1. Introduction
The mouth is a central feature of our face, facilitating our most important functions such as eating, breathing and speech. An important event in mouth formation is the creation of “hole” which connects the digestive system and the external environment. This hole, which has also been called the primary or embryonic mouth in vertebrates [
1,
2,
3], appears in humans during the fourth week of pregnancy. At this time the surrounding face is still in the early stages of development comprised of the frontonasal and paired maxillary and mandibular prominences. These prominences will grow, converge, and develop into the tissues such as bones and muscles that make up the jaw and palate (see reviews [
4,
5,
6,
7,
8]). As the craniofacial complex matures the remnants of the embryonic mouth comes to reside deep inside the oral cavity at the level of the tonsils. Due to the transient nature and the difficulty to study the embryonic mouth in mammals, the importance of the structure has been under appreciated. However, a revival of embryological and molecular studies focusing on the formation of the mouth in other developmental models has revealed its importance in craniofacial development. For example, the region that gives rise to the embryonic mouth, called the Extreme Anterior Domain, has been shown to contain signals that pattern the surrounding face in
Xenopus [
9,
10,
11]. On the other hand, important aspects of the mouth opening have been proposed but little studied. For example, the actual hole formed during embryonic mouth formation could provide the physical separation of the growing prominences, yet this has not been rigorously tested. In mammals, the opening to the digestive system is also thought to be required for ingestion of amniotic fluid which aids in development of both the digestive and immune systems [
12]. However, this role is very difficult to study. Thus, it is clear we need more comprehensive studies of the anatomy and formation of the mouth.
Xenopus has been integral to furthering our basic understanding of how the mouth is formed. We know that the Extreme Anterior Domain gives rise to the mouth and this region undergoes several morphological changes or processes before a hole is formed [
1,
2,
9,
13,
14] Such processes include cell death and cellular reorganizations that eventually form a thin covering over the embryonic mouth, called the buccopharyngeal membrane. This buccopharyngeal membrane is actually 1-2 cell layers which is continuous with the facial epidermis and lining of the oral cavity [
15,
16,
17,
18,
19]. The cells of the buccopharyngeal membrane are unique in that they do not secrete an ECM and are not derived from mesodermal nor neural crest cells [
9]. An important last step in embryonic mouth development is the rupture or perforation of this buccopharyngeal membrane which then creates the opening to the digestive system.
When the buccopharyngeal membrane does not rupture, it results in a medical condition called persistent buccopharyngeal membrane [
20,
21,
22,
23]. While rare on its own, this condition also appears to be present in a number of craniofacial syndromes such as 1p36 deletion syndrome (OMIM #607872), Hypomandibular faciocranial dysostosis (OMIM #241310) Agnathia-otocephaly complex (OMIM #202650), microphthalmia, syndromic 5 (OMIM # 610125) and Holzgreve-Wagner-Rehder syndrome (OMIM # 236110) [
24,
25]. A persistent buccopharyngeal membrane can also be accompanied by cleft palate [
26]. Importantly, a persistent buccopharyngeal membrane has also been proposed to be a possible cause for more common oral obstruction type malformations [
27,
28,
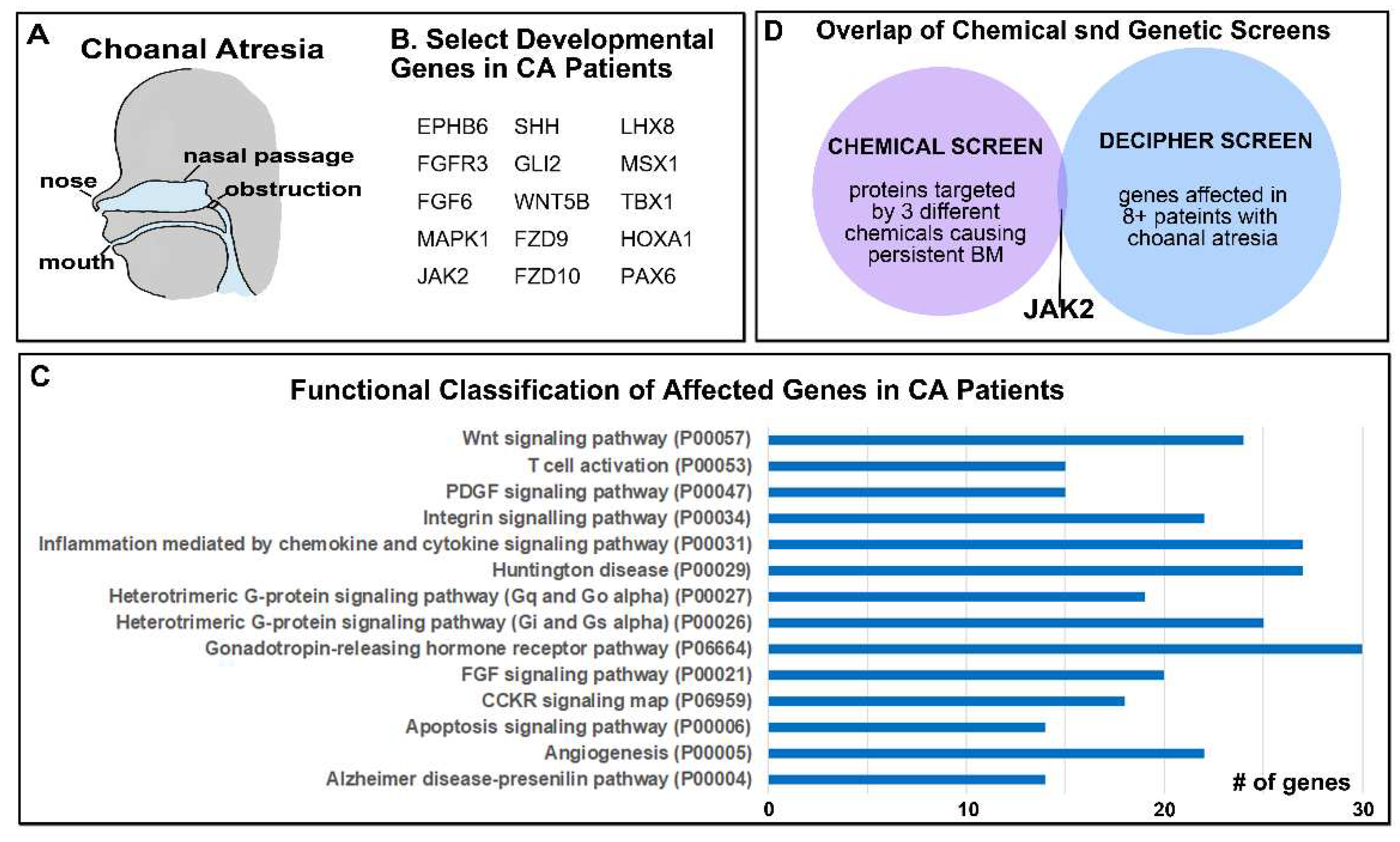
29]. In particular one such condition, Choanal Atresia, occurs when there is a physical blockage between nasal and oral cavities at birth [
30]. This condition can cause respiratory distress in newborns and when undiagnosed has been postulated to lead to sudden infant death syndrome (SIDS) [
31]. Choanal Atresia is difficult to correct and even with surgery patients have long lasting problems in their airways that affect their quality of life [
30]. Strikingly, in a search of the OMIM database, Choanal Atresia is present in 82 different congenital disorders (Suppl Excel File 1). Human and mouse studies have revealed that disruptions of retinoic acid and FGF signaling can result in Choanal Atresia [
32,
33,
34]. Such studies also indicate that disruptions to other craniofacial processes such as primitive choana formation and cranial vault development can lead to this craniofacial malformation. Thus, if a persistent buccopharyngeal membrane is responsible for even a subset of the multitude of disorders accompanied by Choanal Atresia there is an even more compelling reason to understand mechanisms that affect the last stages of mouth development.
In summary, a collation of case studies and descriptions of craniofacial disorders has revealed that persistent buccopharyngeal membrane may be more pervasive and detrimental than initially believed. But we know very little about the mechanisms governing the process that leads to its rupture of this structure. The goals of this study are to begin to rectify a gap in our knowledge of mouth development and uncover potential mechanisms that drive the perforation of the buccopharyngeal membrane. We use the vertebrate model
Xenopus laevis. This is an ideal model for studies of buccopharyngeal membrane rupture since the embryonic or primary mouth is well conserved with mammals [
35] but unlike mammals it is easy to view and study. We use comprehensive chemical and genetic screens combined with classic embryological methods in this model to demonstrate that rupture of the buccopharyngeal membrane requires mechanical forces generated by the jaw muscles.
2. Materials and Methods
Gathering embryos and staging
Xenopus laevis were housed in the Biology Department Aquatics facility and maintained using protocols approved by VCU IACUC. Embryos were obtained and cultured using standard methods [
59]. To ensure that results were not clutch specific we gathered eggs from two to three females into a petri dish of egg media (high salt 1X Modified Barth’s Solution). The media was removed and one half a testis (previously removed from a male frog) was cut up and spread around the dish of eggs. This was followed by adding frog embryo media (0.1X Modified Barth’s Solution). Once the embryos were fertilized, they were immersed in a 2% cysteine solution (ph 7.8) and swirled until the jelly coats were dissolved. Embryos were then washed in frog media and maintained in this solution in a 15°C incubator until desired stage for experiments. Staging was performed according to Nieuwkoop and Faber (Nieuwkoop and Faber, 1967).
Chemical screen
At stage 22-24, six embryos were placed in 2ml of frog embryo media in each well of 12 well culture dishes. Stock solutions (10mM) dissolved in DMSO of each chemical from the NCI Cancer Diversity set II were added directly to the wells so that the final concentrations of all chemicals were 30uM. The culture dishes were gently swirled and then the dish was placed in an incubator set to 23°C. Controls consisted of sibling embryos that were incubated with equivalent volumes of DMSO in the same volume of media. At stage 40-41 the media and chemicals were removed and replaced with 4% paraformaldehyde (PFA) to fix the embryos. The faces of embryos were imaged as described below. Characteristics that were recorded were whether the embryos had 1) stomodeum, 2) an open mouth, 3) a cleft-like appearance of the open mouth or 4) no observable sign of a mouth. All chemicals that caused embryos to have a stomodeum and persistent buccopharyngeal membrane were retested using the same protocol. The PubChem (
https://pubchem.ncbi.nlm.nih.gov) database was used to identify candidate targets of chemicals that caused buccopharyngeal membrane persistence. Genecards (
www.genecards.org) was used to determine the function and corresponding gene symbol of each target. A functional classification of all the targets was performed using PANTHER (
www.pantherdb.org) [
60]. Data is publically available and hosted here (
https://drive.google.com/drive/folders/1XYI1iE54YD0ZEHlXLicsUxl9d5cg61rx?usp=sharing).
