Submitted:
05 April 2023
Posted:
06 April 2023
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study site, patient population, and specimen collection
2.2. Xpert® MTB/RIF assay
2.3. Phenotypic drug susceptibility testing (DST)
2.4. Genotypic DST
2.5. Evaluation and interpretation of results
2.6. Spoligotyping
3. Results
3.1. Profile of the isolates
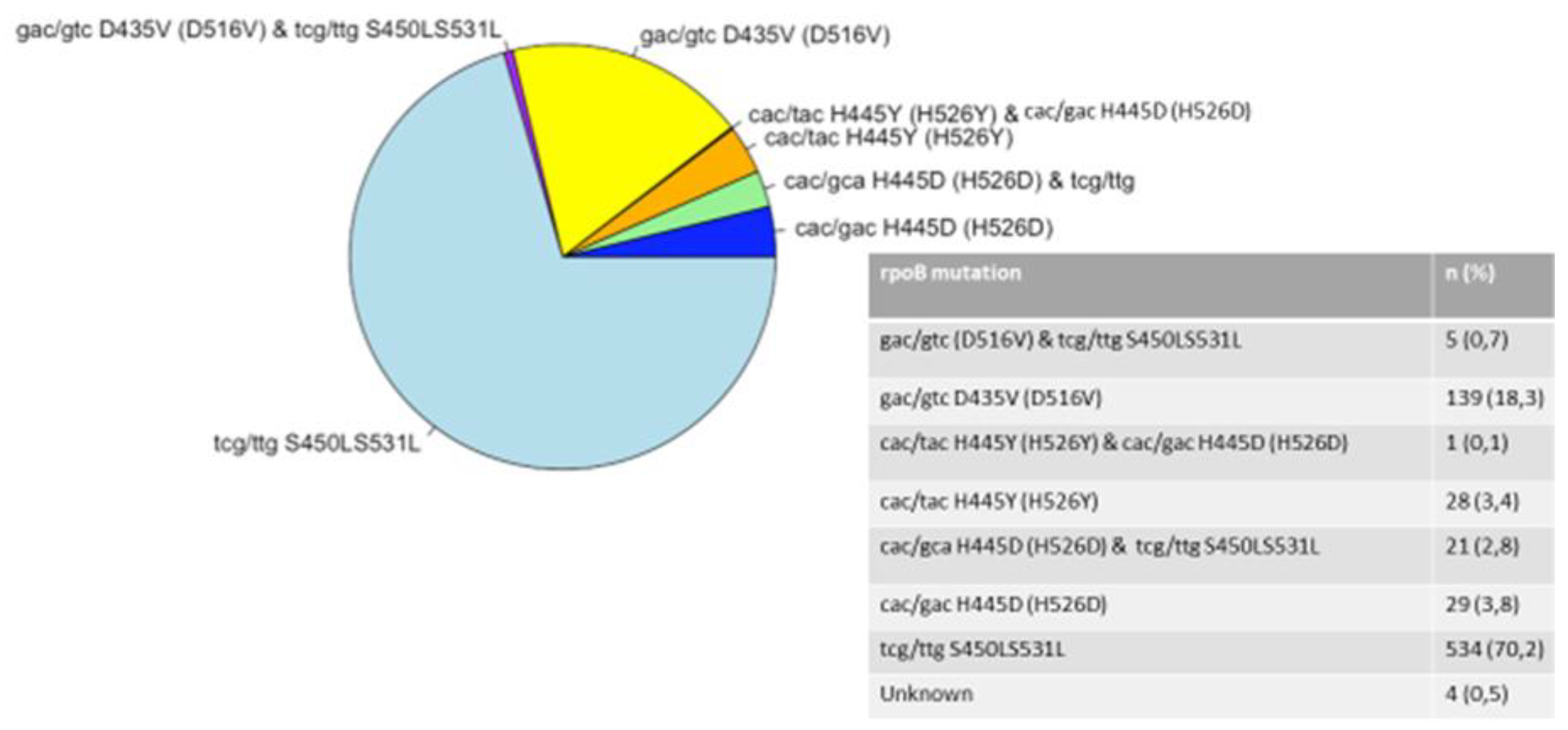
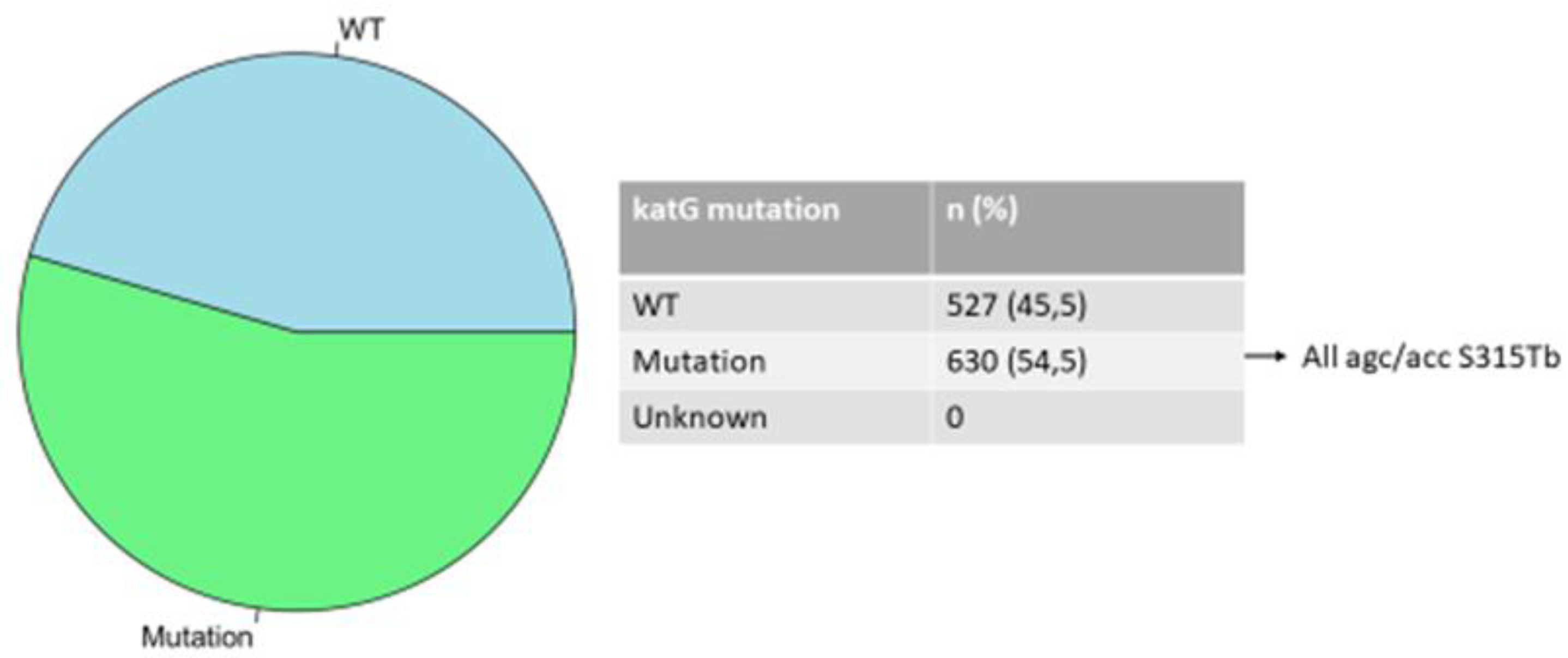
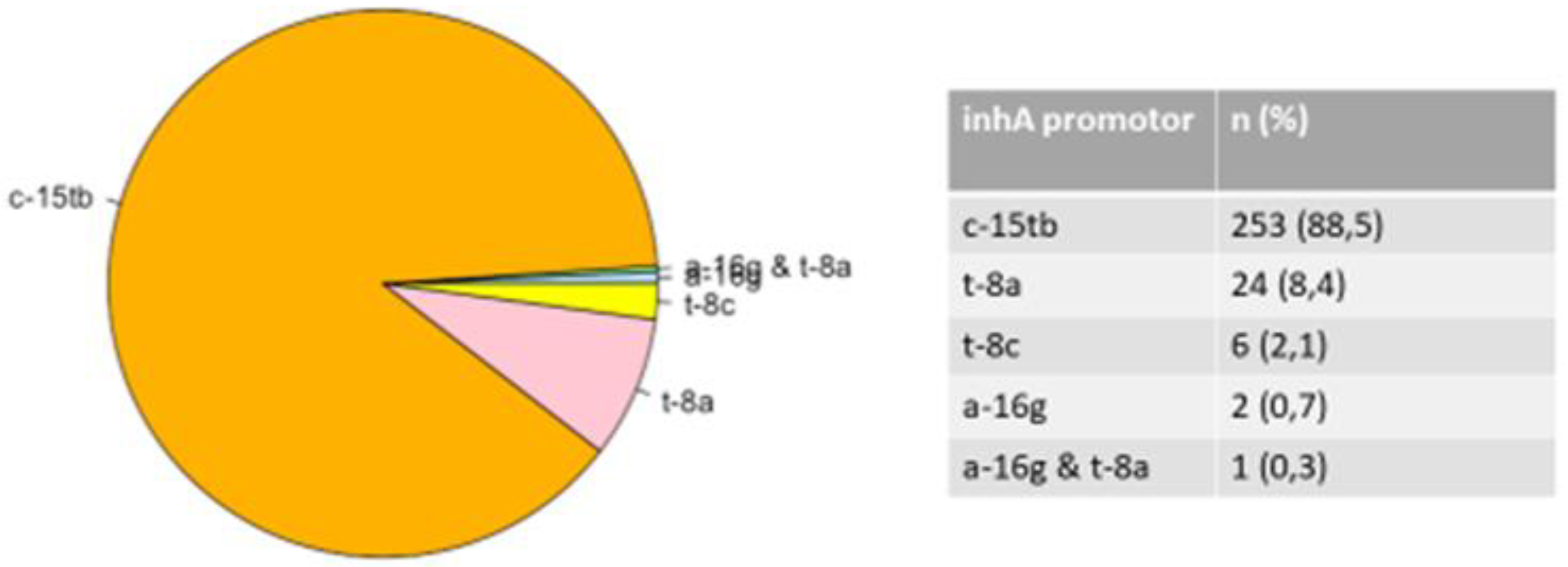
3.2. Mutations in the rpoB, katG, and inhA genes
3.3. Heteroresistance mutations
3.4. Spoligotyping
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bwalya, P.; Yamaguchi, T.; Solo, E.S.; Chizimu, J.Y.; Mbulo, G.; Nakajima, C.; Suzuki, Y. Characterization of Mutations Associated with Streptomycin Resistance in Multidrug-Resistant Mycobacterium tuberculosis in Zambia. Antibiotics 2021, 10, 1169. [CrossRef]
- World Health Organization. Global tuberculosis report, 2020 World Health Organization. Geneva, Switzerland, available at https://apps.who.int/ iris/handle/10665/336069 [accessed August 6, 2022].
