Submitted:
11 April 2023
Posted:
12 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Terminology
2.3. Materials
2.3. Methods
3. Results
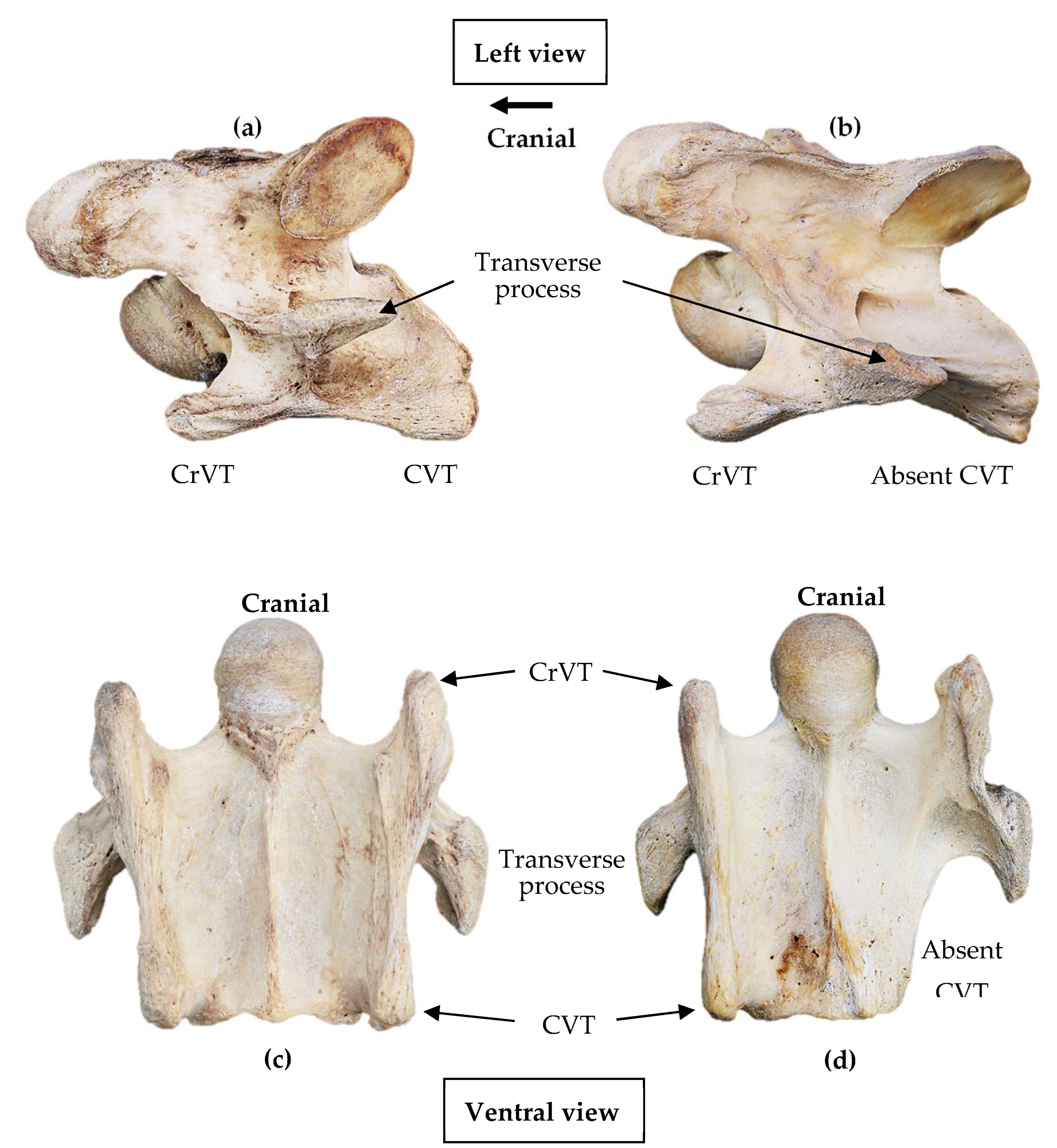
3.1. General Anatomy
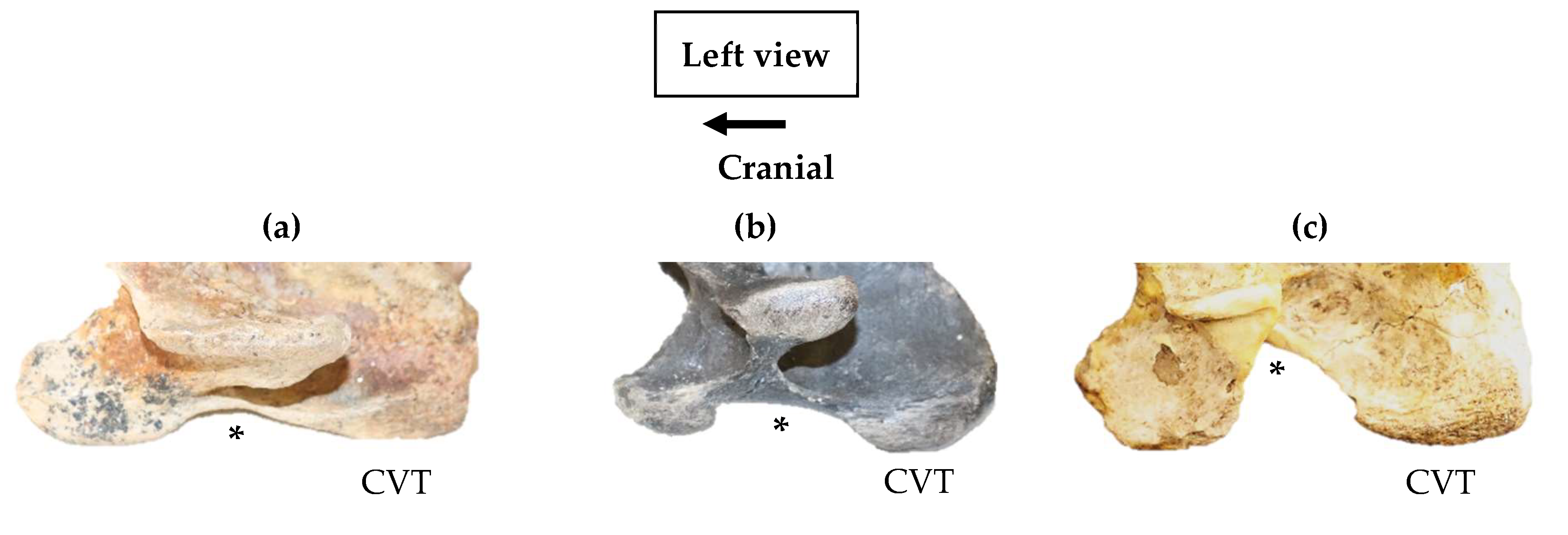
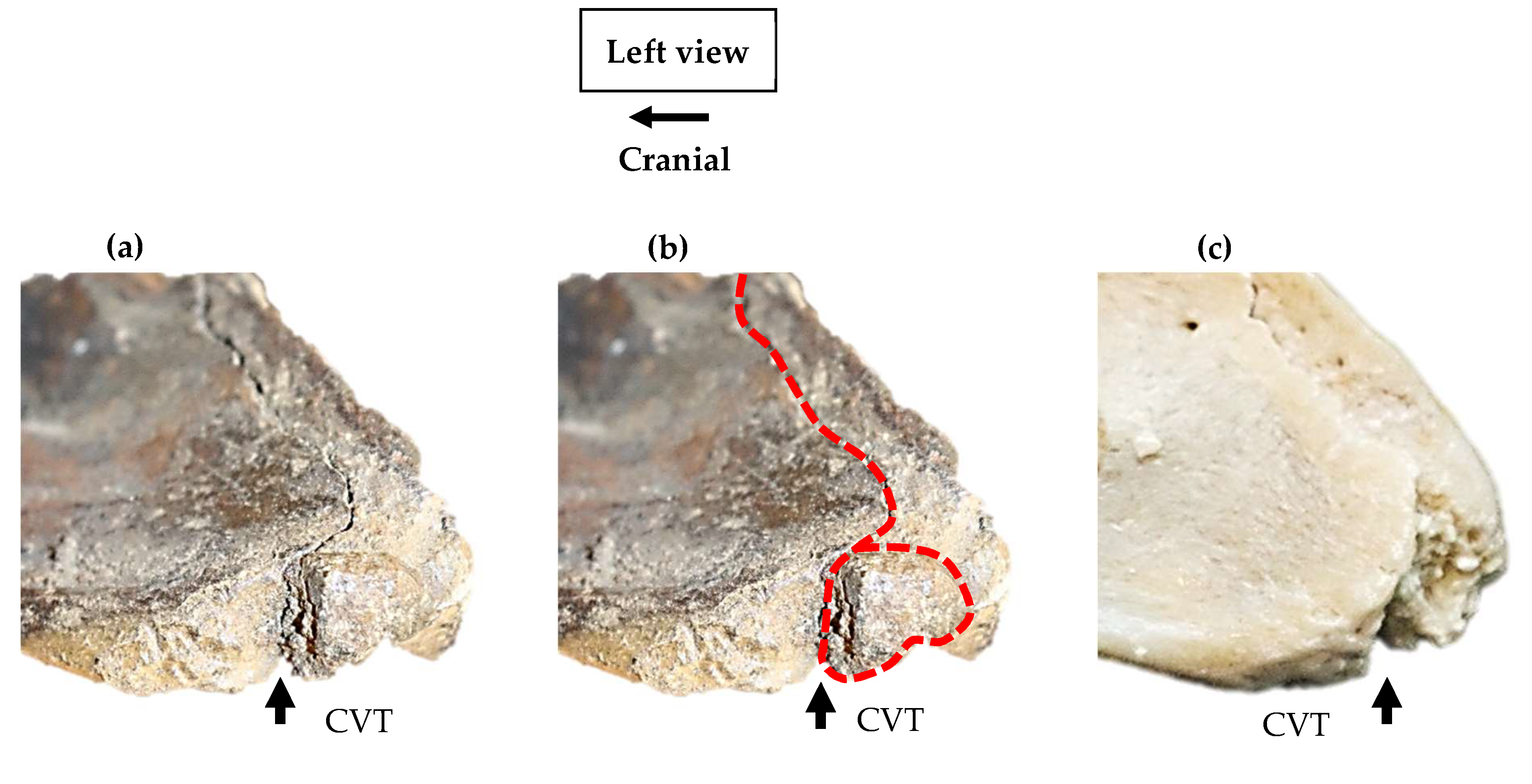
3.2. Lateral profile of the ventral process
3.3. Variations of the Epiphyseal
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arnold, P. Evolution of the mammalian neck and development, morpho-functional, and paleontological perspectives. J. Mamm. Evol. 2021, 28, 173–183. [Google Scholar] [CrossRef]
- Arnold, P.; Amson, E.; Fischer, M.S. Differential scaling patterns of vertebrae and the evolution of the neck length in mammals. Evo. 2017, 71, 587–599. [Google Scholar] [CrossRef]
