1. Introduction
Avian adenoviruses cause variety of diseases in different bird species including chickens, ducks, quails, ostriches, falcons, raptors, psittacines and parrots [
1]. Adenovirus infection (Inclusion body hepatitis) was first reported in broilers in the USA in 1963 [
2]. Up to date, fowl adenoviruses (FAdVs) have been detected in chickens as causative agents of inclusion body hepatitis (IBH), hydropericardium hepatitis syndrome (HHS), adenoviral gizzard erosion (AGE), avian adenoviral splenomegaly (AAS) and egg drop syndrome (EDS) [
3,
4,
5]. Recently, IBH and HHS have been frequently reported in commercial chickens from several countries causing significant economical losses [
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17].
FAdVs are non-enveloped double-stranded DNA viruses, which belong to the family
Adenoviridae, composed of 720 hexons arranged in 240 trimers and 12 vertex pentons [
1,
18,
19,
20]. Three main structural proteins of FAdVs capsids are hexon, fiber and penton base. The hexon gene is prone to mutations and used for serotyping as it harbors the major neutralizing epitopes [
21]. The family adenoviridae contains six genera named as mastadenovirus, aviadenovirus, atadenovirus, siadenovirus and ichtadenovirus in addition to recently proposed testadenovirus of turtles and tortoises. Adenoviruses from three genera (aviadenovirus, siadenovirus, and atadenovirus) can infect birds [
1,
5] FAdVs are classified into five different species (FAdV-A to FAdV-E) based on their molecular structure and also into 12 serotypes (FAdV-1-8a, 8b-11), as a result of cross-neutralization tests [
1,
22]. At least 12 genotypes were identified within the five FAdV species based on the hexon gene sequences [
21,
23]. FAdV-D (FAdV-2 and FAdV-11) and FAdV-E (FAdV-8a and FAdV-8b) commonly associated with IBH while HHS caused by FAdV-C (FAdV-4). FAdV-A (FAdV-1) has been isolated from most cases of gizzard erosion [
1,
22,
24].
There are several methods to diagnose and identify FAdVs in chickens. Real time PCR for rapid diagnosis and PCR for sequencing the hexon gene which allows the differentiation of field isolates to species. In addition, serological tests like serum neutralisation are used to investigate the serotypes of FAdVs (Schachner et al., 2016; Cizmecigil et al., 2020). FAdVs are transmitted vertically and horizontally via all excretions, but the highest titers are found in feces and therefore fecal-oral transmission is very efficient way of transmission [
19]. Because of rapid spread via feces and emergence of hypervirulent strain in China, outbreaks have been reported in the Middle east, Africa, Asia and recently in the USA [
8,
9,
10,
11,
12,
13,
14,
15,
16,
17]. However, there is no report about the occurrence of this disease complex in Azerbaijan at present. Also commercial vaccines are not being used against FAdVs in Azerbaijan due to lack of knowledge about disease frequency and circulating viruses. The aim of this study was to investigate outbreaks of adenoviral disease causing mortalities in broiler flocks to determine circulating strains and genetic diversity of FAdVs in Azerbaijan in relation to clinico-pathological signs.
2. Materials and Methods
2.1. Farms and Study Population
Mortality up to %20 was observed in broiler breeder flocks (Ross 308) and layer breeder flocks (Hyline-Sonja) in Azerbaijan in December 2022. Broiler flock consisted of 40,000 birds and layer flock 12,000 birds. The age of broiler breeder flock was 124 days and layer breeder flock 113 days. Hygienic conditions of the farms were good and biosecurity measurements were applied. According to flock records, birds were vaccinated with live Newcastle disease virus and IBV vaccines via spray as well as ILT vector MDV vaccines by the subcutaneous route in the hatchery and followed by NDV, IBV, IBDV, ARTV live vaccines via spray/drinking water and poxvirus vaccine via wing web, AEV via drinking water on farm. Flocks were not vaccinated with adenovirus vaccine.
2.2. Necropsy
Necropsy of the 20 chickens was performed on-site in sick birds. Samples of the liver, heart and spleen embedded to FTA cards were taken from necropsied animals from both broiler breeder and layer breeder flocks and submitted to the Department of Virology of the Veterinary Faculty of Istanbul University-Cerrahpasa. The tests performed in this study were in the context of routine diagnosis and research activities, and no experimental studies were performed during the study. Therefore, no ethical issue is the concern of this study.
2.3. Nucleic Acid Extraction and Reverse Transcription for RNA Viruses
Three punch samples (about 2 mm) were taken from each FTA card which have different tissues from different chickens. 200 μL of QIAcard FTA wash buffer (QIAcard FTA Wash Buffer (Cat. No. WB120112, Qiagen) were added on the samples and incubated for 5 minutes by vortexing. 100 mikrolitre Nuclease-free water was added to each sample. Viral DNA and RNA were extracted from these suspensions to detect FAdVs by using a commercial DNA/RNA extraction kit (innuPREP virus DNA/RNA kit, 854 KS, IST Innuscreen) according to the manufacturer’s protocol (IST Innuscreen, Germany). DNA/RNA was eluted in 30 μL of elution buffer and stored at −20°C until used. Reverse transcription was performed by using a high-capacity cDNA Reverse Transcription Kit (Applied Biosystems, Waltham, MA, USA) following the manufacturer’s instructions.