Copy number variant screen in patients with Choanal Atresia
Imaging the Facial Features
At stage 40-41, embryos were anesthetized in 1% tricaine for 10 minutes and then fixed in 4% paraformaldehyde overnight at 4°C. Embryos were washed in phosphate buffered saline with 0.1% Tween 20 (PBT) and placed in plastic petri dishes. A sterile disposable No. 15 scalpel (VWR, cat. no: 82029-856) and Dumont No. 5 forceps (Fisher, cat. no: NC9404145) were used to make two cuts to isolate the head: first- at the posterior end of the gut and then second caudal to the gills. Isolated heads were mounted in small holes or depressions in either agarose or clay-lined dishes containing PBT. The faces were imaged using a Discovery V8 stereoscope fitted with an Axiovision digital camera (Zeiss).
Morpholinos
Antisense
jak2 morpholino (CTCTCTGTTTGTGTCACTGTCAAAG),
myod morpholino GGCAAGAGCTCCATAGAAACAGCCG [
41] and standard control morpholino were purchased from Gene Tools. Microinjections were carried out using an Eppendorf microinjector and a Zeiss stereoscope.
Phalloidin labeling, immunofluorescence auto fluorescent rendering
To examine F-actin, embryos were fixed in 4% PFA, washed in PBT and incubated in Alexa Fluor 488 labeled Phalloidin (1:50, Invitrogen) for 2-5 days at 4°C. Laminin and muscle specific antigen (12/101) were visualized by immunofluorescence. Briefly, embryos were fixed in 4% PFA then dehydrated in a methanol series (50%,70%, 80%, 90% and 100%) where they were then stored in the freezer (-20°C) for 1 day to 2 weeks. Embryos were then rehydrated by reversing the same series of methanol solutions and then either processed whole or in tissue sections (see below). The embryos or tissues were washed in PBT and blocked/permeabilized overnight in 1% BSA, 1% serum and 1% triton-X 100. Samples were incubated in phospho-JAK2 (Sigma, 1:100), laminin antibody (1:1000, Sigma) or 12/101 (1:25, Developmental Studies Hybridoma Bank) for 48 hours and then washed in PBT. Secondary detection was performed with goat-anti mouse or goat anti-rabbit Alexa Fluor 488 or 568 (1:500, Invitrogen) combined with 2 drops of Dapi (NucBlue, Invitrogen), for 24 hours. After washing in PBT, samples were immersed in 90% glycerol in PBT for imaging.
Embryos were rendered auto fluorescent by immersing in Bouin’s fixative for one day to two weeks. After washing in 100% ethanol, embryos were then immersed in a solution of two parts benzyl benzoate and one part benzyl alcohol and imaged on glass slides.
All fluorescent images were collected with a C2 Nikon confocal microscope (VCU Biology microscopy core) using 0.5-1.0 micron steps and compiled using the maximum intensity function to compress the z stacks. All experimental images were adjusted for brightness and color balance in the same way as controls to adjust for variations in fluorescent intensity between biological replicates.
Tissue sectioning
Embryos were sectioned using two different methods 1) thick agarose section and 2) thin plastic sections. Thick agarose sections were created with embryos fixed in 4% PFA and then washed in PBT. Embryos were then immersed in 4-5% low melt agarose in a small petri dish. A square of agarose was cut, containing the embryos, and attached using superglue to the mounting block. 200 micron sections were created with a 5000 series Leica vibratome. For thin plastic sections embryos were fixed in 2% PFA and 2% glutaraldehyde in PBT buffer for 24 hours and then embedded in plastic resin (JB-4 Plus) and sectioned at 5μm using a tungsten carbide knife on a rotary microtome. Sections were stained with Giemsa (1:20, Fisher Scientific) for 1 hour followed by a 10 second acetic acid (0.05%) differentiation wash. Slides were dried and covered with Permount and imaged on a Nikon compound microscope fitted with a digital camera (VCU Biology microscopy core).
Micro incisions in the face
Prior to all surgeries embryos were anesthetized in 0.1% tricaine (in 0.1X MBS) for 15 minutes and then moved into clay lined dissection dishes containing 0.01% tricaine. The embryos were placed vertically into custom fit holes made in the clay and gently secured by modeling the clay around the face. Glass knives, created from pulled out capillary tubes using a Sutter needle puller, were used to sever the tissue. To assess effects on buccopharyngeal membrane perforation embryos were maintained for 3-5 hours (until complete rupture in all control embryos) and imaged. To assess buccopharyngeal membrane structure, embryos were fixed in 4% PFA, 30 minutes after surgery and labeled with phalloidin as described above.
3. Results
3.1. Buccopharyngeal membrane perforation in Xenopus laevis
The embryonic mouth forms in a series of morphological steps, the last of which is the perforation of the buccopharyngeal membrane. The first indication of perforation occurred during stage 39 (~56-60 hpf at 23°C) when a small hole was observed in the buccopharyngeal membrane (
Figure 1A-C’). This first hole became larger and then a second hole often formed (
Figure 1D,D’). As each of the holes increased in size, the cells appeared to stretch across the embryonic mouth (
Figure 1E, E’). Eventually, the holes coalesced to form a single hole (
Figure 1F,F’). Finally, this larger hole increased in size until the entire buccopharyngeal membrane disappeared. The entire process, from the first small hole to the elimination of the buccopharyngeal membrane, took approximately 5-6 hours at 23°C.
3.2. Chemical screen identified known and new candidate regulators of buccopharyngeal membrane perforation.
To uncover mechanisms regulating buccopharyngeal perforation and general mouth development an unbiased chemical screening approach was employed. Embryos were exposed to 1200 different chemicals from the Cancer Diversity Set II and Natural Product Set I over the period of mouth development (
Figure 1G). We determined that exposure to 41 different chemicals resulted in embryos with a persistent buccopharyngeal membrane but also an observable stomodeum (Suppl Excel File 2), suggesting a more specific effect later in mouth development (
Figure 1H-O, shows a subset). To find candidate targets of the identified chemicals, we turned to the biological activity database hosted in PubChem. From this, 176 different biological targets were identified, some chemicals having one target while others could target many proteins. We then determined each target’s function and corresponding gene symbol by interrogating GeneCards (Suppl Excel File 2). By manually scrutinizing the targets, several signaling molecules or transcription factors that are known to have important roles during embryonic development were identified (for example, members of the retinoic acid, BMP, WNT, and JAK/STAT signaling pathways, Fig 1P). Next, a functional classification of all the biological targets was performed using PANTHER and categories were identified with 5 or more genes. Two such categories are known to be required for embryonic mouth development, WNT signaling and Apoptosis [
2,
14]. These results reflected the validity of the screen but additionally also allowed us to identify new candidate pathways to investigate. For example, enriched categories included opioid, dopamine and serotonin signaling which could point to an importance of neurotransmission. Alternatively, ion channels and neurotransmitters have also been shown to have non-neural roles in craniofacial development [
36,
37]. Future work could further investigate how these neurotransmitter and other signaling pathways could be involved in buccopharyngeal membrane rupture.
3.3. Human genetic data from patients with choanal atresia identified potential regulators of buccopharyngeal membrane perforation.
In parallel, a complimentary but very different screening approach was utilized to uncover regulators of buccopharyngeal rupture with the hopes to narrow down the list from the chemical screen. Since the embryonic mouth develops in a similar way in mammals and amphibians, we hypothesized that the same factors regulate human and
Xenopus buccopharyngeal membrane perforation. In humans, persistent buccopharyngeal membrane on its own is rare and therefore there is no genetic data for this birth defect. There is genetic data for patients with Choanal Atresia, a condition that could be caused by a persistent buccopharyngeal membrane (
Figure 2A). Thus, we used copy number variation data from patients with this birth defect to identify candidate regulators of buccopharyngeal membrane perforation. Using the DECIPHER database [
38] 83 patients were identified with Choanal Atresia and having copy number deletions (Suppl Excel File 3). Some of the deletions spanned large regions of chromosomes and included many different genes. The total number of identified genes in this analysis was 2202 and these were compiled (Suppl Excel File 3). First, we manually scrutinized the gene list for important developmental regulators. From this we identified genes in the WNT, FGF, SHH, and JAK/STAT signaling pathways (
Figure 2B). Notably, disruption of FGF signaling in particular has been reported in a connection between Choanal Atresia and Craniosynostosis [
32]. In addition, in this DECIPHER screen we also identified transcription factors known to be important in craniofacial development, such as LHX3, PAX8 and MSX1. A Panther functional classification of the affected genes was also undertaken as in
Section 2. Strikingly, we noted that seven of the functional groups identified in the chemical screen were also identified in this DECIPHER screen (
Figure 2C). Such common categories included the known pathways important in embryonic mouth development: Apoptosis and WNT signaling. In addition, Heterotrimeric G-protein signaling (Gi/Gs, Gq/Go), CCKR signaling, Huntington disease and Inflammation categories with identified in both screens. These results support the possibility that both screening approaches were effective in identifying known and new regulators of buccopharyngeal membrane rupture.
3.4. Overlap in chemical and genetic screens identifies JAK2 as a candidate regulator of buccopharyngeal membrane perforation.
In an attempt to focus our screening methods, the lists of genes identified in both approaches were narrowed down and compared. To select for genes most likely important in Choanal Atresia we choose to focus on genes affected in approximately 10% of the patients (Suppl Excel File 4) which narrowed the list to 35 genes. In addition, to select for the most important targets in our chemical screen we chose to focus on the proteins targeted by at least three different chemicals causing a persistent buccopharyngeal membrane. This narrowed this list to 28 different proteins. An overlap in these two smaller focused lists produced only one gene/protein in common, Janus Kinas 2 (JAK2) (
Figure 2D). Therefore, we decided to test whether Jak2 could be a regulator of buccopharyngeal membrane perforation in
X. laevis.
3.5. Deficient Jak2 causes a persistent buccopharyngeal membrane.
To determine whether Jak2 is important for buccopharyngeal membrane perforation, modified antisense oligos (Morpholinos, Genetools) were first utilized to reduce this protein in developing embryos. The
jak2 MO targeted the transcriptional start sites of both
jak2 homeologs (
jak2.L and
jak2.S, Genetools, suppl
Figure 1A). A range of
jak2 MO concentrations were injected into the one-cell stage and the faces visualized at stage 40-41 after the buccopharyngeal membrane ruptured in control embryos. The morphant embryos had craniofacial defects that increased in severity with morpholino concentration. We determined that approximately 20ng of MO/embryo resulted in 70% of embryos having a partially or fully intact buccopharyngeal membrane (not shown). At 40ng of MO/embryo, 81.7% of embryos had either a fully or partially intact buccopharyngeal membrane (
Figure 3A-A’’,B-B’’ and F). Finally, at 60 ng/embryo, 93.3% of embryos with either a fully or partially intact buccopharyngeal membrane (
Figure 3C-C’’ and F). When one-cell at the two-cell stage was injected with 30-40ng of
jak2 MO the buccopharyngeal membrane persisted on the injected side in 86.7% of these half morphants (
Figure 3D-D’’ and F) compared to 0% of the control half morphants. Also, in these half-injected embryos we noted a decrease in Jak2 protein on the injected half compared to the control half (see below). Thus, we provide the first evidence that Jak2 is required for buccopharyngeal membrane rupture.