- Buchholtz, E.A.; Bailin, H.G.; Laves, S.A.; Yang, J.T.; Chan, M-Y. ; Drozd, L.E. Fixed cervical count and the origin of the mammalian diaphragm. Evol. Dev. 2012, 5, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Willmore, K.E. The Body Plan Concept and Its Centrality in Evo-Devo. Evo Edu Outreach. 2012, 5, 219–230. [Google Scholar] [CrossRef]
- Flower, W.H. An Introduction to the Osteology of the Mammalia, 3rd ed.; Gadow, H., Ed.; Macmillion: London, United Kingdom, 1885; pp. 12–32. [Google Scholar]
- Chauveau, A. The Comparative Anatomy of the Domesticated Animals., 2nd ed.; Fleming, G. D. Appleton and Company: New York, USA, 1905; pp. 7–167. [Google Scholar]
- Getty, R. Equine Osteology. In The Anatomy of the Domestic Animals, 5th ed,; Sisson, S., Grossman, J. D., Eds.; Saunders: Philadelphia, USA, 1975; pp. 255–348. [Google Scholar]
- Frandson, R.D.; Wilke, W.L.; Fails, A.D. Anatomy and Physiology of Farm Animals. 7th ed,; Wiley-Blackwell: Ames, Iowa, USA, 2009; pp. 59–71. [Google Scholar]
- Janis, C.M. New ideas in ungulate phylogeny and evolution. Trends Ecol. Evol. 1988, 11, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Prothero, D.R. Evolutionary transitions in the fossil record of terrestrial hoofed mammals. Evo. Edu. Outreach. 2009, 289–302. [Google Scholar] [CrossRef]
- Narita, Y.; Kuratani, S. Evolution of the vertebral formulae in mammals: A perspective on developmental constraints. J Exp. Zool. 2005, 304, 91–106. [Google Scholar] [CrossRef]
- Sisson, S. Osteology. In A Textbook of Veterinary Anatomy. W.B. Saunders Company: Philadelphia, USA, 1910; pp. 31–35.
