Introduction
The increasing global population has resulted in increased consumption of global energy. The growing energy demand is covered by 85% of fossil fuels, which are finite and non-renewable[
1]. Besides being scarce and expensive, fossil fuels such as oil constitute to one of the major causes of human-driven climate change. Further, fossil fuel reserves are localized in few countries and thus, this contributes to the most common causes of international conflicts threatening human existence. The limitations of overreliance on fossil fuels have driven researchers to explore alternative energy sources. As a result, fermentative technologies are currently receiving a revived interest as an economically viable means of producing renewable energy carriers, such as hydrogen, ethanol, and methane, from waste material or cheap biomass [
2]. Among the renewable energy carriers, hydrogen holds a promising potential because of its beneficial features. Hydrogen is a clean energy carrier since its combustion products are water and oxygen which do not pollute the environment. In addition, water has a high energy density that can be converted to electricity, and can be produced from biologically degradable waste, which is in tandem with energy recycling [
3].
The challenges of hydrogen as an energy carrier lie in its sustainable production. Several commercial hydrogen production methods include coal gasification, steam reformation, and water electrolysis. Currently, steam-reforming methane and water electrolysis is regarded as the practical way of producing hydrogen [
4]. However, its production is very costly for the equivalent amount of energy with gasoline. Therefore, renewable hydrogen production sources can be the keys to unlocking sustainable fuels. One of the sustainable means of producing hydrogen is from organic biomass.
Organic biomass comprises all masses of living organisms, including plants, animals, fungi, bacteria, and their remains [
3]. Hydrogen can be obtained from organic biomass by naturally occurring bacterial communities. Dark fermentation is a bioprocess, where anaerobic bacteria ferment organic materials to release hydrogen gas [
5]. Dark fermentation (DF) is a promising and cheaper way of producing biohydrogen because it achieves two goals simultaneously, the production of clean energy and, at the same time reduction of environmental burden. Moreover, DF can use a complex variety of waste biomass as feedstock and produces biochemical byproducts of economic interest. Other excellent properties of DF include having the potential to produce hydrogen day and night since it doesn't depend on light. The DF can produce hydrogen at a higher rate than biophotolysis and photo-fermentation. Also, it can utilize existing reactors and thus can be easily scaled up by designing the bioreactor[
5].
Despite being a promising method, DF still has a major limitation of low hydrogen yield making it difficult for industrial production [
6]. The challenge of low yield can be overcome by optimizing the fermentative production process and screening for better bacterial strains. This paper will illuminate the current status of biohydrogen production through dark fermentation, challenges, and opportunities for its production.
Factors influence hydrogen production by Dark Fermentation
Temperature
Temperature has an effect on the production rate of hydrogen and activities of hydrogen producing bacteria. The dark fermentative hydrogen production can be operated at different temperatures depending on the microbial strain under consideration. It can be operated at mesophilic temperature (25-40
oC), thermophilic (40-65
oC), extreme thermophilic (65-80
oC) and hyperthemophilic (above 80
oC) [
7]. Currently, most DF are run at a temperature of 35-55
oC. Extreme thermophilic temperature range is preferred owing to numerous advantages compared to mesophilic range. Under extreme thermophilic temperature, a higher yield of hydrogen can be achieved. At the same time, majority of pathogenic and hydrogen consuming microbes such as methanogens are destroyed at extreme temperatures [
7].
Medium pH
The levels of pH have a huge impact on enzyme activity for each microbe since each enzyme has a specific pH and can only be reach maximum activity at optimal pH. The hydrogen production pathway under DF is sensitive to pH. Various research has shown that control of pH was paramount for optimal production of hydrogen under DF[
8]. Operating under suboptimal pH shifts hydrogen fermentation towards solvent production. Some studies have reported optimal pH for hydrogen production to be in the range of 5.0 to 5.5 for mesophilic microbes [
9]. However, for extreme thermophilic temperature a pH range of between 6.5 -7.5 is recommended [
8].
Hydraulic Retention Time (HRT)
Hydraulic retention time (HRT) measures the average time a soluble compound remains in a bioreactor. In most hydrogen bioreactors, such as continuous stirred tank reactors (CSTR), a short HRT is preferred since it helps wash away slow-growing methanogens that can easily consume the hydrogen produced [
10]. Importantly, short HRT results in low pH. An HRT of less than three days is preferred since methanogens such as archaea require more than three days to grow [
10].
Hydrogen Partial Pressure
The concentration of hydrogen in the liquid phase, which relates to hydrogen partial pressure, is an important determinant in hydrogen production through DF. The synthesis of hydrogen through dark fermentation is sensitive to hydrogen concentration. As hydrogen concentration increases, hydrogen synthesis decreases, and the overall metabolic pathway shifts to the production of reduced substrates such as butanol, acetate, and ethanol. However, an increase in temperature will likely not affect hydrogen synthesis in DF[
11].