3.6. Jak2 is specifically required for the buccopharyngeal membrane rupture rather than earlier steps in embryonic mouth development.
One possible reason to explain why
jak2 morphants had a persistent buccopharyngeal membrane was that the MOs caused defects in earlier developmental events that non-specifically affected buccopharyngeal membrane rupture. To test for this possibility, we exposed embryos to a JAK2 inhibitor, Ruxolitinib, just prior to buccopharyngeal membrane perforation. Indeed 81.7% of embryos exposed to this chemical had a partially or fully intact buccopharyngeal membrane, phenocopying the
jak2 morphants (
Figure 3 E-E’’ and F). These results are also evidence that the MOs are specifically affecting Jak2 levels in the embryos.
In addition, we also tested whether earlier events in embryonic mouth development were affected in
jak2 morphants by assessing the thickness of the buccopharyngeal membrane. During embryonic mouth development the stomodeum becomes thinner to form the 1-2 cell layer thick buccopharyngeal membrane. Thus, it could be argued that earlier effects of Jak2 knockdown could affect buccopharyngeal membrane thinning which in turn could physically prevent its rupture. If this was true, then we expected to observe a thickened buccopharyngeal membrane in the
jak2 morphants. To view the buccopharyngeal membrane, embryos were rendered auto fluorescent and transparent and then optical lateral sections were captured using confocal microscopy. Results indicated that the buccopharyngeal membrane was actually thin in 100% of the jak2 morphants analyzed with a persistent buccopharyngeal membrane (
Figure 3G,H). These results suggest decreased Jak2 did not affect earlier thinning of the stomodeum.
Together, the observations of buccopharyngeal membrane thickness and the late stage JAK2 inhibitor treatments suggested that the reduction in Jak2 had a specific effect on the rupture of the buccopharyngeal membrane rather than on earlier events in embryonic mouth development.
3.7. Jak2 is required for cranial muscle development.
To uncover how Jak2 regulates buccopharyngeal membrane perforation we first determined where Jak2 was acting during mouth development. We used a JAK2 antibody, created against the phosphorylated (tyrosine 1007/1008) human protein. Phosphorylation at this site is an indicator of high activity of the JAK2 protein. Further, this region of the protein is 100% similar to the corresponding sequence of the
X. laevis Jak2 protein (suppl
Figure 1). Robust labeling with the phosho-JAK2 antibody was observed in regions adjacent to the oral cavity just prior and during buccopharyngeal membrane perforation (
Figure 3I). This labeling was eliminated in
jak2 morphants (suppl
Figure 1). Such labeling appeared to be in the developing cranial muscles and indeed when we double labeled, using a muscle specific antibody, we observed overlap in phospho-JAK2 and muscle filaments (
Figure 3I-L, arrows).
Since we observed active Jak2 in the cranial muscles of the developing embryos we hypothesized that this protein is then required for cranial muscle formation. When the cranial muscles were examined in the
jak2 morphants, 96.7% of the embryos had a partial or complete reduction of muscles anterior to the eyes (
Figure 3M-O). On the other hand, the trunk muscles did not appear affected in these morphant embryos, suggesting that Jak2 might have a specific role in cranial muscle development (
Figure 3M,N). Certainly, cranial and trunk muscles are regulated in part by different developmental programs [
39].
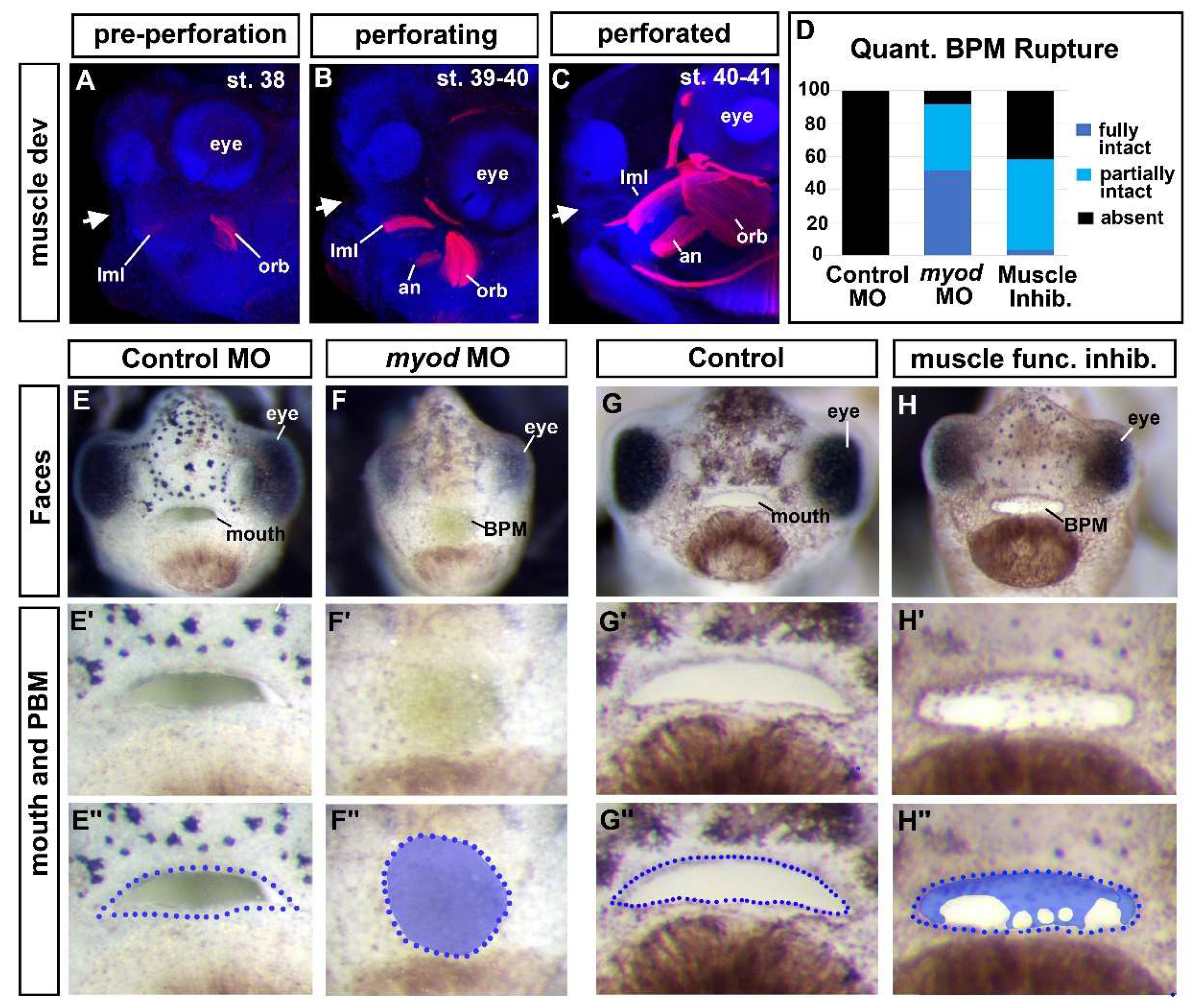
3.8. The cranial muscles are required for buccopharyngeal membrane rupture.
Our results thus far suggested that the Jak2 was required for both cranial muscle development and buccopharyngeal membrane perforation. We, therefore, hypothesized that the development of the buccopharyngeal membrane rupture was dependent on the muscles surrounding the oral cavity. Supporting this hypothesis is that the cranial muscles developed over the same time as buccopharyngeal membrane perforation (
Figure 4 A-C; [
40]). At stage 38, just prior to buccopharyngeal membrane perforation both the levator mandibulae longus (lml) and orbithyoidus (orb) muscles could be observed (Fig 4A). During perforation these muscles increased in size and were joined by the angularis (an) muscles (
Figure 4B). At the end of perforation, the cranial muscles were larger (
Figure 4C) however the mouth did not yet appear to move.
To test whether cranial muscles are required for buccopharyngeal membrane perforation, independent of Jak2, we performed experiments to perturb muscle development directly. To do this we first knocked down an important regulator of muscle development, MyoD. Embryos were injected with 40-50ng/embryo of a previously validated
myod1 MO [
41] at the one cell stage. Results indicated that indeed 92% of
myod1 morphant embryos had a fully or partially intact buccopharyngeal membrane (
Figure 4D and E-F’’).
To further demonstrate that muscles have a role in buccopharyngeal membrane perforation, we induced muscle paralysis. During this time the jaws do not yet move, however paralysis might perturb muscle tone and/or eliminate muscle twitching which could in turn affect muscle development. Embryos were exposed to N-Benzyl-p-toluenesulfonamide (BTS) which inhibits the ATPase activity of skeletal muscle myosin II and thereby reversibly blocks gliding motility and suppresses force and twitch production in fast skeletal muscle [
42]. Embryos exposed to 100um of BTS indeed resulted in paralysis. We found that this concentration was not lethal within 24 hours since the effects could be reversed when embryos were removed from BTS (not shown). Results indicated that 58% of embryos treated with BTS from stage 37 until stage 40 had a fully or partially intact buccopharyngeal membrane (
Figure 4D and G-H’’). The effects of BTS were not highly penetrant which could indicate that contractibility of fast twitch muscles itself is not the sole reason muscles are important for buccopharyngeal membrane rupture. It is also possible that the drug does not affect all individuals equally using a bath application.
One might also argue that the effects of the BTS on the buccopharyngeal membrane are secondary to its effect on heartbeat and circulation. To test whether a functional heart was required for buccopharyngeal membrane rupture we removed the developing heart at stage 35-37. While this resulted in craniofacial differences, the buccopharyngeal membrane ruptured in 100% of the embryos (Suppl. Fig 2). These results are further evidence that it is the developing cranial muscles that are important for buccopharyngeal membrane rupture during mouth development.
One possible reason for the importance of muscles is that they give the face its shape and size during craniofacial development. Thus, increasing cranial growth could in turn be structurally important for buccopharyngeal membrane perforation. If this is the case, then the buccopharyngeal membrane would not rupture in embryos with narrower and/or smaller faces (no matter the cause). To evaluate this possibility, we reexamined the images of embryos from the chemical screen (
Figure 1, section 2). There were several examples of embryos with narrower heads but also with a ruptured buccopharyngeal membrane (Suppl
Figure 3). Whether the buccopharyngeal membrane ruptured also did not seem to depend on size or shape of the mouth since even mouths that were small, and round could have an absent buccopharyngeal membrane. These observations suggest that buccopharyngeal membrane rupture does not depend on size or shape of the head and mouth further supporting a more specific role of muscles in the process.
