Submitted:
29 March 2023
Posted:
30 March 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
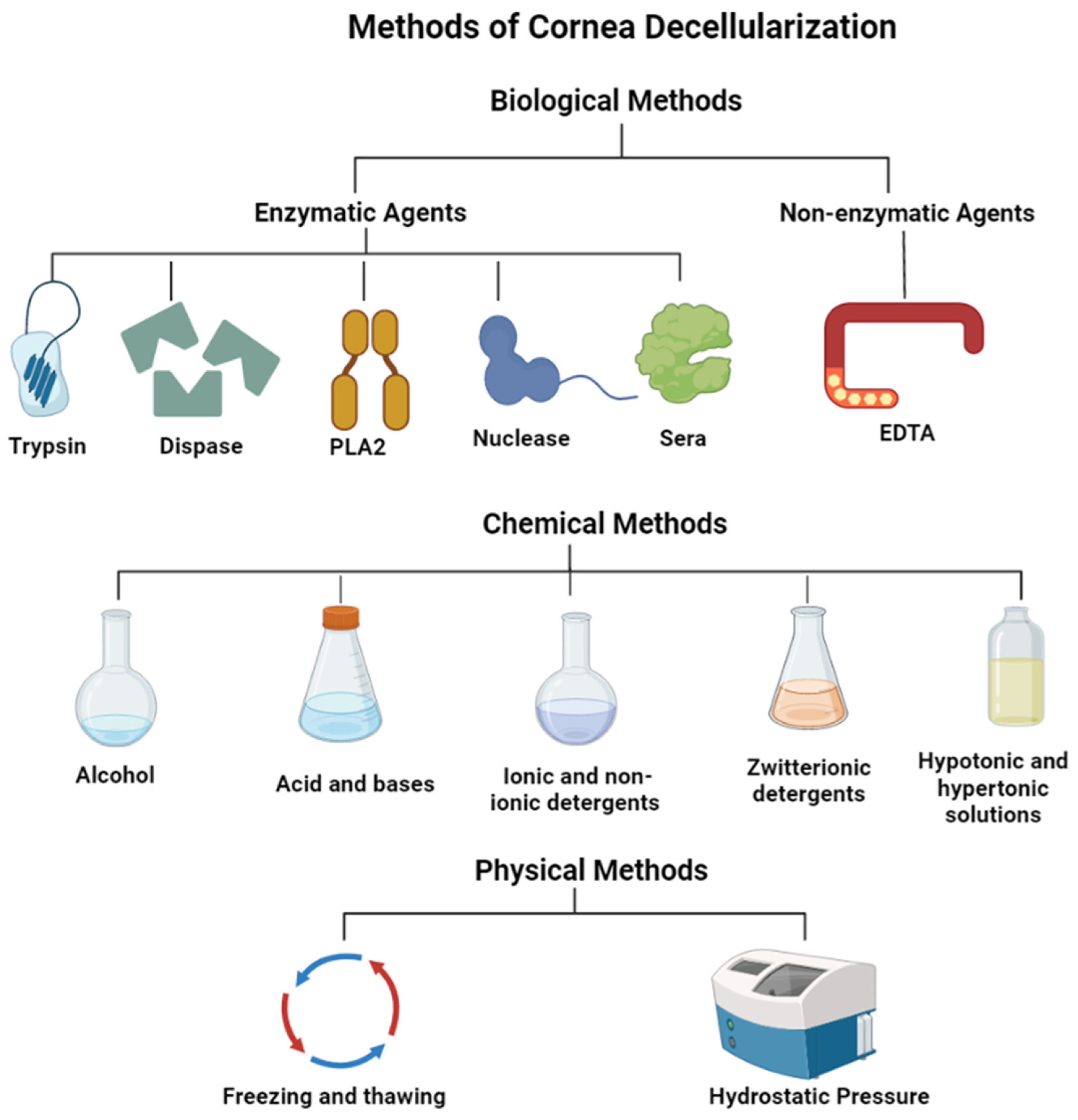
2. Methods of corneal decellularization
2.1. Biological techniques for the decellularization of cornea
2.1.1. Enzymatic agents
2.1.2. Non-enzymatic agents
| Methods/Techniques | Mechanism of Action | Advantages | Disadvantages |
|---|---|---|---|
| Biological | |||
| Enzymatic Agents | |||
| Trypsin [35,39,62] | Hydrolyzes protein and disrupts protein-protein interactions | Breaks cell-matrix interactions | An extended exposure can disrupt the collagen structure |
| Dispase [56] | Cleaves peptides associated with basement membrane proteins | Can aid the decellularization process by initially removing epithelium and endothelium | May cause damage to the basement membrane |
| Phospholipases A2 (PLA2) [42] | Hydrolyzes phospholipid components of cells | Effective at the removal of DNA and residual cellular components that tend to adhere to ECM proteins Helps maintain collagen and proteoglycans in the corneal tissue |
|
| Nucleases (RNase and DNase) [63] | Cleaves nucleic acids and aid in their removal | Effective at the removal of DNA and residual cellular components that tend to adhere to the stroma’s ECM proteins | Incomplete removal of the enzymes may impede recellularization and successful transplantation |
| Sera [42] | Serum nucleases degrade DNA and RNA. | Effectively removes cells while maintaining tissue transparency | The use of non-human sera carries a risk of cross-species transmission of pathogens |
| Non-enzymatic Agents | |||
| EDTA [64] | Dissociates cells by separating metal ions | Can be used for effective when combined with other agents | Ineffective at cell removal when used unaccompanied |
| Chemical | |||
| Alcohols | |||
| Ethanol [10,65] | Dehydrates and lyses cells. Removes lipids from tissues. |
More effective in removing lipids from tissues than lipase Antimicrobial, antifungal, and antiviral properties |
Can cause damage to the ultrastructure of tissue |
| Glycerol [20,66] | Dehydrates and lyses cells Removes lipids from tissues |
Can maintain or restore corneal transparency Cryoprotectant for long-term cornea storage |
Can cause damage to the ultrastructure of tissue |
| Acids and Alkalis | |||
| Peracetic acid [62,65] | Solubilizes cytoplasmic components of cells Removes nucleic acids via hydrolytic degradation |
Acts to simultaneously sterilize tissue | Ineffective decellularization that can also disrupt the ECM |
| Ammonium hydroxide [67,68] | Hydrolytic degradation of biomolecules | Results in complete DC with little effect on collagen architecture | Can eliminate GFs and reduce mechanical properties |
| Ionic Detergents | |||
| Sodium dodecyl sulfate(SDS) [60,65] | Solubilizes cell membranes and dissociates DNA from protein Disrupts protein-protein interactions |
Complete removal of cells can be achieved | Can be highly detrimental to ECM structure including disorganization of collagen fibrils and loss of GAGs Loss of tissue transparency |
| Sodium deoxycholate [20,43,63] | Solubilizes cell membranes and dissociates DNA from protein Disrupts protein-protein interactions |
Complete removal of cells can be achieved when used with other agents | Less effective at removal of cells |
| Non-ionic Detergents | |||
