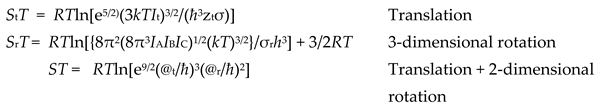3.1. Revising the Carnot cycle as a basis for a Gibbs action field
Equation (7) shows that the total heat content needed is the sum of the internal energy (c
vT ) plus the absolute energy of the field (-
gt), that becomes more positive as its quantum state increases. Removing temperature (
T) in Equation (7) gives the absolute entropy per molecule of the quantum state under the current environmental conditions of temperature and pressure.
Table 1 reproduces and extends data from our earlier article [
2]; shown are relationships between matter and quantum fields relevant to all four stages proposed by Carnot as reversible, determining the most efficient generation of power in the heat engine cycle. The table shows that Carnot’s formal explanation of the cycle using caloric is consistent with quantum theory, with its modern surrogate shown as negative Gibbs energy (-
gt). Carnot specifically indicated that the maximum possible work was equal to the second differences of
a–
a’ or
b’–
b, where
a,
b’,
a’ and
b were primary differences between absolute Gibbs energy values that we calculated for argon and nitrogen shown in
Table 1. For two working materials as ideal gases, we make the following conclusions from the heat engine cycle, considering the impulsive quantum properties of the working fluid as causal. Most formulae such as the Schrödinger wave equation estimate quanta absorbed or emitted as the difference between states, but
Table 1 gives their absolute mean values. Note that these values for translational action (@
t) are corrected here for a simple programming error in reference [
2], that underestimated action by a factor of 1.47.
At all four stages of the cycle, the relative action (@) of the working fluid calculated indicates its entropy state, according to Equation (7). We remind you that Gibbs energy (gt) is always zero or negative, decreasing from minimum action near absolute zero K. Uniquely, action mechanics quantifies the Gibbs field here as mean numbers of virtual quanta needed per molecule to sustain their temperature and pressure.
Atmospheric pressure is not relevant to the enclosed Carnot cycle, so from Equation (7) all effects of changes in pressure in the cycle can be calculated as changes in Gibbs energy calculated from macroscopic temperature and pressure, given these are equivalent [
2,
6]. Shown in
Table 1, the field of virtual quanta (Σ
hv) contains almost 10 times as much field energy (largely provided in melting and vaporization) as the kinetic energy of the material particles, sustaining molecular torques (
mv2) and material pressures.
Each turn of the Carnot cycle shown in
Table 1 is assumed to absorb
kT of heat from the hot source quantized appropriately for the temperature and pressure and the same quantity
kT removed at the colder sink as different quanta of lower frequency.
The pressure values shown in the table also produce the ratio of torque intensity mv2/3a3 or kT/a3 to the negative Gibbs energy (-gt/a3) or mean density of virtual quanta held within the mean volume a3 occupied by each molecule. For argon, this energy ratio is constant for transitions in adiabatic or isentropic states with no change in heat content. Where isothermal processes at constant temperature (or torque) occur, there is a change in this ratio, as heat is added or removed.
For nitrogen, the interaction between quantum cells for translation and rotation requires that the product of the quantum densities, shown as (nt3 x jr2) respectively in the table, must remain constant for adiabatic processes that are isentropic exhibiting constant action.
The ratio for wavelength of virtual quanta and the material radial motion (λ/2πr; r=a/2) of about 105 for the gases is indicative of the ratio between the speed of light (λν = rω) and speed of the Brownian spiral of gas molecules. This can be visualised as the frequency of the conjugate quanta being of a similar order to that of the orbital frequency of the molecules, though with the photon’s impulse cycling on a much longer radius, proportional to the ratio of speeds (c/v).
Table 1 also illustrates the correspondence for both argon (mass 40) and nitrogen (mass 28) of the ratio of the cumulative quantum impulse (n
h/λ) and the dynamic impulse (Σ
mv=
mrω) per molecule. This is a factor near 1x10
-5, the inverse of the ratio of the speed of light to that of the molecules. In calculating translational action of molecules [
2] we found it necessary to make two corrections. One, a factor of 1/1.09, corrects root mean square velocity from the Maxwell distribution (3
kT=
mv2) to mean velocity. The second corrects action for symmetry, to avoid double counting of molecules (1/2). For cubic translation, this is an overall factor of 1/10.2297). This correction then allows the entropy calculated to match that for third law experiments in the literature. This correction factor (z
t) was initially established empirically [
1], then interpreted rationally [
2]. Overall, it allows the density of quanta needed to sustain the system to fall by a factor of 2.3205.
