Submitted:
03 April 2023
Posted:
04 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. The Experimental Trial
2.3. Chemical Analyses
2.4. Leaf Phenology Scoring
2.5. Statistical Analyses
3. Results
3.1. Substrate Nutrient Content Differences between the Treatments
3.2. Leaf Mineral Nutrition Variations
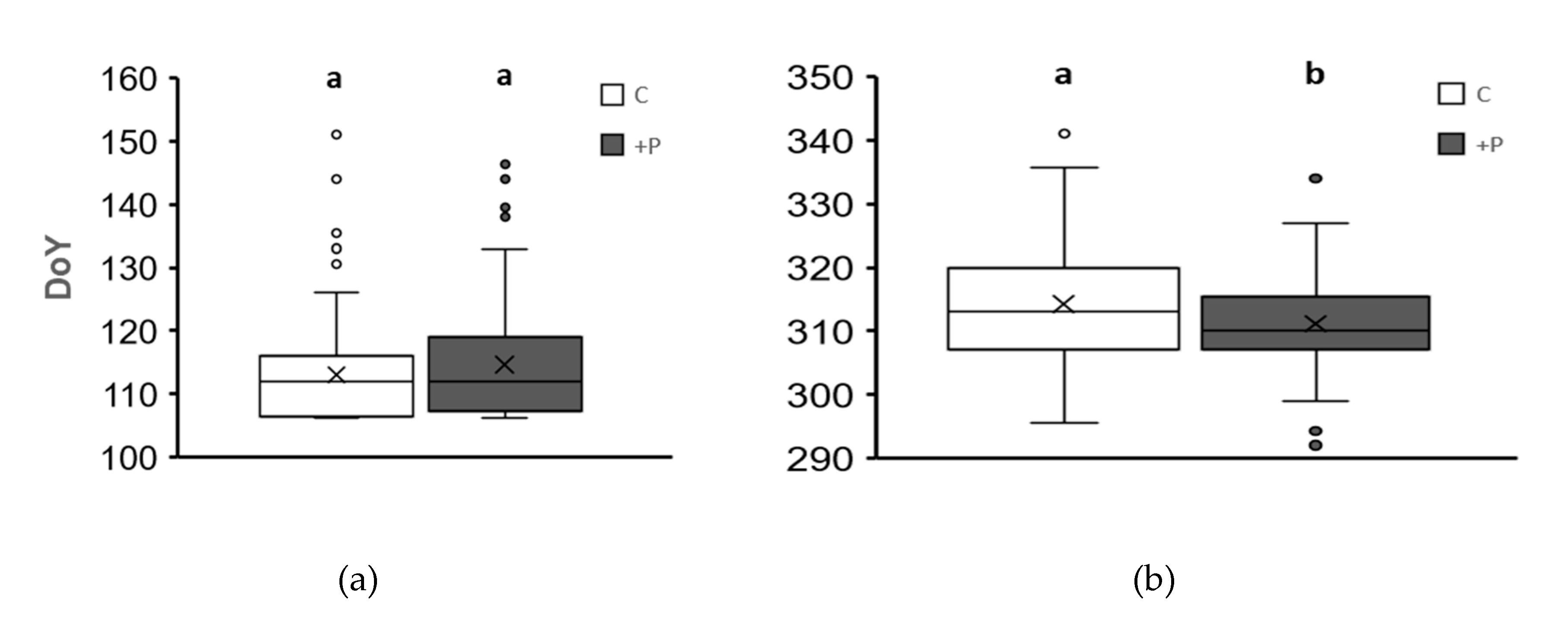
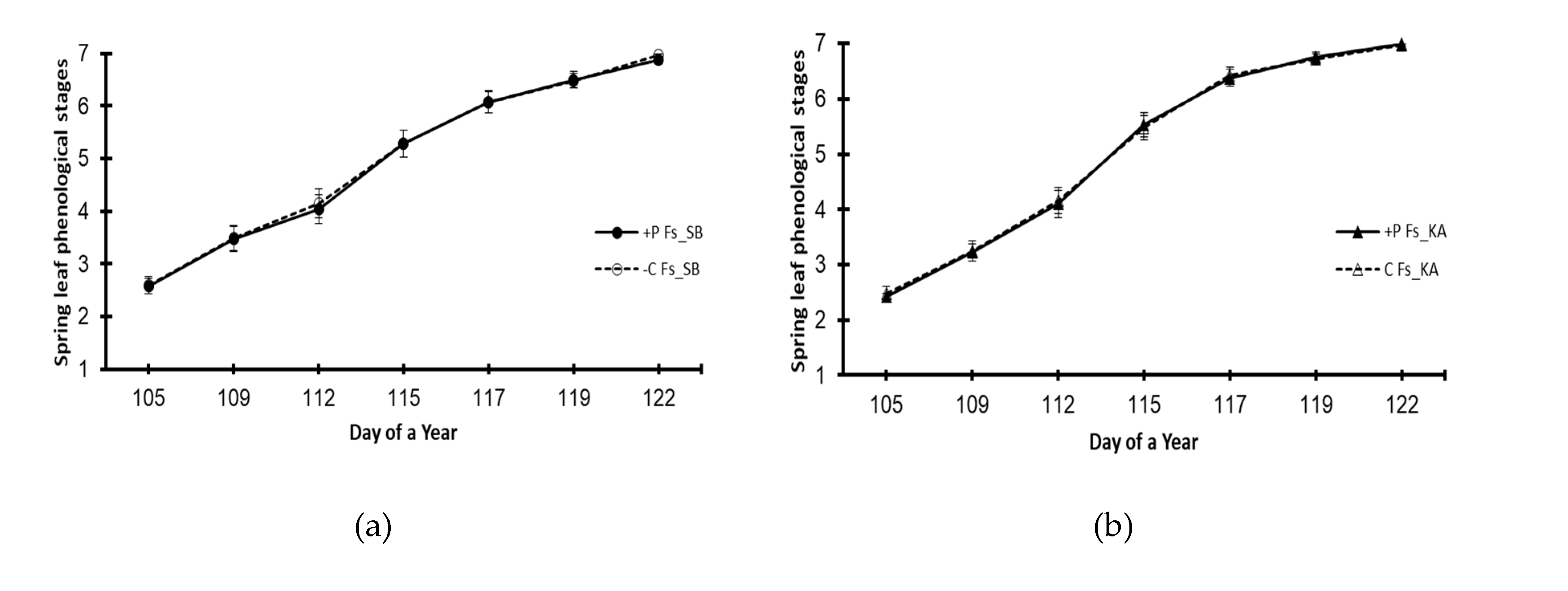
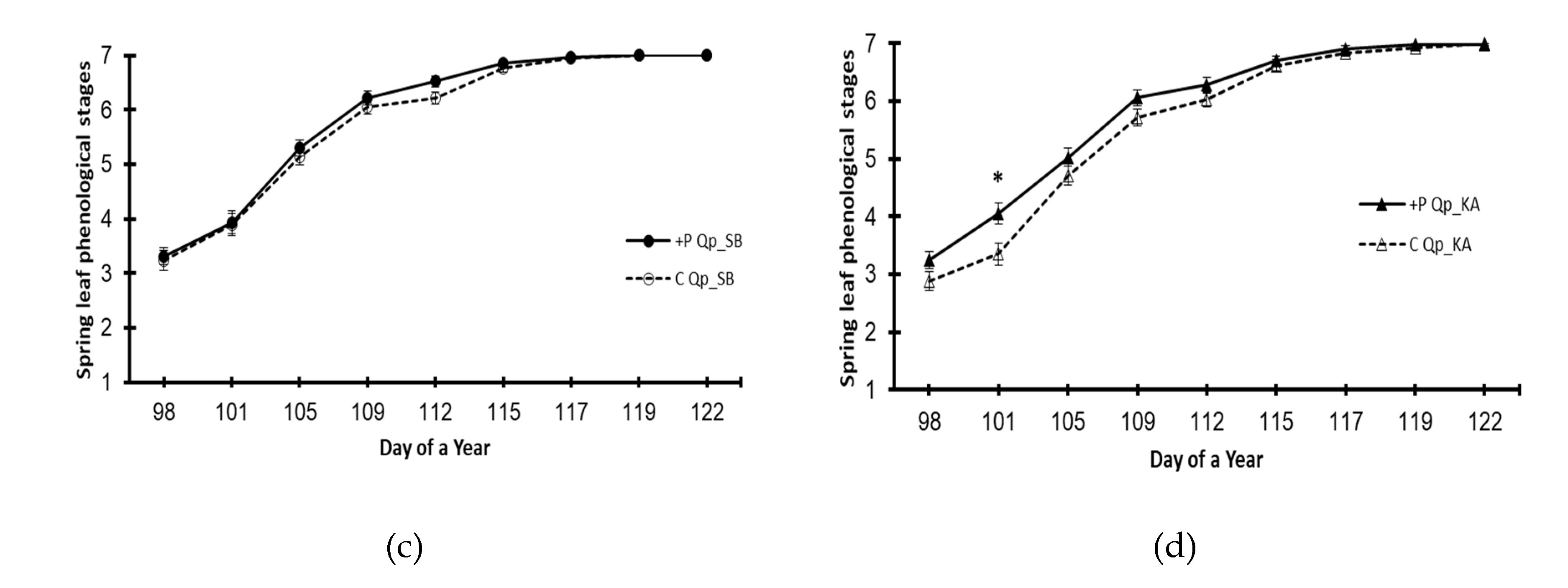
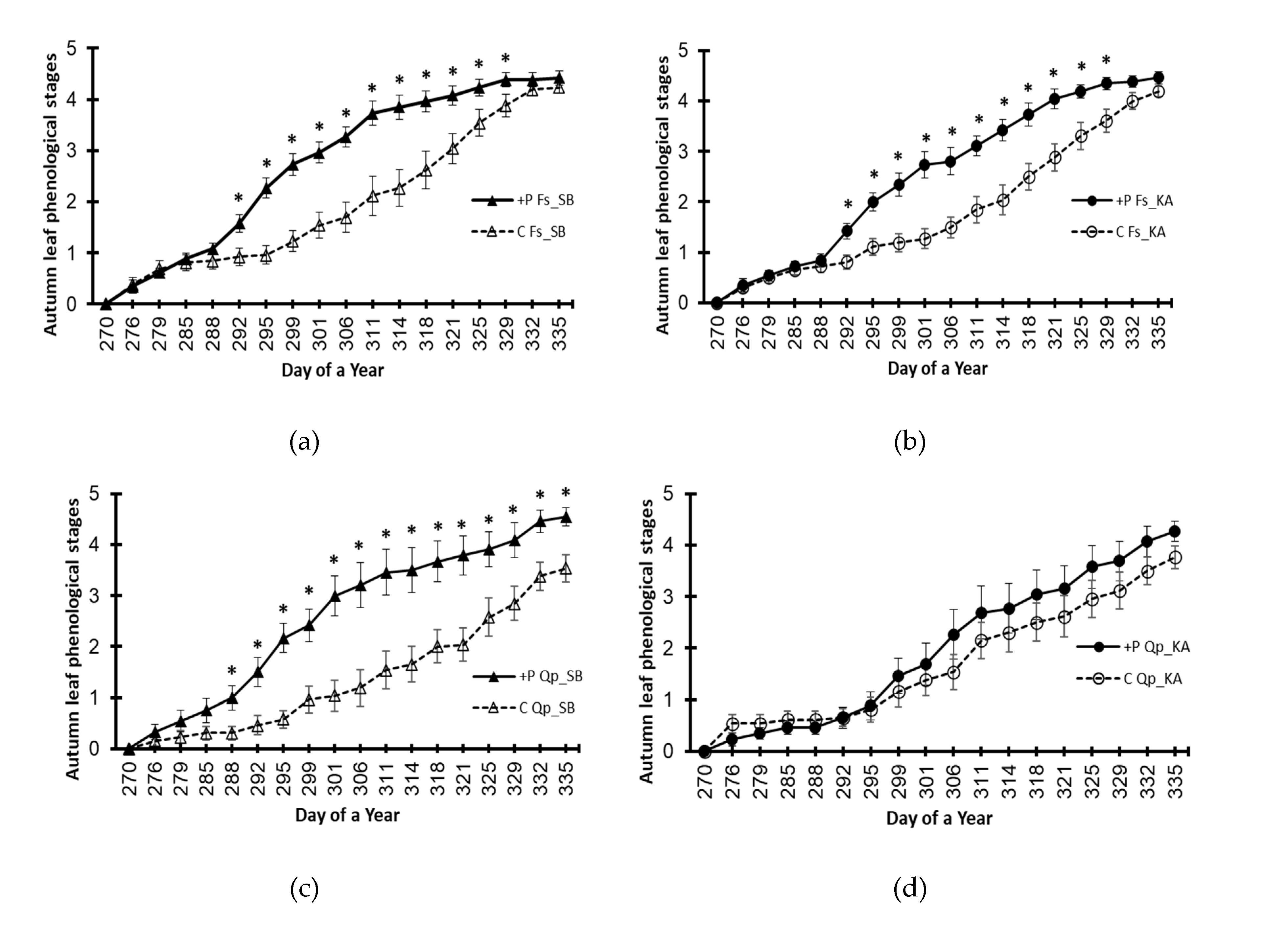
3.3. Variations Caused by the Elevated Phosphorus Treatment (+P)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Polgar, C.A.; Primack, R.B. Leaf-out phenology of temperate woody plants: from trees to ecosystems. New Phytol. 2011, 191, 926–941. [Google Scholar] [CrossRef] [PubMed]
- Way, D.A.; Montgomery, R.A. Photoperiod constraints on tree phenology, performance and migration in a warming world. Plant, Cell Environ. 2015, 38, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Kuster, T.M.; Arend, M.; Günthardt-Goerg, M.S.; Schulin, R. Root growth of different oak provenances in two soils under drought stress and air warming conditions. Plant Soil 2012, 369, 61–71. [Google Scholar] [CrossRef]