| Triton X-100 [63] | Breaks up lipid-lipid and lipid-protein interactions | Mild and non-denaturing | Less effective than ionic detergent treatments Can cause damage to the ECM |
| Zwitterionic Detergents | |||
| CHAPS [64,69] | Has properties of non-ionic and ionic detergents | Better cell removal than non-ionic detergents Improved preservation of the ECM ultrastructure than ionic detergents |
Poor cellular removal Very disruptive to stromal architecture |
| Hypo- and Hypertonic Solutions | |||
| Sodium Chloride (NaCl) [10,64,70] | Detaches DNA from proteins | Can maintain optically clarity Ability to maintain the stromal architecture and retain GAG content |
Does not remove cellular residues Mixed reports on the success of cell removal efficiency |
| Tris-HCl [10,64] | Lyses cells by osmotic shock | Reduces decellularization time | Mixed reports on cell removal |
| Physical | |||
| Freeze-thawing [20,32] | Ice crystal formation causes cell lysis | Effectively destroys tissue and organ cells | Expensive Needs subsequent treatment to remove cells Enhanced pore formation and disruptions to ECM |
| Hydrostatic Pressure [10,20,32,62] | Increase in pressure results in cell lysis | Effectively decellularizes whilst maintaining collagen fibril structure Kills bacteria and viruses |
Expensive |
| Sonication and Mechanical Agitation [71] | Cell lysis and removal | Does not remove DNA remnants from the corneal tissue | Only effective with enzymatic treatments |
2.2. Chemical techniques for the decellularization of cornea
2.2.1. Acid and alkali treatment
2.2.2. Alcohols
2.2.3. Surfactants
2.2.4. Hypotonic and hypertonic solutions
2.3. Physical techniques for the decellularization of cornea
2.3.1. Freeze-thaw cycles
2.3.2. High hydrostatic pressure
2.3.3. Sonication and mechanical agitation
3. Characterization of decellularized cornea prostheses
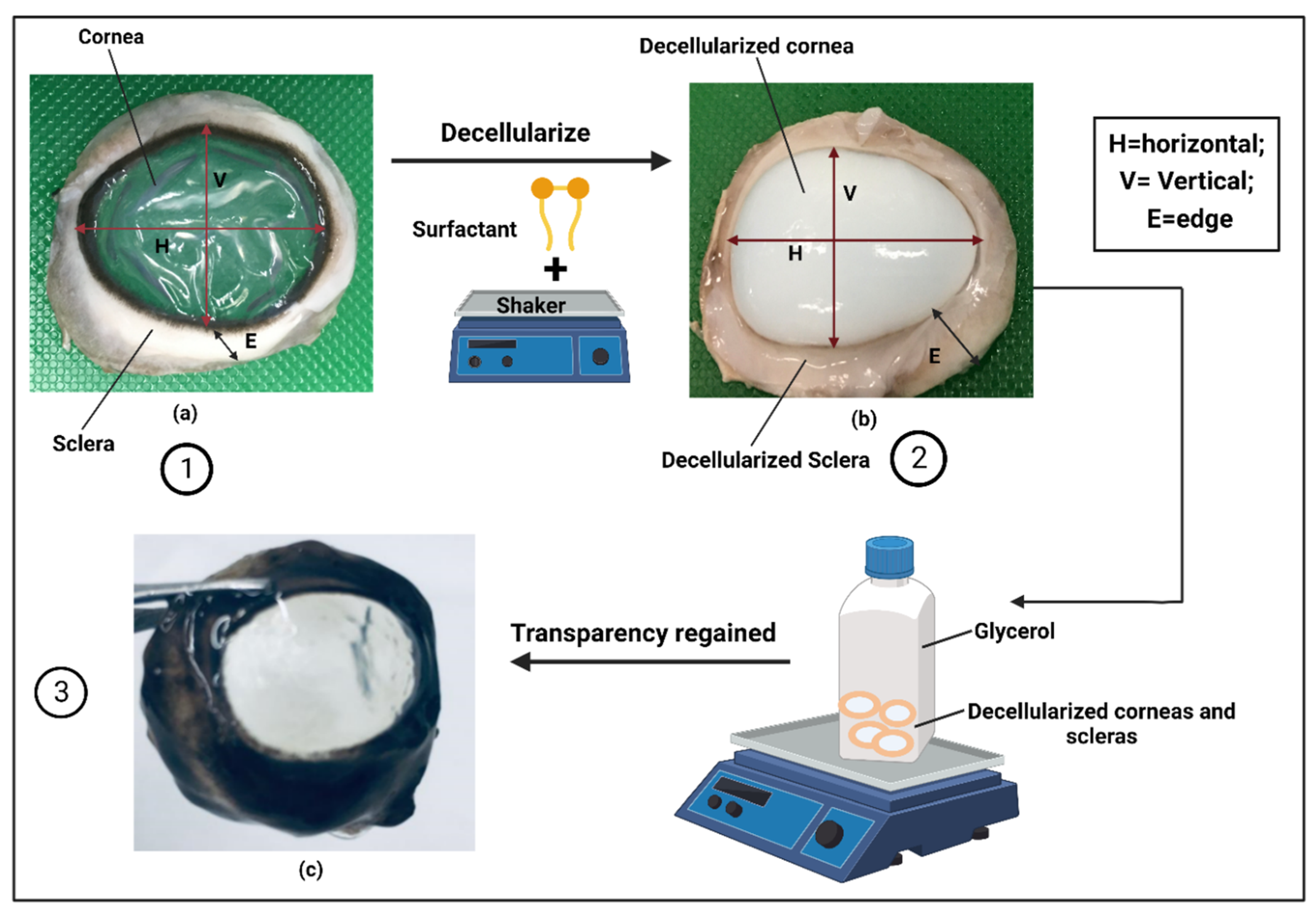
3.1. Removal of cellular materials
3.2. Biocompatibility evaluation of corneal scaffolds
3.3. Preservation of innate biological attributes post-decellularization
3.3.1. ECM architecture preservation
3.3.2. Transparency
3.3.3. Biomechanical properties
| Mechanical techniques | Application | Advantages | Disadvantages |
|---|---|---|---|
| Compression testing [128] |
The tissue is placed under two plates and compressed The test is used to determine the mechanical behavior of the tissue under the crushing load |
It can measure the mechanical behavior of the tissue and ductile fracture limits of the tissue It also gives you a detailed assessment of the tissue’s load-bearing capacity and elasticity properties |
It flattens the tissue and damages the structural architecture Due to the corneal curvature shape, the test may not distribute the pressure equally |
| Holographic interferometry [129] |
Is a tool that uses a laser to trace the changes in the tissue and perform interferometric measurements | It is a precise method that can detect residual stresses and cracks on the tissue without mechanical contact | There is no fixed distance so the location of the structure cannot be obtained |
| Bulge and inflation testing [128] |
Is a tool that is used to biomechanical test corneal tissue by inflating the tissue and measuring the displacement | It is a reliable tool that demonstrates the intrinsic properties of the cornea layers and resembles Intraocular pressure | The inflation capacity is difficult to control and it could affect viscoelasticity |
| Corvis STL Tonometer/Pachymeter [130] |