Another possible source for lack of correspondence in matching action impulses between quanta and molecules is that phase space for position and momentum can never exactly match true action space. The ideal coordinate system may not be Cartesian phase space since this separates variables (
mv and
r) that must be combined when quantized. A radial or polar system (
r,
ϕ, θ) is needed [
7] but also one that recognizes that changes in position in 3-dimensions is absolutely quantized as jumps in the space of objects from one locus to another. There is no such thing as a smooth curve in nature for translation of rotation except by perception within the space of views, as explained a century ago by Jean Nicod [
10].
Our algorithm obviously gives good correspondence between potential impulse rates of quantum and dynamic molecules in the field, though not exact. There is plainly an effect in the case of the nitrogen molecule an effect of rotation, responsible for about 20% of the quantum energy density. The number of quantum states for translation increases with lower temperature and pressure but that for rotation does the opposite. We continue to analyze the significance of this matter.
It is unfortunate that the text of Carnot’s monograph [
4] was lost for many years after his premature death from cholera in 1832. Caloric was clearly regarded by Carnot as causal for the power (
puissance motrice) of heat engines. Lord Kelvin and Clausius assumed for convenience that the heat consumed in Carnot’s cycle reappeared directly as the external work performed by the heat engine. This was despite Clausius originally speculating that heat was concerned with performing internal work of the fluid needed to support external work. Later, this process of internal work that he named the system’s
ergal, suggesting this term could replace Rankine’s new definition of potential energy [
2], was neglected; the heat consumed was judged to be transformed directly to external work, not that it was required internally to support the external work, as we now find [
2].
We coined the term radial action to indicate the complementary relationship between maintaining the action of a particle and the action of its complementary quantum, of similar frequency. The wavelength of the quantum is always much larger than the radial motion of the particle, by orders of magnitude. However, it is relevant that impulses from particles such as quanta and molecules exert torques counted as kinetic energy proportional to their radius of action. The impulse of the quanta on their conjugate wavelength exerting torque is equal to that of the molecules on their radius (r).
3.2. Thermodynamic stabilization with altitude for atmospheric gases by quantum fields of molecules in air
Assuming that translational action of molecules in air refers to non-linear or erratically curved motion in the dimensional form
mr2ω as used for calculating translational entropy in heat engines, shown in
Table 1, we can derive the following relations by equating thermodynamic and gravitational pressure with altitude. In Equation (8),
a represents the mean length of the side of a cube occupied by each different molecule and
r, the mean radial separation, half this value. Then
M is equal to N
m, the total mass of
n molecules in the atmospheric column above a square with base of side
a cm, of weight N
mg, assuming the value of gravity (
g) is invariant in the troposphere. It is assumed that the inertial force
provides the internal pressure on the six faces of the cube of side
a, tending to equilibrate with the gravitational pressure of the atmosphere.
Thus, the instantaneous gravitational force on average necessarily exerted by each molecule is one-sixth of the centrifugal or inertial force exerted in each 6-faced cell of side
a by the translational motion of each molecule. The primary gravitational pressure from the weight of air is exerted only downwards and not to all six cardinal points. The thermodynamic relationship is statistical ─ according to the Maxwell-Boltzmann distribution, with the molecular velocities having values statistically varying around the root mean square velocity, characteristic of the temperature. Incidentally, the hydrostatic or isobaric requirement that the pressure is a function of density is only true for an isothermal atmosphere. In a real atmosphere, with a tropospheric temperature gradient with altitude a little more than 6.5 K per km [
7], pressure also varies as a function of temperature.
We challenged the assumption that the decline in temperature with altitude is an adiabatic response to expansion of atmospheric gases as may occur in a cylinder with increasing volume. Instead, the decline in temperature with altitude indicates the operation of the virial theorem for gases of differing gravitational potential and thermal energy [
7]. Changes in potential energy indicate changes in both quantum state and field as well as changes in kinetic energy. According to the virial theorem, the absorption of a quantum of gravitational energy causes a decrease in kinetic energy of the same magnitude, meaning that the increase in potential energy is twice either the decrease in the kinetic energy or the increase in the field gravitational energy. Thermal and gravitational fields are quite separate.
