Introduction
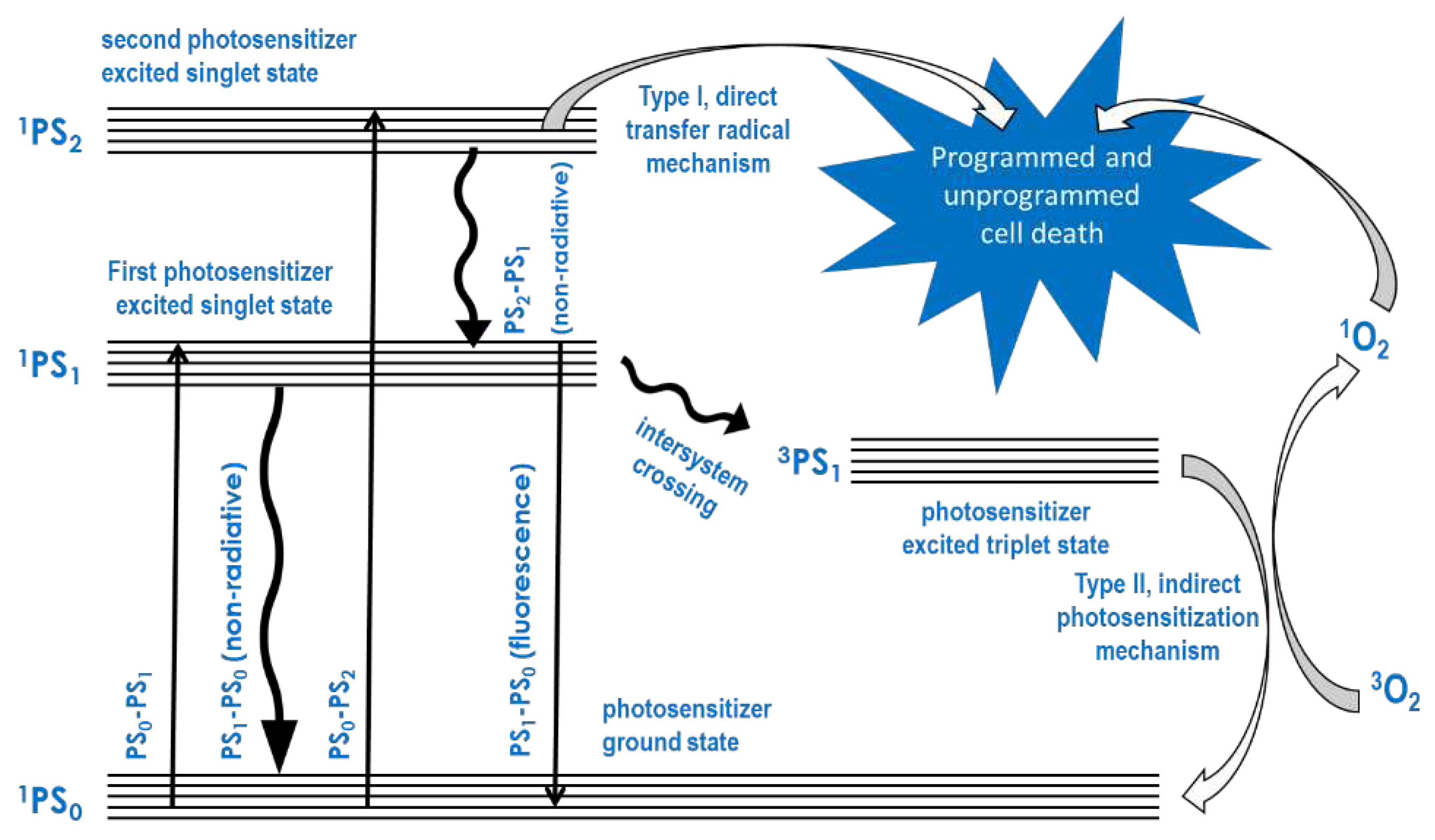
Several compounds known as photosensitizers absorb light energy and transfer it to oxygen in the ground state to produce reactive oxygen in the triplet state. Following this, a series of other reactive oxygen species are produced in biological media. Upon excitation with light energy, most of these compounds can also react directly with biological molecules such as cell membrane and cytoplasmic components, including monosaccharides, nucleic and amino acid components of DNA, RNA, glycan, and protein molecules. Photodynamic therapy (PDT) is based on the initial absorption of light by the photosensitizer (PS) and the subsequent direct and oxygen-mediated reactions with the cell membrane and cytoplasmic cell components, because these reactions initiate the death of cells by apoptosis or their rapid destruction by necrosis. Thus, PDT requires an accumulation of the PS through targeted delivery to the disease cells, which either does not occur in normal healthy cells or does so to a relatively lower degree compared to the disease cells. The foregoing mechanism of PDT may be illustrated using a Jablonski diagram, as shown in
Figure 1.