2.4. PCR Amplification of the Hexon Loop-1 Region of Fowl Adenovirus
The hexon gene was partially amplified by using PCR to confirm the presence of adenoviral DNA and for sequencing as described previously [
10]. One set of primers binding to the hex loop 1 (L1) gene was used to amplify L1. The primers were as follows: Hex L1-F 5′-ATGGGAGCSACCTAYTTCGACAT-3′ (301–323) as the forward primer and Hex L1-R 5′-AAATTGTCCCKRAANCCGATGTA-3′ (890–868) as the reverse primer [
10,
21,
25] Briefly, in an optimised PCR reaction, a total volume of 25 μL of reaction mixture containing 2 μL (10 μM) of each forward and reverse primer, 12.5 μL of Maxima Hot Start PCR Master Mix (Thermo Scientific, Waltham, MA, USA), 4.5 μL of nuclease-free water, 2 μL of MgCl2, and 2 μL of DNA were used to amplify 590 base pair (bp) of hexon gene under the protocol described previously [
10,
21]. In all PCR reactions, positive and negative controls were included. A known positive field sample was used as the positive control, while nuclease-free water was included as the negative control in place of the DNA template. Following 1.5% agarose gel electrophoresis, amplified PCR products from the liver samples were sent for sequencing to a commercial company (MedSanTek, Turkey).
Samples were also analysed by PCR for the presence of infectious bronchitis virus (IBV), avian metapneumovirus (aMPV), Marek disease virus (MDV) and infectious bursal disease virus (IBDV) as described previously [
26,
27,
28,
29] and infectious laryngotracheitis virus (ILTV) by using
in house method as part of routine diagnostic work.
2.5. Sequencing and Phylogenetic Analysis
Nucleotide sequences of the partial hexon genes (590bp) of FAdV were edited, aligned, and used for phylogenetic analysis using the MAFFT version 7 (online version) [
30]. To compare the genotypic relationship between FAdV strains of this study and other FAdV strains detected in other countries, multiple alignments of partial hexon gene sequences of the FAdV data available in the National Centre for Biotechnology Information were made using the MEGA-X software [
31] Phylogentic tree was generated by using Maximum Likelihood method and Hasegawa-Kishino-Yano (HKY) model with 1000 Bootstrap replicates by using the MEGA-X [
31] Comparative percentage homology was performed by doing heatmap using Geneious prime software (Version 2023.0.1). Two FAdV field strains (representative of broiler and layer breeder flocks) detected in this study (in Azerbaijan) were submitted to GenBank under the submission numbers (OQ160972 and OQ160973).
3. Results
3.1. Clinical Findings
The mortality up to 20 % was the first prominent clinical findings in the suspected fowl adenovirus-4 infected chickens. There was a slight increase in mortality by the 7 and 9 weeks of age in both broiler and layer breeder flocks, respectively. In addition, lethargy, ruffled feathers, depression, decreased feed intake and egg production were also observed.
3.2. Postmortem Findings
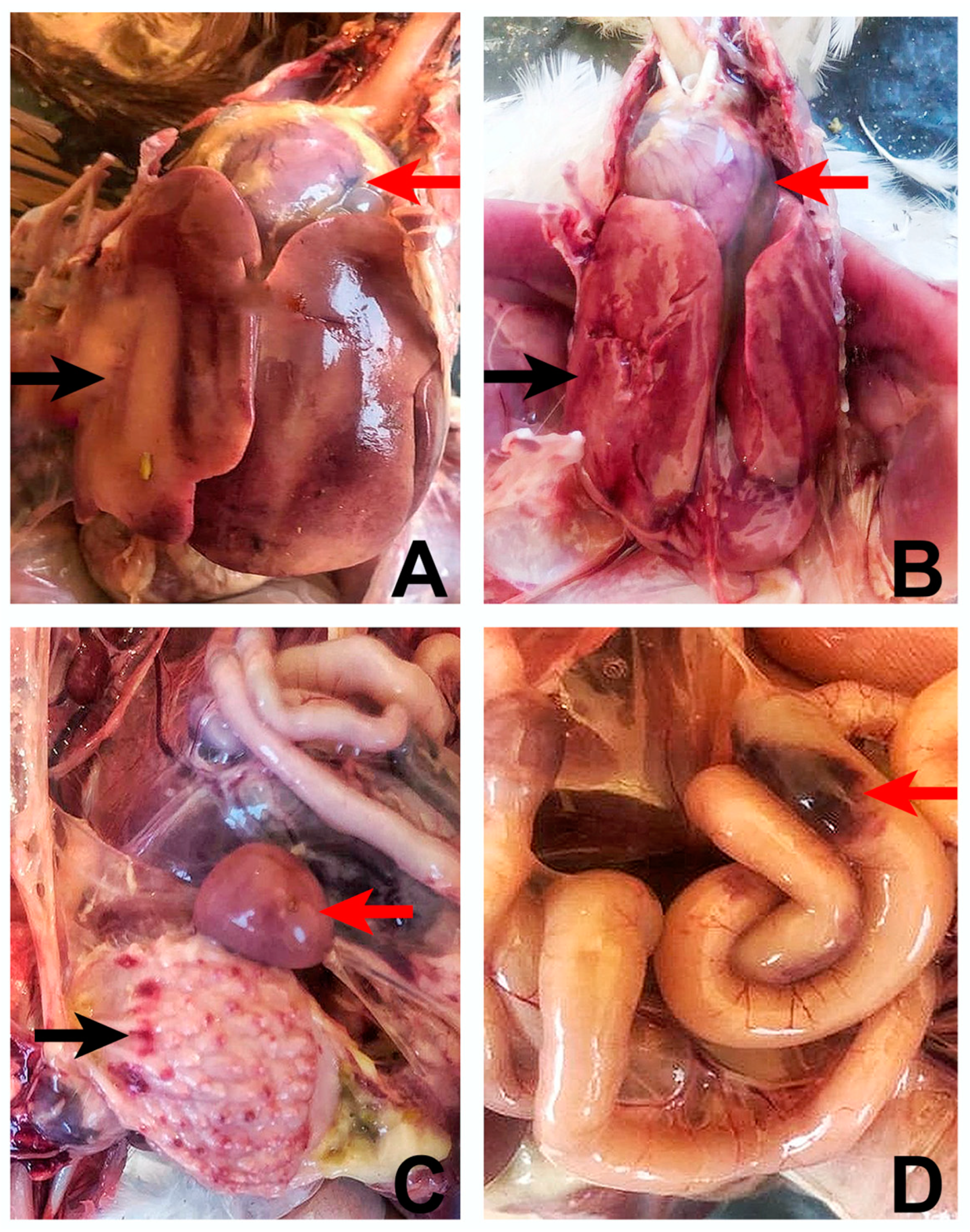
Postmortem findings of both broiler and layer breeder flocks were similar and the most affected organs were the liver-hepatitis and the heart-hydropericardium. The livers were enlarged, friable, and pale with petechial and/or ecchymotic haemorrhages (
Figure 1A,B). Although not observed in all the chickens which had hepatitis, hydropericardium was distinct with an accumulation of clear to straw-colored, watery or jelly-like fluid in the pericardial sac, giving the heart a misshapen and flabby appearance (
Figure 1A,B). The kidneys were swollen and haemorrhagic in about 60% of birds.The spleens showed small white foci on the surface with splenomegaly signs. There were petechial hemorrhages in the mucosa of the proventriculus of around 20% percent of chickens (
Figure 1C). Congestion and ecchymotic hemorrhages in the intestines were also remarkable (
Figure 1D).
