1. Introduction
In 2015 the National Institute of Child Health and Human Development expert panel recommended the use of the term “Triple I” or TI (Intrauterine Inflammation, Infection or both) to replace the term of chorioamnionitis, in order to refine the definition and management of a heterogeneous array of conditions characterized by infection and inflammation in pregnancy [
1].
The TI definition is suggested because single clinical variables are not able to predict clinical outcomes and have a low diagnostic performance if considered alone, but the combination of them seems to be more related to both maternal and fetal outcomes.
Maternal fever, which represents the main diagnostic criteria, has a very low diagnostic accuracy if considered alone, since it can be caused by extra-uterine infection/inflammation, by the use of epidural anesthesia or by prostaglandins to induce labor.
Fetal tachycardia may be present also in absence of chorioamnionitis, for example related to some drugs use (i.e. ephedrine and beta agonists) or to transient fetal hypoxia [
2]. Maternal fever and fetal tachycardia together have been described as risk factors for maternal and neonatal adverse outcomes [
3,
4,
5].
Maternal leukocytosis has a low specificity (5%-30%) as a sign of intra-amniotic infection (IAI), since it can be caused by corticosteroids administration, labor pain, and labor duration [
6]; moreover, maternal leukocytosis at admission can be even associated with severe adverse infant neurodevelopmental outcomes [
7].
Meconium stained and/or foul smelling amniotic fluid seem more specific signs and in particular they can predict funisitis in case of maternal fever [
8]; purulent amniotic fluid is more frequent in patients with severe or prolonged infection [
9]. Both these conditions should alert the physician to the potential for infection and increased perinatal morbidity in that infant [
10].
“Histological chorioamnionitis” (HCA) refers to histopathological inflammation with or without clinical or microbiological findings associated with acute infection, introducing the possibility of sterile inflammation of tissues [
11]. Prevalence of HCA is inversely related to gestational age, occurring in 20% of deliveries at term after spontaneous labor and up to 50% of preterm births [
12]. Histological data retrospectively support the diagnosis of Triple I according to the definition of this condition.
The association between placental histopathologic findings and adverse clinical outcomes have been described in literature [
13]: deepness of placental invasion by polymorphonucleated define the grading and staging of HCA, which are related to the severity of the process and consequently to adverse maternal and neonatal outcomes. An advanced HCA stage leads to a worse prognosis, such as the occurrence of neonatal sepsis. Maternal fever in term deliveries worsens maternal and neonatal outcomes in the presence of HCA [
14], [
15]. Even worse perinatal outcomes (i.e. respiratory distress syndrome, neonatal sepsis, pneumonia, necrotizing enterocolitis) can occur if the inflammatory response involves the umbilical vein, the arteries and the chorionic plate surface vessels, defining the funisitis scenario [
16,
17,
18].
On maternal side, HCA and in particular funisitis are associated with labor dystocia, postpartum hemorrhage, postpartum fever, endometritis/miometritis, and even sepsis [
19,
20,
21].
Some studies have been investigating the role of TI criteria in predicting the diagnosis of intra-amniotic infection (IAI) and demonstrated some controversies; therefore, more studies about the clinical implication of TI definition need to be performed [
22], [
23].
In particular, it has been unclear if the clinical signs or the histology pattern better relate to adverse perinatal and maternal outcomes and how the combination between these two tools can impact on maternal and neonatal prognosis.
The primary aim of our study is to assess the relation between clinical antenatal signs supporting or not the complete TI spectrum and perinatal and maternal outcomes.The second aim is to evaluate the relation between clinical criteria of TI and placental histology and to investigate the ability of placental histologic findings to predict perinatal outcomes.