Carbon (IV) oxide (CO2) Partial Pressure
A high concentration of carbon (IV) oxide favors the production of reduced substrates such as succinate and fumarate. Fumarates and succinates consume electrons, thereby reducing the production of hydrogen. Some studies have pointed out that the removal of CO2 from the reaction can lead to improved hydrogen production. However, the study [
12] reported findings with doubling hydrogen after carbon dioxide removal. .
Substrate Composition
Most previous studies have focused on using simple sugars such as sucrose and glucose as model substrates. Fewer researchers have used substrates such as agricultural wastes, industrial wastewater, industrial waste, and those from food processing in dark fermentation.
Substrates rich in carbohydrates are ideal for hydrogen-producing microbes[
13]. As a result, wastes from factories such as potato processing industries and sugar plants are rich in carbohydrates and offer a good choice for anaerobic fermentation. During hydrolysis, substrates rich in carbohydrates are broken down by hydrolytic bacteria into low molecular weight sugars such as xylose and glucose. The bacteria further break down these low molecular weight sugars to produce biohydrogen [
13]. The primary sources of lipids include food from processing wastes, dairy products, and oil products. Hydrolysis of lipids is facilitated by lipase enzymes found in some bacteria. The hydrolysis of lipids produces free fatty acids and glycerols, which can further be hydrolyzed to acetyl-COA, acetate, and hydrogen[
14]. However, the production process of hydrogen from lipids is relatively slower than carbohydrates due to the formation of volatile fatty acids, which inhibits biohydrogen production.
Proteins are compounds rich in polypeptides of amino acids. Some of the protein-rich substrate sources include food processing wastes from fish, cheese, eggs, and meat. During hydrogen production, bacteria break down proteins to their simpler forms with the help of the protease enzyme[
15]. Some of the proteins' low molecular weight products, such as amino acids, are further broken into volatile fatty acids, carbon dioxide, and hydrogen gas. Agricultural wastes are majorly composed of cellulose, hemicellulose, and lignocellulose components. This means they comprise different sugars that can be harnessed for biohydrogen production. However, the problem with these materials is that cellulose is hardly biodegradable by most microbes. Therefore, a pretreatment stage is required to utilize these materials for hydrogen production, including chemical treatment, alkaline or acidic treatment, and steam explosion to release sugars for hydrogen production[
15].
Types of Seed Culture
Production of hydrogen can be impacted by the type of seed culture used, which can either be pure or mixed culture. A pure culture fermentation involves a fermentative process that uses a single strain of a microbe throughout the process, while a mixed culture is a fermentative process achieved by several strains of microbial culture [
16]. The advantage of pure culture is that it lacks interference from hydrogen consuming bacteria such as methanogenic archaea and sulphate-reducing bacteria [
16]. Nevertheless, pure culture can easily be contaminated by other strains; hence aseptic technique must be observed. On the other hand, mixed culture contains diverse microbes which can utilize different substrates and this makes it suitable for diverse applications.
Organic Loading Rate
The organic loading rate (OLR) is the volume of organic feedstock fed into a reactor over a specific period. In industrial applications, a high OLR is regarded as advantageous, since it reduces the energy needed for operation. Further, a high OLR could increase the hydrogen production rate (HPR) if well managed [
17]. However, increased substrate concentration leads to a build-up of volatile fatty acids and hydrogen partial pressure, inhibiting HPR. Therefore, a high HPR may be achieved at a low substrate concentrations [
17].