3.3. PCR Findings of Other Viral Pathogens of Chickens
When samples were screened for the presence of possible mixed viral infections by PCR, only IBDV-RNA was detected in 2 samples taken from the broiler breeders and layer breeders. The sequence and phylogenetic analysis of these viruses revealed that they were belong to very virulent strain of IBDV.
3.4. Genotype Findings of Fowl Adenoviruses
When DNA extrated from tissue samples on the FTA cards were subjected to PCR to amplify partial hexon gene of fowl adenovirus-4, a DNA band of PCR product 590 bp observed on the agarose gel. Sequence analysis and phylogenetic analyses were performed to detemine the phylographic realtionship of obsevered sequences.
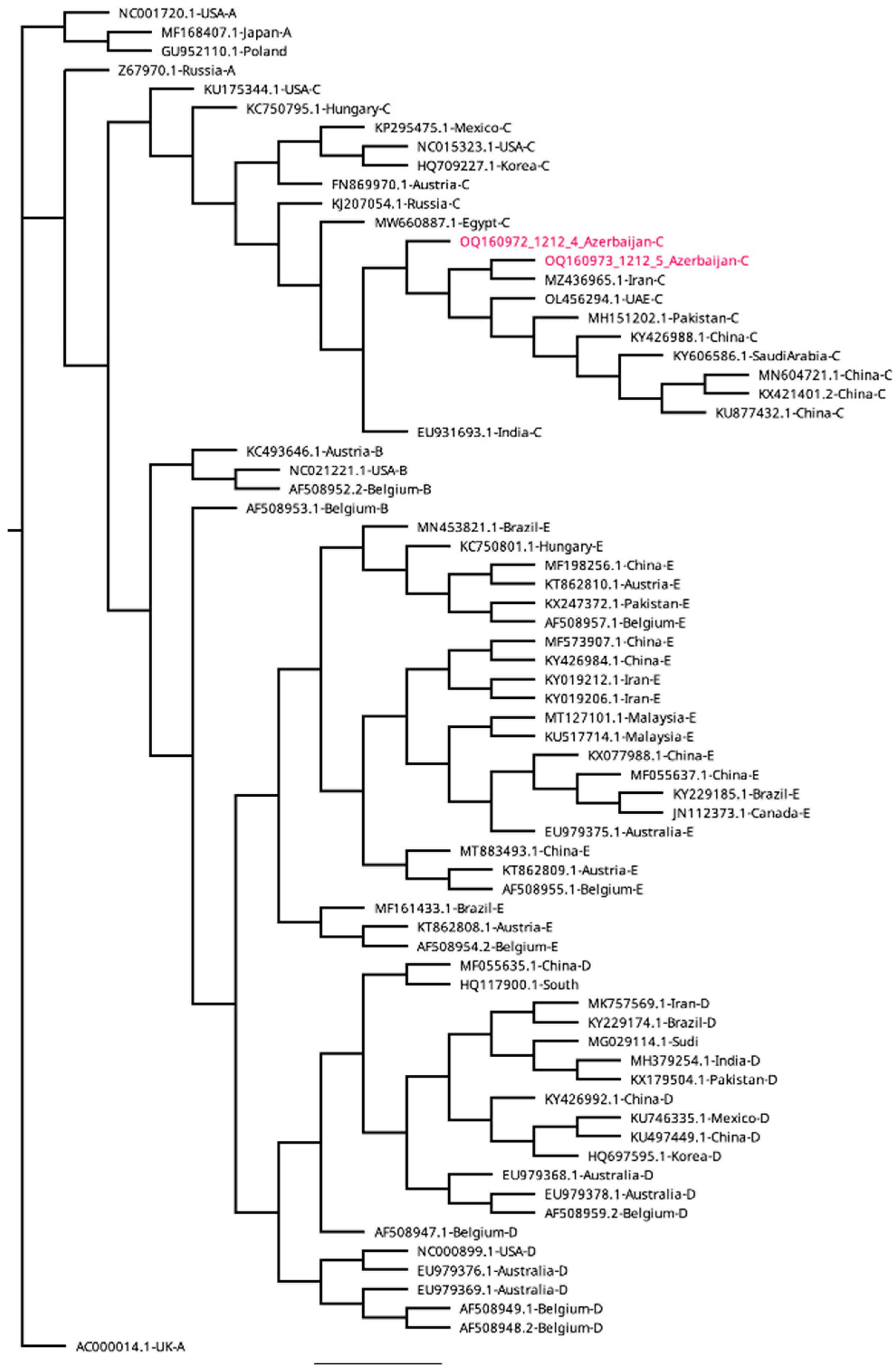
A phylogenetic tree, based on the sequences of the 507 bp
hexon genes, generated five distinct clusters of fowl adenovirus namely A, B, C, D and E (
Figure 2). The FAdVs detected in this study were clustered in the species FAdV-C with 100% nucleodite sequence homology within the amplified hexon gene of fowl adenovirus-4 (
Figure 2). The sequences of FAdV-C obtained in this study shared 100% nucleotide identity to each other (broiler and layer breeders) and between 94.8% and 100% identity with the previously published sequences from other countries (BLAST, NCBI
http://blast.ncbi.nlm.nih.gov/Blast.cgi.). Since all the sequences were similar, only two sequences representing broiler and layer breeder flocks were submitted to GenBank (OQ160972 and OQ160973) and those sequences were used for phylogenetic analyses. All the sequences obtained from the liver and heart were also 100% identical.
