1. Introduction
Cannabis (Cannabaceae) is an annual flowering plant genus, whose taxonomy has always been quite complex and troublesome. According to the most accepted interpretation proposed by Small and Cronquist (1976), the genus is monospecific (Cannabis sativa L.), and includes two subspecies [Cannabis sativa L. subsp. sativa and Cannabis sativa L. subsp. indica (Lam.)] which are in turn characterized by several varieties, conferring a relevant variability to the genus (Faeti et al., 1996; Rapa et al., 2019). Such a genetic diversity has played a key role in the long history of domestication of C. sativa L. and has contributed to provide the crop with special traits, such as great versatility, environmental sustainability, prompt adaptation to different environmental conditions, and application in several fields (Cerino et al., 2021). In such a complex scenario, the main discrimination factor among the varieties is the content of the psychoactive molecule (-)-trans-Δ9-tetrahydrocannabinol (Δ9-THC, or simply THC), which allows to discriminate the drug-type specimens (i.e., marijuana) from the fiber-type plants (i.e., industrial hemp) (Sgrò et al., 2021). Whereas market has notoriously focused on industrial hemp to produce fiber and hurds for various applications (e.g., textiles, paper, green building, bioplastics, bioenergy etc.) (Ranalli & Venturi, 2004), hempseeds -whose estimated production is of 6000 t per year in the EU (Karus and Vogt, 2004)- have been undervalued and treated as a waste product (Karus and Vogt, 2004; Farinon et al., 2020). However, hempseeds are a valuable source of essential fatty acids, minerals, dietary fiber, vitamins, phenolics and, not least, essential aminoacids contained in the highly digestible proteins edestin and albumin (Callaway, 2004; Kriese et al., 2014; Farinon et al., 2020; Sorrentino, 2021). Hence, encouraged by the growing awareness of their unique nutritional profile and health benefits, new niche markets have recently emerged, including the segment of the hempseed-based food (Karus and Vogt, 2004).
The cold-pressed hempseed oil successfully fits with the growing consumer interest in those natural cold-pressed oils which are often considered as functional foods, for the various health benefits provided over the refined counterparts (Kostadinovic & Mitrev, 2013; Verhé et al., 2006). Furthermore, the production of such seed oil turns out to be in line with the uprising trend of the oil industry of seeking for processes that can minimize the environmental impact, decrease toxic residues, and use by-products more efficiently without compromising the nutritional and organoleptic quality of the final product (Cicero et al., 2018; Albergamo et al., 2020). According to the literature, this edible oil exhibits up to 80% of polyunsaturated fatty acids (PUFA), mainly including the ω-6 linoleic acid and the ω-3 α-linolenic acid (Siudem et al., 2019), as well as the γ-linolenic acid, which is typical only of certain seed oils, and the stearidonic acid, which occurs only in few plant families (Matthäus et al., 2008), and whose dietary intake is beneficial to the consumer health, as it promotes the synthesis of the eicosapentaenoic acid more effectively than α-linolenic acid (Lemke 2013). Minor bioactive compounds retained during the cold pressing (e.g., fat-soluble vitamins, phytosterols, polyphenols, pigments, inorganic elements, etc.) have been also highlighted. Depending on the type of compound, they may be beneficial to consumer’s health (Blasi et al., 2022), improve the antioxidative status of the oil (Izzo et al., 2020; Castelo-Branco et al., 2016; Liu et al., 2020), as well as accelerate its oxidation and quality deterioration (Liang et al., 2018).
Indeed, owing to the low degree of processing of the raw material, cold-pressed oils contain antioxidants (i.e., tocopherols, polyphenols, and squalene) effective against the lipid oxidation caused by the high PUFA content (Grosshagauer et al., 2019; Blasi et al., 2020), as well as pro-oxidant components (i.e., metals, chlorophylls, and lipid peroxides) responsible for a lower oxidative stability of such oils compared to their refined counterparts (Chloe et al., 2006). On this basis, the cold-pressed hempseed oil tends to have an even higher initial oxidation state than other cold-pressed seed oils (e.g., rapeseed and sunflower), due to a very high unsaturation degree and a large amount of photosensitizer chlorophylls, making it more susceptible to the formation of off-flavors and substances harmful to consumer health (Matthäus et al., 2008; Grosshagauer et al., 2019). For these reasons, the cold-pressed hempseed oil necessitates storage in dark or opaque containers. However, to enhance its appeal and consumer acceptance, it is generally marketed in transparent bottles, which inevitably lead not only to an unpleasant color change but also to a faster oxidative deterioration and rancid flavor (Matthäus & Brühl, 2008, Aachary 2016).
Few recent attempts focused on ameliorating the oxidative stability of the cold-pressed hempseed oil and they spanned from the addition of natural antioxidants to the oil (Moczkowska,), the optimization of the storage conditions (Tura et al., 2022), to the improvement of the oil processing (Özdemir et al., 2021Aachary et al. (2016) Liang et al. (2018). With regard to the last aspect, ultrasound bleaching has proven to be convenient, because it prevents the lipid oxidation catalyzed by pigments, by removing the chlorophylls typically abundant in the hempseed oil. However, according to the Codex Alimentarius Standard 210-1999 and Amendments (2003/2005), the cold-pressed oil should be obtained only by mechanical processes, such as squeezing or pressing, without the application of heat, and it should be purified only by washing with water, settling, filtering, or centrifuging. Therefore, as an alternative, sustainable and quick approach to pigment removal, the cold-pressed hempseed oil may go through filtration.
To the best knowledge of the authors, the effect of filtration on the compositional characteristics and the oxidative status of cold-pressed oils has been unfairly underexplored (Van Hoed et al., 2011). Hence, aim of this study was to comprehensively evaluate the effectiveness of filtration on the oxidative stability and minor compounds of the cold-pressed hempseed oil during a 12 week storage in transparent glass bottles. To this purpose, the hydrolytic and oxidative status, fatty acid (FA) composition, tocopherols, pigments, phenols, squalene, and metals were monitored in experimental filtered and non-filtered oils over the study period, statistically elaborated, and discussed to evaluate the effectiveness of the filtration process on the cold-pressed hemp oil, as well as its convenience for future commercial applications.
2. Materials and Methods
2.1. Samples
Virgin cold-pressed hempseed oils were provided in 2022 by Sativa Molise (Italy) and they were obtained from seeds of C. sativa L. subsp. Sativa cv. Finola. The seeds were mechanically separated from inflorescences (~95% pure seed and ~5% dockage), dried at 25±2°C up to a humidity of ~8–10%, packaged in polyethylene bags, and stored in a dry room (21±3 °C, 20-30% RH) for a maximum of two weeks. Then, seeds were cold pressed in an expeller press with a mean temperature of 50 °C and, in the case of filtered oil samples, they were filtered within 24h of pressing through a filter press equipped with cellulose acetate membranes (thickness: 0.81 mm) and operating with a pressure of 6 bar. Non-filtered and filtered hempseed oils (respectively, NF-HO and F-HO) were separately blotted in triplicate by employing transparent glass bottles of 100 mL each with screw caps (~3% headspace), for a total of n=12 NF-HO bottles and n=12 F-HO bottles. Under these experimental conditions, the oil oxidation may be attributable to the autoxidation -occurring just with atmospheric oxygen (3O2) - and photooxidation -related to the presence of light, sensitizers, and 3O2.
Changes in the oxidative state and minor compounds of various oil samples were monitored during 12 weeks of storage by keeping all bottles in a controlled environment at room temperature (22±1.2 °C), under a 12/12 h light/dark regime and rotating them every 10 days (Presca et al., 2014; Tura et al., 2022). Hence, three bottles from every treatment were considered at the beginning of the experimental trial (T0) and every 4 weeks (T4, T8 and 12), and the oil from each bottle was analyzed in triplicate.
2.2. Materials and reagents
Solvents with reagent grade (i.e., n-heptane, n-hexane, diethyl ether, and methanol) were purchased from J.T. Baker (Phillipsburg, NJ, USA), while solvents with HPLC grade (i.e., n-hexane, ethyl acetate, methanol, and water) were supplied by LiChrosolv (Merk, Darmstadt, Germany). Reagents with a trace metal analysis grade [i.e., H2O2 (30% v/v) and HNO3 (65% v/v)] and ultrapure water (resistivity of 10 mΩ cm) were purchased from J.T. Baker (Milan, Italy). The Folin–Ciocalteu reagent was from Sigma-Aldrich (Steinheim, Germany). Fatty acid methyl esters (FAMEs) reference standards (C4–C24) and commercial standards of single tocopherols (i.e., α-tocopherol, γ-tocopherol, and δ-tocopherol, 98% purity each), squalene, gallic acid (≥99% purity), squalene (≥98% purity) were purchased from Supelco (Bellefonte, PA, USA). Stock solutions of Na, Mg, K, Fe, Cu, Mn, Zn, Se, Ni, Cr, Al, As, Cd, and Pb (1000 mg/L in 2% HNO3, each) were provided by Fluka (Milan, Italy). Depending on the targeted analyte, the internal standards employed for the normalization of calibration procedure were: tetradecane (99% purity, Sigma Aldrich), and rhenium (1000 µg/mL in 5% HNO3, LGC Standards)
2.3. Physicochemical properties
For the determination of free acidity and peroxide value, the procedures already reported in Costa and colleagues (2016) were followed. For the acidity, 90 mL of a solution of ethyl alcohol/diethyl ether (1:2,
v/
v) was mixed with few drops of 1% phenolphthalein and subsequently neutralized with 0.1N KOH. The mixture was then added with 5 g of oil sample and titrated with 0.1N KOH until color changed. The acidity was calculated according to the following equation and expressed as % of oleic acid:
where 𝑉 is the volume of titrant (mL of KOH), 𝑁 is the normality of KOH (0.1), MW
OA is the molecular weight of oleic acid (282 g/mol), and Ws is the weight of oil sample (g).
For the determination of peroxide value, 25 mL of a solution of glacial acetic acid/chloroform (3:2,
v/
v) was mixed with 500 𝜇L of a saturated KI solution. After vigorously shaking, the solution was allowed to stand in the dark for ~5 min. Then, 75 mL of distilled water and starch indicator were added to the mixture and a titration with 0.01N Na
2S
2O
3 was conducted until color changed. The peroxide value, defined as milliequivalents of reactive oxygen content per 1 kg of oil sample (mEq/O
2/Kg), was derived by following the equation:
where 𝑉 is the volume of titrant (mL of Na
2S
2O
3), 𝑁 is the normality of Na
2S
2O
3 (0.01), and Ws is the weight of oil sample (g).
Finally, the spectrophotometric exam was conducted by measuring specific UV absorbances at 232 and 270 nm and expressing them as extinction coefficients K232 and K270.