Methodology
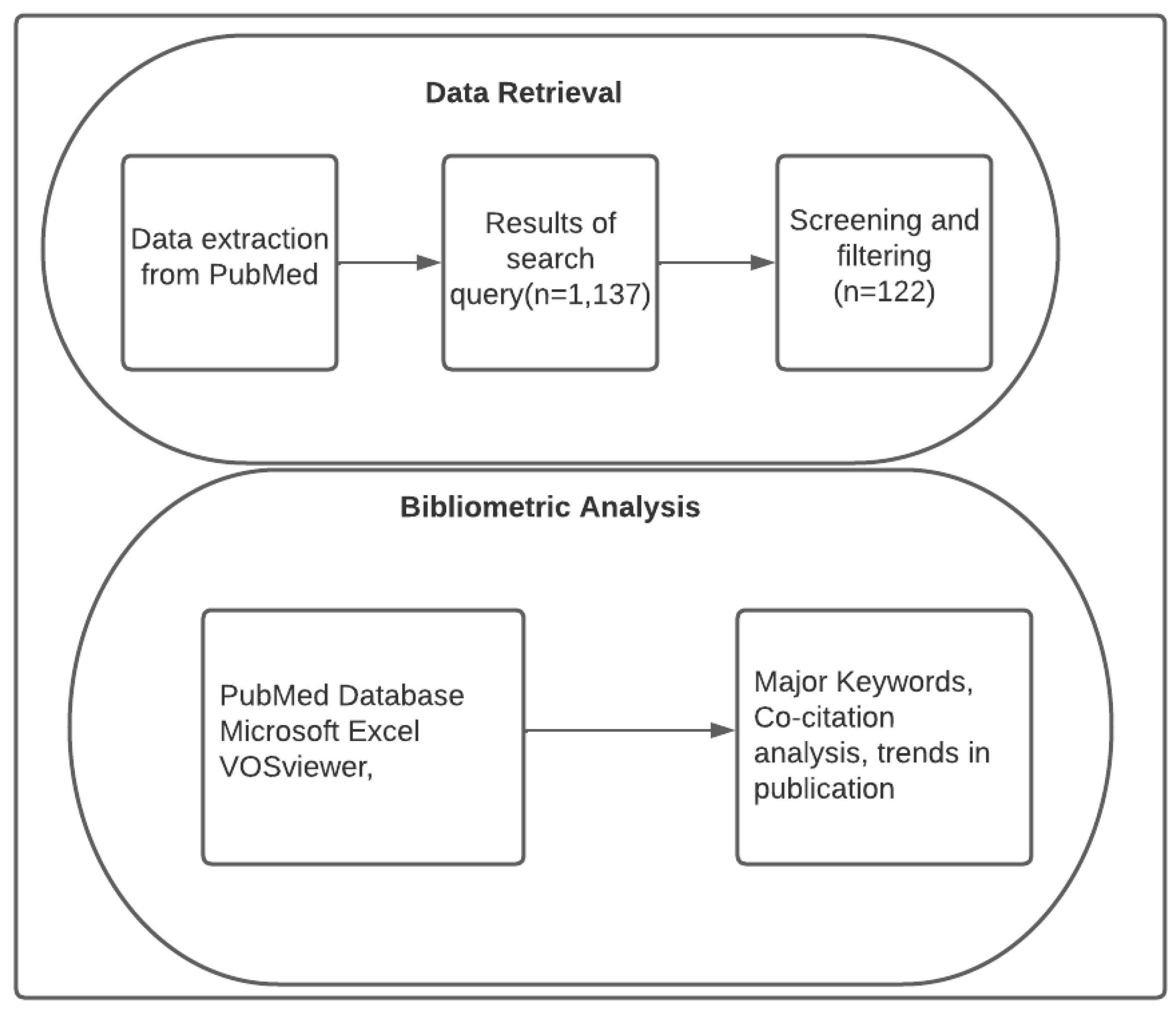
The findings presented in this paper were obtained from a bibliometric analysis of articles published in the last ten years (2013 to 2023) on biohydrogen production through dark fermentation. The analysis used published articles to arrive at the current trends in the dark fermentative production of hydrogen and the existing knowledge gap. The PubMed database was used in obtaining the articles for analysis as shown in
Figure 1.
Data collection
The relevant literature was identified through PubMed database search. The PubMed database is one of the reliable sources of scholarly peer-reviewed scholarly articles with more inclusive articles, making it a preferred database for this paper [
18]. The search for relevant literature was performed between 2013 to 2023 mainly because this period depicts changes in the literature on Dark fermentation. Therefore, performing literature analysis in the past ten years yielded significant research contributions. The literature search performed on PubMed was based on keywords including dark fermentation, biohydrogen production, hydrogen, and biohythane. The articles were only limited to those written in English. The initial search yielded 1,137 articles, but further screening and filtering to remove irrelevant literature produced 122 articles.
Data Analysis
A PubMed database was used in extracting articles in dark fermentation with the highest citation and in extracting keywords. The evaluation of the articles was based on total number of publications. Importantly, the total number of publication indicates the contribution of each research institution in different countries to the selected research theme. Visualization and co-occurrences of keywords was performed on VOSviewer software.
Data Management
The VOSviewer (version 1.6.19.0) and Microsoft Excel (version 2302) software were used in data visualization and qualitative analysis, respectively. VOSviewer is good at the graphical presentation of bibliometric maps in a manner that is easy to interpret [
19]. The data were imported into the VOSviewer software, and bibliometric analysis was performed.