Action mechanics [
1,
2] combines the macroscopic variations in volume (N
a3 = 8N
r3) or density and temperature of molecules as action (
mrvδϕ), illustrated in
Section 3.1. In
Table 2, estimates are given for thermodynamic properties of atmospheric N
2 as translational (n
t) and rotational (j
r) molecular action. Activation of vibrational states for N
2 in the atmosphere are negligible, as shown previously [
1] given the high frequency and energies involved. If required, vibrational Gibbs energy can be estimated from the statistical component of vibrational entropy together with the zero-point energy
Nhν/2 of 14.115 kJ per mol. More than 75% of the energy content indicated for N
2 is required to sustain its translational Gibbs field, shown in
Table 2, with resonant quanta in the range 3.8 to 2.2x10
-22 J of frequency 5.7x10
11 to 3.32x10
11 Hz and wavelengths from 523 to 904 μm. N
2 contributes almost nothing [
1] to the thermal emission to space in the infrared and far infrared up to 100 μm wavelength, unlike water and other greenhouse gases.
However, both the translational and rotational fields contain large numbers of quanta, of wavelengths longer than 520 μm and 100 μm respectively. We have described in detail how these values are easily calculated and employed [
1,
2,
6]. In our opinion, too much emphasis is placed on the calculus of the Maxwell relations in teaching thermodynamics and too little on mechanical methods of computation for real world molecules. Equation (7) has the advantage of yielding mean quantum values, with the radial action needed to provide torques sustaining the dynamic motion of the molecules indicated by the temperature (
T). The use of the form
ST equals
H -
G emphasizes the fact that entropy or its product with temperature is not a single property that stands alone but consists of the sum of the internal energy or enthalpy and a statistical field containing quantum information required for the molecular configuration of the system.
Given that the Gibbs energy defined by Equation (7) is regarded as responsible for the configuration of the molecular system as well as positions of equilibrium in chemical reactions [
6] it is surprising that this field receives so little attention. In proposing its importance in the atmosphere, we need to consider more the likelihood that this energy field for translation and rotation can also contribute to radiation lost to space. Much information is collected on surface radiation from Earth by satellites, but this is usually restricted to the thermal region of vibrational infrared wavelengths of less than 100 μm wavelength than of longer microwaves or radio waves that will be released from the upper atmosphere when less caged by matter. Any such field energy losses will be continuously replenished with thermal energy from the surface, as predicted by the equipartition principle, subject to quantum restrictions at lower temperatures.
For translation and rotation of N
2 molecules,
Table 2 shows estimates with altitude for entropy per mole (
S), absolute Gibbs quantum state levels (n
t), the total heat energy required to reach the state (
ST) and the mean value of virtual field quanta (
hv). Vibrational energy for N
2 is negligible. To estimate peak wavelengths of quanta in the field energy, the virtual quantum value (
hv) is divided by
h to obtain frequency (
v), then inverted and multiplied by the speed of light
c to obtain wavelength. Thus, at the surface temperature and pressure we estimate that some 1.14833x10
26 quanta per mol of peak translational energy 3.809x10
-22 J support the kinetic activity of N
2 with a frequency of 5.74848x10
11 (574.849 GHz) with a peak frequency at a wavelength of 521.52 μm. At 12 km of altitude, the peak wavelength is almost twice as long at 901.704 μm, though there are more quanta per molecule. The table also shows absolute values for the Gibbs field energy per mol, a property that is always negative and becomes more so as spontaneous processes occur that increase the entropy.
Gibbs energy is often referred to as Gibbs free energy or chemical potential. It can be made positive in value when expressed in the usual form at atmospheric pressure as G = H – ST, but it must be appreciated that ST contains the Gibbs energy (G) and the enthalpy (H) or internal energy (E). Little attention is paid to this major reservoir of internal quantum state energy in the atmosphere with the kinetic energy and macroscopic pressure-volume work at atmospheric pressure usually given prominence.