- Vitasse, Y.; Lenz, A.; Kã¶Rner, C. The interaction between freezing tolerance and phenology in temperate deciduous trees. Front. Plant Sci. 2014, 5, 541–541. [Google Scholar] [CrossRef] [PubMed]
- Lenz, A.; Hoch, G.; Körner, C.; Vitasse, Y. Convergence of leaf-out towards minimum risk of freezing damage in temperate trees. Funct. Ecol. 2016, 30, 1480–1490. [Google Scholar] [CrossRef]
- ehulić, I.; Sever, K.; Katičić Bogdan, I.; Jazbec, A.; Škvorc, Ž.; Bogdan, S. Drought Impact on Leaf Phenology and Spring Frost Susceptibility in a Quercus Robur L. Provenance Trial. Forests 2019, 10, 50. [Google Scholar] [CrossRef]
- Murray, M.B.; Smith, R.I.; Leith, I.D.; Fowler, D.; Lee, H.S.J.; Friend, A.D.; Jarvis, P.G. Effects of elevated CO2, nutrition and climatic warming on bud phenology in Sitka spruce (Picea sitchensis) and their impact on the risk of frost damage. Tree Physiol. 1994, 14, 691–706. [Google Scholar] [CrossRef]
- Fløistad, I.S.; Kohmann, K. Influence of nutrient supply on spring frost hardiness and time of bud break in Norway spruce (Picea abies (L.) Karst.) seedlings. New For. 2004, 27, 1–11. [Google Scholar] [CrossRef]
- Luoranen, J.; Rikala, R. Nutrient loading of Norway spruce seedlings hastens bud burst and enhances root growth after outplanting. Silva Fenn. 2011, 45. [Google Scholar] [CrossRef]
