Submitted:
03 April 2023
Posted:
03 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
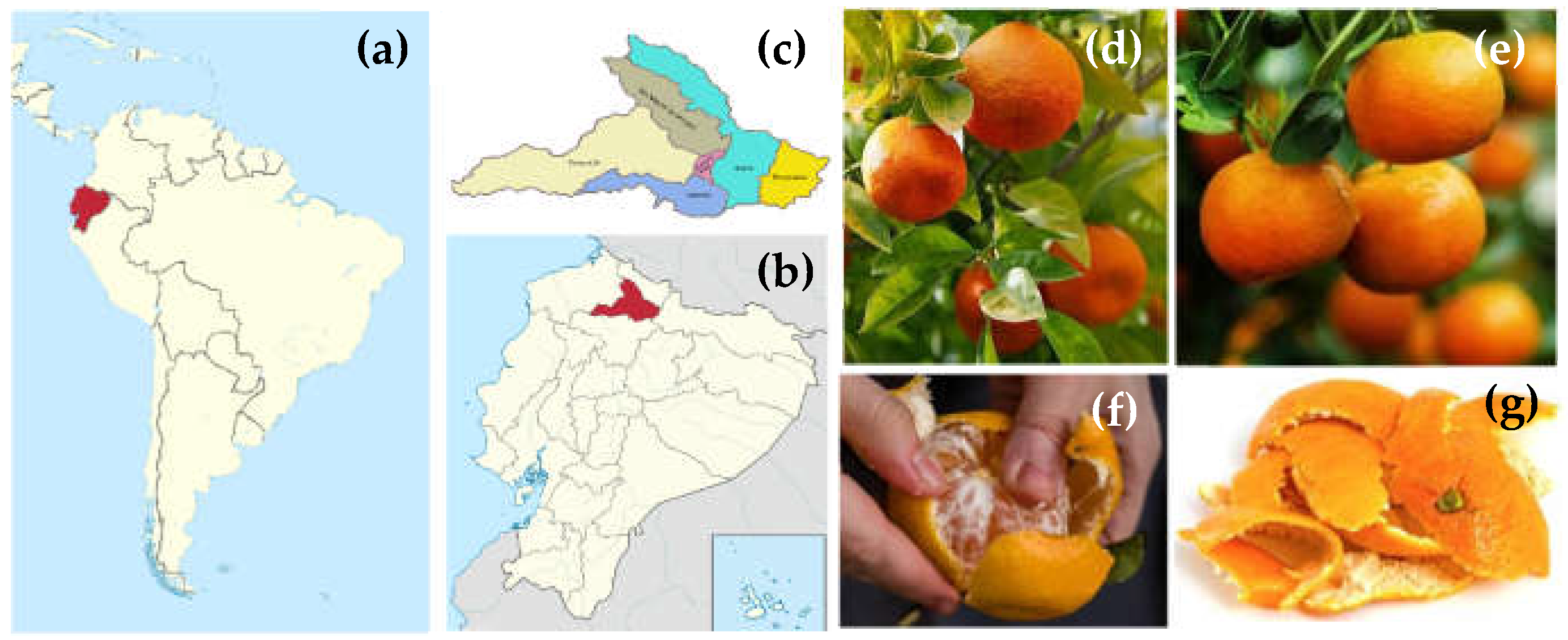
2.1. Location and Origin of the Tangerine Fruits Used
2.2. Solvent Extraction at Laboratory Scale
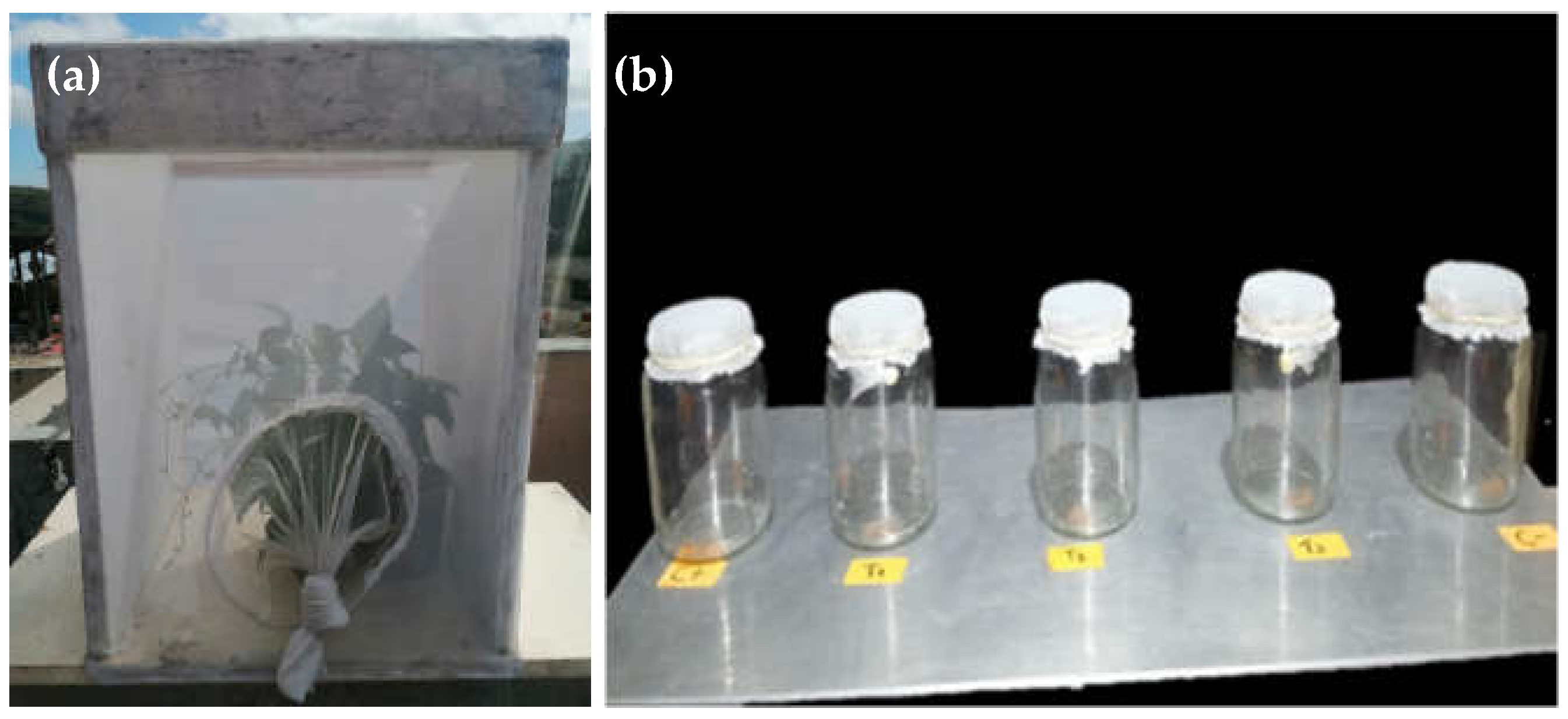
2.3. Determination of the Rate of Mortality of the Greenhouse Whitefly
2.4. Statistical Analysis and Comparison between Treatments
2.5. FTIR Analysis of EOEs from Tangerine Peels
3. Results
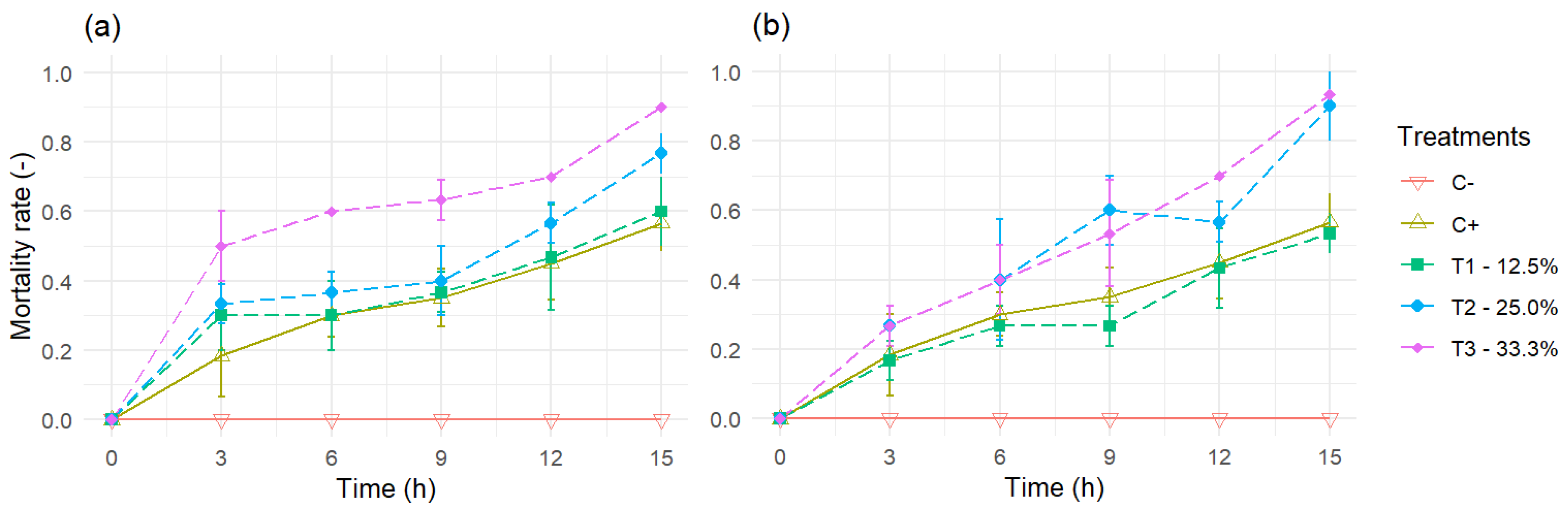
3.1. Mortality Rate Experiments
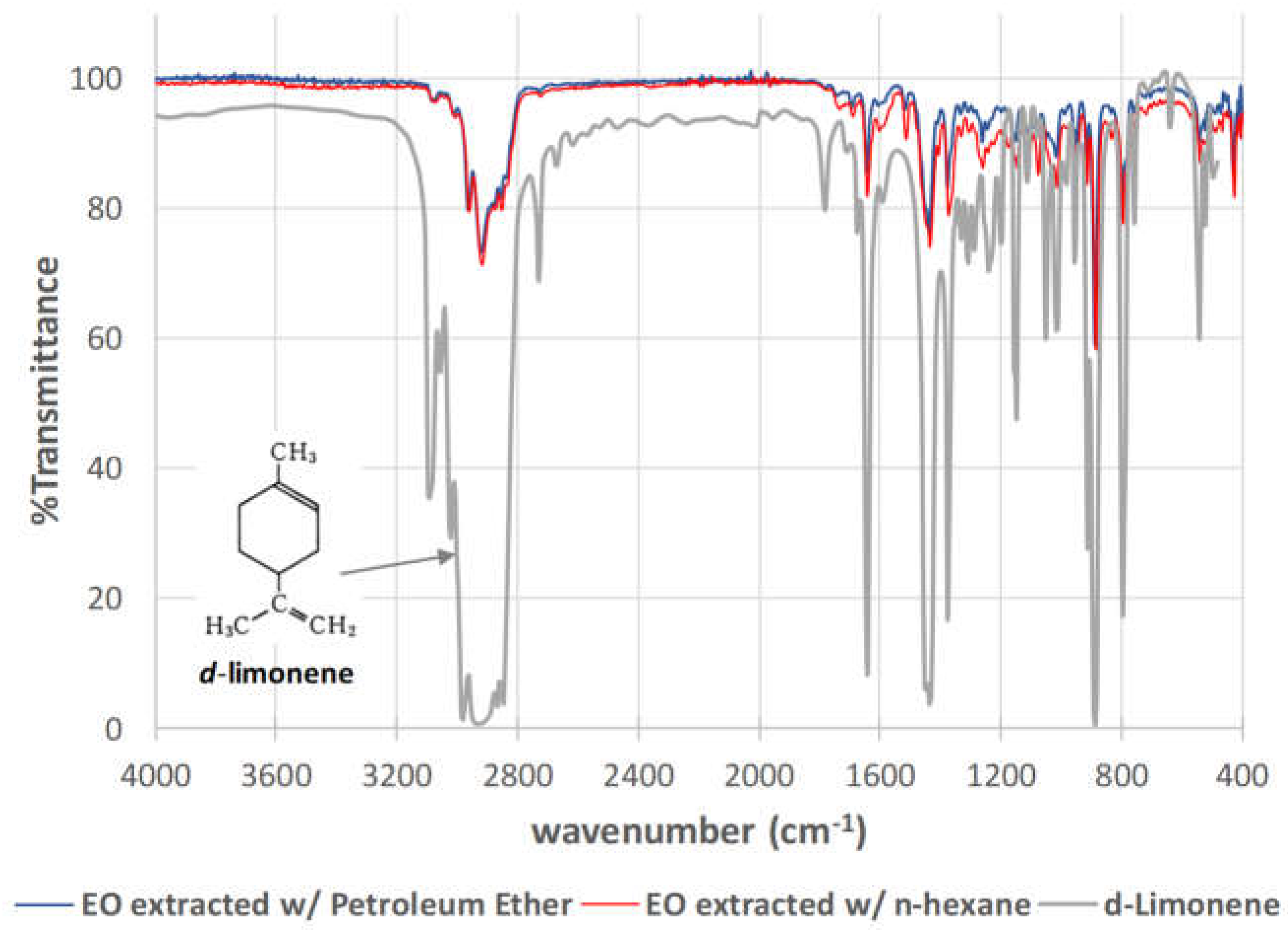
3.2. FTIR-Characterisation of EOEs from Tangerine Peels
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nayak, P.; Solanki, H. PESTICIDES AND INDIAN AGRICULTURE- A REVIEW. International Journal of Research -GRANTHAALAYAH 2021, 9. [Google Scholar] [CrossRef]
- Davydov, R.; Sokolov, M.; Hogland, W.; Glinushkin, A.; Markaryan, A. The Application of Pesticides and Mineral Fertilisers in Agriculture. In Proceedings of the MATEC Web of Conferences; 2018; Vol. 245. [Google Scholar]
