Submitted:
29 March 2023
Posted:
30 March 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Auditory Stream Segregation and Hearing Assistive Technology
Previous Studies on SPIN Performance in the ASD Population
Previous HAT Studies in the ASD Population
Overview of the Present Study
Methods
Participants
Target Measures
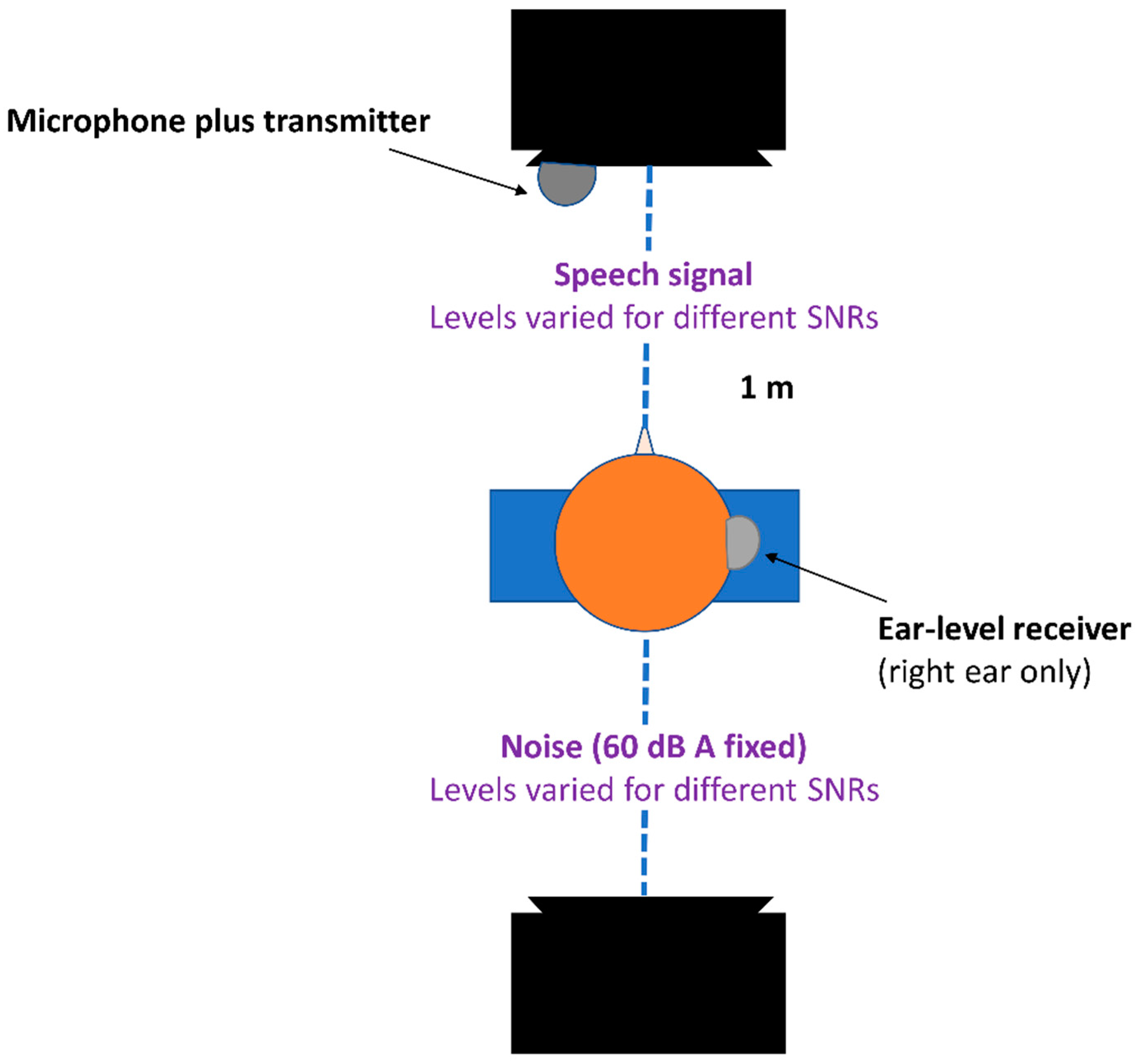
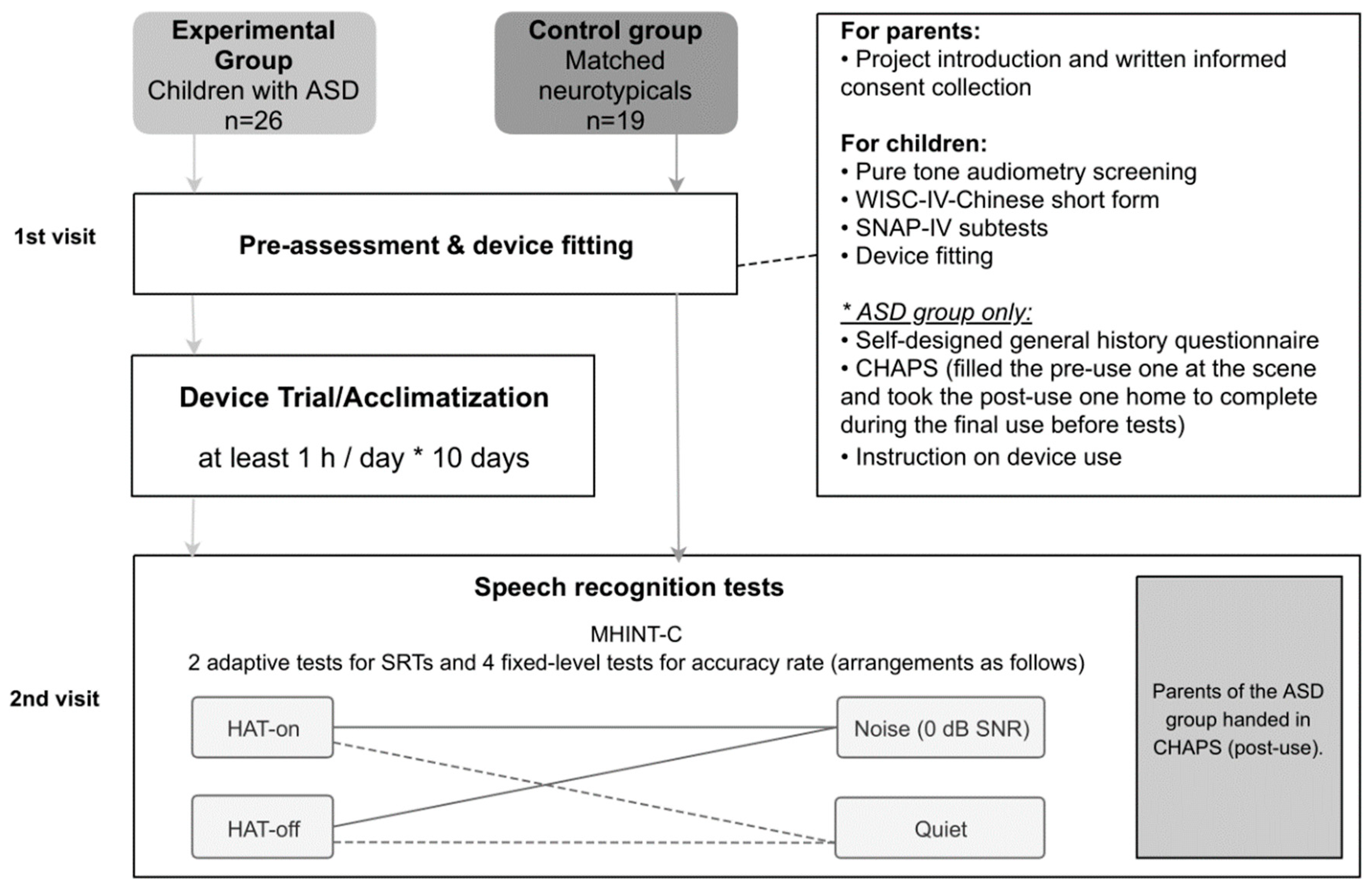
Experimental Procedures
Data Analysis
Results
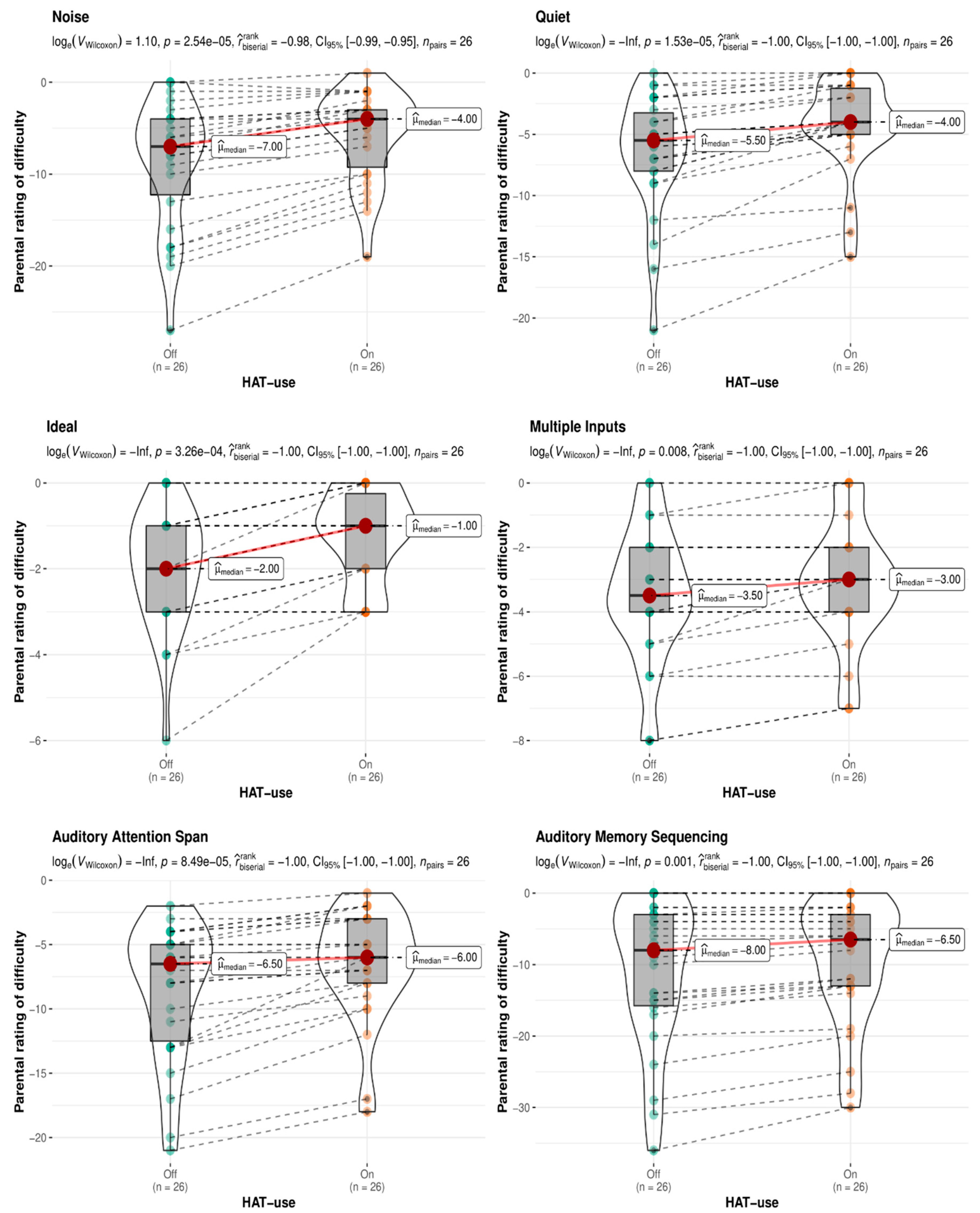
Impact of HAT Use on Behavioral Ratings of Listening Difficulties
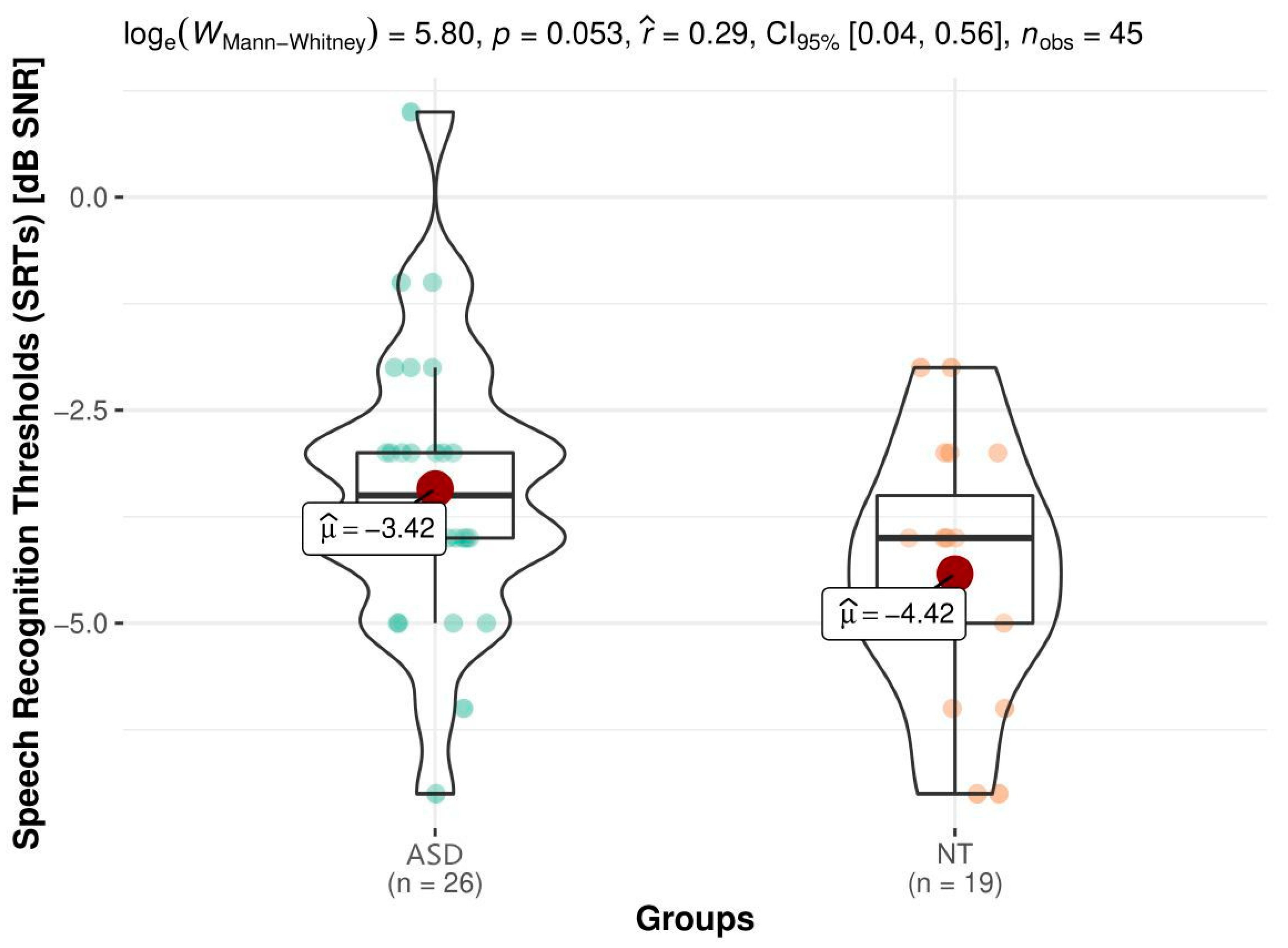
Baseline SPIN Data
Impact of HAT on SPIN Accuracy
Discussion
SPIN Issues under Energetic Maskers
Effectiveness of HAT
Limitations
Conclusion
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alcántara, J. I., Cope, T. E., Cope, W., & Weisblatt, E. J. (2012). Auditory temporal-envelope processing in high-functioning children with autism spectrum disorder. Neuropsychologia, 50(7), 1235-1251. [CrossRef]
- Alcántara, J. I., Weisblatt, E. J., Moore, B. C., & Bolton, P. F. (2004). Speech-in-noise perception in high-functioning individuals with autism or Asperger's syndrome. Journal of Child Psychology and Psychiatry, 45(6), 1107-1114. [CrossRef] [PubMed]
- American Academy of Audiology. (2011). Supplement B: Classroom audio distribution systems – Selection and verification.
