Submitted:
27 March 2023
Posted:
28 March 2023
You are already at the latest version
Abstract
Keywords:
1. Background
2. Pathophysiology of COVID-induced PF
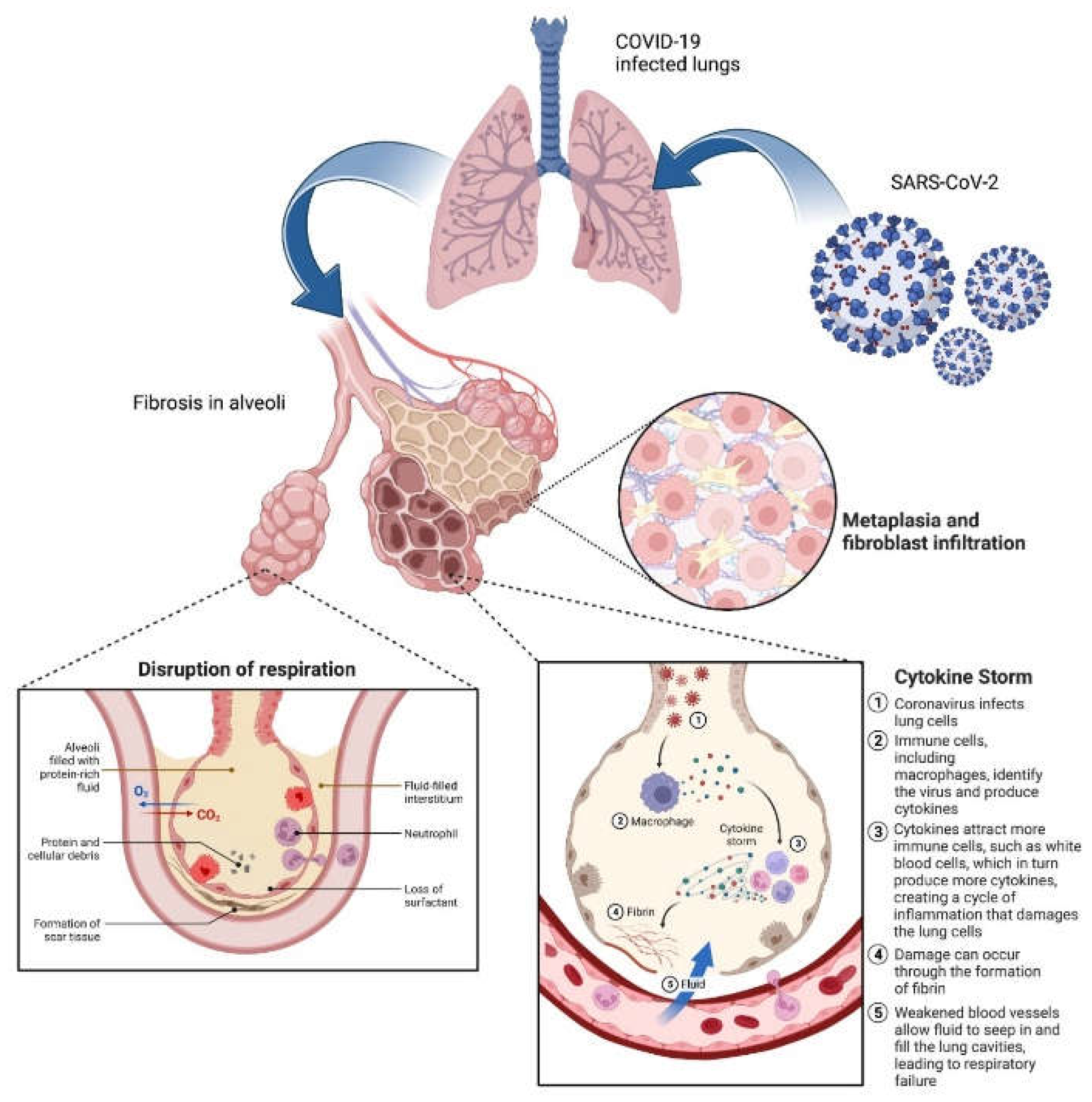
3. Current Status of COVID-induced PF therapy
4. Nano-therapeutic approaches to treat COVID induced PF
4.1. Cell-mimicking nanodecoys
4.2. CD-24 exosomes
4.3. Mannosylated albumin-siRNA NPs
4.4. Nanostructured hydroxychloroquine
4.5. PLGA-PEG-G0-C14-IL11 siRNA NPs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
References
- P. M. George, C. M. Patterson, A. K. Reed, and M. Thillai, “Lung transplantation for idiopathic pulmonary fibrosis,” Lancet Respir. Med., vol. 7, no. 3, pp. 271–282, 2019. [CrossRef]
- K. C. Meyer, “Pulmonary fibrosis, part I: epidemiology, pathogenesis, and diagnosis,” Expert Rev. Respir. Med., vol. 11, no. 5, pp. 343–359, May 2017. [CrossRef]
- T. E. King Jr, A. Pardo, and M. Selman, “Idiopathic pulmonary fibrosis,” Lancet, vol. 378, no. 9807, pp. 1949–1961, Dec. 2011. [CrossRef]
- W. D. Travis et al., “An Official American Thoracic Society/European Respiratory Society Statement: Update of the International Multidisciplinary Classification of the Idiopathic Interstitial Pneumonias,” Am. J. Respir. Crit. Care Med., vol. 188, no. 6, pp. 733–748, Sep. 2013. [CrossRef]
- M. A. Seibold et al., “A Common MUC5B Promoter Polymorphism and Pulmonary Fibrosis,” N. Engl. J. Med., vol. 364, no. 16, pp. 1503–1512, Apr. 2011. [CrossRef]
- C. A. Newton et al., “Telomere-related lung fibrosis is diagnostically heterogeneous but uniformly progressive,” Eur. Respir. J., vol. 48, no. 6, pp. 1710 LP – 1720, Dec. 2016. [CrossRef]
- V Yang, “Epigenomics of idiopathic pulmonary fibrosis,” Epigenomics, vol. 4, no. 2, pp. 195–203, Mar. 2012. [CrossRef]
- Tzouvelekis and N. Kaminski, “Epigenetics in idiopathic pulmonary fibrosis,” Biochem. Cell Biol., vol. 93, no. 2, pp. 159–170, Jan. 2015. [CrossRef]
- Caminati, F. Madotto, G. Cesana, S. Conti, and S. Harari, “Epidemiological studies in idiopathic pulmonary fibrosis: pitfalls in methodologies and data interpretation,” Eur. Respir. Rev., vol. 24, no. 137, pp. 436 LP – 444, Sep. 2015. [CrossRef]
- G. Raghu et al., “Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001–11,” Lancet Respir. Med., vol. 2, no. 7, pp. 566–572, Jul. 2014. [CrossRef]
- S. E. Tanni et al., “Pulmonary fibrosis secondary to COVID-19: a narrative review,” Expert Rev. Respir. Med., vol. 15, no. 6, pp. 791–803, Jun. 2021. [CrossRef]
- J.-N. Zou et al., “The characteristics and evolution of pulmonary fibrosis in COVID-19 patients as assessed by AI-assisted chest HRCT,” PLoS One, vol. 16, no. 3, p. e0248957, Mar. 2021, [Online]. [CrossRef]
- M. Fisher et al., “Predicting Life Expectancy for Pirfenidone in Idiopathic Pulmonary Fibrosis,” J. Manag. Care Spec. Pharm., vol. 23, no. 3-b Suppl, pp. S17–S24, Mar. 2017. [CrossRef]
- L. Richeldi et al., “Efficacy and Safety of Nintedanib in Idiopathic Pulmonary Fibrosis,” N. Engl. J. Med., vol. 370, no. 22, pp. 2071–2082, May 2014. [CrossRef]