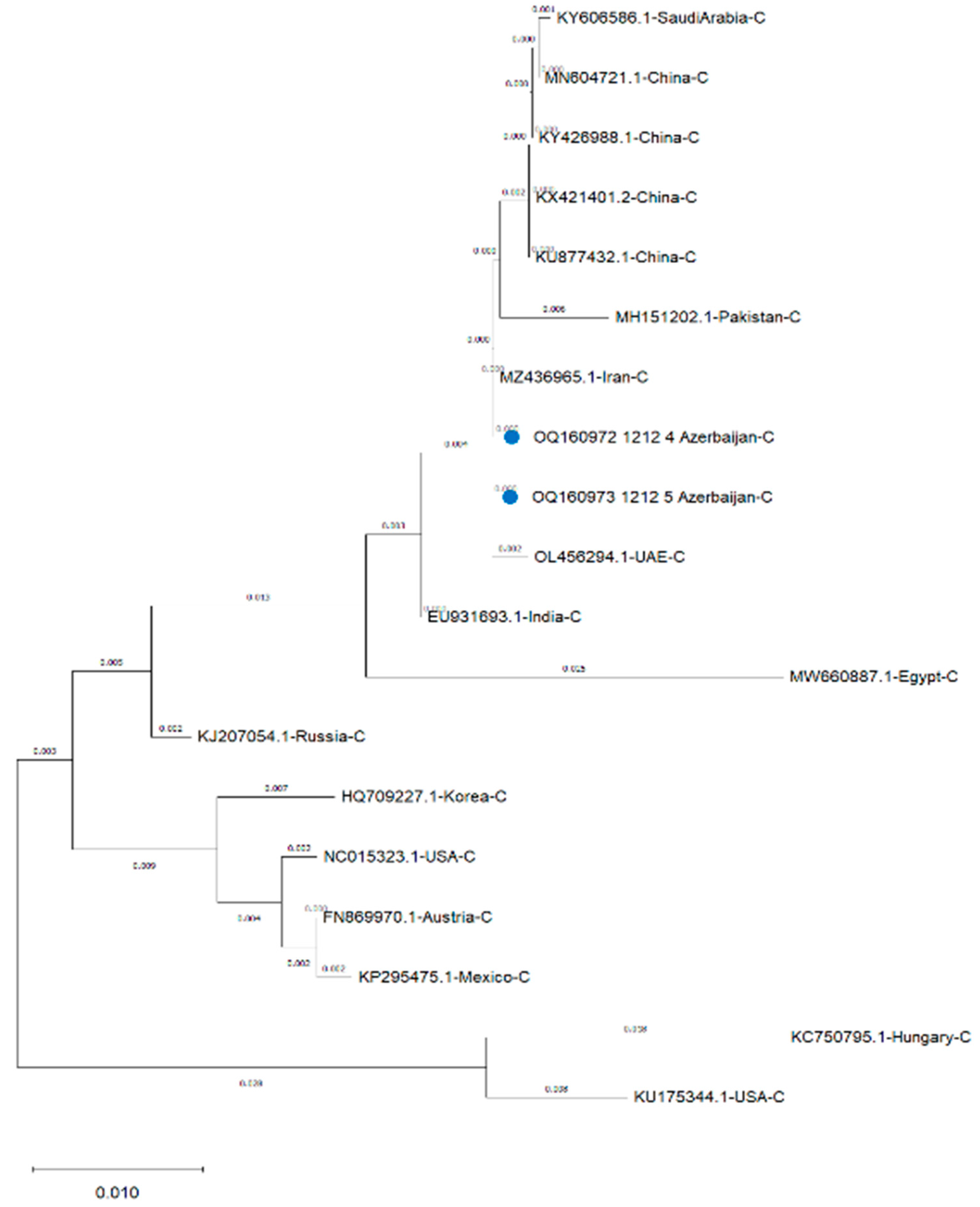
Comparative percentage homology was determined using bioinformatic program (Geneious Prime, Version 2023.0.1) and results indicated that the strains detected in this study had 100% homologous identity with the FAdV-4 reported from Iran (MZ436965) (
Table 1). the sequences also had 99% homology with FAdV-4 virulent strains reported from China (KX421401, KU877432, MN604721, KY426988) and United Arab Emirates (OL456294), 99.6% from India (EU931693) and Saudi Arabia (KY606586), 99.3% from Pakistan (MH151202) 98% from Russia (KJ207054), 97.3 % from Egypt (MW660887), 96.9 % from Austria (FN869970), 96.7 from Mexico (KP295475), 96.5 % from USA (NC015323), 95.9% from Korea (HQ709227), 94.8% from USA (KU175344) and Hungary (KC750795) (
Table 1). The phylogenetic analysis of the hexon gene of FAdV-4 strains against reference strains of FAdV-4 in
Figure 2 revealed high proximity with FAdV-4 strains reported from Iran, United Arab Emirates, China, Saudi Arabia and Pakistan but showed diversity from FadV-4 strains of India, Russia, Egypt, Korea, USA, Austria, Mexico and Hungary (
Figure 2).