2. Materials and Methods
2.1. Population
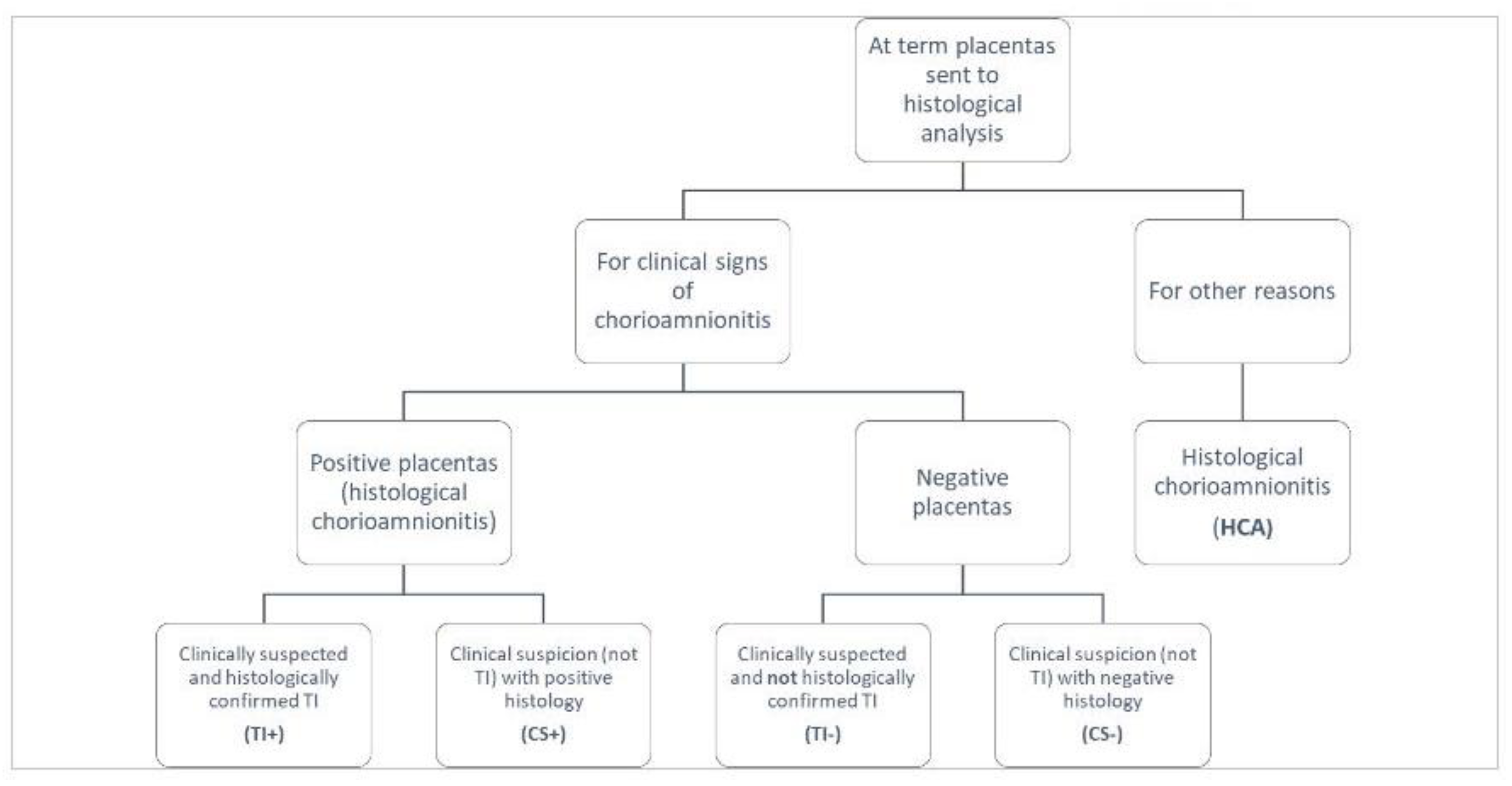
We conducted a retrospective cohort study including term pregnancies (≥37 g.a.) with clinical suspicion and/or histological diagnosis of IAI and/or inflammation. Cases in the study originated from the histological analysis of all at term placentas sent to the pathologist Unit because of clinical suspicion of IAI or for other reasons (
Figure 1) in the period January 2014 - July 2017.
All these groups were divided in diagnostic categories, based on clinical and histological data found in Maternal Unit records.
Suggestive data for suspected and confirmed TI were collected: body temperature, white blood cells count, fetal heart rate, qualitative amniotic fluid characteristics and placental histology, which could be either maternal, fetal or both side inflammation.
Data collection also included: maternal age, Body Mass Index (BMI), parity, gestational age, Group B Streptococcus assay (GBS), Premature Rupture of Membranes (PROM), time from PROM to delivery, induction of labor, use of oxytocin, epidural anesthesia, duration of labor, number of vaginal examinations, mode of delivery (vaginal or caesarean), blood loss, antibiotic therapy during labour and in the postpartum.
We considered TI diagnostic criteria according to NICHD definition; maternal temperature 38.0 °C or greater plus any of the followings:
- -
Baseline fetal tachycardia (greater than 160 beats per min for 10 min or longer, excluding accelerations, decelerations, and periods of marked variability);
- -
Maternal white blood cell count greater than 15,000 per mm3 in the absence of corticosteroids;
- -
Definite purulent fluid from the cervical os.
Based on clinical and histological data, population on study was divided into five all-inclusive and mutually exclusive groups:
Suspected TI (TI-): cases with clinical signs respecting all TI diagnostic criteria not confirmed by histological analysis.
Confirmed TI (TI+): cases with clinical signs respecting all TI criteria plus positive placental histology revealing diagnostic features of infection and/or inflammation. To define TI+ we considered only placental data and not laboratory findings (amniotic fluid analysis) included in the definition of confirmed TI proposed by NICHD.
Positive histology with clinical suspicion (CS+): positive placental histology in suspected cases not respecting all TI diagnostic criteria (i.e. isolate maternal fever or maternal leukocytosis with meconium stained amniotic fluid).
Negative histology with clinical suspicion (CS-): negative placental histology in suspected cases of IAI or intra-amniotic inflammation not respecting all TI diagnostic criteria.
Histological chorioamnionitis without clinical signs (HCA): cases of acute infection documented by histological examination but in absence of fever, symptoms or signs of chorioamnionitis.
Composite maternal outcome included blood loss >1000 mL and/or postpartum fever and/or endometritis and/or postpartum antibiotic therapy and/or re-admission.
Composite neonatal outcome included Apgar score at 5 min <7 and/or C-Reactive Protein (CRP) > 1 mg/dl and/or positive hemoculture and/or antibiotic therapy at discharge and/or subsequent hospital re-admission.