- T. E. King et al., “A Phase 3 Trial of Pirfenidone in Patients with Idiopathic Pulmonary Fibrosis,” N. Engl. J. Med., vol. 370, no. 22, pp. 2083–2092, May 2014. [CrossRef]
- J. Fadista et al., “Shared genetic etiology between idiopathic pulmonary fibrosis and COVID-19 severity,” eBioMedicine, vol. 65, Mar. 2021. [CrossRef]
- D. Wendisch et al., “SARS-CoV-2 infection triggers profibrotic macrophage responses and lung fibrosis,” Cell, vol. 184, no. 26, pp. 6243-6261.e27, Dec. 2021. [CrossRef]
- W. J. Wiersinga, A. Rhodes, A. C. Cheng, S. J. Peacock, and H. C. Prescott, “Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review,” JAMA, vol. 324, no. 8, pp. 782–793, Aug. 2020. [CrossRef]
- T. Parimon, M. Espindola, A. Marchevsky, R. Rampolla, P. Chen, and C. M. Hogaboam, “Potential mechanisms for lung fibrosis associated with COVID-19 infection,” QJM An Int. J. Med., p. hcac206, Aug. 2022. [CrossRef]
- F. Rendeiro et al., “The spatial landscape of lung pathology during COVID-19 progression,” Nature, vol. 593, no. 7860, pp. 564–569, 2021. [CrossRef]
- Flaifel et al., “Pulmonary Pathology of End-Stage COVID-19 Disease in Explanted Lungs and Outcomes After Lung Transplantation,” Am. J. Clin. Pathol., vol. 157, no. 6, pp. 908–926, Jun. 2022. [CrossRef]
- P. Cheresh, S.-J. Kim, S. Tulasiram, and D. W. Kamp, “Oxidative stress and pulmonary fibrosis,” Biochim. Biophys. Acta - Mol. Basis Dis., vol. 1832, no. 7, pp. 1028–1040, 2013. [CrossRef]
- S. Tran, A. Ksajikian, J. Overbey, P. Li, and Y. Li, “Pathophysiology of Pulmonary Fibrosis in the Context of COVID-19 and Implications for Treatment: A Narrative Review,” Cells, vol. 11, no. 16. 2022. [CrossRef]
- K. Sehlmeyer, J. Ruwisch, N. Roldan, and E. Lopez-Rodriguez, “Corrigendum: Alveolar Dynamics and Beyond – the Importance of Surfactant Protein C and Cholesterol in Lung Homeostasis and Fibrosis ,” Frontiers in Physiology , vol. 11. 2020, [Online]. Available: https://www.frontiersin.org/articles/10.3389/fphys.2020.00943. [CrossRef]
- M. A. Seibold et al., “The Idiopathic Pulmonary Fibrosis Honeycomb Cyst Contains A Mucocilary Pseudostratified Epithelium,” PLoS One, vol. 8, no. 3, p. e58658, Mar. 2013, [Online]. [CrossRef]
- F. Conforti et al., “Paracrine SPARC signaling dysregulates alveolar epithelial barrier integrity and function in lung fibrosis,” Cell Death Discov., vol. 6, no. 1, p. 54, 2020. [CrossRef]
- M. J. Evans, L. S. Van Winkle, M. V Fanucchi, and C. G. Plopper, “The Attenuated Fibroblast Sheath of the Respiratory Tract Epithelial–Mesenchymal Trophic Unit,” Am. J. Respir. Cell Mol. Biol., vol. 21, no. 6, pp. 655–657, Dec. 1999. [CrossRef]
- Ghazavi, A. Ganji, N. Keshavarzian, S. Rabiemajd, and G. Mosayebi, “Cytokine profile and disease severity in patients with COVID-19,” Cytokine, vol. 137, p. 155323, 2021. [CrossRef]
- Colarusso et al., “Post-COVID-19 Patients Who Develop Lung Fibrotic-like Changes Have Lower Circulating Levels of IFN-β but Higher Levels of IL-1α and TGF-β,” Biomedicines, vol. 9, no. 12. 2021. [CrossRef]
- J. C. Melms et al., “A molecular single-cell lung atlas of lethal COVID-19,” Nature, vol. 595, no. 7865, pp. 114–119, 2021. [CrossRef]
