4.1. Recovery of Time-Dependent Smoothed Conductivity in the Examined Samples
The basic relationship describing the voltage response
of the examined samples under affect of the constant direct current
can be written in the following form:
Here we neglect small variations of the total capacitance of the system “sample + connecting cables” along the runs. Considering the trend components
of the “voltage drop – time lapse” dependencies, we arrive to the following expression for evaluation of the smoothed time-dependent conductivity:
In this case, the term "smoothed conductivity" means evaluation of
at the moment using the corresponding values of the voltage and its first derivative estimated for the trend component
. As an example,
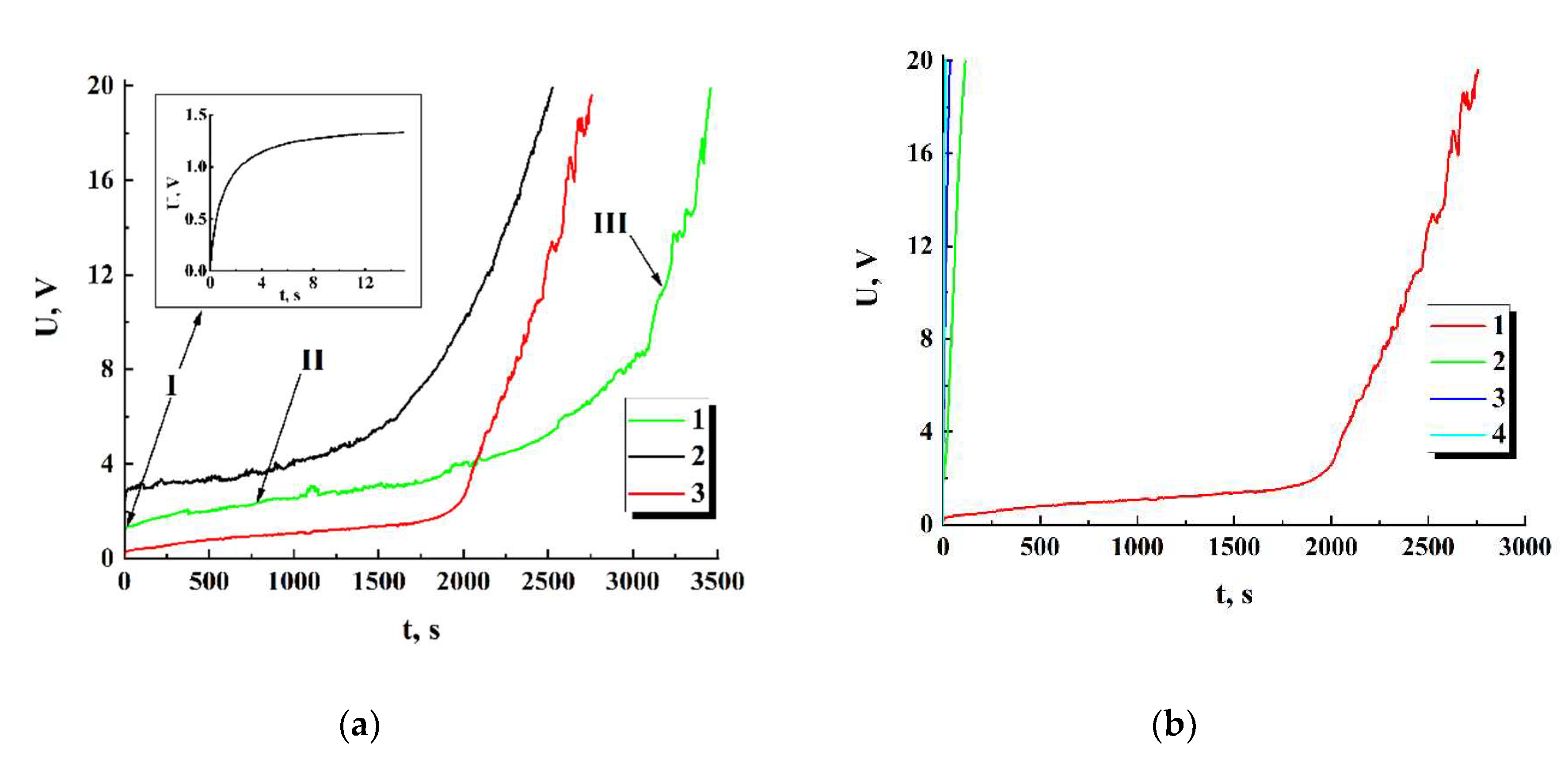
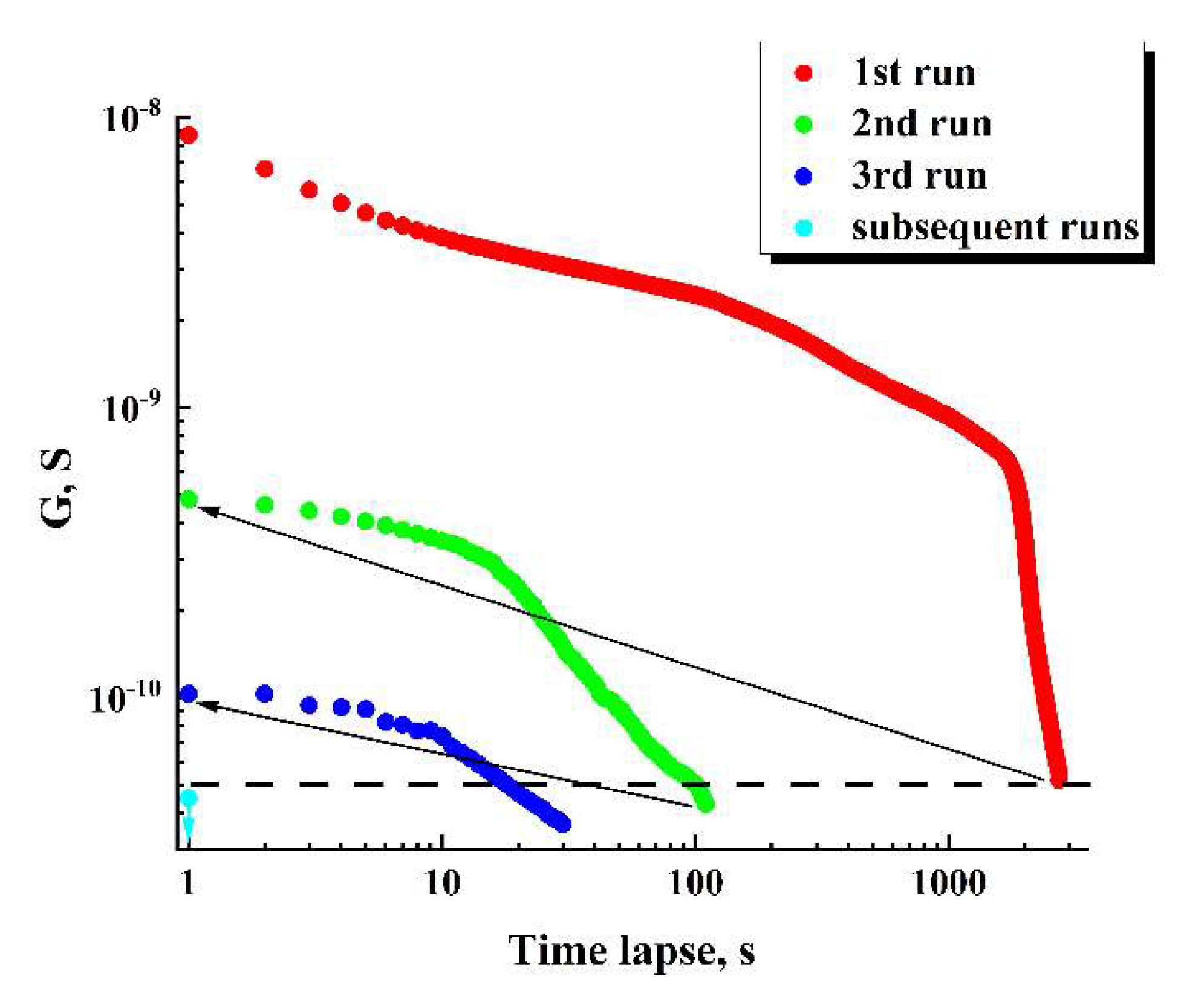
Figure 10 displays the values of
against the time lapse recovered for the series of sequential runs (for the initial datasets see
Figure 8, b). The recovered dataset
for the first run exhibits the behavior at the final stage of the run, which is typical for percolation systems near the threshold.
The horizontal dashed line corresponds to the minimal value of “pure” ohmic conductivity (5⋅10-11 S) achieved under = 20 V in the case of = 0. During the first runs, the achievable maximal values of the displacement current in the samples are many times less than the set value of the total current (1 nA). On subsequent runs, contribution of the displacement current to the total current gradually becomes dominant. Accordingly, the recovered values are below the dotted threshold line on the graph.
The arrows indicate partial recovery of ohmic conductivity in the examined sample during 90-minute time intervals between the first-second and second-third runs. However, it should be noted that this recovery under the used conditions (in particular, at room temperature) is subtle and decreases from run to run. In the subsequent runs, ohmic conductivity occurs vanishingly small (the single cyan marker).
