1. Introduction
Acute gastroenteritis (AGE) is still a leading cause of childhood morbidity and mortality worldwide, accounting for nearly 500,000 deaths of children <5 years of age in 2015, mostly in low-income countries [
1,
2]. Enteric viruses are the most frequent common pathogens causing AGE, responsible for approximately 50% of cases. Rotavirus A (RVA), norovirus (NoV), human adenovirus (HAdV), sapovirus (SaV), and astrovirus (AstV) are the most common viral agents worldwide [
3]. However, in a significant number of AGE cases (up to 50%), the causative agent can remain undiagnosed with laboratory methods [
4,
5].
Human bocavirus (HBoV), which belongs to Parvoviridae family, Bocaparvovirus genus, was first discovered in 2005 in children with acute respiratory tract infection [
6,
7]. Later, HBoV was identified in human stool samples from children with AGE [
8,
9,
10]. HBoV is currently considered to be an emerging virus and it is still unclear that could be associated with cases of AGE due to the high rate of the co-infection pattern of HBoV with other gastroenteric viruses in symptomatic patients, as well as its frequent detection in asymptomatic individuals [
11,
12].
HBoV is a small non-enveloped icosahedral virus (20 nm) with a single-stranded DNA genome containing about 5.3 kilobases. Its genome is organized into three open reading frames (ORFs) that encoding for two nonstructural proteins (NS1 and NP1) and two viral capsid proteins (VP1 and VP2) [
12,
13]. HBoV is divided into four genotypes (HBoV-1 to HBoV-4), based on the genomic analysis of VP1 and VP2 proteins [
6,
8,
14]. HBoV-1 is frequently associated with respiratory tract infections; however, it has been detected in AGE cases. On the other hand, the other genotypes are found mainly in stool samples of children and adults with gastrointestinal symptoms [
11,
15,
16].
Therefore, this study aimed to investigate the prevalence of HBoV infection in children up to 5 years with or without AGE symptoms in Acre, Northern Brazil. In order, we screened the positive HBoV samples for other major gastroenteric viruses, such as RVA, NoV, and HAstV to determine whether the causative agent of diarrhea was in the collected samples. Additionally, we investigated the correlation between HBoV detection and epidemiological and clinical features.
2. Materials and Methods
2.1. Clinical Specimens and Ethics Aspects
A cross-sectional, retrospective study was carried out in Rio Branco, Acre between January and December 2012. A total of 480 stool samples were collected from children aged <5 years with (226 children) or without (254 children) AGE symptoms admitted to a local hospital (collections made up to 48 hours from admission) or outpatient clinic treatment. Standard questionnaires were filled out with epidemiological and clinical information. Fecal samples had already been tested for RVA, NoV, and HAstV previously [
17,
18,
19]. This study was approved by the Evandro Chagas Institute Ethical Research Committee (protocol number 3.383.249) in accordance with National Health Council’s Resolution 466/2012.
2.2. Nucleic Acid Extraction
Viral nucleic acids (DNA and RNA viruses) were extracted from 10% fecal suspensions with Tris-calcium buffer (pH = 7.2) using silica glass powder [
20]. The isolated nucleic acid was kept frozen at −70 ◦C until the molecular analysis. In each extraction procedure, RNAse/DNAse-free water was used as a negative control.
2.3. HBoV Molecular Detection
HBoV detection was performed by a PCR followed by a Nested-PCR using two sets of primers targeting a variable VP1/VP2 region as described previously [
14]. The PCR products were detected by electrophoresis on 1.5% agarose gel. The presence of HBoV was determined through a specific-sized amplicon corresponding to the second round nested-PCR of 576 bp, respectively, after staining with a nucleic acid staining solution and visualized under a UV-transilluminator.
2.4. Molecular Characterization and Phylogenetic Analysis
The amplicons obtained were purified using ExoSAP-IT® enzymes (Applied Biosystems), according to the manufacturer’s recommendations. The purified PCR products were then subjected to direct sequencing using the same primers of Nested-PCR. The sequencing reaction was performed with a commercial BigDye Terminator v.3.1 Cycle Sequencing Kit (Applied Biosystems, CA, USA) in an ABI Prism 3130xl automated sequencer (Applied Biosystems).