2.4. FA composition
For the elucidation of the FA profile of hemp oil samples, the protocol already employed by Sdiri et al (2020) was considered. Approximately, 0.1 g of hemp oil was mixed with 2 mL of n-heptane and 0.2 mL of methanolic KOH solution for 30 s at room temperature, and decantated. Then, the upper layer containing FAMEs was injected into a gas chromatograph (GC) (Dani Master GC1000) equipped with a split/splitless injector and a flame ionization detector (FID) (Dani Instrument, Milan, Italy). For the chromatographic separation, a SLB-IL100 capillary column (60 m × 0.25 mm ID, 0.20 μm film thickness, Supelco, Sigma Aldrich, USA) was employed with the operating conditions: column oven temperature from 165 °C to 210 °C at 2 °C/min (10 min hold); injector and detector temperatures 250 °C; He gas at a linear velocity of 30 cm/s (constant); injection volume was 1 μl, with a split ratio of 1:100. Data acquisition and handling was performed using Clarity Chromatography Software v4.0.2. FAMEs of nutritional interest were identified by direct comparison with the retention times of reference compounds and expressed as relative percent area of the total chromatogram.
2.5. Tocopherols
For the determination of α-, γ-, and δ-tocopherols, ~200 mg of oil sample was diluted in 1.8 mL of n-hexane, filtered through a 0.20 μm PTFE syringe filter and analyzed by a high-performance liquid chromatography system coupled to fluorescence detector (HPLC/FD, Shimadzu, Milan, Italy) according to the conditions already reported by Albergamo et al. 2022. Specifically, the chromatographic separation was carried out by a LiChrosorb® Si60 column (250 mm × 4.6 mm I.D., 5 µm particle size, Merck, Darmstadt, Germany), protected by a LiChroCART 4-4 guard column with the same stationary phase (Merck, Darmstadt, Germany), and by exploiting a mobile phase consisting of n-hexane/ethyl acetate (90:10 v/v), under isocratic conditions. HPLC/FD analyses were performed at 40 °C, with an injection volume of 20 µL and a flow rate was 0.8 mL/min. Data processing occurred by the LabSolutions software ver. 5.10.153 (Shimadzu). The identification of tocopherols was carried out by a direct comparison with the retention time of relative commercial standards at respective excitation and emission wavelengths of 295 nm and 330 nm. The quantitative analysis was performed by constructing appropriate external calibration curves for every investigated tocopherol.
2.6. Pigments and polyphenols
For the determination of chlorophyll (Chl) a and chlorophyll (Chl) b and total carotene the protocol of Blasi et. al, 2022 was considered. Briefly, 1 g of every oil sample were mixed with 50 mL diethyl ether, vortexed and sonicated for 1 min. The absorbance of solutions was measured by an UV spectrophotometer (UV-2401 PC, Shimadzu, Milan, Italy). Chl a and Chl b showed the maximum absorbances at 663 nm (A663) and 640 nm (A640), respectively, while total carotene content was determined at 470 nm (A470). The concentration (μg/mL) of these pigments was calculated according to the formulas proposed by Izzo et al., 2020:
For the extraction of polyphenols, approximately 6 g of oil sample were mixed with 6 mL of a methanol/water solution (80:20, v/v), stirred for 2 min and kept at room temperature until phase separation. Then, the colorimetric assay was conducted according to what already reported by Aghraz et al., 2020. Specifically, 0.2 mL of the upper part of the mixture was collected and 1.8 mL of distilled water was added along with 8mL of Na2CO3 (20%) and 10 mL of Folin–Ciocalteu reagent. The mixture was kept in the dark for 30 min and read at 700 nm with an UV-visible spectrophotometer (UV-2401 PC, Shimadzu, Milan, Italy). The quantification procedure occurred through an external calibration curve of gallic acid, and the total phenol content was calculated as milligrams of gallic acid equivalent in 1 L of hemp oil (mg GAE/Kg).
2.7. Squalene
Squalene was extracted from every oil sample by means of a solid phase extraction (SPE) exploiting Supelco Discovery DSC-Si Silica cartridges and n-hexane and analysed by a gas chromatography system (GC-2010, Shimadzu, Japan) coupled to a single quadrupole mass spectrometer (QP-2010 Plus, Shimadzu, Japan) according to the protocol reported in Vadalà et al. 2023. Chromatographic separations occurred on a SPB-5MS capillary column (30 m × 0.25 mm i.d. × 0.25 μm film thickness, Supelco, USA). The oven temperature program was: from 80 °C (1 min hold) to 140 °C at 20 °C/min, and finally to 290 °C (2 min hold) at 5 °C/min. The injection port temperature was set at 250 °C and the injection volume was 1 μL, with a split ratio 1:10. The MS conditions were: EI source temperature 230 °C; ionization energy and emission current, 70 eV and 250 μA respectively; interface temperature 290°C. The identification occurred in full scan (mass range: 40–400 m/z) by comparing both retention time and mass spectrum with those of commercial standard; while quantification was performed in selected ion monitoring (SIM), by monitoring four characteristic mass fragments (121, 137,161 and 175 m/z). Hence, the amount of the compound was derived by considering the relative base peak ion and exploiting the internal standard normalization with the internal standard tetradecane.
2.8. Element analysis
Around 0.5 g of each oil sample were mineralized with 8 mL of HNO3 and 2 mL of H2O2 by a microwave digestion system (Ethos 1, Milestone, Bergamo, Italy) with a temperature of 0–200 °C in 10 min (step 1), and 200 °C held for 10 min (step 2), and a power of 1000 W. Digested samples were cooled down at room temperature and properly diluted with the internal standard Re in ultrapure water. Elemental analyses were carried out by a quadrupole ICP-MS iCAP Q (Thermo Scientific, Waltham, MA, USA), equipped with an ASX-520 autosampler (Cetac Technologies Inc., Omaha, NE, USA). Samples were screened in triplicate for selected elements according to the procedure reported in Ben Amor et al (2021), Bua et al. (2017), by using the following operating parameters: incident radio frequency power equal to 1500Wand plasma, auxiliary, and carrier gases (Ar) at respective flow rates of 15 L/min, 0.9 L/min and 1.10 L/min. The instrument operated in He collision mode (4 mL/min) and with a spray chamber set at +2 °C. The injection volume and the sample introduction flow rate were respectively, 200 µL and 1 mL/min. Spectra acquisition was performed in full scan mode (dwell time 0.5 or 0.01 s/point, depending on the analyte). For quantification purposes, an external calibration procedure combined with an internal standard normalization was exploited. Instrumental control and data acquisition were performed by Thermo Scientific Qtegra™ Intelligent Scientific Data System software.
2.9. Statistical analysis
Data were statistically analyzed by R studio v. 3.6.1 (Boston, MA, USA) for Windows. A descriptive analysis, including mean and standard deviation, was conducted for all the experimental data obtained from this study. After running a Shapiro–Wilk test to verify the normal distribution of experimental data, every parameter analyzed in all oil samples was statistically elaborated by i) the one way-ANOVA followed by a post-hoc Tukey’s HSD to study the effect of storage and highlight significant differences in NF-HO samples (or F-HO samples) during T0-T12, and ii) two-tailed Studentʼs t-test for unpaired data to evaluate the effectiveness of filtration and point out significant differences between NF-HO and F-HO samples. Statistical significance was accepted at p≤ 0.05.
3. Results
Although the virgin cold-pressed hempseed oil is already available on the market and increasingly appreciated by consumers, its regulatory framework in the EU is ambiguous due to the lack of regulation on quality and compositional requirements for its marketing (Izzo et al., 2020). To date, only the Codex Alimentarius (CODEX STAN 19-1981) has established certain guidance values, but they relate to the wide and varied category of edible fats and oils not covered by individual standards, and they are expressly intended for voluntary application by commercial partners, not by governmental agencies.
3.1. Hydrolytic and oxidative status of cold-pressed hempseed oils
The hydrolytic and oxidative status of cold-pressed hempseed oil was explored in NF-HO and F-HO samples (
Figure 1) and compared with the available quality parameters fixed for edible fats and oils not covered by individual standards by the Codex Alimentarius for free acidity (2% of oleic acid or 4 mg KOH/g of oil) and peroxides (15 mEqO
2/kg of oil).
Free fatty acids are more susceptible to autoxidation than esterified fatty acids and they also stimulate the hydrolysis of phenolics (Choe and Min, 2006, Lercker, Frega, Bocci, & Servidio, 1994), thus, contributing to the deterioration of shelf life of the edible oil. At the beginning of the experimental trial, fresh NF-HO and F-HO samples were characterized by similar and acceptable acidities (respectively 0.87% and 0.90%). By exploiting the highest formation rate during the first 4 weeks of storage (NF-HO: +54%; F-HO: +49%), free fatty acids became higher, but still within the Codex limits, in both types of oil (NF-HO: 0.87-1.87%, p<0.05; F-HO: 0.90-1.77%, p<0.05), in agreement with the recent literature, which, however, has also reported fresh oils with a very high acidity (Tura et al., 2022, 2023; Lucini et al., Izzo et al.). With the advancement of storage (T4-T12), lower formation rates were recorded and only NF-HO reported acidities higher than the Codex guide value, the free acidity of NF-HO and F-HO samples being respectively from 1.87% to 4.30% (p<0.05) and from 1.77% to 1.93% (p<0.05) (
Figure 1a,b). Overall, the NF-HO showed a worse hydrolytic quality than F-HO (mean acidities: 2.52% and 1.60%, p<0.05), thus suggesting an ameliorative effect of filtration on the oil acidity (
Figure 1c).
Notoriously, proper seed storage conditions -in terms of humidity, light and temperature- as well as a good seed quality -in terms of moisture level and percent dockage- are essential requirements to produce an oil with a favorable hydrolytic status (Jian et al., 2019). However, according to the results of this study, also the filtration had a positive effect on the acidity of the cold-pressed hempseed oil during storage, probably because less water was dispersed in filtered oil samples. This would be consistent with a recent work of Tura and colleagues (2022), where stable and compliant acidities were recorded in cold-pressed hempseed oils subjected in laboratory to cotton gauze filtration and centrifugation before the storage in amber glass bottles for 90 days. Also, Fregapane et al. (2006), reported that filtration reduced the rate of hydrolysis of the triacylglycerol matrix with positive effects on the oxidative stability of the virgin olive oil. However, on the other hand, Frega et al. (1999) suggested that filtration increased the oil susceptibility to oxidative degradation by removing suspended solid materials. From their point of view, suspended solids and free fatty acids may react to form a precipitated residue that is not capable of oxidative reaction.