- American National Standard. (2010). Acoustical performance criteria, design requirements, and guidelines for schools.
- American National Standards Institute. (1996). Specifications for audiometers (ANSI S3.6-1996). American National Standards Institute.
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). American Psychiatric Publishing.
- Arunachalam, S., & Luyster, R. J. (2016). The integrity of lexical acquisition mechanisms in autism spectrum disorders: A research review. Autism Research, 9(8), 810-828. [CrossRef] [PubMed]
- Ashburner, J., Rodger, S., & Ziviani, J. (2008). Sensory processing and classroom emotional, behavioural, and educational outcomes in children with autism spectrum disorder. American Journal of Occupational Therapy, 62(5), 564-573. [CrossRef] [PubMed]
- Benevides, T. W., & Lane, S. J. (2015). A review of cardiac autonomic measures: Considerations for examination of physiological response in children with autism spectrum disorder. Journal of autism and developmental disorders, 45(2), 560–575. [CrossRef] [PubMed]
- Benitez-Barrera, C. R., Skoe, E., Huang, J., & Tharpe, A. M. (2022). Evidence for a musician speech-perception-in-noise advantage in school-age children. Journal of Speech, Language, and Hearing Research, 65(10), 3996-4008. [CrossRef] [PubMed]
- Bhatara, A., Babikian, T., Laugeson, E., Tachdjian, R., & Sininger, Y. S. (2013). Impaired timing and frequency discrimination in high-functioning autism spectrum disorders. Journal of Autism and Developmental Disorders, 43(10), 2312-2328. [CrossRef] [PubMed]