3.3. Vortical action as high level atmospheric thermodynamics in anticyclones
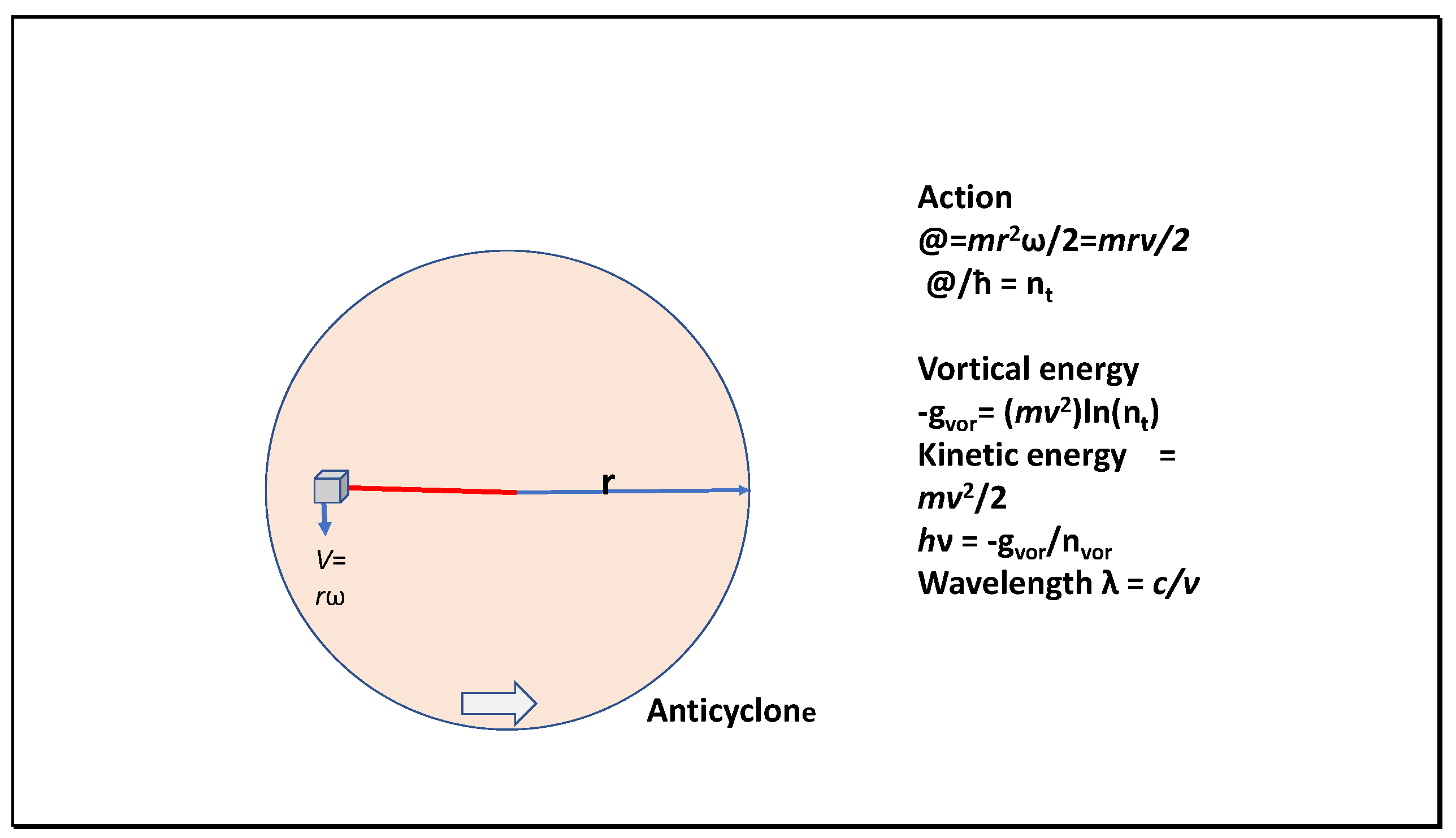
Our hypothetical introduction of vortical energy [
2] for a molecular field in concerted motion is estimated by a similar method to that used for the Carnot cycle (
Figure 1). The translational action (@
w) of air molecules in anticyclones concerted in motion as wind velocity is estimated as
mrv from a knowledge of the mass of a material particle, the radial separation
r equal to a/2, where
a is the radial coordinate diameter of the anticyclone (
Figure 1 and
Figure 2). The number of quantum levels is estimated using division by the reduced Planck’s constant of action (1.054x10
-34) (n
t =
mrv/
ћ), with a symmetry factor of 2 for symmetrical partners. The Gibbs vortical energy per matter cell
a3 is then estimated using the logarithm of the quantum number multiplied by the appropriate torque factor. Frequency and wavelength is then easily determined. Just as in the case of the Carnot cycle, the ratio of mean quantum wavelength and that of the radius to the centre of the anticyclone is of the same magnitude as the ratio of the mean velocity of the molecule to the speed of light. The total vortical Gibbs energy is obtained from the product of number of molecules per cubic metre and the number of molecules.
