4. Discussion
- Determination of Statistical Risk: as previously mentioned, a Machine Learning classification algorithm is used to determine the Statistical Risk, more specifically a Bagged Tree built on the basis of an initial dataset that has been encoded, normalized and balanced using SMOTE-NC. All this process prior to the construction of the model has been carried out with the aim of ensuring that the initial dataset used for the construction of the model is coherent and adequate. An attempt has been made to achieve a sufficient representativeness regarding the possible casuistry, as well as to aim for normality in the data distributions, in order to guarantee the subsequent obtaining of robust and reliable classifiers. As mentioned before, in this work a Bagged Trees algorithm was chosen, however, the use of one algorithm or another is not relevant because any other Machine Learning approach could provide plausible results. In any case, it should be noted that for this to be true, the datasets used in the model training and validation processes must have been obtained under similar circumstances, with common diagnostic criteria. The same circumstances should apply when it is desired to analyze data from new patients. In relation to the treatment of uncertainty, in this case it is achieved using a purely probabilistic approach.
- Determination of the Symbolic Risk: concurrently to the calculation of the Statistical Risk, the Symbolic Risk is determined using a series of expert systems, which are perhaps the most representative models for symbolic reasoning in the field of artificial intelligence and allow diversifying and formalizing the knowledge of the specialists. In this particular case, the formalization of knowledge has been achieved through the definition of an architecture of expert systems arranged in cascade. The diversification of information is possible through the definition of a series of declarative rules in each of the expert systems that model the knowledge of events that have occurred in similar circumstances. Thus, there is a clear and undeniable dependence between the way in which the expert system performs its reasoning and who defines its knowledge base, which implies assuming a certain degree of doubt and error in the process, and therefore, the presence of uncertainty in the generation of the rules. The formalization of knowledge is an inherent characteristic of expert systems, and it is possible to do it in this case through the definition of a cascade-based architecture. This allows the gradual integration of the consequents of the previous levels, all of them considered as technical variables representing the risk of suffering from OSA. These consequents are treated, in turn, as qualitative variables when acting as antecedents of the expert systems in the next level. As discussed in the work by Casal-Guisande et al. [17], the distinction that the same variable may be treated as antecedent or consequent of a rule makes a clear difference in the very fuzziness of the variable, which is related to the uncertainty associated with its numerical representation. In addition, the cascaded expert systems architecture allows for simpler logical constructs, through which it is possible to represent knowledge. That also results in a better control and in the progressive reduction of uncertainty throughout the different stages of the cascade. It is because of all those reasons that the intelligent system, in its symbolic aspect, has capabilities to manage uncertainty.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramachandran, A.; Karuppiah, A. A Survey on Recent Advances in Machine Learning Based Sleep Apnea Detection Systems. Healthcare 2021, Vol. 9, Page 914 2021, 9, 914. [Google Scholar] [CrossRef]
- Benjafield, A. v.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.L.D.; et al. Estimation of the Global Prevalence and Burden of Obstructive Sleep Apnoea: A Literature-Based Analysis. Lancet Respir Med 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Watson, N.F. Health Care Savings: The Economic Value of Diagnostic and Therapeutic Care for Obstructive Sleep Apnea. Journal of Clinical Sleep Medicine 2016, 12, 1075–1077. [Google Scholar] [CrossRef]