- BKB-SIN. (2005). Bamford-Kowal-Bench speech in noise test. Elk Grove, IL: Etymotic Research.
- Brattberg, G. (2003). Enastående: självbiografisk berättelse om livet med högfungerande autism (Exceptional: autobiographical story of life with high functioning autism) (2nd ed. ed.). Värkstaden AB.v.
- Chandni, J., Vipin Ghosh, P. G., Chetak, K. B., & Aishwarya, L. (2020, Dec). Maturation of speech perception in noise abilities during adolescence. International Journal of Pediatric Otorhinolaryngology, 139, 110459. [CrossRef] [PubMed]
- Chen, F., Zhou, J., Wang, Y., Yu, K., Arshad, S., Khawaji, A., & Conway, D. (2016). Robust Multimodal Cognitive Load Measurement. Springer International Publishing.
- Chen, Y., & Wong, L. L. N. (2020). Development of the mandarin hearing in noise test for children. International Journal of Audiology, 1-6. [CrossRef] [PubMed]
- China Ministry of Education. (2020, October 7). Overview of educational achievements in China in 2019. Retrieved January 25 from http://en.moe.gov.cn/documents/reports/202102/t20210209_513095.html.
- Cooperative Research Centre for Living with Autism. (2018). Australian autism educational needs analysis – What are the needs of schools, parents and students on the autism spectrum? https://www.autismcrc.com.au/australian-education-needsanalysis.