- Bradley, O. The Topical Anatomy of the Head and Neck of the Horse., 2nd ed.; Green and sons: Edinburgh, 1947. [Google Scholar]
- Denoix,J-M.; Pailloux, J-P. Anatomy and Basic Biomechanical Concepts. In Physical Therapy and Massage for the Horse, 2nd ed.; Trafalgar Square Publishing: North Pomfret, USA, 2001: pp. 19–69.
- Bainbridge, D. The Normal Anatomy of the Neck. In Equine Neck and Back Pathology, 2nd ed.; Henson., F.M.D., Ed.; John Wiley and Sons LTD: Hoboken, UK, 2018; pp. 1–9. [Google Scholar]
- Rhombach, N.; Stubbs, N.; Clayton, H. Gross anatomy of the deep perivertebral musculature in horses. J. Amer. Vet. Res. 2014, 75, 433–440. [Google Scholar] [CrossRef]
- Arnold, P.; Esteve-Altava, B.; Fischer, M.S. Musculoskeletal networks reveal topological disparity in mammalian neck evolution. BMC Evol Biol. 2017, 17, 251. [Google Scholar] [CrossRef]
- Davies, Z. Equine Science, 2nd ed,; John and Sons inc., Hoboken, New Jersey USA, 2018, pp 80–135.
- Haussler, K.K. Functional anatomy and clinical biomechanics of the equine cervical spine. Proceedings of the Association of American Equine Practitioners, 360˚ Pain in the Neck Conference. Functional Anatomy and Clinical Biomechanics of the Equine Cervical Soine. Retrieved from https://www.researchgate.net/publication/316053036_. 3160.
- Popesko, P. Atlas of Topographical Anatomy of the Domestic Animals, 4th ed.; W.B. Saunders: Philadelphia, USA, 1985; Volume 4, p. 185. [Google Scholar]
- Ballou, W.R. A Compendium of Equine Anatomy and Physiology. P. Blakiston’s Son and Co: Philadelphia USA, 1907; Replicated by Kessinger Legacy Reprints.
- Ashdown, R.R.; Done, S.H. Color atlas of veterinary anatomy., 2nd ed.; Mosby-Wolfe: London UK, 2000. [Google Scholar]
- Budras, K-D. ; Sack, W.O.; Röck, S. Anatomy of the Horse., 5th ed.; Schlütersche Verlagsgesellschaft & Co: Hannover Germany, 2009. [Google Scholar]
- 19, de Lahunta, A. Veterinary neuroanatomy and clinical neurology., 4th ed.; Elsevier: St. Louis USA, 2015. [Google Scholar]
- Dyce, K.M.; Sack, W.O.; Wensing, C. J. Textbook of veterinary anatomy., 5th ed.; Elsevier: Pennsylvania USA, 2018. [Google Scholar]
- Fails, A.D. Functional anatomy of the equine musculoskeletal system. In Adams and Stashak’s Lameness in Horses., 7th ed.; Ed. Gary Baxter. Blackwell Publishing: Oxford UK, 2020; pp. 1–65. [Google Scholar]
- Hodgins, J.E.; Haskett, T.H. The anatomy and diseases and treatment of domestic animals. The Veterinary Science Company: Detroit USA, 1905.
- Percivall, W. Anatomy, physiology, and pathology of the horse. 1826; retrieved from https://archive.org/.
- Steel, J.H. STEEL, J. H. (1876). Outlines of Equine Anatomy: A Manual for the use of Veterinary Students in the Dissection Room. William Wood and Co: New York USA, 1876. Replicated by Hardpress Publishing.