Results and Discussion
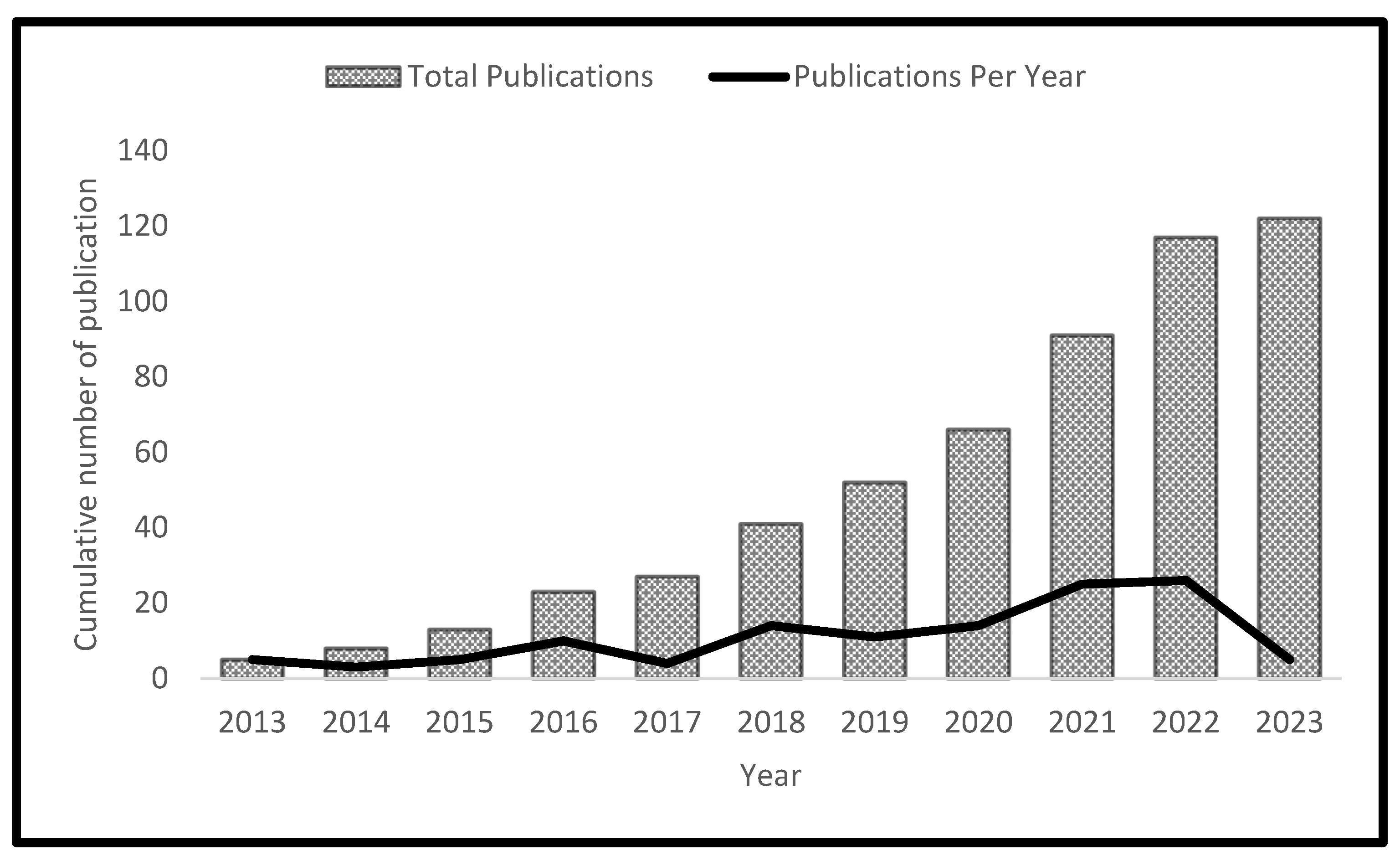
There has been a growing interest in biohydrogen production through dark fermentation over the past decade, as shown in figure 2. Only nine articles were published between 2013 and 2014. However, there was an upward trend in publications starting from 2015 onwards, reaching a peak in 2021 and 2022. In a similar study,[
20] noted an increase in the global trend in biohydrogen production, majorly due to an increase in government incentives toward studies on the domain of biohydrogen production. Another reason for a progressive increase in studies on biohydrogen production could be an increased demand for alternative energy that is ecologically friendly and sustainable. Moreover, research by [
21]concluded that there was an upward trend in publications on the biohydrogen economy with a focus on multidisciplinary facets such as the production, storage, application, and transportation of hydrogen. Notably, there was a decline in publications between 2019 and 2020. The decline in publications between 2019 and 2020 could be attributable to the lockdown occasioned by the COVID-19 pandemic and the consequent shutting down of universities and research institutions. The decline in publication observed in this paper agrees with the findings of [
22],who noted an 18% decrease in publication on non-COVID-19 research during the pandemic period. Overall, the results of the publication trend indicate a growing interest in dark fermentation, resulting from a growing demand for green renewable energy and sustainable carrier.
Figure 2.
A summary of the number of articles published on dark fermentation over the past 10 years.
Figure 2.
A summary of the number of articles published on dark fermentation over the past 10 years.