4.2. Scaling Behavior of
and the Conductivity Critical Exponent
At the final stages of the first runs, when conducting systems in the studied samples approach their critical states, contributions of the displacement current
to the total current are small and the trend components of
are mainly governed by
:
. On the other hand, the general property of percolation systems is power-law dependence of the system conductivity on detuning the governing parameter
from its critical value
:
, where
. For the examined systems, which consist of large sets of statistically independent random conduction channels (percolation clusters), the control parameter
is associated, for example, with the number of blocked nodes or bonds in these percolation clusters. Accordingly, when
,
falls down (
should have a positive value). The trend component of the voltage drop
should exhibit a similar behavior near the threshold of ohmic conductance:
(
is negative). The first derivative
can be expressed as
. This expression can be rewritten in the following form:
. We can assume that, at a rather short stage of rapid growth in
, the dependence
admits a linear approximation (
and, accordingly,
. Under these assumptions, we arrive to the following reduced relationship between the current values of
and corresponding first derivatives:
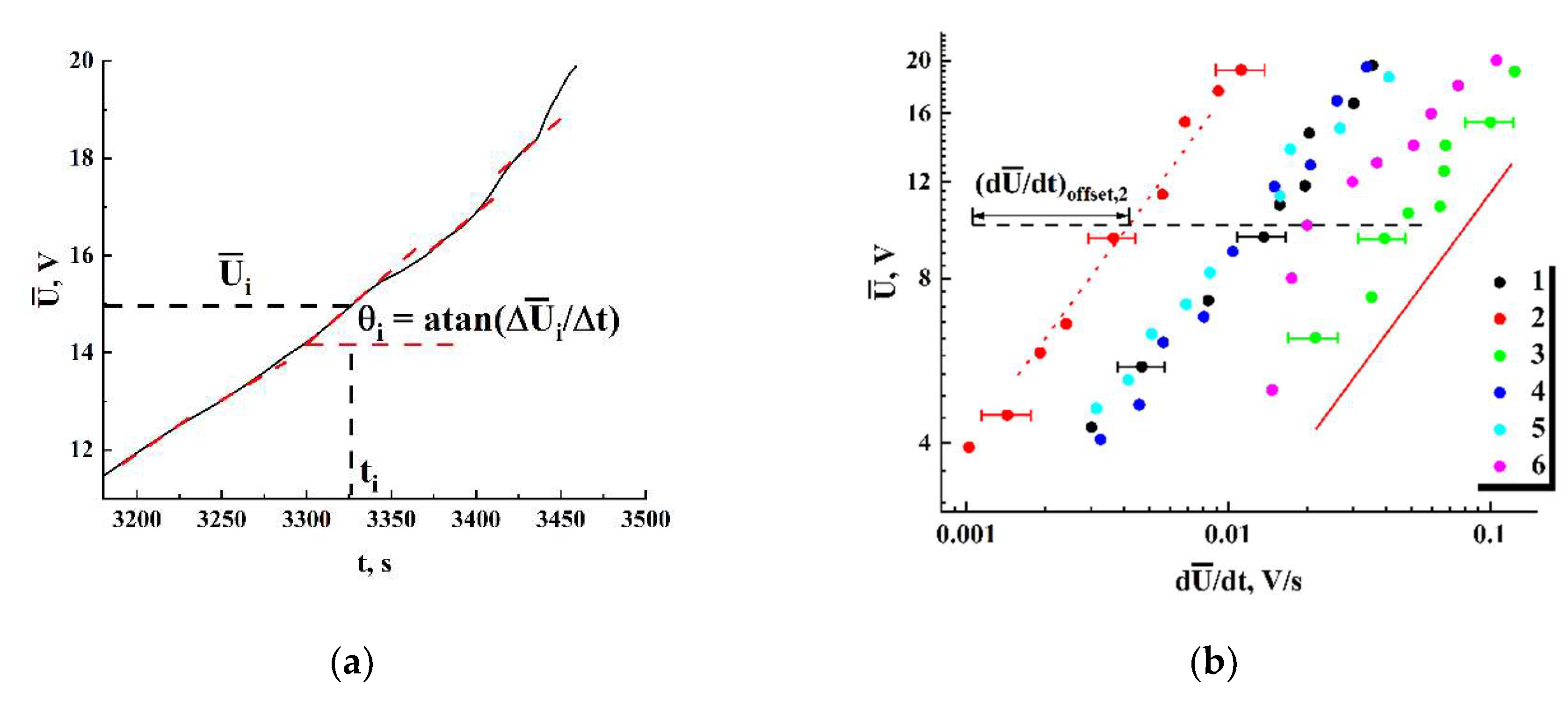
Figure 11, a illustrates the procedure for estimating
and
values from the obtained trend curves
.
Figure 10, b displays in the logarithmic coordinates the sets of pairs
corresponding to the final stages of the fist runs for various examined samples. Note that, despite a remarkable scattering of these datasets across the
coordinate plane, they exhibit common power-law trends with the close values of slopes in logarithmic coordinates. The red dashed line with the slope of ≈ 0.615 ± 0.029 indicates this common trend for all the datasets and serves as a guide for the eye. Selectively shown error bars for the values of
correspond to the confidence level of 0.9. Note that for this data presentation scheme (
is an argument, and
is a function), the slope of the trend line is determined by the ratio
; thus, the critical exponent for the trend value of the voltage drop is estimated as ≈ -1.597.
According to Eq. 4, mutual shifts of the datasets in the coordinate plane can be due to different values of the parameter
for these samples. As follows from the above consideration, this parameter characterizes the rate in the increase of the number of blocked nodes or bonds in the model percolation system that mimics the studied ensembles of interelectrode conductive bridges. Larger values of
should lead to larger values of the first derivative
for the same values of
. Note that there is a certain correlation between the shifts of the datasets towards large values of
and
,
values characterizing the studied samples. The parameter
can be considered as a measure of the total cross-section of interelectrode bridges in the sample. Accordingly, the average current density in the examined samples is proportional to the ratio
.