- Frost & Sullivan. Hidden Health Crisis Costing America Billions Underdiagnosing and Undertreating Obstructive Sleep Apnea Draining Healthcare System. American Academy of Sleep Medicine 2016. [Google Scholar]
- Ye, L.; Li, W.; Willis, D.G. Facilitators and Barriers to Getting Obstructive Sleep Apnea Diagnosed: Perspectives from Patients and Their Partners. Journal of Clinical Sleep Medicine 2022, 18, 835–841. [Google Scholar] [CrossRef]
- Douglas, N.J.; Thomas, S.; Jan, M.A. Clinical Value of Polysomnography. The Lancet 1992, 339, 347–350. [Google Scholar] [CrossRef]
- Rundo, J.V.; Downey, R. Polysomnography. In Handbook of Clinical Neurology; Elsevier, 2019; Vol. 160, pp. 381–392. [CrossRef]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. Journal of Clinical Sleep Medicine 2017, 13, 479–504. [Google Scholar] [CrossRef]
- Punjabi, N.M. The Epidemiology of Adult Obstructive Sleep Apnea. Proc Am Thorac Soc 2008, 5, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, S.S.; Mendonça, F.; Ravelo-García, A.G.; Morgado-Dias, F. A Systematic Review of Detecting Sleep Apnea Using Deep Learning. Sensors 2019, Vol. 19, Page 4934 2019, 19, 4934. [Google Scholar] [CrossRef] [PubMed]
- Prisant, L.M.; Dillard, T.A.; Blanchard, A.R. Obstructive Sleep Apnea Syndrome. The Journal of Clinical Hypertension 2006, 8, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.L.; Brown, R.H.; Woo, H.; Brooker, A.C.; Paulin, L.M.; Schneider, H.; Schwartz, A.R.; Diette, G.B.; Wise, R.A.; Hansel, N.N.; et al. Obstructive Sleep Apnea and Airway Dimensions in Chronic Obstructive Pulmonary Disease. Ann Am Thorac Soc 2020, 17, 116–118. [Google Scholar] [CrossRef]
- Pevernagie, D.A.; Gnidovec-Strazisar, B.; Grote, L.; Heinzer, R.; McNicholas, W.T.; Penzel, T.; Randerath, W.; Schiza, S.; Verbraecken, J.; Arnardottir, E.S. On the Rise and Fall of the Apnea−hypopnea Index: A Historical Review and Critical Appraisal. J Sleep Res 2020, 29, e13066. [Google Scholar] [CrossRef] [PubMed]
- Casal-Guisande, M.; Comesaña-Campos, A.; Cerqueiro-Pequeño, J.; Bouza-Rodríguez, J.-B. Design and Development of a Methodology Based on Expert Systems, Applied to the Treatment of Pressure Ulcers. Diagnostics 2020, 10, 614. [Google Scholar] [CrossRef] [PubMed]
- Comesaña-Campos, A.; Casal-Guisande, M.; Cerqueiro-Pequeño, J.; Bouza-Rodríguez, J.B. A Methodology Based on Expert Systems for the Early Detection and Prevention of Hypoxemic Clinical Cases. Int J Environ Res Public Health 2020, 17, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Cerqueiro-Pequeño, J.; Comesaña-Campos, A.; Casal-Guisande, M.; Bouza-Rodríguez, J.-B. Design and Development of a New Methodology Based on Expert Systems Applied to the Prevention of Indoor Radon Gas Exposition Risks. Int J Environ Res Public Health 2020, 18, 269. [Google Scholar] [CrossRef] [PubMed]
- Casal-Guisande, M.; Comesaña-Campos, A.; Dutra, I.; Cerqueiro-Pequeño, J.; Bouza-Rodríguez, J.-B. Design and Development of an Intelligent Clinical Decision Support System Applied to the Evaluation of Breast Cancer Risk. Journal of Personalized Medicine 2022, Vol. 12, Page 169 2022, 12, 169. [Google Scholar] [CrossRef] [PubMed]
- Casal-Guisande, M.; Bouza-Rodríguez, J.-B.; Cerqueiro-Pequeño, J.; Comesaña-Campos, A. Design and Conceptual Development of a Novel Hybrid Intelligent Decision Support System Applied towards the Prevention and Early Detection of Forest Fires. Forests 2023, Vol. 14, Page 172 2023, 14, 172. [Google Scholar] [CrossRef]
- Mamdani, E.H.; Assilian, S. An Experiment in Linguistic Synthesis with a Fuzzy Logic Controller. Int J Man Mach Stud 1975, 7, 1–13. [Google Scholar] [CrossRef]
- Mamdani, E.H. Advances in the Linguistic Synthesis of Fuzzy Controllers. Int J Man Mach Stud 1976, 8, 669–678. [Google Scholar] [CrossRef]
- Mamdani, E.H. Application of Fuzzy Logic to Approximate Reasoning Using Linguistic Synthesis. IEEE Transactions on Computers 1977, C–26, 1182–1191. [CrossRef]
- Ross, T.J. Fuzzy Logic with Engineering Applications: Third Edition; Third edit.; John Wiley & Sons, Ltd: Chichester, UK, 2010; ISBN 9781119994374.