- Crane, L., Goddard, L., & Pring, L. (2009). Sensory processing in adults with autism spectrum disorders. Autism, 13(3), 215-228. [CrossRef]
- Danneels, M., Degeest, S., Dhooge, I., & Keppler, H. (2021). Central auditory processing and listening effort in normal-hearing children: A pilot study. International Journal of Audiology, 60(10), 739-746. [CrossRef]
- Davis, D. (1991). Utilizing amplification devices in the regular classroom. Hearing Instruments, 42(7), 18-23.
- DePape, A. M., Hall, G. B., Tillmann, B., & Trainor, L. J. (2012). Auditory processing in high-functioning adolescents with autism spectrum disorder. PLoS One, 7(9). [CrossRef]
- Dockrell, J. E., & Shield, B. (2012). The impact of sound-field systems on learning and attention in elementary school classrooms. Journal of Speech, Language, and Hearing Research, 55(4), 1163-1176. [CrossRef] [PubMed]
- Dryden, A., Allen, H. A., Henshaw, H., & Heinrich, A. (2017). The association between cognitive performance and speech-in-noise perception for adult listeners: A systematic literature review and meta-Analysis. Trends in Hearing, 21, 2331216517744675. [CrossRef] [PubMed]
- Edgar, J. C., Blaskey, L., Green, H. L., Konka, K., Shen, G., Dipiero, M. A., Berman, J. I., Bloy, L., Liu, S., McBride, E., Ku, M., Kuschner, E. S., Airey, M., Kim, M., Franzen, R. E., Miller, G. A., & Roberts, T. P. L. (2020). Maturation of auditory cortex neural activity in children and implications for auditory clinical markers in diagnosis. Frontiers in Psychiatry, 11, 584557. [CrossRef]
- Eigsti, I. M., & Fein, D. A. (2013). More is less: pitch discrimination and language delays in children with optimal outcomes from autism. Autism Research, 6(6), 605-613. [CrossRef] [PubMed]
- Elliott, L. L. (1979). Performance of children aged 9 to 17 years on a test of speech intelligibility in noise using sentence material with controlled word predictability. Journal of the Acoustical Society of America, 66(3), 651–653.
- Elsabbagh, M., Divan, G., Koh, Y. J., Kim, Y. S., Kauchali, S., Marcin, C., Montiel-Nava, C., Patel, V., Paula, C. S., Wang, C., Yasamy, M. T., & Fombonne, E. (2012). Global prevalence of autism and other pervasive developmental disorders. Autism Research, 5(3), 160-179. [CrossRef] [PubMed]
- Feldman, J. I., Thompson, E., Davis, H., Keceli-Kaysili, B., Dunham, K., Woynaroski, T., Tharpe, A. M., & Picou, E. M. (2022). Remote microphone systems can improve listening-in-noise accuracy and listening effort for youth with autism. Ear and Hearing, 43(2), 436-447. [CrossRef] [PubMed]
- Foxe, J. J., Molholm, S., Del Bene, V. A., Frey, H. P., Russo, N. N., Blanco, D., Saint-Amour, D., & Ross, L. A. (2015). Severe multisensory speech integration deficits in high-functioning school-aged children with Autism Spectrum Disorder and their resolution during early adolescence. Cerebral Cortex, 25(2), 298-312. [CrossRef] [PubMed]
- Gau, S. S., Lin, C. H., Hu, F. C., Shang, C. Y., Swanson, J. M., Liu, Y. C., & Liu, S. K. (2009). Psychometric properties of the Chinese version of the Swanson, Nolan, and Pelham, Version IV Scale-Teacher Form. Journal of Pediatric Psychology, 34(8), 850-861. [CrossRef]
- Groen, W. B., van Orsouw, L., Huurne, N. t., Swinkels, S., van der Gaag, R.-J., Buitelaar, J. K., & Zwiers, M. P. (2009). Intact spectral but abnormal temporal processing of auditory stimuli in autism. Journal of Autism and Developmental Disorders, 39(5), 742-750. [CrossRef]
- Hornickel, J., Chandrasekaran, B., Zecker, S., & Kraus, N. (2011). Auditory brainstem measures predict reading and speech-in-noise perception in school-aged children. Behavioural Brain Research, 216(2), 597-605. [CrossRef]
- Hornickel, J., Zecker, S. G., Bradlow, A. R., & Kraus, N. (2012). Assistive listening devices drive neuroplasticity in children with dyslexia. Proceedings of the National Academy of Sciences of the United States of America, 109(41), 16731-16736. [CrossRef]
- Hrabok, M., Brooks, B. L., Fay-McClymont, T. B., & Sherman, E. M. (2014). Wechsler Intelligence Scale for Children-fourth edition (WISC-IV) short-form validity: A comparison study in pediatric epilepsy. Child Neuropsychology, 20(1), 49-59. [CrossRef]
- Huang, A. X., Jia, M., & Wheeler, J. J. (2012). Children with autism in the People’s Republic of China: Diagnosis, legal issues, and educational services. Journal of Autism and Developmental Disorders, 43(9), 1991-2001. [CrossRef]
- Huang, D., Yu, L., Wang, X., Fan, Y., Wang, S., & Zhang, Y. (2018). Distinct patterns of discrimination and orienting for temporal processing of speech and nonspeech in Chinese children with autism: An event-related potential study. European Journal of Neuroscience, 47(6), 662-668. [CrossRef]
- James, P., Schafer, E., Wolfe, J., Matthews, L., Browning, S., Oleson, J., Sorensen, E., Rance, G., Shiels, L., & Dunn, A. (2022). Increased rate of listening difficulties in autistic children. Journal of Communication Disorders, 99, 106252. [CrossRef]
- Jiang, J., Liu, F., Wan, X., & Jiang, C. (2015). Perception of melodic contour and intonation in autism spectrum disorder: Evidence from Mandarin speakers. Journal of Autism and Developmental Disorders, 45(7), 2067-2075. [CrossRef]
- Jones, P. R., Moore, D. R., & Amitay, S. (2015). Development of auditory selective attention: Why children struggle to hear in noisy environments. Developmental Psychology, 51(3), 353-369. [CrossRef]
- Keith, W. J., & Purdy, S. C. (2014). Assistive and therapeutic effects of amplification for auditory processing disorder. Seminars in Hearing, 35(1), 27–38.