- Agerholm, J.S.; Bendixen, C.; Andersen, O.; Arnbjerg, J. Complex vertebral malformation in Holstein calves. J. Vet. Diagn. Invest. 2001, 13, 283–289. [Google Scholar] [CrossRef]
- Agerholm, J.S. Complex vertebral malformation Syndrome in Holstein cattle: The Story so Far. Acta. Vet. Scan. 2007, 49, S5. [Google Scholar] [CrossRef]
- Nielsen US, Aamand GP, Andersen O, Bendixen C, Nielsen VH, Agerholm JS: Effects of complex vertebral malformation on fertility traits in Holstein cattle. Livestock Prod. Sci. 2003, 79: 233-238. 10.1016/S0301-6226(02)00170-7.
- May-Davis, S. The occurrence of a congenital malformation in the sixth and seventh cervical vertebrae predominantly observed in thoroughbred horses. J. Equine. Vet. Sci. 2014, 18, 1313–1317. [Google Scholar] [CrossRef]
- DeRouen, A.; Spriet, M.; Aleman, M. Prevalence of anatomical variation of the sixth cervical vertebra and association with vertebral canal stenosis and articular process osteoarthritis in the horse. Vet. Radiol. Ultrasound. 2016, 57, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Santinelli, I.; Beccati, F.; Arcelli, R. Anatomical variation of the spinous and transverse processes in the caudal cervical vertebrae and the first thoracic vertebra in horses. Equine Vet. J. 2016, 48, 45–49. [Google Scholar] [CrossRef]
- Veraa, S.; Bergmann, W.; van den Belt, A-J. ; Wijnberg, I,; Back, W. Ex vivo computed tomographic evaluation of morphology variations in equine cervical vertebrae. Vet. Rad. Ultra. 2016, 0, 1–7. [Google Scholar]
- Veraa, S.; de Graaf, K.; Wijnberg, I.D.; Back, W.; Vernooij, H. ; Nielen, M; Belt, A,J, Caudal Cervical Vertebral Morphological Variation is not Associated with Clinical Signs in Warmblood Horses. Equine Vet. J. 2019, 52, 210–224. [Google Scholar]
- Beccati, F.; Pepe, M.; Santinelli, I.; Gialletti, R.; Di Meo, A.; Romero, J.M. Radiographic findings and anatomical variations of the caudal cervical area in horses with neck pain and ataxia: case–control study on 116 horses. Vet. Rec. 2020, 187, e79. [Google Scholar] [CrossRef]
- May-Davis, S. Congenital Malformations of the 1st Sternal Rib. J. Equine Vet. Sci. 2014, 49, 92–100. [Google Scholar] [CrossRef]
- May-Davis, S.; Walker, C. Variations and Implications of the Gross Morphology in the Longus colli Muscle in Thoroughbred and Thoroughbred Derivative Horses Presenting with a Congenital Malformation of the Sixth and Seventh Cervical Vertebrae. J. Equine Vet. Sci, 2015, 35, 560–568. [Google Scholar] [CrossRef]
- Witzmann, F.; Haridy, Y.; Hilger, A.; Manke, I.; Asbach, P. Rarity of congenital malformation and deformity in the fossil record of vertebrates – A non-human perspective. Int J Paeopatho. 2021, 33, 30–42. [Google Scholar] [CrossRef]
- van der Geer, A.A.E.; Galis, F. High incidence of cervical ribs indicates vulnerable condition in Late Pleistocene woolly rhinoceroses. PeerJ. 2017, e3684. [Google Scholar] [CrossRef] [PubMed]
- Reumer, J.W.F.; ten Broek, C.M.A.; Galis, F. Extraordinary incidence of cervical ribs indicates vulnerable condition in Late Pleistocene mammoths. PeerJ. 2014, 2:318. [CrossRef]
- Varela-Lasheras, I.; Bakker, A.J.; van der Mije, S.D.; Metz, J.A.J.; van Alphen, J.; Galis, F. Breaking evolutionary and pleiotropic constraints in mammals: On sloths, manatees and homeotic mutations. EvoDevo, 2011, 2:11.
- Gasse, H., W. Van Den Broeck. and P. Simeons. Nomina Anatomica Veterinaria. 6th ed. Published by the Editorial Committee for the World Association of Veterinary Anatomists. Hanover, Ghent, Columbia MO and Rio de Janeiro. 2017; pp. 17.