The peroxide value is indicative of the total peroxidic bonded oxygen present in an oil, and, consequently, of its oxidative status especially at the early stages of storage. As a result, lipid peroxyl radicals and hydroperoxides are typically defined as primary oxidation products. This parameter can vary in relation to the oxygen partial pressure in the headspace, the type of oxygen (i.e.,
1O
2 is much more reactive with lipids than
3O
2), temperature (i.e., the solubility of oxygen in oil decreases as the temperature increases), light (i.e., light promotes production of
1O
2 in presence of sensitizer pigments and
3O
2), and minor oil components (i.e., metals, free fatty acids, mono- and diacylglycerols, phospholipids generally accelerate oil oxidation). Fresh NF-HO and F-HO samples had similar and acceptable peroxides values (respectively 7.98 mEqO
2/kg and 8.21 mEqO
2/kg). However, similarly to the acidity, they grew within 4 weeks of storage in both sets of samples (NF-HO: 7.98-14.39 mEqO
2/kg, p<0.05, and F-HO: 8.21-12.64 mEqO
2/kg, p<0.05), while still remaining lower than the Codex value. Values from our fresh hempseed oils can be hardly compared with literature, since peroxides quickly react to generate other radical forms, thus, showing a considerable fluctuation even in the same oil (Tura et al., 2022, + acc. Storage +23; Preska et a Izzo et al. 2020). Interestingly, during the first 4 weeks of storage, the highest rate of formation of peroxides was observed both in NF-HO (+45%) and F-HO (+35%) samples. This can be interpreted as a trigger effect generated by the initial and variable level of
3O
2 dissolved in the oil during the production and bottling phases (Parenti et al. (2007).With the advancement of storage (T4-T12), however, peroxides of NF-HO samples became higher than the Codex guidance value, the peroxide value of NF-HO and F-HO samples varying respectively from 12.82 mEqO
2/Kg to 16.85 mEq O
2/Kg (p<0.05) and from 7.64 mEqO
2/Kg to 13.38 mEq O
2/Kg (p>0.05) (
Figure 1a,b). Overall, the effectiveness of filtration on oil peroxides is supported also by the fact that F-HO showed a better oxidative status than NF-HO (mean peroxides: 12.18 mEqO
2/Kg and 15.49 mEqO
2/Kg, p<0.05) (
Figure 1c).
Specific extinction coefficients (K) at the UV wavelengths of 232 nm and 270 nm are helpful in studying the progress of autoxidation of a vegetable oil. In fact, when hydroperoxides are formed, double bond shifting and isomerization occur, producing primary oxidation products such as conjugated dienes, that exhibit an intense absorption at 232–234 nm, and subsequently conjugated trienes, that typically adsorb at 268–270 nm (Lukesova et al 2009). At this point, the decomposition of conjugated systems into secondary oxidation products (i.e., aldehydes, ketones, alcohols, and short-chain hydrocarbons) is commonly observed. According to our results, the extinction K
232 raised from 1.88 to 4.10 (p<0.05) in NF-HO and from 2.41 to 2.87 (p<0.05) in F-HO. Similarly, K
270 of NF-HO and F-HO samples increased respectively in the range 0.74-0.81 (p<0.05) and 0.53-0.71 (p<0.05). In line with previous studies on the hempseed oil, both NF-HO and F-HO were characterized by K
232 directly correlated with the evolution of peroxides (Tura et al., 2022; Liang et al 2008). Moreover, similarly to peroxides, K
232 and K
270 showed the highest increase during the first 4 weeks of storage both in NF-HO (+43% and +36%) and F-HO (+32% and +30%) samples. Subsequently, a steady state of oxidation was observed during storage. In fact, K
232 and K
270 were not significantly different both in NF-HO and F-HO during T4-T8 (p>0.05), probably due to the establishment of an equilibrium between the formation of diene systems and their decomposition into secondary products, such as hexanal, 2-decenal, or 2-heptenal potentially responsible for the off-flavour of the oxidized oils (Nyam et al., 2013). From the discussed data, a weak effect of filtration on the formation of dienes and trienes in the hempseed oil during storage may be argued. This is also suggested by the fact that NF-HO had slightly higher conjugated dienes (mean values: K
232= 3.25 vs. 3.12, p>0.05) and trienes (mean values: K
270=0.74 vs. 0.70, p>0.05) than F-HO (
Figure 1c). A possible explanation of the different oxidative behavior of hempseed oils is that filtration may reduce all those minor oil components that may cause the initial increase in peroxides and conjugated systems by various mechanisms, such as autoxidation (i.e., free fatty acids), increase of the rate of oil oxidation (i.e., transition metals), and increase of the diffusion rate of oxygen from the headspace into the oil (i.e., free fatty acids, mono- and diacylglycerols, and phospholipids) (Choe and Min, 2006). Consequently, a greater presence of these compounds in NF-HO samples would be indicative of a lower stability against the oxidative degradation. Additionally, NF-HO would be more affected by the light from the use of transparent bottles than F-HO, since light may trigger
1O
2 oxidation pathways and, consequently, further increase the level of primary oxidation products (Choe and Min, 2006, Okogeri et al., 2002).
Conflicting conclusions on the influence of filtration and storage on the oxidative stability of the oil can be drawn from literature. A study from Shendi et al., 2020 was consistent with our findings, since peroxides, K232 and K268 significantly differed in filtered and non-filtered olive oils during two years of storage in amber glass bottles, with the highest values observed in non-filtered oil samples at early months of storage. Liang and colleagues (2008) did not employ filtration but an ultrasonic bleaching to reduce the amount of prooxidant components in the hempseed oil, and they found that such a treatment had an impact on the oxidative oil stability, as it significantly delayed the increase both in peroxide value and conjugated diene systems, compared to untreated control oils during accelerated storage in colored glass containers. However, filtration also showed to have no effect on oil peroxides, dienes and trienes (Tsimidou et al., 2005, Guerrini et al., 2020; Fregapane et al., 2006; Brkić Bubola et al., 2017) or even accelerate the formation rate of such compounds (Fortini et al 2016; Sacchi et al., 2015; Brkić Bubola et al., 2017) in olive oils.
3.2. FA composition
Table 1 reports the profile of the most nutritionally relevant FAs present in NF-HO and F-HO samples stored during 12 weeks in transparent glass bottles.
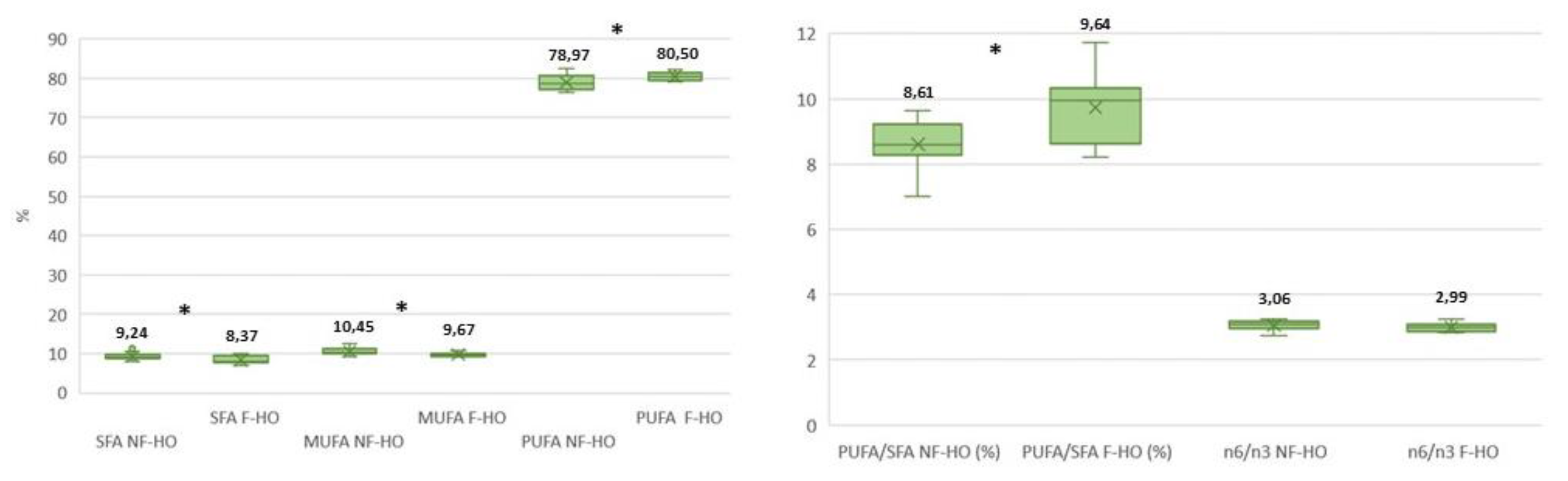
Minimal differences were recorded in single FAs of NF-HO and F-HO samples at T0. However, major classes of SFA and MUFA were more concentrated in NF-HO than F-HO (SFA: 10.44% and 9.72%, MUFA: 11.35% and 10.18%); conversely, PUFA were higher in F-HO than NF-HO (81.87% and 80.74%). During storage of NF-HO samples, no significant variations were detected between the initial (T0) and final (T4) percent content of most of single FAs. In fact, only the palmitic and linoleic acids decreased significantly over 12 weeks of storage (i.e., 7.20-5.87% and 55.56-52.36%, p<0.05) and, in parallel, total SFA and PUFA reduced significantly from 10.44% to 8.27% (p<0.05) and from 80.74% to77.71% (p<0.05). Accordingly, the n-6/n-3 ratio lowered from 3.07 to 2.07 (
Table 1). Conversely, in F-HO samples, while most FAs decreased, the linoleic acid remained stable (56.27-54.29%, p>0.05) and the stearidonic acid increased (1.02-1.76, p<0.05) during the study period. Accordingly, SFA, MUFA, PUFA and n-6/n-3 significantly decreased in the respective ranges 9.72-7.80% (p<0.05), 10.18-9.29% (p<0.05), 81.87-80.06% (p<0.05) and 3.05-2.84 (p<0.05).
As shown in
Figure 2, the filtration process impacted the FA composition. In fact, SFA and MUFA were on average more abundant in NF-HO than F-HO (respectively, 9.24% and 8.37%, p<0.05; 10.45% and 9.67%, p<0.05). Conversely, PUFA and PUFA/SFA were lower in NF-HO and higher in F-HO (respectively, 78.97% and 80.50%, p<0.05; 8.61% vs. 9.64%, p<0.05).
Overall, the FA composition of the cold-pressed hempseed oil, revealed in both NF-HO and F-HO sample, was within the ranges reported by recent literature, which already highlighted high levels of linoleic (50-80%) and α-linolenic (15-25%) acids in the hempseed oil (Baldini et al., 2018; Esmaeilzadeh Kenari and Dehghan, 2020; Farinon et al., 2020), as well as a peculiar amount of the stearidonic acid (0.5-1.5%) (Mikulcová et al., 2017). In addition, oil from this study belonged to the Finola hemp variety, which has already been shown to contain up to 2% stearidonic acid and up to 4% γ-linolenic acid. [Golimowski 22 23, Oomah 2022).