- Mencar, C.; Gallo, C.; Mantero, M.; Tarsia, P.; Carpagnano, G.E.; Foschino Barbaro, M.P.; Lacedonia, D. Application of Machine Learning to Predict Obstructive Sleep Apnea Syndrome Severity. Health Informatics J 2020, 26, 298–317. [Google Scholar] [CrossRef]
- Sun, L.M.; Chiu, H.W.; Chuang, C.Y.; Liu, L. A Prediction Model Based on an Artificial Intelligence System for Moderate to Severe Obstructive Sleep Apnea. Sleep and Breathing 2010 15:3 2010, 15, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, J.; Keeran, N.; Sagahyroon, A.; Aloul, F. Towards Validating the Effectiveness of Obstructive Sleep Apnea Classification from Electronic Health Records Using Machine Learning. Healthcare 2021, Vol. 9, Page 1450 2021, 9, 1450. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Santos, D.; Rodrigues, P.P. A Clinical Risk Matrix for Obstructive Sleep Apnea Using Bayesian Network Approaches. Int J Data Sci Anal 2019, 8, 339–349. [Google Scholar] [CrossRef]
- Zoroglu, C.; Turkeli, S. Fuzzy Expert System for Severity Prediction of Obstructive Sleep Apnea Hypopnea Syndrome. The journal of cognitive systems 2017, 2. [Google Scholar]
- Matthews, J.M.; Kwiatkowska, M.; Matthews, L.R. A Preliminary Fuzzy Model for Screening Obstructive Sleep Apnea. Proceedings of the 2013 Joint IFSA World Congress and NAFIPS Annual Meeting, IFSA/NAFIPS 2013 2013, 187–191. [CrossRef]
- Wasserman, L. All of Statistics : A Concise Course in Statistical Inference. In; Springer: New York, 2004; Vol. 26 ISBN 978-0-387-21736-9.
- Hevner, A.R.; March, S.T.; Park, J.; Ram, S. Design Science in Information Systems Research. MIS Q 2004, 28, 75–105. [Google Scholar] [CrossRef]
- Hevner, A.R.; Chatterjee, S. Design Research in Information Systems: Theory and Practice; Springer: New York, NY, USA, 2010; ISBN 978-1-4419-6107-5. [Google Scholar]
- App Designer. Available online: https://www.mathworks.com/products/matlab/app-designer.html (accessed on 18 October 2022).
- Classification Learner. Available online: https://www.mathworks.com/help/stats/classificationlearner-app.html (accessed on 18 October 2022).
- Fuzzy Logic Toolbox - MATLAB. Available online: https://www.mathworks.com/products/fuzzy-logic.html (accessed on 1 November 2022).
- Imbalanced-Learn. Available online: https://imbalanced-learn.org/dev/index.html (accessed on 18 October 2022).
- Agresti, A. Categorical Data Analysis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002; ISBN 0471360937. [Google Scholar]
- Powers, D.; Xie, Y. Statistical Methods for Categorical Data Analysis; Emerald Group Publishing, 2008.
- Mohammed, A.J.; Hassan, M.M.; Kadir, D.H. Improving Classification Performance for a Novel Imbalanced Medical Dataset Using Smote Method. International Journal of Advanced Trends in Computer Science and Engineering 2020, 9, 3161–3172. [Google Scholar] [CrossRef]
- Chawla, N. v.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic Minority Over-Sampling Technique. Journal of Artificial Intelligence Research 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Refaeilzadeh, P.; Tang, L.; Liu, H. Cross-Validation. Encyclopedia of Database Systems 2009, 532–538. [Google Scholar] [CrossRef]
- Deborah, L. Thurston Utility Function Fundamentals. In Decision Making in Engineering Design; ASME Press: New York, USA, 2006. [Google Scholar]
- Sundar Krishnamurty Normative Decision Analysis in Engineering Design. In Decision Making in Engineering Design; ASME Press: New York, USA, 2006.
