Submitted:
31 March 2023
Posted:
31 March 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
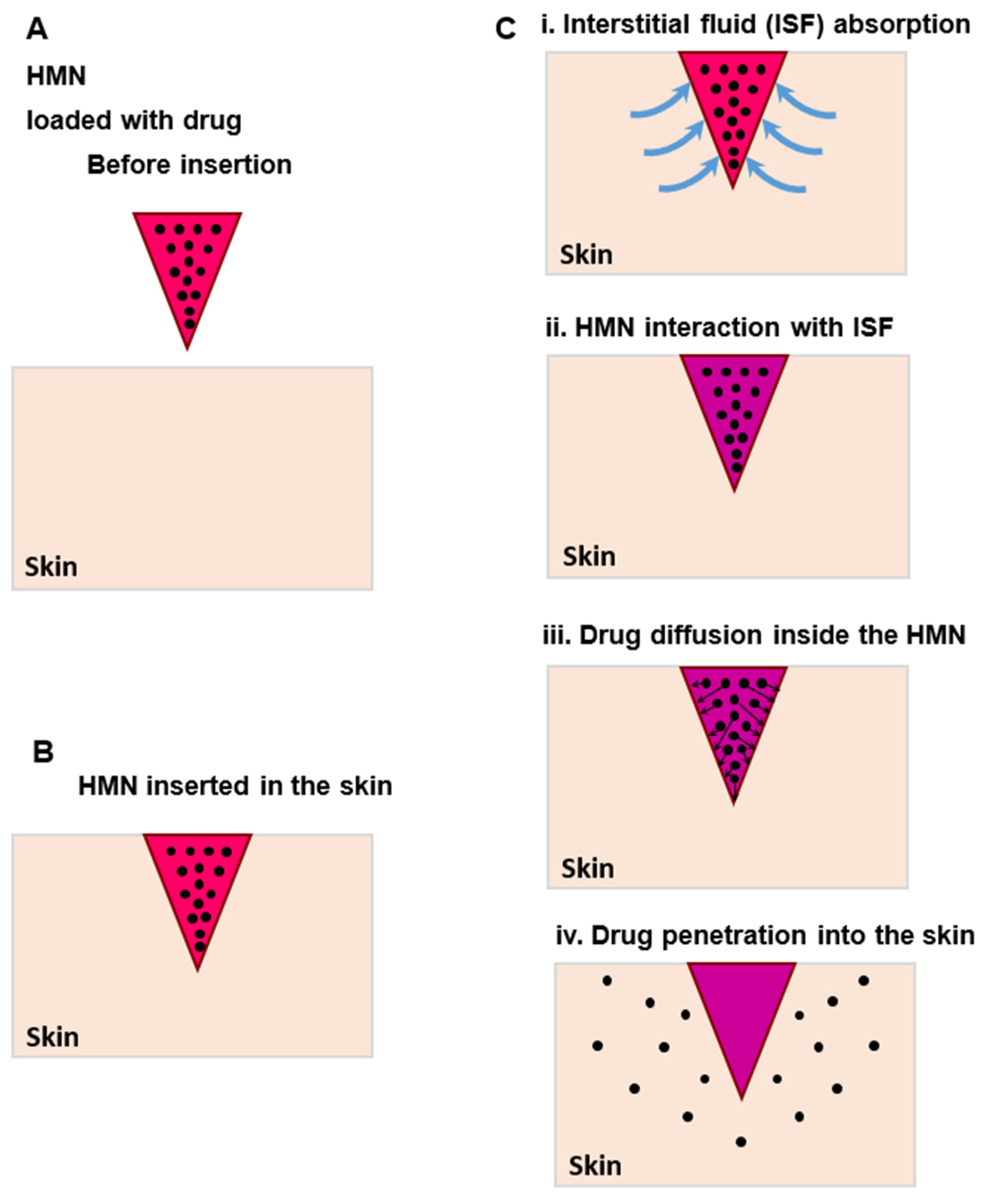
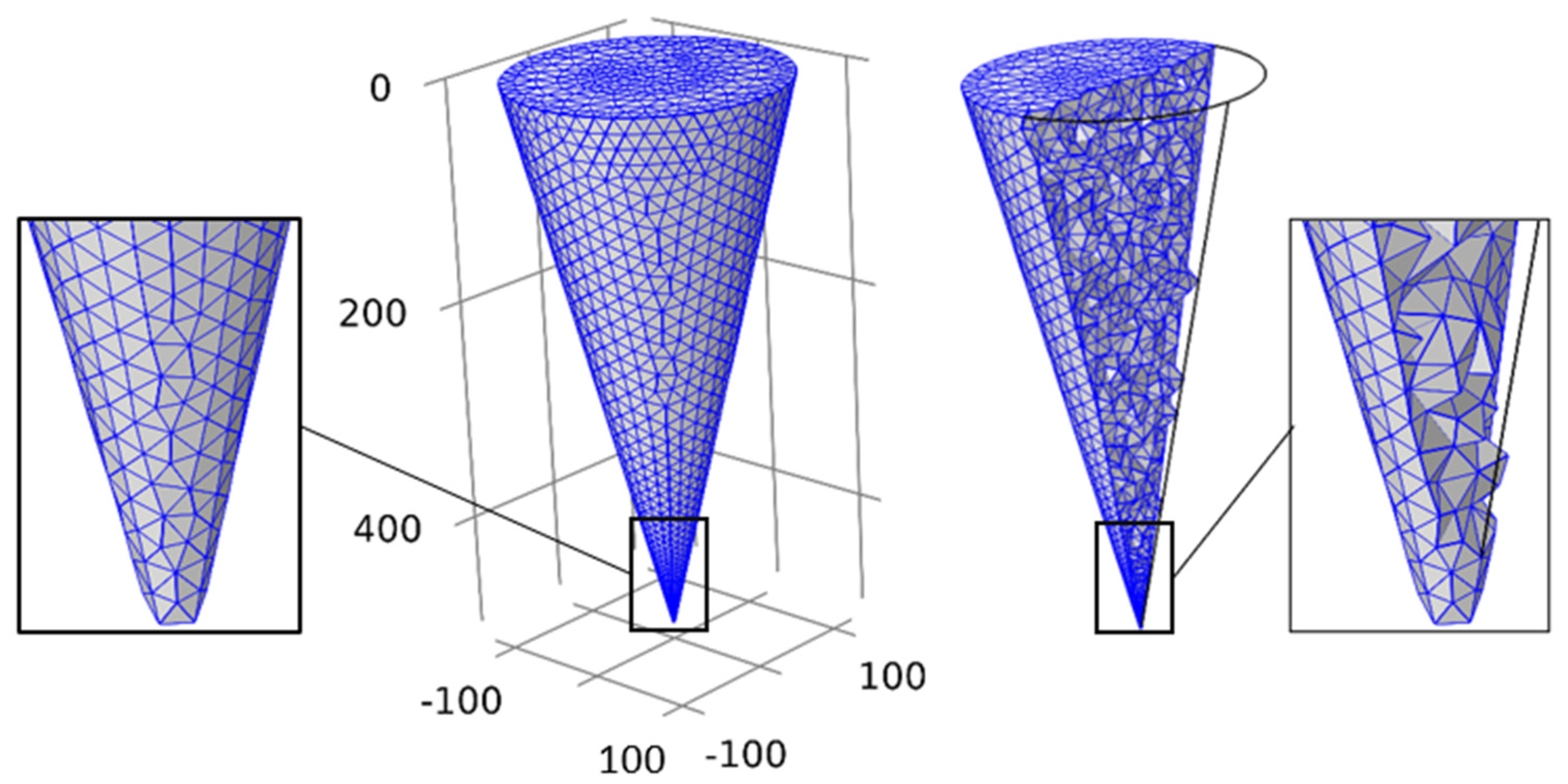