- Lanz, B.; Dietz, S.; Swanson, T. The Expansion of Modern Agriculture and Global Biodiversity Decline: An Integrated Assessment. Ecological Economics 2018, 144. [Google Scholar] [CrossRef]
- McLennon, E.; Dari, B.; Jha, G.; Sihi, D.; Kankarla, V. Regenerative Agriculture and Integrative Permaculture for Sustainable and Technology Driven Global Food Production and Security. Agron J 2021, 113. [Google Scholar] [CrossRef]
- Carvalho, F.P. Agriculture, Pesticides, Food Security and Food Safety. Environ Sci Policy 2006, 9. [Google Scholar] [CrossRef]
- Arora, S.; Sahni, D. Pesticides Effect on Soil Microbial Ecology and Enzyme Activity- An Overview. Journal of Applied and Natural Science 2016, 8. [Google Scholar] [CrossRef]
- Sharma, N.; Singhvi, R. Effects of Chemical Fertilizers and Pesticides on Human Health and Environment: A Review. International Journal of Agriculture, Environment and Biotechnology 2017, 10. [Google Scholar] [CrossRef]
- Margni, M.; Rossier, D.; Crettaz, P.; Jolliet, O. Life Cycle Impact Assessment of Pesticides on Human Health and Ecosystems. Agric Ecosyst Environ 2002, 93. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front Public Health 2016, 4. [Google Scholar] [CrossRef]