Figure 11 displays the dataset shifts
along the
axis (
Figure 10) against
; the shifts were estimated with respect to the reference point (
= 1.0⋅10
-3 V/s;
= 10 V, see
Figure 11, b). The values of
are associated with the introduced rate parameter
up to a constant factor (see Eq. 4); thus, it can be concluded that lower average current densities in the samples at stage III cause larger rates of their approach to the percolation threshold. This conclusion may seem rather trivial, but the established features of the influence of geometry of systems, described in terms of the parameter
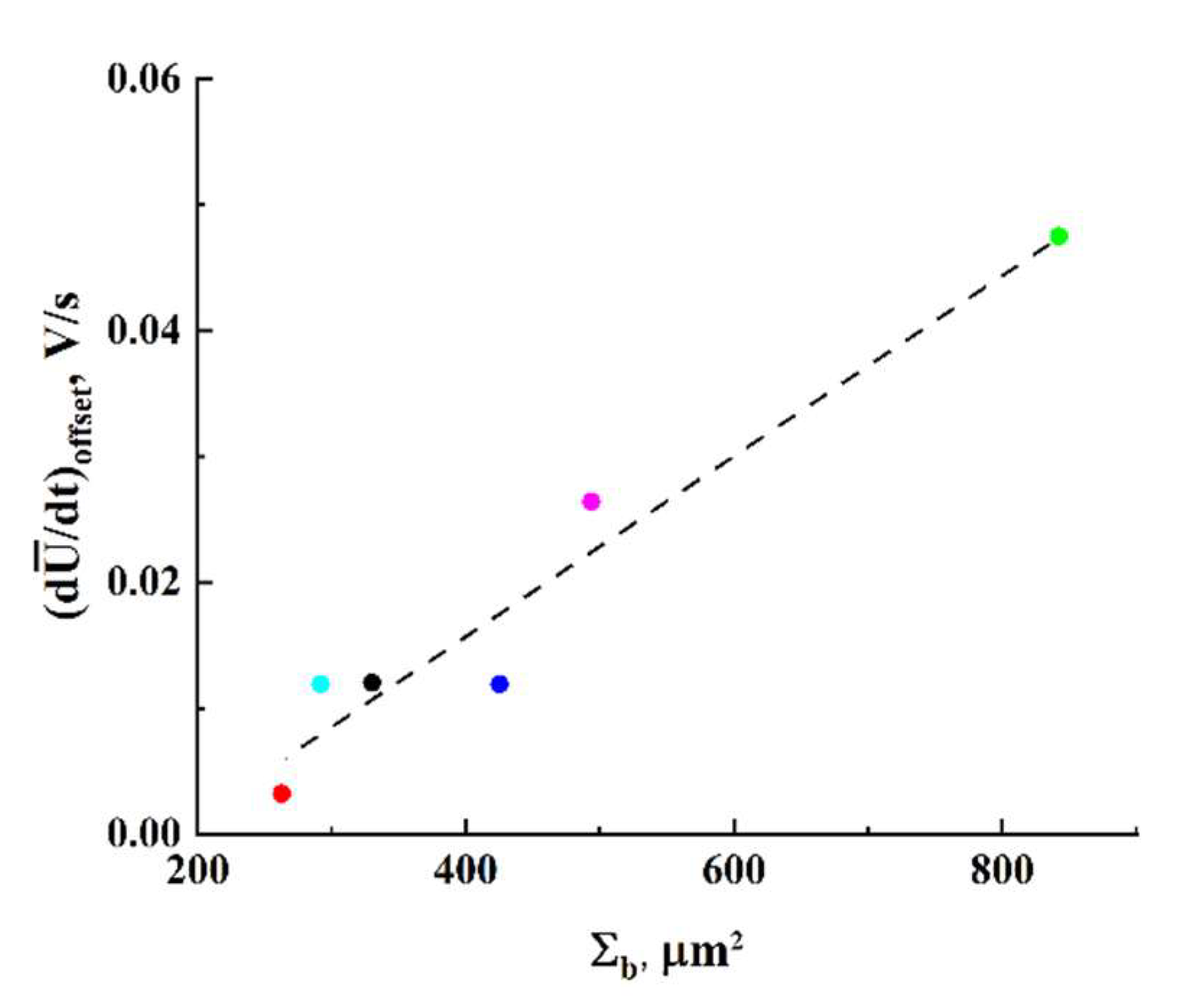
, on the decay of ohmic conductivity can be useful for a deeper understanding of the behavior of such systems near the percolation threshold. In particular, one of these features is a close-to-linear relationship between
and
(
Figure 12).
It is interesting to compare the obtained value of the critical exponent for ohmic conductivity with a variety of currently known similar values, obtained mainly using statistical modeling or other theoretical approaches. Numerous theoretical studies of percolation in 3D resistive grids carried out since the nineties of the last century gave the value of the critical conductivity exponent close to 2.0 (see, e.g. [
20,
21,
22]). These values significantly exceed the value established in accordance with the method under discussion. On the other hand, modeling of percolation in two-dimensional lattices leads to the values of the critical conductivity exponent of the order of 1.3 (see, e.g., [
23,
24]). When comparing the value obtained from our experimental results and the critical exponents obtained for the model 3D and 2D percolation systems, it must be taken into account that the conducting structures (interelectrode bridges) studied in our case are rather transitional between two-dimensional and three-dimensional conducting systems (the form factor
is very small). It should also be noted that a rigorous theoretical analysis of the "conductor-insulator" transition using the transfer matrix approach was carried out in [
25], gave a value of the critical conductivity exponent of the order of 1.54 ± 0.08, which is fairly close to our result.