4. Discussion
Hydropericardium hepatitis syndrome (HHS), formerly called hydropericardium syndrome (HPS) and Angara disease, was first described in 1987 in broiler chickens in Angara Goth, Pakistan [
3]. Since 2015, after the detection of hypervirulent strain of FAdV-4 in China [
32,
38] HHS cases have increased and severe outbreaks of HHS have been reported in chicken flocks in many countries like Iran, India, China, Egypt, United Arab Emirates, Poland and USA causing economical losses especially in 3–5 weeks old broilers with mortality rate up to 100% [
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17]. After the report of hypervirulent FAdV-4 strain and its rapid spread in China [
32,
38] the emergent novel FAdV-4 serotype became very important virus to investigate in terms of molecular epidemiology and vaccine design. Since there is no report on the adenoviral diseases in chickens in Azerbaijan at present, outbreaks of HHS in broilers and layer breeders were investigated in this study to determine circulating strains and genetic diversity in relation to clinico-pathological signs.
Hexon and fiber proteins of FAdV are important structural proteins in virulence and host immune response to FAdVs and have been used for molecular characterisation by many investigators [
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
33]. In the present study, the hexon gene was used to investigate the phylogeny of the FAdVs. The phylogenetic analysis of hexon gene has shown that all the FadVs detected in this study clustered within FAdV-C serotype FAdV-4. All the sequences were similar with 100 % homology and therefore only two sequences to represent each broiler and layer breeder flock were submitted to GenBank. They also showed 100 % homology with the strain detected in a neighbouring country Iran, and this might indicate that common ancestor virus is circulating because of the trade between these two countries. In addition, the phylogenetic analysis of the hexon gene of FAdV-4 strains have revealed that high proximity with FAdV-4 strains reported from Iran, United Arab Emirates, China, Saudi Arabia and Pakistan was found but sequences showed diversity from FadV-4 strains of India, Russia, Egypt, Korea, USA, Austria, Mexico and Hungary. These results suggest that strains detected in this study might have been deriven from a common ancestor FAdV-4 virus circulating amongst neighboring regions. Similarly, it is possible that strains detected in this study might have been originated from the virulent strain detected in China in 2015 [
32,
38] since the 99% homology was observed with virulent strain of China [
38] as well as the severity of clinical signs and pathological lesions observed in this study. However, the epidemiological relationship is unclear at present and warrants further investigations.
Although the HHS mostly reported from the broiler flocks after 2 weeks of hatch, breeding and laying flocks can also be affected with less frequency [
7] as we have seen in this study. The mortality rates in broilers may reach up to 100% [
16,
17]. However, mortality rate is lower when chickens get older since FAdV-4 infections are found to be age related [
39]. There is a clear age effect with avian adenoviruses, as the age of the host increases, the degree of multiplication of the viruses within the host is restricted and the mortality decreases. In a recent study, the pathogenicity of the hypervirulent (hvFAdV-4 strain GD616) in chickens were investigated and it was found that chickens younger than 59-day-old showed 100% morbidity and mortality, while 180-day-old chickens still exhibited a hydropericardium syndrome with 60% morbidity and 20% mortality [
39]. Similarly, the highest mortality rate observed in this study was 20%. However, Chen and others [
7] reported an HHS outbreak that occurred in a 100-day-old replacement pullet flock with 60% mortality.
The clinical symnptoms ruffled feathers, depression, dullness, varrying degrees of diarrhea, reduced feed intake, reduced performance and lack of uniformity seen in the present study were similar to those reported previously [
15,
16,
17]. It has been well documented that hapatic lesions seen in IBH and HHS cases are similar but the only distinguishing feature between these two diseases is the presence of cardiac lesions and accumulation of fluid in the pericardial sac [
34]. In the present stuy, lesions were detected in liver, heart, kidneys, spleen, proventriculus and intestine but the most affected organs were liver, heart and kidneys. The typical pathological findings at necrropsy like clear, straw-colored fluid accumulation in the pericardial sac, enlarged, friable and pale yellow liver with multiple haemorrhages, enlarged spleen with necrotic foci on their surface and swollen haemorrhagic kidneys were also similar to those reported previously [
7,
11,
15,
16,
17,
34] However, petechial haemorrhages seen in the proventriculus have been reported in HHS cases although the adenoviral gizzard erosions (AGE) has been reported due to adenovirus serotype 1 (FAdV-1) infections in broiler chickens [
35]. However, swelling of the proventriculus was seen in experiementally infected Specified pathogen free (SPF) chickens and proventricular bleeding observed in commercial chickens [
37]. All of these findings indicate that FAdV-4 might be affecting glandular stomach [
4,
37]. However, lesions seen in proventriculus is most lkely to be the consequence of vvIBDV infection dtected in this study since it has been previously reported in vvIBDV infection (36).