- Reshma, J.; Vinaya, C.; Linu, M. Agricultural Applications of Endophytic Microflora. In Seed Endophytes: Biology and Biotechnology; 2019.
- Gunasekhar, V.; Pooja, G.S.; Thippeswamy, T. Effect of Endophytic Bacteria on the Rhizosphere Microflora of Mulberry (Morus Alba L.) Inoculated with Root Rot Pathogen Rhizoctonia Bataticola (Taub.) Butler. Indian Journal of Sericulture 2014, 53. [Google Scholar]
- Sabatier, P.; Poulenard, J.; Fangeta, B.; Reyss, J.L.; Develle, A.L.; Wilhelm, B.; Ployon, E.; Pignol, C.; Naffrechoux, E.; Dorioz, J.M.; et al. Long-Term Relationships among Pesticide Applications,Mobility, and Soil Erosion in a Vineyard Watershed. Proc Natl Acad Sci U S A 2014, 111. [Google Scholar] [CrossRef]
- Boardman, J. Soil Erosion in Britain: Updating the Record. Agriculture (Switzerland) 2013, 3. [Google Scholar] [CrossRef]
- Anderson, J.P.E.; Armstrong, R.A.; Smith, S.N. Methods to Evaluate Pesticide Damage to the Biomass of the Soil Microflora. Soil Biol Biochem 1981, 13. [Google Scholar] [CrossRef]