- Yao et al., “Cell-Type-Specific Immune Dysregulation in Severely Ill COVID-19 Patients,” Cell Rep., vol. 34, no. 1, Jan. 2021. [CrossRef]
- Bharat et al., “Lung transplantation for patients with severe COVID-19,” Sci. Transl. Med., vol. 12, no. 574, p. eabe4282, Dec. 2020. [CrossRef]
- S. W. Aesif et al., “Pulmonary Pathology of COVID-19 Following 8 Weeks to 4 Months of Severe Disease: A Report of Three Cases, Including One With Bilateral Lung Transplantation,” Am. J. Clin. Pathol., vol. 155, no. 4, pp. 506–514, Apr. 2021. [CrossRef]
- J. C. T. Horowitz Victor J, “Epithelial-Mesenchymal Interactions in Pulmonary Fibrosis,” Semin Respir Crit Care Med, vol. 27, no. 06, pp. 600–612, 2006. [CrossRef]
- T. M. Maher, A. U. Wells, and G. J. Laurent, “Idiopathic pulmonary fibrosis: multiple causes and multiple mechanisms?,” Eur. Respir. J., vol. 30, no. 5, pp. 835 LP – 839, Nov. 2007. [CrossRef]
- D. Uhal, I. Joshi, W. F. Hughes, C. Ramos, A. Pardo, and M. Selman, “Alveolar epithelial cell death adjacent to underlying myofibroblasts in advanced fibrotic human lung,” Am. J. Physiol. Cell. Mol. Physiol., vol. 275, no. 6, pp. L1192–L1199, Dec. 1998. [CrossRef]
- L. Yao et al., “Paracrine signalling during ZEB1-mediated epithelial–mesenchymal transition augments local myofibroblast differentiation in lung fibrosis,” Cell Death Differ., vol. 26, no. 5, pp. 943–957, 2019. [CrossRef]
- P. Jiang et al., “Ineffectual Type 2–to–Type 1 Alveolar Epithelial Cell Differentiation in Idiopathic Pulmonary Fibrosis: Persistence of the KRT8hi Transitional State,” Am. J. Respir. Crit. Care Med., vol. 201, no. 11, pp. 1443–1447, Feb. 2020. [CrossRef]
- M. Cassandras et al., “Gli1+ mesenchymal stromal cells form a pathological niche to promote airway progenitor metaplasia in the fibrotic lung,” Nat. Cell Biol., vol. 22, no. 11, pp. 1295–1306, 2020. [CrossRef]
- Prasse et al., “BAL Cell Gene Expression Is Indicative of Outcome and Airway Basal Cell Involvement in Idiopathic Pulmonary Fibrosis,” Am. J. Respir. Crit. Care Med., vol. 199, no. 5, pp. 622–630, Aug. 2018. [CrossRef]
- T. S. Blackwell et al., “Future Directions in Idiopathic Pulmonary Fibrosis Research. An NHLBI Workshop Report,” Am. J. Respir. Crit. Care Med., vol. 189, no. 2, pp. 214–222, Oct. 2013. [CrossRef]
- K. Koli et al., “Bone Morphogenetic Protein-4 Inhibitor Gremlin Is Overexpressed in Idiopathic Pulmonary Fibrosis,” Am. J. Pathol., vol. 169, no. 1, pp. 61–71, Jul. 2006. [CrossRef]
- J. K. Keski-Oja Katri; Lohi, Jouko; Laiho, Marikki, “Growth Factors in the Regulation of Plasminogen-Plasmin System in Tumor Cells,” Semin Thromb Hemost, vol. 17, no. 03, pp. 231–239, 1991. [CrossRef]
- R. Raghow, A. E. Postlethwaite, J. Keski-Oja, H. L. Moses, and A. H. Kang, “Transforming growth factor-beta increases steady state levels of type I procollagen and fibronectin messenger RNAs posttranscriptionally in cultured human dermal fibroblasts.,” J. Clin. Invest., vol. 79, no. 4, pp. 1285–1288, Apr. 1987. [CrossRef]
- J. M. Shannon and B. A. Hyatt, “Epithelial-Mesenchymal Interactions in the Developing Lung,” Annu. Rev. Physiol., vol. 66, no. 1, pp. 625–645, Feb. 2004. [CrossRef]