- Mutz, J.; McClory, R.; van Dijk, L.J.A.; Ehrlén, J.; Tack, A.J.M. Pathogen infection influences the relationship between spring and autumn phenology at the seedling and leaf level. Oecologia 2021, 197, 447–457. [Google Scholar] [CrossRef]
- Maurer, S.; Egli, P.; Spinnler, D.; Körner, C. Carbon and water fluxes in Beech-Spruce model ecosystems in response to long-term exposure to atmospheric CO2 enrichment and increased nitrogen deposition. Funct. Ecol. 1999, 13, 748–755. [Google Scholar] [CrossRef]
- Nord, E.A.; Lynch, J.P. Plant phenology: a critical controller of soil resource acquisition. J. Exp. Bot. 2009, 60, 1927–1937. [Google Scholar] [CrossRef] [PubMed]
- Sigurdsson, B.D. Elevated [CO2] and nutrient status modified leaf phenology and growth rhythm of young Populus trichocarpa trees in a 3-year field study. Trees 2001, 15, 403–413. [Google Scholar] [CrossRef]
- Wang, P.; Fu, C.; Wang, L.; Yan, T. Delayed autumnal leaf senescence following nutrient fertilization results in altered nitrogen resorption. Tree Physiol. 2022, 42, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.H.; Piao, S.; Delpierre, N.; Hao, F.; Hänninen, H.; Geng, X.; Peñuelas, J.; Zhang, X.; A Janssens, I.; Campioli, M. Nutrient availability alters the correlation between spring leaf-out and autumn leaf senescence dates. Tree Physiol. 2019, 39, 1277–1284. [Google Scholar] [CrossRef]