- Statistics South Africa. Mortality and causes of death in South Africa. Findings from death notification. Pretoria, South Africa. Stats SA’s June 2021 report [accessed 21 January 2022].
- World Health Organization. Global tuberculosis report 2021: supplementary material [accessed August 8, 2022].
- Kabir, S.; Junaid, K.; Rehman, A. Variations in rifampicin and isoniazid resistance associated genetic mutations among drug naïve and recurrence cases of pulmonary tuberculosis. Int. J. Infect. Dis. 2021, 103, 56-61. [CrossRef]
- Libiseller-Egger, J.; Phelan, J.; Campino, S.; Mohareb, F.; Clark, T.G. Robust detection of point mutations involved in multidrug-resistant Mycobacterium tuberculosis in the presence of co-occurrent resistance markers. PLoS Comput. Biol. 2020, 16(12), e1008518. [CrossRef]
- Wan, L.; Liu, H.; Li, M.; Jiang, Y.; Zhao, X.; Liu, Z.; Wan, K.; Li, G.; Guan, C-x. Genomic Analysis Identifies Mutations Concerning Drug-Resistance and Beijing Genotype in Multidrug-Resistant Mycobacterium tuberculosis Isolated From China. Front. Microbiol. 2020, 11, 1444. [CrossRef]
- Pitso, L.; Potgieter, S.; Van der Spoel van Dijk, A. Prevalence of isoniazid resistance-conferring mutations associated with multidrug-resistant tuberculosis in Free State Province, South Africa. S Afr. Med. J. 2019, 109(9), 659-664. [CrossRef]
- Solo, E.S.; Nakajima, C.; Kaile, T.; Bwalya, P.; Mbulo, G.; Fukushima, Y.; Chila, S.; Kapata, N.; Shah, Y.; Suzuki, Y. Mutations in rpoB and katG genes and the inhA operon in multidrug-resistant Mycobacterium tuberculosis isolates from Zambia. J. Glob. Antimicrob. Res. 2020, 22, 302-307. [CrossRef]
- Diriba, G.; Kebede, A.; Tola, H.H.; Alemu, A.; Yenew, B.; Moga, S.; Addise, D.; Mohammed, Z.; Getahun, M.; Fantahun, M.; Tadesse, M. Utility of line probe assay in detecting drug resistance and the associated mutations in patients with extra pulmonary tuberculosis in Addis Ababa, Ethiopia. SAGE Open Med. 2022, 10, 20503121221098241.