- Gidley, J.W. A new three-toed horse. J. Morhol. 1903, 29, 465–476. [Google Scholar]
- Wood, A.R.; Bebej, R.M.; Manz, C.L; Begun, D.L; Gingerich, P.D. Postcranial functional morphology of Hyracotherium (Equidae, Perissodactyla) and locomotion in the earliest horse. J. Mammal. Evol. 2011, 18, 1–32. [Google Scholar] [CrossRef]
- Franzen, J.F.; Habersetzer, J. Complete skeleton of Eurohippus messelensis (Mammalia, Perissodactyla, Equoidea) from the early middle Eocene of Grube Messel (Germany). Palaeobio. Palaeoenv. 2017, 97, 807–832. [Google Scholar] [CrossRef]
- Chauveau, M.D. (1908). The Comparative Anatomy of the Domesticated Animals. 2nd ed. Translation George Fleming. Appleton and Company: New York, USA, 1908; pp. 53–80.
- MacFadden, B.J. Fossil Horses: Systematics, Paleobiology, and Evolution of the Family Equidae. Cambridge University Press: Cambridge UK, 1992.
- Randau, M.; Goswani, A. Morphological modularity in the vertebral column of Felidae (Mammalia, Carnivora). BMC Evol. Biol. 2017, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Schlosser, G.; Wagner, G.P. Modularity in Development and Evolution. University of Chicago Press: Chicago, USA, 2004.
- Figueirido, B.; Pérez-Ramos, A.; Martín-Serra, A. Intravertebral vs. intervertebral integration and modularity in the vertebral column of mammalian carnivorans. J. Anat. 2022, 4, 642–656. [Google Scholar] [CrossRef] [PubMed]
- Vidal, P.P.; Graf, W.; Berthoz, A. The orientation of the cervical vertebral column in unrestrained awake animals. Exp Brain Res, 1986, 61, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Graf, W.; de Waele, C.; Vidal, P.; Wang, D.; Evinger, C. The orientation of the cervical vertebral column in unrestrained awake animals. II. Movement strategies. Brain Behav. Evol. 1995, 45, 209–231. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, C.M.; Wright, L.; Kristoffersen, M.; Puchalski, S.M. Computed tomography and myelography of the equine cervical spine: 180 cases (2013–2018). EVJ. 2020, 9, 475–483. [Google Scholar] [CrossRef]


| Species (n) and Geologic | Institute (n=8) |
Collection/ Catalogue No. | Adult or Sub adult |
|---|---|---|---|
| Equus Sp (4) | NHMB | MB. Ma. 45354 | Adult |
| MB. Ma. 24107 | Sub adult | ||
| MB. Ma. 24410 | Adult | ||
| MB. Ma. 24463 | Adult | ||
|
Equus mosbachensis (4) 80–600kya |
MMHBE | GO 374 3' 8P 197 | Adult |
| Scho 13 II 4 95 690 22 14 | |||
| Scho 13 II 4 95 690 18 45 | |||
| Scho 13 II 4 96 692 27 | |||
| Equus sp 10–40kya (1) | Ko 100-108 | Sub adult | |
| Equus occidentalis (20) 10–40kya | TPMRLB | 2-T22,8'9 | Adult |
| E-2,9 | Sub adult | ||
| 3 E2,16 | Sub adult (left side only) | ||
| 3,E-1,17.5 | Adult | ||
| 3,E-2, 12 | |||
| 3,F4,12 | Sub adult | ||
| 3,F-4,12 | Adult |
||
| 4 B2 M-19 | |||
| 4 B5,20 | |||
| 4 C-2,17-19 | |||
| 4 C-4 13-15 | |||
| 4 D2-4,8,17 | |||
| 4 D2-99 | |||
| 4, C5, 17 246 | Sub adult | ||
| 60 D-12,14-16 | Adult | ||