- K. C. Meyer, “Pulmonary fibrosis, part II: state-of-the-art patient management,” Expert Rev. Respir. Med., vol. 11, no. 5, pp. 361–376, May 2017. [CrossRef]
- M. Kreuter, “Pirfenidone: an update on clinical trial data and insights from everyday practice,” Eur. Respir. Rev., vol. 23, no. 131, pp. 111 LP – 117, Mar. 2014. [CrossRef]
- H. Taniguchi et al., “Pirfenidone in idiopathic pulmonary fibrosis,” Eur. Respir. J., vol. 35, no. 4, pp. 821 LP – 829, Apr. 2010. [CrossRef]
- P. W. Noble et al., “Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials,” Lancet, vol. 377, no. 9779, pp. 1760–1769, May 2011. [CrossRef]
- P. Spagnolo, T. M. Maher, and L. Richeldi, “Idiopathic pulmonary fibrosis: Recent advances on pharmacological therapy,” Pharmacol. Ther., vol. 152, pp. 18–27, 2015. [CrossRef]
- S. King and S. D. Nathan, “Practical considerations in the pharmacologic treatment of idiopathic pulmonary fibrosis,” Curr. Opin. Pulm. Med., vol. 21, no. 5, 2015, [Online]. Available: https://journals.lww.com/co-pulmonarymedicine/Fulltext/2015/09000/Practical_considerations_in_the_pharmacologic.10.aspx. [CrossRef]
- Wani et al., “Surface PEGylation of Mesoporous Silica Nanorods (MSNR): Effect on loading, release, and delivery of mitoxantrone in hypoxic cancer cells,” Sci. Rep., vol. 7, no. 1, p. 2274, 2017. [CrossRef]
- R. Roggers, S. Kanvinde, S. Boonsith, and D. Oupický, “The practicality of mesoporous silica nanoparticles as drug delivery devices and progress toward this goal,” AAPS PharmSciTech, vol. 15, no. 5, pp. 1163–1171, Oct. 2014. [CrossRef]
- Y. Zhu et al., “Self-immolative polycations as gene delivery vectors and prodrugs targeting polyamine metabolism in cancer,” Mol. Pharm., vol. 12, no. 2, pp. 332–341, Feb. 2015. [CrossRef]
- S. Deodhar et al., “Transformation of dolutegravir into an ultra-long-acting parenteral prodrug formulation,” Nat. Commun., vol. 13, no. 1, p. 3226, 2022. [CrossRef]
- M. Moss and M. Siccardi, “Optimizing nanomedicine pharmacokinetics using physiologically based pharmacokinetics modelling,” Br. J. Pharmacol., vol. 171, no. 17, pp. 3963–3979, Sep. 2014. [CrossRef]
- N. Gautam et al., “Lipophilic nanocrystal prodrug-release defines the extended pharmacokinetic profiles of a year-long cabotegravir,” Nat. Commun., vol. 12, no. 1, p. 3453, 2021. [CrossRef]
- T. A. Kulkarni et al., “A year-long extended release nanoformulated cabotegravir prodrug,” Nat. Mater., vol. 19, no. 8, pp. 910–920, 2020. [CrossRef]
- S. Beyerstedt, E. B. Casaro, and É. B. Rangel, “COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection,” Eur. J. Clin. Microbiol. Infect. Dis., vol. 40, no. 5, pp. 905–919, 2021. [CrossRef]
- W. Ni et al., “Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19,” Crit. Care, vol. 24, no. 1, p. 422, 2020. [CrossRef]
- J. Yang et al., “Molecular interaction and inhibition of SARS-CoV-2 binding to the ACE2 receptor,” Nat. Commun., vol. 11, no. 1, p. 4541, 2020. [CrossRef]