- Valafar, S.J. Systematic review of mutations associated with isoniazid resistance points to continuing evolution and subsequent evasion of molecular detection, and potential for emergence of multidrug resistance in clinical strains of Mycobacterium tuberculosis. Antimicrob. Agents. Chem. 2021, 65(3), e02091-20. [CrossRef]
- Liu, L.; Zhao, X.; Wu, X.; Li, S.; Liu, B.; Rajaofera, M.J.; Zeng, Y.; Dong, S.; Bei, Z.; Pei, H.; Xia, Q. Prevalence and molecular characteristics of drug-resistant Mycobacterium tuberculosis in Hainan, China: from 2014 to 2019. BMC. Microbiol. 2021, 21, 185. [CrossRef]
- Bodmer, T.; Ströhle, A. Diagnosing pulmonary tuberculosis with the Xpert MTB/RIF test. J.Vis. Exp. 2012, 62, e3547.
- World Health Organization. The impact of the roll-out of rapid molecular diagnostic testing for tuberculosis on empirical treatment in Cape Town, South Africa, Bulletin WHO, 2017, 95, 545-608.
- Hain Lifescience. Company history and product releases. 2016. https://www.hain-lifescience.de/en/company/history.html. (Accessed 11 February 2021).
- Ogari, C.O.; Nyamache, A.K.; Nonoh, J.; Amukoye, E. Prevalence and detection of drug resistant mutations in Mycobacterium tuberculosis among drug naïve patients in Nairobi, Kenya. BMC Infect. Dis. 2019, 19, 279. [CrossRef]
- Couvin, D.; David, A.; Zozio, T.; Rastogi, N. Macro-geographical specificities of the prevailing tuberculosis epidemic as seen through SITVIT2, an updated version of the Mycobacterium tuberculosis genotyping database. Infect. Genet.Evol. 2019, 72, 31-43. [CrossRef]
- Bhembe, N.L.; Green, E. Characterization of mutations in the rpoB gene conferring rifampicin resistance in Mycobacterium tuberculosis complex isolated from lymph nodes of slaughtered cattle from South Africa. Braz. J. Microbiol. 2020, 51(4), 1919-1927. [CrossRef]
- Otchere, I.D.; Asante-Poku, A.; Osei-Wusu, S.; Baddoo, A.; Sarpong, E.; Ganiyu, A.H.; Aboagye, S.Y.; Forson, A.; Bonsu, F.; Yahayah, A.I.; Koram, K. Detection and characterization of drug-resistant conferring genes in Mycobacterium tuberculosis complex strains: a prospective study in two distant regions of Ghana. Tuberculosis (Edinb). 2016, 99, 147-154. [CrossRef]
- Jia, H.; Xu, Y.; Sun, Z. Analysis on Drug-Resistance-Associated Mutations among Multidrug-Resistant Mycobacterium tuberculosis Isolates in China. Antibiotics. 2021, 10, 1367. [CrossRef]
- Isakova, J.; Sovkhozova, N.; Vinnikov, D.; Goncharova, Z.; Talaibekova, E.; Aldasheva, N.; Aldashev, A. Mutations of rpoB, katG, inhA and ahp genes in rifampicin and isoniazid-resistant Mycobacterium tuberculosis in Kyrgyz Republic. BMC Microbiol. 2018, 18, 22. [CrossRef]
- Uddin, M.K.; Rahman, A.; Ather, M.F.; Ahmed, T.; Rahman, S.M.; Ahmed, S.; Banu, S. Distribution and frequency of rpoB mutations detected by Xpert MTB/RIF assay among Beijing and non-Beijing rifampicin resistant Mycobacterium tuberculosis isolates in Bangladesh. Infect. Drug Resist. 2020, 13, 789. [CrossRef]
- Meftahi, N.; Namouchi, A.; Mhenni, B.; Brandis, G.; Hughes, D.; Mardassi, H. Evidence for the critical role of a secondary site rpoB mutation in the compensatory evolution and successful transmission of an MDR tuberculosis outbreak strain. J. Antimicrob. Chemother. 2016, 71, 324-332.