2.2. Placental Analysis
Placental samples were collected and analyzed by the pathologist Unit of ASST-Brianza according to the classification proposed by Redline, where placental lesions are divided into Maternal and Fetal Inflammatory Response and are respectively staged as 1, 2 or 3 [
14].
On maternal side, stage 1 is represented by the presence of neutrophils in the subchorial intervillous space or beneath the chorion laeve layer (acute subchorionitis or chorionitis), stage 2 stands as the involvement of both the chorion and amnion and stage 3 as necrotizing chorioamnionitis. Fetal Inflammatory Response includes stage 1 as umbilical phlebitis, stage 2 as the involvement of the umbilical vein and one or more umbilical arteries, and stage 3 as necrotizing funisitis.
We defined WPLI (Whole Placental Involvement) as a concurrent inflammatory response of maternal and fetal side of the placenta (acute chorioamnionitis plus Fetal Inflammatory Response).
2.3. Statistical Analysis
Categorical variables were reported as numbers and percentages and continuous variables as mean and standard deviation. Categorical variables were analysed by Chi-square test and continuous ones by ANOVA test. A p-value<0.005 and an Odds Ratio (OR) with 95% CI were considered statistically significant.
The sensibility, specificity, Positive Predictive Value (PPV), Negative Predictive Value (NPV), diagnostic accuracy and Likelihood Ratio (LR) of TI and WPLI as predictive factors of composite neonatal outcome were calculated.
A multivariate logistic regression was performed to assess the independent effect of histological damage and clinical signs on composite adverse neonatal outcome.
Statistical analyses were performed using SPSS version 22.0.
3. Results
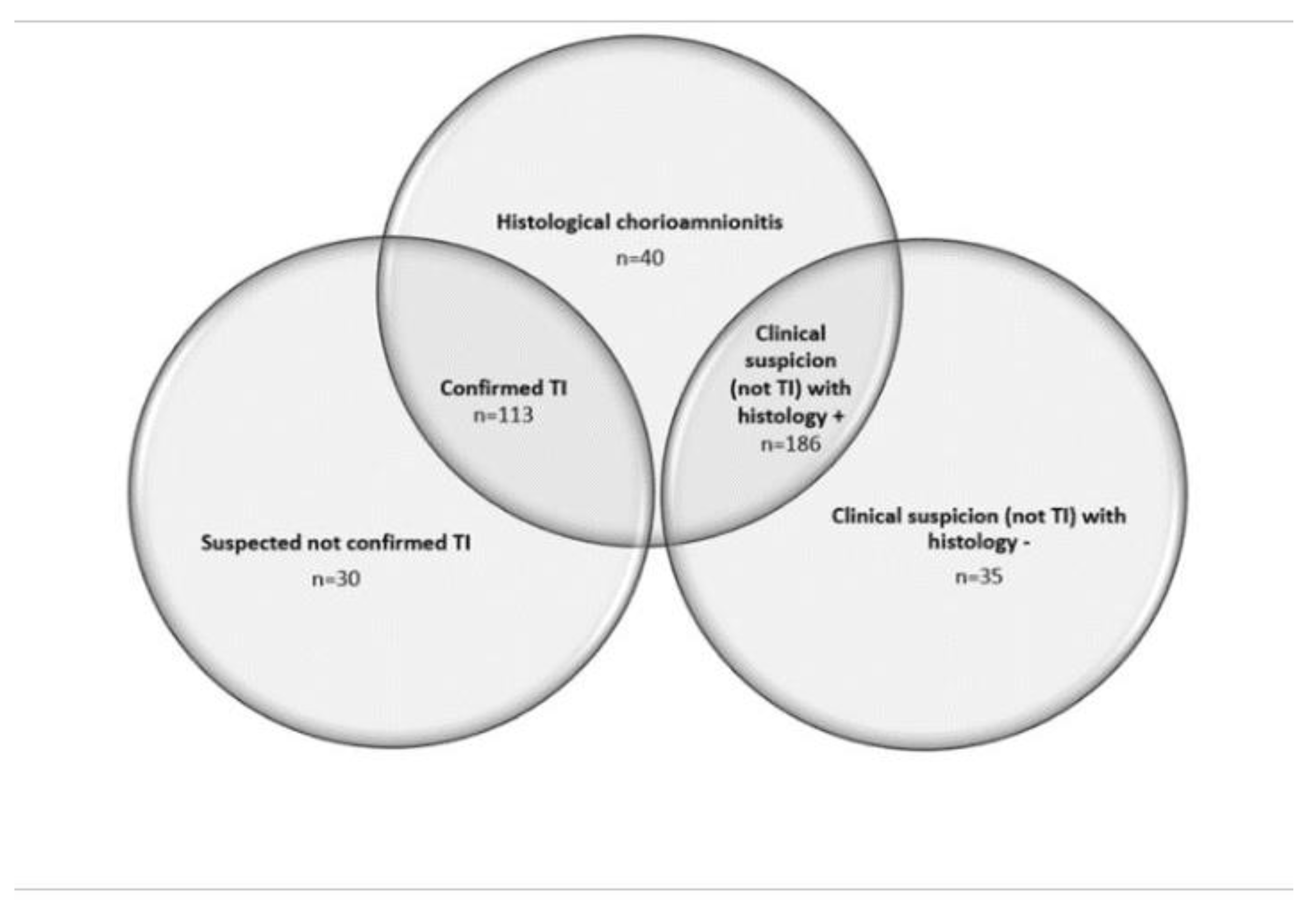
Between January 2014 and July 2017 in the Maternity Unit of Carate Brianza Hospital, Vittorio Emanuele III, out of 6,962 term deliveries, 1,122 placentas were sent for histological analysis. Population on study was composed of 404 term deliveries (364 cases presenting symptoms and/or signs suggestive for inflammation/infection plus 40 isolated HCA without clinical signs).