The sequences obtained in sequencing analysis were assembled by De novo and reference mapping methods, edited, and curated using the Geneious software (v.7) [
21]. The sequence alignment together with other related sequences available on GenBank (
www.ncbi.nlm.nih.gov) was performed using the MAFFT algorithm [
22]. For the construction of the phylogenetic tree of the HBoV VP1/VP2 partial genes, they were constructed using the Maximum Likelihood Method using FastTree v.2.1.11 software, including the GTR+Gamma+F nucleotide substitution model. Bootstrap values of the nodes indicate support of 1000 replicas, obtaining reproducible results and providing greater reliability to the clusters. Nucleotide distances were generated from an identity matrix in MEGA v.X software. Partial nucleotide sequences from this study were deposited in the GenBank database (
http://www.ncbi.nlm.nih.gov) under the access numbers OQ695608-OQ695654.
2.5. Statistical analysis
All statistical analyses were performed in a database created by the Statistical Package Software for Social Sciences-SPSS (version 20.0). Baseline data were submitted to the descriptive analysis to obtain the prevalence of the investigated outcomes. Bivariate analysis was carried out to verify the association between independent variables and HBoV infection using the chi-square test (x2). A p-value ≤ 0.05 was considered statistically significant.
3. Results
Overall HBoV-positivity was 10% (48/480), being HBoV-positivity rates of 8.4% (19/226) and 11.4% (29/254) recorded among diarrheic and non-diarrheic children, respectively. No statistical significance was observed among the analyzed groups.
No correlation was shown between epidemiological aspects and the risk of HBoV infection; however, the most affected age group was between 7 and 24 months, corresponding to 50% of cases. HBoV infection was similar between females (47.9%) and males (52.1%). Regarding epidemiological characteristics, it was reported that infection by HBoV was more frequent in children who lived in urban areas (85.4%), used water from the public network (56.2%), and lived with adequate sewage facilities (50%). Concerning to family income, 52.1% received up to one monthly minimum wage (
Table 1). None of these parameters showed statistical significance.
With respect to clinical features, most children infected with HBoV (60.4%, 29/48) sought care for other clinical causes; they belonged to the non-diarrheic group (
Table 2). Considering the analyzed symptoms, it was reported in the non-diarrheic group that 75.9% (22/29) and 96.6% (28/29) of children did not present fever and vomiting episodes, respectively, with a statistical difference for these symptoms (p ≤ 0.05). It is noteworthy that was not register most clinical symptoms in the questionnaires.