Corvis ST is a device that uses a high-speed Scheimpflug camera to record the cornea movement | Corvis ST device evaluates the central cornea thickness, corneal stiffness, and intraocular pressure | Very expensive |
| Ultrasound | It is a device that uses sound waves to get a very detailed image of structures | It is a non-invasive technique that shows detailed surface imaging of the cornea | It depends on the user’s skill |
| Ocular Response Analyzer [131] |
Is a non-invasive device that uses rapid air pulse to make an indentation in the cornea | It measures the cornea biomechanical properties such as corneal hysteresis, intraocular pressure, and corneal resistance factor | Very expensive |
| Indentation testing [10] |
Is a test that measures the indentation left behind in the cornea after it was compressed | Determine the hardness of the cornea with minimal destruction | Doesn’t assess tensile strength |
3.4. Evaluation of recellularization performance
4. Preclinical and clinical applications
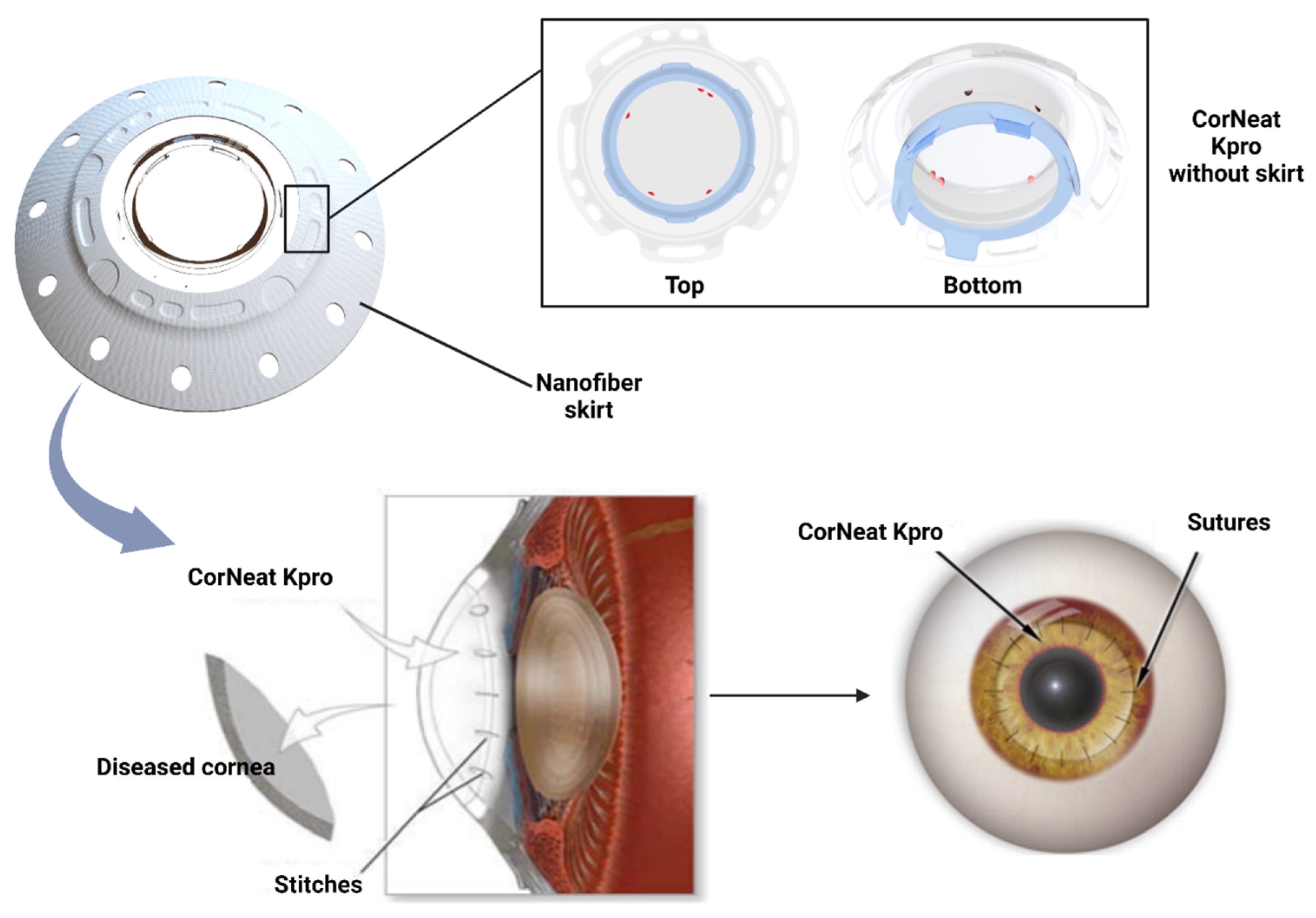
5. Conclusion and perspectives
6. Hypothesis of proposed model for keratoprostheses
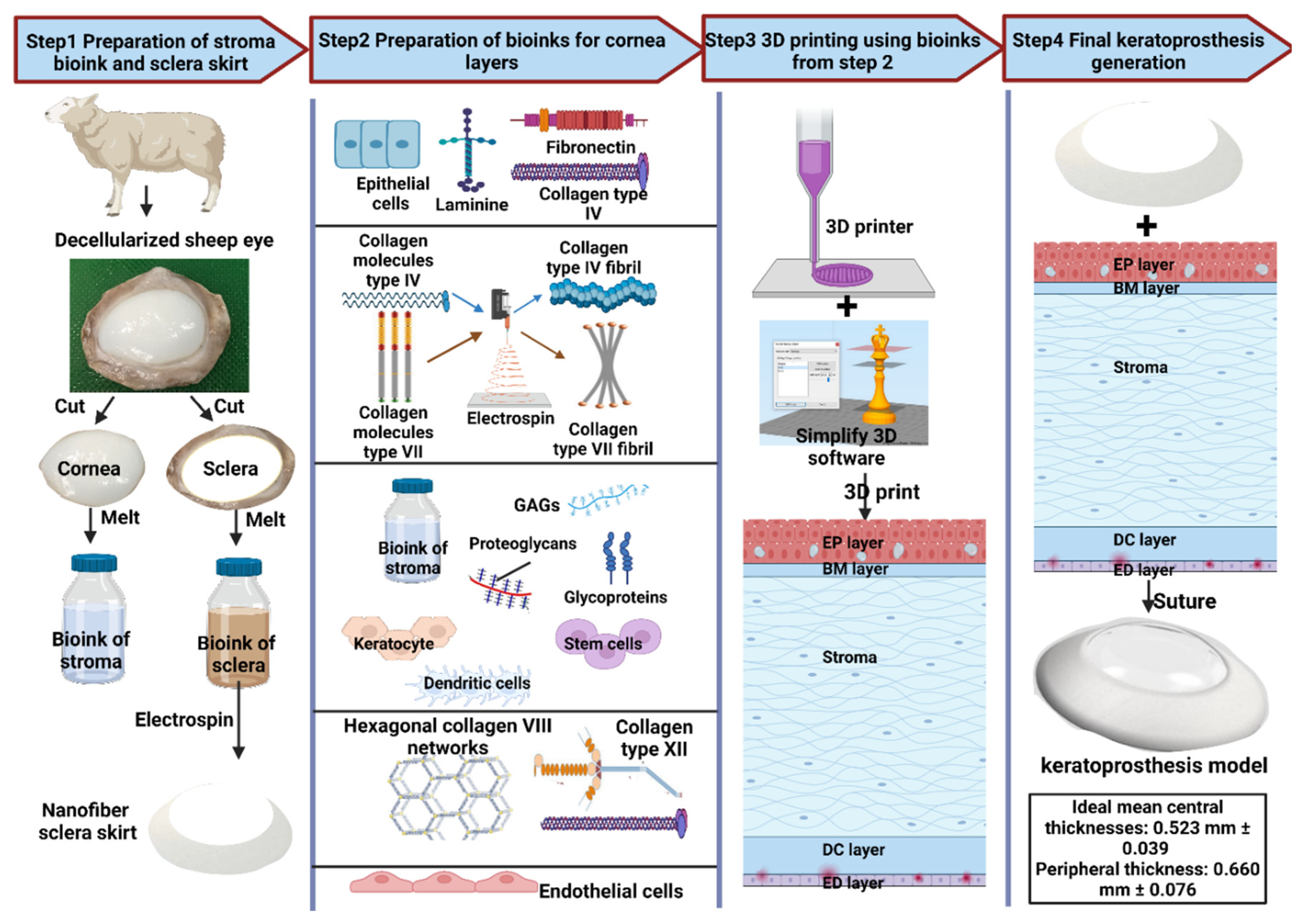
Author Contributions
Funding
Acknowledgments
References
- P. Gain et al., “Global Survey of Corneal Transplantation and Eye Banking,” (in eng), JAMA ophthalmology, vol. 134, no. 2, pp. 167-73, Feb 2016. [CrossRef]
- M. J. Burton, “Prevention, treatment and rehabilitation,” (in eng), Community eye health, vol. 22, no. 71, pp. 33-5, Dec 2009.
- W. J. Armitage et al., “High-risk Corneal Transplantation: Recent Developments and Future Possibilities,” Transplantation, vol. 103, no. 12, pp. 2468-2478, 2019. [CrossRef]
- H. Gao et al., “Survey report on keratoplasty in China: A 5-year review from 2014 to 2018,” (in eng), PloS one, vol. 15, no. 10, p. e0239939, 2020. [CrossRef]
- S. L. Wilson, L. E. Sidney, S. E. Dunphy, H. S. Dua, and A. Hopkinson, “Corneal Decellularization: A Method of Recycling Unsuitable Donor Tissue for Clinical Translation?,” (in eng), Curr Eye Res, vol. 41, no. 6, pp. 769-82, Jun 2016. [CrossRef]
- B. Gurnani, C. N. Czyz, N. Mahabadi, and S. J. Havens, “Corneal Graft Rejection,” in StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC., 2022.
- G. Holland et al., “Artificial Cornea: Past, Current, and Future Directions,” (in eng), Frontiers in medicine, vol. 8, p. 770780, 2021. [CrossRef]
- R. Sc, T. I, and T. D. T, “Osteo-odonto keratoprosthesis in Stevens-Johnson syndrome: a case report,” (in eng), International journal of ophthalmology, vol. 4, no. 2, pp. 212-5, 2011. [CrossRef]
- M. Vacalebre et al., “Current State of the Art and Next Generation of Materials for a Customized IntraOcular Lens according to a Patient-Specific Eye Power,” Polymers, vol. 15, no. 6, p. 1590, 2023. [Online]. Available: https://www.mdpi.com/2073-4360/15/6/1590.
- S. L. Wilson, L. E. Sidney, S. E. Dunphy, J. B. Rose, and A. Hopkinson, “Keeping an eye on decellularized corneas: a review of methods, characterization and applications,” (in eng), J Funct Biomater, vol. 4, no. 3, pp. 114-61, Jul 10 2013. [CrossRef]
- J. C. Igor Pantic, Svetlana Valjarevic et al., “Computational approaches for evaluating morphological changes in the corneal stroma associated with decellularization,” PREPRINT (Version 1) available at Research Square [https://doi.org/10.21203/rs.3.rs-2480023/v1], 16 January 2023.