Within cases with clinical signs suggestive for infection and/or inflammation, 299 placentas (82.1%) presented positive histological findings, while 65 (17.9%) were negative.
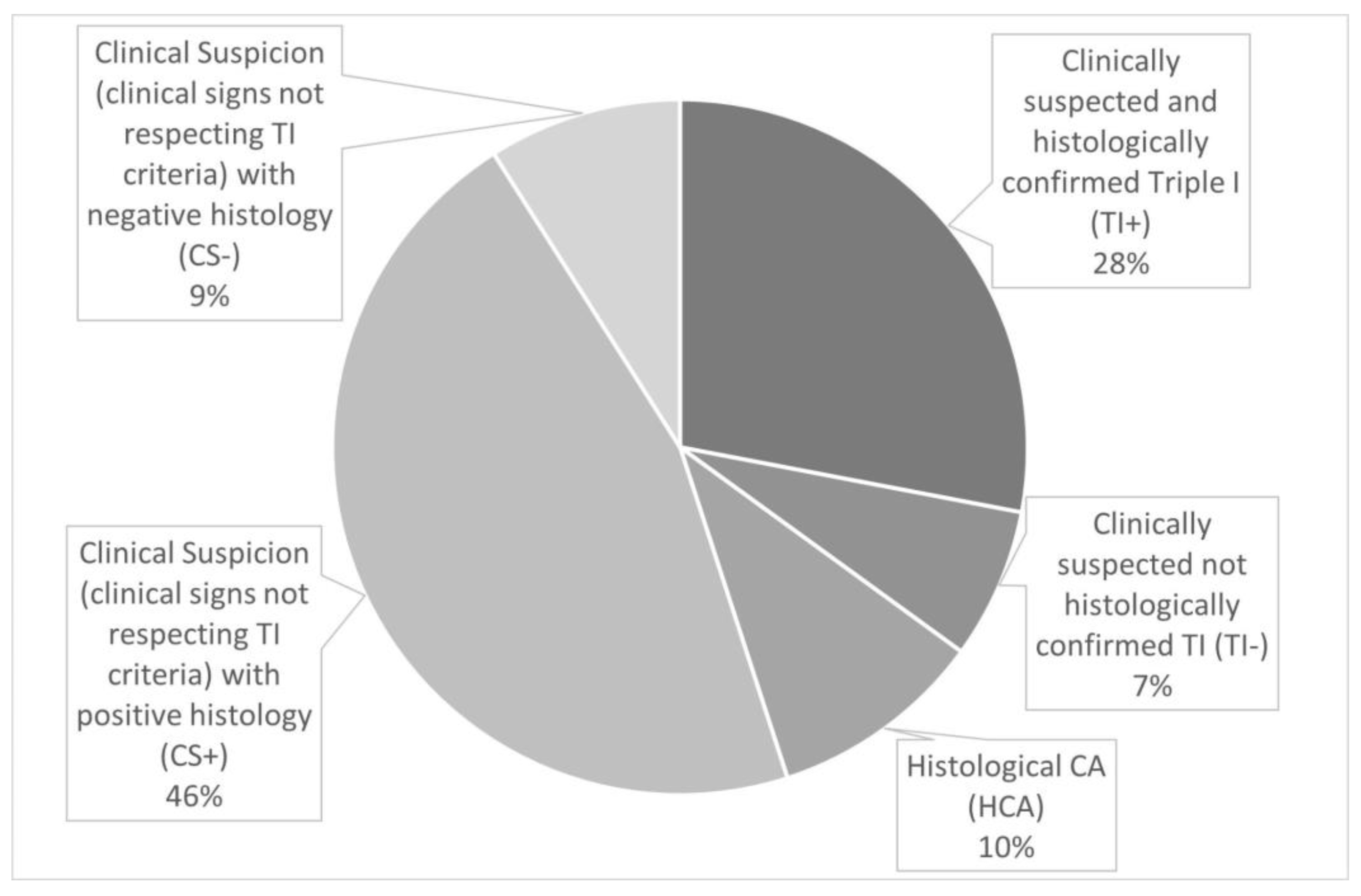
The five groups were divided in the population on study as follows (
Figure 2 and
Figure 3): TI+ (n=113, 28%), TI- (n=30, 7%), CS+ (n=186, 46%), CS- (n=35, 9%), HCA (n=40, 10%).