2. Materials and Methods
2.1. Governing equation and boundary conditions
3. Results and Discussion
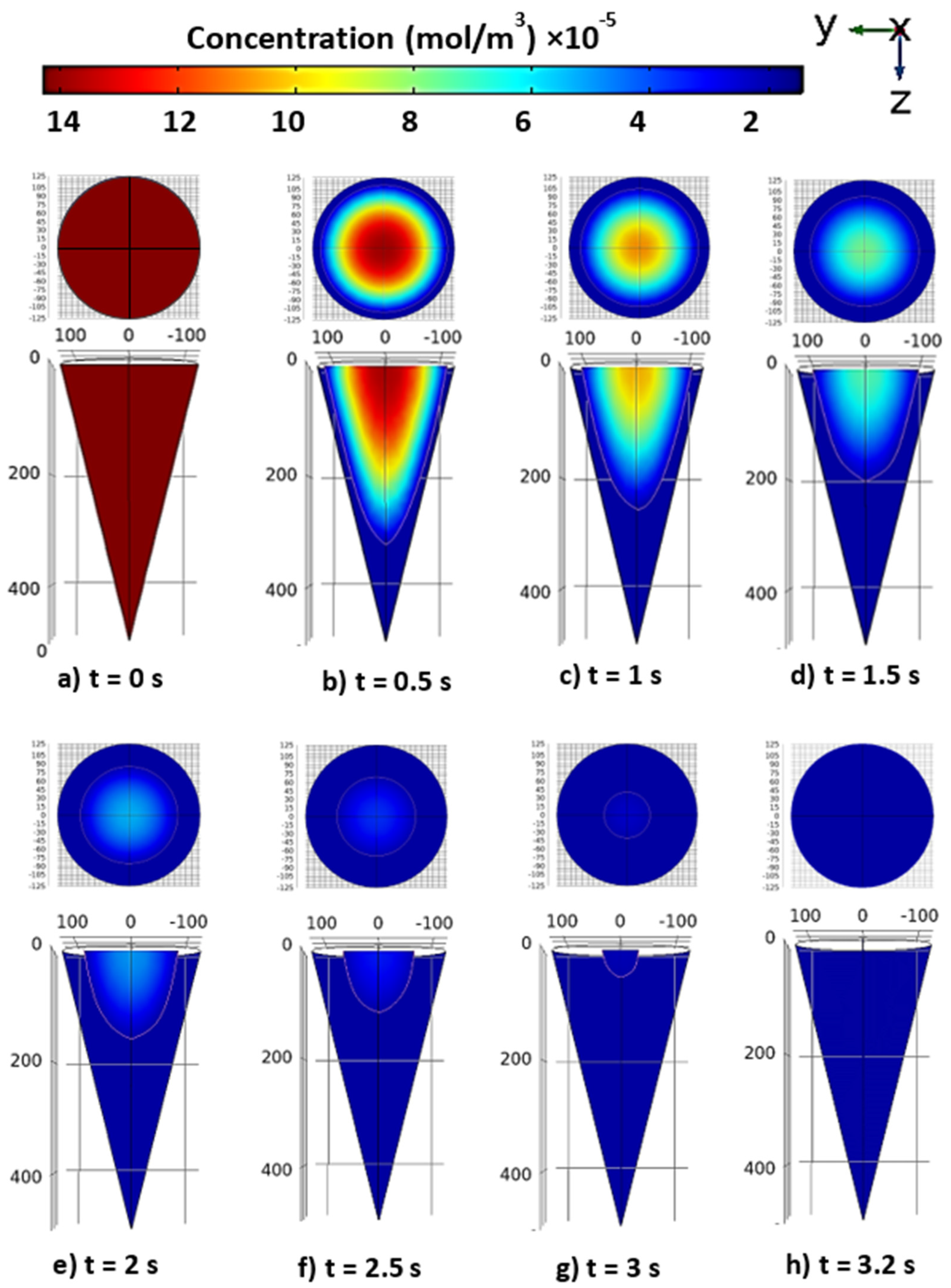
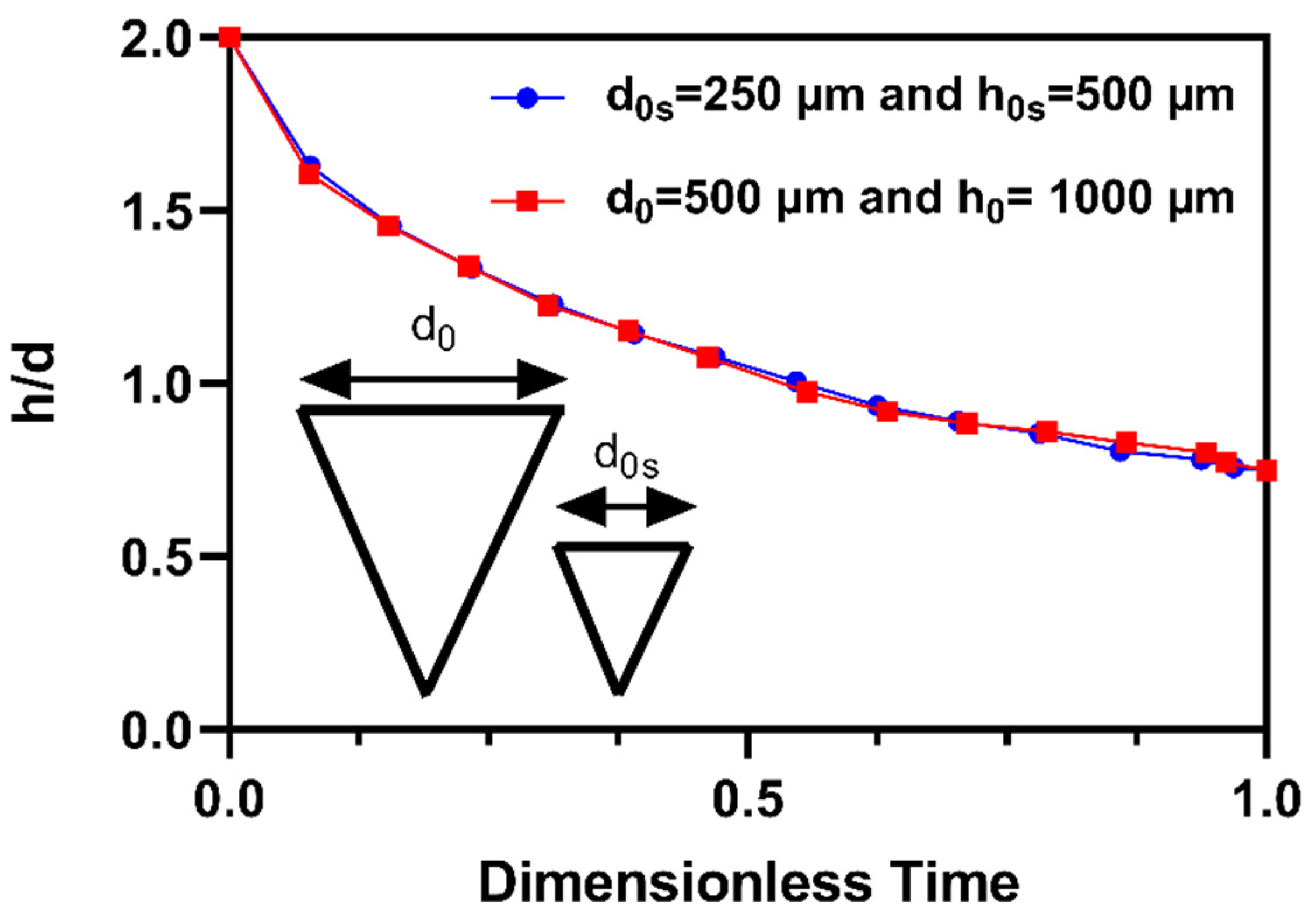