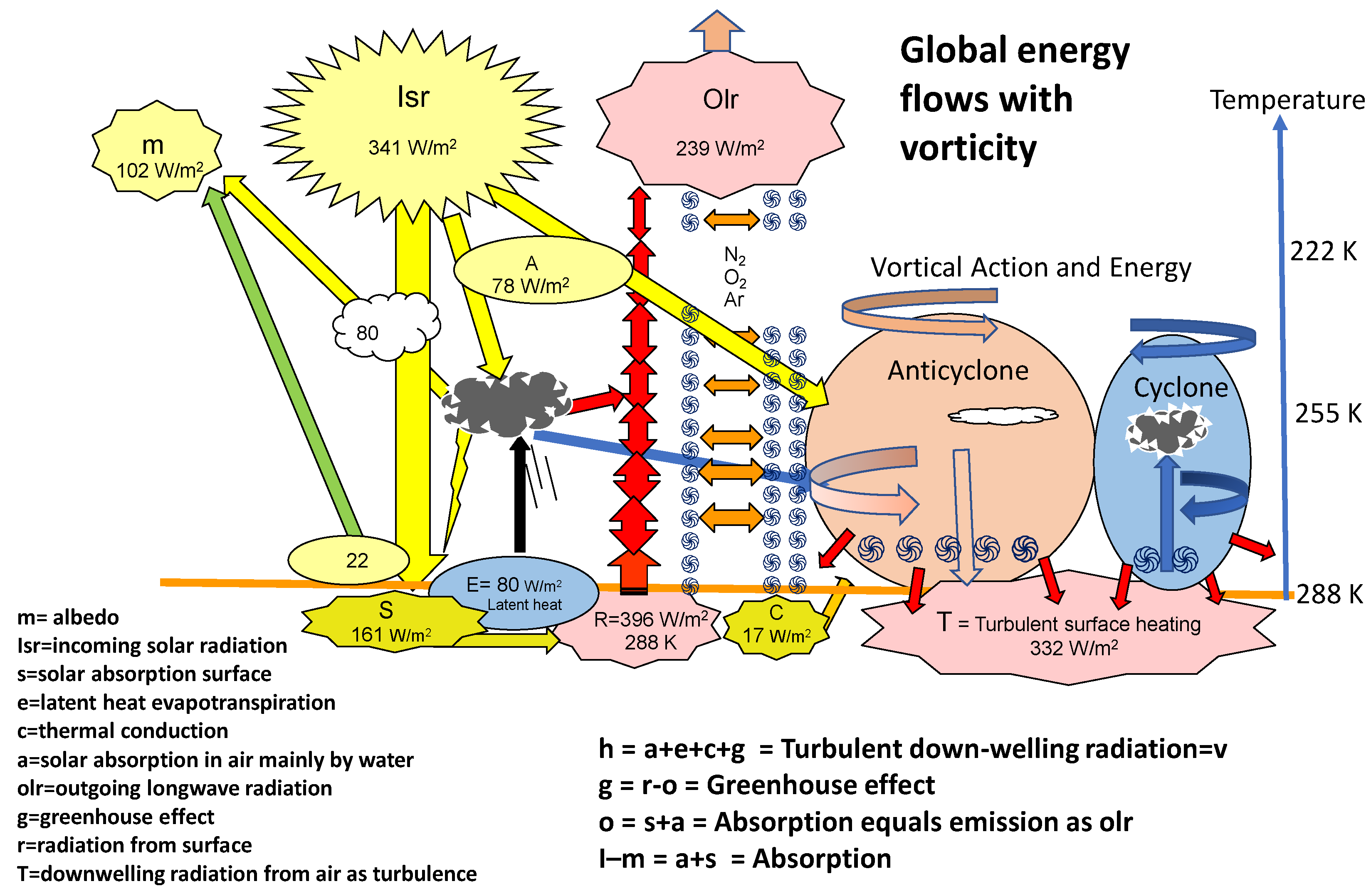
Figure 1 integrates the vortical rotational energy in anticyclones and cyclones with the global Trenberth heat flow budget [
11] for radiation. The Kiehl-Trenberth budget proposes that 332 W per m
2 of downwelling radiation is returned from the atmosphere, needed to explain the blackbody temperature of the Earth’s surface. We propose instead of net radiation from higher in a colder atmosphere to the surface that vortical action in anticyclones is generated as work processes in air, facilitated by Coriolis accelerations in each hemisphere. This work process requires significant absorption of heat radiated from the surface in greenhouse processes involving mainly water and carbon dioxide without rises in temperature. As shown in the Carnot cycle, any increase in freedom of relative translational motion of molecules increases the heat capacity of the gas phase. For anticyclones, this allows turbulent friction processes nearer the surface to release heat in the boundary layer of the lower atmosphere (v), to the extent (h=a+e+c+g=v) of about 330 watts per m
2 [
2] as a global average, rather than by radiation from a colder atmosphere to the surface, in accordance with the second law of thermodynamics. The decreasing wind speed near the surface regarded as vorticity represents the loss of power with wind speed, warming air and causing spectral radiation proportional to temperature.
Our model for greenhouse warming in
Figure 1 (g=r-o) involves the difference between black body radiation from the surface (r=
aT4) and outgoing longwave radiation (o), with the atmosphere warmed by solar absorption by water in air (a), latent heat of evapotranspiration at the surface followed by condensation under convection (e), thermal conduction from the surface (c) and the greenhouse effect itself (r-o). The work of vortical action and energy (v) provides a mechanism for turbulent release of heat as radiation near the surface. No conflict with the second law of thermodynamics is required, solving an objection voiced by some. As shown in