4.3. Comments on DC Conductivity and Permittivity of the Studied Systems
We consider the following qualitative model of conductivity evolution in the ensembles of interelectrode bridges under the influence of direct current:
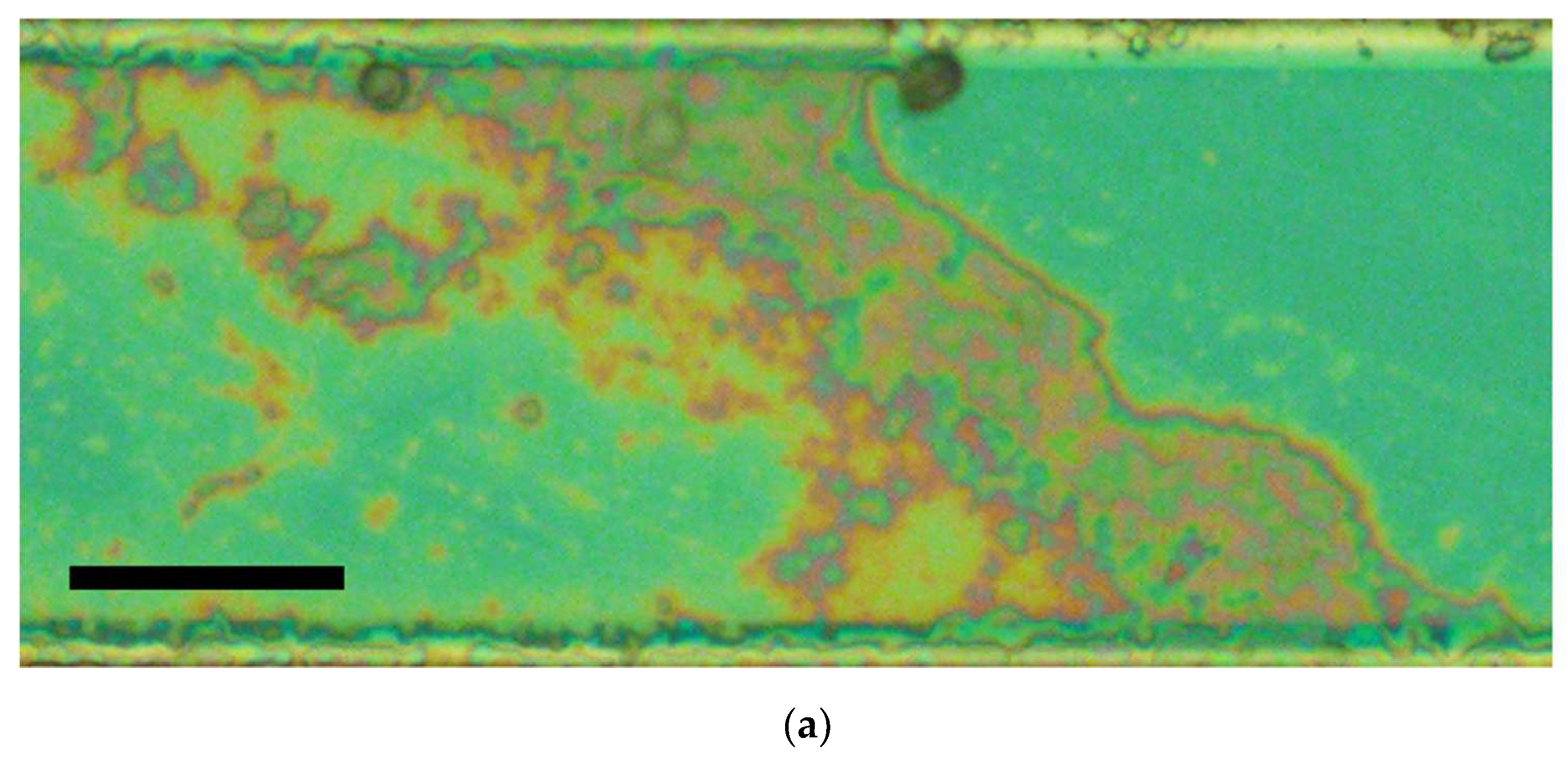
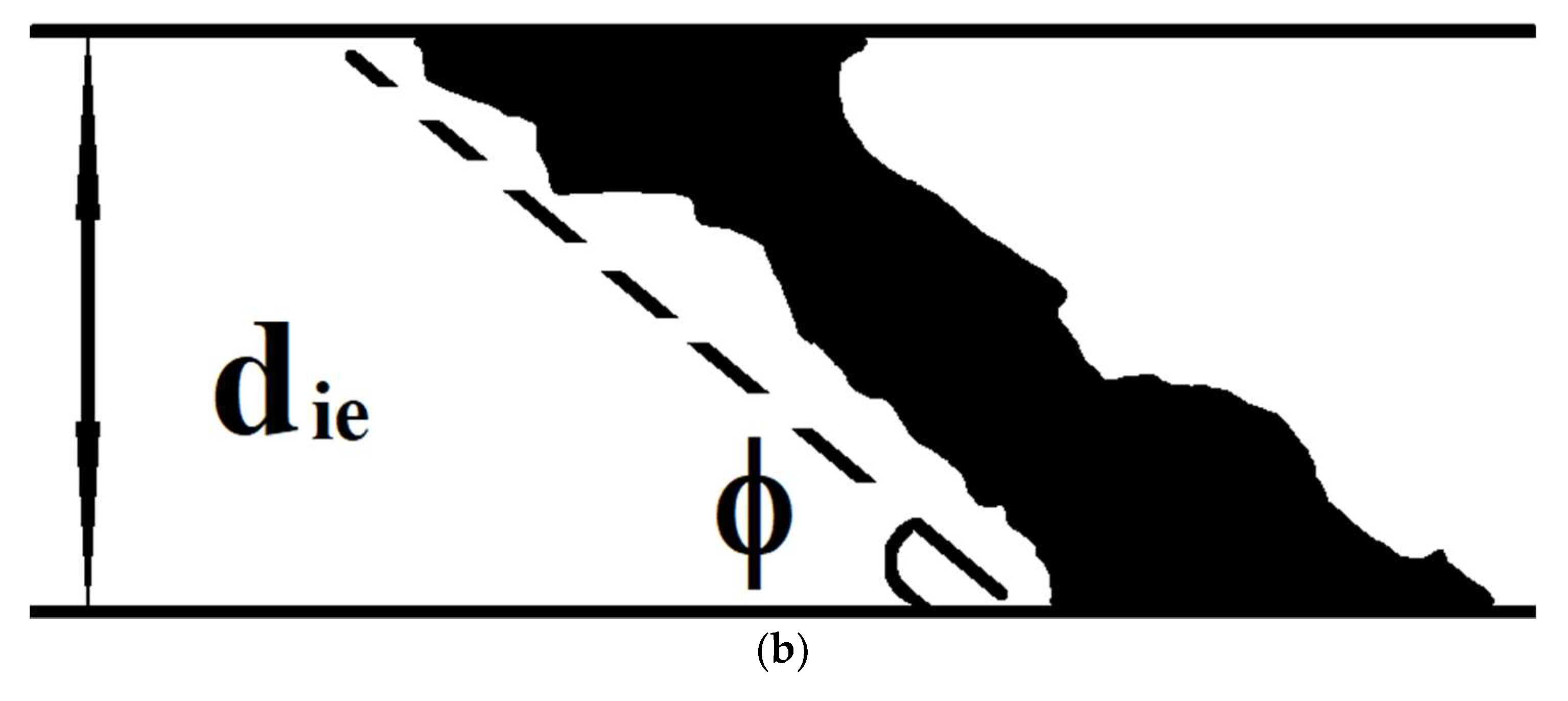
1) during a short-term stage I (see inset in
Figure 8, a), sets of conduction channels (percolation clusters) are formed in the interelectrode bridges (
Figure 13);
2) a long-term quasi-stationary stage II is characterized by a gradual decrease in ohmic conductivity of the bridges (see the dataset 1 in
Figure 10) due to decrease in the number of conduction channels previously formed at a stage I; this effect can be considered in terms of accumulating decrease in the concentration of electrons due to their capture by deep traps in anatase nanoparticles during electron transport in the bridges;
3) as the number of conduction channels in the bridges approaches a critical value corresponding to the percolation threshold, a rapid decrease in ohmic conductivity and, accordingly, an abrupt increase in voltage drops across the studied samples occurs (stage III).
It can be assumed that the characteristic formation time for the sets of conduction channels in the bridges at stage I should correlate with the characteristic time of electron transfer through the bridges. This assumption makes it possible to roughly estimate the electron mobility in the studied structures of close-packed anatase nanoparticles.
Accordingly, we can write the following approximate expression for the relationship between
and the interelectrode distance
:
As
, we take the time interval when the derivative
at stage I decreases (see inset in
Figure 8, a) to a level of 0.05 from the initial value. Thus, electron mobility in the studied structures can be roughly estimated as:
Analysis of initial fragments of
dependencies (stage I) for a group of 5 samples with various
and
but the same interelectrode distance
≈ 44.9 µm gave a range of
as ≈ (6.18 ± 3.69) V⋅s. Accordingly,
for the studied samples was roughly estimated as ≈ (3.72 ± 2.16)⋅10
-6 cm
2/V⋅s. It should be noted that this estimation approach is simplified and does not take into account a variety of influencing factors (e.g., non-uniformity of the electric field in the interelectrode space, and so on). Therefore, strictly speaking, its applicability is limited to estimates of the order of magnitude. Nevertheless, the obtained value of the electron mobility satisfactorily agrees with the value
≈ 5.0⋅10
-6 cm
2/V⋅s for nanoporous anatase, obtained using the measurements of the photoresponse under laser irradiation and presented in [
26]. The specimens studied in this work were obtained by annealing a mixture of 16-nm particles of anatase and turpentine oil on glass substrates and were characterized by the anatase volume fraction of about 50%.