- Evans, J.; Stead, M.C.; Nicol, M.P.; Segal, H. Rapid genotypic assays to identify drug-resistant Mycobacterium tuberculosis in South Africa. J. Antimicrob. Chemother. 2009, 63, 11-16. [CrossRef]
- Ssengooba, W.; Meehan, C.J.; Lukoye, D.; Kasule, G.W.; Musisi, K.; Joloba, M.L.; Cobelens, F.G.; de Jong, B.C. Whole genome sequencing to complement tuberculosis drug resistance surveys in Uganda. Infect. Genet. Evol. 2016, 40, 8-16. [CrossRef]
- Salvato, R.S.; Schiefelbein, S.; Barcellos, R.B.; Praetzel, B.M.; Anusca, I.S.; Esteves, L.S.; Halon, M.L.; Unis, G.; Dias, C.F.; Miranda, S.S.; de Almeida, I.N.. Molecular characterisation of multidrug-resistant Mycobacterium tuberculosis isolates from a high-burden tuberculosis state in Brazil. Epidemiol. Infec. 2019, 147.
- Tajbakhsh, A.; Ghasemi, F.; Mirbagheri, S.Z.; Heravi, M.M.; Rezaee, M.; Meshkat, Z. Investigation of the rpoB mutations causing rifampin resistance by rapid screening in Mycobacterium tuberculosis in North-East of Iran. Iran. J. Pathol. 2018, 13(4), 429.
- Bollela, V.R., Namburete, E.I.; Feliciano, C.S.; Macheque, D.; Harrison, L.H.; Caminero, J.A. Detection of katG and inhA mutations to guide isoniazid and ethionamide use for drug-resistant tuberculosis. Int. J. Tuberc. Lung Dis. 2016, 20(8), 1099-1104. [CrossRef]
- Verza, M.; Scheffer, M.C.; Salvato, R.S.; Schorner, M.A.; Barazzetti, F.H.; Machado, H.D.; Medeiros, T.F.; Rovaris, D.B.; Portugal, I.; Viveiros, M.; Perdigão, J. Genomic epidemiology of Mycobacterium tuberculosis in Santa Catarina, Southern Brazil. Sci. Rep. 2020, 10, 12891.
- Jagielski, T.; Grzeszczuk, M.; Kamiński, M.; Roeske, K.; Napiórkowska, A.; Stachowiak, R.; Augustynowicz-Kopeć, E.; Zwolska, Z.; Bielecki, J. Identification and analysis of mutations in the katG gene in multidrug-resistant Mycobacterium tuberculosis clinical isolates. Adv. Resp. Med. 2013, 81(4), 298-307. [CrossRef]
- Abanda, N.N.; Djieugoué, J.Y.; Lim, E.; Pefura-Yone, E.W.; Mbacham, W.; Vernet, G.; Penlap, V.M.; Eyangoh, S.I.; Taylor, D.W.; Leke, R.G. Diagnostic accuracy and usefulness of the Genotype MTBDRplus assay in diagnosing multidrug-resistant tuberculosis in Cameroon: A cross-sectional study. BMC infect. Dis. 2017, 17, 379. [CrossRef]
- Tseng, S.T.; Tai, C.H.; Li, C.R.; Lin, C.F.; Shi, Z.Y. The mutations of katG and inhA genes of isoniazid-resistant Mycobacterium tuberculosis isolates in Taiwan. J. Microbiol. Immunol. Infect. 2015, 48(3), 249-255. [CrossRef]
- Charoenpak, R.; Santimaleeworagun, W.; Suwanpimolkul, G.; Manosuthi, W.; Kongsanan, P.; Petsong, S.; Puttilerpong, C. Association between the phenotype and genotype of isoniazid resistance among Mycobacterium tuberculosis isolates in Thailand. Infect. Drug Resist. 2020, 13, 627.