Table 3, a wind speed of 10 m per sec contains vortical energy of 1.47x10
3 J per m
3 of air in wind, many times greater than the kinetic energy, with an additional to 2.4 MJ per m
3 of thermal energy required for air to be heated from 0 K to 298 K [
1].
3.4. Estimation of power produced by wind turbines from vortical energy in anticyclones
In our new theory to estimate wind power based on action mechanics [
8] the rate of impulsive action or torque is estimated for both windward and leeward surfaces of wind turbines. Using the difference in these torques, power can then be estimated using the angular velocity of the turbine rotors (Ω). Current blade element momentum theory estimates the potential wind power available by using the rate of kinetic energy of wind flowing through the area swept by the rotor blades, despite most of air passing through not impacting the turbine blades. By analogy to
Figure 1 showing winds in anticyclones, we regard the impulsive power of the energy in air in vortical action striking turbine blades as a better model of turbine function. In
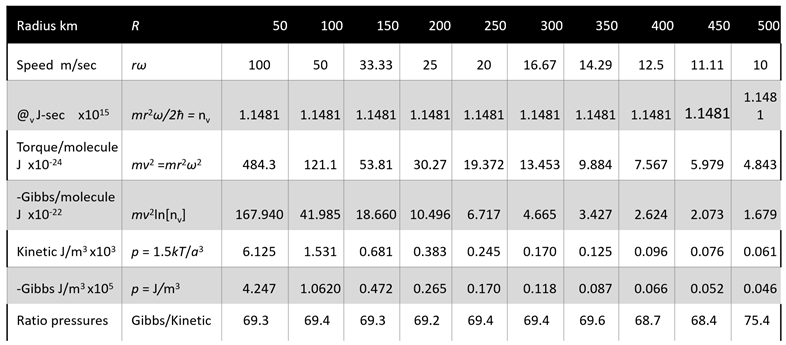
Table 3, we produce radial action estimates at different speeds for a wind turbine with 83 m rotor diameter. This value is also corrected to the kinetic energy actually impacting the blades, reducing the power estimate to a fraction of that actually achieved. We therefore reject the idea that the wind power is derived from the quantity of kinetic energy of the wind, except to provide the molecular pressure sustained in turn by the field quanta.