Based on the obtained approximate value of
and typical voltage drops
at the beginning of a quasi-stationary stage II (
Figure 8, a), we can roughly estimate the initial concentration
of conduction electrons in the formed bridge-like structures. Depending on
and
, these voltage drops for the samples of the second group range from 1 to 3 V. The value of
can be expressed as
, where
is the electron charge. Accordingly,
(1.31 ± 0.52)⋅10
18 cm
-3. Taking into account the factor
of filling the volume of bridge-like structures with nanoparticles, we can estimate electron concentration in the nanophase. The approximate value
0.45 was obtained on the basis of volumetric estimations and measurements of
and
. As a result, we arrive to the following estimate for the anatase nanophase:
(2.92 ± 1.15)⋅10
18 cm
-3. This value can be compared to the published experimental data on the concentration of conduction electrons in the undoped anatase (see numerous references and corresponding Table 2 in [
27]). It should be noted that, depending on the preparation technique, these data are strongly scattered in the range from ~1.0⋅10
16 cm
-3 to ~ 9⋅10
19 cm
-3, though, most results fall within the range of ≈ 3.2⋅10
17 cm
-3 to ≈ 2.0⋅10
19 cm
-3. Thus, the obtained value of
seems quite reasonable.
It is interesting to estimate an average number of conduction electrons per one nanoparticle; in our case, this value occurs approximately equal to
24. On the other hand, each nanoparticle is characterized by a certain number of deep traps capable of arresting conduction electrons. According to the photoconductivity data for the nanostructured anatase, the estimated number of traps
per one nanoparticle with the average diameter of 16 nm is of the order of 28 [
28]; so a large value is reasonably explained by a great amount of surface states in anatase nanoparticles. In our case, the average number of traps per particle presumably exceeds this value due to a larger average surface of particles. It is obvious that the ratio between
and
has a crucial influence on degradation of ohmic conductivity in the process of electron transfer in the bridge-like structures. A detailed quantitative analysis of this influence is beyond the scope of this work and is the object of further study.
Consideration of a small but systematic increase in the capacitance of samples resulted from formation of ensembles of interelectrode bridges (see
Section 2) makes it possible to estimate the real part of their initial effective permittivity
. A rigorous quantitative analysis of the influence of
on
is a complicated problem, which is far beyond the scope of this work. Therefore, we applied an approximate approach based on the assumption of a close-to-linear dependence of the capacitance increment
on the ratio of the area covered by the bridges (
) to the total area covered by the electrodes (
). Within this approach, the relative capacitance increment
can be expressed as
. In this expression, the factor
is equal to the ratio of the average height of the bridges to the height of electrodes and takes into account a partial filling of the interelectrode space along the height with the nanoparticles. For a confidence level of 0.9, the ratios
for the samples of the first group are in the range from 0.00524 to 0.01212, and the mean value is ≈ 0.0087. Similarly, the
factor is characterized by the range from 0.0514 to 0.0801 and the mean value of 0.0658. With the average value of
approximately equal to 0.03, we obtained effective permittivity of the examined anatase bridges as ≈ 56.0 ± 17.4. Note that such high permittivity values for the anatase-based structures is not surprising. To verify the obtained data, low-frequency permittivity (in the range from 1 Hz to 10
3 Hz) of the layers of densely packed nanoparticles (the same product #637254 of Sigma Aldrich Inc. as applied for the sample preparation) was measured using the Novocontrol Alpha AN impedance measuring system. The layers were tightly pressed up to the values of the anatase volume fraction of the order of 0.7. The obtained value of the real part of low-frequency permittivity is approximately equal to 70. Applying the Maxwell Garnet model of an effective medium [
29] and considering the anatase phase in the prepared layers as the matrix substance with air inclusions, we can recover permittivity of the bulk anatase as ≈ 115. Evaluation of effective permittivity for the substance of bridges with 0.45 volume fraction of anatase carried out using the Maxwell Garnet model gave
41. This estimate is remarkably less than the above presented mean value (≈ 56) resulted from the capacitance increment analysis. However, it should be noted that systematic errors of the Maxwell Garnet model rise up when the volume fractions of the matrix and inclusions are comparable and their permittivity values strongly differ from each other. In addition, taking into account an abundance of assumptions in the capacitance analysis, we can suggest a satisfactory agreement between these data.
It should be noted that the effect of an anomalous increase in the permittivity of percolation systems near the percolation threshold discussed in a number of works (see, e.g., [
30,
31,
32]) was not observed in our experiments. On the contrary, at the end of the first runs, when ohmic conductivity of the examined samples decreases over short time intervals by two orders of magnitude or more (
Figure 10, a), there is a significant (up to 2–3 times) decrease in
. Accordingly, effective permittivity of the interelectrode bridges falls down by the same number of times. This effect is a direct consequence of localization of a significant number of conduction electrons on the traps, which leads to a significant decrease in polarizability of nanoparticles and, accordingly, of the entire substance forming the bridges. A similar effect associated with localization of photoinduced electrons on the traps during a long-term laser action on anatase nanoparticles in the fundamental absorption band was discussed in [
17].