The global prevalence of coinfection involving HBoV and other viruses was 1.7% (8/480). However, when analyzing only HBoV and other viruses positive specimens, coinfection prevalence was 16.7% (8/48). It was reported that the most prevalent coinfection was RVA+ HBoV (50%, 4/8), followed by NoV+HBoV (37.5%, 3/8) and HAstV+HBoV (12.5%, 1/8). Co-infection with HBoV and at least one virus was shown in diarrheic and non-diarrheic children (
Table 3).
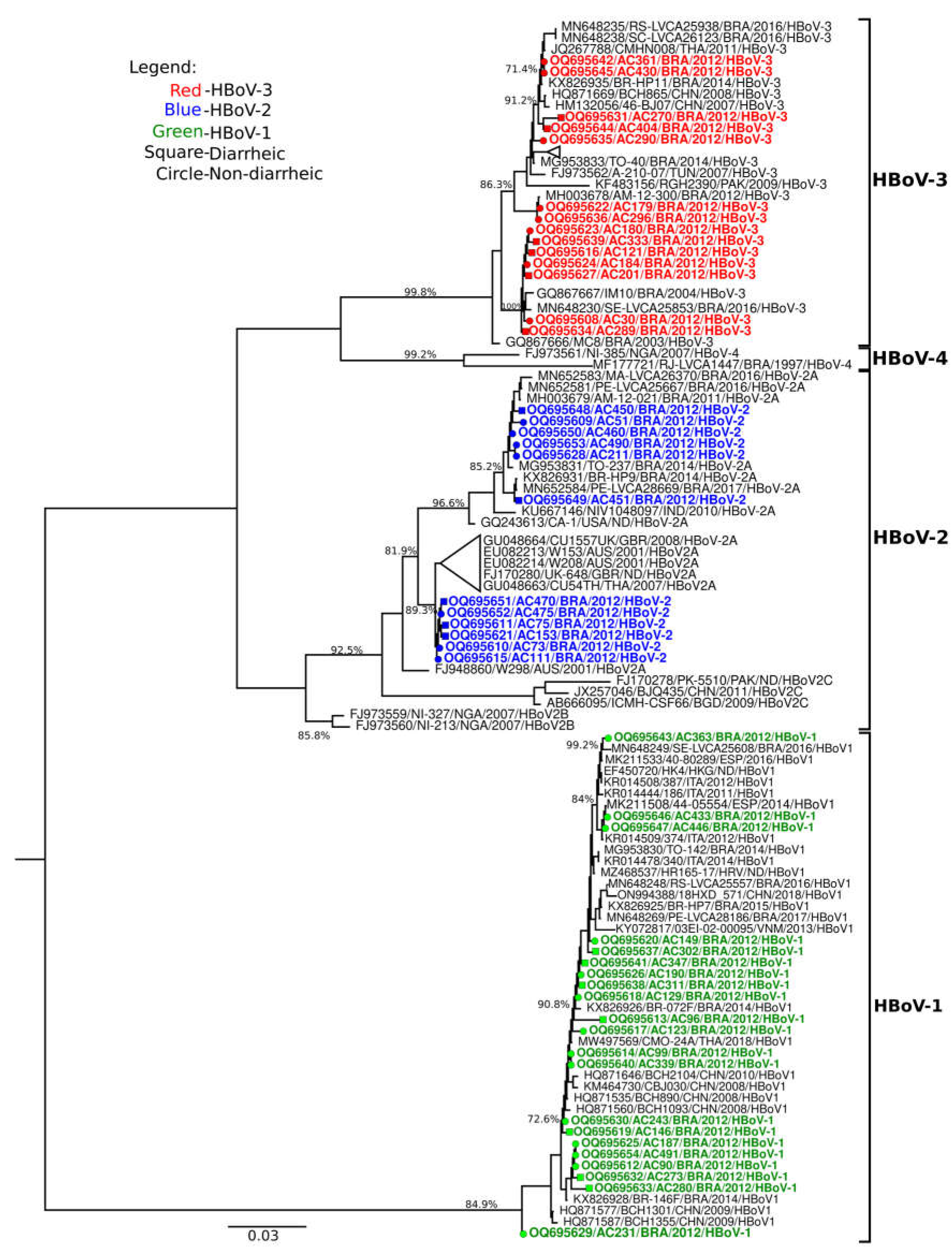
Concerning HBoV characterization, 47 positive samples were genotyped. HBoV-1 was the most frequent species detected, responsible for 43.8% (21/48) of cases, followed by HBoV-3 (29.2%, 14/48) and HBoV-2 (25%, 12/48). Regarding phylogenetic inference of VP1/VP2 gene showed that HBoV-1 strains grouped with isolates from Brazil, China, Thailand and European strans, that circulated during 2008 to 2018, showing bootstrap support values ranging from 72.6% to 99.2% and had high nucleotide (98.6–100%) similarities with contemporaneous strains. HBoV-2 samples grouped into lineage A and formed two clusters, the first cluster grouped with Brazillian isolates detected in 2011, 2014, 2016 and 2017 (85.2% bootstrap value), while the second cluster grouped with strains from Australia (2001), United Kingdom (2007) and Thailand (2007), with a bootstrap value of 89.3%. Despite of Brazilian HBoV-2 strains grouped into two clusters, nucleotide similarity ranged from 91-99% among them. For HBoV-3 strains, they clustered with isolates from Brazil (2003-2016), China (2007-2008), Thailand (2011), Tunisia (2007) and Pakistan (2009), showing bootstrap value ranging from 71.4-100%. Suchlike Brazilian HBoV-2 strains, HBoV-3 strains formed two clusters and grouped with nucleotide similarity ranging from 89-100%. The HBoV-4 type was not identified (
Figure 1).
4. Discussion
Since the first identification of HBoV in 2005, this virus has been found to infect symptomatic and asymptomatic individuals for AGE in different prevalence, including studies carried out in the northern region of Brazil [
16,
23,
24,
25]. In the present study, overall HBoV-positivity was 10% and similar rates were detected in diarrheic children (8,4%) and non-diarrheic children (11.4%). Our results corroborate with data from a study conducted in Finland, where HBoV was detected in 9.2% of children aged 0–15 years with and without AGE symptoms. On the other hand, in that study HBoV was the most frequent in diarrheic (9,7%), than in non-diarrheic (5.4%) children, however, no statistical significance was observed among the analyzed groups [
26]. Otherwise, the global HBoV frequency reported in a study conducted in Russia was 1.1%, where HBoV rates from children with and without AGE symptoms were 1.2% and 0.3%, respectively [
27]. Divergence of HBoV frequencies among studies can be related to different methodologies used; the period of stool samples collection; the age of the analyzed population; and socioeconomic, environmental, and sanitation features of each setting [
13,
24].