- Zani, D.; Crowther, T.W.; Mo, L.; Renner, S.S.; Zohner, C.M. Increased growing-season productivity drives earlier autumn leaf senescence in temperate trees. Science 2020, 370, 1066–1071. [Google Scholar] [CrossRef]
- Asshoff, R.; Zotz, G.; Körner, C. Growth and phenology of mature temperate forest trees in elevated CO2. Glob. Chang. Biol. 2006, 12, 848–861. [Google Scholar] [CrossRef]
- Ueda, M.U.; Mizumachi, E.; Tokuchi, N. Allocation of nitrogen within the crown during leaf expansion in Quercus serrata saplings. Tree Physiol. 2009, 29, 913–919. [Google Scholar] [CrossRef]
- Netzer, F.; Mueller, C.W.; Scheerer, U.; Grüner, J.; Kögel-Knabner, I.; Herschbach, C.; Rennenberg, H. Phosphorus Nutrition of Populus$\times$ Canescens Reflects Adaptation to High P-Availability in the Soil. Tree Physiol. 2018, 38, 6–24. [Google Scholar] [CrossRef]
- Netzer, F.; Herschbach, C.; Oikawa, A.; Okazaki, Y.; Dubbert, D.; Saito, K.; Rennenberg, H. Seasonal Alterations in Organic Phosphorus Metabolism Drive the Phosphorus Economy of Annual Growth in F. sylvatica Trees on P-Impoverished Soil. Front. Plant Sci. 2018, 9, 723. [Google Scholar] [CrossRef]