- Riaz, M.A.; Chandor-Proust, A.; Dauphin-Villemant, C.; Poupardin, R.; Jones, C.M.; Strode, C.; Régent-Kloeckner, M.; David, J.P.; Reynaud, S. Molecular Mechanisms Associated with Increased Tolerance to the Neonicotinoid Insecticide Imidacloprid in the Dengue Vector Aedes Aegypti. Aquatic Toxicology 2013, 126. [Google Scholar] [CrossRef]
- Karatolos, N.; Denholm, I.; Williamson, M.; Nauen, R.; Gorman, K. Incidence and Characterisation of Resistance to Neonicotinoid Insecticides and Pymetrozine in the Greenhouse Whitefly, Trialeurodes Vaporariorum Westwood (Hemiptera: Aleyrodidae). Pest Manag Sci 2010, 66. [Google Scholar] [CrossRef] [PubMed]
- Conboy, N.J.A.; McDaniel, T.; George, D.; Ormerod, A.; Edwards, M.; Donohoe, P.; Gatehouse, AMR; Tosh, C. R. Volatile Organic Compounds as Insect Repellents and Plant Elicitors: An Integrated Pest Management (IPM) Strategy for Glasshouse Whitefly (Trialeurodes Vaporariorum). J Chem Ecol 2020, 46. [Google Scholar] [CrossRef] [PubMed]
- 18. Trends in Integrated Insect Pest Management.