HBoV was mostly detected in children aged 7 to 24 months years, corresponding to 50% of cases, followed by those aged 25 to 60 months (39.6%). Previous studies described that children aged ≤ 2 years old were found to be most susceptible to HBoV infection [
28,
29]. Two studies with hospitalized children presenting AGE symptoms from Brazil reported a higher HBoV positivity rate in children aged ≤ 5 years old. Soares et al. (2019)[
23] investigated 225 fecal samples from children aged less than 10 years with AGE from Northern Brazil and reported that 66.6% of HBoV cases affected children aged 7 to 24 months years. Suchlike, a study conducted by Malta et al. (2020)[
25] analyzed stool samples from children up to two years of age from ten Brazilian states and demonstrated that HBoV infected 15.7% of children aged 12 to 24 months. These results corroborate that children are protected from HBoV infections by maternal antibodies, which decline at 6 months and after children are usually exposed to viral infections [
30,
31].
Concerning the epidemiological profile, HBoV was more frequent in children who lived in urban areas (85.4%), used water from the public network (56.2%), and lived with adequate sewage facilities (50%), although no correlation was showed these features and the risk of HBoV infection. These features are considered once housing quality, sanitation aspects, and/or lifestyle conditions are important factors influencing health and disease in a population, including waterborne disease, like reported by Neves et al. (2016)[
18] that investigated the presence of RVA in the same population and related the association between public water supply, inadequate sewage facilities, and RVA infection.
Regarding economic conditions, most of the children infected by HBoV had a family income of up to three minimum wages (87.5%). Similar data were reported by Castro et al. [2019][24] who investigated the occurrence of RVA and HBoV in immunosuppressed patients from Northern Brazil and showed 90% of patients affected by HBoV had the same monthly income. Low household incomes reduce access to adequate amounts and good quality foods; consequently, children are prone to the development of viral infections, including diarrhea.
With respect to clinical profile, in the present study, most of the children infected with HBoV were asymptomatic for AGE (60.4% of cases). Diarrhea, fever, and vomiting symptoms affected 39.6%, 37.5%, and 16.7% of infected children, respectively. In a study performed in Pune, West India, with 418 fecal samples from children with AGE, it was observed that the same symptoms were more prevalent in individuals infected with HBoV; these included diarrhea (100%), fever (90%), and vomiting (58%) [
32]. Such symptoms are commonly observed in individuals HBoV positive and affected by AGE, mostly involving coinfection with other enteric viruses. Although in the present study, the evaluation of the clinical profile of HBoV infections was limited, further studies on these aspects are warranted.
Concerning co-infection related HBoV and gastroenteric viruses, HBoV was detected mostly as a single viral pathogen (83.3%) and the most prevalent coinfection was RVA+ HBoV (50%), followed by NoV+HBoV (37.5%) and HAstV+HBoV (12.5%). Distinct findings were reported by Malta et al. (2020) [
25] that found HBoV monoinfection in 20.9% of strains. This difference could be associated that the present study included individuals with and without AGE symptoms, furthermore the survey for a few gastroenteric viruses.
Regarding the genotypic analysis, it was observed that the prevalence of HBoV-1 was 45.8% (22/48), followed by other types detected (HBoV-2 and HBoV-3) in the study. This genotype was found as the only HBoV strain associated with viral diarrhea in children in Paraguay, with positivity of 10.6% (37/349) [
33]. Although, in most studies, HBoV-1 is the species most commonly related to respiratory infections, its presence in gastrointestinal infections is not null. However, there is no scientific proof whether its presence in feces is associated with infection secondary to respiratory tract infections or if it directly afflicts the gastrointestinal tract [
34].