- Poirier, Y.; Bucher, M. Phosphate Transport and Homeostasis in Arabidopsis. Arab. Book 2002, 1, e0024–e0024. [Google Scholar] [CrossRef]
- Malhotra, H.; Sharma, S.; Pandey, R. Phosphorus nutrition: Plant growth in response to deficiency and excess. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore. 2018; pp. 171–190. [CrossRef]
- Yang, N.; Zavišić, A.; Pena, R.; Polle, A. Phenology, Photosynthesis, and Phosphorus in European Beech (Fagus Sylvatica L. ) in Two Forest Soils with Contrasting P Contents. J. Plant Nutr. Soil Sci. 2016, 179, 151–158. [Google Scholar]
- Kolström, M.; Lindner, M.; Vilén, T.; Maroschek, M.; Seidl, R.; Lexer, M.J.; Netherer, S.; Kremer, A.; Delzon, S.; Barbati, A.; et al. Reviewing the Science and Implementation of Climate Change Adaptation Measures in European Forestry. Forests 2011, 2, 961–982. [Google Scholar] [CrossRef]
- Brang, P.; Spathelf, P.; Larsen, J.B.; Bauhus, J.; Ina, A.B.; Chauvin, C.; Drössler, L.; García-Güemes, C.; Heiri, C.; Kerr, G.; et al. Suitability of close-to-nature silviculture for adapting temperate European forests to climate change. For. Int. J. For. Res. 2014, 87, 492–503. [Google Scholar] [CrossRef]
- Forest Europe 2020. State of Europe’s Forests 2020. Available online: https://foresteurope.org/state-of-europes-forests/ (accessed on 3 January 2023).