There are some repeorts that co-infections with immunosuppressive chicken viruses like Marek’s disease virus
(MDV), infectious bursal disease virus, (IBDV), chicken anemia virus (CAV), Avian metapneumovirus (AMPV) or infectious laryngotracheitis virus (ILTV), may exacerbate the FAdV pathogensis in chickens [
6,
19,
40] For this reasion, we also analysed the the presence of these viruses (MDV, IBV, IBDV aMPV, and ILTV) in both flocks. Presence of vvIBDV infection was determined indicating that IBDV may have affected the disease severity. In conrast, there are reports showing the occurence of severe FAdV infections in the absence of immunosuppressive viruses [
12,
18,
41].
The farms analysed in this study were vaccinated against major chicken viruses such as NDV, IB, and others. However, they were not vaccinated for fowl adenoviruses. Therefore, increase in biosecurity measurements and vaccination strategies needed to apply for prevention and control of fowl adenovirus infections in Azerbaijan. Vaccines against HHS, particularly novel genotype, play a curicial role and will be the most effective tool to prevent and control of FAdV-4 infections [
38].
In conclusion, this is the first report detailing the genetic composition of FAdVs and the HHS disease with severe hepatitis and hydropericardium caused by FAdV-4 in broiler and layer breeders in Azerbaijan. The results provide an evidence that continued prevlence of virulent strains of FadVs in chickens flocks is becoming a serious concerns for the poultry production in the central Asian countries Increased diseases burden along with severe economical loses requires a an effective diseases control stratagies including availability of efficacious. The data on disease burden, epidemiological studies togather from genotype to phenotype of prevaling fowl adenoviruses and their association with overall dammage to poultry production in Azerbaijan is important in the development of frame work for implematation of disease preventative measures (diagnostics and vaccination). Our data will contribute in this effort for the development and implementation of appropriate effective vaccines to prevent and control the IBH and HHS diseases in chickens in Azerbaijan.
Author Contributions
Conceptualization: E.B., H.Y., A.Y., and N.T.; Data curation: A.Y., O.A., H.E.T., S,G.Y., N.T., A.O., E.B., O.E.B., and M.K.; Formal analysis: A.Y., O.A., H.E.T., S,G.Y., N.T., and E.B.,; Funding acquisition: H.Y., and M.I; Investigation: A.Y., O.A., H.E.T., S,G.Y., N.T., A.O., E.B., O.E.B., and M.K.; Methodology: M.I., A.Y., N.T., J-R.S., P.C., H.Y., and E.B.; Project administration: HY, M.I.; Resources: H.Y., N.T., A.Y., and M.I.; Software: E.B., A.Y., J-R.S., and P.C.; Supervision: A.Y., J-R. S., P.C., H.Y., and M.I.; Validation: A.Y., and O.A., Writing—original draft: E.B., A.Y., H.Y., and M.I.; Writing—review & editing: E.B., A.Y., H.Y., J-R.S., P.C., and M.I.
Funding
This work was funded by the Istanbul University-Cerrahpasa (BAP-Project No: 27243). The funding from UK Research and Innovation (UKRI) Biotechnology and Biological Sciences Research Council (BBSRC) Grants (BBS/E/I/00007034, BBS/E/I/00007035, BB/T013087/1, BB/W003325/1, BB/S011269/1) also contributed to this study.
Institutional Review Board Statement
All subjects gave their informed consent for inclusion before they participated in the study.
Informed Consent Statement
Informed consent was taken from the patients
Data Availability Statement
The data of this study are included in the manuscript. The data is available up on request from the corresponding author.
Conflicts of Interest
The authors declared that there is no conflicts of interest.
References
- Hess, M.; Aviadenovirus infections. In D.E. Swayne, M. Bouliann, C.M. Logue, L.R. McDougald, V. Nair, D.L. Suarez, S. de Wit, T. Grimes, D. Johnson, M. Kromm, T.Y. Prajitno, I. Rubinoff & G. Zavala (Eds.), Diseases of poultry. 2020, (14th ed., pp. 322–332). Hoboken, NJ: Wiley-Blackwell.
- Helmboldt, C.F.; Frazier, M.N; Avian hepatic inclusion bodies of unknown significance. Avian Dis. 1963, 7(4), 446-50.
- Anjum, A.D.; Sabri, M.A.; Iqbal, Z. Hydropericarditis syndrome in broiler chickens in Pakistan. Vet. Rec. 1989, 124, 247– 248. [CrossRef]
- Li, P.H.; Zheng, P.P.; Zhang, T.F.; Wen, G.Y; Shao, H.B.; Luo, Q.P. Fowl adenovirus serotype 4: Epidemiology, pathogenesis, diagnostic detection, and vaccine strategies. Poult Sci. 2017, 96(8), 2630-2640.
- El-Shall, N.A.; El-Hamid, H.S.A.; Elkady, M.F.; Ellakany, H.F.; Elbestawy, A.R.; Gado, A.R.; Geneedy, A.M.; Hasan, M.E.; Jaremko, M.; Selim, S.; El-Tarabily, K.A.; El-Hack, M.E.A. Corrigendum: Epidemiology, pathology, prevention, and control strategies of inclusion body hepatitis and hepatitis-hydropericardium syndrome in poultry: A comprehensive review. Front Vet Sci. 2022, 9, 1075948.