Vegetable oils with a high unsaturation degree are characterized by a greater formation rate and amount of primary oxidation compounds accumulated over time. The rate of autoxidation and 1O2 oxidation depend on the rate of the lipid alkyl radical formation, which in turn depends mainly on the type of FA (Choe Min). In this respect, unsaturated FAs, such as oleic, linoleic, and linolenic acids, are particularly prone to oxidation and consequently more easily degraded in an oil (Madhujith and Sivakanthan, 2018). This would explain the reduction of MUFA and PUFA observed in the hempseed oils during 12 weeks of storage and, in general, the lower oxidative stability of high PUFA oils (Prescha et al., 2014). The factor “light” must also be considered during storage, since it is well-known that the oxidation of unsaturated FAs is accelerated by exposure to light especially when pigments, such as chlorophylls, are present in the oil (Rastrelli et al). The influence of light on the FA composition of hempseed oil has not been investigated in any study. However, Rastrelli and coworkers (..) found out that the sum of linoleic and α-linolenic acids decreased more significantly in the clear glass oil bottles than the in the dark ones, during 1 year of storage. Therefore, under our experimental conditions, both the oxygen dissolved in the oil and the light may contribute to the decreasing stability of unsaturated FAs in the hempseed oil.
Considering the filtration process, no significant differences in single FAs were revealed between F-HO and NF-HO, thus, confirming that oil treatments do not cause drastic changes in the FA profile of a vegetable oil (Pal, Pestana,Aluyor). However, Golimowsky () recently applied a low-temperature bleaching to the cold-pressed hempseed oils with positive effects on the major classes of FAs rather than single FAs, as the oils displayed a reduced SFA proportion and a consequent growth in the proportion of PUFA and SFA/PUFA ratio. Accordingly, results from this study pointed out that filtration may better preserve MUFA and PUFA of the cold-pressed hempseed oil during storage.
3.3. Tocopherols
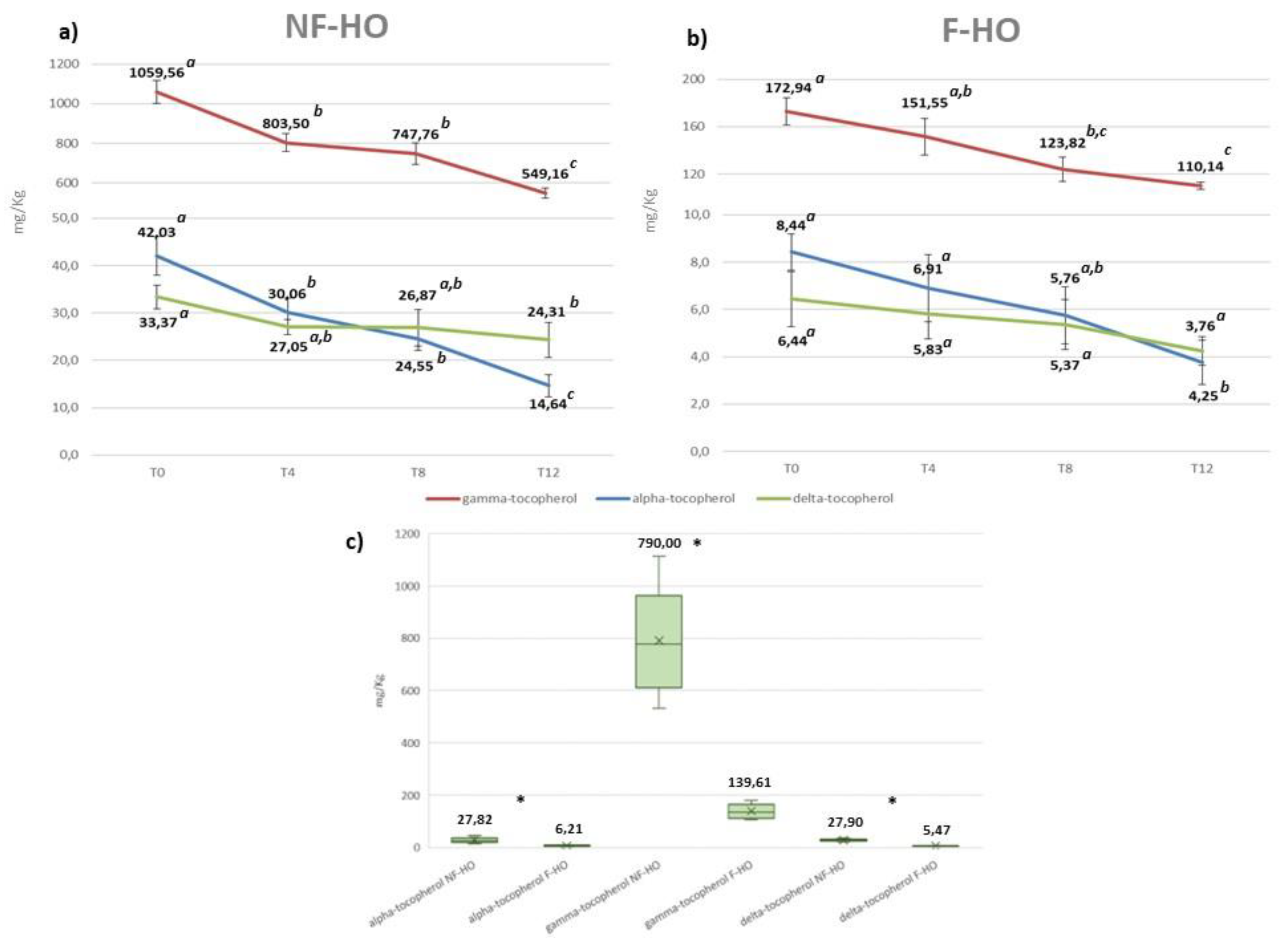
Tocopherols are the most important antioxidants present in a vegetable oil since they reduce the extent of oil autoxidation by competing with unsaturated FAs for alkoxyl and peroxyl radicals in synergistic action with polyphenols (Grajzer et al 2020). In the present study, the content of α-, γ-, and δ-tocopherol in NF-HO and F-HO samples stored during 12 weeks in transparent glass bottles is illustrated in
Figure 3. As expected, fresh NF-HO and F-HO displayed very different contents of α- (42.03 mg/Kg and 8.44 mg/Kg) γ- (1059.56 mg/Kg and 172.94 mg/Kg) and δ- (33.37 mg/Kg and 6.44 mg/Kg) tocopherols. Additionally, a further reduction in all isomers occurred in all oils with increasing storage time, especially at the initial stage (T0-T4). Specifically, γ-tocopherol decreased in NF-HO from 1059.56 mg/Kg to 549.16 mg/Kg (p<0.05) over the study period, with a 24% reduction observed during T0-T4; while in F-HO it showed a reduction from 172.94 mg/Kg to 110.14 mg/Kg (p<0.05), with a 12% loss observed during the first 4 weeks of storage. A-tocopherol varied in NF-HO from 42.03 mg/Kg to 24.31 mg/Kg (p<0.05) during 12 weeks of storage, lowering by 28% during T0-T4. The same isomer reduced in F-HO from 8.44 mg/Kg to 3.76 mg/Kg (p<0.05), with a 18% decrease during T0-T4. Δ-tocopherol lowered in the range 33.37-14.64 mg/Kg (p<0.05) and 6.44-4.25 mg/Kg (p>0.05), respectively in NF-HO and F-HO samples stored over T0-T12, with respective reduction rates of 19% and 9% during the first 4 weeks of storage (
Figure 3a,b). Differently from other isomers, α-tocopherol showed higher degradation rates in NF-HO along with statistically significant quantitative changes (p<0.05) observed at every storage step. Conversely, F-HO samples displayed a lower consumption rate of such antioxidant together with relatively stable contents during T4-T12 (
Figure 3a,b).
Overall, the filtration procedure demonstrated to markedly affect the content of tocopherols in oil. In fact, NF-HO samples showed higher mean tocopherol contents than F-HO (γ-tocopherol: 790.00 mg/Kg vs. 139.61 mg/Kg, p<0.05; α-tocopherol: 27.82 mg/Kg vs. 6.21 mg/Kg, p<0.05; δ-tocopherol: 27.90 mg/Kg vs. 5.47 mg/Kg, p<0.05) (
Figure 3c). Additionally, from the data discussed above, it is evident that filtration affected the degradation rate of tocopherols, since it was generally more pronounced in NF-HO than F-HO samples. Cold-pressed hempseed oils from this study shared the highest content of γ-tocopherol followed by α- and δ- isomers, thus, resulting in line with previous literature (Tura et al., 2022; Blasi et al., Izzo). However, while the freshly produced NF-HO samples were marked by tocopherol levels consistent with most recent studies dealing with non-treated hempseed oils (
Table 2), the F-HO samples had much lower amounts of such antioxidants. However, differently from this study, Tura and colleagues (2023) did not find significant differences between filtered and non-filtered commercial hempseed oils. Additionally, the cotton gauze filtration and centrifugation of fresh hempseed oil performed on laboratory scale did not lower the tocopherol content typically observed in the oil.
Considering the evolution of tocopherols during storage, our findings are consistent with previous literature on vegetable oils, including hempseed oil. Tura and colleagues (2022) recorded a non-statistically significant reduction of γ- and α-tocopherols and a statistically significant reduction of the δ-isomer in cold-pressed hempseed oils subjected in laboratory to filtration and centrifugation before the storage in amber glass bottles for three months. In another work, however, Tura and coworkers () demonstrated that α-tocopherol was present in the oil only before starting an accelerated oxidation test, while γ-tocopherol significantly reduced, with the main decrease noticed during the first 6 days of test (corresponding to 6 months of storage at room temperature). Interestingly, Rastrelli and colleagues () assessed the change in minor compounds of extra-virgin olive oil stored at different conditions and for 12 months, and they pointed out that α-tocopherol decreased by 92-93% in half-empty clear bottles and 24-25% in filled clear bottles, thus, confirming that the contribution of light to the direct decomposition of such tocopherol was negligible.
The decrease in tocopherols observed in NF-HO and F-HO over storage time may be attributed to the role of these antioxidants in counteracting the process of lipid autoxidation (Choe Min). Additionally, under the experimental conditions of the study, their degradation may be accelerated by their protective action against photooxidation processes induced by sensitizer pigments in presence of light (Gliszczynska-Swiglo). Indeed, tocopherols cannot be excited by visible light, but they can scavenge 1O2 by combining physical and chemical quenching (Mukai et al, 1991). Particularly, the tocopherol oxidation rate would vary according to the isomer type, following the order α > γ > δ (Abramovic et al 2007; Kim et al., 2006; Reische and others 2002). This was also confirmed by our study, since δ-tocopherol was consumed at a slower rate than γ- and α-isoforms in both NF-HO and F-HO, thus resulting more stable against oxidation. However, as previously mentioned, greater tocopherol deterioration rates were recorded in NF-HO than F-HO. This may be because high concentrations of tocopherols are capable of being consumed via their radical forms also in side reactions, which may result in a prooxidant effect. In fact, the tocopheroxy radical -especially the α-tocopheroxy radical- subtracts hydrogen from the lipid matrix and produces tocopherol and lipid alkyl radicals, which would accelerate the lipid oxidation (Zuta et al 2007). It has been also suggested that such an effect may be promoted by a synergistic action with high concentrations of prooxidant transition metals (Fuster 1998). From this point of view, filtration would be beneficial as it would prevent oxidation processes mediated by high levels of tocopherols. However, contrarily to our findings, Fregapane and coworkers observed similar degradation rates of α-tocopherol in non-filtered and filtered extra-virgin olive oils stored under accelerated conditions for 8 months, thus, concluding that filtration had no effect on its degradation over time.