Among 339 placentas (30.2%) presenting histological chorioamnionitis, 204 (60.2%) presented only Maternal Inflammatory Response, 2 (0.6%) only Fetal Inflammatory Response and 133 (39.2%) WPLI.
Main characteristics of the population in study are summarized in
Table 1.
Antibiotic therapy during labor was administered in 60.5% cases: ampicillin was used in 31.3% of cases, amoxicillin+clavulanic in 12.9% and in 16.4% broad spectrum antibiotics were administered. MSAF was observed in 51% of our cases, of which 82% of cases with suspected IAI confirmed by histology. Excluding cases with HCA without clinical suspicion, the presence of MSAF was very common (56.5%), even if more frequent in cases with negative compared to cases with positive histology (67% vs 54%, p-value=0.13).
Maternal and neonatal outcomes are described in
Table 2. Composite maternal adverse outcomes occurred in 192 patients (47.5%). Neonatal composite outcome occurred in 114 cases (28.2%) and Early Onset Sepsis (EOS) in only 4 cases (0.9%), all in TI+ cases. None of the newborns were re-hospitalized after discharge. Interestingly, in TI+ and TI- the incidence of maternal and neonatal composite outcome was similar, except for EOS, which occurred only in TI+.
Maternal side involvement of the placenta occurred with a similar frequency (98.2% vs 98.9%) in TI+ and CS+, while fetal side inflammation was present in 51.3% of TI+ and in 35.5% of CS+ (p-value=0.008). Comparing TI+ with CS+, TI+ was more related to WPLI (51.3% vs 34.4%; p-value=0.005) and to neonatal and maternal composite outcome (respectively 45.1% and 25.3%, p-value=0.001; 72.6% and 36.6%; p-value <0.001). WPLI was significantly related both to maternal (37.0% vs 29.2%; p-value=0.046) and to neonatal composite outcome (47.4% vs 27.2%; p-value <0.001).
At logistic regression, both WPLI and TI+ proved to be independent predictors of neonatal composite outcome, with OR respectively 2.23 and 2.21 (
Table 3).
The diagnostic accuracy of WPLI and TI was calculated, as shown in
Table 4: specificity of TI was 84.8% and its LR+ 3.7, while the WPLI specificity was 68.5% and LR+ 1.61.
4. Discussion
Early diagnosis of IAI or intra-amniotic inflammation is crucial to reduce adverse outcomes, however antenatal isolated clinical signs do not show reliable diagnostic accuracy [
22]. Hence, the proposal of the TI criteria to diagnose an IAI and/or inflammation and to predict neonatal and maternal adverse outcomes [
1].
Although TI criteria were introduced by NICHD in 2015, they have not been implemented in clinical practice and several recent studies have questioned their utility. Ona S. et al [
24] concluded that applying TI criteria to guide clinical diagnosis of IAI or inflammation may misdiagnose women at risk for adverse infectious outcomes, since the sensitivity and specificity of suspected TI for an adverse infectious outcome were 67.6% and 38.1% respectively. Maki Y. et al [
25] investigated the diagnostic performance of TI criteria in predicting IAI in preterm labor and found them to be insufficiently sensitive (15.4%) and found no differences in the incidence of composite outcome between cases respecting TI criteria compared to isolated maternal fever. Both studies have many limitations, such as including preterm pregnancies and the small number of studied cases (only 99 in the second study).
In contrast, our study revealed that both TI+ and TI- are better predictors of adverse outcomes at term, compared to CS. Therefore, even in cases of negative placental histology, clinical criteria of TI can be useful as a predictive test; furthermore, all 4 EOS cases -the most significant outcome- occurred in TI+ group. Of interest, in our series the incidence of EOS at term was similar to that reported by literature (0.1% vs 0.04%)[
26].
A recent review suggests the implementation of TI criteria in current clinical guidelines, in order to avoid overexposure of newborns to broad-spectrum antibiotics and to their potential short- and long-term adverse effects. Neonatal management should be guided by the clinical category (isolated maternal fever, suspected Triple I or confirmed Triple I): in case of isolated fever and suspected TI before 34 weeks or confirmed Triple I, neonates should be treated, while in case of Isolated maternal fever or suspected TI and delivery over 34 weeks, clinical condition should be evaluated. Therefore, well-appearing term and late preterm neonates who are asymptomatic can be closely observed without antibiotics [
27].
Placental histology is an important tool for improving perinatal care and needs of a proper classification system for an adequate and reliable diagnosis [
28]. Information from placental histology can be useful to confirm the diagnosis of IAI and to predict early and late adverse outcomes. Our analysis suggests that in the context of suspected IAI, also in the absence of severe signs leading to a TI diagnosis, WPLI independently predicts perinatal adverse outcomes. Therefore, placental analysis could be incorporated to differentiate cases at risk.
Some authors investigated the utility of frozen section fast placental analysis for the early diagnosis of chorioamnionitis or funisitis as a sensitive screening for evaluation of placenta, which can be performed directly in the delivery room by pathologist or trained clinicians [
29], [
30]. The results of such a technique are obtained in less than 20 minutes that, compared to 7 days required for a traditional method, seem very precious to prompt direct clinical management. Hence, fast placental analysis in cases with clinical signs not respecting all the TI criteria could represent a reliable predictor to drive the clinical management.