- Niczyporuk, J.S. Phylogenetic and geographic analysis of fowl adenovirus field strains isolated from poultry in Poland. Arch. Virol. 2016, 161(1), 33-42. [CrossRef]
- Chen, L.; Yin, L.; Zhou, Q. Epidemiological investigation of fowl adenovirus infections in poultry in China during 2015–2018. BMC Vet Res. 2019, 15, 271. [CrossRef]
- Wang, K.; Sun, H.; Li, Y. Characterization and pathogenicity of fowl adenovirus serotype 4 isolated from eastern China. BMC Vet Res. 2019, 15, 373. [CrossRef]
- Wibowo, M.H.; Sahesty, A.; Mahardika, B.K.; Purwanto, B.; Lestariningsih, C.L.; Kade Suardana, I.B.; Oka Winaya, I.B.; Irine, I.; Suryanggono, J.; Jonas, M.; et al. Epizootiology, Clinical Signs, and Phylogenetic Analysis of Fowl Adenovirus in Chicken Farms in Indonesia from 2018 to 2019. Avian Dis. 2019, 63(4), 619-624. [CrossRef]
- Cizmecigil, U.Y.; Umar, S.; Yilmaz, A.; Bayraktar, E.; Turan, N.; Tali, B.; Aydin, O.; Tali, H.E.; Yaramanoglu, M.; Yilmaz, S.G.; et al. Characterisation of Fowl Adenovirus (FAdV-8b) Strain Concerning the Geographic Analysis and Pathological Lesions Associated with Inclusion Body Hepatitis in Broiler Flocks in Turkey. J Vet Res. 2020, 64(2),231-237. [CrossRef]
- Yuming, F.; Sheng, Y.; Wenyu, D.; Shihong, C.; Wenfeng, L.; Wenjing, H.; Xiaowen, L.; El-Ashram, S.; Mei, K.; Jinyue, G.; et al. Molecular characterization and phylogenetic analysis of fowl adenovirus serotype-4 from Guangdong Province, China. Vet World. 2020, 13(5), 981-986. [CrossRef]
- Chitradevi, S.; Sukumar, K.; Suresh, P.; Balasubramaniam, G.A.; Kannan, D. Molecular typing and pathogenicity assessment of fowl adenovirus associated with inclusion body hepatitis in chicken from India. Trop Anim Health Prod. 2021, 53(4), 412. [CrossRef]
- Lai, V.D.; Min, K.; Lai, H.T.L.; Mo, J. Epidemiology of fowl adenovirus (FAdV) infections in South Korean chickens during 2013-2019 following introduction of FAdV-4 vaccines. Avian Pathol. 2021, 50(2), 182-189. [CrossRef]
- Mete, A.; Armien, A.G.; Rejmanek, D.; Mott, M.; Crossley, B.M. Emergence of fowl aviadenovirus C-4 in a backyard chicken flock in California. J Vet Diagn Invest. 2021, 33(4), 806-809. [CrossRef]
- Sultan, H.; Arafa, A.E.; Adel, A.; Selim, K.; Hossiny, M.; Talaat, S. Molecular Detection of a Novel Fowl Adenovirus Serotype-4 (FadV-4) from an Outbreak of Hepatitis Hydropericardium Syndrome in Commercial Broiler Chickens in Egypt. Avian Dis. 2021, 65(3), 385-390. [CrossRef]
- Ishag, H.Z.A.; Terab, A.M.A.; El Tigani-Asil, E.T.A.; Bensalah, O.K.; Khalil, N.A.H.; Khalafalla, A.I.; Al Hammadi, Z.M.A.H.; Shah, A.A.M.; Al Muhairi, S.S.M. Pathology and Molecular Epidemiology of Fowl Adenovirus Serotype 4 Outbreaks in Broiler Chicken in Abu Dhabi Emirate, UAE. Veterinary Sciences. 2022, 9(4), 154. [CrossRef]
- Toroghi, R.; Sodavari, S.; Tabatabaeizadeh, S.E.; Sharghi, A.S.; Irankhah, N.; Fakhraee, M.; Farzin, H.R.; Sarani, M.; Khayyat, S.H.; Ashouri, M.; et al. The First Occurrence of Hepatitis-Hydropericardium Syndrome in Iran and Effective Applied Control Measures in the Affected Commercial Broiler Flock. Avian Dis. 2022, 66(2), 213-219. [CrossRef]
- Steer, P.A.; Kirkpatrick, N.C.; O’Rourke, D.; Noormohammadi, A.H. Classification of fowl adenovirus serotypes by use of high-resolution melting-curve analysis of the hexon gene region. J Clin Microbiol. 2009, 47(2), 311-321. [CrossRef]
- Schachner, A.; Matos, M.; Grafl, B.; Hess, M. Fowl adenovirus-induced diseases and strategies for their control—a review on the current global situation. Avian Pathol. 2018, 47(2), 111-126. [CrossRef]
- Benkő, M.; Aoki, K.; Arnberg, N.; Davison, A.J.; Echavarría, M.; Hess, M.; Jones, M.S.; Kaján, G.L.; Kajon, A.E.; Mittal, S.K.; et al. ICTV Virus Taxonomy Profile: Adenoviridae 2022. J Gen Virol. 2022, 103(3), 001721. [CrossRef]
- Schachner, A.; Marek, A.; Grafl, B.; Hess, M. Detailed molecular analyses of the hexon loop-1 and fibers of fowl aviadenoviruses reveal new insights into the antigenic relationship and confirm that specific genotypes are involved in field outbreaks of inclusion body hepatitis. Vet Microbiol. 2016, 186, 13-20. [CrossRef]
- Schachner, A.; Hess, M. Special Issue: Avian Adenoviruses. Viruses. 2022, 14(4):680. [CrossRef]