- Hair, J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E. Multivariate Data Analysis; Prentice Hall, 2009; Vol. 87; ISBN 9780138132637.
- Cooper, J.C.B. Artificial Neural Networks versus Multivariate Statistics: An Application from Economics. http://dx.doi.org/10.1080/02664769921927 2010, 26, 909–921. [CrossRef]
- Wang, C.Y.; Lee, T.F.; Fang, C.H.; Chou, J.H. Fuzzy Logic-Based Prognostic Score for Outcome Prediction in Esophageal Cancer. IEEE Trans Inf Technol Biomed 2012, 16, 1224–1230. [Google Scholar] [CrossRef]
- Yazdanbakhsh, O.; Dick, S. Forecasting of Multivariate Time Series via Complex Fuzzy Logic. IEEE Trans Syst Man Cybern Syst 2017, 47, 2160–2171. [Google Scholar] [CrossRef]
- Egrioglu, E.; Aladag, C.H.; Yolcu, U.; Uslu, V.R.; Basaran, M.A. A New Approach Based on Artificial Neural Networks for High Order Multivariate Fuzzy Time Series. Expert Syst Appl 2009, 36, 10589–10594. [Google Scholar] [CrossRef]
- Smithson, M. Multivariate Analysis Using ‘and’ and ‘Or. ’ Math Soc Sci 1984, 7, 231–251. [Google Scholar] [CrossRef]
| Subgroup | Data | Data type | Commentary |
|
Sleep time |
Hours of sleep | Numerical | - |
| Minutes until falling asleep |
Numerical | - | |
| Prolonged intra-sleep awakenings | Categorical | No/Occasionally/Often | |
| Subgroup | Data | Data type | Commentary |
|
Unrefreshing sleep |
Feeling of unrefreshing sleep | Categorical | No/Occasionally/Often |
| Daytime tiredness | Categorical | No/Occasionally/Often | |
| Morning dullness | Categorical | No/Occasionally/Often | |
| Subgroup | Data | Data type | Commentary |
|
Complicating sleep factors |
Unjustified multiple awakenings | Categorical | Yes/No |
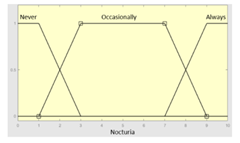
| Nocturia | Categorical | No/Occasionally/Often | |
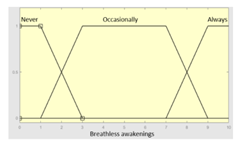
| Breathless awakenings | Categorical | No/Occasionally/Often | |
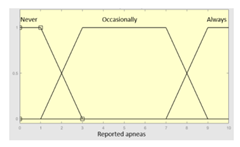
| Reported apneas | Categorical | No/Occasionally/Often | |
|
Snores |
Snorer | Categorical | No/In supine position only/Yes |
| High intensity snorer | Categorical | Yes/No | |
| Snore related awakenings |
Categorical | No/Occasionally/Often |
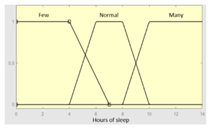
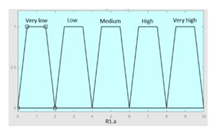
| Inference system associated to the ‘sleep time’ data group | |||
|---|---|---|---|
| Input Data | Range | Output Risk | Range |
| Hours of sleep | 0–14 hours | R1.a | 0–10 |
 |
 |
||
| Minutes until falling asleep |
0–240 minutes | Initial configuration | |
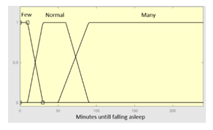 |