Table 3 compares the power available from kinetic energy and from vortical potential energy for a parcel of air (per m
3) located 1000 km from an anticyclone centre (
Figure 1 and
Figure 2). The analysis considers the wind impacting the rotor blades as well as that passing through the circle of blade rotations. Only in the latter case does the kinetic energy available to the blades exceed the radial action estimate. By comparison, the maximum vortical power estimated is more than five times greater, restricted to the blade area.
As shown in
Table 4, the virtual Gibbs field contains about 70 times as much energy as the density of kinetic energy of the molecules. We conclude that this field configurational energy sustains the kinetic energy. We challenge the idea that all air passing through the circle swept by the turbines but mainly never contacting the turbine blades could have sufficient solidity to have a significant effect on power output. This defies reason. We prefer the idea that the impulsive radial action must generate torques strictly exerted on the blades. Some proposals regarding the nature of vortical energy per paired molecule are given in
Table 4. Using the torque generated per molecule (
kT =
mv2) at 1000 km radius estimates vortical energy available almost two orders of magnitude greater than that of kinetic energy.
A feature of the action mechanics model for quantum fields is that it allows absolute values of Gibbs energy content per molecule to be calculated, rather than transitional values (δg).
For example, for wind at 10 m per sec, the mean virtual quantum (
hv) has the value of 7.46018x10
-38 J with a wavelength of 2.6627x10
12 m. This wavelength is more than 10
6 greater than the material radius. This means that the curvature of the oscillating longitudinal motion is relatively linear and the action velocity for the molecules is about 10
6 less than the velocity of light. Differences in Gibbs energy per molecule with wind speed are easily calculated. We have proposed methods in our articles [
2,
6,
8] to test the vortical energy field hypothesis using appropriate sensors allowing release of this energy under turbulent conditions. If confirmed, this could be an important source of regional warming and land dehydration [
8], possibly raising fire risk from wind farms. We have estimated that a 100 MW windfarm could raise the temperature of air downwind by turbulent release of vortical energy up to 2
0C, increasing evapotranspiration for many km distance. This prediction is recommended [
8] to be tested as a matter of due diligence regarding the location of windfarms.
3.5. Power in tropical cyclones from action mechanics of heat of volatilization on the ocean surface and convective condensation of water at the eyewalls
Tropical cyclones are known to be fully energized by the heat of vaporization of water at warm ocean surfaces in low latitudes. Condensation by strong spiral convection near the eyewall releases radiant infrared energy as directly resonant quanta, from H-bonding between water molecules in the formation of water droplets [
12]. Yet it is also well known that the kinetic energy of the cyclonic motion is only a small percentage of the total energy and power dissipated in the path of a cyclone’s trajectory.
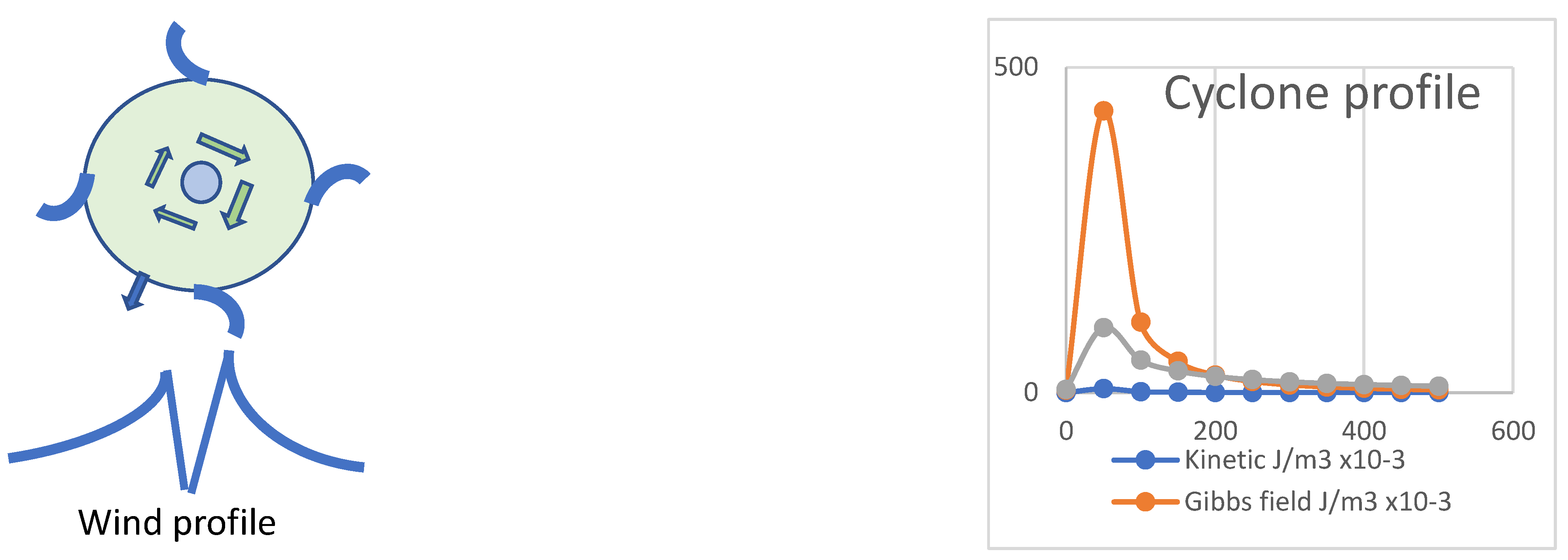