3.9. Jaw muscles are connected to the oral epithelium by laminin.
To better understand how cranial muscles could influence buccopharyngeal membrane perforation we next performed a more detailed examination of muscles with respect to the mouth and oral cavity. The muscles were labeled with the muscle specific antibody (12/101) in transverse sections of the head during and just prior to and after buccopharyngeal membrane perforation. Further, these sections were counterstained with laminin which outlined the oral cavity and muscle compartments (
Figure 5A-H). Intriguingly, in this analysis we noticed that laminin connected the jaw muscle compartments to the basal lamina lining the oral cavity (
Figure 5B,C,F,G, white arrows). Similarly, intimate association between the oral cavity and muscles were also observed in histological sections made with 5um plastic sections and prepared with a general histological stain (
Figure 5D,H; pink arrows). The epithelium of the oral cavity is continuous with the buccopharyngeal membrane. Therefore, these results suggest the possibility that jaw muscles might physically influence the oral cavity and thus the buccopharyngeal membrane.
Next, we performed a similar analysis of laminin in both the
jak2 and
myod morphants. These results revealed the predicted absence or reduction in muscle fibers (reduction or lack of red labeling in
Figure 5K,L). However, the laminin lined compartments that would normally contain muscles were still present, although the laminin labeling appeared disjointed (
Figure 5J-L, white arrows). Importantly, the compartments normally containing muscle were not joined to the oral cavity as in the controls. Such results might suggest that these connections might be integral to buccopharyngeal membrane rupture.
3.10. Incisions around the oral cavity results in a persistent buccopharyngeal membrane
To test whether the ECM connections between the muscle and oral cavity are indeed required for buccopharyngeal membrane rupture we next attempted to sever such connections. Embryos were exposed to a low dose of tricaine in embryo media to decrease movement. Then embryos were inserted tail first into holes made in clay lined dishes. The clay was pinched around the head of each embryo to hold tightly. A fine capillary needle was used to make incisions around the edges of the embryonic mouth just prior to perforation (
Figure 5M). The depth of the incisions was such that the connections between the mouth and muscle could likely be eliminated. Sham operations of embryos in the same clay lined dish were performed, where the capillary knife only poked the epidermis. Following the surgeries, 50% of the media was exchanged to further reduce the concentration of tricaine and embryos were observed over 5 hours. In 88% of the operated embryos, the buccopharyngeal membrane failed to perforate (
Figure 5Q-S’). On the other hand, the buccopharyngeal membrane ruptured in all of the sham control embryos (
Figure 5N-P’). Interestingly, approximately 30 minutes after the surgical incisions, the embryonic mouth seemed to collapse and become smaller (
Figure 5R, R’). Therefore, in a subset of the experiments some of the embryos were fixed 30 minutes post-surgery, sectioned and labeled with fluorescently labeled phalloidin. In these labeled embryos the buccopharyngeal membrane appeared to have folded over or buckled in embryos that had undergone the surgery. On the other hand, in the sham controls the cells appeared as a normal linear structure covering the oral cavity (compare
Figure 5T and 5U). These observations prompted the hypothesis that connections between the oral cavity and surrounding muscle generate force across the buccopharyngeal membrane.
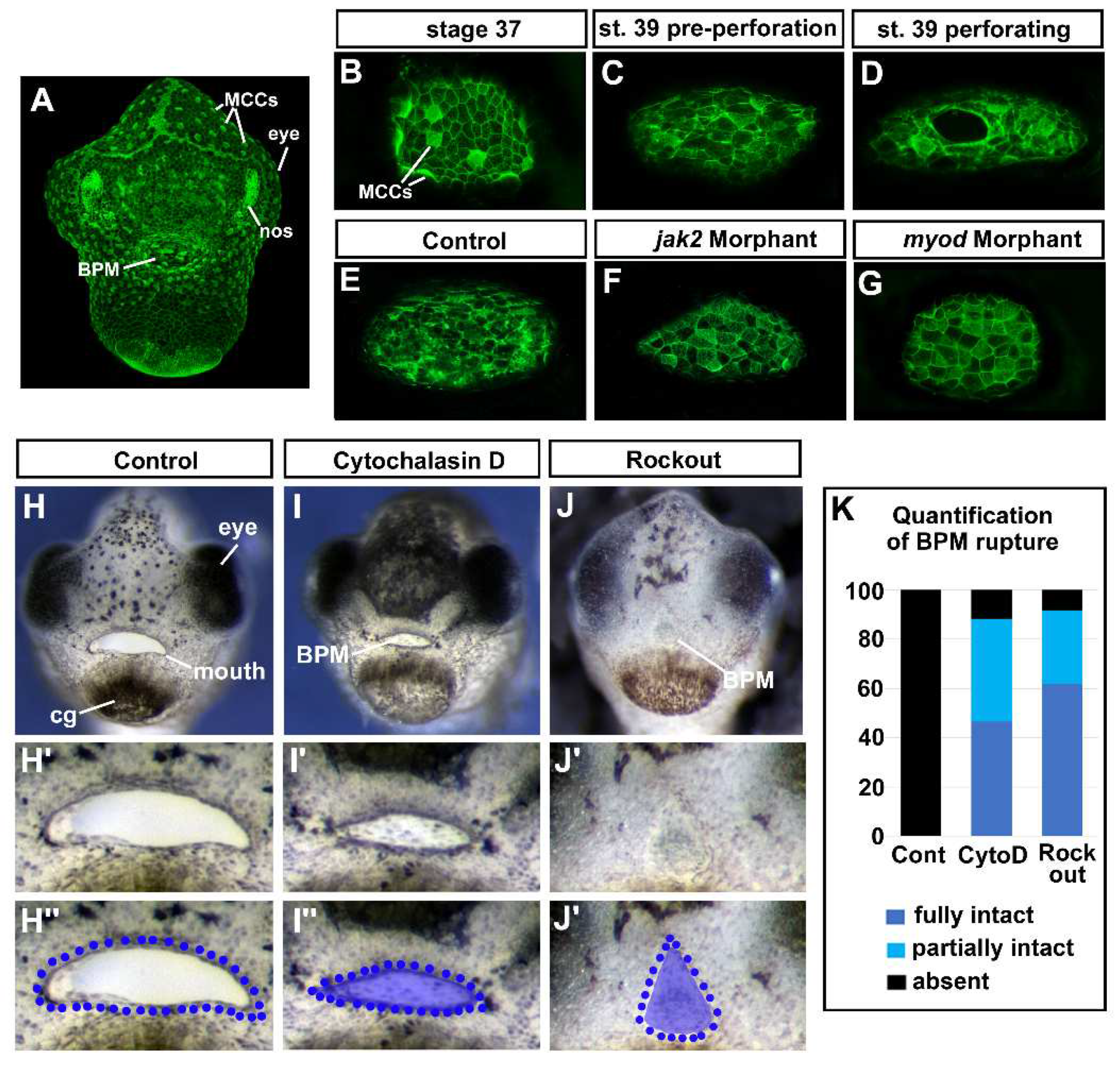
3.12. F-actin puncta was observed in the cells of the buccopharyngeal membrane prior to rupture.
F-actin has been proposed to function as a dynamic tension sensor [
43]. Therefore, if the cells of the buccopharyngeal membrane are under tension then we hypothesized that we would observe changes in F-actin as the buccopharyngeal membrane perforates and ruptures. F-actin, labeled with fluorescently tagged phalloidin, lined the inner membrane of all epidermal cells and was enriched at the surface of ciliated cells (
Figure 6A, B). At stage 37, the cells that will form the buccopharyngeal membrane appeared similar to the surrounding epidermis (
Figure 6B). However, at stage 39 just prior to perforation, we noted punctate accumulations of F-actin in cells of the buccopharyngeal membrane (
Figure 6C). Later during perforation, F-actin additionally appeared to be enriched around the perforating holes (
Figure 6D). Thus, these results do indicate that there are changes in F-actin that could indicate that the buccopharyngeal membrane was under tension.
We next hypothesized that if the changes in the actin cytoskeleton of cells comprising the buccopharyngeal membrane are indeed due to tension across the structure, then embryos lacking cranial muscles would not display such changes. Thus, we examined F-actin in both
jak2 and
myod morphants, that have major reductions in jaw muscles. Results indicated that indeed 90% of the
jak2 and 80% of the
myod morphant embryos appeared to have little or no F-actin positive puncta like accumulations in the buccopharyngeal membrane compared to controls (
Figure 6E-G). These results indicate that the presence of F-actin puncta in the cells of the buccopharyngeal membrane correlates with the presence of cranial muscles.
3.13. Perturbing actin dynamics results in a persistent buccopharyngeal membrane.
If the changes in the actin cytoskeleton were required for cells to sense or facilitate a response to tension, then we predicted that perturbing actin dynamics just before and during buccopharyngeal membrane perforation (stage 37-40) would prevent its rupture. First, actin polymerization was inhibited using cytochalasin D (1um) and we noted that 88.3% of embryos exposed to this compound had a fully or partially persistent buccopharyngeal membrane compared to 0% of the controls (Fig 6H-I’’, K). Interestingly, the mouth also appeared smaller, similar to the effects of the surgical incisions around the oral cavity (see
Figure 5R). Secondly, actin dynamics was also perturbed by inhibiting Rho associated kinase (ROCK) which is critical to regulating actin organization [
44]. We treated embryos with the ROCK inhibitor (ROCKOUT, 100uM). Indeed, we determined that 91.7% of embryos exposed to this inhibitor resulted in a fully or partially persisting buccopharyngeal membrane (
Figure 6J-K). Notably, this treatment had dramatic effects on mouth development and resulted in changes in the mouth shape similar to what we observed in the
jak2 morphants (see
Figure 3C). These experiments may provide evidence that actin dynamics is required for the rupture of the buccopharyngeal membrane. However, we cannot rule out that the effects we observed in response to perturbing actin and ROCK are actually due to effect on muscle development or function.
Taken together, these results point toward a model where the cranial muscles, connected to the oral epithelium, are required to generate tension on the buccopharyngeal membrane which is in turn required for its rupture.