- Oguh, C.E.; Okpaka, C.O.; Ubani, C.S.; Okekaji, U.; PS, J. ; Amadi, EU View of Natural Pesticides (Biopesticides) and Uses in Pest Management- A Critical Review. Asian Journal of Biotechnology and Genetic Engineering 2019, 2. [Google Scholar]
- Smith, C.J.; Perfetti, T.A. A Comparison of the Persistence, Toxicity, and Exposure to High-Volume Natural Plant-Derived and Synthetic Pesticides. Toxicology Research and Application 2020, 4. [Google Scholar] [CrossRef]
- Ngegba, P.M.; Cui, G.; Khalid, M.Z.; Zhong, G. Use of Botanical Pesticides in Agriculture as an Alternative to Synthetic Pesticides. Agriculture 2022, Vol. 12, Page 600 2022, 12, 600. [Google Scholar] [CrossRef]
- Sindhu, R.; Binod, P.; Pandey, A.; Gnansounou, E. Agroresidue-Based Biorefineries. In Refining Biomass Residues for Sustainable Energy and Bioproducts: Technology, Advances, Life Cycle Assessment, and Economics; R. Praveen Kumar, Edgard Gnansounou, Raman, J.K., Gurunathan Baskar, Eds.; Academic Press: London, UK, 2020 ISBN 9780128189962.
- Shahzad, K.; Nawaz, S.; Ahmad, R.; Akram, N.; Iqbal, Z. Evaluation of Antbacterial, Antifungal and Antioxidant Activity of Essentail Oil of Citrus Reticulata Fruit (Tangerine Fruit Peel). Pharmacologyonline 2009, 3. [Google Scholar]
- Sreepian, A.; Sreepian, P.M.; Chanthong, C.; Mingkhwancheep, T.; Prathit, P. Antibacterial Activity of Essential Oil Extracted from Citrus Hystrix (Kaffir Lime) Peels: An in Vitro Study. Trop Biomed 2019, 36. [Google Scholar]
- Kholaf, G.M.; Gomaa, E.G.; Ziena, H.M. Antimicrobial Activity of Some Egyptian Citrus Peels Extracts. Alexandria Science Exchange Journal 2017, 38. [Google Scholar] [CrossRef]
- Kamal, G.M.; Ashraf, M.Y.; Hussain, A.I.; Shahzadi, A.; Chughtai, M.I. Antioxidant Potential of Peel Essential Oils of Three Pakistani Citrus Species: Citrus Reticulata, Citrus Sinensis and Citrus Paradisii. Pak J Bot 2013, 45. [Google Scholar]
- Mahmoud, E. Essential Oils of Citrus Fruit Peels Antioxidant, Antibacterial and Additive Value as Food Preservative. Journal of Food and Dairy Sciences 2017, 8. [Google Scholar] [CrossRef]
- Nair S, A.; SR, RK; Nair, A. S.; Baby, S. Citrus Peels Prevent Cancer. Phytomedicine 2018, 50. [Google Scholar] [CrossRef] [PubMed]
- Khumukcham, N.; Ajungla, T.; Singh, C.B. GCMS BASED METABOLIC PROFILING OF ESSENTIAL OIL OF CITRUS MACROPTERA MONTRUZ. LEAVES AND PEEL, ASSESSMENT OF IN VITROANTIOXIDANT AND ANTI-INFLAMMATORY ACTIVITY. Int J Pharm Pharm Sci 2017, 9. [Google Scholar] [CrossRef]
- El Sawi, S.A.; Ibrahim, M.E.; El-Rokiek, K.G.; El-Din, S.A.S. Allelopathic Potential of Essential Oils Isolated from Peels of Three Citrus Species. Annals of Agricultural Sciences 2019, 64. [Google Scholar] [CrossRef]
- Aripin, D.; Julaeha, E.; Dardjan, M.; Cahyanto, A. Chemical Composition of Citrus Spp. and Oral Antimicrobial Effect of Citrus Spp. Peels Essential Oils against Streptococcus Mutans. Padjadjaran Journal of Dentistry 2015, 27. [Google Scholar] [CrossRef]
- Liu, N.; Li, X.; Zhao, P.; Zhang, X.; Qiao, O.; Huang, L.; Guo, L.; Gao, W. A Review of Chemical Constituents and Health-Promoting Effects of Citrus Peels. Food Chem 2021, 365. [Google Scholar] [CrossRef] [PubMed]
- Karoui, I.J.; Wannes, W.A.; Marzouk, B. Refined Corn Oil Aromatization by Citrus Aurantium Peel Essential Oil. Ind Crops Prod 2010, 32. [Google Scholar] [CrossRef]
- Gültepe, N. Protective Effect of D-Limonene Derived from Orange Peel Essential Oil against Yersinia Ruckeri in Rainbow Trout. Aquac Rep 2020, 18. [Google Scholar] [CrossRef]
- de Brito, W.A.; Siquieroli, A.C.S.; Andaló, V.; Duarte, J.G.; de Sousa, R.M.F.; Felisbino, J.K.R.P.; da Silva, G.C. Botanical Insecticide Formulation with Neem Oil and D-Limonene for Coffee Borer Control. Pesqui Agropecu Bras 2021, 56. [Google Scholar] [CrossRef]
- Karr, L.L. Toxic Properties of D-Limonene in Insects and the Earthworm Eisenia Fetida. Iowa State University 2014, 13. [Google Scholar]
- Tripathi, A.K.; Prajapati, V.; Khanuja, S.P.S.; Kumar, S. Effect of D-Limonene on Three Stored-Product Beetles. J Econ Entomol 2003, 96. [Google Scholar] [CrossRef]
- Feng, J.; Wang, R.; Chen, Z.; Zhang, S.; Yuan, S.; Cao, H.; Jafari, S.M.; Yang, W. Formulation Optimization of D-Limonene-Loaded Nanoemulsions as a Natural and Efficient Biopesticide. Colloids Surf A Physicochem Eng Asp 2020, 596. [Google Scholar] [CrossRef]
- John, I.; Muthukumar, K.; Arunagiri, A. A Review on the Potential of Citrus Waste for D-Limonene, Pectin, and Bioethanol Production. Int J Green Energy 2017, 14. [Google Scholar] [CrossRef]
- Sun, J. D-Limonene: Safety and Clinical Applications. Alternative Medicine Review 2007, 12. [Google Scholar]
- Anandakumar, P.; Kamaraj, S.; Vanitha, M.K. D-Limonene: A Multifunctional Compound with Potent Therapeutic Effects. J Food Biochem 2021, 45. [Google Scholar] [CrossRef]
- Bährle-Rapp, M. Citrus Reticulata. In Springer Lexikon Kosmetik und Körperpflege; 2007.