Fuzzy structure: Mamdani-type. Membership function type: trapezoidal. Defuzzification method: centroid [22]. Implication method: MIN. Aggregation method: MAX. Number of fuzzy rules: 46 |
||
| Prolonged intra-sleep awakenings |
0–10 | Subset as an example of the 46 fuzzy rules | |
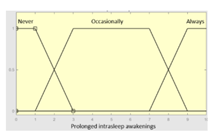 |
1. IF (Hours_of_sleep is Few) AND (Minutes_until_falling_asleep is Few) AND (Prolonged_intra-sleep_awakenings is Never) THEN (R1.a is Low). 2. IF (Hours_of_Sleep is Few) AND (Minutes_until_falling_asleep is Few) AND (Prolonged_intra-sleep_awakenings is Never) THEN (R1.a is Medium). |
||
| Graphical example of fuzzy rules 1 and 2 | |||
 | |||
| Inference system associated to the ‘unrefreshing sleep’ data group | |||
|---|---|---|---|
| Input Data | Range | Output Risk | Range |
| Feeling of unrefreshing sleep |
0–10 | R1.b | 0 – 10 |
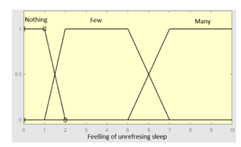 |
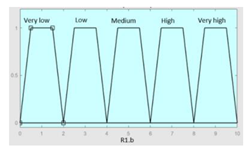 |
||
| Daytime tiredness | 0–10 | Initial configuration | |
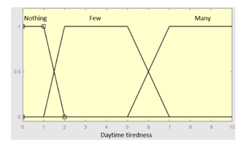 |
Fuzzy structure: Mamdani-type. Membership function type: trapezoidal. Defuzzification method: centroid [22]. Implication method: MIN. Aggregation method: MAX. Number of fuzzy rules: 45 |
||
| Morning dullness | 0–10 | Subset as an example of the 45 fuzzy rules | |
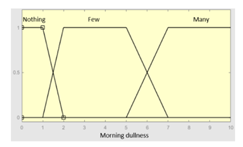 |
1. IF (Feeling_of_unrefreshing_sleep is Nothing) AND (Daytime_tiredness is Nothing) AND (Morning_dullness is Nothing) THEN (R1.b is Very_low). 2. IF (Feeling_of_unrefreshing_sleep is Nothing) AND (Daytime_tiredness is Nothing) AND (Morning_dullness is Few) THEN (R1.b is Very_low). |
||
| Graphical example of fuzzy rules 1 and 2 | |||
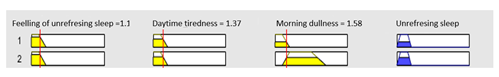 | |||
| Inference system associated to the ‘complicating sleep factors’ data group | |||
|---|---|---|---|
| Input Data | Range | Output Risk | Range |
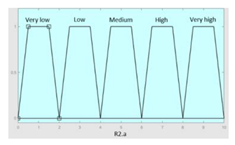
| Unjustified multiple awakenings | 0–1 | R2.a | 0 – 10 |
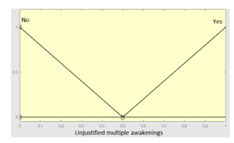 |
 |
||
| Nocturia | 0–10 | Initial configuration | |
 |
Fuzzy structure: Mamdani-type. Membership function type: trapezoidal. Defuzzification method: centroid [22]. Implication method: MIN. Aggregation method: MAX. Number of fuzzy rules: 78 |
||
| Breathless awakenings | 0–10 | ||
 | |||
| Reported apneas | 0–10 | Subset as an example of the 78 fuzzy rules | |
 |
IF (Reported_apneas is Always) THEN (R2.a is Very_high) IF (Unjustified_multiple_awakenings is No) AND (Nocturia is Never) AND (Beathless_awakenings is Never) AND (Reported_apneas is Never) THEN (R2.a is Very_low) |
||
| Graphical example of fuzzy rule 1 | |||
 | |||