- A. Neishabouri, A. Soltani Khaboushan, F. Daghigh, A. M. Kajbafzadeh, and M. Majidi Zolbin, “Decellularization in Tissue Engineering and Regenerative Medicine: Evaluation, Modification, and Application Methods,” Front Bioeng Biotechnol, vol. 10, p. 805299, 2022. [CrossRef]
- P. R. Corridon, “In vitro investigation of the impact of pulsatile blood flow on the vascular architecture of decellularized porcine kidneys,” Sci Rep, vol. 11, no. 1, p. 16965, Aug 20 2021. [CrossRef]
- P. R. Corridon, “Intravital microscopy datasets examining key nephron segments of transplanted decellularized kidneys,” Sci Data, vol. 9, no. 1, p. 561, Sep 10 2022. [CrossRef]
- P. R. Corridon, “Still finding ways to augment the existing management of acute and chronic kidney diseases with targeted gene and cell therapies: Opportunities and hurdles,” (in English), Frontiers in Medicine, Mini Review vol. 10, 2023-March-07 2023. [CrossRef]
- P. R. Corridon, I. K. Ko, J. J. Yoo, and A. Atala, “Bioartificial Kidneys,” Curr Stem Cell Rep, vol. 3, no. 2, pp. 68-76, Jun 2017. [CrossRef]
- V. Pantic, A. Shakeel, G. A. Petroianu, and P. R. Corridon, “Analysis of Vascular Architecture and Parenchymal Damage Generated by Reduced Blood Perfusion in Decellularized Porcine Kidneys Using a Gray Level Co-occurrence Matrix,” Front Cardiovasc Med, vol. 9, p. 797283, 2022. [CrossRef]
- A. Shakeel and P. R. Corridon, “Mitigating challenges and expanding the future of vascular tissue engineering-are we there yet?,” Front Physiol, vol. 13, p. 1079421, 2022. [CrossRef]
- X. Wang, V. Chan, and P. R. Corridon, “Acellular Tissue-Engineered Vascular Grafts from Polymers: Methods, Achievements, Characterization, and Challenges,” Polymers (Basel), vol. 14, no. 22, Nov 9 2022. [CrossRef]
- X. Wang, V. Chan, and P. R. Corridon, “Decellularized blood vessel development: Current state-of-the-art and future directions,” Front Bioeng Biotechnol, vol. 10, p. 951644, 2022. [CrossRef]
- P. Corridon, “Enhancing the expression of a key mitochondrial enzyme at the inception of ischemia-reperfusion injury can boost recovery and halt the progression of acute kidney injury,” ed: Research Square, 2023.
- A. Wilson, J. Jones, and J. Marshall, “Biomechanical Evaluation of Decellularized and Crosslinked Corneal Implants Manufactured From Porcine Corneas as a Treatment Option for Advanced Keratoconus,” (in eng), Frontiers in bioengineering and biotechnology, vol. 10, p. 862969, 2022. [CrossRef]
- M. M. Islam et al., “Effects of gamma radiation sterilization on the structural and biological properties of decellularized corneal xenografts,” (in eng), Acta Biomater, vol. 96, pp. 330-344, Sep 15 2019. [CrossRef]
- S. Sharifi et al., “Toward electron-beam sterilization of a pre-assembled Boston keratoprosthesis,” (in eng), The ocular surface, vol. 20, pp. 176-184, Apr 2021. [CrossRef]
- D. K. Cooper et al., “Alpha1,3-galactosyltransferase gene-knockout pigs for xenotransplantation: where do we go from here?,” (in eng), Transplantation, vol. 84, no. 1, pp. 1-7, Jul 15 2007. [CrossRef]
- H. Hara and D. K. Cooper, “Xenotransplantation--the future of corneal transplantation?,” (in eng), Cornea, vol. 30, no. 4, pp. 371-8, Apr 2011. [CrossRef]
- R. L. Khan, A. A. Khraibi, L. F. Dumée, and P. R. Corridon, “From waste to wealth: Repurposing slaughterhouse waste for xenotransplantation,” (in English), Frontiers in Bioengineering and Biotechnology, Perspective vol. 11, 2023-February-03 2023. [CrossRef]
- A.S. Xinyu Wang, Ahmed E. Salih et al., “A scalable corneal xenograft platform: simultaneous opportunities for tissue engineering and circular economic sustainability by repurposing slaughterhouse waste,” PREPRINT (Version 1) available at Research Square [https://doi.org/10.21203/rs.3.rs-2480068/v1], 16 January 2023.
- E. Yoeruek et al., “Decellularization of porcine corneas and repopulation with human corneal cells for tissue-engineered xenografts,” Acta Ophthalmologica, vol. 90, no. 2, pp. e125-e131, 2012. [CrossRef]
- E. Yoeruek et al., “Reconstruction of corneal stroma with decellularized porcine xenografts in a rabbit model,” Acta Ophthalmologica, vol. 90, no. 3, pp. e206-e210, 2012. [CrossRef]
- S. Chen, M. J. Mienaltowski, and D. E. Birk, “Regulation of corneal stroma extracellular matrix assembly,” (in eng), Experimental eye research, vol. 133, pp. 69-80, Apr 2015. [CrossRef]
- P. M. Crapo, T. W. Gilbert, and S. F. Badylak, “An overview of tissue and whole organ decellularization processes,” (in eng), Biomaterials, vol. 32, no. 12, pp. 3233-43, Apr 2011. [CrossRef]
- S. Amano, N. Shimomura, S. Yokoo, K. Araki-Sasaki, and S. Yamagami, “Decellularizing corneal stroma using N2 gas,” (in eng), Molecular vision, vol. 14, pp. 878-82, May 14 2008.
- D. Gusnard and R. H. Kirschner, “Cell and organelle shrinkage during preparation for scanning electron microscopy: effects of fixation, dehydration and critical point drying,” (in eng), Journal of microscopy, vol. 110, no. 1, pp. 51-7, May 1977. [CrossRef]
- A. Isidan et al., “Decellularization methods for developing porcine corneal xenografts and future perspectives,” (in eng), Xenotransplantation, vol. 26, no. 6, p. e12564, Nov 2019. [CrossRef]
- U. Mendibil, R. Ruiz-Hernandez, S. Retegi-Carrion, N. Garcia-Urquia, B. Olalde-Graells, and A. Abarrategi, “Tissue-Specific Decellularization Methods: Rationale and Strategies to Achieve Regenerative Compounds,” (in eng), International journal of molecular sciences, vol. 21, no. 15, Jul 30 2020. [CrossRef]
- M. Yang, C. Z. Chen, X. N. Wang, Y. B. Zhu, and Y. J. Gu, “Favorable effects of the detergent and enzyme extraction method for preparing decellularized bovine pericardium scaffold for tissue engineered heart valves,” (in eng), Journal of biomedical materials research. Part B, Applied biomaterials, vol. 91, no. 1, pp. 354-61, Oct 2009. [CrossRef]
- E. Rieder et al., “Decellularization protocols of porcine heart valves differ importantly in efficiency of cell removal and susceptibility of the matrix to recellularization with human vascular cells,” The Journal of thoracic and cardiovascular surgery, vol. 127, no. 2, pp. 399-405, 2004.
- C. Zhang et al., “Survival and integration of tissue-engineered corneal stroma in a model of corneal ulcer,” (in eng), Cell and tissue research, vol. 329, no. 2, pp. 249-57, Aug 2007. [CrossRef]
- F. Wang, Z. Wang, X. Sun, F. Wang, X. Xu, and X. Zhang, “Safety and efficacy of dispase and plasmin in pharmacologic vitreolysis,” (in eng), Investigative ophthalmology & visual science, vol. 45, no. 9, pp. 3286-90, Sep 2004. [CrossRef]
- Prasertsung, S. Kanokpanont, T. Bunaprasert, V. Thanakit, and S. Damrongsakkul, “Development of acellular dermis from porcine skin using periodic pressurized technique,” (in eng), Journal of biomedical materials research. Part B, Applied biomaterials, vol. 85, no. 1, pp. 210-9, Apr 2008. [CrossRef]
- Z. Wu et al., “The use of phospholipase A(2) to prepare acellular porcine corneal stroma as a tissue engineering scaffold,” (in eng), Biomaterials, vol. 30, no. 21, pp. 3513-22, Jul 2009. [CrossRef]
- M. Blum et al., “Tissue engineered vascular grafts transform into autologous neovessels capable of native function and growth,” Communications Medicine, vol. 2, no. 1, p. 3, 2022/01/10 2022. [CrossRef]
- R. S. S. Azevedo et al., “In situ immune response and mechanisms of cell damage in central nervous system of fatal cases microcephaly by Zika virus,” (in eng), Sci Rep, vol. 8, no. 1, p. 1, Jan 8 2018. [CrossRef]
- N. Li et al., “Tectonic lamellar keratoplasty with acellular corneal stroma in high-risk corneal transplantation,” (in eng), Molecular vision, vol. 17, pp. 1909-17, 2011.