| 4, G + H-2+3,8 | Sub adult | ||
| 67 H-9,15-18.5 | |||
| 77 E-9,11 | Adult | ||
| Circle 61 | Sub adult | ||
| GIZ, 14.5-16.5 | Adult (right side only) | ||
| Pliohippus (1) | YHM | Mounted replica | Adult |
| Hyracotherium (1) | |||
| Mesohippus (1) | |||
| Hyracotherium (1) 55mya | AMNH | 15428 | Adult (right side only) |
| Merychippus isonesus (1) 15–5mya | ESP. 5263638 | ||
| Mesohippus bairdii (1) 37–32mya | 1477 | Adult | |
| Miohippus doliquideus (1) 32–25mya | 39115 | Adult (left side only) | |
| Pliohippus mirabilis (1) 12–6mya | 60810 | ||
| Pliohippus pernix (1) 12–6mya | 60803 | Adult | |
| Mesohippus (cast) (1) | OUMNH | Mounted Replica | Adult |
| Pliohippus (1) 12–6mya | LACM | (CIT) 210 | Adult |
| Equus Sp (6) | LACM | (CIT) 138 120 | Sub adult |
| (CIT) 138 138 | Adult (left side only) | ||
| (CIT) 138 699 | Adult | ||
| 192 L8 | Adult (right side only) | ||
| LACM San Josecito |
(CIT) 192 | Adult (right side only) | |
| LACM Row XIX |
(CIT) 192 (duplicate) | Adult (right side only) | |
|
Equus simplicidens (15) 5.3–1.8mya |
USNM | USNM 13791 | Adult |
| USNM 12573 | |||
| USNM PAL 785552 | Sub adult | ||
| USNM V 12575 | Adult | ||
| USNM PAL 785561 | |||
| USNM 785560 | |||
| USNM PAL 785553 | |||
| USNM 78559 | |||
| USNM 12155 | |||
| USNM 12580 | |||
| USNM PAL 785557 | |||
| USNM PAL 785556 | |||
| USNM PAL 785559 | |||
| USNM PAL 785555 | |||
| USNM PAL 785554 | |||
| Equus sp. (7) | UF | Leisey 1A UF 82283 | Adult |
| Leisey 1A UF 151092 | |||
| Leisey 1A UF 151020 | Adult (right side only) | ||
| UF 242520 | |||
| UF 242521 | Adult | ||
| Leisey 1A UF 151094 | Adult (Left side only) | ||
| UF 242522 | Adult (right side only) |
| Species (n) | Institute | Collection/Catalogue No. | Adult or Sub adult |
|---|---|---|---|
| Equus africanus (1) | NBC | N.A.M. 28.-11-1972 |
Adult |
| Equus hermionus (1) | No. 509 | ||
| Equus sp. (1) | Equus sp mounted skeleton | ||
| Equus asinus (3) | ACEPT | Donald | Adult |
| Sebastian | |||
| Kam | |||
| Equus przewalskii (5) | ES | Heteni | |
| Rideg | Sub adult | ||
| YHM | YHM – Live Pony Park | Adult | |
| NHMB | MB. Ma. 16464 | ||
| MB. Ma. 45373 | |||
| Equus quagga boehmi (1) | ES | Kimberley – Zoo bred |
| Species (n) | Institute | Collection/Catalogue No. | Adult or Sub adult |
|---|---|---|---|
| Neohipparion whitneyi (1) 13.6–4.9mya [46] | AMNH | 9815 | Adult |
| Hyracotherium grangeri (1) 55mya [47] | UM | 115547 | |
| Eurohippus messelensis (1) 56–33.9mya [48] | SMF | SMF ME 11034 |
| Species (n) and Geologic | Institute (n=8) |
Collection Catalogue No. | Size of Convexity |
|---|---|---|---|
| Hyracotherium (1) | YHM | Mounted replica | Large |
| Hyracotherium (1) 55mya | AMNH | 15428 | Large |
| Hyracotherium grangeri (1) 55mya [47] | UM | 115547 | Large |
| Eurohippus messelensis (1) 56–33.9mya [48] | SMF | SMF ME 11034 | * |
| Mesohippus (cast) (1) | OUMNH | Mounted Replica | Large |
| Mesohippus bairdii (1) 37–32mya | AMNH | 1477 | Large |