- Marek, A.; Schulz, E.; Hess, C.; Hess, M. Comparison of the fibers of Fowl adenovirus A serotype 1 isolates from chickens with gizzard erosions in Europe and apathogenic reference strains. J Vet Diagn Invest. 2010, 22(6), 937-941. [CrossRef]
- Raue, R.; Gerlach, H.; Müller, H. Phylogenetic analysis of the hexon loop 1 region of an adenovirus from psittacine birds supports the existence of a new psittacine adenovirus (PsAdV). Arch Virol. 2005, 150(10), 1933-43. [CrossRef]
- Pilkington, P.; Brown, T.; Villegas, P.; McMurray, B.; Page, R.K.; Rowland, G.N.; Thayer, S.G. Adenovirus-Induced Inclusion Body Hepatitis in Four-Day-Old Broiler Breeders. Avian Diseases, 1997, 41(2), 472–474. [CrossRef]
- Yilmaz, H.; Altan, E.; Cizmecigil, U.Y.; Gurel, A.; Ozturk, G.Y.; Bamac, O.E.; Aydin, O.; Britton, P.; Monne, I.; Cetinkaya, B.; et al. Phylogeny and S1 Gene Variation of Infectious Bronchitis Virus Detected in Broilers and Layers in Turkey. Avian Dis. 2016, 60(3), 596-602. [CrossRef]
- Bayraktar, E.; Umar, S.; Yilmaz, A.; Turan, N.; Franzo, G.; Tucciarone, C.M.; Cecchinato, M.; Cakan, B.; Iqbal, M.; Yilmaz, H. First Molecular Characterization of Avian Metapneumovirus (aMPV) in Turkish Broiler Flocks. Avian Dis. 2018, 62(4), 425-430. [CrossRef]
- Yilmaz, A.; Turan, N.; Bayraktar, E.; Gurel, A.; Cizmecigil, U.Y.; Aydin, O.; Bamac, O.E.; Cecchinato, M.; Franzo, G.; Tali, H.E.; et al. Phylogeny and evolution of infectious bursal disease virus circulating in Turkish broiler flocks. Poult Sci. 2019, 98(5), 1976-1984. [CrossRef]
- Yilmaz, A.; Turan, N.; Bayraktar, E.; Tali, H.E.; Aydin, O.; Umar, S.; Cakan, B.; Sadeyen, J.R.; Baigent, S.; Iqbal, M.; et al. Molecular characterisation and phylogenetic analysis of Marek’s disease virus in Turkish layer chickens. Br Poult Sci. 2020, 61(5), 523-530. [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874.
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [CrossRef]
- Ye, J.; Liang, G.; Zhang, J.; Wang, W.; Song, N.; Wang, P.; Zheng, W.; Xie, Q.; Shao, H.; Wan. Z.; et al. Outbreaks of serotype 4 fowl adenovirus with novel genotype, China. Emerg Microbes Infect. 2016, 5(5), e50. [CrossRef]
- Zhang, Y.; Liu, R.; Tian, K.; Wang, Z.; Yang, X.; Gao, D.; Zhang, Y.; Fu, J.; Wang, H.; Zhao, J. Fiber2 and hexon genes are closely associated with the virulence of the emerging and highly pathogenic fowl adenovirus 4. Emerg Microbes Infect. 2018, 7(1), 199. [CrossRef]
- Niu, Y.J.; Sun, W.; Zhang, G.H.; Qu, Y.J.; Wang, P.F.; Sun, H.L.; Xiao, Y.H.; Liu, S.D. Hydropericardium syndrome outbreak caused by fowl adenovirus serotype 4 in China in 2015. J Gen Virol. 2016, 97(10), 2684-2690. [CrossRef]
- Mirzazadeh, A.; Grafl, B.; Abbasnia, M.; Emadi-Jamali, S.; Abdi-Hachesoo, B.; Schachner, A.; Hess, M. Reduced Performance Due to Adenoviral Gizzard Erosion in 16-Day-Old Commercial Broiler Chickens in Iran, Confirmed Experimentally. Front Vet Sci. 2021, 8, 635186. [CrossRef]
- Islam, M.T.; Samad, M.A. Clinico-pathological studies on natural and experimental infectious bursal dis- ease in broiler chickens. Bangladesh J. Vet. Med., 2004, 2: 31-35.
- Jiang, Z.; Liu, M.; Wang, C.; Zhou, X.; Li, F.; Song, J.; Pu, J.; Sun, Y.; Wang, M.; Shahid M.; et al. Characterization of fowl adenovirus serotype 4 circulating in chickens in China. Vet Microbiol. 2019, 238, 108427. [CrossRef]
- Liu, A.; Zhang, Y.; Cui, H.; Wang, X.; Gao, Y.; Pan, Q. Advances in Vaccine Development of the Emerging Novel Genotype Fowl Adenovirus 4. Front Immunol. 2022, 13, 916290. [CrossRef]
- Yuan, F.; Song, H.; Hou, L.; Wei, L.; Zhu, S.; Quan, R.; Wang, J.; Wang, D.; Jiang, H.; Liu, H.; et al. Age-dependence of hypervirulent fowl adenovirus type 4 pathogenicity in specific-pathogen-free chickens. Poult Sci. 2021, 100(8), 101238. [CrossRef]
- Choi, K.S.; Kye, S.J.; Kim, J.Y.; Jeon, W.J.; Lee, E.K.; Park, K.Y.; Sung, H.W. Epidemiological investigation of outbreaks of fowl adenovirus infection in commercial chickens in Korea. Poult Sci. 2012, 91(10), 2502-6. [CrossRef]
- Gomis, S.; Goodhope, A.R.; Ojkic, A.D.; Willson, P. Inclusion body hepatitis as a primary disease in broilers in Saskatchewan, Canada. Avian Dis. 2006, 50(4), 550-505.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).