3.1. Drug diffusion inside the tapered MN
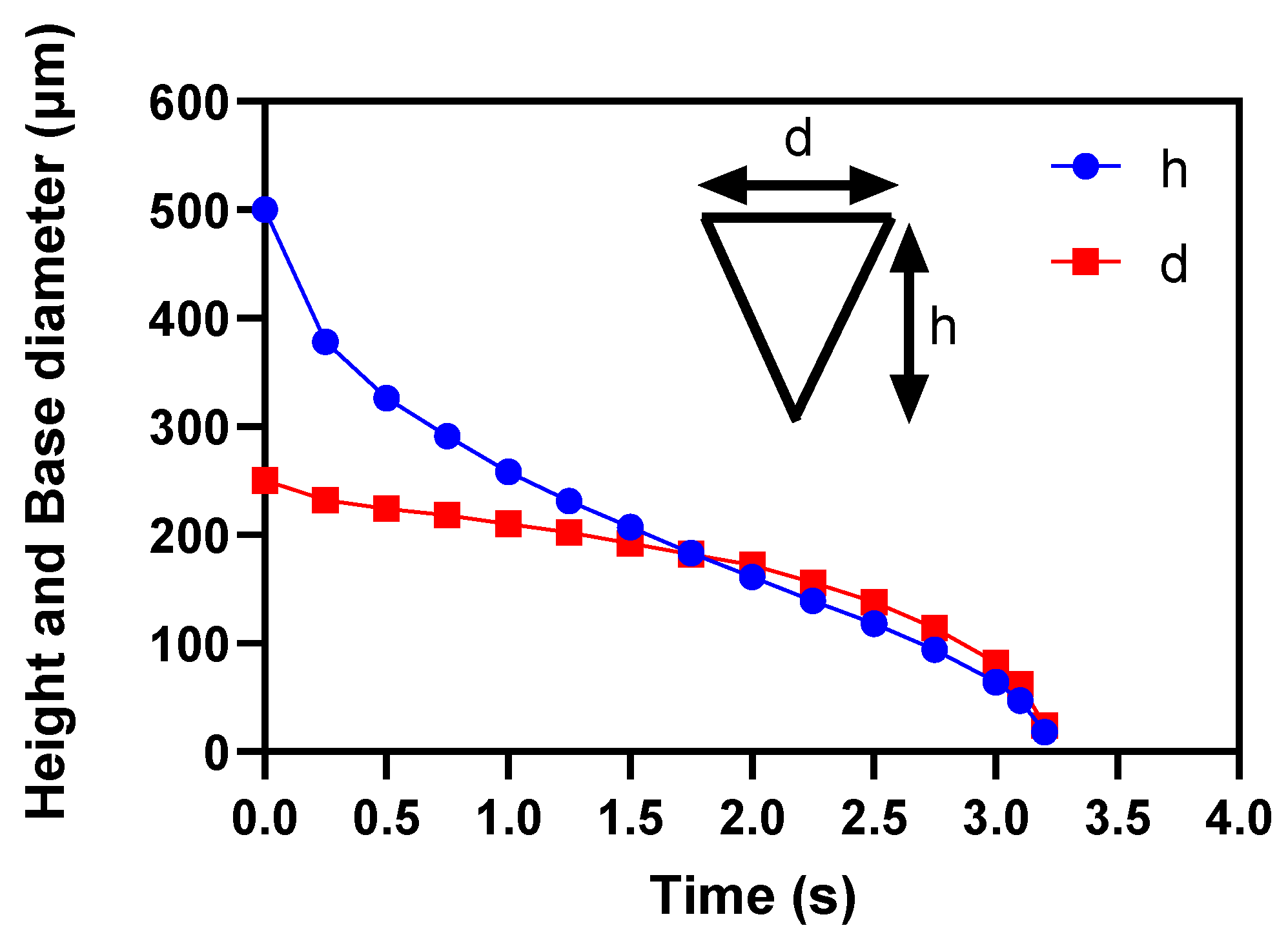
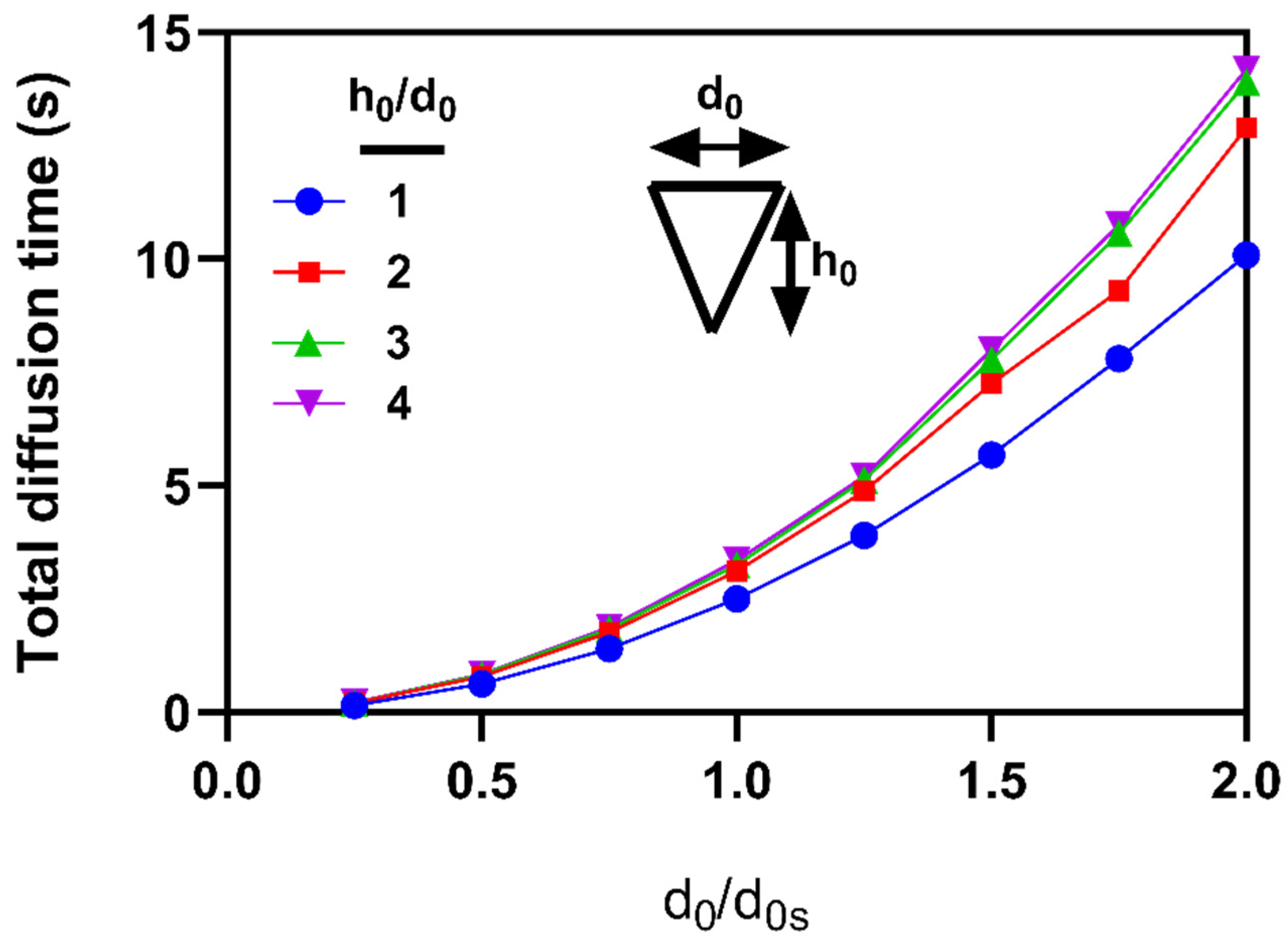
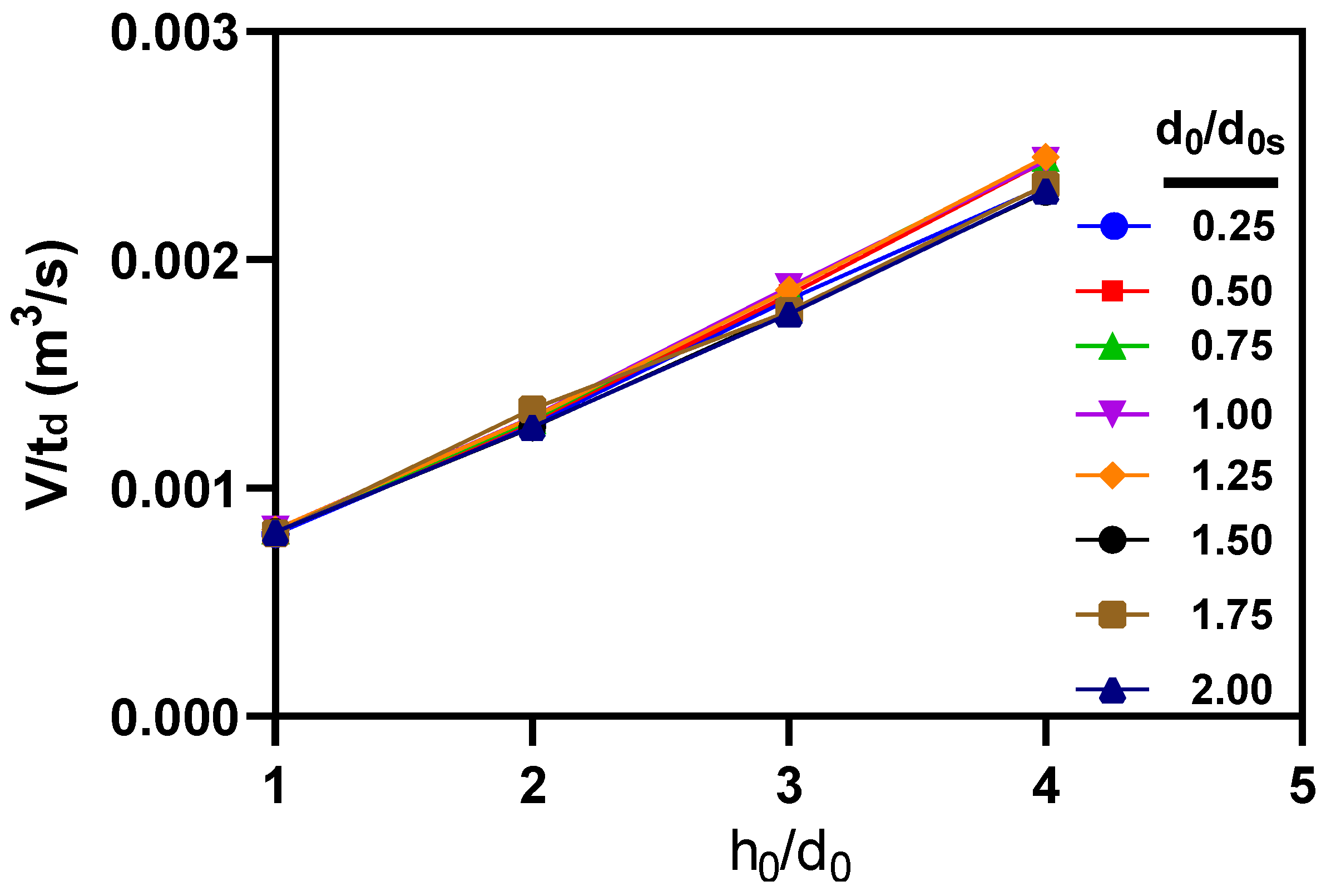