4. Discussion
Rupture of the buccopharyngeal membrane is a critical event in mouth development and establishes the first connection between the external environment and the inside of the embryo. Without this process, basic functions such as eating and breathing would not be possible in free living embryos. In humans, failure of buccopharyngeal membrane rupture would disrupt swallowing of amniotic fluid which could also have multiple effects on embryonic development. Importantly, in all vertebrates a lack of mouth opening could have additional complications in craniofacial development. For example, a persistent buccopharyngeal membrane has been proposed to lead to the formation of oral obstructions such as choanal atresia [
1,
30]. Therefore, understanding how the buccopharyngeal membrane disappears could lead to novel insights into such craniofacial birth defects. Moreover, by studying embryonic mouth development we provide a deeper understanding of how craniofacial structures are integrated during development. This knowledge is not only critical for unraveling the complexity of craniofacial defects but also the function and evolution of the face.
We have uncovered that buccopharyngeal membrane rupture is intimately integrated with the formation of the cranial muscles. In particular, we demonstrated that disrupted jaw muscle development also perturbed buccopharyngeal membrane perforation. Muscles are also required for the development of other craniofacial structures such as cartilage and tendons [
45,
46]. Further support of a role for jaw muscle function in craniofacial development comes from the observations that in humans and animal models with muscular dystrophy can also have a host of craniofacial malformations [
47,
48,
49]. Thus, evidence is mounting to support a mechanical role for jaw muscles in various processes during craniofacial development [
50].
How do the cranial muscles regulate buccopharyngeal membrane rupture? The first step to unraveling this question was the observation that the cranial muscles are physically connected to the oral cavity via extracellular matrix (ECM). Specifically, laminin appeared to bridge jaw muscles with the oral epithelium that lines the oral cavity. Since the oral epithelium is continuous with the buccopharyngeal membrane, we hypothesized that the laminin connections allow for the muscles to influence this structure. To test this, we surgically severed the connections and determined that indeed they were important for buccopharyngeal membrane perforation. Importantly, when we did this, we also noticed that the mouth collapsed, and cells of the buccopharyngeal membrane buckled. Based on this data we have formulated a model where the connections between the muscles and oral epithelium create tension on the buccopharyngeal membrane and this in turn is necessary to stimulate its perforation. Such a hypothesis is supported by the fact that the tension sensor, F-actin [
43], accumulated in puncta the buccopharyngeal membrane cells during perforation. Moreover, this accumulation was reduced in embryos lacking cranial muscles and perturbation of actin dynamics caused a persistent buccopharyngeal membrane. Of course, there are the caveats such as the surgical manipulations could have altered muscle function and actin puncta accumulations are due to cell movements or apoptosis. Future experiments to test our hypothesis could include performing more targeted disruption of the ECM and measuring tension in the buccopharyngeal membrane directly. While no studies have specifically shown a connection between jaw muscles and buccopharyngeal membrane rupture in mammals, cranial muscles are in close association of the oral epithelium in mice [
51] presenting the possibility that this mechanism is conserved.
How do the mechanical forces across the buccopharyngeal membrane stimulate its perforation? Our futures goals are also to delve into this question. In other developmental events, mechanical forces can activate several different signals such as the Hippo pathway or Piezo regulated calcium channels to initiate cytoskeletal changes in the cells. Such changes then can in turn promote events such as apoptosis [
52,
53] and down regulation of adherens junctions [
54], processes we know are critical in buccopharyngeal membrane rupture [
1,
2,
55]. Alternatively, the forces generated by the muscles could physically pull apart the cells of the buccopharyngeal membrane. These hypotheses remain interesting avenues to pursue.
In this study we demonstrate the power of using human genetic data associated with birth defects combined with animal studies to uncover novel developmental mechanisms. We combined a chemical screen in
Xenopus embryos with an analysis of human genetics associated with a birth defect that may be caused by persistent buccopharyngeal membrane. This analysis uncovered the potential importance of JAK2 in mouth development. JAK2 is a Janus Kinase which acts as a cell surface receptor that can bind many different ligands including cytokines and growth factors and therefore can in turn regulate numerous processes in the embryo [
56]. Mutations in Jak2 are associated with blood disorders and knockout mice are not viable due to the effects on hematopoiesis [
57]. Because of this effect, many of the other developmental functions of JAK2 may be hidden or are not as well studied. Here, by using titratable tools in
Xenopus embryos before blood has a major role in craniofacial development, we have uncovered potentially a novel role for Jak2. Specifically, JAK2 is required for the differentiation of jaw muscles. Consistently, JAK2 has been shown to regulate the differentiation of myoblasts which is partially mediated by muscle specific transcription factors MyoD and MEF2 [
58]. It will be interesting to determine how JAK2 is integrated into the muscle developmental program during craniofacial development.
5. Conclusions
This work uncovers a novel role for Jak2 in regulating cranial muscle development which in turn is required for the final step in embryonic mouth development, buccopharyngeal membrane perforation. We hypothesize that muscles generate tension across the buccopharyngeal membrane which is necessary for its rupture and to create the mouth opening.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Suppl Excel File 1: OMIM search results of all conditions mentioning Choanal Atresia, Suppl Excel File 2: Chemical screen, Suppl Excel File 3: Decipher screen, Suppl Excel File 4: Overlap in screens identifies JAK2, Suppl
Figure 1: Jak2 protein sequence and validation of the morpholino, Suppl.
Figure 2: Removing the heart has no effect on buccopharyngeal membrane rupture.
Author Contributions
Dickinson coordinated and performed the majority of the experiments and wrote the manuscript. However, many students helped perform preliminary trials, quantifications or collated data. This work was therefore not possible without a large number of VCU undergraduate students. See Acknowledgments for more details.
Funding
This research was funded by National Sciences Foundation Career award (IOS1349668) as well as the Jeffress Memorial (Virginia, USA) Fund to A. Dickinson.
Data Availability Statement
Acknowledgments
I would like to thank first and foremost Jeremy Thompson and Molly Allen for performing and analyzing the first round of the chemical screen. This was a tremendous amount of work and many hours of pipetting and imaging embryos. I would like to thank two undergraduate classes of BIOZ 491 (VCU) for preliminary work and discussion that lead to the hypothesis presented in this manuscript. In addition, many students enrolled in Biol 492 (Independent Study, VCU) performed the preliminary experiments or quantified final experiments presented in the manuscript. I would also like to thank exchange student Thiseas Eleftheriou who collated genes from a subset of patients with Choanal Atresia using the DECIPHER database. Finally, I would especially like to thank our lab manager and animal technician Deborah Howton who made all these experiments possible. This study makes use of data generated by the DECIPHER community. A full list of centres who contributed to the generation of the data is available from
https://deciphergenomics.org/about/stats and via email from contact@deciphergenomics.org. Funding for the DECIPHER project was provided by Wellcome [grant number WT223718/Z/21/Z].
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dickinson: A.J. Using frogs faces to dissect the mechanisms underlying human orofacial defects. Semin Cell Dev Biol 2016, 51, 54-63. [CrossRef]
- Dickinson, A.J.; Sive, H. Development of the primary mouth in Xenopus laevis. Dev Biol 2006, 295, 700-713. [CrossRef]
- Soukup, V.; Horácek, I.; Cerny, R. Development and evolution of the vertebrate primary mouth. J Anat 2013, 222, 79-99. [CrossRef]
- Richman, J.M.; Lee, S.H. About face: signals and genes controlling jaw patterning and identity in vertebrates. BioEssays : news and reviews in molecular, cellular and developmental biology 2003, 25, 554-568. [CrossRef]
- Chai, Y.; Maxson, R.E., Jr. Recent advances in craniofacial morphogenesis. Dev Dyn 2006, 235, 2353-2375. [CrossRef]
- Francis-West, P.H.; Robson, L.; Evans, D.J.R. Craniofacial development : the tissue and molecular interactions that control development of the head; Springer: Berlin ; New York, 2003; pp. vi, 144 p.
- Gitton, Y.; Heude, E.; Vieux-Rochas, M.; Benouaiche, L.; Fontaine, A.; Sato, T.; Kurihara, Y.; Kurihara, H.; Couly, G.; Levi, G. Evolving maps in craniofacial development. Semin Cell Dev Biol 2010, 21, 301-308. [CrossRef]
- Helms, J.A.; Cordero, D.; Tapadia, M.D. New insights into craniofacial morphogenesis. Development 2005, 132, 851-861. [CrossRef]
- Chen, J.; Jacox, L.A.; Saldanha, F.; Sive, H. Mouth development. Wiley Interdiscip Rev Dev Biol 2017, 6. [CrossRef]
- Jacox, L.; Chen, J.; Rothman, A.; Lathrop-Marshall, H.; Sive, H. Formation of a “Pre-mouth Array” from the Extreme Anterior Domain Is Directed by Neural Crest and Wnt/PCP Signaling. Cell reports 2016, 16, 1445-1455. [CrossRef]
- Jacox, L.; Sindelka, R.; Chen, J.; Rothman, A.; Dickinson, A.; Sive, H. The extreme anterior domain is an essential craniofacial organizer acting through Kinin-Kallikrein signaling. Cell reports 2014, 8, 596-609. [CrossRef]
- Mulvihill, S.J.; Stone, M.M.; Debas, H.T.; Fonkalsrud, E.W. The role of amniotic fluid in fetal nutrition. J Pediatr Surg 1985, 20, 668-672. [CrossRef]
- Dickinson, A.; Sive, H. Positioning the extreme anterior in Xenopus: cement gland, primary mouth and anterior pituitary. Semin Cell Dev Biol 2007, 18, 525-533. [CrossRef]
- Dickinson, A.J.; Sive, H.L. The Wnt antagonists Frzb-1 and Crescent locally regulate basement membrane dissolution in the developing primary mouth. Development 2009, 136, 1071-1081. [CrossRef]
- Watanabe, K.; Sasaki, F.; Takahama, H. The ultrastructure of oral (buccopharyngeal) membrane formation and rupture in the anuran embryo. Anat Rec 1984, 210, 513-524.
- Waterman, R.E. Ultrastructure of oral (buccopharyngeal) membrane formation and rupture in the hamster embryo. Dev Biol 1977, 58, 219-229.
- Waterman, R.E. Formation and perforation of closing plates in the chick embryo. Anat Rec 1985, 211, 450-457.
- Waterman, R.E.; Balian, G. Indirect immunofluorescent staining of fibronectin associated with the floor of the foregut during formation and rupture of the oral membrane in the chick embryo. Anat Rec 1980, 198, 619-635.
- Waterman, R.E.; Schoenwolf, G.C. The ultrastructure of oral (buccopharyngeal) membrane formation and rupture in the chick embryo. Anat Rec 1980, 197, 441-470.
- Agarwal, R.; Kumar, P.; Kalra, G.S.; Bhushan, S.; Chandra, R. Persistent buccopharyngeal membrane: a report of two cases. Plastic and reconstructive surgery 1996, 98, 866-868.
- Arcand, P.; Haikal, J. Persistent buccopharyngeal membrane. J Otolaryngol 1988, 17, 125-127.