3.4. Chlorophylls, carotenes, and polyphenols,
Figure 4 illustrates the trend of total chlorophylls (intended as sum of chlorophyll a+b) and total carotenes recorded in NF-HO and F-HO samples over 12 weeks of storage in transparent glass bottles.
The filtration induced a consistent pigment decrease in fresh F-HO compared to the NF-HO counterpart (chlorophylls: 65.44 mg/Kg and 18.93 mg/Kg; carotenes 52.70 mg/Kg and 37.41 mg/Kg). A further reduction was observed with increasing storage weeks. Specifically, total chlorophylls and carotenoids reduced from 65.44 mg/Kg to 9.23 mg/Kg (p<0.05) and from 52.70 mg/Kg to 34.98 mg/Kg (p<0.05) in NF-HO samples, with the greatest reduction rates at T0-T4 (respectively, -59% and -17%). A downward trend was also observed in the F-HO samples, as chlorophylls a+b and carotenoids decreased in the range 18.93-7.23 mg/Kg (p<0.05) and 37.41-28.15 mg/Kg (p<0.05), with the highest deterioration rates equal to -42% and -6% observed after 4 weeks of storage. For both types of pigment, high degradation rates were recorded in NF-HO samples along with statistically significant quantitative changes (p<0.05) during T4-T12. Conversely, F-HO samples displayed lower consumption rates together with relatively stable pigments contents after 4 weeks of storage (
Figure 4a,b). Additionally, although a proper color assessment could not be made, fresh NF-HO samples were characterized by a deep and cloudy green color that turned brown at the end of storage, while fresh F-HO samples had a more stable color that varied from a brilliant yellow to a slightly darker hue after 12 weeks of storage. As expected, filtration had a great impact on such pigments, as all NF-HO and F-HO samples showed a mean chlorophyll level amounting to 29.13 mg/Kg and 11.34 mg/Kg (p<0.05) and a mean carotene content equal to 44.18 mg/Kg and 32.93 mg/Kg (p<0.05) (
Figure 4c).
Freshly produced NF-HO samples were marked by a chlorophyll content consistent with non-treated hempseed oils characterized in other recent studies (
Table 3). On the other hand, fresh F-HO samples had slightly higher pigment contents than hempseed oils treated by ultrasound bleaching or refining process (
Table 3), thus, suggesting that the filtration has a milder effect on such compounds. However, recent literature showed variable and lower levels of carotenoids with respect to our study, whether or not the hemp seed oil had received any treatment (
Table 2). Only Aladić and colleagues () obtained similar carotenoid levels (31.5 mg/Kg) by producing the cold-pressed hempseed oil on a laboratory scale.
To the best knowledge of the authors, the evolution of pigments in the cold-pressed hempseed oil during storage has not yet been investigated. However, our findings are consistent with the general degradation of these pigments observed during the storage of other vegetable oils (Cichelli et al., 2015 Choe Min).
Total chlorophylls tend to degrade to pheophytins and pheophorbides, with greater rates observed during the initial stages of storage and upon exposure to light (Giuliani et al 2015; Petrović et al 2017). Chlorophylls and derivatives are primarily involved in the photooxidation process, since they can act as sensitizers in the presence of light and 3O2 to produce 1O2, thus, causing a faster deterioration of the oxidative stability, colour change, and an inevitable reduction in the nutritional and economic value of the oil (Choe Min; Giuliani et al 2015). On the other hand, carotenoids slow down photooxidation by light filtering, 1O2 quenching, sensitizer inactivation, and free radical scavenging (Choe Min). Among carotenoids, β-carotene could be protected from degradation by α-tocopherol, to prevent both the autoxidation and the photooxidation of an oil with a synergistic effect (Choe and Min Palozza and Krinsky 1992). In presence of chlorophylls, carotenoids counteract the progress of the photooxidation by physical or chemical 1O2 quenching. However, while the physical quenching doesn’t alter their structure, the chemical quenching leads to their degradation and conversion into epoxide or carbonyl derivatives. As a result, an overall reduction in carotenoids in the oil, as well as its discoloration, can be expected to occur during storage and in the presence of light (Rotkiewicz, D. 2002).
Results from this study also pointed out that filtration lowered the pigment content of the fresh hempseed oil and at the same reduce their consumption during storage. Indeed, greater pigment deterioration rates were recorded in NF-HO than F-HO samples over the study period. This may be explained by the higher content of chlorophylls in non-filtered oils which, reacting with the atmospheric oxygen (3O2) dissolved in the oil, triggered the process of photooxidation to a greater extent, especially at the initial stages of storage. As a result, carotenoids were also consumed at higher rates in non-filtered oils to counteract the oxidation process. Therefore, oil filtration become highly desirable not only to limit the photooxidation process by chlorophylls and derivatives, but also to slow down the consumption of antioxidant carotenoids over time. As proof, Liang and colleagues () found that peroxides were significantly inversely correlated with total chlorophylls in non-treated hempseed oils; while no correlation was observed in the oils treated by ultrasound bleaching, which were characterized by lower and stable chlorophylls during accelerated storage.
Similarly to pigments, fresh NF-HO showed a higher level of total phenols than fresh F-HO samples, and an overall reduction in such secondary metabolites was observed in both series of hempseed oil over storage. Specifically, total polyphenols decreased in the range 82.88-12.97 mg/Kg (p<0.05) and 57.55-28.30 mg/Kg (p<0.05) respectively in NF-HO and F-HO samples (
Figure 4a,b), with higher oxidation rates recorded in NF-HO than F-HO samples, especially in the early storage (T0-T4: -71% and -23%, respectively). However, both arrays of oil samples shared a non-statistically significant reduction in total polyphenols at every storage step analyzed during T4-T12 (p>0.05,
Figure 4a,b). Interestingly, F-HO was characterized by a non-statistically significant higher total phenol content than NF-HO (respectively, 41.39 mg/Kg and 34.63 mg/Kg, p>0.05) (
Figure 4c).
The activity of polyphenols is notably supported by the presence of tocopherols in the oil. In fact, a clear synergism between polyphenols, acting both as metal chelators and radical scavengers, and tocopherols, acting as radical scavengers, has been already displayed in previous studies dealing with cold-pressed oils with high-PUFA levels (Grajzer et al 2020, Pava Martin). However, the antioxidant activity of phenolics is strictly related to the compound family and content, which in turn depend on genotype, geographical origin, cultivation practices of the hemp, as well as extraction and storage conditions of the oil (Faugno et al; Smeriglio, Babiker 2021; Calzolari).
While considering that total polyphenols by Folin–Ciocalteu method might be biased by several interfering non-phenolic compounds—such as amino acids, ascorbic acid, reducing sugars, and transition metals (amarena)-, highly variable quantitative ranges have been found in recent literature on cold-pressed hempseed oils (
Table 3). Consequently, the total polyphenol content of fresh oils from our study would be more similar to that reported by Ciano and coworkers and Tura and colleagues (). On the other hand, Smeriglio and colleagues reported much higher and non-comparable total phenolics (267500 mg/Kg) in cold-pressed hempseed oil obtained from the same cultivar used in this study.
Considering the trend of polyphenols during oil storage, a recent work of Tura et al (22) has already pointed out a degradation of such antioxidants in the cold-pressed hempseed oil subjected to accelerated storage. In line with our results, polyphenol deterioration resulted also strongly linked to the progress of oxidation during the storage of virgin olive oil (DASKALAKI et al 2009, Okogeri, Morello et al., 2004, Tsimidou, Georgiou, Koidis, and Boskou (2005) ), camelina oil (Abramovic), and cold-pressed rapeseed oil (Koski et al 2002). Indeed, these studies revealed that the oxidation of phenolic compounds was linearly related to the formation of primary oxidation products over storage, probably because they were increasingly involved in the scavenging of hydroperoxide radicals. The influence of the light on the degradation rate of polyphenols was studied in the olive oil stored in dark and transparent glass bottles and non-significant changes were observed in the content of such secondary metabolites over time (). Therefore, it can be concluded that, under our experimental conditions, the oxygen dissolved in the oil and the content primary oxidation products would most affect the stability of polyphenols (daskalaki et al,2009).
The filtration process had a clear effect on the cold-pressed hempseed oil. From the obtained results, it is evident that filtration can lower the water-soluble phenolic content in the fresh hempseed oil and at the same reduce their deterioration rate during storage. This was probably because filtration improved the oxidative stability of the hempseed oil as discussed in paragraph…, with relevant implications for a reduced consumption and better stability of the polyphenol content during storage. On the other hand, the lower oxidative stability of non-filtered oils resulted in a faster consumption of these antioxidants.
Literature showed contradictory results on the influence of filtration and storage on the polyphenol content of a vegetable oil. Some studies confirmed the progressive decrease of polyphenols during the storage of virgin olive oils. However, higher deterioration rates were recorded in filtered than non-filtered oils, which consequently showed higher total phenol contents (Shendi et al. Timidou et al). Conversely, other research highlighted minimal or no differences in total phenols of filtered and non-filtered olive oils during storage (Brkić Bubola et al., 2017, Fortini et al. (2016) and Sacchi et al 2015).