3.3. Effects of microneedle’s height and base diameter on drug diffusion
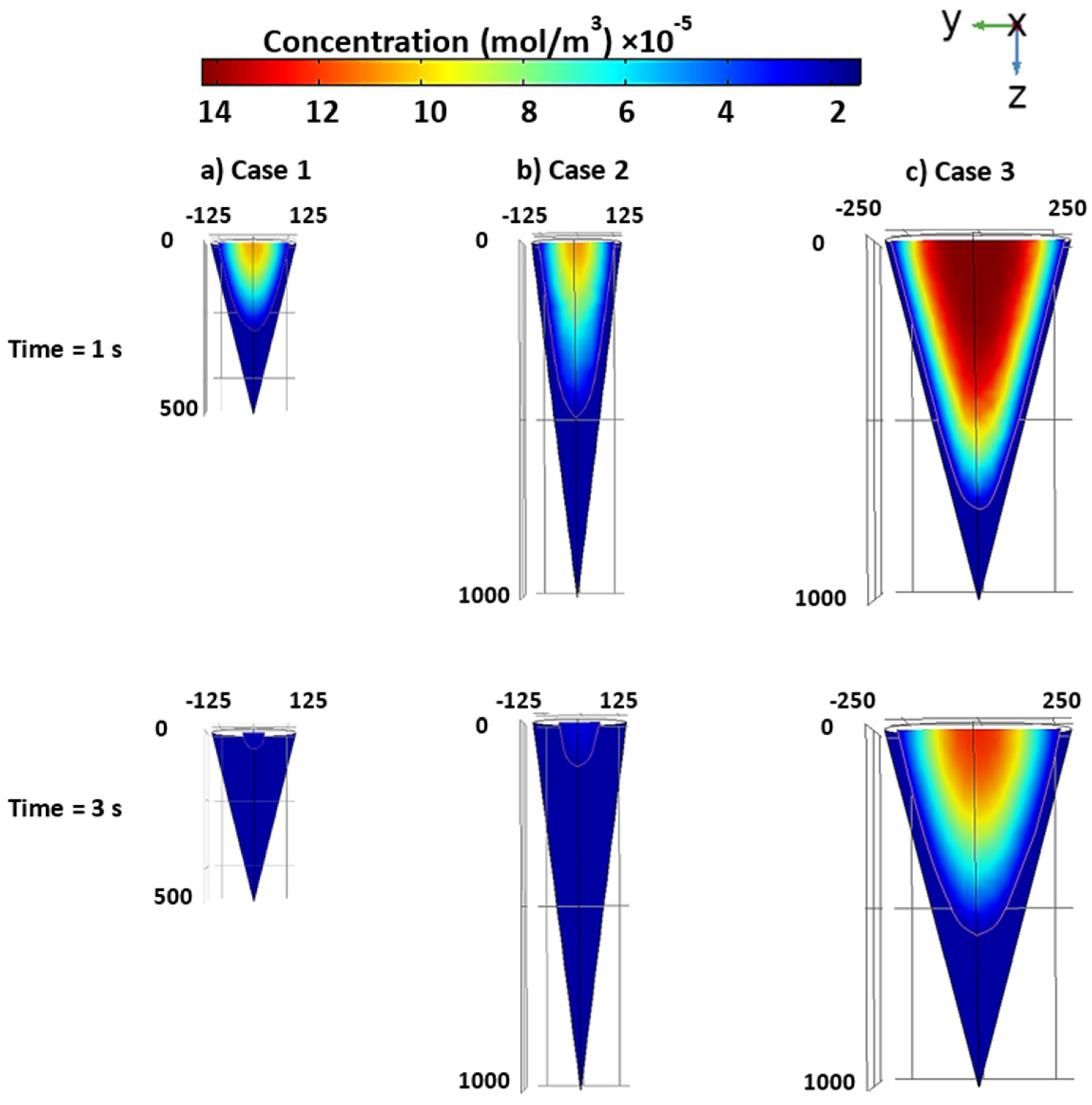
3.4. Case studies
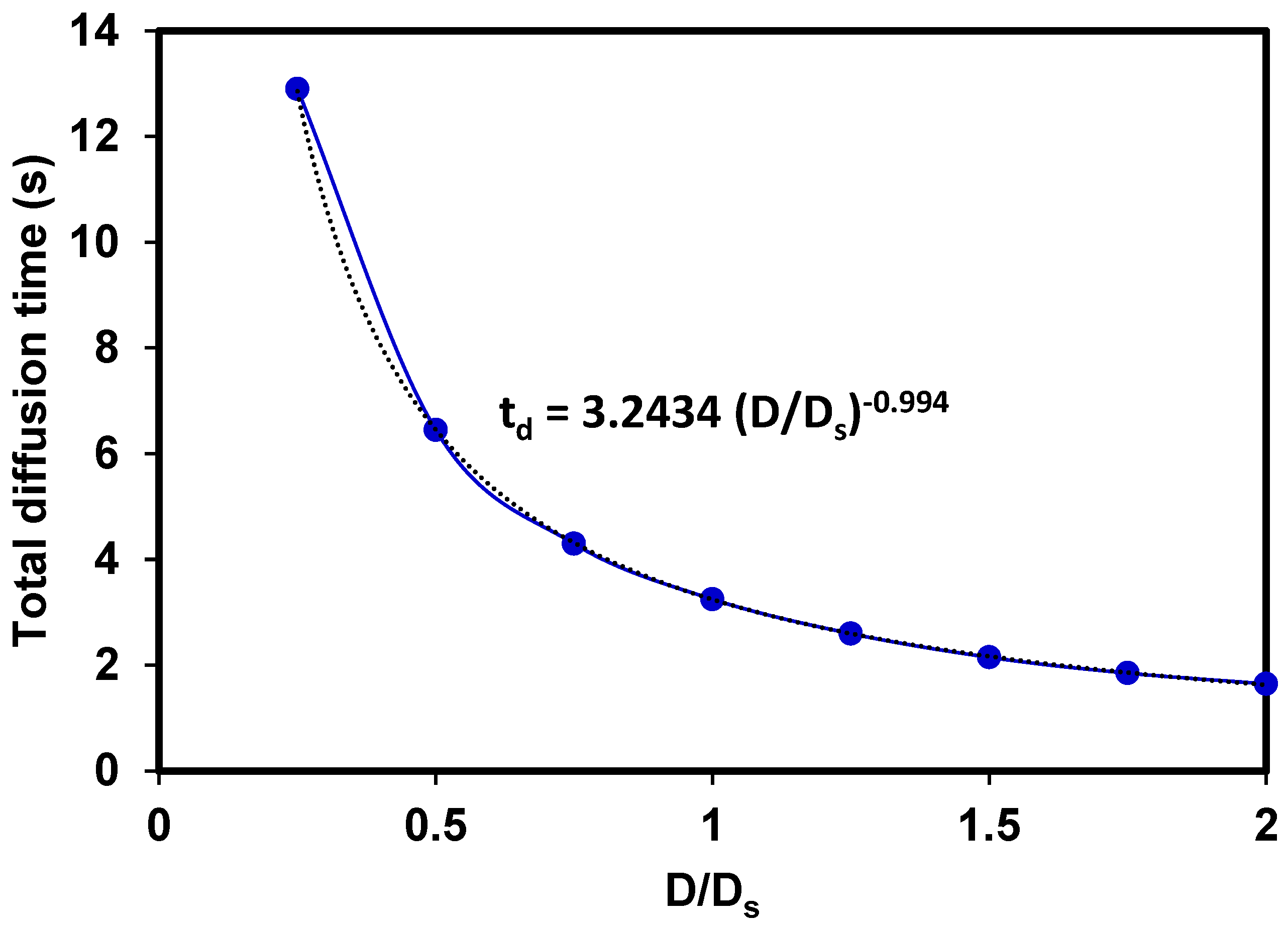
3.5. Effects of the diffusion coefficient
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bilal, M., et al., Microneedles in smart drug delivery. Advances in wound care, 2021. 10(4): p. 204-219. [CrossRef]
- Choi, S.-O. and J.-H. Park, Microneedle technologies. 2021, Springer. p. 1-2. [CrossRef]
- Kashaninejad, N., E. Moradi, and H. Moghadas, Chapter Two - Micro/nanofluidic devices for drug delivery, in Progress in Molecular Biology and Translational Science, A. Pandya and V. Singh, Editors. 2022, Academic Press. p. 9-39.