| Mesohippus (1) | YHM | Mounted replica | Large |
| Miohippus doliquideus (1) 32–25mya | AMNH | 39115 | Large |
| Merychippus isonesus (1) 15–5mya | AMNH | ESP. 5263638 | Large |
| Neohipparion whitneyi (1) 13.6–4.9mya [46] | AMNH | 9815 | Large^ |
| Pliohippus mirabilis (1) 12–6mya | AMNH | 60810 | Large |
| Pliohippus pernix (1) 12–6mya | AMNH | 60803 | Large |
| Pliohippus (1) | YHM | Mounted replica | Large |
| Pliohippus (1) 12–6mya | LACM | (CIT) 210 | Large |
|
Equus simplicidens (15) 5.3–1.8mya |
USNM | USNM 13791 | Small |
| USNM 12573 | Small | ||
| USNM PAL 785552 | Small | ||
| USNM V 12575 | Small | ||
| USNM PAL 785561 | Small | ||
| USNM 785560 | Small | ||
| USNM PAL 785553 | Medium | ||
| USNM 785559 | Medium | ||
| USNM 12155 | Medium | ||
| USNM 12580 | Small | ||
| USNM PAL 785557 | Medium | ||
| USNM PAL 785556 | Small | ||
| USNM PAL 785559 | Small | ||
| USNM PAL 785555 | Medium | ||
| USNM PAL 785554 | Small | ||
| Equus occidentalis (20) 10–40kya | TPMRLB | 2-T22,8'9 | Small |
| E-2,9 | Small | ||
| 3 E2,16 | Small | ||
| 3,E-1,17.5 | Medium | ||
| 3,E-2, 12 | Medium | ||
| 3,F4,12 | Medium | ||
| 3,F-4,12 | Small | ||
| 4 B2 M-19 | Small | ||
| 4 B5,20 | Small | ||
| 4 C-2,17-19 | Small | ||
| 4 C-4 13-15 | Small | ||
| 4 D2-4,8,17 | Small | ||
| 4 D2-99 | Small | ||
| 4, C5, 17 246 | Small | ||
| 60 D-12,14-16 | Small | ||
| 4, G + H-2+3,8 | Small | ||
| 67 H-9,15-18.5 | Small | ||
| 77 E-9,11 | Medium | ||
| Circle 61 | Medium | ||
| GIZ, 14.5-16.5 | Small | ||
|
Equus mosbachensis (4) 80–600kya |
MMHBE | GO 374 3' 8P 197 | Small |
| Scho 13 II 4 95 690 22 14 | Small | ||
| Scho 13 II 4 95 690 18 45 | Small | ||
| Scho 13 II 4 96 692 27 | Small | ||
| Equus sp 10–40kya (1) | Ko 100-108 | Small | |
| Equus Sp (4) | NHMB | MB. Ma. 24107 | Small |
| MB. Ma. 24410 | Medium | ||
| MB. Ma. 24463 | Medium | ||
| MB. Ma. 45354 | Medium | ||
| Equus Sp (6) | LACM | (CIT) 138 120 | Small |
| (CIT) 138 138 | Small | ||
| (CIT) 138 699 | Medium | ||
| 192 L8 | Medium | ||
| LACM San Josecito |
(CIT) 192 | Small | |
| LACM Row XIX |
(CIT) 192 (duplicate) | Small | |
| Equus sp. (7) | UF | Leisey 1A UF 82283 | Small |
| Leisey 1A UF 151092 | Medium | ||
| Leisey 1A 151120 | Medium | ||
| UF 242520 | Small | ||
| UF 242521 | Small | ||
| Leisey 1A UF 151094 | Medium | ||
| UF 242522 | Medium |
| Species (n) | Institute | Collection/Catalogue No. | Adult or Sub adult |
|---|---|---|---|
| Equus africanus (1) | NBC | N.A.M. 28.-11-1972 |
Small |
| Equus hermionus (1) | No. 509 | ||
| Equus sp. (1) | Equus sp mounted skeleton | ||
| Equus asinus (3) | ACEPT | Donald | Small |
| Sebastian | |||
| Kam | |||
| Equus przewalskii (7) | ES | Heteni | |
| Rideg | Small | ||
| YHM | YHM – Live Pony Park | Small | |
| NHMB | MB. Ma. 16464 | ||
| MB. Ma. 45373 | |||
| Equus quagga boehmi (1) | ES | Kimberley |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