- Chandra, R.; Yadava, V.N.; Sharma, R.N. Persistent buccopharyngeal membrane. Case report. Plastic and reconstructive surgery 1974, 54, 678-679.
- Cotton, R.T. Persistent buccopharyngeal membrane. J Otolaryngol 1988, 17, 260.
- Ferril, G.R.; Barham, H.P.; Prager, J.D. Novel airway findings in a patient with 1p36 deletion syndrome. Int J Pediatr Otorhinolaryngol 2014, 78, 157-158. [CrossRef]
- Legius, E.; Moerman, P.; Fryns, J.P.; Vandenberghe, K.; Eggermont, E. Holzgreve-Wagner-Rehder syndrome: Potter sequence associated with persistent buccopharyngeal membrane. A second observation. American journal of medical genetics 1988, 31, 269-272. [CrossRef]
- Pillai, K.G.; Kamath, V.V.; Kumar, G.S.; Nagamani, N. Persistent buccopharyngeal membrane with cleft palate. A case report. Oral Surg Oral Med Oral Pathol 1990, 69, 164-166. [CrossRef]
- Gartlan, M.G.; Davies, J.; Smith, R.J. Congenital oral synechiae. Ann Otol Rhinol Laryngol 1993, 102, 186-197. [CrossRef]
- Assanasen, P.; Metheetrairut, C. Choanal atresia. J Med Assoc Thai 2009, 92, 699-706.
- Chia, S.H.; Carvalho, D.S.; Jaffe, D.M.; Pransky, S.M. Unilateral choanal atresia in identical twins: case report and literature review. Int J Pediatr Otorhinolaryngol 2002, 62, 249-252. [CrossRef]
- Kwong, K.M. Current Updates on Choanal Atresia. Front Pediatr 2015, 3, 52. [CrossRef]
- Katz, E.S.; Mitchell, R.B.; D’Ambrosio, C.M. Obstructive sleep apnea in infants. Am J Respir Crit Care Med 2012, 185, 805-816. [CrossRef]
- Lesciotto, K.M.; Heuzé, Y.; Jabs, E.W.; Bernstein, J.M.; Richtsmeier, J.T. Choanal Atresia and Craniosynostosis: Development and Disease. Plastic and reconstructive surgery 2018, 141, 156-168. [CrossRef]
- Kurosaka, H.; Mushiake, J.; Saha, M.; Wu, Y.; Wang, Q.; Kikuchi, M.; Nakaya, A.; Yamamoto, S.; Inubushi, T.; Koga, S.; et al. Synergistic role of retinoic acid signaling and Gata3 during primitive choanae formation. Human molecular genetics 2021, 30, 2383-2392. [CrossRef]
- Kurosaka, H.; Wang, Q.; Sandell, L.; Yamashiro, T.; Trainor, P.A. Rdh10 loss-of-function and perturbed retinoid signaling underlies the etiology of choanal atresia. Human molecular genetics 2017, 26, 1268-1279. [CrossRef]
- Soukup, V.; Horacek, I.; Cerny, R. Development and evolution of the vertebrate primary mouth. J Anat 2013, 222, 79-99. [CrossRef]
- Sullivan, K.G.; Levin, M. Neurotransmitter signaling pathways required for normal development in Xenopus laevis embryos: a pharmacological survey screen. J Anat 2016, 229, 483-502. [CrossRef]
- Adams, D.S.; Uzel, S.G.; Akagi, J.; Wlodkowic, D.; Andreeva, V.; Yelick, P.C.; Devitt-Lee, A.; Pare, J.F.; Levin, M. Bioelectric signalling via potassium channels: a mechanism for craniofacial dysmorphogenesis in KCNJ2-associated Andersen-Tawil Syndrome. J Physiol 2016, 594, 3245-3270. [CrossRef]
- Firth, H.V.; Richards, S.M.; Bevan, A.P.; Clayton, S.; Corpas, M.; Rajan, D.; Van Vooren, S.; Moreau, Y.; Pettett, R.M.; Carter, N.P. DECIPHER: Database of Chromosomal Imbalance and Phenotype in Humans Using Ensembl Resources. American journal of human genetics 2009, 84, 524-533. [CrossRef]
- Michailovici, I.; Eigler, T.; Tzahor, E. Craniofacial Muscle Development. Current topics in developmental biology 2015, 115, 3-30. [CrossRef]
- Schmidt, J.; Schuff, M.; Olsson, L. A role for FoxN3 in the development of cranial cartilages and muscles in Xenopus laevis (Amphibia: Anura: Pipidae) with special emphasis on the novel rostral cartilages. J Anat 2011, 218, 226-242. [CrossRef]
- Della Gaspera, B.; Armand, A.S.; Lecolle, S.; Charbonnier, F.; Chanoine, C. Mef2d acts upstream of muscle identity genes and couples lateral myogenesis to dermomyotome formation in Xenopus laevis. PloS one 2012, 7, e52359. [CrossRef]
- Shaw, M.A.; Ostap, E.M.; Goldman, Y.E. Mechanism of inhibition of skeletal muscle actomyosin by N-benzyl-p-toluenesulfonamide. Biochemistry 2003, 42, 6128-6135. [CrossRef]
- Galkin, V.E.; Orlova, A.; Egelman, E.H. Actin filaments as tension sensors. Curr Biol 2012, 22, R96-101. [CrossRef]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; et al. Tensional homeostasis and the malignant phenotype. Cancer cell 2005, 8, 241-254. [CrossRef]
- Shwartz, Y.; Farkas, Z.; Stern, T.; Aszódi, A.; Zelzer, E. Muscle contraction controls skeletal morphogenesis through regulation of chondrocyte convergent extension. Dev Biol 2012, 370, 154-163. [CrossRef]
- Subramanian, A.; Kanzaki, L.F.; Galloway, J.L.; Schilling, T.F. Mechanical force regulates tendon extracellular matrix organization and tenocyte morphogenesis through TGFbeta signaling. eLife 2018, 7. [CrossRef]
- Matsuyuki, T.; Kitahara, T.; Nakashima, A. Developmental changes in craniofacial morphology in subjects with Duchenne muscular dystrophy. Eur J Orthod 2006, 28, 42-50. [CrossRef]
- Lightfoot, P.S.; German, R.Z. The effects of muscular dystrophy on craniofacial growth in mice: a study of heterochrony and ontogenetic allometry. J Morphol 1998, 235, 1-16. [CrossRef]
- Jones, D.C.; Zelditch, M.L.; Peake, P.L.; German, R.Z. The effects of muscular dystrophy on the craniofacial shape of Mus musculus. J Anat 2007, 210, 723-730. [CrossRef]
- Du, W.; Bhojwani, A.; Hu, J.K. FACEts of mechanical regulation in the morphogenesis of craniofacial structures. Int J Oral Sci 2021, 13, 4. [CrossRef]
- Grimaldi, A.; Parada, C.; Chai, Y. A Comprehensive Study of Soft Palate Development in Mice. PloS one 2015, 10, e0145018. [CrossRef]
- Friedland, F.; Babu, S.; Springer, R.; Konrad, J.; Herfs, Y.; Gerlach, S.; Gehlen, J.; Krause, H.J.; De Laporte, L.; Merkel, R.; et al. ECM-transmitted shear stress induces apoptotic cell extrusion in early breast gland development. Front Cell Dev Biol 2022, 10, 947430. [CrossRef]
- Zeng, Y.; Du, X.; Yao, X.; Qiu, Y.; Jiang, W.; Shen, J.; Li, L.; Liu, X. Mechanism of cell death of endothelial cells regulated by mechanical forces. J Biomech 2022, 131, 110917. [CrossRef]
- Vorisek, C.; Weixler, V.; Dominguez, M.; Axt-Fliedner, R.; Hammer, P.E.; Lin, R.Z.; Melero-Martin, J.M.; Del Nido, P.J.; Friehs, I. Mechanical strain triggers endothelial-to-mesenchymal transition of the endocardium in the immature heart. Pediatr Res 2022, 92, 721-728. [CrossRef]
- Houssin, N.S.; Bharathan, N.K.; Turner, S.D.; Dickinson, A.J. Role of JNK during buccopharyngeal membrane perforation, the last step of embryonic mouth formation. Dev Dyn 2017, 246, 100-115. [CrossRef]
- Bousoik, E.; Montazeri Aliabadi, H. “Do We Know Jack” About JAK? A Closer Look at JAK/STAT Signaling Pathway. Frontiers in oncology 2018, 8, 287. [CrossRef]
- Park, S.O.; Wamsley, H.L.; Bae, K.; Hu, Z.; Li, X.; Choe, S.W.; Slayton, W.B.; Oh, S.P.; Wagner, K.U.; Sayeski, P.P. Conditional deletion of Jak2 reveals an essential role in hematopoiesis throughout mouse ontogeny: implications for Jak2 inhibition in humans. PloS one 2013, 8, e59675. [CrossRef]
- Jang, Y.N.; Baik, E.J. JAK-STAT pathway and myogenic differentiation. Jakstat 2013, 2, e23282. [CrossRef]
- Sive, H.; Grainger, R.; Harland, R. Early Development of Xenopus laevis: a laboratory manual; Cold Spring Harbor Laboratory Press: 2000.
- Thomas, P.D.; Ebert, D.; Muruganujan, A.; Mushayahama, T.; Albou, L.P.; Mi, H. PANTHER: Making genome-scale phylogenetics accessible to all. Protein Sci 2022, 31, 8-22. [CrossRef]
Figure 1.
Buccopharyngeal membrane rupture and chemical screen. A) Frontal view of the face of an embryo at stage 39 prior to the perforation of the buccopharyngeal membrane. B-F’) magnified images of the mouth at progressive points of buccopharyngeal membrane perforation. In the prime labeled images, the buccopharyngeal membrane is shaded blue. Each image is from a different embryo that represents the most common appearance at each stage (from over 200 embryos examined in 10 biological replicates). G) Schematic outlining the chemical screen. H-O) A subset of representative embryos treated with select chemicals causing a persistent buccopharyngeal membrane but with a stomodeum present. The National Service Center Number identifier is shown above each embryo. P) Gene symbols of select targets of chemicals causing a persistent buccopharyngeal membrane. Q) The top functional categories identified from the targets of chemicals causing a persistent buccopharyngeal membrane. The GO identifier is in brackets.
Figure 1.
Buccopharyngeal membrane rupture and chemical screen. A) Frontal view of the face of an embryo at stage 39 prior to the perforation of the buccopharyngeal membrane. B-F’) magnified images of the mouth at progressive points of buccopharyngeal membrane perforation. In the prime labeled images, the buccopharyngeal membrane is shaded blue. Each image is from a different embryo that represents the most common appearance at each stage (from over 200 embryos examined in 10 biological replicates). G) Schematic outlining the chemical screen. H-O) A subset of representative embryos treated with select chemicals causing a persistent buccopharyngeal membrane but with a stomodeum present. The National Service Center Number identifier is shown above each embryo. P) Gene symbols of select targets of chemicals causing a persistent buccopharyngeal membrane. Q) The top functional categories identified from the targets of chemicals causing a persistent buccopharyngeal membrane. The GO identifier is in brackets.