- Khan, I.; Shah, Z.A.; Saeed, M.; Shah, H.U. Physicochemical Analysis of Citrus Sinensis, Citrus Reticulata and Citrus Paradisi. Journal of the Chemical Society of Pakistan 2010, 32. [Google Scholar]
- Caicedo Jiménez, D.I. Evaluación de Pérdidas Poscosecha En Mandarina (Citrus Reticulata), En El Cantón Patate. Bachelor Degree in Engineering Science, Universidad Central del Ecuador: Quito, 2021.
- Panwar, D. ; Panesar, PS; Chopra, H.K. Recent Trends on the Valorization Strategies for the Management of Citrus By-Products. Food Reviews International.
- Punvichai, T.; Pioch, D. Co-Valorization of Agro-Industry by-Products: Effect of Citrus Oil on the Quality of Soap Derived from Palm Fatty Acid Distillate and Spent Bleaching Clay. Letters in Applied NanoBioScience 2019, 8. [Google Scholar] [CrossRef]
- Sahraoui, N.; Vian, M.A.; El Maataoui, M.; Boutekedjiret, C.; Chemat, F. Valorization of Citrus By-Products Using Microwave Steam Distillation (MSD). Innovative Food Science and Emerging Technologies 2011, 12. [Google Scholar] [CrossRef]
- Mandal, S.; Mandal, M. Tangerine (Citrus Reticulata L. Var.) Oils. Essential Oils in Food Preservation, Flavor and Safety. [CrossRef]
- Knapp, G. Mountain Agriculture for Global Markets: The Case of Greenhouse Floriculture in Ecuador. In Mountains: Physical, Human-Environmental, and Sociocultural Dynamics; 2019.
- Nazeeh, N.; Suárez-López, J.R. Summary Data of Home Proximity to the Nearest Greenhouse (Floricultural) Crops and Areas of Greenhouse Crops around Various Distances from Homes in Agricultural Settings in Ecuador. Data Brief 2020, 31. [Google Scholar] [CrossRef] [PubMed]
- Yagual, A.; Lovato, S.; Mite, M. Importancia de La Exportación de Flores Sobre Total Exportaciones FOB No Tradicionales En Ecuador 2012-2016 Importance of the Export of Flowers on Total Non-Traditional FOB Exports in Ecuador 2012-2016. Revista Espacio 2018, 39. [Google Scholar]
- Yulan Negrete, H.; Garcia Regalado, J.; Medina Zambrano, D.; Limones Salazar, A. Analysis of Rose Exports to the U.S. and Their Impact on Ecuadorian GDP Period 2015-2019. Universidad Ciencia y Tecnología 2021, 25. [Google Scholar] [CrossRef]
- Rincon, D.F.; Vasquez, D.F.; Rivera-Trujillo, H.F.; Beltrán, C.; Borrero-Echeverry, F. Economic Injury Levels for the Potato Yellow Vein Disease and Its Vector, Trialeurodes Vaporariorum (Hemiptera: Aleyrodidae), Affecting Potato Crops in the Andes. Crop Protection 2019, 119. [Google Scholar] [CrossRef]
- SCOTTA, R.R.; SÁNCHEZ, D.A.E.; ARREGUI, M.C. DETERMINACIÓN DE LAS PÉRDIDAS CAUSADAS POR LA MOSCA BLANCA DE LOS INVERNADEROS (Trialeurodes Vaporariorum) EN CULTIVOS DE TOMATE BAJO INVERNADERO. FAVE Sección Ciencias Agrarias 2015, 13. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, R.; Zeng, X.; Wang, X.; Wang, D.; Jia, H.; Xu, W.; Gao, Y. Exposure to Neonicotinoid Insecticides and Their Characteristic Metabolites: Association with Human Liver Cancer. Environ Res 2022, 208. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, Z.; Xiao, Q.; Li, Z.; Jia, X.; Hu, W.; Liu, K.; Lu, S. Urinary Neonicotinoid Insecticides in Children from South China: Concentrations, Profiles and Influencing Factors. Chemosphere 2022, 291. [Google Scholar] [CrossRef]
- 57. Godfray, HCJ; Blacquière, T.; Field, LM; Hails, R.S.; Petrokofsky, G.; Potts, S.G.; Raine, N.E.; Vanbergen, AJ; McLean, A.R. A Restatement of the Natural Science Evidence Base Concerning Neonicotinoid Insecticides and Insect Pollinators. Proceedings of the Royal Society B: Biological Sciences.