- Norouzi, F.; Moghim, S.; Farzaneh, S.; Fazeli, H.; Salehi, M.; Esfahani B.N. Significance of the coexistence of non-codon 315 katG, inhA, and oxyR-ahpC intergenic gene mutations among isoniazid-resistant and multidrug-resistant isolates of Mycobacterium tuberculosis: a report of novel mutations. Pathog. Glob. Health. 2022, 116(1), 22-29.
- Tessema, B.; Beer, J.; Emmrich, F.; Sack, U.; Rodloff, A.C. Analysis of gene mutations associated with isoniazid, rifampicin and ethambutol resistance among Mycobacterium tuberculosis isolates from Ethiopia. BMC Infect. Dis. 2012, 12, 37.
- Gliddon, H.D.; Frampton, D.; Munsamy, V.; Heaney, J.; Pataillot-Meakin, T.; Nastouli, E.; Pym, A.S.; Steyn, A.J.; Pillay, D.; McKendry, R.A. A Rapid Drug Resistance Genotyping Workflow for Mycobacterium tuberculosis, Using Targeted Isothermal Amplification and Nanopore Sequencing. Microbiol. Spectr. 2021, 9(3), e00610. [CrossRef]
- Abate, D.; Tedla, Y.; Meressa, D.; Ameni, G. Isoniazid and rifampicin resistance mutations and their effect on second-line anti-tuberculosis treatment. Int. J. Tuberc. Lung Dis. 2014, 18(8), 946-951. [CrossRef]
- Lempens, P.; Meehan, P.J.; Vandelannoote, K.; Fissette, K.; de Rijk, P.; Van Deun, A.; Rigouts, L.; de Jong, B.C. Isoniazid resistance levels of Mycobacterium tuberculosis can largely be predicted by high confidence resistance-conferring mutations. Sci. Rep. 2018, 8:3246. [CrossRef]
- Sarin, R.; Bhalla, M.; Kumar, G.; Singh, A.; Myneedu, V.P.; Singhal, R. Correlation of inhA mutations and ethionamide susceptibility: Experience from national reference center for tuberculosis. Lung India. 2021, 38(6), 520-523. [CrossRef]
- Maitre, T.; Morel, F.; Brossier, F.; Sougakoff, W.; Jaffre, J.; Cheng, S.; Veziris, N.; Aubry, A.; NRC-MyRMA. How a PCR Sequencing Strategy Can Bring New Data to Improve the Diagnosis of Ethionamide Resistance. Microorganisms 2022, 10(7), 1436. [CrossRef]
- Seifert, M.; Catanzaro, D.; Catanzaro, A.; Rodwell, T.C. Genetic Mutations Associated with Isoniazid Resistance in Mycobacterium tuberculosis: A Systematic Review. PLoS ONE, 2015, 10(3), e0119628. [CrossRef]
- Hofmann-Thiel, S.; van Ingen, J.; Feldmann, K.; Turaev, L.; Uzakova, G.T.; Murmusaeva, G.; van Soolingen, D.; Hoffmann, H. Mechanisms of heteroresistance to isoniazid and rifampin of Mycobacterium tuberculosis in Tashkent, Uzbekistan. Eur. Respir. J. 2009, 33, 368–374. [CrossRef]
- Shin, S.S.; Modongo, C.; Baik, Y.; Allender, C.; Lemmer, D.; Colman, R.E.; Engelthaler, D.M.; Warren, R.M.; Zetola, N.M. Mixed Mycobacterium tuberculosis–strain infections are associated with poor treatment outcomes among patients with newly diagnosed tuberculosis, independent of pretreatment heteroresistance. J. Infect. Dis. 2018, 218(12), 1974-1982.
- Rinder, H.; Mieskes, K.T.; Löscher, T. Heteroresistance in Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 2001, 5(4), 339-345.