- Jansen, S.; Konrad, H.; Geburek, T. Crossing borders – European forest reproductive material moving in trade. J. Environ. Manag. 2019, 233, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Sever, K.; Bogdan, S.; Škvorc. ; Sever, M.Z.O.; Franjić, J. Estimation of leaf nitrogen concentrations in Quercus robur L. using the CCM-200 portable chlorophyll meter for different patterns of vegetative growth and acorn production. New For. 2016, 47, 513–527. [Google Scholar] [CrossRef]
- Sever, K.; Bogdan, S.; Škvorc. Response of photosynthesis, growth, and acorn mass of pedunculate oak to different levels of nitrogen in wet and dry growing seasons. J. For. Res. 2022, 34, 167–176. [Google Scholar] [CrossRef]
- Schmal, J.L.; Jacobs, D.F.; O’reilly, C. Nitrogen budgeting and quality of exponentially fertilized Quercus robur seedlings in Ireland. Eur. J. For. Res. 2011, 130, 557–567. [Google Scholar] [CrossRef]
- Walters, M.B.; Reich, P.B. Seed Size, Nitrogen Supply, and Growth Rate Affect Tree Seedling Survival in Deep Shade. Ecology 2000, 81, 1887–1901. [Google Scholar] [CrossRef]
- Villar-Salvador, P.; Puértolas, J.; Peñuelas, J.L.; Planelles, R. Effect of nitrogen fertilization in the nursery on the drought and frost resistance of Mediterranean forest species. 2005, 14, 408. [CrossRef]
- Villar-Salvador, P.; Heredia, N.; Millard, P. Remobilization of acorn nitrogen for seedling growth in holm oak (Quercus ilex), cultivated with contrasting nutrient availability. Tree Physiol. 2010, 30, 257–263. [Google Scholar] [CrossRef]
- Villar-Salvador, P.; Penuelas, J.L.; Nicolás-Peragón, J.L.; Benito, L.F.; Domínguez-Lerena, S. Is Nitrogen Fertilization in the Nursery a Suitable Tool for Enhancing the Performance of Mediterranean Oak Plantations? New For. 2013, 44, 733–751. [Google Scholar] [CrossRef]
- Shi, W.; Villar-Salvador, P.; Li, G.; Jiang, X. Acorn size is more important than nursery fertilization for outplanting performance of Quercus variabilis container seedlings. Ann. For. Sci. 2019, 76, 22. [Google Scholar] [CrossRef]
- Vitra, A.; Lenz, A.; Vitasse, Y. Frost hardening and dehardening potential in temperate trees from winter to budburst. New Phytol. 2017, 216, 113–123. [Google Scholar] [CrossRef]
- Geilfus, C.-M.; Carpentier, S.C.; Zavišić, A.; Polle, A. Changes in the Fine Root Proteome of Fagus Sylvatica L. Trees Associated with P-Deficiency and Amelioration of P-Deficiency. J. Proteomics 2017, 169, 33–40. [Google Scholar]
- Pan, Y.; Song, Y.; Zhao, L.; Chen, P.; Bu, C.; Liu, P.; Zhang, D. The Genetic Basis of Phosphorus Utilization Efficiency in Plants Provide New Insight into Woody Perennial Plants Improvement. Int. J. Mol. Sci. 2022, 23, 2353. [Google Scholar] [CrossRef] [PubMed]
- Bolte, A.; Czajkowski, T.; Kompa, T. The north-eastern distribution range of European beech a review. For. Int. J. For. Res. 2007, 80, 413–429. [Google Scholar] [CrossRef]
- Maleki, K.; Zeller, L.; Pretzsch, H. Oak often needs to be promoted in mixed beech-oak stands - the structural processes behind competition and silvicultural management in mixed stands of European beech and sessile oak. iForest - Biogeosciences For. 2020, 13, 80–88. [Google Scholar] [CrossRef]
- Marchi, M.; Castellanos-Acuña, D.; Hamann, A.; Wang, T.; Ray, D.; Menzel, A. ClimateEU, scale-free climate normals, historical time series, and future projections for Europe. Sci. Data 2020, 7, 1–9. [Google Scholar] [CrossRef]
- Sever, K.; Vukmirović, A.; Hodak, L.; Bogdan, S.; Katičić Bogdan, I.; Krstonošić, D.; Karažija, T.; Franjić, J.; Škvorc, Ž. Funkcionalna Prilagodba Prirodnog Pomlatka Hrasta Kitnjaka i Obične Bukve Na Različite Stanišne Prilike. Šumar. List 2022, 146, 293–307 (In Croatian with English summary), (In Croatian with English summary). [Google Scholar] [CrossRef]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H. Methods of Soil Analysis, Part 3: Chemical Methods; John Wiley & Sons, 2020; Vol. 14.