3.5. Squalene
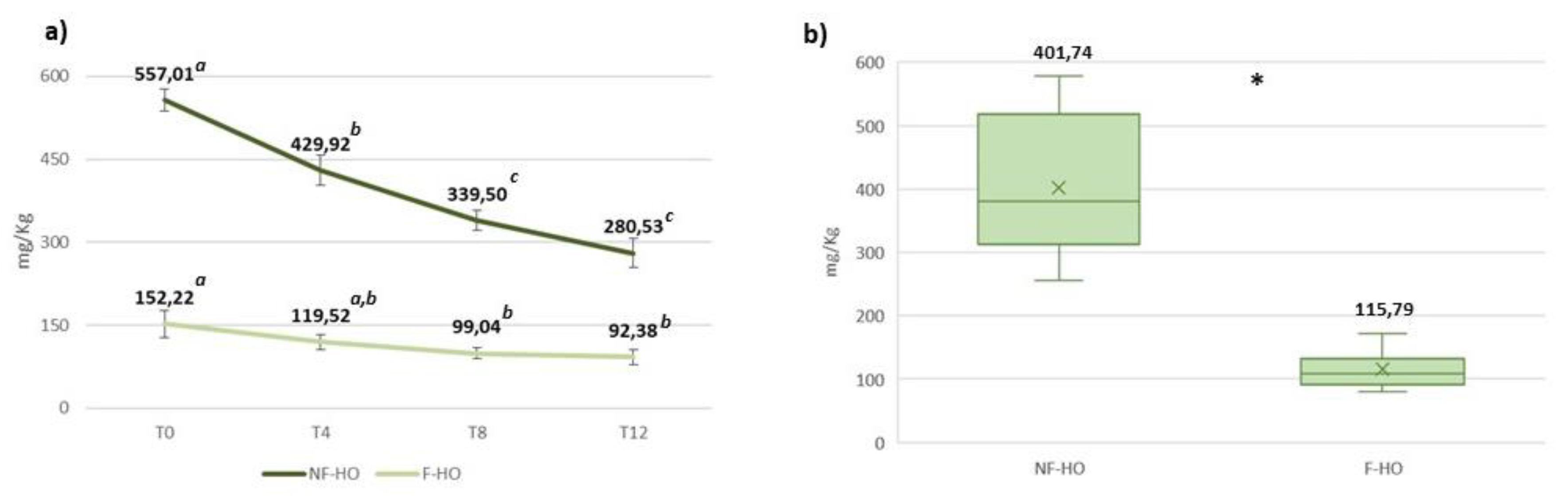
Figure 5 reports the content of squalene in NF-HO and F-HO samples over the considered storage period. As expected, fresh NF-HO showed a higher amount of squalene than fresh F-HO (557.01 mg/Kg and 152.22 mg/Kg). However, squalene decreased over storage in both NF-HO and F-HO within the respective ranges 557.01-280.53 mg/Kg (p<0.05) and 152.22-92.38 mg/Kg (p<0.05), again with more pronounced reduction rates observed in NF-HO than F-HO samples, especially during the first 4 weeks of storage (respectively, -23% and -21%) (
Figure 5a,b). On average, NF-HO was characterized by a higher amount of squalene than F-HO (respectively, 401.74 mg/Kg and 117.71 mg/Kg, p<0.05) (
Figure 5c).
Little and recent literature has addressed the squalene in the hempseed oil, reporting levels of this antioxidant not comparable to those found in this study (
Table 4), because of the influence of genetic, environmental, and agronomic factors (Shi et al 2019). Additionally, oil refining, if present, significantly reduced the content of such phytochemical in vegetable oils (Nergiz 2011).
To the best knowledge of the authors, the influence of filtration and storage on the content of squalene has not yet explored in minor cold pressed oils. However, the consumption of this antioxidant typically occurs during the storage of virgin olive oil, and the diffuse lighting of the oil did not seem to play a major role in the degradation process (Rastrelli). As a result, the consumption of this compound observed in the hempseed oils during storage would be attributable to the scavenging of peroxyl radicals (Dessì et al 2002), and the consequent conversion into quite stable degradation products that are not involved in further propagation reactions (Psomiadou and Tsimidou 2002). As proof, the olive oil refined and subsequently enriched with squalene showed lower acidity, peroxide value and absorbances at 237 nm and 270 nm with respect to the crude olive oil during storage, thus confirming the role of such antioxidant in the delay of the autoxidation (Rigane).
3.6. Inorganic elements
The profile of inorganic elements of NF-HO and F-HO samples recorded during storage is reported in
Table 5.
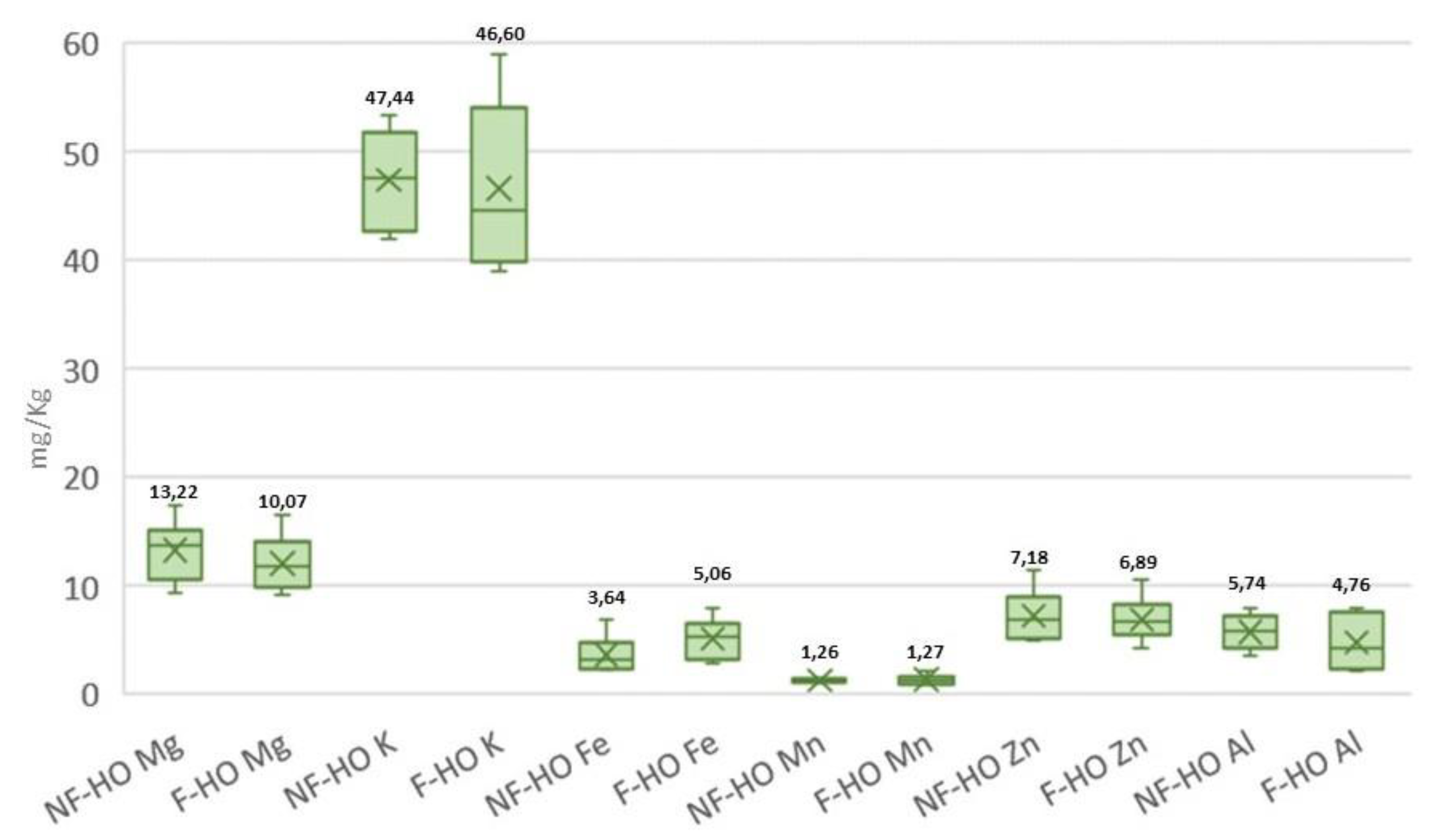
Freshly produced NF-HO and F-HO samples were characterized by similar element profiles. Specifically, Na was found at the highest content (NF-HO: 238.95 mg/Kg and F-HO: 254.14 mg/Kg), followed by other major elements, such as K (NF-HO: 46.40 mg/Kg and F-HO: 45.39 mg/Kg), and Mg (NF-HO: 13.99 mg/Kg and F-HO: 12.92 mg/Kg). Among essential trace elements, the most abundant elements were Fe (NF-HO: 4.11 mg/Kg and F-HO: 5.28 mg/Kg) and Zn (NF-HO: 7.86 mg/Kg and F-HO: 7.95 mg/Kg). Considering the potentially toxic trace metals, Al was the most concentrated metal (NF-HO: 5.74 mg/Kg and F-HO: 4.58 mg/Kg), followed by a much lower amount of Pb (NF-HO: 0.26 mg/Kg and F-HO: 0.41 mg/Kg). Additionally, both fresh NF-HO and F-HO samples were marked by levels of Pb and As lower than the limit fixed by the Codex Alimentarius [] for such heavy metals (0.1 mg/Kg).
For greater convenience, only the data from T0 and T12 were reported in
Table 5, as both NF-HO and F-HO samples showed overlapping and non-statistically different element contents (p>0.05) from the start to the end of the storage period. Not only the storage in clear glass bottles, but also the filtration process did not alter these minor components in the cold-pressed hempseed oil, since, as reported in
Figure 6, all NF-HO and F-HO samples showed non-significantly different mean concentrations of each investigated analyte (p>0.05).
To the best knowledge of the authors, the effect of storage and filtration process on the inorganic elements of the cold-pressed hempseed oil during storage has not been addressed elsewhere, nor is there any comparative literature on other vegetable oils. The stable and comparable element profile obtained during the storage of both NF-HO and F-HO may be related i) to the absence of inorganic matter decomposition during the storage of the oils, and not least, ii) the non-responsiveness of these minor compounds to the filtration process.
However, this compositional aspect should be worthy of more in-depth investigation, as the oxidative stability of edible oils is influenced not only by storage conditions, energy inputs (e.g., light), oxygen type, triacylglycerol matrix, but also by a variety of minor compounds, including metals.
In fact, metals catalyze the initiation step in the autoxidation, and, not least, they produce 1O2 and hydroxy radicals respectively from 3O2 and hydrogen peroxides, which inevitably accelerate the overall oil oxidation. Among elements, transition elements such as Fe and Cu are known to accelerate these processes (Choe Min). Additionally, Fe also causes decomposition of phenolics, thus, further deteriorating the oil oxidative stability (Keceli and Gordon 2002).
Figure 1.
Evolution of free acidity, peroxide value, K232, and K270 of non- filtered (NF-HO, a) and filtered (F-HO, b) cold-pressed hempseed oil during 12 weeks of storage in transparent glass bottles. Data are expressed as mean±sd of n=3 oil bottles, each analyzed in triplicate. a-d: different superscript letters in the same line indicate significantly different values for a given parameter (p<0.05 by post hoc Tukey’s HSD test); same superscript letters in the same line indicate not significantly different values (p>0.05 by post hoc Tukey’s HSD test). Figure (c) illustrates the free acidity, peroxides, K232, and K270 of total NF-HO and F-HO samples. Data are expressed as mean±sd of n=12 oil bottles, each analyzed in triplicate. In each box, “×” indicates the average value. In the comparison between NF-HO and F-HO samples, “*” indicates significantly different values (p< 0.05 by Studentʼs t-test) for a given parameter; conversely, the absence of “*” indicates non-significantly different values (p>0.05, by Studentʼs t-test).
Figure 1.