Keyword Analysis
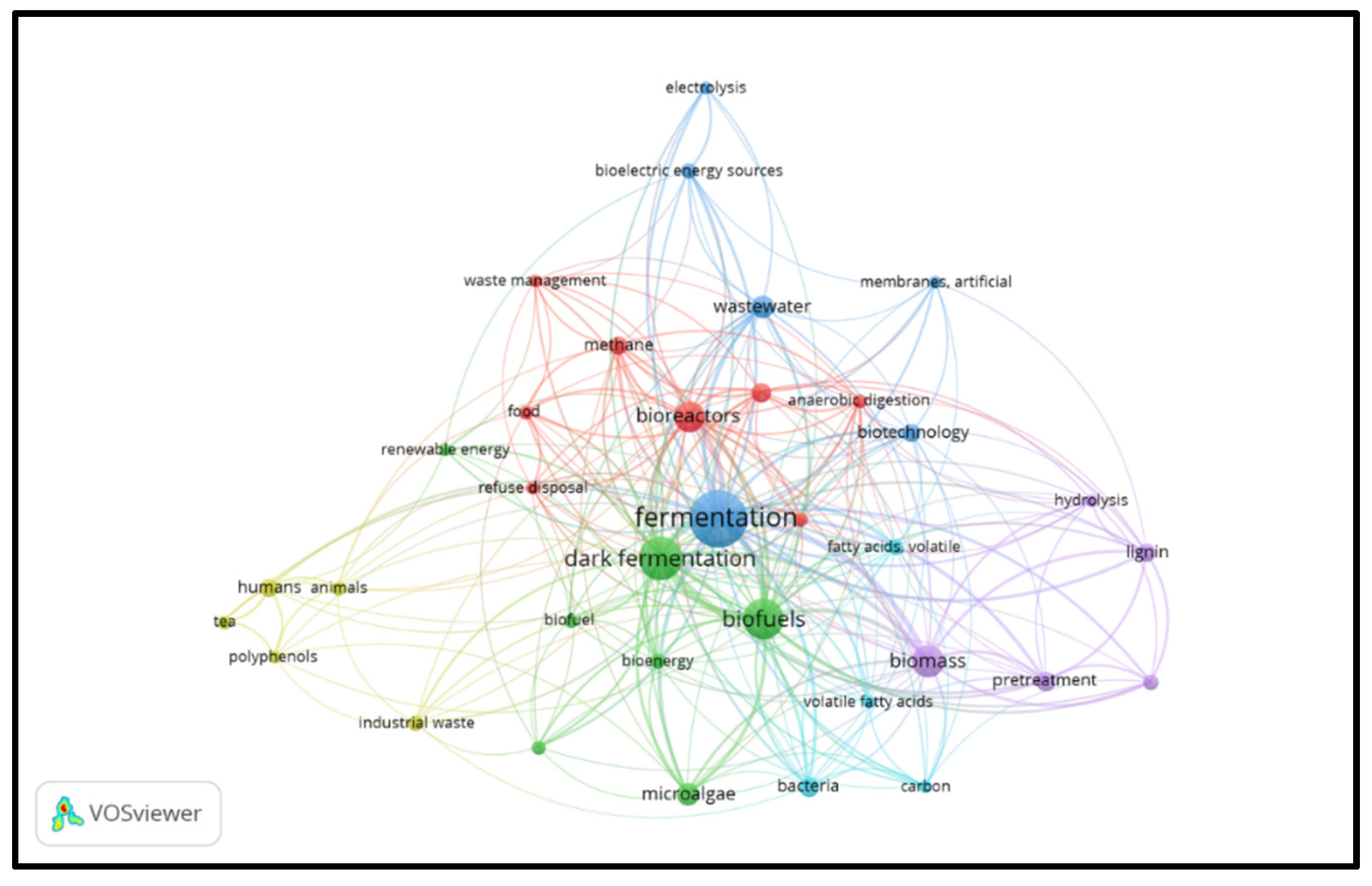
Figure 3 illustrates the results of keyword analysis generated from VOSviewer using co-occurrence analysis. The threshold used in the study was a minimum of five occurrences. It was noted that out of 554 keywords, only 38 met the threshold. Common keywords associated with biohydrogen production were removed so that the study could focus on unexpected keywords. Such keywords included “hydrogen,” “hydrogen production,” and “biohydrogen.” The finding yielded five clusters based on the similarities, each representing a specific area.