- González-Andrade, F.; López-Pulles, R.; Estévez, E. Acute Pesticide Poisoning in Ecuador: A Short Epidemiological Report. J Public Health (Bangkok) 2010, 18. [Google Scholar] [CrossRef]
- Breilh, J.; Pagliccia, N.; Yassi, A. Chronic Pesticide Poisoning from Persistent Low-Dose Exposures in Ecuadorean Floriculture Workers: Toward Validating a Low-Cost Test Battery. Int J Occup Environ Health 2012, 18. [Google Scholar] [CrossRef]
- Skomal, A.E.; Zhang, J.; Yang, K.; Yen, J.; Tu, X.; Suarez-Torres, J.; Lopez-Paredes, D.; Calafat, A.M.; Ospina, M.; Martinez, D.; et al. Concurrent Urinary Organophosphate Metabolites and Acetylcholinesterase Activity in Ecuadorian Adolescents. Environ Res 2022, 207. [Google Scholar] [CrossRef]
- Friedman, E.; Hazlehurst, M.F.; Loftus, C.; Karr, C.; McDonald, K.N.; Suarez-Lopez, J.R. Residential Proximity to Greenhouse Agriculture and Neurobehavioral Performance in Ecuadorian Children. Int J Hyg Environ Health 2020, 223. [Google Scholar] [CrossRef]
- Grandjean, P.; Harari, R.; Barr, D.B.; Debes, F. Pesticide Exposure and Stunting as Independent Predictors of Neurobehavioral Deficits in Ecuadorian School Children. Pediatrics 2006, 117. [Google Scholar] [CrossRef]
- Phillips, S.; Suarez-Torres, J.; Checkoway, H.; Lopez-Paredes, D.; Gahagan, S.; Suarez-Lopez, J.R. Acetylcholinesterase Activity and Thyroid Hormone Levels in Ecuadorian Adolescents Living in Agricultural Settings Where Organophosphate Pesticides Are Used. Int J Hyg Environ Health 2021, 233. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Ko, KY; Kim, I. H. Optimization of D-Limonene Extraction from Tangerine Peel in Various Solvents by Using Soxhlet Extractor. Korean Chemical Engineering Research 2015, 53. [Google Scholar] [CrossRef]
- Angeles-Martínez, O.; García-Mateos, Ma.R.; Rodríguez-Pérez, E.; Sánchez-Alvarez, E.; Soto-Hernández, M. Toxicity of Plant Extracts for Control of Trialeurodes Vaporariorum W. (Homoptera: Aleyrodidae) in Laboratory and Greenhouse Tomatoes. The Journal of Agriculture of the University of Puerto Rico 2021, 95. [Google Scholar] [CrossRef]
- Muthuthantri, S.; Clarke, A.R.; Hayes, R.A.; Kevin, J. Effect of Citrus Peel Chemicals on Bactrocera Tryoni Larval Survival. Acta Hortic 2015, 1105. [Google Scholar] [CrossRef]
- Bailão, E.F.L.C.; Pereira, D.G.; Romano, C.A.; Paz, A.T. de S.; Silva, T.M. e.; Paula, J.R. de; Gomes, C.M.; Borges, L.L. Larvicidal Effect of the Citrus Limettioides Peel Essential Oil on Aedes Aegypti. South African Journal of Botany 2022, 144. [Google Scholar] [CrossRef]
- Azmy, R.M.; El Gohary, E.G.E.; Salem, D.A.M.; Mahmoud, D.M.; Salama, M.S.; Abdou, M.A. Evaluation of the Larvicidal Activity of Nanoemulsion from Citrus Aurantifolia (Christm) Swingle Peel on Culex Pipiens l. (Diptera: Culicidae) and the Induced Morphological Aberrations. Egypt J Aquat Biol Fish 2021, 25. [Google Scholar] [CrossRef]
- Delkhoon, S.; Fahim, M.; Hosseinzadeh, J.; Panahi, O. Effect of Lemon Essential Oil on the Developmental Stages of Trialeurodes Vaporariorum West (Homoptera: Aleyrodidae). Archives of Phytopathology and Plant Protection 2013, 46. [Google Scholar] [CrossRef]
- Dehghani, M.; Ahmadi, K. Anti-Oviposition and Repellence Activities of Essential Oils and Aqueous Extracts from Five Aromatic Plants against Greenhouse Whitefly Trialeurodes Vaporariorum Westwood (Homoptera: Aleyrodidae). Bulgarian Journal of Agricultural Science 2013, 19. [Google Scholar]