- Klatte, M., Hellbrück, J., Seidel, J., & Leistner, P. (2017). Effects of classroom acoustics on performance and well-being in elementary school children: A field study. Environment and Behavior, 42(5), 659-692. [CrossRef]
- Krug, D. A., Arick, J., & Almond, P. (1980). Behavior checklist for identifying severely handicapped individuals with high levels of autistic behavior. Journal of Child Psychology and Psychiatry, 21(3), 221-229. [CrossRef]
- Kuhl, P. K., Conboy, B. T., Coffey-Corina, S., Padden, D., Rivera-Gaxiola, M., & Nelson, T. (2008). Phonetic learning as a pathway to language: New data and native language magnet theory expanded. Philosophical Transactions of the Royal Society B: Biological Sciences, 363(1493), 979-1000. [CrossRef] [PubMed]
- Kujala, T., Kuuluvainen, S., Saalasti, S., Jansson-Verkasalo, E., Wendt, L. V., & Lepisto, T. (2010). Speech-feature discrimination in children with Asperger syndrome as determined with the multi-feature mismatch negativity paradigm. Clinical Neurophysiology, 121(9), 1410-1419. [CrossRef] [PubMed]
- Leekam, S. R., Nieto, C., Libby, S. J., Wing, L., & Gould, J. (2007). Describing the sensory abnormalities of children and adults with autism. Journal of Autism and Developmental Disorders, 37(5), 894-910. [CrossRef] [PubMed]
- Lenth, R. V. (2020). emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.5.3. https://CRAN.R-project.org/package=emmeans.
- Lepistö, T., Silokallio, S., Nieminen-von Wendt, T., Alku, P., Naatanen, R., & Kujala, T. (2006). Auditory perception and attention as reflected by the brain event-related potentials in children with Asperger syndrome. Clinical Neurophysiology, 117(10), 2161-2171. [CrossRef] [PubMed]
- Li, J., Zhong, J., Cai, L., Chen, Y., & Zhou, M. (2005). Clinical application of the Childhood Autism Rating Scale. Chinese Journal of Child Health Care, 13(3), 267-268. (in Chinese). [CrossRef]
- Li, L., Li, M., Lu, J., Ge, X., Xie, W., Wang, Z., Li, X., Li, C., Wang, X., Han, Y., Wang, Y., Zhong, L., Xiang, W., Huang, X., Chen, H., & Yao, P. (2018). Prenatal progestin exposure is associated with autism spectrum disorders. Frontiers in Psychiatry, 9, 611. [CrossRef] [PubMed]
- Li, Y., Xing, H., Zhang, L., Shu, H., & Zhang, Y. (2021). How visual word decoding and context-driven auditory semantic integration contribute to reading comprehension: A test of additive vs. multiplicative models. Brain Sciences, 11(7), 830. [CrossRef] [PubMed]
- Lo, C. Y., Looi, V., Thompson, W. F., & McMahon, C. M. (2020). Music training for children with sensorineural hearing loss improves speech-in-noise perception. Journal of Speech, Language, and Hearing Research, 63(6), 1990-2015. [CrossRef] [PubMed]
- Lord, C., Risi, S., Lambrecht, L., Cook, E. H., Jr, L., B. L., DiLavore, P. C., Pickles, A., & Rutter, M. (2000). The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30(3), 205–223.
- Lord, C., Rutter, M., DiLavore, P. C., Risi, S., Gotham, K., & Bishop, S. L. (2012). Autism Diagnostic Observation Schedule (2nd ed.). Western Psychological Services.
- Lord, C., Rutter, M., Goode, S., Heemsbergen, J., Jordan, H., Mawhood, L., & Schopler, E. (1989). Austism Diagnostic Observation Schedule: A standardized observation of communicative and social behavior. Journal of Autism and Developmental Disorders, 19, 185–212. [CrossRef]
- Lord, C., Rutter, M., & Le Couteur, A. (1994). Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24, 659–685.
- Lu, J., Yang, Z., Shu, M., & Su, L. (2004). Reliability, validity analysis of the Childhood Autism Rating Scale. China Journal of Modern Medicine, 14(13), 119–121. (in Chinese).
- Maamor, N., & Billings, C. J. (2017, Jan 1). Cortical signal-in-noise coding varies by noise type, signal-to-noise ratio, age, and hearing status. Neuroscience Letters, 636, 258-264. [CrossRef]
- Mair, K. R. (2013). Speech perception in autism spectrum disorder: Susceptibility to masking and interference [Dissertation, University College London]. London.
- Manfredi, M., Cohn, N., Sanchez Mello, P., Fernandez, E., & Boggio, P. S. (2020). Visual and verbal narrative comprehension in children and adolescents with autism spectrum disorders: An ERP study. Journal of Autism and Developmental Disorders, 50(8), 2658-2672. [CrossRef]
- Millman, R. E., & Mattys, S. L. (2017). Auditory verbal working memory as a predictor of speech perception in modulated maskers in listeners with normal hearing. Journal of Speech Language and Hearing Research, 60(5), 1236-1245.
- Nettelbeck, T., & Burns, N. R. (2010). Processing speed, working memory and reasoning ability from childhood to old age. Personality and Individual Differences, 48(4), 379-384. [CrossRef]
- Nilsson, M., Soli, S. D., & Sullivan, J. A. (1994). Development of the Hearing in Noise Test for the measurement of speech reception thresholds in quiet and in noise. Journal of the Acoustical Society of America, 95(2), 1085–1099. [CrossRef] [PubMed]
- Nittrouer, S., Krieg, L. M., & Lowenstein, J. H. (2018). Speech recognition in noise by children with and without dyslexia: How is it related to reading? Research in Developmental Disabilities, 77, 98-113.
- O'Connor, K. (2012). Auditory processing in autism spectrum disorder: A review. Neuroscience and Biobehavioral Reviews, 36(2), 836-854. [CrossRef]
- Palmer, C. M. (1998). Quantification of the ecobehavioral impact of a soundfield loudspeaker system in elementary classrooms. Journal of Speech, Language, and Hearing Research, 41, 819-833.
- Perrone-Bertolotti, M., Tassin, M., & Meunier, F. (2017). Speech-in-speech perception and executive function involvement. PLoS One, 12(7), e0180084. [CrossRef] [PubMed]
- Peterson, G. E., & Lehiste, I. (1962). Revised CNC lists for auditory tests. Journal of Speech and Hearing Disorders, 27(1), 62–70.
- Plaisted, K., Saksida, L., Alcantara, J., & Weisblatt, E. (2003). Towards an understanding of the mechanisms of weak central coherence effects: experiments in visual configural learning and auditory perception. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 358(1430), 375–386. [CrossRef] [PubMed]
- Pollack, I. (1975). Auditory informational masking. The Journal of the Acoustical Society of America, 57(S5). [CrossRef]
- R Core Team. (2020). R: A language and environment for statistical computing. https://www.R-project.org/.