5. Conclusions
In conclusion, in the present study, HBoV infections are not associated with AGE, as most HBoV cases belonged to the non-diarrheal group without AGE symptoms. However, we cannot assert that HBoV is directly associated with asymptomatic cases since there are other factors associated with viral permanence in the organism. Although HBoV is related to respiratory infections, few studies have demonstrated its relationship with gastroenteric diseases. Therefore, future studies are warranted to determine the role of HBoV in causing acute diarrhea disease
Author Contributions
Conceptualization: FD'TBT, JDPM, LSS; Data curation: FD'TBT, SFSG, PSL, JFC, LSS; Investigation: FD'TBT, ESFR, SFSG, ETPJ, PSL, DAMB, MAONK, MCMS, LSS; Methodology: FD'TBT, SFSG, ETPJ, PSL, DAMB, MAONK, ESFR, JAAA, JD'PM, LSS; Writing – Original Draft Preparation: FD'TBT; Writing – Review & Editing: SFSG, PSL, LSS. Supervision: LSS.
Funding
This research received no external funding. FD'TBT and ESFR were the recipients of National Council for Scientific and Technological Development (CNPq) fellowships.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee and Research of the Evandro Chagas Institute (protocol code 3.383.249, date of approval: June 11, 2019).
Informed Consent Statement
The Informed Consent Form (FICF) was applied for permission from the parents and/or guardians of the children, to provide knowledge and clarification of the general and specific objectives of the study.
Acknowledgments
The authors would like to acknowledge the staff of the Virology Section of the Evandro Chagas Institute and the children/mothers who agreed to participate in this study as volunteers and permitted the analysis of their relevant biological material. The authors are also thankful to the National Council for Scientific and Technological Development – CNPq for granting a master's scholarship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- GBD 2016 Diarrhoeal Disease Collaborators Estimates of the Global, Regional, and National Morbidity, Mortality, and Aetiologies of Diarrhoea in 195 Countries: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1211–1228. [CrossRef]
- Liu, L.; Oza, S.; Hogan, D.; Chu, Y.; Perin, J.; Zhu, J.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, Regional, and National Causes of under-5 Mortality in 2000-15: An Updated Systematic Analysis with Implications for the Sustainable Development Goals. Lancet Lond. Engl. 2016, 388, 3027–3035. [CrossRef]
- Bányai, K.; Estes, M.K.; Martella, V.; Parashar, U.D. Viral Gastroenteritis. The Lancet 2018, 392, 175–186. [CrossRef]
- Simpson, R.; Aliyu, S.; Iturriza-Gómara, M.; Desselberger, U.; Gray, J. Infantile Viral Gastroenteritis: On the Way to Closing the Diagnostic Gap. J. Med. Virol. 2003, 70, 258–262. [CrossRef]
- Vocale, C.; Rimoldi, S.G.; Pagani, C.; Grande, R.; Pedna, F.; Arghittu, M.; Lunghi, G.; Maraschini, A.; Gismondo, M.R.; Landini, M.P.; et al. Comparative Evaluation of the New xTAG GPP Multiplex Assay in the Laboratory Diagnosis of Acute Gastroenteritis. Clinical Assessment and Potential Application from a Multicentre Italian Study. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2015, 34, 33–37. [CrossRef]
- Allander, T.; Tammi, M.T.; Eriksson, M.; Bjerkner, A.; Tiveljung-Lindell, A.; Andersson, B. Cloning of a Human Parvovirus by Molecular Screening of Respiratory Tract Samples. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 12891–12896. [CrossRef]
- Current ICTV Taxonomy Release | ICTV Available online: https://ictv.global/taxonomy (accessed on 23 March 2023).