Evolution of free acidity, peroxide value, K232, and K270 of non- filtered (NF-HO, a) and filtered (F-HO, b) cold-pressed hempseed oil during 12 weeks of storage in transparent glass bottles. Data are expressed as mean±sd of n=3 oil bottles, each analyzed in triplicate. a-d: different superscript letters in the same line indicate significantly different values for a given parameter (p<0.05 by post hoc Tukey’s HSD test); same superscript letters in the same line indicate not significantly different values (p>0.05 by post hoc Tukey’s HSD test). Figure (c) illustrates the free acidity, peroxides, K232, and K270 of total NF-HO and F-HO samples. Data are expressed as mean±sd of n=12 oil bottles, each analyzed in triplicate. In each box, “×” indicates the average value. In the comparison between NF-HO and F-HO samples, “*” indicates significantly different values (p< 0.05 by Studentʼs t-test) for a given parameter; conversely, the absence of “*” indicates non-significantly different values (p>0.05, by Studentʼs t-test).
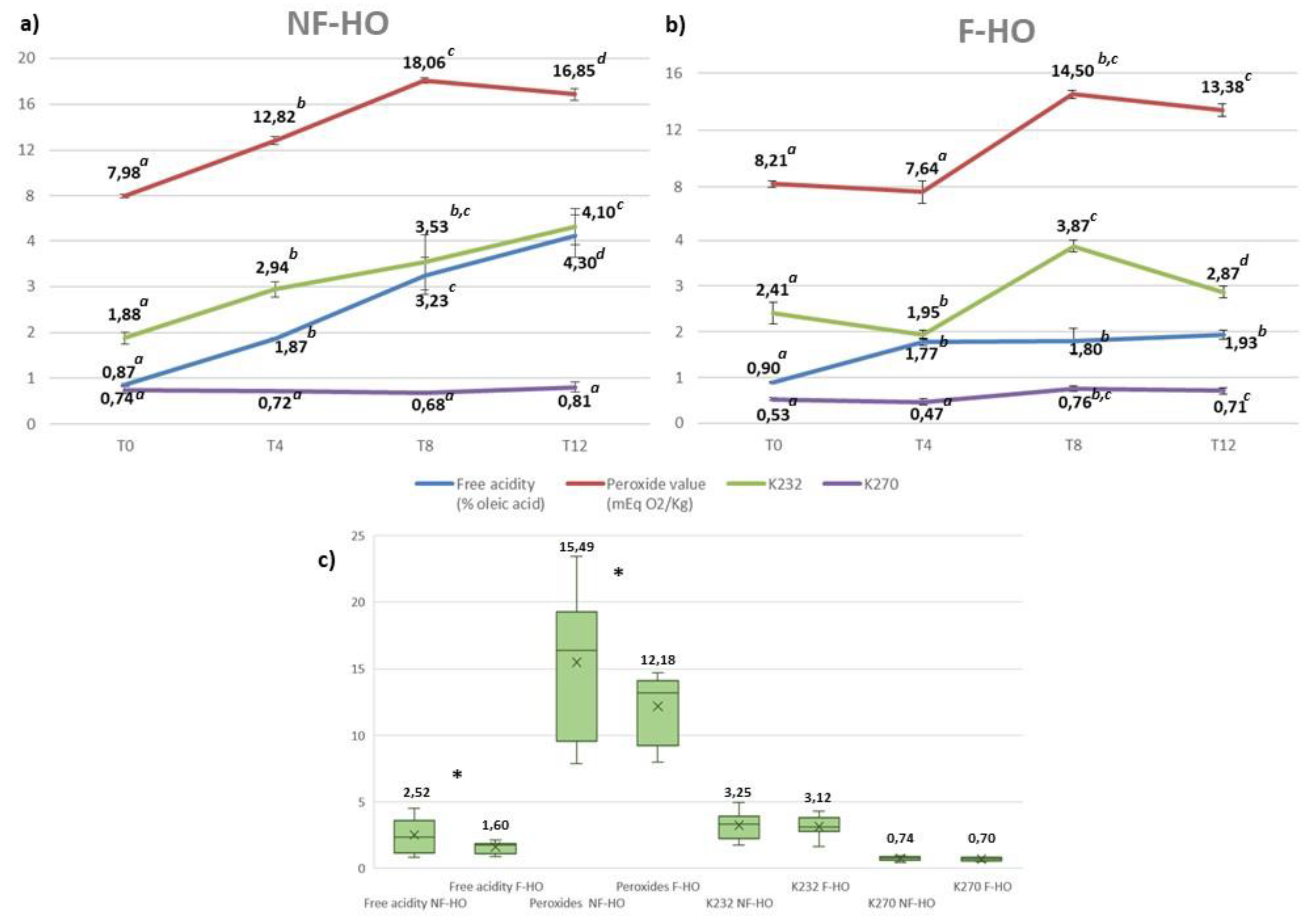
Figure 2.
Main figures of the FA composition from total non-filtered (NF-HO) and filtered (F-HO) cold-pressed hempseed oil samples. Data are expressed as mean±sd of n=12 oil bottles, each analyzed in triplicate. In every box, “×” indicates the average value, whereas the outlier points display the outlier data lying above the upper whisker. In the comparison between NF-HO and F-HO samples, “*” indicates significantly different values (p< 0.05 by Studentʼs t-test) for a given parameter; conversely, the absence of “*” indicates non-significantly different values (p>0.05, by Studentʼs t-test).
Figure 2.
Main figures of the FA composition from total non-filtered (NF-HO) and filtered (F-HO) cold-pressed hempseed oil samples. Data are expressed as mean±sd of n=12 oil bottles, each analyzed in triplicate. In every box, “×” indicates the average value, whereas the outlier points display the outlier data lying above the upper whisker. In the comparison between NF-HO and F-HO samples, “*” indicates significantly different values (p< 0.05 by Studentʼs t-test) for a given parameter; conversely, the absence of “*” indicates non-significantly different values (p>0.05, by Studentʼs t-test).
Figure 3.
Evolution of the content of α- γ- and δ-tocopherol in non- filtered (NF-HO, a) and filtered (F-HO, b) cold-pressed hempseed oil during 12 weeks of storage in transparent glass bottles. Data are expressed as mean±sd of n=3 oil bottles, each analyzed in triplicate. a-d: different superscript letters in the same line indicate significantly different values for a given parameter (p<0.05 by post hoc Tukey’s HSD test); same superscript letters in the same line indicate not significantly different values (p>0.05 by post hoc Tukey’s HSD test). Figure (c) illustrates the α- γ- and δ-tocopherol of total NF-HO and F-HO samples. Data are expressed as mean±sd of n=12 oil bottles, each analyzed in triplicate. In each box, “×” indicates the average value. In the comparison of every analyte between NF-HO and F-HO samples, “*” indicates significantly different values (p< 0.05 by Studentʼs t-test).
Figure 3.
Evolution of the content of α- γ- and δ-tocopherol in non- filtered (NF-HO, a) and filtered (F-HO, b) cold-pressed hempseed oil during 12 weeks of storage in transparent glass bottles. Data are expressed as mean±sd of n=3 oil bottles, each analyzed in triplicate. a-d: different superscript letters in the same line indicate significantly different values for a given parameter (p<0.05 by post hoc Tukey’s HSD test); same superscript letters in the same line indicate not significantly different values (p>0.05 by post hoc Tukey’s HSD test). Figure (c) illustrates the α- γ- and δ-tocopherol of total NF-HO and F-HO samples. Data are expressed as mean±sd of n=12 oil bottles, each analyzed in triplicate. In each box, “×” indicates the average value. In the comparison of every analyte between NF-HO and F-HO samples, “*” indicates significantly different values (p< 0.05 by Studentʼs t-test).
Figure 4.
Evolution of the content of chlorophill a+b, total carotenes and total polyphenols in non- filtered (NF-HO, a) and filtered (F-HO, b) cold-pressed hempseed oil during 12 weeks of storage in transparent glass bottles. Data are expressed as mean±sd of n=3 oil bottles, each analyzed in triplicate. a-d: different superscript letters in the same line indicate significantly different values for a given parameter (p<0.05 by post hoc Tukey’s HSD test); same superscript letters in the same line indicate not significantly different values (p>0.05 by post hoc Tukey’s HSD test). Figure (c) illustrates chlorophills a+b, total carotenes and total polyphenols of all NF-HO and F-HO samples. Data are expressed as mean±sd of n=12 oil bottles, each analyzed in triplicate. In each box, “×” indicates the average value. In the comparison of every analyte between NF-HO and F-HO samples, “*” indicates significantly different values (p< 0.05 by Studentʼs t-test); conversely, the absence of “*” indicates non-significantly different values (p>0.05, by Studentʼs t-test).
Figure 4.
Evolution of the content of chlorophill a+b, total carotenes and total polyphenols in non- filtered (NF-HO, a) and filtered (F-HO, b) cold-pressed hempseed oil during 12 weeks of storage in transparent glass bottles. Data are expressed as mean±sd of n=3 oil bottles, each analyzed in triplicate. a-d: different superscript letters in the same line indicate significantly different values for a given parameter (p<0.05 by post hoc Tukey’s HSD test); same superscript letters in the same line indicate not significantly different values (p>0.05 by post hoc Tukey’s HSD test). Figure (c) illustrates chlorophills a+b, total carotenes and total polyphenols of all NF-HO and F-HO samples. Data are expressed as mean±sd of n=12 oil bottles, each analyzed in triplicate. In each box, “×” indicates the average value. In the comparison of every analyte between NF-HO and F-HO samples, “*” indicates significantly different values (p< 0.05 by Studentʼs t-test); conversely, the absence of “*” indicates non-significantly different values (p>0.05, by Studentʼs t-test).

Figure 5.
Evolution of the content of squalene in non- filtered (NF-HO) and filtered (F-HO) cold-pressed hempseed oil during 12 weeks of storage in transparent glass bottles (a). Data are expressed as mean±sd of n=3 oil bottles, each analyzed in triplicate. a-d: different superscript letters in the same line indicate significantly different values for a given parameter (p<0.05 by post hoc Tukey’s HSD test); same superscript letters in the same line indicate not significantly different values (p>0.05 by post hoc Tukey’s HSD test). Figure (b) illustrates the mean content of squalene in all NF-HO and F-HO samples. Data are expressed as mean±sd of n=12 oil bottles, each analyzed in triplicate. In each box, “×” indicates the average value. In the comparison of squalene between NF-HO and F-HO samples, “*” indicates significantly different values (p< 0.05 by Studentʼs t-test).
Figure 5.
Evolution of the content of squalene in non- filtered (NF-HO) and filtered (F-HO) cold-pressed hempseed oil during 12 weeks of storage in transparent glass bottles (a). Data are expressed as mean±sd of n=3 oil bottles, each analyzed in triplicate. a-d: different superscript letters in the same line indicate significantly different values for a given parameter (p<0.05 by post hoc Tukey’s HSD test); same superscript letters in the same line indicate not significantly different values (p>0.05 by post hoc Tukey’s HSD test). Figure (b) illustrates the mean content of squalene in all NF-HO and F-HO samples. Data are expressed as mean±sd of n=12 oil bottles, each analyzed in triplicate. In each box, “×” indicates the average value. In the comparison of squalene between NF-HO and F-HO samples, “*” indicates significantly different values (p< 0.05 by Studentʼs t-test).