- R. W. Grauss, M. G. Hazekamp, F. Oppenhuizen, C. J. van Munsteren, A. C. Gittenberger-de Groot, and M. C. DeRuiter, “Histological evaluation of decellularised porcine aortic valves: matrix changes due to different decellularisation methods,” (in eng), Eur J Cardiothorac Surg, vol. 27, no. 4, pp. 566-71, Apr 2005. [CrossRef]
- D. E. Heath, “A Review of Decellularized Extracellular Matrix Biomaterials for Regenerative Engineering Applications,” Regenerative Engineering and Translational Medicine, vol. 5, no. 2, pp. 155-166, 2019/06/01 2019. [CrossRef]
- A. Neishabouri, A. Soltani Khaboushan, F. Daghigh, A.-M. Kajbafzadeh, and M. Majidi Zolbin, “Decellularization in Tissue Engineering and Regenerative Medicine: Evaluation, Modification, and Application Methods,” (in English), Frontiers in Bioengineering and Biotechnology, Review vol. 10, 2022-April-25 2022. [CrossRef]
- Y. Oh, M. K. Kim, H. J. Lee, J. H. Ko, W. R. Wee, and J. H. Lee, “Processing porcine cornea for biomedical applications,” (in eng), Tissue engineering. Part C, Methods, vol. 15, no. 4, pp. 635-45, Dec 2009. [CrossRef]
- R. Bochert, A. Zhivov, R. Kraak, J. Stave, and R. Guthoff, “Contribution to comprehension of image formation in confocal microscopy of cornea with Rostock cornea module,” Br J Ophthalmol, vol. 89, pp. 1351-5, 11/01 2005. [CrossRef]
- Hedhly, Y. Wang, S. Zeng, F. Ouerghi, J. Zhou, and G. Humbert, “Highly Sensitive Plasmonic Waveguide Biosensor Based on Phase Singularity-Enhanced Goos–Hänchen Shift,” Biosensors, vol. 12, no. 7, p. 457, 2022. [Online]. Available: https://www.mdpi.com/2079-6374/12/7/457.
- Polisetti et al., “A decellularized human corneal scaffold for anterior corneal surface reconstruction,” Scientific Reports, vol. 11, no. 1, p. 2992, 2021/02/04 2021. [CrossRef]
- Gupta and P. Upadhyay, “Use of glycerol-preserved corneas for corneal transplants,” Indian J Ophthalmol, vol. 65, no. 7, pp. 569-573, Jul 2017. [CrossRef]
- S. Chaurasia, S. Das, and A. Roy, “A review of long-term corneal preservation techniques: Relevance and renewed interests in the COVID-19 era,” (in eng), Indian J Ophthalmol, vol. 68, no. 7, pp. 1357-1363, Jul 2020. [CrossRef]
- H. C. Lin, S. J. Ong, and A. N. Chao, “Eye preservation tectonic graft using glycerol-preserved donor cornea,” Eye, vol. 26, no. 11, pp. 1446-1450, 2012/11/01 2012. [CrossRef]
- M. Gonzalez-Andrades, J. de la Cruz Cardona, A. M. Ionescu, A. Campos, M. Del Mar Perez, and M. Alaminos, “Generation of bioengineered corneas with decellularized xenografts and human keratocytes,” (in eng), Investigative ophthalmology & visual science, vol. 52, no. 1, pp. 215-22, Jan 5 2011. [CrossRef]
- L. Gui, S. A. Chan, C. K. Breuer, and L. E. Niklason, “Novel utilization of serum in tissue decellularization,” (in eng), Tissue engineering. Part C, Methods, vol. 16, no. 2, pp. 173-84, Apr 2010. [CrossRef]
- Y. Shao et al., “Evaluation of novel decellularizing corneal stroma for cornea tissue engineering applications,” (in eng), International journal of ophthalmology, vol. 5, no. 4, pp. 415-8, 2012. [CrossRef]
- T. Bayyoud et al., “Decellularized bovine corneal posterior lamellae as carrier matrix for cultivated human corneal endothelial cells,” (in eng), Current eye research, vol. 37, no. 3, pp. 179-86, Mar 2012. [CrossRef]
- L. Du and X. Wu, “Development and characterization of a full-thickness acellular porcine cornea matrix for tissue engineering,” (in eng), Artificial organs, vol. 35, no. 7, pp. 691-705, Jul 2011. [CrossRef]
- E. Yoeruek et al., “Decellularization of porcine corneas and repopulation with human corneal cells for tissue-engineered xenografts,” (in eng), Acta ophthalmologica, vol. 90, no. 2, pp. e125-31, Mar 2012. [CrossRef]
- A. Gilpin and Y. Yang, “Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications,” Biomed Res Int, vol. 2017, p. 9831534, 2017. [CrossRef]
- S. Cebotari et al., “Detergent decellularization of heart valves for tissue engineering: toxicological effects of residual detergents on human endothelial cells,” (in eng), Artificial organs, vol. 34, no. 3, pp. 206-10, Mar 2010. [CrossRef]
- M. S. Alhamdani, C. Schröder, J. Werner, N. Giese, A. Bauer, and J. D. Hoheisel, “Single-step procedure for the isolation of proteins at near-native conditions from mammalian tissue for proteomic analysis on antibody microarrays,” (in eng), Journal of proteome research, vol. 9, no. 2, pp. 963-71, Feb 5 2010. [CrossRef]
- S. Ponce Márquez et al., “Decellularization of bovine corneas for tissue engineering applications,” (in eng), Acta biomaterialia, vol. 5, no. 6, pp. 1839-47, Jul 2009. [CrossRef]
- A. P. Lynch and M. Ahearne, “Strategies for developing decellularized corneal scaffolds,” (in eng), Experimental eye research, vol. 108, pp. 42-7, Mar 2013. [CrossRef]
- S. Choi et al., “Bioengineering endothelialized neo-corneas using donor-derived corneal endothelial cells and decellularized corneal stroma,” (in eng), Biomaterials, vol. 31, no. 26, pp. 6738-45, Sep 2010. [CrossRef]
- Y. Dai et al., “Characterizing the effects of VPA, VC and RCCS on rabbit keratocytes onto decellularized bovine cornea,” (in eng), PloS one, vol. 7, no. 11, p. e50114, 2012. [CrossRef]
- T. J. Keane, I. T. Swinehart, and S. F. Badylak, “Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance,” (in eng), Methods (San Diego, Calif.), vol. 84, pp. 25-34, Aug 2015. [CrossRef]
- B. Ekser et al., “Clinical xenotransplantation: the next medical revolution?,” (in eng), Lancet (London, England), vol. 379, no. 9816, pp. 672-83, Feb 18 2012. [CrossRef]
- Y. G. Xu, Y. S. Xu, C. Huang, Y. Feng, Y. Li, and W. Wang, “Development of a rabbit corneal equivalent using an acellular corneal matrix of a porcine substrate,” (in eng), Molecular vision, vol. 14, pp. 2180-9, 2008.