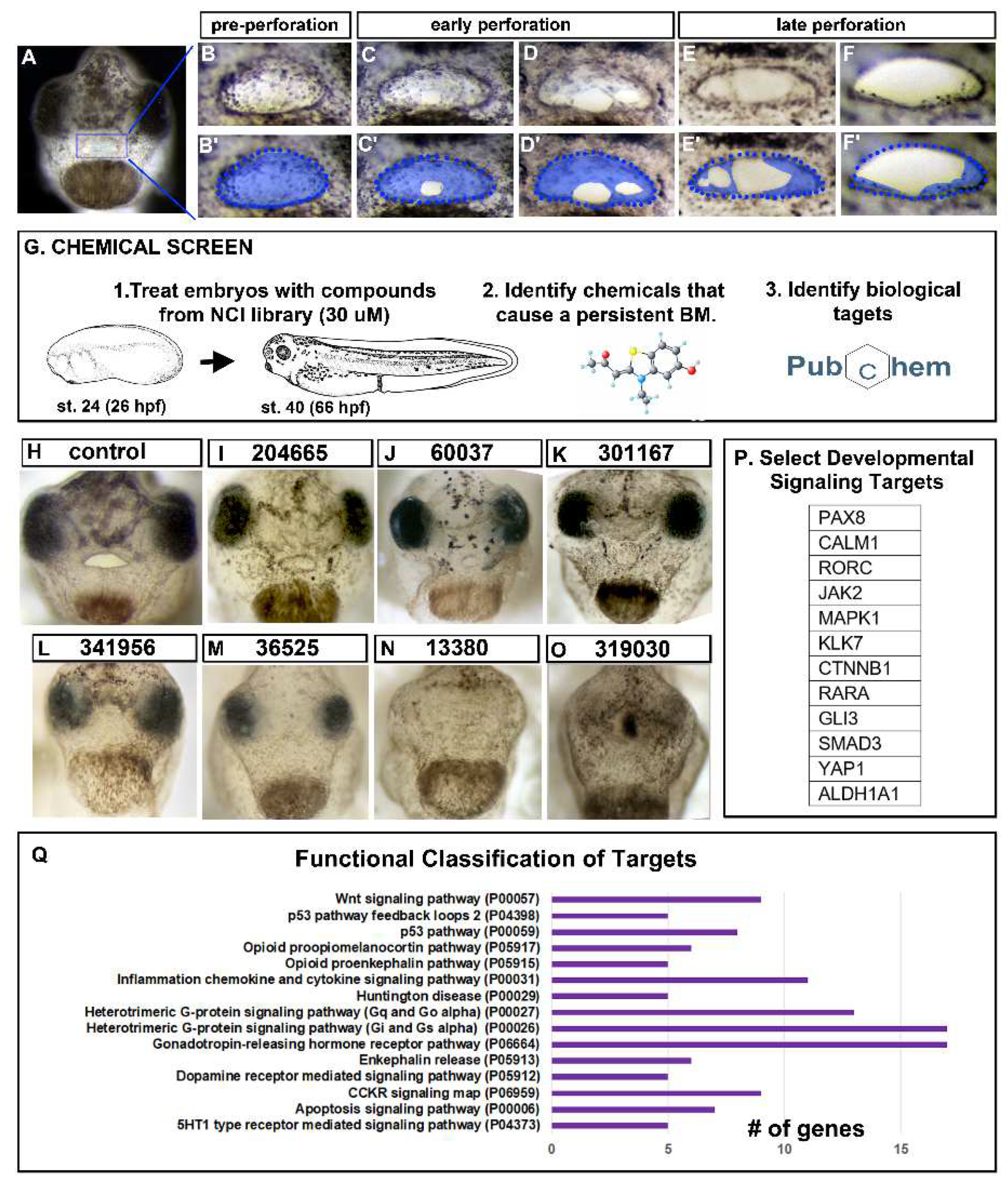
Figure 2.
A) Schematic showing where the airway blockage can occur in Choanal Atresia. B) Select genes with copy number deletions identified in patients with Choanal Atresia known to be important in development. C) The top functional categories identified from copy number deletions in patients with Choanal Atresia. The GO identifier is in brackets. D) Overlap in genes identified in the chemical and genetic screens identifies JAK2.
Figure 2.
A) Schematic showing where the airway blockage can occur in Choanal Atresia. B) Select genes with copy number deletions identified in patients with Choanal Atresia known to be important in development. C) The top functional categories identified from copy number deletions in patients with Choanal Atresia. The GO identifier is in brackets. D) Overlap in genes identified in the chemical and genetic screens identifies JAK2.
Figure 3.
Jak2 knockdown affects buccopharyngeal membrane and cranial muscles. A-E’’) Frontal view of the face of representative embryos at stage 40-41 showing a ruptured or persisting buccopharyngeal membranes (images from n=60, 3 biological replicates for each treatment). In the bottom right corner shows an image of the injection site/stage. Prime labeled images show magnified images of the mouth and the double prime labeled images show the same images with the buccopharyngeal membrane shaded blue. F) Shows relative percentages of embryos with an intact, partially intact or absent buccopharyngeal membrane (n=60 for each group, 3 biological replicates). G-H) Optical sagittal section through the head of representative controls and jak2 morphants. The buccopharyngeal membrane is absent in control and is present but thin in the jak2 morphants (n=12, 2 biological replicates). I-L) Representative thick agarose section through the face showing phosphor-‘JAK2 and 12/101 immunofluorescence. I) phospho-Jak2 (red), J) 12/101=muscle specific antibody (green), K) DAPI (blue), L) merge. M,N) Lateral views of representative embryos showing 12/101 muscle labeling (red) in control and jak2 morphants and counterstained with DAPI (blue). White arrows point to the location of the mouth. O) Quantification of the presence of cranial muscles in control and jak2 morphants (n=60, 2 biological reps). Abbreviations; BPM= buccopharyngeal membrane, nos=nostril, MO=morpholino.
Figure 3.
Jak2 knockdown affects buccopharyngeal membrane and cranial muscles. A-E’’) Frontal view of the face of representative embryos at stage 40-41 showing a ruptured or persisting buccopharyngeal membranes (images from n=60, 3 biological replicates for each treatment). In the bottom right corner shows an image of the injection site/stage. Prime labeled images show magnified images of the mouth and the double prime labeled images show the same images with the buccopharyngeal membrane shaded blue. F) Shows relative percentages of embryos with an intact, partially intact or absent buccopharyngeal membrane (n=60 for each group, 3 biological replicates). G-H) Optical sagittal section through the head of representative controls and jak2 morphants. The buccopharyngeal membrane is absent in control and is present but thin in the jak2 morphants (n=12, 2 biological replicates). I-L) Representative thick agarose section through the face showing phosphor-‘JAK2 and 12/101 immunofluorescence. I) phospho-Jak2 (red), J) 12/101=muscle specific antibody (green), K) DAPI (blue), L) merge. M,N) Lateral views of representative embryos showing 12/101 muscle labeling (red) in control and jak2 morphants and counterstained with DAPI (blue). White arrows point to the location of the mouth. O) Quantification of the presence of cranial muscles in control and jak2 morphants (n=60, 2 biological reps). Abbreviations; BPM= buccopharyngeal membrane, nos=nostril, MO=morpholino.
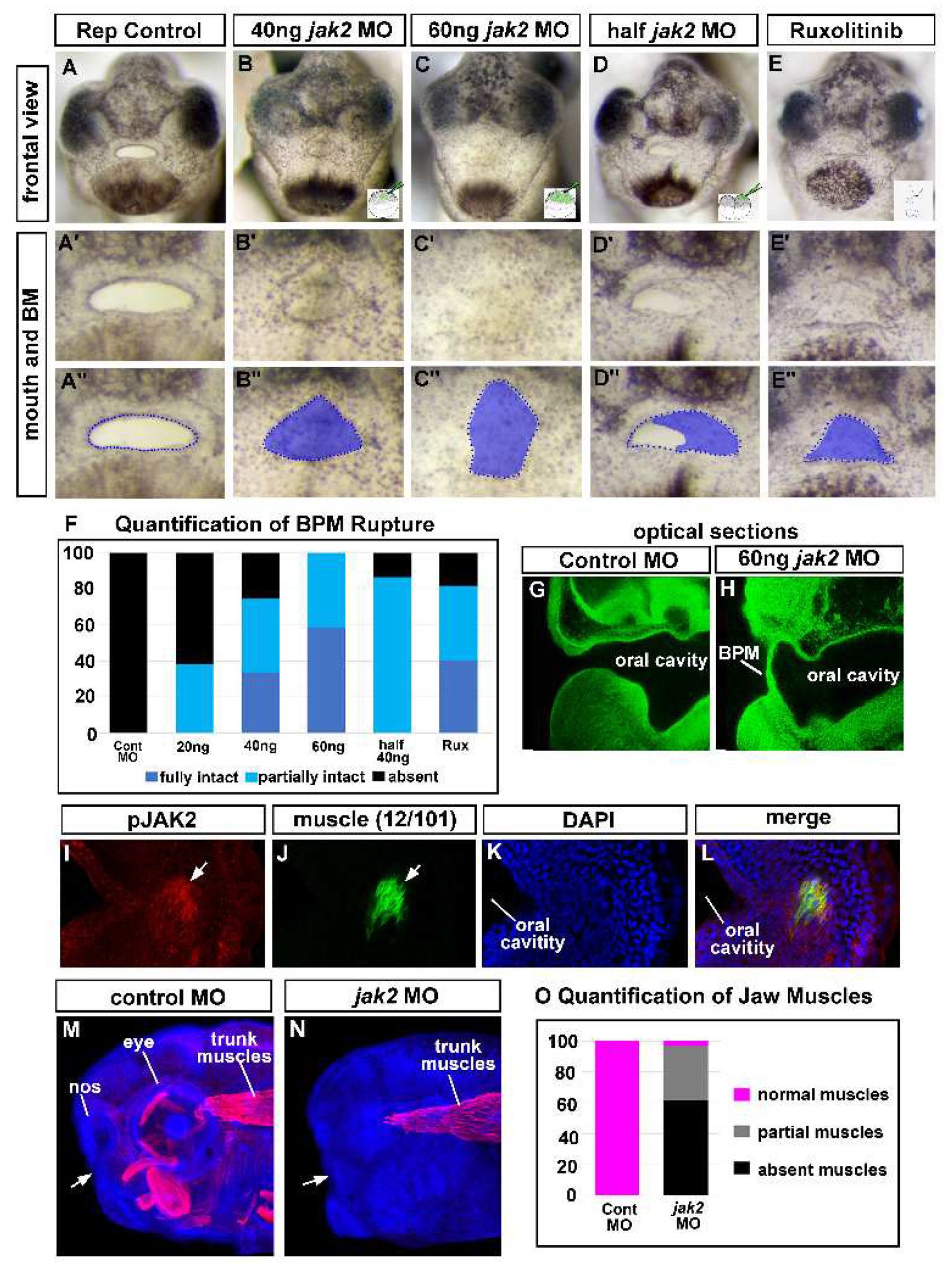
Figure 4.