Figure 6.
Mean content (mg/Kg) of the most abundant elements present in from total non-filtered (NF-HO) and filtered (F-HO) cold-pressed hempseed oil samples. Data are expressed as mean±sd of n=12 oil bottles, each analyzed in triplicate. For each element, “×” indicates the average value. In the comparison of each element between NF-HO and F-HO samples, “*” indicates significantly different values (p< 0.05, by Studentʼs t-test). Conversely, the absence of “*” indicates non-significantly different values (p>0.05, by Studentʼs t-test).
Figure 6.
Mean content (mg/Kg) of the most abundant elements present in from total non-filtered (NF-HO) and filtered (F-HO) cold-pressed hempseed oil samples. Data are expressed as mean±sd of n=12 oil bottles, each analyzed in triplicate. For each element, “×” indicates the average value. In the comparison of each element between NF-HO and F-HO samples, “*” indicates significantly different values (p< 0.05, by Studentʼs t-test). Conversely, the absence of “*” indicates non-significantly different values (p>0.05, by Studentʼs t-test).
Table 1.
Evolution of the FA composition (%) in non-filtered (NF-HO) and filtered (F-HO) cold-pressed hempseed oil during 12 weeks of storage in transparent glass bottles. Data are expressed as mean±sd of n=3 oil bottles, each analyzed in triplicate.
Table 1.
Evolution of the FA composition (%) in non-filtered (NF-HO) and filtered (F-HO) cold-pressed hempseed oil during 12 weeks of storage in transparent glass bottles. Data are expressed as mean±sd of n=3 oil bottles, each analyzed in triplicate.
| |
NF-HO |
F-HO |
| T0 |
T4 |
T8 |
T12 |
T0 |
T4 |
T8 |
T12 |
| C16:0 |
7.20±0.45a
|
6.30±0.44a,b
|
6.82±0.33a,b
|
5.87±0.31b
|
6.77±0.23a
|
5.62±0.53b
|
5.57±0.32b
|
5.53±0.19b
|
| C18:0 |
3.24±0.29a
|
2.94±0.08a
|
2.18±0.34a
|
2.40±0.67a
|
2.95±0.08a
|
2.58±0.61a
|
2.18±0.12a
|
2.27±0.08a
|
| SFA |
10.44±0.74a
|
9.24±0.39a,b
|
9.00±0.03b,c
|
8.27±0.48b,c
|
9.72±0.24a
|
8.20±1.14a,b
|
7.75±0.38b
|
7.80±0.12b
|
| C18:1 n-9 |
10.42±1.08a
|
9.41±1.03a
|
9.04±0.23a
|
9.06±0.67a
|
9.18±0.33a
|
8.75±0.21a,b
|
8.80±0.33a,b
|
8.42±0.19b
|
| C18:1 n-7 |
0.93±0.13a
|
1.13±0.44a
|
0.96±0.22a
|
0.87±0.08a
|
1.00±0.12a
|
0.88±0.08a
|
0.81±0.03a
|
0.87±0.09a
|
| MUFA |
11.35±1.21a
|
10.54±1.46a
|
10.01±0.15a
|
9.93±0.74a
|
10.18±0.42a
|
9.63±0.13a,b
|
9.61±0.35a,b
|
9.29±0.21b
|
| C18:2 n-6 |
55.56±0.61a
|
55.18±1.62a,b
|
52.74±0.90b,c
|
52.36±0.87c
|
56.27±0.40a
|
56.13±1.25a
|
54.61±0.64a
|
54.29±0.49a
|
| C18:3 n-6 |
5.34±0.95a
|
5.47±0.46a
|
5.86±0.37a
|
5.40±0.83a
|
5.18±0.36a
|
4.89±0.14a
|
4.71±0.17a
|
4.94±0.06a
|
| C18:3 n-3 |
18.85±0.75a
|
17.92±0.34a
|
18.37±0.76a
|
18.70±0.35a
|
19.18±0.40a
|
18.63±0.68a
|
18.80±0.33a
|
19.07±0.19a
|
| C18:4 n-3 |
0.99±0.12a
|
0.90±0.21a
|
1.00±0.23a
|
1.25±0.38a
|
1.02±0.13a
|
0.99±0.24a
|
1.33±0.34a,b
|
1.76±0.11b
|
| PUFA |
80.74±2.20a
|
79.47±2.30a
|
77.97±1.38a
|
77.71±1.44a
|
81.87±0.41a
|
80.64±0.78a,b
|
79.44±0.23b
|
80.06±0.72b
|
| PUFA/SFA |
7.77±0.75a
|
8.62±0.59a,b
|
8.67±0.13a,b
|
9.41±0.37b
|
8.43±0.18a
|
9.98±1.54a
|
10.27±0.55a
|
10.27±0.09a
|
| n-6/n-3 |
3.07±0.10a
|
3.22±0.03a,b
|
3.03±0.12a
|
2.90±0.13a,c
|
3.05±0.05a
|
3.11±0.13a,b
|
2.95±0.13a
|
2.84±0.02a,c
|
Table 2.
Recent literature on the content (mg/Kg) of α-, γ -, and δ-tocopherols revealed in non-treated and treated hempseed oils.
Table 2.
Recent literature on the content (mg/Kg) of α-, γ -, and δ-tocopherols revealed in non-treated and treated hempseed oils.
| Sample information |
α-tocopherol |
γ-tocopherol |
δ-tocopherol |
Reference |
| Commercial cold-pressed oils |
38.6-77.6 |
625.3-1013.2 |
14.0-35.1 |
Blasi 2022 |
| Commercial hot/cold-pressed oils |
20.8-74.8 |
99.9-576.7 |
16.6-49.1 |
Ciano |
| Array of filtered and non-filtered cold-pressed oils |
14.6-53.0 |
594-967 |
19.6-50.3 |
Tura 2023 comm |
| Commercial cold-pressed oils |
21.02-65.92 |
376.28-906.63 |
- |
Tura accel. Sto. |
| Commercial cold-pressed oils |
39.2-47.7 |
774.3-924.5 |
3.2-4.0 |
Occhiuto |
| Commercial cold-pressed oils subjected to cotton filtration and centrifugation in laboratory |
38.73 |
794.66 |
29.22 |
Tura storage |
Table 3.
Recent literature on the content (mg/Kg) of chlorophylls a+b, total carotenes, and level (mg GAE/Kg) of total polyphenols revealed in non-treated and treated hempseed oils.
Table 3.
Recent literature on the content (mg/Kg) of chlorophylls a+b, total carotenes, and level (mg GAE/Kg) of total polyphenols revealed in non-treated and treated hempseed oils.
| Sample information |
Chlorophylls a+b |
Total carotenes |
Total polyphenols |
Reference |
| Commercial cold-pressed oils |
34.8-76.4 |
2.61-1.78 |
- |
Blasi |
| Commercial refined oils |
0.41-2.64 |
0.29-1.73 |
22100-160800 |
Izzo |
| Commercial hot/cold-pressed oils |
- |
2.37-52.15 |
8.32-200.42 |
Ciano |
| Commercial cold-pressed oils |
33.5-67.8 |
- |
162.5 |
Aachari |
| Commercial cold-pressed oils subjected to ultrasound bleaching |
0.4-11.5 |
- |
106.0-118.1 |
| Commercial cold-pressed oils |
56.3 |
23.4 |
- |
Liang |
|
Commercial cold-pressed oilssubjected to ultrasound bleaching
|
7.8-14.8 |
2.3-4.0 |
- |
| Array of filtered and non-filtered commercial cold-pressed oils |
0.78-75-7 |
2.53-3.93 |
- |
Tura 23 |
| Commercial cold-pressed oils |
- |
- |
12.08-186.78 |
Tura 22 |
| Commercial cold-pressed oils |
- |
- |
290320-384520 |
Occhiuto |
Table 4.
Recent literature on the content (mg/Kg) of squalene revealed in hempseed oils.
Table 4.
Recent literature on the content (mg/Kg) of squalene revealed in hempseed oils.
| Sample information |
Squalene |
Reference |
| Commercial oil |
ND |
Gutierrez-Luna |
| Commercial hot/cold-pressed oils |
521.4-30594.90 |
Ciano |
| Refined commercial oil |
80.52 |
Montserrat de la plaz |
| Commercial oil |
13.9 |
Feng |
Table 5.
Element profile (mg/Kg) of non- filtered (NF-HO) and filtered (F-HO) cold-pressed hempseed oils before the experimental trial (T0) and after 12 weeks of storage in transparent glass bottles (T12). Data are expressed as mean±sd of n=3 oil bottles, each analyzed in triplicate. Limit of Detection (LOD) of Se: 0.002 mg/Kg.
Table 5.
Element profile (mg/Kg) of non- filtered (NF-HO) and filtered (F-HO) cold-pressed hempseed oils before the experimental trial (T0) and after 12 weeks of storage in transparent glass bottles (T12). Data are expressed as mean±sd of n=3 oil bottles, each analyzed in triplicate. Limit of Detection (LOD) of Se: 0.002 mg/Kg.
| |
NF-HO |
F-HO |
| T0 |
T12 |
T0 |
T12 |
| Na |
238.95±17.54a
|
233.95±21.91a
|
254.14±6.71a
|
246.83±13.30a
|
| Mg |
13.99±3.22a
|
12.45±2.76a
|
12.92±3.71a
|
11.22±1.11a
|
| K |
46.40±4.67a
|
48.47±5.25a
|
45.39±6.31a
|
47.80±10.28a
|
| Fe |
4.11±2.36a
|
3.17±0.89a
|
5.28±2.54a
|
4.84±1.42a
|
| Cu |
0.028±0.026a
|
0.071±0.035a
|
0.047±0.025a
|
0.070±0.019a
|
| Mn |
1.17±0.20a
|
1.25±0.19a
|
1.12±0.23a
|
1.43±0.58a
|
| Zn |
7.86±3.28a
|
6.49±1.46a
|
7.95±2.42a
|
5.83±1.50a
|
| Se |
<LOD |
<LOD |
<LOD |
<LOD |
| Ni |
0.023±0.009a
|
0.014±0.004a
|
0.016±0.004a
|
0.020±0.009a
|
| Cr |
0.008±0.003a
|
0.006±0.002a
|
0.006±0.004a
|
0.007±0.003a
|
| Al |
5.74±2.15a
|
5.74±1.28a
|
4.58±2.60a
|
4.94±2.88a
|
| Cd |
0.08±0.004a
|
0.011±0.005a
|
0.015±0.004a
|
0.012±0.006a
|
| Pb |
0.26±0.08a
|
0.33±0.07a
|
0.41±0.24a
|
0.38±0.14a
|
| As |
0.08±0.004a
|
0.009±0.001a
|
0.009±0.003a
|
0.009±0.007a
|