- T. W. Gilbert, T. L. Sellaro, and S. F. Badylak, “Decellularization of tissues and organs,” (in eng), Biomaterials, vol. 27, no. 19, pp. 3675-83, Jul 2006. [CrossRef]
- M. C. Jamur and C. Oliver, “Cell fixatives for immunostaining,” (in eng), Methods in molecular biology (Clifton, N.J.), vol. 588, pp. 55-61, 2010. [CrossRef]
- W. Chen et al., “Comparison of fresh corneal tissue versus glycerin-cryopreserved corneal tissue in deep anterior lamellar keratoplasty,” (in eng), Investigative ophthalmology & visual science, vol. 51, no. 2, pp. 775-81, Feb 2010. [CrossRef]
- A. Isidan et al., “Comparison of porcine corneal decellularization methods and importance of preserving corneal limbus through decellularization,” (in eng), PLoS One, vol. 16, no. 3, p. e0243682, 2021. [CrossRef]
- Y. Zhou et al., “Development and characterization of acellular porcine corneal matrix using sodium dodecylsulfate,” (in eng), Cornea, vol. 30, no. 1, pp. 73-82, Jan 2011. [CrossRef]
- M. González-Andrades et al., “Effects of Detergent-Based Protocols on Decellularization of Corneas With Sclerocorneal Limbus. Evaluation of Regional Differences,” (in eng), Transl Vis Sci Technol, vol. 4, no. 2, p. 13, Apr 2015. [CrossRef]
- Du, X. Wu, K. Pang, and Y. Yang, “Histological evaluation and biomechanical characterisation of an acellular porcine cornea scaffold,” (in eng), The British journal of ophthalmology, vol. 95, no. 3, pp. 410-4, Mar 2011. [CrossRef]
- A. Shafiq, R. A. Gemeinhart, B. Y. Yue, and A. R. Djalilian, “Decellularized human cornea for reconstructing the corneal epithelium and anterior stroma,” (in eng), Tissue engineering. Part C, Methods, vol. 18, no. 5, pp. 340-8, May 2012. [CrossRef]
- Rabbani, N. Zakian, and N. Alimoradi, “Contribution of Physical Methods in Decellularization of Animal Tissues,” (in eng), Journal of medical signals and sensors, vol. 11, no. 1, pp. 1-11, Jan-Mar 2021. [CrossRef]
- Pulver, A. Shevtsov, B. Leybovich, I. Artyuhov, Y. Maleev, and A. Peregudov, “Production of organ extracellular matrix using a freeze-thaw cycle employing extracellular cryoprotectants,” Cryo Letters, vol. 35, no. 5, pp. 400-6, Sep-Oct 2014. [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed/25397955.
- Q. Xing, K. Yates, M. Tahtinen, E. Shearier, Z. Qian, and F. Zhao, “Decellularization of fibroblast cell sheets for natural extracellular matrix scaffold preparation,” (in eng), Tissue Eng Part C Methods, vol. 21, no. 1, pp. 77-87, Jan 2015. [CrossRef]
- C. Azuma, H. Tohyama, H. Nakamura, F. Kanaya, and K. Yasuda, “Antibody neutralization of TGF-beta enhances the deterioration of collagen fascicles in a tissue-cultured tendon matrix with ex vivo fibroblast infiltration,” (in eng), Journal of biomechanics, vol. 40, no. 10, pp. 2184-90, 2007. [CrossRef]
- K. H. Hussein, K. M. Park, K. S. Kang, and H. M. Woo, “Biocompatibility evaluation of tissue-engineered decellularized scaffolds for biomedical application,” (in eng), Materials science & engineering. C, Materials for biological applications, vol. 67, pp. 766-778, Oct 1 2016. [CrossRef]
- S. Nagata, R. Hanayama, and K. Kawane, “Autoimmunity and the clearance of dead cells,” (in eng), Cell, vol. 140, no. 5, pp. 619-30, Mar 5 2010. [CrossRef]
- B. N. Brown, J. E. Valentin, A. M. Stewart-Akers, G. P. McCabe, and S. F. Badylak, “Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component,” (in eng), Biomaterials, vol. 30, no. 8, pp. 1482-91, Mar 2009. [CrossRef]
- E. M. Espana and D. E. Birk, “Composition, structure and function of the corneal stroma,” (in eng), Experimental eye research, vol. 198, p. 108137, Sep 2020. [CrossRef]
- S. Sridhar, “Anatomy of cornea and ocular surface,” (in eng), Indian journal of ophthalmology, vol. 66, no. 2, pp. 190-194, Feb 2018. [CrossRef]
- R. Corridon, X. Wang, A. Shakeel, and V. Chan, “Digital Technologies: Advancing Individualized Treatments through Gene and Cell Therapies, Pharmacogenetics, and Disease Detection and Diagnostics,” Biomedicines, vol. 10, no. 10, Sep 30 2022. [CrossRef]
- L. M. Davidovic et al., “Gray-Level Co-occurrence Matrix Analysis for the Detection of Discrete, Ethanol-Induced, Structural Changes in Cell Nuclei: An Artificial Intelligence Approach,” Microsc Microanal, vol. 28, no. 1, pp. 265-271, Feb 2022. [CrossRef]
- Pantic, J. Cumic, S. Dugalic, G. A. Petroianu, and P. R. Corridon, “Gray level co-occurrence matrix and wavelet analyses reveal discrete changes in proximal tubule cell nuclei after mild acute kidney injury,” Scientific Reports, vol. 13, no. 1, p. 4025, 2023/03/10 2023. [CrossRef]
- Pantic, J. Paunovic, J. Cumic, S. Valjarevic, G. A. Petroianu, and P. R. Corridon, “Artificial neural networks in contemporary toxicology research,” Chemico-Biological Interactions, vol. 369, p. 110269, 2023/01/05/2023. [CrossRef]
- R. Corridon, “Capturing effects of blood flow on the transplanted decellularized nephron with intravital microscopy,” bioRxiv, p. 2021.02.10.430561, 2023. [CrossRef]
- L. C. Keong and A. S. Halim, “In vitro models in biocompatibility assessment for biomedical-grade chitosan derivatives in wound management,” (in eng), International journal of molecular sciences, vol. 10, no. 3, pp. 1300-1313, Mar 2009. [CrossRef]
- M. M. Singer and R. S. Tjeerdema, “Fate and effects of the surfactant sodium dodecyl sulfate,” (in eng), Reviews of environmental contamination and toxicology, vol. 133, pp. 95-149, 1993. [CrossRef]
- A. M. Kajbafzadeh, N. Javan-Farazmand, M. Monajemzadeh, and A. Baghayee, “Determining the optimal decellularization and sterilization protocol for preparing a tissue scaffold of a human-sized liver tissue,” (in eng), Tissue engineering. Part C, Methods, vol. 19, no. 8, pp. 642-51, Aug 2013. [CrossRef]
- E. Murray, C. García Godoy, and F. García Godoy, “How is the biocompatibilty of dental biomaterials evaluated?,” (in eng), Medicina oral, patologia oral y cirugia bucal, vol. 12, no. 3, pp. E258-66, May 1 2007.