- Z. Li et al., “Cell-mimicking nanodecoys neutralize SARS-CoV-2 and mitigate lung injury in a non-human primate model of COVID-19,” Nat. Nanotechnol., vol. 16, no. 8, pp. 942–951, 2021. [CrossRef]
- S. Shapira et al., “A novel platform for attenuating immune hyperactivity using EXO-CD24 in COVID-19 and beyond,” EMBO Mol. Med., vol. 14, no. 9, p. e15997, Sep. 2022. [CrossRef]
- X. Fang, P. Zheng, J. Tang, and Y. Liu, “CD24: from A to Z,” Cell. Mol. Immunol., vol. 7, no. 2, pp. 100–103, 2010. [CrossRef]
- S. Gurung, D. Perocheau, L. Touramanidou, and J. Baruteau, “The exosome journey: from biogenesis to uptake and intracellular signalling,” Cell Commun. Signal., vol. 19, no. 1, p. 47, 2021. [CrossRef]
- H. Chen et al., “Exosomes, a New Star for Targeted Delivery ,” Frontiers in Cell and Developmental Biology , vol. 9. 2021, [Online]. Available: https://www.frontiersin.org/articles/10.3389/fcell.2021.751079. [CrossRef]
- K. Herrmann, M. J. A. Wood, and G. Fuhrmann, “Extracellular vesicles as a next-generation drug delivery platform,” Nat. Nanotechnol., vol. 16, no. 7, pp. 748–759, 2021. [CrossRef]
- P.-U. C. Dinh et al., “Inhalation of lung spheroid cell secretome and exosomes promotes lung repair in pulmonary fibrosis,” Nat. Commun., vol. 11, no. 1, p. 1064, 2020. [CrossRef]
- S. Shapira, D. Kazanov, S. Weisblatt, A. Starr, N. Arber, and S. Kraus, “The CD24 Protein Inducible Expression System Is an Ideal Tool to Explore the Potential of CD24 as an Oncogene and a Target for Immunotherapy in vitro and in vivo,” J. Biol. Chem., vol. 286, no. 47, pp. 40548–40555, Nov. 2011. [CrossRef]
- Wick, A. Backovic, E. Rabensteiner, N. Plank, C. Schwentner, and R. Sgonc, “The immunology of fibrosis: innate and adaptive responses,” Trends Immunol., vol. 31, no. 3, pp. 110–119, Mar. 2010. [CrossRef]
- L. Zhang, Y. Wang, G. Wu, W. Xiong, W. Gu, and C.-Y. Wang, “Macrophages: friend or foe in idiopathic pulmonary fibrosis?,” Respir. Res., vol. 19, no. 1, p. 170, 2018. [CrossRef]
- K. Tsuchiya et al., “Macrophage Mannose Receptor CD206 Predicts Prognosis in Community-acquired Pneumonia,” Sci. Rep., vol. 9, no. 1, p. 18750, 2019. [CrossRef]
- K. M. Roach et al., “Evaluation of Pirfenidone and Nintedanib in a Human Lung Model of Fibrogenesis,” Frontiers in Pharmacology, vol. 12. 2021, [Online]. Available: https://www.frontiersin.org/articles/10.3389/fphar.2021.679388. [CrossRef]
- J. A. Ardura, G. Rackov, E. Izquierdo, V. Alonso, A. R. Gortazar, and M. M. Escribese, “Targeting Macrophages: Friends or Foes in Disease? ,” Frontiers in Pharmacology , vol. 10. 2019, [Online]. Available: https://www.frontiersin.org/articles/10.3389/fphar.2019.01255. [CrossRef]
- Singh et al., “Nanoparticle targeting of de novo profibrotic macrophages mitigates lung fibrosis,” Proc. Natl. Acad. Sci., vol. 119, no. 15, p. e2121098119, Apr. 2022. [CrossRef]
- M. Patel et al., “The Immunopathobiology of SARS-CoV-2 Infection,” FEMS Microbiol. Rev., vol. 45, no. 6, p. fuab035, Nov. 2021. [CrossRef]
- R. I. Fox, “Mechanism of action of hydroxychloroquine as an antirheumatic drug,” Semin. Arthritis Rheum., vol. 23, no. 2, Supplement 1, pp. 82–91, 1993. [CrossRef]