The primary keyword used, dark fermentation, is the green-colored cluster connected to other significant keywords associated with biohydrogen production, such as “microalgae” and “photo fermentation.” Moreover, other keywords such as “biofuels,” “bioenergy,” and “renewable energy” are in the same cluster, which indicates that the product of the process of dark fermentation process produces a clean, renewable energy carrier. The violet cluster links various keywords such as “biomass,” “lignin,” “pretreatment,” and “hydrolysis,” which are factors that affect dark fermentation [
23]. The red cluster connects the bioreactor to various important components such as “methane,” “waste management”, and “refuse disposal.” Importantly, the type of bioreactor used affects the dark fermentation process; at the same time, methane gas can be produced in a bioreactor (N. Ren et al., 2011). From the analysis of the keywords, it can be concluded that the keywords such as “dark fermentation,” “fermentation,” “bioreactor,” and “biomass” were used by many authors, which shows the significance of hydrogen production by these methods.
Institutional Collaborations
Institutions in the Republic of Korea and Norway eclipsed others in the number of articles published related to dark fermentation (
Table 1). These findings contradict [
20], which established that the institutions in Germany and China were leading in a publication related to biohydrogen production. The difference between our findings and other studies was the fact that the present study focused on dark fermentative hydrogen production, compared to other researchers who focused on biohydrogen production through electrolysis. The plausible reason for high publication on the theme of dark fermentation is that countries are looking for a sustainable method of producing clean energy, and dark fermentation is one of the promising methods. Further, Institutional collaboration was conducted with a minimum of 3 co-authored publications per institution. Out of 418 organizations, only 8 organizations met the minimum threshold set, as shown in
Table 1. The larger the link strength, the stronger the collaboration among the authors. The results show a stronger collaboration between authors at Yonsei University in the Republic of Korea, followed by the University of Stavanger in Norway, with total link strengths of 5 and 6, respectively. Other institutions domiciled in Taiwan and Vietnam reported low collaboration among co-authorships in the field of dark fermentation. [
19] noted the same trend of low international collaboration in studies related to biohydrogen production. The low collaboration could be attributed to difficulties in connecting potential collaborators in different countries, acquiring funding for the projects, and evaluating and sustaining the projects between collaborating international institutions are some of the barriers impeding successful international collaborations.[
24].
Authorship Analysis
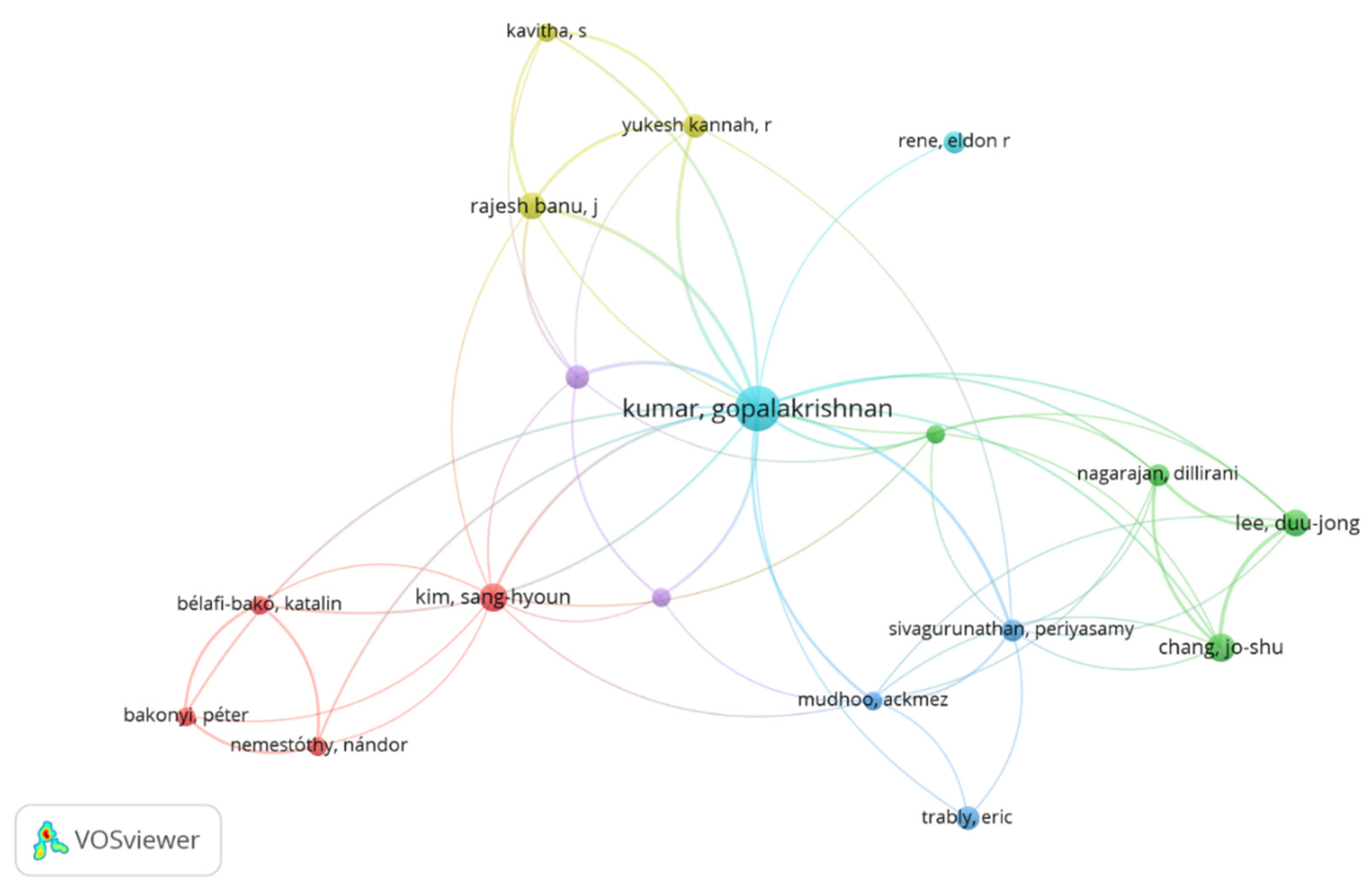
The map in