Moreover, in our study HCA occurred in 9.9% of cases and such data are consistent with previous reports that described the incidence of HCA at around 10% [
10], confirming the reliability of our analysis.
Our analysis found that WPLI and TI+ are independent predictors of neonatal adverse outcomes. WPLI and TI+ revealed similar sensitivity (50.7% and 56.1%) in predicting perinatal adverse outcomes, while they differed in specificity: 84.8% for TI+ versus 68.5% for WPLI.
In accordance with our data, some studies investigated the relation between TI and histological chorioamnionitis with funisitis at term and concluded that the sensitivity of TI to identify funisitis is low, but its specificity is excellent [
31]. In contrast to our results, a recent study concluded that sensitivity of TI criteria in the prediction of clinical adverse infectious outcomes is higher than specificity (67.6% vs 38.1%) [
24]. Furthermore, TI+ appeared related to a more frequent fetal side placental involvement and maternal adverse outcomes compared to clinical suspicion of IAI and/or inflammation where TI diagnostic criteria are not fully respected.
MSAF is a predictor of increased perinatal and neonatal morbidity and mortality and it can be caused by an adverse intrauterine environment, such as chorioamnionitis, which can lead to hypoxia and subsequent premature defecation by the fetus. As MSAF is not rare, complicating 8%–20% of term deliveries, recent studies proposed to pay special attention to term deliveries complicated by MSAF if associated with strong predictors of complications, such as intrapartum fever, polyhydramnios, gestational diabetes and fetal heart rate alterations [
32]. In our population, excluding cases with HCA without clinical suspicion, the presence of MSAF was very common (56.5%), even if more frequent in cases with negative histology (67% vs 54%, p-value=0.13). Other studies report that histologic acute inflammation is more frequent in MSAF than in clear amniotic fluid and this relation was observed in particular for WPLI (p=0.008) [
33], [
34].
There are several limitations in our study: the retrospective nature of the research, the absence of a clinically and histologically negative control group to be compared with our population and the difficult analysis of neonatal outcomes independently from therapeutic decisions (i.e antibiotic therapy); in the future perspective, it is necessary to perform new studies without the bias of widely antibiotics administration, as happened so far, in order to analyze composite outcomes. Moreover, since at term intrauterine infection and sterile inflammation can both occur, even amniotic fluid analysis, such as white blood cells count or IL-6 and glucose concentration could be used in the future to differentiate these two conditions [
35].
In addition, the independent role of fetal inflammatory involvement in relation to composite neonatal outcome has not been evaluated due to the low incidence of this complication.
Another limitation is the use of Redline’s criteria to define placental histology, as it has been recently updated by the Amsterdam Placental Workshop Group Consensus Statement in 2016. This update only recognizes stages 2 and 3 on the maternal side as representing fully developed histologic acute chorioamnionitis, which has to be specified with or without Fetal Inflammatory Response [
36]. Despite using Redline’s criteria in our analysis, maternal stage I alone did not contribute in any case to determining WPLI in our series; hence, WPLI occurred only with maternal stage II and III positive placental histology plus Fetal Inflammatory Response.
The strengths are represented by the peculiarity of the investigated topic - Triple I at term of pregnancy- on which there has been little literature published so far, as well as the homogeneity in neonatal and maternal care management, based on our standardized Maternal-Fetal Unit protocols. Other strengths are the high percentage of vaginal deliveries in our delivery rooms (85%) which allows to study the clinical evolution of at term labor, as well as the large number of available histological data.
6. Conclusion
Since the discrepancy between clinical signs possibly suggesting IAI or intra-amniotic inflammation and the low incidence of perinatal adverse outcomes in term labor, TI criteria seem to be independent and reliable predictors of perinatal complications and also of positive histological findings. In presence of antenatal clinical signs not respecting TI criteria, we suggest to send the placenta for histological analysis since the diagnosis of WPLI can be integrated with clinical presentation due to its ability to independently predict composite neonatal outcome. Even in cases of TI+, WPLI can improve diagnostic accuracy of the clinical presentation.
Future perspectives of research are the application of diagnostic-therapeutic protocols which include the TI criteria and the fast placental analysis as early perinatal tests for outcomes prediction.
Author Contributions
The following statements should be used Conceptualization, AL, SC, AP, FM; data curation VV, IVT, FM, PC; writing—original draft preparation ES, SC, AL; supervision, SC, AL. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and Ethical review and approval were not necessary for this study due to its retrospective monocentric nature
Acknowledgments
We acknowledge Dr. paolo Colombo for is contribution
Conflicts of Interest
The authors declare no conflict of interest.