- Arthur, J.L.; Higgins, G.D.; Davidson, G.P.; Givney, R.C.; Ratcliff, R.M. A Novel Bocavirus Associated with Acute Gastroenteritis in Australian Children. PLoS Pathog. 2009, 5, e1000391. [CrossRef]
- Campos, G.S.; Silva Sampaio, M.L.; Menezes, A.D.L.; Tigre, D.M.; Moura Costa, L.F.; Chinalia, F.A.; Sardi, S.I. Human Bocavirus in Acute Gastroenteritis in Children in Brazil. J. Med. Virol. 2016, 88, 166–170. [CrossRef]
- Kapoor, A.; Slikas, E.; Simmonds, P.; Chieochansin, T.; Naeem, A.; Shaukat, S.; Alam, M.M.; Sharif, S.; Angez, M.; Zaidi, S.; et al. A New Bocavirus Species in Human Stool. J. Infect. Dis. 2009, 199, 196–200. [CrossRef]
- Guido, M.; Tumolo, M.R.; Verri, T.; Romano, A.; Serio, F.; De Giorgi, M.; De Donno, A.; Bagordo, F.; Zizza, A. Human Bocavirus: Current Knowledge and Future Challenges. World J. Gastroenterol. 2016, 22, 8684–8697. [CrossRef]
- Ong, D.S.Y.; Schuurman, R.; Heikens, E. Human Bocavirus in Stool: A True Pathogen or an Innocent Bystander? J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2016, 74, 45–49. [CrossRef]
- Khamrin, P.; Malasao, R.; Chaimongkol, N.; Ukarapol, N.; Kongsricharoern, T.; Okitsu, S.; Hayakawa, S.; Ushijima, H.; Maneekarn, N. Circulating of Human Bocavirus 1, 2, 3, and 4 in Pediatric Patients with Acute Gastroenteritis in Thailand. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2012, 12, 565–569. [CrossRef]
- Kapoor, A.; Simmonds, P.; Slikas, E.; Li, L.; Bodhidatta, L.; Sethabutr, O.; Triki, H.; Bahri, O.; Oderinde, B.S.; Baba, M.M.; et al. Human Bocaviruses Are Highly Diverse, Dispersed, Recombination Prone, and Prevalent in Enteric Infections. J. Infect. Dis. 2010, 201, 1633–1643. [CrossRef]
- Chow, B.D.W.; Ou, Z.; Esper, F.P. Newly Recognized Bocaviruses (HBoV, HBoV2) in Children and Adults with Gastrointestinal Illness in the United States. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2010, 47, 143–147. [CrossRef]
- Paloniemi, M.; Lappalainen, S.; Salminen, M.; Kätkä, M.; Kantola, K.; Hedman, L.; Hedman, K.; Söderlund-Venermo, M.; Vesikari, T. Human Bocaviruses Are Commonly Found in Stools of Hospitalized Children without Causal Association to Acute Gastroenteritis. Eur. J. Pediatr. 2014, 173, 1051–1057. [CrossRef]
- Bitencurt, E.L.R.; Siqueira, J.A.M.; Medeiros, T.B.; Bandeira, R. da S.; de Souza Oliveira, D.; de Paula Souza E Guimarães, R.J.; da Silva Soares, L.; Macarenhas, J.D.P.; Teixeira, D.M.; Silva, R.S.U.; et al. Epidemiological and Molecular Investigation of Norovirus and Astrovirus Infections in Rio Branco, Acre, Northern Brazil: A Retrospective Study. J. Med. Virol. 2019, 91, 997–1007. [CrossRef]
- Neves, M.A.O.; Pinheiro, H.H.C.; Silva, R.S.U.; Linhares, A.C.; Silva, L.D.; Gabbay, Y.B.; Silva, M.C.M.; Loureiro, E.C.B.; Soares, L.S.; Mascarenhas, J.D.P. High Prevalence of G12P[8] Rotavirus Strains in Rio Branco, Acre, Western Amazon, in the Post-Rotavirus Vaccine Introduction Period. J. Med. Virol. 2016, 88, 782–789. [CrossRef]
- Silva, L.D. da; Rodrigues, E.L.; Lucena, M.S.S. de; Lima, I.C.G. de; Oliveira, D. de S.; Soares, L.S.; Mascarenhas, J.D.P.; Linhares, A. da C.; Gabbay, Y.B. Detection of the Pandemic Norovirus Variant GII.4 Sydney 2012 in Rio Branco, State of Acre, Northern Brazil. Mem. Inst. Oswaldo Cruz 2013, 108, 1068–1070. [CrossRef]