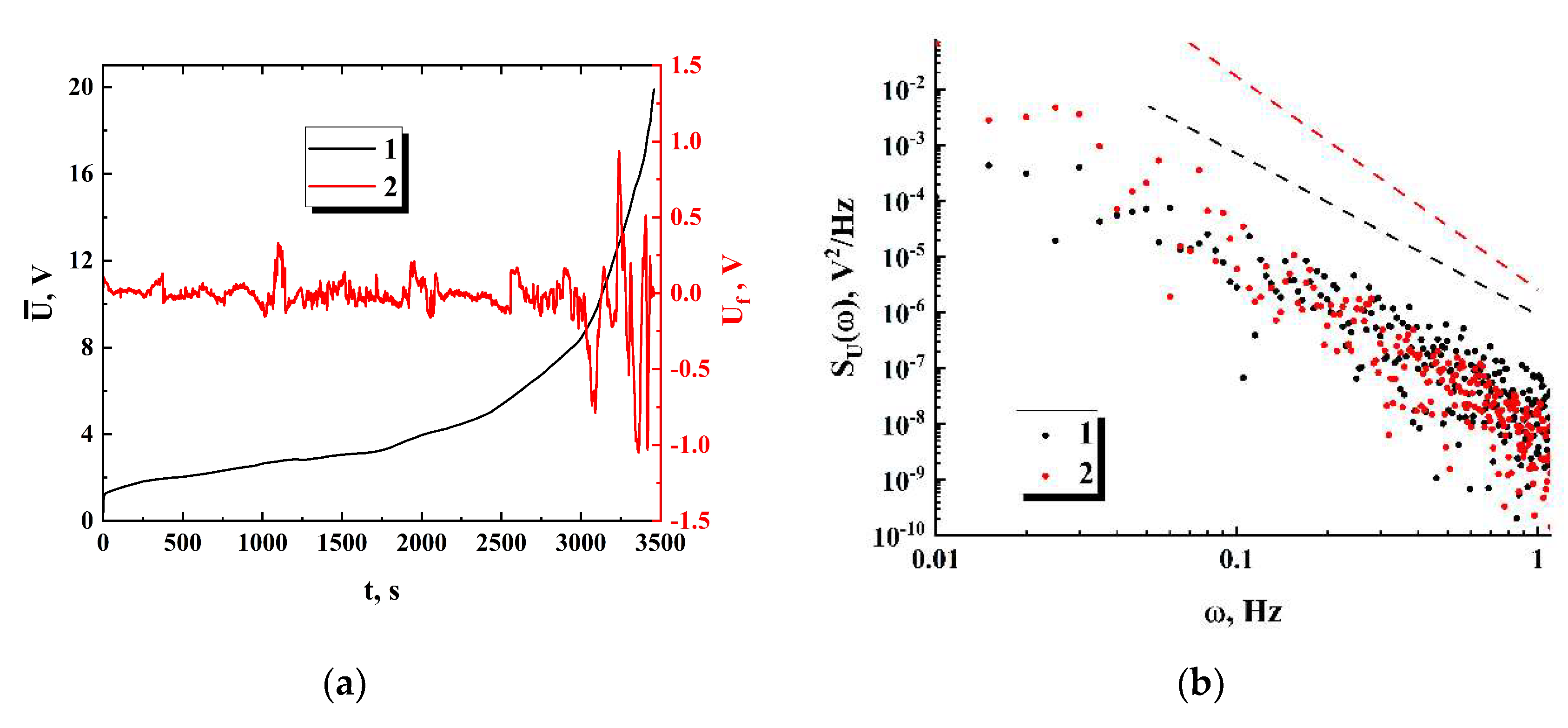
4.4. Spectral Properties of the Noise of Conduction Current
Eq. 2 can be rewritten in the following form:
Here,
is an instantaneous value of conduction current associated with a transfer of conduction electrons between the systems of electrodes of opposite signs. Differentiating Eq. 7 and considering spectral densities of the conduction current, the displacement current and their derivatives, and taking into account the relationships between them, we obtain:
Thus, the spectral density of conduction current fluctuations can be recovered from the spectral density of voltage drop fluctuations applying the renormalization factor
. The power spectra of the voltage drop fluctuations
typically exhibit a decay, which is close to the power law
in the frequency range from 0.01 Hz to 1 Hz (see
Figure 9, b). Accordingly, the spectral exponent of conduction current fluctuations
is in the range of (-1.0 ÷ -0.5) at the quasi-stationary stage and decreases down to (-1.5 ÷ -2.0) at the final stages of the first runs. The behavior
is associated with a classical flicker noise observed in a variety of conducting systems. At the same time, a remarkably high noise level for the examined systems should be noted; the normalized root-mean-square values of conduction current fluctuations at the quasi-stationary stage are typically in the range from ≈ 5⋅10
-3 to ≈ 1.2⋅10
-2. This feature is presumably due to the quasi-stationary dynamics of ensembles of conducting channels in all bridges. Such dynamics manifests itself in the opening of new and blocking of some of the existing channels with minor changes in their total number.
A decrease in the spectral exponent
of conduction current fluctuations down to the values close to -2 indicates emergence of a new mechanism that controls a transfer of mobile charge carriers at the stage of significant conductivity decay. Also, considering a significant increase in the root-mean-square values of
at this stage (
Figure 9, a), it can be assumed appearance of avalanche breakdowns in groups of mutually connected previously blocked channels with a rapid increase in the voltage drop across the samples.