- Rance, G., Chisari, D., Saunders, K., & Rault, J. L. (2017). Reducing listening-related stress in school-aged children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 47(7), 2010-2022. [CrossRef] [PubMed]
- Rance, G., Saunders, K., Carew, P., Johansson, M., & Tan, J. (2014). The use of listening devices to ameliorate auditory deficit in children with autism. Journal of Pediatrics, 164(2), 352-357. [CrossRef]
- Richards, C., Flexer, C., Brandy, W., & Wray, D. (1993). Signal-to-noise enhancing devices can improve kids' reading skills. Hearing Instruments, 44(11), 12-15.
- Schafer, E. C., Amlani, A. M., Paiva, D., Nozari, L., & Verret, S. (2011). A meta-analysis to compare speech recognition in noise with bilateral cochlear implants and bimodal stimulation. International Journal of Audiology, 50(12), 871-880. [CrossRef]
- Schafer, E. C., Bryant, D., Sanders, K., Baldus, N., Algier, K., Lewis, A., Traber, J., Layden, P., & Amin, A. ( 2014). Fitting and verification of frequency modulation systems on children with normal hearing. Journal of the American Academy of Audiology, 25(6), 529–540. [CrossRef]
- Schafer, E. C., Gopal, K. V., Mathews, L. A., Kaiser, K., Canale, E., & Creech, A. (2019). Verification and validation of remote-microphone technology on children and college-age adults who have autism spectrum disorder. Journal of Educational, Pediatric and (Re)Habilitative Audiology(24), 2-7.
- Schafer, E. C., Mathews, L., Mehta, S., Hill, M., Munoz, A., Bishop, R., & Moloney, M. (2013). Personal FM systems for children with autism spectrum disorders (ASD) and/or attention-deficit hyperactivity disorder (ADHD): An initial investigation. Journal of Communication Disorders, 46(1), 30-52. [CrossRef] [PubMed]
- Schafer, E. C., Traber, J., Layden, P., Amin, A., Sanders, K. H., Bryant, D., & Baldus, N. (2014). Use of wireless technology for children with auditory processing disorders, attention-deficit hyperactivity disorder, and language disorders. Seminars in Hearing, 35, 193-205. [CrossRef]
- Schafer, E. C., Wright, S., Anderson, C., Jones, J., Pitts, K., Bryant, D., Watson, M., Box, J., Neve, M., Mathews, L., & Reed, M. P. (2016). Assistive technology evaluations: Remote-microphone technology for children with autism spectrum disorder. Journal of Communication Disorders, 64, 1-17. [CrossRef] [PubMed]
- Schelinski, S., & Kriegstein, K. (2020). Brief report: Speech-in-noise recognition and the relation to vocal pitch perception in adults with autism spectrum disorder and typical development. Journal of Autism and Developmental Disorders, 50, 356-363. [CrossRef]
- Schopler, E., Reichler, R. J., DeVellis, R. F., & Daly, K. (1980). Toward objective classification of childhood autism: Childhood Autism Rating Scale (CARS). Journal of Autism and Developmental Disorders, 10(1), 91–103. [CrossRef]
- Seep, B., Glosemeyer, R., Hulce, E., Linn, M., & Aytar, P. (2000). Classroom acoustics I: A resource for creating learning environments with desirable listening conditions. Melville, NY: Acoustical Society of America.
- Shan, L., Feng, J. Y., Wang, T. T., Xu, Z. D., & Jia, F. Y. (2021). Prevalence and developmental profiles of Autism Spectrum Disorders in children with global developmental delay. Frontiers in Psychiatry, 12, 794238. [CrossRef]
- Slater, J., Skoe, E., Strait, D. L., O'Connell, S., Thompson, E., & Kraus, N. (2015). Music training improves speech-in-noise perception: Longitudinal evidence from a community-based music program. Behavioural Brain Research, 291, 244-252. [CrossRef] [PubMed]
- Slater, M. (2019). The 100 Largest China City Economies by GDP. Retrieved Jan 25 from https://www.chinacheckup.com/blog/china-city-economies.
- Smaldino, J. J., Crandell, C. C., Kreisman, B. M., John, A. B., & Kreisman, N. V. (2008). Room acoustics for listeners with normal hearing and hearing impairment. In M. Valente, H. Hosford-Dunn, & R. Roeser (Eds.), Audiology Treatment (pp. 418–451). New York, NY: Thieme Medical Publishers.
- Smits, C. (2017). Improving the efficiency of speech-in-noise hearing screening tests. Ear and Hearing, 38(6), e385-e388. [CrossRef]
- Smoski, W., Brunt, M. A., & Tannahill, J. C. (1998). Children’s auditory performance scale (CHAPS). Educational Audiology Association.
- Studebaker, G. A., Mcdaniel, D. M., & Sherbecoe, R. L. (1995). Evaluating relative speech recognition performance using the proficiency factor and rationalized arcsine differences. Journal of The American Academy of Audiology, 6(2), 173-182.
- Sun, X., Allison, C., Auyeung, B., Baron-Cohen, S., & Brayne, C. (2013). A review of healthcare service and education provision of Autism Spectrum Condition in mainland China. Research in Developmental Disabilities, 34(1), 469-479. [CrossRef]
- Sun, X., Allison, C., Matthews, F. E., Sharp, S. J., Auyeung, B., Baron-Cohen, S., & Brayne, C. (2013). Prevalence of autism in mainland China, Hong Kong and Taiwan: A systematic review and meta analysis. Molecular Autism, 4(7). [CrossRef]
- Talarico, M., Abdilla, G., Aliferis, M., Balazic, I., Giaprakis, I., Stefanakis, T., Foenander, K., Grayden, D. B., & Paolini, A. G. (2007). Effect of age and cognition on childhood speech in noise perception abilities. Audiology and Neuro-Otology, 12(1), 13-19. [CrossRef] [PubMed]
- Tharpe, A. M., Ricketts, T., & Sladen, D. P. (2004). FM systems for children with minimal to mild hearing loss. In D. Fabry & C. D. Johnson (Eds.), ACCESS: Achieving Clear Communication Employing Sound Solutions (pp. 191–197). Phonak AG.