- D. Granchi et al., “Adhesive protein expression on human endothelial cells after in vitro contact with woven Dacron,” (in eng), Biomaterials, vol. 19, no. 1-3, pp. 93-8, Jan-Feb 1998. [CrossRef]
- E. Cenni et al., “Established cell lines and primary cultures in testing medical devices in vitro,” (in eng), Toxicology in vitro: an international journal published in association with BIBRA, vol. 13, no. 4-5, pp. 801-10, Aug-Oct 1999. [CrossRef]
- M. S. Rao et al., “Comparison of RNA-Seq and Microarray Gene Expression Platforms for the Toxicogenomic Evaluation of Liver From Short-Term Rat Toxicity Studies,” (in eng), Frontiers in genetics, vol. 9, p. 636, 2018. [CrossRef]
- M. G. Hayat, N. Farahani, E. Safdarian, A. Roointan, and A. Sahebkar, “Gene Delivery Using Lipoplexes and Polyplexes: Principles, Limitations and Solutions,” (in eng), Crit Rev Eukaryot Gene Expr, vol. 29, no. 1, pp. 29-36, 2019. [CrossRef]
- M. G. Michaels, F. J. Jenkins, K. St George, M. A. Nalesnik, T. E. Starzl, and C. R. Rinaldo, Jr., “Detection of infectious baboon cytomegalovirus after baboon-to-human liver xenotransplantation,” (in eng), Journal of virology, vol. 75, no. 6, pp. 2825-8, Mar 2001. [CrossRef]
- A. Fishman, L. Scobie, and Y. Takeuchi, “Xenotransplantation-associated infectious risk: a WHO consultation,” (in eng), Xenotransplantation, vol. 19, no. 2, pp. 72-81, Mar-Apr 2012. [CrossRef]
- F. Naso, A. Gandaglia, L. Iop, M. Spina, and G. Gerosa, “Alpha-Gal detectors in xenotransplantation research: a word of caution,” (in eng), Xenotransplantation, vol. 19, no. 4, pp. 215-20, Jul-Aug 2012. [CrossRef]
- F. Groell, O. Jordan, and G. Borchard, “In vitro models for immunogenicity prediction of therapeutic proteins,” (in eng), European journal of pharmaceutics and biopharmaceutics: official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, vol. 130, pp. 128-142, Sep 2018. [CrossRef]
- M. Park, S. M. Park, S. R. Yang, S. H. Hong, and H. M. Woo, “Preparation of immunogen-reduced and biocompatible extracellular matrices from porcine liver,” (in eng), Journal of bioscience and bioengineering, vol. 115, no. 2, pp. 207-15, Feb 2013. [CrossRef]
- H. Mirmalek-Sani, D. C. Sullivan, C. Zimmerman, T. D. Shupe, and B. E. Petersen, “Immunogenicity of decellularized porcine liver for bioengineered hepatic tissue,” (in eng), The American journal of pathology, vol. 183, no. 2, pp. 558-65, Aug 2013. [CrossRef]
- G. Orlando et al., “Production and implantation of renal extracellular matrix scaffolds from porcine kidneys as a platform for renal bioengineering investigations,” (in eng), Annals of surgery, vol. 256, no. 2, pp. 363-70, Aug 2012. [CrossRef]
- Musselmann, B. Kane, B. Alexandrou, and J. R. Hassell, “Stimulation of collagen synthesis by insulin and proteoglycan accumulation by ascorbate in bovine keratocytes in vitro,” (in eng), Investigative ophthalmology & visual science, vol. 47, no. 12, pp. 5260-6, Dec 2006. [CrossRef]
- M. Meek and C. Knupp, “Corneal structure and transparency,” (in eng), Progress in retinal and eye research, vol. 49, pp. 1-16, Nov 2015. [CrossRef]
- W. Teng et al., “Multiphoton autofluorescence and second-harmonic generation imaging of the ex vivo porcine eye,” (in eng), Investigative ophthalmology & visual science, vol. 47, no. 3, pp. 1216-24, Mar 2006. [CrossRef]
- Morishige et al., “Second-harmonic imaging microscopy of normal human and keratoconus cornea,” (in eng), Investigative ophthalmology & visual science, vol. 48, no. 3, pp. 1087-94, Mar 2007. [CrossRef]
- Jalbert, F. Stapleton, E. Papas, D. F. Sweeney, and M. Coroneo, “In vivo confocal microscopy of the human cornea,” (in eng), The British journal of ophthalmology, vol. 87, no. 2, pp. 225-36, Feb 2003. [CrossRef]
- Cai, A. C. Lai, K. Liao, P. R. Corridon, D. J. Graves, and V. Chan, “Recent Advances in Fluorescence Recovery after Photobleaching for Decoupling Transport and Kinetics of Biomacromolecules in Cellular Physiology,” Polymers (Basel), vol. 14, no. 9, May 7 2022. [CrossRef]
- A. Collett et al., “Hydrodynamic Isotonic Fluid Delivery Ameliorates Moderate-to-Severe Ischemia-Reperfusion Injury in Rat Kidneys,” J Am Soc Nephrol, vol. 28, no. 7, pp. 2081-2092, Jul 2017. [CrossRef]
- R. Corridon, “Enhancing the expression of a key mitochondrial enzyme at the inception of ischemia-reperfusion injury can boost recovery and halt the progression of acute kidney injury,” Front Physiol, vol. 14, p. 1024238, 2023. [CrossRef]
- R. Corridon et al., “A method to facilitate and monitor expression of exogenous genes in the rat kidney using plasmid and viral vectors,” (in eng), Am J Physiol Renal Physiol, vol. 304, no. 9, pp. F1217-29, May 1 2013. [CrossRef]
- A.M. Hall, G. J. Rhodes, R. M. Sandoval, P. R. Corridon, and B. A. Molitoris, “In vivo multiphoton imaging of mitochondrial structure and function during acute kidney injury,” Kidney Int, vol. 83, no. 1, pp. 72-83, Jan 2013. [CrossRef]
- L. Kolb et al., “Exogenous Gene Transmission of Isocitrate Dehydrogenase 2 Mimics Ischemic Preconditioning Protection,” J Am Soc Nephrol, vol. 29, no. 4, pp. 1154-1164, Apr 2018. [CrossRef]
- Shaya et al., “Design, photophysical properties, and applications of fluorene-based fluorophores in two-photon fluorescence bioimaging: A review,” Journal of Photochemistry and Photobiology C: Photochemistry Reviews, vol. 52, p. 100529, 2022/09/01/2022. [CrossRef]
- R. Corridon, S. H. Karam, A. A. Khraibi, A. A. Khan, and M. A. Alhashmi, “Intravital imaging of real-time endogenous actin dysregulation in proximal and distal tubules at the onset of severe ischemia-reperfusion injury,” Scientific Reports, vol. 11, no. 1, p. 8280, 2021/04/15 2021. [CrossRef]
- A. Torricelli, V. Singh, V. Agrawal, M. R. Santhiago, and S. E. Wilson, “Transmission electron microscopy analysis of epithelial basement membrane repair in rabbit corneas with haze,” (in eng), Investigative ophthalmology & visual science, vol. 54, no. 6, pp. 4026-33, Jun 10 2013. [CrossRef]
- Goswami et al., “Gene Therapy Leaves a Vicious Cycle,” Front Oncol, vol. 9, p. 297, 2019. [CrossRef]
- W. Drexler, U. Morgner, R. K. Ghanta, F. X. Kärtner, J. S. Schuman, and J. G. Fujimoto, “Ultrahigh-resolution ophthalmic optical coherence tomography,” (in eng), Nature medicine, vol. 7, no. 4, pp. 502-7, Apr 2001. [CrossRef]
- M. Corridon, R. Ascázubi, C. Krest, and I. Wilke, “Time-domain terahertz spectroscopy of artificial skin,” in Society of Photo-Optical Instrumentation Engineers (SPIE) Conference Series, February 01, 2006 2006, vol. 6080, p. 608007, doi: 10.1117/12.646632. [Online]. Available: https://ui.adsabs.harvard.edu/abs/2006SPIE.6080E..07C.
- He, D. Wang, and Y. Jiang, “Overview of Ultrasound Biomicroscopy,” (in eng), Journal of current glaucoma practice, vol. 6, no. 1, pp. 25-53, Jan-Apr 2012. [CrossRef]
- L. Alexander et al., “A systematic review of ultrasound biomicroscopy use in pediatric ophthalmology,” (in eng), Eye (London, England), vol. 35, no. 1, pp. 265-276, Jan 2021. [CrossRef]
- J. A. Stammen, S. Williams, D. N. Ku, and R. E. Guldberg, “Mechanical properties of a novel PVA hydrogel in shear and unconfined compression,” (in eng), Biomaterials, vol. 22, no. 8, pp. 799-806, Apr 2001. [CrossRef]
- J. L. Calkins, B. F. Hochheimer, and W. J. Stark, “Corneal wound healing: holographic stress-test analysis,” (in eng), Investigative ophthalmology & visual science, vol. 21, no. 2, pp. 322-34, Aug 1981.