- Ibáñez, M.D.; Sanchez-Ballester, N.M.; Blázquez, M.A. Encapsulated Limonene: A Pleasant Lemon-like Aroma with Promising Application in the Agri-Food Industry. A Review. A Review. Molecules 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- K, R.; S, S.; Bhaskar S A, V.; S M, V.; Sesha, Mr.N. Extraction of Essential Oil D-Limonene from Sweet Orange Peels by Simple Distillation. IOSR Journal of Applied Chemistry 2016, 09. [Google Scholar] [CrossRef]
- Mirdha, K.; Routray, C. Extraction and Characterization of D-Limonene Oil from Orange Peels Using Different Solvents. Mukt Shabd Journal 2020. [Google Scholar]
- Filho, CA; Silva, C. M.; Quadri, M.B.; Macedo, E.A. Tracer Diffusion Coefficients of Citral and D-Limonene in Supercritical Carbon Dioxide. Fluid Phase Equilib 2003, 204. [Google Scholar] [CrossRef]
- Auta, M.; Musa, U.; Tsado, D.G.; Faruq, A.A.; Isah, A.G.; Raji, S.; Nwanisobi, C. Optimization of Citrus Peels D-Limonene Extraction Using Solvent-Free Microwave Green Technology. Chem Eng Commun 2018, 205. [Google Scholar] [CrossRef]
- Khandare, R.D.; Tomke, P.D.; Rathod, V.K. Kinetic Modeling and Process Intensification of Ultrasound-Assisted Extraction of d-Limonene Using Citrus Industry Waste. Chemical Engineering and Processing - Process Intensification 2021, 159. [Google Scholar] [CrossRef]
- Droby, S.; Eick, A.; Macarisin, D.; Cohen, L.; Rafael, G.; Stange, R.; McColum, G.; Dudai, N.; Nasser, A.; Wisniewski, M.; et al. Role of Citrus Volatiles in Host Recognition, Germination and Growth of Penicillium Digitatum and Penicillium Italicum. Postharvest Biol Technol 2008, 49. [Google Scholar] [CrossRef]
- Dong, Z.B.; Shao, W.Y.; Liang, Y.R. Isolation and Characterization of Essential Oil Extracted from Tangerine Peel. Asian Journal of Chemistry 2014, 26. [Google Scholar] [CrossRef]
- Sharma, P.; Vishvakarma, R.; Gautam, K.; Vimal, A.; Kumar Gaur, V.; Farooqui, A.; Varjani, S.; Younis, K. Valorization of Citrus Peel Waste for the Sustainable Production of Value-Added Products. Bioresour Technol 2022, 351. [Google Scholar] [CrossRef]
- Satari, B.; Karimi, K. Citrus Processing Wastes: Environmental Impacts, Recent Advances, and Future Perspectives in Total Valorization. Resour Conserv Recycl 2018, 129. [Google Scholar] [CrossRef]
- Campos, E.V.R.; Proença, P.L.F.; Oliveira, J.L.; Bakshi, M.; Abhilash, P.C.; Fraceto, L.F. Use of Botanical Insecticides for Sustainable Agriculture: Future Perspectives. Ecol Indic 2019, 105. [Google Scholar] [CrossRef]
- Ahmed, N.; Alam, M.; Saeed, M.; Ullah, H.; Iqbal, T.; Awadh Al-Mutairi, K.; Shahjeer, K.; Ullah, R.; Ahmed, S.; Abd Aleem Hassan Ahmed, N.; et al. Botanical Insecticides Are a Non-Toxic Alternative to Conventional Pesticides in the Control of Insects and Pests. In Global Decline of Insects; 2022.

| Treatment | The volume of AC (µL) 1 | Characteristic of AC |
|---|---|---|
| Positive control (C+) | 0.178 | C9H10ClN5O2 |
| T1 - 12.50 % (v/v) | 22.2 | EOEs 2 w/PET or HEX |
| T2 - 25.00 % (v/v) | 44.4 | EOEs w/PET or HEX |
| T3 - 33.33 % (v/v) | 59.3 | EOEs w/PET or HEX |
| Negative control (C-) | none | Water |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