References
- Peng, C.-C.; Chang, J.-H.; Lin, H.-Y.; Cheng, P.-J.; Su, B.-H. Intrauterine inflammation, infection, or both (Triple I): A new concept for chorioamnionitis. Pediatr. Neonatol. 2018, 59, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Chaemsaithong, P.; Korzeniewski, S.J.; Kusanovic, J.P.; Docheva, N.; Martinez-Varea, A.; Ahmed, A.I.; Yoon, B.H.; Hassan, S.S.; Chaiworapongsa, T.; et al. Clinical chorioamnionitis at term III: how well do clinical criteria perform in the identification of proven intra-amniotic infection? jpme 2015, 44, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Dior, U.P.; Kogan, L.; Eventov-Friedman, S.; Gil, M.; Bahar, R.; Ergaz, Z.; Porat, S.; Calderon-Margalit, R. Very High Intrapartum Fever in Term Pregnancies and Adverse Obstetric and Neonatal Outcomes. Neonatology 2015, 109, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Ashwal, E.; Salman, L.; Tzur, Y.; Aviram, A.; Bashi, T.B.-M.; Yogev, Y.; Hiersch, L. Intrapartum fever and the risk for perinatal complications – the effect of fever duration and positive cultures. J. Matern. Neonatal Med. 2017, 31, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Ghi, T.; Di Pasquo, E.; Dall’asta, A.; Commare, A.; Melandri, E.; Casciaro, A.; Fieni, S.; Frusca, T. Intrapartum fetal heart rate between 150 and 160 bpm at or after 40 weeks and labor outcome. Acta Obstet. et Gynecol. Scand. 2020, 100, 548–554. [Google Scholar] [CrossRef]
- R. Romero et al., “Clinical Chorioamnionitis at Term: New Insights into the Etiology, Microbiology, and the Fetal, Maternal and Amniotic Cavity Inflammatory Responses.,” Nogyogy. es szuleszeti Tovabbk. Szle., vol. 20, no. 3, 2018. [PubMed]
- J. C. Pasquier et al., “Maternal Leukocytosis After Preterm Premature Rupture of Membranes and Infant Neurodevelopmental Outcome: A Prospective,Population-Based Study (Décrire L’ouverture des Membranes Inopinée le Nouveau-né et l’Organisation des Soins [DOMINOS] Study),” J. Obstet. Gynaecol. Canada, vol. 29, no. 1, 2007. [CrossRef]
- Suzuki, S. Association between clinical chorioamnionitis and histological funisitis at term. J. Neonatal-Perinatal Med. 2019, 12, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Tita, A.T.; Andrews, W.W. Diagnosis and Management of Clinical Chorioamnionitis. Clin. Perinatol. 2010, 37, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Pavlova, Z.; Incerpi, M.H.; Ramanathan, R. Meconium-Stained Amniotic Fluid and Neonatal Morbidity in Near-Term and Term Deliveries with Acute Histologic Chorioamnionitis and/or Funisitis. J. Perinatol. 2001, 21, 537–540. [Google Scholar] [CrossRef] [PubMed]
- S. Sagay, “Histological Chorioamnionitis.,” J. West African Coll. Surg., vol. 6, no. 3, pp. x–xiii, 2016. [PubMed]
- Roberts, D.J.; Celi, A.C.; Riley, L.E.; Onderdonk, A.B.; Boyd, T.K.; Johnson, L.C.; Lieberman, E. Acute Histologic Chorioamnionitis at Term: Nearly Always Noninfectious. PLOS ONE 2012, 7, e31819. [Google Scholar] [CrossRef] [PubMed]
- R. W. Redline, “Placental pathology: Is it time to get serious?,” Contemp. Ob. Gyn., vol. 59, no. 2, 2014.
- Redline, R.W.; Faye-Petersen, O.; Heller, D.; Qureshi, F.; Savell, V.; Vogler, C. Amniotic Infection Syndrome: Nosology and Reproducibility of Placental Reaction Patterns. Pediatr. Dev. Pathol. 2003, 6, 435–448. [Google Scholar] [CrossRef]
- Ge, Y.; Zhang, C.; Cai, Y.; Huang, H. Adverse Maternal and Neonatal Outcomes in Women With Elevated Intrapartum Temperature Complicated by Histological Chorioamnionitis at Term: A Propensity-Score Matched Study. Front. Pediatr. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Mittal, N.; Byard, R.W.; Dahlstrom, J.E. A practical guide to placental examination for forensic pathologists. Forensic Sci. Med. Pathol. 2019, 16, 295–312. [Google Scholar] [CrossRef] [PubMed]
- C. Beck, K. Gallagher, L. A. Taylor, J. A. Goldstein, L. B. Mithal, and A. D. Gernand, “Chorioamnionitis and Risk for Maternal and Neonatal Sepsis: A Systematic Review and Meta-analysis,” Obstet. Gynecol., vol. 137, no. 6, 2021. [CrossRef]
- C. J. Kim, R. Romero, P. Chaemsaithong, N. Chaiyasit, B. H. Yoon, and Y. M. Kim, “Acute chorioamnionitis and funisitis: Definition, pathologic features, and clinical significance,” American Journal of Obstetrics and Gynecology, vol. 213, no. 4. 2015. [CrossRef]