- Y. S. Chhonker, S. Kanvinde, R. Ahmad, A. B. Singh, D. Oupický, and D. J. Murry, “Simultaneous Quantitation of Lipid Biomarkers for Inflammatory Bowel Disease Using LC–MS/MS,” Metabolites , vol. 11, no. 2. 2021. [CrossRef]
- S. S. Kesharwani et al., “Site-directed non-covalent polymer-drug complexes for inflammatory bowel disease (IBD): Formulation development, characterization and pharmacological evaluation,” J. Control. Release, vol. 290, pp. 165–179, 2018. [CrossRef]
- S. Kanvinde et al., “Pharmacokinetics and efficacy of orally administered polymeric chloroquine as macromolecular drug in the treatment of inflammatory bowel disease,” Acta Biomater., vol. 82, pp. 158–170, Dec. 2018. [CrossRef]
- S. Ali, M. G. Alrashedi, O. A. Ahmed, and I. M. Ibrahim, “Pulmonary Delivery of Hydroxychloroquine Nanostructured Lipid Carrier as a Potential Treatment of COVID-19,” Polymers, vol. 14, no. 13. 2022. [CrossRef]
- X. Bai et al., “Inhaled siRNA nanoparticles targeting IL11 inhibit lung fibrosis and improve pulmonary function post-bleomycin challenge,” Sci. Adv., vol. 8, no. 25, p. eabn7162, Feb. 2023. [CrossRef]
- K. Y. Fung et al., “Emerging roles for IL-11 in inflammatory diseases,” Cytokine, vol. 149, p. 155750, 2022. [CrossRef]
- D. M. Walters and S. R. Kleeberger, “Mouse Models of Bleomycin-Induced Pulmonary Fibrosis,” Curr. Protoc. Pharmacol., vol. 40, no. 1, pp. 5.46.1-5.46.17, Mar. 2008. [CrossRef]
- V Raveendran, R. Jayadevan, and S. Sashidharan, “Long COVID: An overview,” Diabetes Metab. Syndr. Clin. Res. Rev., vol. 15, no. 3, pp. 869–875, 2021. [CrossRef]
- S. Lopez-Leon et al., “More than 50 long-term effects of COVID-19: a systematic review and meta-analysis,” Sci. Rep., vol. 11, no. 1, p. 16144, 2021. [CrossRef]
- R. L. Levine, “Addressing the Long-term Effects of COVID-19,” JAMA, vol. 328, no. 9, pp. 823–824, Sep. 2022. [CrossRef]
- M. Suran, “Autopsies Reveal Lung Damage Patterns From COVID-19,” JAMA, vol. 326, no. 24, p. 2463, Dec. 2021. [CrossRef]
- F. McGroder et al., “Pulmonary fibrosis 4 months after COVID-19 is associated with severity of illness and blood leucocyte telomere length,” Thorax, vol. 76, no. 12, pp. 1242 LP – 1245, Dec. 2021. [CrossRef]
- E. Bazdyrev, P. Rusina, M. Panova, F. Novikov, I. Grishagin, and V. Nebolsin, “Lung Fibrosis after COVID-19: Treatment Prospects,” Pharmaceuticals, vol. 14, no. 8. 2021. [CrossRef]
- J. Hama Amin et al., “Post COVID-19 pulmonary fibrosis; a meta-analysis study,” Ann. Med. Surg., vol. 77, p. 103590, 2022. [CrossRef]
- Mohammadi et al., “Post-COVID-19 Pulmonary Fibrosis,” Cureus, vol. 14, no. 3, p. e22770, 2022. [CrossRef]
- T. A. Wynn, “Integrating mechanisms of pulmonary fibrosis,” J. Exp. Med., vol. 208, no. 7, pp. 1339–1350, Jul. 2011. [CrossRef]
- S. Kanvinde, T. Kulkarni, S. Deodhar, D. Bhattacharya, and A. Dasgupta, “Non-Viral Vectors for Delivery of Nucleic Acid Therapies for Cancer,” BioTech, vol. 11, no. 1. 2022. [CrossRef]
- S. Soares, J. Sousa, A. Pais, and C. Vitorino, “Nanomedicine: Principles, Properties, and Regulatory Issues ,” Frontiers in Chemistry , vol. 6. 2018, [Online]. Available: https://www.frontiersin.org/articles/10.3389/fchem.2018.00360. [CrossRef]