- J. Wang et al., “Effect of travoprost, latanoprost and bimatoprost PGF2α treatments on the biomechanical properties of in-vivo rabbit cornea,” (in eng), Experimental eye research, vol. 215, p. 108920, Feb 2022. [CrossRef]
- Kaushik and S. S. Pandav, “Ocular Response Analyzer,” (in eng), Journal of current glaucoma practice, vol. 6, no. 1, pp. 17-19, Jan-Apr 2012. [CrossRef]
- R. Joseph, O. P. Srivastava, and R. R. Pfister, “Modeling Keratoconus Using Induced Pluripotent Stem Cells,” (in eng), Investigative ophthalmology & visual science, vol. 57, no. 8, pp. 3685-97, Jul 1 2016. [CrossRef]
- G. A. Villalona et al., “Cell-seeding techniques in vascular tissue engineering,” (in eng), Tissue engineering. Part B, Reviews, vol. 16, no. 3, pp. 341-50, Jun 2010. [CrossRef]
- J. Fernández-Pérez and M. Ahearne, “Decellularization and recellularization of cornea: Progress towards a donor alternative,” (in eng), Methods (San Diego, Calif.), vol. 171, pp. 86-96, Jan 15 2020. [CrossRef]
- Nouri Barkestani, S. Naserian, G. Uzan, and S. Shamdani, “Post-decellularization techniques ameliorate cartilage decellularization process for tissue engineering applications,” Journal of Tissue Engineering, vol. 12, p. 2041731420983562, 2021/01/01 2021. [CrossRef]
- S. Avadhanam, H. E. Smith, and C. Liu, “Keratoprostheses for corneal blindness: a review of contemporary devices,” (in eng), Clin Ophthalmol, vol. 9, pp. 697-720, 2015. [CrossRef]
- G. Holland et al., “Artificial Cornea: Past, Current, and Future Directions,” (in English), Frontiers in Medicine, Review vol. 8, 2021-November-12 2021. [CrossRef]
- Gulati, S. K. Salaria, V. Anand, N. Jain, and S. Pandey, “Periodontium bestows vision!!,” (in eng), Journal of Indian Society of Periodontology, vol. 20, no. 3, pp. 349-51, May-Jun 2016. [CrossRef]
- V. Chirila, “An overview of the development of artificial corneas with porous skirts and the use of PHEMA for such an application,” (in eng), Biomaterials, vol. 22, no. 24, pp. 3311-7, Dec 2001. [CrossRef]
- G. J. Crawford et al., “The Chirila Keratoprosthesis: phase I human clinical trial,” (in eng), Ophthalmology, vol. 109, no. 5, pp. 883-9, May 2002. [CrossRef]
- Fagerholm et al., “A biosynthetic alternative to human donor tissue for inducing corneal regeneration: 24-month follow-up of a phase 1 clinical study,” (in eng), Science translational medicine, vol. 2, no. 46, p. 46ra61, Aug 25 2010. [CrossRef]
- J. M. Hackett et al., “Biosynthetic corneal implants for replacement of pathologic corneal tissue: performance in a controlled rabbit alkali burn model,” (in eng), Investigative ophthalmology & visual science, vol. 52, no. 2, pp. 651-7, Feb 3 2011. [CrossRef]
- Brunette et al., “Alternatives to eye bank native tissue for corneal stromal replacement,” (in eng), Progress in retinal and eye research, vol. 59, pp. 97-130, Jul 2017. [CrossRef]
- D. F. Duarte Campos et al., “Corneal bioprinting utilizing collagen-based bioinks and primary human keratocytes,” (in eng), Journal of biomedical materials research. Part A, vol. 107, no. 9, pp. 1945-1953, Sep 2019. [CrossRef]
- A. Isaacson, S. Swioklo, and C. J. Connon, “3D bioprinting of a corneal stroma equivalent,” (in eng), Experimental eye research, vol. 173, pp. 188-193, Aug 2018. [CrossRef]
- El-Massry, O. Ibrahim, M. Abdalla, I. Osman, and S. Mahmoud, “Safety and Indicative Effectiveness of Porcine Corneal Lenticular Implants in Patients with Advanced Keratoconus and Post Lasik Ectasia: A Retrospective Clinical Study,” (in eng), Clinical ophthalmology (Auckland, N.Z.), vol. 15, pp. 3165-3171, 2021. [CrossRef]
- Elsheikh, D. Alhasso, and P. Rama, “Biomechanical properties of human and porcine corneas,” (in eng), Experimental eye research, vol. 86, no. 5, pp. 783-90, May 2008. [CrossRef]
- H. S. Dua, L. A. Faraj, D. G. Said, T. Gray, and J. Lowe, “Human corneal anatomy redefined: a novel pre-Descemet’s layer (Dua’s layer),” (in eng), Ophthalmology, vol. 120, no. 9, pp. 1778-85, Sep 2013. [CrossRef]
- D. E. Birk, J. M. Fitch, and T. F. Linsenmayer, “Organization of collagen types I and V in the embryonic chicken cornea,” (in eng), Investigative ophthalmology & visual science, vol. 27, no. 10, pp. 1470-7, Oct 1986.
- K. Gordon, J. W. Foley, D. E. Birk, J. M. Fitch, and T. F. Linsenmayer, “Type V collagen and Bowman’s membrane. Quantitation of mRNA in corneal epithelium and stroma,” (in eng), The Journal of biological chemistry, vol. 269, no. 40, pp. 24959-66, Oct 7 1994.
- K. Gipson, S. J. Spurr-Michaud, and A. S. Tisdale, “Anchoring fibrils form a complex network in human and rabbit cornea,” (in eng), Investigative ophthalmology & visual science, vol. 28, no. 2, pp. 212-20, Feb 1987.
- Y. Komai and T. Ushiki, “The three-dimensional organization of collagen fibrils in the human cornea and sclera,” (in eng), Investigative ophthalmology & visual science, vol. 32, no. 8, pp. 2244-58, Jul 1991.
- A.J. Quantock et al., “From nano to macro: studying the hierarchical structure of the corneal extracellular matrix,” (in eng), Experimental eye research, vol. 133, pp. 81-99, Apr 2015. [CrossRef]
- C. Davies, S. J. Jenkins, J. E. Allen, and P. R. Taylor, “Tissue-resident macrophages,” (in eng), Nature immunology, vol. 14, no. 10, pp. 986-95, Oct 2013. [CrossRef]
- Kawakita, E. M. Espana, H. He, W. Li, C. Y. Liu, and S. C. Tseng, “Intrastromal invasion by limbal epithelial cells is mediated by epithelial-mesenchymal transition activated by air exposure,” (in eng), The American journal of pathology, vol. 167, no. 2, pp. 381-93, Aug 2005. [CrossRef]
- M. Fitch, D. E. Birk, C. Linsenmayer, and T. F. Linsenmayer, “The spatial organization of Descemet’s membrane-associated type IV collagen in the avian cornea,” (in eng), The Journal of cell biology, vol. 110, no. 4, pp. 1457-68, Apr 1990. [CrossRef]
- S. Penn, A. Madan, R. B. Caldwell, M. Bartoli, R. W. Caldwell, and M. E. Hartnett, “Vascular endothelial growth factor in eye disease,” (in eng), Progress in retinal and eye research, vol. 27, no. 4, pp. 331-71, Jul 2008. [CrossRef]
- R. Hassell and D. E. Birk, “The molecular basis of corneal transparency,” (in eng), Experimental eye research, vol. 91, no. 3, pp. 326-35, Sep 2010. [CrossRef]
- D. M. Maurice, “The structure and transparency of the cornea,” (in eng), The Journal of physiology, vol. 136, no. 2, pp. 263-86, Apr 30 1957. [CrossRef]
- M. Meek and D. W. Leonard, “Ultrastructure of the corneal stroma: a comparative study,” (in eng), Biophysical journal, vol. 64, no. 1, pp. 273-80, Jan 1993. [CrossRef]
- D. E. Birk and R. L. Trelstad, “Extracellular compartments in matrix morphogenesis: collagen fibril, bundle, and lamellar formation by corneal fibroblasts,” (in eng), The Journal of cell biology, vol. 99, no. 6, pp. 2024-33, Dec 1984. [CrossRef]
- M. Griffith, R. d. Cataldo, and K. H. Fogarty, “Do-It-Yourself: 3D Models of Hydrogenic Orbitals through 3D Printing,” Journal of Chemical Education, vol. 93, no. 9, pp. 1586-1590, 2016/09/13 2016. [CrossRef]
- Z. Gu, J. Fu, H. Lin, and Y. He, “Development of 3D bioprinting: From printing methods to biomedical applications,” (in eng), Asian journal of pharmaceutical sciences, vol. 15, no. 5, pp. 529-557, Sep 2020. [CrossRef]
- J. Kort-Mascort et al., “Decellularized ECM hydrogels: prior use considerations, applications, and opportunities in tissue engineering and biofabrication,” Biomaterials Science, 10.1039/D2BM01273A vol. 11, no. 2, pp. 400-431, 2023. [CrossRef]
- M. Giraud Guille, G. Mosser, C. Helary, and D. Eglin, “Bone matrix like assemblies of collagen: from liquid crystals to gels and biomimetic materials,” (in eng), Micron (Oxford, England: 1993), vol. 36, no. 7-8, pp. 602-8, 2005. [CrossRef]
- Hemmavanh, M. Koch, D. E. Birk, and E. M. Espana, “Abnormal corneal endothelial maturation in collagen XII and XIV null mice,” (in eng), Investigative ophthalmology & visual science, vol. 54, no. 5, pp. 3297-308, May 7 2013. [CrossRef]
- K. Olivero and L. T. Furcht, “Type IV collagen, laminin, and fibronectin promote the adhesion and migration of rabbit lens epithelial cells in vitro,” (in eng), Investigative ophthalmology & visual science, vol. 34, no. 10, pp. 2825-34, Sep 1993.
- E.-L. Martola and J. L. Baum, “Central and Peripheral Corneal Thickness: A Clinical Study,” Archives of Ophthalmology, vol. 79, no. 1, pp. 28-30, 1968. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