Cranial muscles are required for buccopharyngeal membrane rupture. A-C) Lateral views of embryos showing cranial muscles (red) and counterstained with DAPI (blue) (best images from 10 embryos imaged at each stage). White arrows show the location of the developing mouth. D) Proportion of embryos that had a fully intact, partially intact, or absent buccopharyngeal membrane after myoD knockdown or treatment with a muscle inhibitor (BTS) compared to controls (n=60, 3 biological replicates for each treatment). E-H’’) Frontal views of representative embryos injected with myoD morpholinos or treated with an inhibitor of muscle function (BTS). Prime labeled images show magnified images of the mouth, and the double prime labeled images show the buccopharyngeal membrane shaded blue. Abbreviations: levator mandibulae longus=lml, orbithyoidus=orb, angularis=an, BPM=buccopharyngeal membrane.
Figure 4.
Cranial muscles are required for buccopharyngeal membrane rupture. A-C) Lateral views of embryos showing cranial muscles (red) and counterstained with DAPI (blue) (best images from 10 embryos imaged at each stage). White arrows show the location of the developing mouth. D) Proportion of embryos that had a fully intact, partially intact, or absent buccopharyngeal membrane after myoD knockdown or treatment with a muscle inhibitor (BTS) compared to controls (n=60, 3 biological replicates for each treatment). E-H’’) Frontal views of representative embryos injected with myoD morpholinos or treated with an inhibitor of muscle function (BTS). Prime labeled images show magnified images of the mouth, and the double prime labeled images show the buccopharyngeal membrane shaded blue. Abbreviations: levator mandibulae longus=lml, orbithyoidus=orb, angularis=an, BPM=buccopharyngeal membrane.
Figure 5.
A-L) Muscle and oral cavity connections. A,E,I) Schematics of the lateral views of embryos showing locations of the corresponding sections. B,C,F,G,J,K,L) Transverse sections labeled with antibodies to detect muscle specific protein (12/101 in red), laminin (green) and counterstained with DAPI (blue). Images were chosen from representative images taken from 20 different embryos in 2 biological replicates. White arrows indicate laminin that bridges the oral cavity and muscle compartments. D,H) Plastic section stained with Giemsa showing representative images of the association between muscle and oral cavities (based on sections of 20 embryos in 2 biological replicates). Pink arrows indicate the connections between the muscle and oral cavity. M) Schematic of a frontal view showing the location of the surgical incisions. N-S’) Shows the embryonic mouth and buccopharyngeal membrane in representative embryos before (0 minutes) and after sham or incisions (at 30 minutes and 5 hours). Prime images show the buccopharyngeal membrane shaded in blue and the mouth outlined in blue dots. Representative images chosen from 50 embryos performed over 5 biological replicates. In R and S, the incised tissue is colored pink. T,U) representative embryos 30 minutes after surgeries (or sham) sectioned and labeled with phalloidin to show the cellular arrangements in the buccopharyngeal membrane. Shows representative images taken from a total of 10 embryos in 2 biological replicates, Abbreviations: oc=oral cavity, BPM=buccopharyngeal membrane, cg=cement gland.
Figure 5.
A-L) Muscle and oral cavity connections. A,E,I) Schematics of the lateral views of embryos showing locations of the corresponding sections. B,C,F,G,J,K,L) Transverse sections labeled with antibodies to detect muscle specific protein (12/101 in red), laminin (green) and counterstained with DAPI (blue). Images were chosen from representative images taken from 20 different embryos in 2 biological replicates. White arrows indicate laminin that bridges the oral cavity and muscle compartments. D,H) Plastic section stained with Giemsa showing representative images of the association between muscle and oral cavities (based on sections of 20 embryos in 2 biological replicates). Pink arrows indicate the connections between the muscle and oral cavity. M) Schematic of a frontal view showing the location of the surgical incisions. N-S’) Shows the embryonic mouth and buccopharyngeal membrane in representative embryos before (0 minutes) and after sham or incisions (at 30 minutes and 5 hours). Prime images show the buccopharyngeal membrane shaded in blue and the mouth outlined in blue dots. Representative images chosen from 50 embryos performed over 5 biological replicates. In R and S, the incised tissue is colored pink. T,U) representative embryos 30 minutes after surgeries (or sham) sectioned and labeled with phalloidin to show the cellular arrangements in the buccopharyngeal membrane. Shows representative images taken from a total of 10 embryos in 2 biological replicates, Abbreviations: oc=oral cavity, BPM=buccopharyngeal membrane, cg=cement gland.
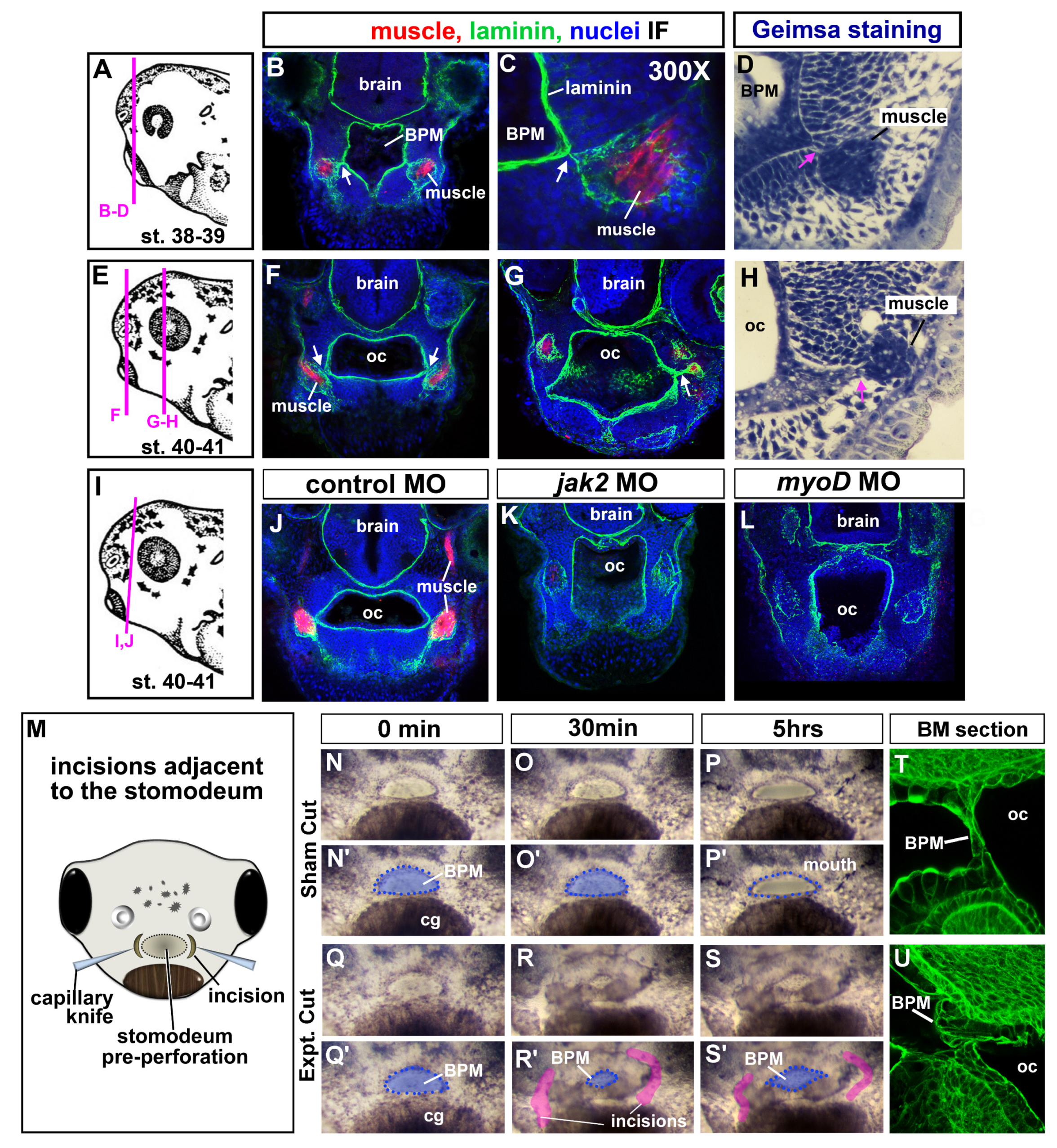
Figure 6.
Actin dynamics and buccopharyngeal perforation. A-G) Actin dynamics revealed by phalloidin labeling during buccopharyngeal membrane perforation. A) Shows a frontal view of the face at stage 39 (representative from 30 embryos in 4 biological replicates). B) The buccopharyngeal membrane at stage 37 when the mouth shape is round. C) The buccopharyngeal membrane at stage 39 just prior to perforation. Note the puncta spots and accumulation of F-actin (white arrows). D) The buccopharyngeal membrane during perforation shows accumulation of F-actin surrounding the holes. E-G) The buccopharyngeal membranes control morphants (E), jak2 morphants (F), and myod morphants (G). H-J) Images of representative embryos after treating with cytochalasin D (1uM) and Rockout (100uM) from stages 37-40. K) Quantification of buccopharyngeal membrane rupture in embryos exposed to actin inhibitors shown in H-J’ (n= 60, 3 biological replicates). .
Figure 6.
Actin dynamics and buccopharyngeal perforation. A-G) Actin dynamics revealed by phalloidin labeling during buccopharyngeal membrane perforation. A) Shows a frontal view of the face at stage 39 (representative from 30 embryos in 4 biological replicates). B) The buccopharyngeal membrane at stage 37 when the mouth shape is round. C) The buccopharyngeal membrane at stage 39 just prior to perforation. Note the puncta spots and accumulation of F-actin (white arrows). D) The buccopharyngeal membrane during perforation shows accumulation of F-actin surrounding the holes. E-G) The buccopharyngeal membranes control morphants (E), jak2 morphants (F), and myod morphants (G). H-J) Images of representative embryos after treating with cytochalasin D (1uM) and Rockout (100uM) from stages 37-40. K) Quantification of buccopharyngeal membrane rupture in embryos exposed to actin inhibitors shown in H-J’ (n= 60, 3 biological replicates). .
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).