- J. Ren et al., “Precision Nanomedicine Development Based on Specific Opsonization of Human Cancer Patient-Personalized Protein Coronas,” Nano Lett., vol. 19, no. 7, pp. 4692–4701, Jul. 2019. [CrossRef]
- C. Velino et al., “Nanomedicine Approaches for the Pulmonary Treatment of Cystic Fibrosis ,” Frontiers in Bioengineering and Biotechnology , vol. 7. 2019, [Online]. Available: https://www.frontiersin.org/articles/10.3389/fbioe.2019.00406. [CrossRef]
- M. Skibba, A. Drelich, M. Poellmann, S. Hong, and A. R. Brasier, “Nanoapproaches to Modifying Epigenetics of Epithelial Mesenchymal Transition for Treatment of Pulmonary Fibrosis ,” Frontiers in Pharmacology , vol. 11. 2020, [Online]. Available: https://www.frontiersin.org/articles/10.3389/fphar.2020.607689. [CrossRef]
- C.-Y. Loo and W.-H. Lee, “Nanotechnology-based therapeutics for targeting inflammatory lung diseases,” Nanomedicine, vol. 17, no. 12, pp. 865–879, Mar. 2022. [CrossRef]
- E. S. White, M. Thomas, S. Stowasser, and K. Tetzlaff, “Challenges for Clinical Drug Development in Pulmonary Fibrosis ,” Frontiers in Pharmacology , vol. 13. 2022, [Online]. Available: https://www.frontiersin.org/articles/10.3389/fphar.2022.823085. [CrossRef]
- R. J. Kaner et al., “Design of Idiopathic Pulmonary Fibrosis Clinical Trials in the Era of Approved Therapies,” Am. J. Respir. Crit. Care Med., vol. 200, no. 2, pp. 133–139, Apr. 2019. [CrossRef]
- S. Harari and A. Caminati, “Idiopathic pulmonary fibrosis: from clinical trials to real-life experiences,” Eur. Respir. Rev., vol. 24, no. 137, pp. 420 LP – 427, Sep. 2015. 2015. [CrossRef]
- L. Lancaster et al., “Safety and survival data in patients with idiopathic pulmonary fibrosis treated with nintedanib: pooled data from six clinical trials,” BMJ Open Respir. Res., vol. 6, no. 1, p. e000397, Mar. 2019. [CrossRef]
- S. Hua, M. B. C. de Matos, J. M. Metselaar, and G. Storm, “Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization ,” Frontiers in Pharmacology , vol. 9. 2018, [Online]. Available: https://www.frontiersin.org/articles/10.3389/fphar.2018.00790. [CrossRef]
- S. Đorđević et al., “Current hurdles to the translation of nanomedicines from bench to the clinic,” Drug Deliv. Transl. Res., vol. 12, no. 3, pp. 500–525, 2022. [CrossRef]
| Clinical Trial ID | Title | Intervention | Sponsor |
|---|---|---|---|
| NCT04607928 | Phase-II Randomized Clinical Trial to Evaluate the Effect of Pirfenidone Compared to Placebo in Post-COVID19 | Drug: Pirfenidone Drug: Placebo |
Institut d'Investigació Biomèdica de Bellvitge |
| NCT04818489 | Impact of Colchicine on the Clinical Outcome of COVID-19 and the Development of Post-COVID-19 Pulmonary Fibrosis: Randomized Controlled Clinical Trial | Drug: Colchicine 0.5 mg Other: the standard protocol only |
ClinAmygate |
| NCT04551781 | Short Term Low Dose Corticosteroids for Management of Post Covid-19 Pulmonary Fibrosis | Drug: 20 mg Prednisone for 14 days Drug: control |
South Valley University |