- Boom, R.; Sol, C.J.; Salimans, M.M.; Jansen, C.L.; Wertheim-van Dillen, P.M.; van der Noordaa, J. Rapid and Simple Method for Purification of Nucleic Acids. J. Clin. Microbiol. 1990, 28, 495–503. [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An Integrated and Extendable Desktop Software Platform for the Organization and Analysis of Sequence Data. Bioinforma. Oxf. Engl. 2012, 28, 1647–1649. [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A Novel Method for Rapid Multiple Sequence Alignment Based on Fast Fourier Transform. Nucleic Acids Res. 2002, 30, 3059–3066. [CrossRef]
- Soares, L.S.; Lima, A.B.F.; Pantoja, K.C.; Lobo, P.S.; Cruz, J.F.; Guerra, S.F.S.; Bezerra, D.A.M.; Bandeira, R.S.; Mascarenhas, J.D.P. Molecular Epidemiology of Human Bocavirus in Children with Acute Gastroenteritis from North Region of Brazil. J. Med. Microbiol. 2019, 68, 1233–1239. [CrossRef]
- Castro, L.R.P.; Calvet, F.C.; Sousa, K.L.; Silva, V.P.; Lobo, P.S.; Penha, E.T.; Guerra, S.F.S.; Bezerra, D.A.M.; Mascarenhas, J.D.P.; Pinheiro, H.H.C.; et al. Prevalence of Rotavirus and Human Bocavirus in Immunosuppressed Individuals after Renal Transplantation in the Northern Region of Brazil. J. Med. Virol. 2019, 91, 2125–2133. [CrossRef]
- Malta, F.C.; Varella, R.B.; Guimarães, M.A.A.M.; Miagostovich, M.P.; Fumian, T.M. Human Bocavirus in Brazil: Molecular Epidemiology, Viral Load and Co-Infections. Pathog. Basel Switz. 2020, 9. [CrossRef]
- Risku, M.; Kätkä, M.; Lappalainen, S.; Räsänen, S.; Vesikari, T. Human Bocavirus Types 1, 2 and 3 in Acute Gastroenteritis of Childhood. Acta Paediatr. Oslo Nor. 1992 2012, 101, e405–e410. [CrossRef]
- Tymentsev, A.; Tikunov, A.; Zhirakovskaia, E.; Kurilschikov, A.; Babkin, I.; Klemesheva, V.; Netesov, S.; Tikunova, N. Human Bocavirus in Hospitalized Children with Acute Gastroenteritis in Russia from 2010 to 2012. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2016, 37, 143–149. [CrossRef]
- Schildgen, O. Human Bocavirus: Lessons Learned to Date. Pathog. Basel Switz. 2013, 2, 1–12. [CrossRef]
- Rikhotso, M.C.; Kabue, J.P.; Ledwaba, S.E.; Traoré, A.N.; Potgieter, N. Prevalence of Human Bocavirus in Africa and Other Developing Countries between 2005 and 2016: A Potential Emerging Viral Pathogen for Diarrhea. J. Trop. Med. 2018, 2018. [CrossRef]
- Liu, L.; Hill, K.; Oza, S.; Hogan, D.; Chu, Y.; Cousens, S.; Mathers, C.; Stanton, C.; Lawn, J.; Robert, B.E. Levels and Causes of Mortality under Age Five Years. In Reproductive, Maternal, Newborn, and Child Health: Disease Control Priorities, Third Edition (Volume 2); Black, R.E., Laxminarayan, R., Temmerman, M., Walker, N., Eds.; The International Bank for Reconstruction and Development / The World Bank: Washington (DC), 2016 ISBN 978-1-4648-0348-2.
- Ali, G.M.; Hussein, A.A.; kadim, J.I.; Aufi, I.M. Detecting and Genotyping of Human Bocavirus among Children with Gastroenteritis in Diyala Governorate. In Proceedings of the Annals of Tropical Medicine and Public Health; 2020; Vol. 23.
- Lasure, N.; Gopalkrishna, V. Molecular Epidemiology and Clinical Severity of Human Bocavirus (HBoV) 1-4 in Children with Acute Gastroenteritis from Pune, Western India. J. Med. Virol. 2017, 89, 17–23. [CrossRef]
- Proenca-Modena, J.L.; Martinez, M.; Amarilla, A.A.; Espínola, E.E.; Galeano, M.E.; Fariña, N.; Russomando, G.; Aquino, V.H.; Parra, G.I.; Arruda, E. Viral Load of Human Bocavirus-1 in Stools from Children with Viral Diarrhoea in Paraguay. Epidemiol. Infect. 2013, 141, 2576–2580. [CrossRef]
- Lekana-Douki, S.E.; Behillil, S.; Enouf, V.; Leroy, E.M.; Berthet, N. Detection of Human Bocavirus-1 in Both Nasal and Stool Specimens from Children under 5 Years Old with Influenza-like Illnesses or Diarrhea in Gabon. BMC Res. Notes 2018, 11. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).