| Inference system associated to the ‘snores’ data group | |||
|---|---|---|---|
| Input Data | Range | Output Risk | Range |
| Snorer | 0–2 | R2.b | 0 – 10 |
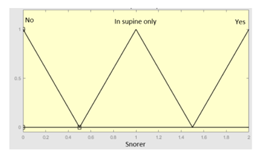 |
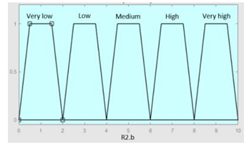 |
||
| High intensity snorer |
0–1 | Initial configuration | |
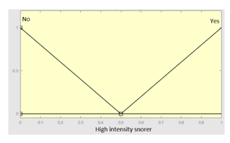 |
Fuzzy structure: Mamdani-type. Membership function type: trapezoidal. Defuzzification method: centroid [22]. Implication method: MIN. Aggregation method: MAX. Number of fuzzy rules: 30 |
||
| Snore related awakenings |
0 – 10 | Subset as an example of the 30 fuzzy rules | |
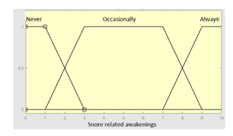 |
1. IF (Snorer is No) THEN (R2.b is Very_low) 2. IF (Snorer is In_dorsal_position_only) AND (High_intensity_snorer is No) AND (Snore_related_awakenings is Never) THEN (R2.b is Very_low) |
||
| Graphical example of fuzzy rule 1 | |||
 | |||
| Inference system for the processing of risks R1.a and R1.b | |||
|---|---|---|---|
| Input Data | Range | Output Risk | Range |
| R1.a | 0–10 | R1 | 0–10 |
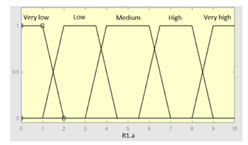 |
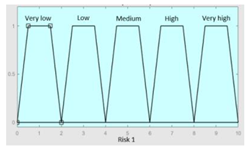 |
||
| R1.b | 0–10 | Initial configuration | |
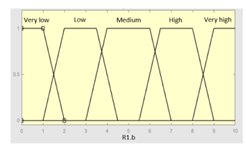 |
Fuzzy structure: Mamdani-type. Membership function type: trapezoidal. Defuzzification method: centroid [22]. Implication method: MIN. Aggregation method: MAX. Number of fuzzy rules: 49 |
||
| Subset as an example of the 49 fuzzy rules | |||
| |||
| Graphical example of fuzzy rules 1, 2 and 3 | |||
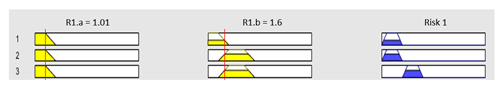 | |||
| Inference system for the processing of risks R2.a and R2.b | |||
|---|---|---|---|
| Input Data | Range | Ouput Risk | Range |
| R2.a | 0–10 | R2 | 0–10 |
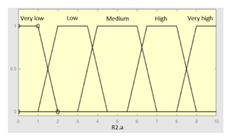 |
 |
||
| R2.b | 0–10 | Initial configuration | |
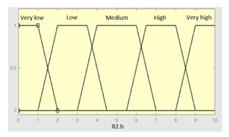 |
Fuzzy structure: Mamdani-type. Membership function type: trapezoidal. Defuzzification method: centroid [22]. Implication method: MIN. Aggregation method: MAX. Number of fuzzy rules: 57 |
||
| Subset as an example of the 57 fuzzy rules | |||
| |||
| Graphical example of fuzzy rules 1, 2 and 3 | |||
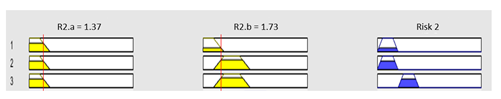 | |||
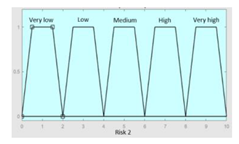
| Inference system for the processing of risks R1 and R2 | |||
|---|---|---|---|
| Input Data | Range | Output Risk | Range |
| R1 | 0–10 | Symbolic Risk | 0–10 |
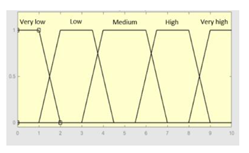 |
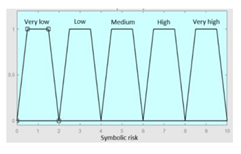 |
||
| R2 | 0–10 | Initial configuration | |
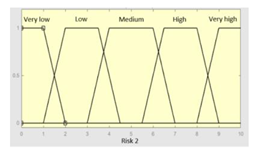 |