| NCT05648734 | Impact of Anti-Inflammatory and Anti-Fibrotic Drugs on Post-acute COVID-19 Pulmonary Fibrosis | Drug: Corticosteroids alone Drug: Corticosteroids + Colchicine Drug: Corticosteroids + Pirfenidone Drug: Corticosteroids + Colchicine + Pirfenidone for ≥ 14 day |
Mansoura University |
| NCT04279197 | Efficacy and Safety of Fuzheng Huayu Tablets in Post-COVID-19 Patients with Pulmonary Inflammation and Fibrosis: A Multicenter Double-blind Randomized Controlled Trial | Drug: Fuzheng Huayu Tablet Drug: Vitamin C tablets Drug: Placebo Other: respiratory function rehabilitation training |
ShuGuang Hospital |
| NCT04541680 | Nintedanib for the Treatment of SARS-Cov-2 Induced Pulmonary Fibrosis" | Drug: Nintedanib 150 mg Other: Placebo |
Assistance Publique - Hôpitaux de Paris |
| NCT04856111 | A Study of the Efficacy and Safety of Pirfenidone vs. Nintedanib in the Treatment of Fibrotic Lung Disease After Coronavirus Disease-19 |
Drug: Pirfenidone Drug: Nintedanib |
Postgraduate Institute of Medical Education and Research |
| NCT04948203 | SECOVID: A Multi-center, Randomized, Dose-ranging Parallel-group Trial Assessing the Efficacy of Sirolimus in Hospitalized Patients With COVID-19 Pneumonia for the Prevention of Post-COVID Fibrosis |
Drug: Sirolimus | University of Chicago |
| NCT05387239 | Safety and Effectiveness of EV-Pure + WJ-Pure Treatment on Pulmonary Fibrosis Secondary to Covid-19 |
Drug: EV-Pure™ and WJ-Pure™ plus standard care Drug: Placebo (Saline plus standard care) |
Vitti Labs, LLC |
| NCT04645368 | Multicenter, Open-label Prospective Cohort Study of the Efficacy and Safety of the Inclusion of Longidaze in the Prevention and Treatment of Post-inflammatory Pulmonary Fibrosis and Interstitial Lung Diseases Caused by COVID-19 | Drug: bovhyaluronidase azoxymer | NPO Petrovax |
| NCT04805086 | Phase I/II MONACO Cell Therapy Study: Monocytes as an Anti-fibrotic Treatment After COVID-19 | Biological: MON002 | Guy's and St Thomas' NHS Foundation Trust |
| NCT04912011 | The Use of a Mineralocorticoid Receptor Antagonist (Spironolactone) in the Treatment of Pulmonary Fibrosis Associated With SARS-CoV-2 Infection | Drug: Canrenoate Potassium Drug: Normal Saline |
Pomeranian Medical University Szczecin |
| NCT04338802 | Efficacy and Safety of Nintedanib Ethanesulfonate Soft Capsule in the Treatment of Pulmonary Fibrosis in Patients with Moderate to Severe COVID-9(COVID 19) : a Single-center, Randomized, Placebo-controlled Study | Drug: Nintedanib 150 mg Other: Placebo |
Tongji Hospital |
| NCT04619680 | Early Nintedanib Deployment in COVID-19 Interstitial Lung Disease | Drug: Nintedanib Drug: Placebo |
Icahn School of Medicine at Mount Sinai |
| NCT04482595 | A Phase 2 Study of BIO 300 Oral Suspension in Discharged COVID-19 Patients | Drug: BIO 300 Oral Suspension Drug: Placebo |
Humanetics Corporation |
| NCT04537130 | Phase Ib Controlled Exploratory Trial for Treatment of Fibrosing Interstitial Lung Disease Patients Secondary to SARS-CoV-2 Infection with IN01 Vaccine (COVINVAC) | Biological: IN01 vaccine | Instituto Oncológico |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