Figure 4 indicates authors with at least three publications on biohydrogen production by dark fermentation. On the map, the size of the node represents the number of publications by the authors, while the curved lines show the collaboration among the authors. Based on the finding, the author Kumar Gopalakrishnan from the University of Stavanger is the leading author with many publications on dark fermentative biohydrogen production. Also, Gopalakrishnan is leading in terms of co-authorship with other authors, as shown in figure 4. Among Gopalakrishnan's notable publications include fermentative biohydrogen production, lignocellulose biohydrogen production, and pretreatment technologies for enhanced hydrogen and methane production. Researchers play an important role in supporting research and development in biohydrogen production. Moreover, researchers are the human resources with knowledge and with support they can provide sustainable and comprehensive development in biohydrogen production[
25]. Therefore, there is a need for more collaboration among researchers in promising methods of biohydrogen production, such as dark fermentation.
Conclusion
The results of bibliometric analysis have revealed that there has been a progressive increase in research on biohydrogen production through dark fermentation since 2013. The outcome of the analysis also spotlighted significant trends in research themes, including pretreatment, and types of biomass, which affect dark fermentation. The greatest collaboration in dark fermentation was institutions in the Republic of Korea, Vietnam, India, and Norway. Moreover, the co-authorship was between institutions in the same countries, which clearly indicates low international collaboration among researchers and institutions working on dark fermentation.
Author Contributions
All authors have made equal and substantial contribution to the Conceptualization, Methodology, Software, Validation, Formal Analysis, Investigation, Resources, Data Curation, writing – Original Draft Preparation, Writing – Review & Editing, and Visualization.
Funding
This material has been developed in absence of any financial support that would be construed as potential conflicts of interest.
Data Availability
Not applicable.
Acknowledgments
Authors acknowledge all the intellectual support from the literature used to obtain datasets used in this material.
Conflicts of interest
Authors declare no conflicts of interest.
References
- M.F. Orhan, I. Dincer, M.A. Rosen, M. Kanoglu, Integrated hydrogen production options based on renewable and nuclear energy sources, Renew. Sustain. Energy Rev. 16 (2012) 6059–6082. [CrossRef]
- V. Gadhamshetty, Y. Arudchelvam, N. Nirmalakhandan, D.C. Johnson, Modeling dark fermentation for biohydrogen production: ADM1-based model vs. Gompertz model, Int. J. Hydrogen Energy. 35 (2010) 479–490. [CrossRef]
- V.L. Martínez, G.L. Salierno, R.E. García, M.J. Lavorante, M.A. Galvagno, M.C. Cassanello, Biological Hydrogen Production by Dark Fermentation in a Stirred Tank Reactor and Its Correlation with the pH Time Evolution, Catalysts. 12 (2022) 1366. [CrossRef]
- T. Smolinka, Fuels–hydrogen production| water electrolysis, (2009).