- AOAC Official Methods of Analysis of AOAC.; International 17th edition.; Association of Analytical Communities Gaithersburg, MD, USA, 2000.
- Sabin, T.E.; Stafford, S.G. Assessing the need for transformation of response variables. For. Res. Lab. Or. State Univ. Corvallis Spec. Publ. 1990, 31 p.
- Mellert, K.H.; Göttlein, A. Comparison of new foliar nutrient thresholds derived from van den Burg’s literature compilation with established central European references. Eur. J. For. Res. 2012, 131, 1461–1472. [Google Scholar] [CrossRef]
- Perry, T.O. Dormancy of Trees in Winter. Science 1971, 171, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Lagercrantz, U.L.F. At the End of the Day: A Common Molecular Mechanism for Photoperiod Responses in Plants? J. Exp. Bot. 2009, 60, 2501–2515. [Google Scholar] [CrossRef]
- Olsson, C.; Jönsson, A.M. A model framework for tree leaf colouring in Europe. Ecol. Model. 2015, 316, 41–51. [Google Scholar] [CrossRef]
- ufar, K.; De Luis, M.; Saz, M.A.; Črepinšek, Z.; Kajfež-Bogataj, L. Temporal Shifts in Leaf Phenology of Beech (Fagus Sylvatica) Depend on Elevation. Trees 2012, 26, 1091–1100. [Google Scholar]
- Delpierre, N.; Vitasse, Y.; Chuine, I.; Guillemot, J.; Bazot, S.; Rutishauser, T.; Rathgeber, C.B.K. Temperate and boreal forest tree phenology: from organ-scale processes to terrestrial ecosystem models. Ann. For. Sci. 2016, 73, 5–25. [Google Scholar] [CrossRef]
- Weih, M. Genetic and environmental variation in spring and autumn phenology of biomass willows (Salix spp.): effects on shoot growth and nitrogen economy. Tree Physiol. 2009, 29, 1479–1490. [Google Scholar] [CrossRef]
- Smart, C.M. Gene expression during leaf senescence. New Phytol. 1994, 126, 419–448. [Google Scholar] [CrossRef]
- Crafts-Brandner, S.J. Phosphorus Nutrition Influence on Leaf Senescence in Soybean. Plant Physiol. 1992, 98, 1128–1132. [Google Scholar] [CrossRef]
- Colomb, B.; Kiniry, J.R.; Debaeke, P. Effect of Soil Phosphorus on Leaf Development and Senescence Dynamics of Field-Grown Maize. Agron. J. 2000, 92, 428–435. [Google Scholar] [CrossRef]
- Zavišić, A.; Polle, A. Dynamics of phosphorus nutrition, allocation and growth of young beech (Fagus sylvatica L.) trees in P-rich and P-poor forest soil. Tree Physiol. 2017, 38, 37–51. [Google Scholar] [CrossRef]
- Zavišić, A.; Yang, N.; Marhan, S.; Kandeler, E.; Polle, A. Forest Soil Phosphorus Resources and Fertilization Affect Ectomycorrhizal Community Composition, Beech P Uptake Efficiency, and Photosynthesis. Front. Plant Sci. 2018, 9, 463. [Google Scholar] [CrossRef] [PubMed]