- Prausnitz, M.R., Engineering microneedle patches for vaccination and drug delivery to skin. Annual review of chemical and biomolecular engineering, 2017. 8: p. 177-200. [CrossRef]
- Jenkins, D., et al., High density and high aspect ratio solid micro-nanoprojection arrays for targeted skin vaccine delivery and specific antibody extraction. Rsc Advances, 2012. 2(8): p. 3490-3495. [CrossRef]
- Arya, J. and M.R. Prausnitz, Microneedle patches for vaccination in developing countries. Journal of Controlled Release, 2016. 240: p. 135-141. [CrossRef]
- Leone, M., et al., Dissolving microneedle patches for dermal vaccination. Pharmaceutical research, 2017. 34(11): p. 2223-2240. [CrossRef]
- Kim, E., et al., Microneedle array delivered recombinant coronavirus vaccines: Immunogenicity and rapid translational development. EBioMedicine, 2020. 55: p. 102743. [CrossRef]
- Queiroz, M.L.B., et al., Microneedles as an alternative technology for transdermal drug delivery systems: a patent review. Expert Opinion on Therapeutic Patents, 2020. 30(6): p. 433-452. [CrossRef]
- Yahyazadeh Shourabi, A., N. Kashaninejad, and M.S. Saidi, An integrated microfluidic concentration gradient generator for mechanical stimulation and drug delivery. Journal of Science: Advanced Materials and Devices, 2021. 6(2): p. 280-290. [CrossRef]
- Rismanian, M., M.S. Saidi, and N. Kashaninejad, A new non-dimensional parameter to obtain the minimum mixing length in tree-like concentration gradient generators. Chemical Engineering Science, 2019. 195: p. 120-126. [CrossRef]
- Ebadi, M., et al., A tool for designing tree-like concentration gradient generators for lab-on-a-chip applications. Chemical Engineering Science, 2020. 212: p. 115339. [CrossRef]
- Vu, H.H., N.-T. Nguyen, and N. Kashaninejad, Re-Entrant Microstructures for Robust Liquid Repellent Surfaces. Advanced Materials Technologies, 2023. 8(5): p. 2201836. [CrossRef]
- Kashaninejad, N. and N.-T. Nguyen, Microfluidic solutions for biofluids handling in on-skin wearable systems. Lab on a Chip, 2023. 23(5): p. 913-937. [CrossRef]
- Han, T. and D.B. Das, A new paradigm for numerical simulation of microneedle-based drug delivery aided by histology of microneedle-pierced skin. Journal of pharmaceutical sciences, 2015. 104(6): p. 1993-2007. [CrossRef]
- Larraneta, E., et al., Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Materials Science and Engineering: R: Reports, 2016. 104: p. 1-32. [CrossRef]
- Nagarkar, R., et al., A review of recent advances in Microneedle technology for transdermal drug delivery. Journal of Drug Delivery Science and Technology, 2020: p. 101923. [CrossRef]
- Kashaninejad, N., et al., Microneedle Arrays for Sampling and Sensing Skin Interstitial Fluid. Chemosensors, 2021. 9(4): p. 83. [CrossRef]
- Choi, I.-J., et al., Live Vaccinia Virus-Coated Microneedle Array Patches for Smallpox Vaccination and Stockpiling. Pharmaceutics, 2021. 13(2): p. 209. [CrossRef]
- Li, X., et al., Gene-coated Microneedle Patch based on Industrialized Ultrasonic Spraying Technology with Polycation Vector to Improve Antitumor Efficacy. Journal of Materials Chemistry B, 2021. [CrossRef]
- Mishra, R., T.K. Maiti, and T.K. Bhattacharyya, Design and scalable fabrication of hollow SU-8 microneedles for transdermal drug delivery. IEEE Sensors Journal, 2018. 18(14): p. 5635-5644. [CrossRef]
- Turner, J.G., et al., Hydrogel-forming microneedles: current advancements and future trends. Macromolecular Bioscience, 2021. 21(2): p. 2000307.
- Kim, K.S., K. Ita, and L. Simon, Modelling of dissolving microneedles for transdermal drug delivery: theoretical and experimental aspects. European Journal of Pharmaceutical Sciences, 2015. 68: p. 137-143. [CrossRef]
- Ronnander, P., et al., Modelling the in-vitro dissolution and release of sumatriptan succinate from polyvinylpyrrolidone-based microneedles. European Journal of Pharmaceutical Sciences, 2018. 125: p. 54-63. [CrossRef]
- Olatunji, O., D.B. Das, and B. Al-Qallaf. Simulation based optimization of microneedle geometry to improve drug permeability in skin. in Proceedings of the 7th Industrial Simulation Conference. 2009. Citeseer.
- Machekposhti, S., et al., Biocompatible polymer microneedle for topical/dermal delivery of tranexamic acid. Journal of Controlled Release, 2017. 261: p. 87-92. [CrossRef]
- Ronnander, P., L. Simon, and A. Koch, Experimental and mathematical study of the transdermal delivery of sumatriptan succinate from polyvinylpyrrolidone-based microneedles. European Journal of Pharmaceutics and Biopharmaceutics, 2020. 146: p. 32-40. [CrossRef]
- Zoudani, E. and M. Soltani, A new computational method of modeling and evaluation of dissolving microneedle for drug delivery applications: Extension to theoretical modeling of a novel design of microneedle (array in array) for efficient drug delivery. European Journal of Pharmaceutical Sciences, 2020. 150: p. 105339. [CrossRef]
- Castilla-Casadiego, D.A., et al., Design, characterization, and modeling of a chitosan microneedle patch for transdermal delivery of meloxicam as a pain management strategy for use in cattle. Materials Science and Engineering: C, 2021. 118: p. 111544. [CrossRef]
- Yadav, P.R., et al., Mathematical modelling, simulation and optimisation of microneedles for transdermal drug delivery: trends and progress. Pharmaceutics, 2020. 12(8): p. 693. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