- van der Kruk, Y., Wilson, W. J., Palghat, K., Downing, C., Harper-Hill, K., & Ashburner, J. (2017). Improved signal-to-noise ratio and classroom performance in children with autism spectrum disorder: A systematic review. Review Journal of Autism and Developmental Disorders, 4(3), 243-253. [CrossRef]
- Wang, X., Wang, S., Fan, Y., Huang, D., & Zhang, Y. (2017). Speech-specific categorical perception deficit in autism: An event-related potential study of lexical tone processing in Mandarin-speaking children. Scientific Reports, 7(1). [CrossRef] [PubMed]
- Wang, X., & Xu, L. (2021). Speech perception in noise: Masking and unmasking. Journal of Otology, 16(2), 109-119. [CrossRef] [PubMed]
- Ward, K. M., & Grieco-Calub, T. M. (2022). Age and hearing ability influence selective attention during childhood. Ear and Hearing, 43(4), 1125-1138. [CrossRef] [PubMed]
- Wilson, W. J., Harper-Hill, K., Armstrong, R., Downing, C., Perrykkad, K., Rafter, M., & Ashburner, J. (2021). A preliminary investigation of sound-field amplification as an inclusive classroom adjustment for children with and without Autism Spectrum Disorder. Journal of Communication Disorders, 93, 106142. [CrossRef] [PubMed]
- Wu, X., Yang, Z., Huang, Y., Chen, J., Li, L., Daneman, M., & Schneider, B. A. (2011). Cross-language differences in informational masking of speech by speech: English versus Mandarin Chinese. Journal of Speech, Language, and Hearing Research, 54(6), 1506-1524. [CrossRef] [PubMed]
- Yang, W., & Bradley, J. S. (2009). Effects of room acoustics on the intelligibility of speech in classrooms for young children. The Journal of the Acoustical Society of America, 125(2), 922-933. [CrossRef] [PubMed]
- Yang, X., Huang, L., Jia, M., & Chen, S. (1993). Validation study of the Chinese version of Autism Behavior Checklist. Chinese Mental Health Journal(7), 279-280. (in Chinese).
- Yu, L., Fan, Y., Deng, Z., Huang, D., Wang, S., & Zhang, Y. (2015). Pitch processing in tonal-language-speaking children with autism: an event-related potential study. Journal of Autism and Developmental Disorders, 45(11), 3656-3667. [CrossRef]
- Yu, L., Huang, D., Wang, S., Wu, X., Chen, Y., & Zhang, Y. (2021). Evidence of altered cortical processing of dynamic lexical tone pitch contour in Chinese children with autism. Neuroscience Bulletin, 37(11), 1605-1608. [CrossRef]
- Yu, Y. H., & Shafer, V. L. (2020). Behavioral and Neurophysiological Evidence of Speech Processing in Chinese-Speaking Individuals with Autism Spectrum Disorder: A Review and Future Directions. In H. M. Liu, F. M. Tsao, & P. Li (Eds.), Speech Perception, Production and Acquisition: Multidisciplinary approaches in Chinese languages (pp. 243-279). Singapore: Springer Singapore.
- Zhang, H. (2009). The revision of WISC-IV Chinese version. Psychological Science, 32(5), 1177-1179. [CrossRef]
- Zhang, J., Meng, Y., Tong, X., Yuan, Z., Wu, C., & Ieong, S. L. (2018). Exploring the neural correlates of lexical stress perception in english among Chinese-English bilingual children with autism spectrum disorder: An ERP study. Neuroscience Letters, 666, 158-164. [CrossRef] [PubMed]
- Zhou, H., Xu, X., Yan, W., Zou, X., Wu, L., Luo, X., Li, T., Huang, Y., Guan, H., Chen, X., Mao, M., Xia, K., Zhang, L., Li, E., Ge, X., Zhang, L., Li, C., Zhang, X., Zhou, Y., Ding, D., Shih, A., Fombonne, E., Zheng, Y., Han, J., Sun, Z., Jiang, Y. H., Wang, Y., & Team, L.-N. S. (2020). Prevalence of autism spectrum disorder in China: A nationwide multi-center population-based study among children aged 6 to 12 years. Neuroscience Bulletin. [CrossRef] [PubMed]
| ASD (n=26) | NT (n=19) | t value | p1 | |
|---|---|---|---|---|
| Age(year) | 6.8-12.1(8.81±1.70) | 6.6-11.2(8.42±1.39) | 1.03 | 0.31 |
| Sex (M/F) | 23/3 | 17/2 | / | 0.71 |
| IQ | 70-130(103.62±18.67) | 96-137(121.16±11.33) | -3.77 | 0.0005 |
| SNAP-IV-sub 1 | 5-13(7.8±3.1) | 6-9(6.9±2.3) | 1.87 | 0.57 |
| SNAP-IV-sub 2 | 4-12(7.5±2.5) | 4-11(6.3±2.1) | 1.63 | 0.60 |
| Inclusion criteria | Exclusion criteria | |
| • Aged 6~12 years • Native speakers of Mandarin • Right-handedness • Normal pure-tone audibility • IQ ≥70 in the WISC-Ⅳ-Chinese short form • Able to iterate simple sentences |
• With cerebral injuries, visual impairments, hearing loss, and comorbid diseases • Having psychotropic medication within three months before the project • Unaccustomed to sustained use of HAT • Clinically significant symptoms of inattention and hyperactivity |
|
| ASD group | NT group | |
| Children meeting the latest diagnostic criteria for ASD | NT children matched by age and gender | |
| Formula code: Accuracy ~ Group+ Listening condition + IQ + Group:Listening condition + Group:IQ + Listening condition:IQ + (1|Subeject) | |||||