- Y. Cao, H. Liu, W. Liu, J. Guo, M. Xian, Debottlenecking the biological hydrogen production pathway of dark fermentation: insight into the impact of strain improvement, Microb. Cell Fact. 21 (2022) 166. [CrossRef] [PubMed]
- W. Han, D.N. Liu, Y.W. Shi, J.H. Tang, Y.F. Li, N.Q. Ren, Biohydrogen production from food waste hydrolysate using continuous mixed immobilized sludge reactors, Bioresour. Technol. 180 (2015) 54–58. [CrossRef] [PubMed]
- Y.M. Wong, T.Y. Wu, J.C. Juan, A review of sustainable hydrogen production using seed sludge via dark fermentation, Renew. Sustain. Energy Rev. 34 (2014) 471–482. [CrossRef]
- M.A. Delavar, J. Wang, Numerical investigation of pH control on dark fermentation and hydrogen production in a microbioreactor, Fuel. 292 (2021) 120355. [CrossRef]
- G. De Gioannis, M. Friargiu, E. Massi, A. Muntoni, A. Polettini, R. Pomi, D. Spiga, Biohydrogen production from dark fermentation of cheese whey: Influence of pH, Int. J. Hydrogen Energy. 39 (2014) 20930–20941. [CrossRef]
- F. Silva-Illanes, E. Tapia-Venegas, M.C. Schiappacasse, E. Trably, G. Ruiz-Filippi, Impact of hydraulic retention time (HRT) and pH on dark fermentative hydrogen production from glycerol, Energy. 141 (2017) 358–367. [CrossRef]
- R. Łukajtis, I. Hołowacz, K. Kucharska, M. Glinka, P. Rybarczyk, A. Przyjazny, M. Kamiński, Hydrogen production from biomass using dark fermentation, Renew. Sustain. Energy Rev. 91 (2018) 665–694. [CrossRef]
- S. Tanisho, M. Kuromoto, N. Kadokura, Effect of CO2 removal on hydrogen production by fermentation, Int. J. Hydrogen Energy. 23 (1998) 559–563. [CrossRef]
- M. Quéméneur, J. Hamelin, S. Benomar, M.-T. Guidici-Orticoni, E. Latrille, J.-P. Steyer, E. Trably, Changes in hydrogenase genetic diversity and proteomic patterns in mixed-culture dark fermentation of mono-, di-and tri-saccharides, Int. J. Hydrogen Energy. 36 (2011) 11654–11665. [CrossRef]
- H.-Y. Ren, B.-F. Liu, F. Kong, L. Zhao, J. Ma, N.-Q. Ren, Favorable energy conversion efficiency of coupling dark fermentation and microalgae production from food wastes, Energy Convers. Manag. 166 (2018) 156–162. [CrossRef]
- Y. Tarazona, A. Vargas, G. Quijano, I. Moreno-Andrade, Influence of the initial proportion of carbohydrates, proteins, and lipids on biohydrogen production by dark fermentation: A multi-response optimization approach, Int. J. Hydrogen Energy. 47 (2022) 30128–30139. [CrossRef]
- N. Khanna, D. Das, Biohydrogen production by dark fermentation, Wiley Interdiscip. Rev. Energy Environ. 2 (2013) 401–421. [CrossRef]
- I. Martins, E. Surra, M. Ventura, N. Lapa, BioH2 from Dark Fermentation of OFMSW: Effect of the Hydraulic Retention Time and Organic Loading Rate, Appl. Sci. 12 (2022) 4240. [CrossRef]
- P.O. Williamson, C.I.J. Minter, Exploring PubMed as a reliable resource for scholarly communications services, J. Med. Libr. Assoc. JMLA. 107 (2019) 16. [CrossRef] [PubMed]
- S.P. Arimbrathodi, M.A. Javed, M.A. Hamouda, A. Aly Hassan, M.E. Ahmed, BioH2 Production Using Microalgae: Highlights on Recent Advancements from a Bibliometric Analysis, Water. 15 (2023) 185. [CrossRef]
- L. Camargo, D. Comas, Y.C. Escorcia, A. Alviz-Meza, G. Carrillo Caballero, I. Portnoy, Bibliometric Analysis of Global Trends around Hydrogen Production Based on the Scopus Database in the Period 2011–2021, Energies. 16 (2022) 87. [CrossRef]
- S.K. Kar, S. Harichandan, B. Roy, Bibliometric analysis of the research on hydrogen economy: An analysis of current findings and roadmap ahead, Int. J. Hydrogen Energy. (2022). [CrossRef]
- M. Raynaud, V. Goutaudier, K. Louis, S. Al-Awadhi, Q. Dubourg, A. Truchot, R. Brousse, N. Saleh, A. Giarraputo, C. Debiais, Impact of the COVID-19 pandemic on publication dynamics and non-COVID-19 research production, BMC Med. Res. Methodol. 21 (2021) 1–10. [CrossRef] [PubMed]
- Y. Chen, Y. Yin, J. Wang, Recent advance in inhibition of dark fermentative hydrogen production, Int. J. Hydrogen Energy. 46 (2021) 5053–5073. [CrossRef]
- R.J. Widmer, J.M. Widmer, A. Lerman, International collaboration: promises and challenges, Rambam Maimonides Med. J. 6 (2015). [CrossRef] [PubMed]
- Y.T. Sin, W.A.N. WM, Industrial and academic collaboration strategies on hydrogen fuel cell technology development in Malaysia, Procedia-Social Behav. Sci. 90 (2013) 879–888. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).