- Pergialiotis, V.; Bellos, I.; Antsaklis, A.; Papapanagiotou, A.; Loutradis, D.; Daskalakis, G. Maternal and neonatal outcomes following a prolonged second stage of labor: A meta-analysis of observational studies. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Kyozuka, H.; Murata, T.; Fukuda, T.; Suzuki, E.; Yazawa, R.; Yasuda, S.; Kanno, A.; Yamaguchi, A.; Hashimoto, Y.; Fujimori, K. Labor dystocia and risk of histological chorioamnionitis and funisitis: a study from a single tertiary referral center. BMC Pregnancy Childbirth 2021, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Azziz, R.; Cumming, J.; Naeye, R. Acute myometritis and chorioamnionitis during cesarean section of asymptomatic women. Am. J. Obstet. Gynecol. 1988, 159, 1137–1139. [Google Scholar] [CrossRef] [PubMed]
- Ona, S.; Easter, S.R.; Prabhu, M.; Wilkie, G.; Tuomala, R.; Diouf, K.; Riley, L. 842: Sensitivity and specificity of the diagnostic criteria for intrauterine inflammation or infection proposed by the 2015 National Institute of Child Health and Human Development workshop. Am. J. Obstet. Gynecol. 2018, 218, S502–S503. [Google Scholar] [CrossRef]
- Cuna, A.; Hakima, L.; Tseng, Y.-A.; Fornier, B.; Islam, S.; Quintos-Alagheband, M.L.; Khullar, P.; Weinberger, B.; Hanna, N. Clinical Dilemma of Positive Histologic Chorioamnionitis in Term Newborn. Front. Pediatr. 2014, 2, 27. [Google Scholar] [CrossRef] [PubMed]
- Ona, S.; Easter, S.R.; Prabhu, M.; Wilkie, G.; Tuomala, R.E.; Riley, L.E.; Diouf, K. Diagnostic Validity of the Proposed Eunice Kennedy Shriver National Institute of Child Health and Human Development Criteria for Intrauterine Inflammation or Infection. Obstetrics & Gynecology 2019, 133, 33–39. [Google Scholar] [CrossRef]
- Maki, Y.; Furukawa, S.; Nakayama, T.; Oohashi, M.; Shiiba, N.; Furuta, K.; Tokunaga, S.; Sameshima, H. Clinical chorioamnionitis criteria are not sufficient for predicting intra-amniotic infection. J. Matern. Neonatal Med. 2020, 35, 52–57. [Google Scholar] [CrossRef] [PubMed]
- K. M. Puopolo, S. Mukhopadhay, A. Frymoyer, and W. E. Benitz, “The Term Newborn: Early-Onset Sepsis,” Clinics in Perinatology, vol. 48, no. 3. 2021. [CrossRef]
- Jain, V.G.; Willis, K.A.; Jobe, A.; Ambalavanan, N. Chorioamnionitis and neonatal outcomes. Pediatr. Res. 2021, 91, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Redline, R.W.; Roberts, D.J.; Parast, M.M.; Ernst, L.M.; Morgan, T.K.; Greene, M.F.; Gyamfi-Bannerman, C.; Louis, J.M.; Maltepe, E.; Mestan, K.K.; et al. Placental pathology is necessary to understand common pregnancy complications and achieve an improved taxonomy of obstetrical disease. Am. J. Obstet. Gynecol. 2023, 228, 187–202. [Google Scholar] [CrossRef] [PubMed]
- E. Mahe, J. Hamid, J. Terry, J. W. Jansen, J. Bourgeois, and J. Arredondo-Marin, “Frozen section of placental membranes and umbilical cord: An aid to early postpartum diagnosis of intra-amniotic infection,” Am. J. Clin. Pathol., vol. 142, no. 2, 2014. [CrossRef]
- Mendilcioglu, I.; Kilicarslan, B.; Zorlu, C.G.; Karaveli, S.; Uner, M.; Trak, B. Placental biopsy by frozen section: Does it have a role in evaluation of fetal well-being? Aust. New Zealand J. Obstet. Gynaecol. 2003, 43, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Salafia, C.; Shen-Schwarz, S.; Guzman, E.; Saade, G.R.; Chauhan, S.P.; Doty, M.S. Histologic Funisitis and Likelihood of Intrauterine Inflammation or Infection: A Case-Control Study. Am. J. Perinatol. 2018, 35, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Levin, G.; Tsur, A.; Shai, D.; Cahan, T.; Shapira, M.; Meyer, R. Prediction of adverse neonatal outcome among newborns born through meconium-stained amniotic fluid. Int. J. Gynecol. Obstet. 2021, 154, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Saeed, H.; Jacques, S.M.; Qureshi, F. Meconium staining of the amniotic fluid and the presence and severity of acute placental inflammation: a study of term deliveries in a predominantly African-American population. J. Matern. Neonatal Med. 2017, 31, 3172–3177. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Pavlova, Z.; Incerpi, M.H.; Ramanathan, R. Meconium-Stained Amniotic Fluid and Neonatal Morbidity in Near-Term and Term Deliveries with Acute Histologic Chorioamnionitis and/or Funisitis. J. Perinatol. 2001, 21, 537–540. [Google Scholar] [CrossRef]
- R. Romero et al., “Clinical chorioamnionitis at term X: Microbiology, clinical signs, placental pathology, and neonatal bacteremia - Implications for clinical care,” J. Perinat. Med., vol. 49, no. 3, 2021. [CrossRef]
- R. W. Redline, “Classification of placental lesions,” American Journal of Obstetrics and Gynecology, vol. 213, no. 4. 2015. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).