| Sentence recognition accuracy (RAU) | |||||
| Fixed effect | Estimate | SE | 95% CI | t value(df = 81) | p value |
| (Intercept) | 3.61 | 1.01 | 1.48 – 5.74 | 3.37 | 0.001 |
| Group: NT | 10.28 | 2.36 | 5.57 – 14.99 | 4.35 | <0.001 |
| Listening condition: Noise | -4.59 | 0.77 | -6.13 – -3.06 | -5.94 | <0.001 |
| IQ | 0.05 | 0.01 | 0.03 – 0.07 | 5.23 | <0.001 |
| Group: NT*Listening condition: Noise | 0.96 | 0.27 | 0.41 – 1.51 | 3.49 | 0.001 |
| Group: NT*IQ | -0.08 | 0.02 | -0.12 – -0.05 | -4.31 | <0.001 |
| Listening condition: Noise*IQ | 0.03 | 0.01 | 0.01 – 0.04 | 3.79 | <0.001 |
| ICC | 0.68 | ||||
| N SUBJ | 45 | ||||
| Observations | 90 | ||||
| Marginal R2 / Conditional R2 | 0.684 / 0.898 | ||||
| Effect | F-ratio | df | p value | η²p |
|---|---|---|---|---|
| Group | 20.77 | 1.07, 41 | <0.001 | 0.34 |
| Listening Condition | 24.09 | 1.05, 42 | < 0.001 | 0.36 |
| IQ | 6.22 | 1.08, 41 | < 0.05 | 0.13 |
| Group × Listening Condition | 12.19 | 1.05, 42 | <0.01 | 0.23 |
| IQ × Group | 18.15 | 1.07, 41 | <0.001 | 0.30 |
| IQ× Listening Condition | 14.36 | 1.05, 42 | <0.001 | 0.25 |
| Pairwise contrasts | Estimate | SE | df | t value | p value | |
|---|---|---|---|---|---|---|
| Quiet ASD – Noise ASD | 1.49 | 0.16 | 42 | 9.08 | <0.001 | |
| Quiet ASD – Quiet NT | -0.77 | 0.37 | 54 | -2.10 | 0.24 | |
| Quiet ASD – Noise NT | -0.24 | 0.36 | 51 | -0.67 | 1.00 | |
| Noise ASD – Quiet NT | -2.26 | 0.36 | 51 | -6.27 | <0.001 | |
| Noise ASD – Noise NT | -1.73 | 0.37 | 54 | -4.71 | <0.001 | |
| Quiet NT – Noise NT | 0.53 | 0.19 | 42 | 2.69 | 0.06 | |
| Estimated marginalmeans | SE | df | 95% CI | |||
| Quiet ASD | 9.56 | 0.21 | 55.8 | 9.15 – 9.98 | ||
| Noise ASD | 8.07 | 0.21 | 55.8 | 7.65 – 8.49 | ||
| Quiet NT | 10.33 | 0.29 | 50.8 | 9.73 – 10.93 | ||
| Noise NT | 9.80 | 0.30 | 50.8 | 9.20 – 10.40 | ||
| Interactions | Trend | SE | 95% CI | t value | p value |
|---|---|---|---|---|---|
| ASD*IQ | 0.07 | 0.01 | 0.05 – 0.09 | 7.07 | < 0.0001 |
| NT*IQ | -0.02 | 0.02 | -0.05 – 0.02 | -1.04 | 0.31 |
| Quiet*IQ | 0.01 | 0.01 | -0.01 – 0.03 | 1.02 | 0.31 |
| Noise*IQ | 0.04 | 0.01 | 0.02 – 0.06 | 3.66 | < 0.001 |
| Pairwise contrasts | Estimate | SE | t value | p value | |
| ASD*IQ – NT*IQ | 0.09 | 0.02 | 4.31 | < 0.0001 | |
| Quiet*IQ – Noise*IQ | -0.03 | 0.007 | -3.79 | < 0.001 | |
| Formula code: Accuracy ~ Group+ HAT-use + IQ + Group:HAT-use + Group:IQ + HAT-use:IQ + (1|Subeject) | |||||
| Sentence recognition accuracy (RAU) | |||||
| Fixed effect | Estimate | SE | 95% CI | t value | p value |
| (Intercept) | -0.94 | 1.15 | -3.23 – 1.35 | -0.82 | 0.42 |
| Group: NT | 11.02 | 2.52 | 6.02 – 16.03 | 4.38 | <0.001 |
| HAT-use: On | 4.81 | 0.89 | 3.04 – 6.58 | 5.40 | <0.001 |
| IQ | 0.08 | 0.01 | 0.06 – 0.10 | 7.37 | <0.001 |
| Group: NT*HAT-use: On | -1.28 | 0.32 | -1.91 – -0.64 | -4.02 | <0.001 |
| Group: NT*IQ | -0.08 | 0.02 | -0.12 – -0.04 | -3.97 | <0.001 |
| HAT-use: On*IQ | -0.03 | 0.01 | -0.04 – -0.01 | -3.00 | 0.004 |
| ICC | 0.63 | ||||
| N SUBJ | 45 | ||||
| Observations | 90 | ||||
| Marginal R2 / Conditional R2 | 0.671 / 0.878 | ||||
| Effect | F-ratio | df | p value | η²p |
|---|---|---|---|---|
| Group | 17.12 | 1.07, 41 | <0.001 | 0.29 |
| HAT-use | 18.67 | 1.05, 42 | < 0.001 | 0.32 |
| IQ | 6.31 | 1.07, 41 | < 0.05 | 0.12 |
| Group × HAT-use | 16.18 | 1.05, 42 | <0.001 | 0.28 |
| HAT-use × IQ | 8.98 | 1.05, 42 | <0.01 | 0.18 |
| Group × IQ | 15.73 | 1.07, 41 | <0.001 | 0.26 |
| Pairwise contrasts | Estimate | SE | df | t value | p value | |
|---|---|---|---|---|---|---|
| ASD off – ASD on | -1.98 | 0.18 | 42.0 | -10.48 | <0.001 | |
| NT off – ASD on | -0.26 | 0.39 | 52.3 | -0.68 | 1.000 | |
| NT off – NT on | -0.71 | 0.23 | 42.0 | -3.12 | <0.05 | |
| Estimated marginalmeans | SE | df | 95% CI | |||
| ASD off | 8.07 | 0.22 | 58.1 | 7.62 – 8.51 | ||
| ASD on | 10.05 | 0.22 | 52.5 | 9.06 – 10.50 | ||
| NT off | 9.79 | 0.31 | 50.8 | 9.14 – 10.43 | ||
| NT on | 10.50 | 0.32 | 52.8 | 9.85 – 11.14 | ||
| Interactions | Trend | SE | 95% CI | t value | p value | ||||
|---|---|---|---|---|---|---|---|---|---|
| HAT-off*IQ | 0.04 | 0.01 | 0.02 – 0.06 | 3.45 | 0.001 | ||||
| HAT-on*IQ | 0.01 | 0.01 | -0.009 – 0.03 | 1.21 | 0.23 | ||||
| ASD*IQ | 0.07 | 0.01 | 0.05 – 0.09 | 6.73 | < 0.001 | ||||
| NT*IQ | -0.02 | 0.02 | -0.05 – 0.02 | -0.83 | 0.41 | ||||
| Pairwise contrasts | Estimate | SE | t value | p value | |||||
| HAT-off*IQ – HAT-on*IQ | 0.03 | 0.008 | 2.99 | < 0.01 | |||||
| ASD*IQ – NT*IQ | 0.08 | 0.02 | 3.97 | < 0.001 | |||||
| 1 | English meaning: “The father brought back a large watermelon.”. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