- de Assis Figueredo, L.J.; de Almeida, I.N.; Augusto, C.J.; Soares, V.M.; Suffys, P.N.; da Silva Carvalho, W.; de Miranda, S.S. Characterization of Mycobacterium tuberculosis heteroresistance by genotyping. Int. J. Mycobacteriol. 2020, 9(4), 368-372. [CrossRef]
- Said, H.; Ratabane, J.; Erasmus, L.; Gardee, Y.; Omar, S.; Dreyer, A.; Ismail, F.; Bhyat, Z.; Lebaka, T.; van der Meulen, M.; Gwala, T.; Adelekan, A.; Diallo, K.; Ismail, N. Distribution and Clonality of drug-resistant tuberculosis in South Africa. BMC Microbiol. 2021, 21, 157. [CrossRef]
- Mokrousov, I.; Ly, H.M.; Otten, T.; Lan, N.N.; Vyshnevskyi, B.; Hoffner, S.; Narvskaya, O. Origin and primary dispersal of the Mycobacterium tuberculosis Beijing genotype: clues from human phylogeography. Genome Res. 2005, 15(10), 1357-1364. [CrossRef]
- María Irene, C.C.; Juan Germán, R.C.; Gamaliel, L.L.; Dulce Adriana, M.E.; Estela Isabel, B.; Brenda Nohemí, M.C.; Payan Jorge, B.; Zyanya Lucía, Z.B.; Myriam, B.D.; Adrian, O.L.; Martha Isabel, M. Profiling the immune response to Mycobacterium tuberculosis Beijing family infection: a perspective from the transcriptome. Virulence 2021, 12(1), 1689-1704. [CrossRef]
- Karmakar, M.; Trauer, J.M.; Ascher, D.B.; Denholm, J.T.; Hyper transmission of Beijing lineage Mycobacterium tuberculosis: systematic review and meta-analysis. J. Infect. 2019, 79(6), 572-581. [CrossRef]
- Maguga-Phasha, N.T.; Munyai, N.S.; Mashinya, F.; Makgatho, M.E.; Mbajiorgu, E.F. Genetic diversity and distribution of Mycobacterium tuberculosis genotypes in Limpopo, South Africa. BMC Infect. Dis. 2017, 17, 764. [CrossRef]
- Chihota, V.N.; Niehaus, A.; Streicher, E.M.; Wang, X.; Sampson, S.L.; Mason, P.; Källenius, G.; Mfinanga, S.G.; Pillay, M.; Klopper, M.; Kasongo, W. Geospatial distribution of Mycobacterium tuberculosis genotypes in Africa. PLoS ONE 2018, 13(8), e0200632. [CrossRef]
- van Soolingen, D.; Qian, L.; De Haas, P.E.; Douglas, J.T.; Traore, H.; Portaels, F.; Qing, H.Z.; Enkhsaikan, D.; Nymadawa, P.; van Embden, J.D. Predominance of a single genotype of Mycobacterium tuberculosis in countries of east Asia. J. Clin. Microbiol. 1995, 33(12), 3234-3238. [CrossRef]
- Pokam, B.D.; Yeboah-Manu, D.; Amiteye, D.; Asare, P.; Guemdjom, P.W.; Yhiler, N.Y.; Morton, S.N.; Ofori-Yirenkyi, S.; Laryea, R.; Tagoe, R.; Asuquo, A.E. Molecular epidemiology and multidrug resistance of Mycobacterium tuberculosis complex from pulmonary tuberculosis patients in the Eastern region of Ghana. Heliyon 2021, 7(10), e08152. [CrossRef]
- Bhembe, N.L.; Nwodo, U.U.; Okoh, A.I.; Obi, C.L.; Mabinya, L.V.; Green, E. Clonality and genetic profiles of drug-resistant Mycobacterium tuberculosis in the Eastern Cape Province, South Africa. Microbiology Open 2019, 8(3), e00449.