Vortical action and entropy generated at the cyclone’s eye-wall near its centre provides the direct connection needed between heat of condensation and the power of the cyclone, with the kinetic pressure and torque being sustained by the impulses of the Gibbs field energy. The field energy pressure is a large multiple of the kinetic energy pressure and can sustain the torques required, at least while the cyclone continues to evaporate water from the sea surface. On land, the energy of the cyclone, no longer sustained by evaporation from the ocean, is soon dissipated by frictional turbulence releasing heat to the surface atmosphere. Such intense regional warming on land is an anecdotal feature of the aftermath of tropical cyclones.
In action mechanics, it is assumed that specific radial action (
r2ω) will be approximately constant across the cyclone, given the inverse square radial distribution of radiation from convective condensation at the eyewall, generating a radially acting Gibbs energy field as
mv2ln[n
v], shown in
Table 5. Furthermore, considering the cyclone as simultaneously rotational and convective, conservation of angular momentum will ensure constant Σ
mr2ω ensuring intensified velocity (
rω) nearer the eye-wall.The heat of vaporization at an estimated rate matches the vortical Gibbs energy and power generated shown in
Table 5. Vortical entropic energy is directly provided by infrared radiation from condensing water in the convective eyewall of a tropical cyclone. The model shown in
Figure 3 exhibits vortical action and energy as shown in
Table 5. Note the 70-fold ratio of energy density in the Gibbs field compared to that of kinetic pressure, similar to that observed for wind driving turbines to develop electrical power (
Table 4).
As discussed in section 3.2, the pressure equation underestimates the kinetic energy density by 50%, adjusted in
Table 5. The greater the vortical energy in the field, the more negative the Gibbs energy of the molecules in the field as the potential to take up thermal energy in the system is exhausted.
This vortical version of the dynamics of a tropical cyclone may be more consistent with the rotating convective model now accepted for best explaining destructive cyclonic intensification [
13]. Previous opinion [
14,
15] favored intensification as caused by large latent heat fluxes from the surface in the core region and that an intensive evaporation-wind feedback process (WISHE) was not needed. Vortical energy would be stored cumulatively during convective processes leading to condensation nearer the eye-wall, or walls if serried in convective configuration. The vortical inertia of the cyclone would require a continuous feed of thermal energy from evaporation and condensation of water but this can be modelled as intensifying as updraft increases, shrinking the radius and providing a higher pressure of vortical field energy nearer the eye wall, with higher wind speeds. The model data in
Table 5 represent a snapshot of the process at one stage in time. Researchers in the dynamics of tropical cyclones are invited to include vortical energy in their models.
We regard the ability of action mechanics to explain the intensifying power of tropical cyclones quantitatively is a major point in favor for the vortical action and energy theory.