Fuzzy structure: Mamdani-type. Membership function type: trapezoidal. Defuzzification method: centroid [22]. Implication method: MIN. Aggregation method: MAX. Number of fuzzy rules: 57 |
||
| Subset as an example of the 57 fuzzy rules | |||
| |||
| Surface | |||
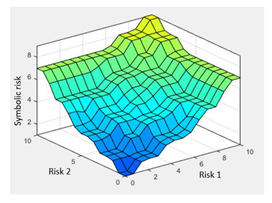 | |||
| Level | Case | Score |
|---|---|---|
| Level 1 | If Risk < Limit 1 (L1) | 0 |
| Level 2 | Limit 1 (L1) ≤ Risk < Limit 2 (L2) | 1 |
| Level 3 | If Risk ≥ Limit 2 (L2) | 2 |
| Case | Recommendation |
|---|---|
| T < 2 | Non-OSA case, do not perform diagnostic studies |
| T = 2 | Doubtful case. Medical team should assess whether further tests or a new medical evaluation after a period of time is necessary to reconsider the patient’s condition. |
| T ≥ 3 | Possible OSA case, perform diagnostic studies |
| Symbolic Risk | |||||
|---|---|---|---|---|---|
| Statistical Risk | Case | Risk < L1 | L1 ≤ Risk < L2 | Risk ≥ L2 | |
| Case | Level & Score | Level 1 (0) | Level 2 (1) | Level 3 (2) | |
| Risk < L1 | Level 1 (0) | 0+0 | 0+1 | 0+2 | |
| Level 1 ≤ Risk < L2 | Level 2 (1) | 1+0 | 1+1 | 1+2 | |
| Risk ≥ L2 | Level 3 (2) | 2+0 | 2+1 | 2+2 | |
| General and anthropometric data | |
| Sex | Male |
| Age | 34 |
| Weight (kg) | 85 |
| Height (cm) | 186 |
| Neck perimeter (cm) | 46 |
| Habits | |
| Smoking habits | No |
| Cigarettes per day | - |
| Years smoking | - |
| Drinking habits | Occasionally |
| Grams of alcohol | - |
| Diagnosed conditions | - |
| Prescribed treatments | - |
| Sleep time group | |
| Hours of sleep | 7 hours |
| Minutes until falling asleep | 20 minutes |
| Prolonged intra-sleep awakenings | Often |
| Unrefreshing sleep group | |
| Feeling of unrefreshing sleep | No |
| Daytime tiredness | No |
| Morning dullness | Occasionally |
| Complicating sleep factors group | |
| Unjustified multiple awakenings | No |
| Nocturia | Often |
| Breathless awakenings | No |
| Reported apneas | Often |
| Snores group | |
| Snorer | Yes |
| High intensity snorer | Yes |
| Snorer related awakenings | Occasionally |
| Level | Case | Level interpretation |
|---|---|---|
| Level 1 | IF Risk < 45 THEN Level 1 | Non-OSA case |
| Level 2 | IF 45 ≤ Risk < 60 THEN Level 2 | Doubtful case |
| Level 3 | IF Risk ≥ 60 THEN Level 3 | Possible OSA case |
| Symbolic Risk | |||||
|---|---|---|---|---|---|
| Statistical Risk | Case | Risk < 45 | 45 ≤ Risk < 60 | Risk ≥ 60 | |
| Case | Level & Score | Level 1 | Level 2 | Level 3 | |
| Risk < 45 | Level 1 | - | X | - | |
| 45 ≤ Risk < 60 | Level 2 | - | - | - | |
| Risk ≥ 60 | Level 3 | - | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