- Van der Spoel van Dijk, A.; Makhoahle, P.M.; Rigouts, L.; Baba, K. Diverse molecular genotypes of Mycobacterium tuberculosis complex isolates circulating in the Free State, South Africa. Int. J. Microbiol. 2016, 2016. [CrossRef]
- Solo, E.S.; Suzuki, Y.; Kaile, T.; Bwalya, P.; Lungu, P.; Chizimu, J.Y.; Shah, Y.; Nakajima, C. Characterization of Mycobacterium tuberculosis genotypes and their correlation to multidrug resistance in Lusaka, Zambia. Int. J. Infect. Dis. 2021, 102, 489-496. [CrossRef]
- Sagonda, T.; Mupfumi, L.; Manzou, R.; Makamure, B.; Tshabalala, M.; Gwanzura, L.; Mason, P.; Mutetwa, R. Prevalence of extensively drug resistant tuberculosis among archived multidrug resistant tuberculosis isolates in Zimbabwe. Tuberc. Res. Treat. 2014, 349141. [CrossRef]
| Year of diagnosis | DR-TB case n (%) |
Annual heteroresistance rate n (%) |
|---|---|---|
| 2018 | 385 (33.3) | 37 (9.6) |
| 2019 | 376 (32.5) | 43 (11.4) |
| 2020 | 396 (34.2) | 127 (32.2) |
| Combined mutation | Number of isolates (%) | Mutation regions | Number of isolates (%) |
|---|---|---|---|
| rpoB and katG | 532 (69.9) | rpoB S315L and katG 531ST | 366 (68,8%) |
| rpoB and inhA | 187 (24.6) | rpoB S315L and inhA c-15tb | 171 (91,4%) |
| rpoB S315L and inhA a-16g | 1 (0,5%) | ||
| rpoB S315L and inhA t-8c | 2 (1,1%) | ||
| rpoB S315L and inhA t-8a | 13 (6,9%) |
| Lineage |
Number of Isolates |
Sub-lineage | Number of Isolates | SIT | Number of Isolates |
|---|---|---|---|---|---|
| Beijing | 185 | 1 | 185 | ||
| LAM | 83 | LAM3 | 62 | 33 | 36 |
| 719 | 17 | ||||
| ORPHAN | 2 | ||||
| 4 | 1 | ||||
| 130 | 1 | ||||
| 376 | 1 | ||||
| 2014 | 1 | ||||
| 2284 | 1 | ||||
| 2302 | 1 | ||||
| not in SITVIT | 1 | ||||
| LAM4 | 15 | 60 | 15 | ||
| LAM5 | 2 | 93 | 1 | ||
| 136 | 1 | ||||
| LAM9 | 2 | 42 | 2 | ||
| LAM11-ZWE | 2 | 811 | 1 | ||
| 1873 | 1 | ||||
| X | 48 | X1 | 18 | 2022 | 10 |
| 2226 | 4 | ||||
| 119 | 3 | ||||
| 336 | 1 | ||||
| X2 | 3 | 18 | 1 | ||
| 137 | 1 | ||||
| 2016 | 1 | ||||
| X3 | 27 | 92 | 21 | ||
| 2286 | 4 | ||||
| 2020 | 2 | ||||
| T | 34 | T1 | 29 | 53 | 13 |
| 926 | 4 | ||||
| 334 | 2 | ||||
| 501 | 2 | ||||
| 156 | 1 | ||||
| 245 | 1 | ||||
| 373 | 1 | ||||
| 519 | 1 | ||||
| 732 | 1 | ||||
| 1122 | 1 | ||||
| 1144 | 1 | ||||
| ORPHAN | 1 | ||||
| T2 | 1 | 52 | 1 | ||
| T2/3 | 2 | 73 | 2 | ||
| T3 | 1 | ORPHAN | 1 | ||
| T5-RUS1 | 1 | 254 | 1 | ||
| T- TUSCANY | 2 | 1737 | 2 | ||
| S | 31 | 34 | 18 | ||
| 789 | 4 | ||||
| 71 | 3 | ||||
| Not in SITVIT | 2 | ||||
| 790 | 1 | ||||
| 1211 | 1 | ||||
| Not in SITVIT | 1 | ||||
| Not in SITVIT | 1 | ||||
| EAI | 16 | EAI1-SOM | 10 | 806 | 6 |
| 48 | 2 | ||||
| 1649 | 2 | ||||
| EAI5 | 5 | 625 | 3 | ||
| ORPHAN | 2 | ||||
| EAI | 1 | Not in SITVIT | 1 | ||
| H | 6 | H1 | 5 | 62 | 2 |
| 2375 | 2 | ||||
| 47 | 1 | ||||
| H3 | 1 | 50 | 1 | ||
| CAS | 5 | CAS1-Kili | 2 | 21 | 2 |
| CAS1-Delhi | 1 | 1092 | 1 | ||
| CAS | 2 | Not in SITVIT | 2 | ||
| unknown | 4 | Unknown | 1 | 2018 | 1 |
| Not in SITVIT | 3 | Not in SITVIT | 1 | ||
| Not in SITVIT | 1 | ||||
| Not in SITVIT | 1 | ||||
| no result | 27 | ||||
| TOTAL | 441 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