- Oliet, J.A.; Salazar, J.M.; Villar, R.; Robredo, E.; Valladares, F. Fall fertilization of Holm oak affects N and P dynamics, root growth potential, and post-planting phenology and growth. Ann. For. Sci. 2011, 68, 647–656. [Google Scholar] [CrossRef]

| Stand/provenance | Mean annual precipitation (mm) | Mean summer precipitation (mm) | Mean annual temperature (°C) | Mean coldest month temperature (°C) | Mean warmest month temperature (°C) |
| KA | 1099 | 508 | 12.3 | 2.0 | 22.4 |
| SB | 848 | 414 | 11.0 | 0.4 | 21.1 |
| Parameter | +P treatment | Control | ||
| Value | Description1 | Value | Description1 | |
| pH (H2O) | 6.8 | Neutral | 7.1 | Neutral |
| P3- (mg/L) | 8.4 | High | 2.7 | Moderate |
| NH4+ (mg/L) | 13.2 | Optimal | 13.8 | Optimal |
| NO3- (mg/L.) | 59.5 | Optimal | 60.0 | Optimal |
| N total (mg/L) | 46.9 | Medium - normal | 49.7 | Medium - normal |
| K+ (mg/L) | 63.0 | Medium - normal | 60.9 | Medium - normal |
| Mg2+ (mg/L) | 61.4 | Moderate | 54.5 | Moderate |
| Ca2+ (mg/L) | 204 | Low | 234 | Low |
| Cl- (mg/L) | 54.8 | Medium -normal | 52.4 | Medium -normal |
| Na+ (mg/L) | 34.79 | Moderate | 36.6 | Moderate |
| E.C. (mS/cm) | 1.185 | Medium - normal | 1.156 | Medium - normal |
| Salt (%) | 0.151 | Medium - normal | 0.147 | Medium - normal |
| Nutrient | Year | Fs_KA | Fs_SB | Qp_KA | Qp_SB | ||||
| +P | C | +P | C | +P | C | +P | C | ||
| P | 2021 | 1.96*±0.04 | 1.31±0.03 | 1.72*±0.09 | 1.24±0.02 | 1.36*±0.08 | 1.08±0.04 | 1.45*±0.02 | 1.06±0.08 |
| 2022 | 1.82±0.16 | 1.64±0.07 | 1.95*±0.10 | 1.28±0.08 | 2.15*±0.35 | 1.22±0.10 | 1.76*±0.15 | 1.31±0.05 | |
| N | 2021 | 21.15±0.45 | 23.00*±0.32 | 21.75±0.19 | 22.50±0.77 | 28.67±0.43 | 27.30±0.60 | 28.08±0.45 | 29.00±0.54 |
| 2022 | 22.34±2.26 | 24.56±1.16 | 22.12±0.98 | 22.25±0.85 | 21.51±0.84 | 22.34±2.26 | 22.34±2.26 | 22.34±2.26 | |
| K | 2021 | 6.38±0.13 | 7.19*±0.30 | 6.57±0.15 | 7.54±0.13 | 7.73±0.09 | 7.73±0.17 | 7.69±0.10 | 7.83±0.11 |
| 2022 | 6.82±0.27 | 8.06*±0.41 | 6.57±0.34 | 7.31±0.34 | 9.67±0.46 | 9.13±0.40 | 9.59±0.28 | 9.15±0.44 | |
| Ca | 2021 | 8.06±0.27 | 8.89±0.43 | 9.18±0.10 | 9.41±0.08 | 6.34±0.27 | 7.72*±0.22 | 6.86±0.16 | 6.59±0.57 |
| 2022 | 7.47±0.62 | 6.71±0.28 | 9.18±0.53 | 8.68±0.63 | 10.41±0.88 | 10.91±0.71 | 10.32±0.76 | 10.98±1.23 | |
| Mg | 2021 | 1.53±0.12 | 1.35±0.06 | 1.58*±0.05 | 1.25±0.08 | 1.87±0.09 | 2.23*±0.04 | 2.00±0.05 | 1.97±0.09 |
| 2022 | 2.19±0.10 | 2.37±0.09 | 2.52±0.11 | 2.31±0.09 | 2.86±0.14 | 3.09±0.21 | 2.89±0.13 | 2.76±0.18 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