Originally, when it was demonstrated that the organic dye PSs of the porphyrin and phthalocyanine type accumulate preferentially in tumor tissue compared to normal host tissue, PDT was described as an anticancer therapeutic technology. However, as the technology progressed through to the clinic, the observed preferential accumulation proved to be insufficient to be of competitive therapeutic value. This triggered an avalanche of research aimed at enhancing the selective delivery of PSs to disease cells and sites compared to normal ones, which is still the main preoccupation of a large population of PDT researchers today. Given that the technology requires oxygen to be present in the disease microenvironment, it was soon realized that the well-documented hypoxia observed in such disease microenvironments as cancerous tumors and bacterial and fungal infections is another major limitation of PDT. This limitation also triggered more research to discover ways to improve oxygenation of the disease microenvironment for the efficacious application of PDT.
Another limitation of PDT is the depth to which light can penetrate human tissue to reach the PS and initiate the PDT reaction, which is no more than 8-10 mm. While most skin tissue and other low-lying diseases are currently the best candidates for effective treatment, diseases that are located deeper than 10 mm or those that are located in dark tissue such as the liver, spleen, pancreas, and bones, cannot be treated using PDT without using optic fiber light delivery, even when using light of frequency in the therapeutic window of the near-infrared region. Sonodynamic therapy (SDT) is a recent iteration of PDT that was developed to overcome the light penetration depth challenge in PDT. Instead of light, the SDT sensitizer, which is known as the sonosensitizer, is activated by ultrasound, which can go through the entire body, thus, reaching through dark tissues and bones. For this reason, much research is dedicated to the application of SDT in periodontal, bone disease, and diseases of dark tissues such as the liver, spleen, and pancreas.
In most clinical PDT, the PS is still administered directly to the patient to reach the diseased tissue after systemic circulation. Nowadays, however, much of the research aimed at enhancing the efficacy and selectivity of PDT uses nanomaterials as vehicles for carrying and delivering the PS to the diseased tissue for precision-targeted delivery and selectivity for the disease over host tissue. Due to their small size and, therefore, high surface area to volume ratio, nanomaterials can adsorb, carry and deliver large quantities of PSs. Like porphyrin and phthalocyanine organic dye PSs, evidence exists to show that the accumulation of nanomaterials in tumor tissue may be driven by the enhanced permeability and retention effect [[
1]]. Therefore nanoparticles have been widely researched for their potential to enhance the efficacy of PDT through enhanced targeted delivery of the PS to the diseased tissue. In addition, nanoparticles have been identified that act as PSs by photosensitizing the production of reactive oxygen species. While these nanomaterials render it unnecessary to load them with organic dye PSs for delivery to the disease site, when loaded with such PSs, the resulting nanoconjugates have been shown to have potent reactive oxygen-generating capabilities and PDT efficacy.
Nanomaterials have been used as a basis for combination therapies involving PDT. This is generally achieved by synthesizing nanoconjugates that possess the characteristics required for PDT and those of the technology that is being combined with PDT in the same nanoconjugate. For example, for combining PDT with magnetic hyperthermia therapy (MGH), magnetic nanoparticles are loaded with the PS. This produces a nanoconjugate that responds to a high-frequency alternating magnetic field by generating heat, thus elevating the temperature beyond the tolerable limit of the disease cells in the microenvironment. Similarly, to combine PDT with photothermal hyperthermia therapy, photothermal nanoparticles are loaded with the PS, generating a nanoconjugate that responds to light irradiation by elevating the temperature beyond the tolerable limit of the disease cells in the microenvironment. However, to combine PDT with chemotherapy, nanoparticles are loaded with a suitable chemotherapy drug as well as the PS to give a functionalized nanoconjugate that delivers the PS and the chemotherapy drug to the disease site and enables simultaneous PDT and chemotherapy.
This paper also discusses the combination of PDT with cold atmospheric pressure plasma (CAP) therapy, which does not necessarily require nanomaterials except to act as a carrier and delivery agent for the PS. A cold flame plasma discharge consisting of the flowing gas and reactive gas species, not unlike the reactive oxygen species generated in PDT, is generated when the gas is passed between electrodes with high voltage direct or alternating current. The electric field removes some of the electrons from the gas species molecules, generating cations, radicals, and electrons, which, upon collision with the gas species, cause further ionization, radical formation, and release of electrons as the gas flows. All this takes place at ambient conditions of temperature and pressure, with minuscule temperature elevation, the makings of CAP, the constituents of which have been shown to be destructive to cells upon contact, especially those of disease. If CAP is seen to generate the species by which PDT destroys disease cells by apoptosis and necrosis, it is reasonable therefore to deduce that its combination with PDT enhances efficacy by increasing the disease-destroying reactive gas species produced by both technologies in the microenvironment.
Anticancer photodynamic Therapy
When applied to cancer, PDT can act by a combination of cancer cell death and by inhibiting the growth of tumors due to cancer cell growth inhibition. It relies on the preferential accumulation of the PS in cancer tissue, where it photosensitizes the generation of reactive oxygen species which kill cancer cells in the microenvironment of the disease. While the origin of the clinical approach is attributed to the direct administration and some level of preferential accumulation of hematoporphyrin and hematoporphyrin derivative followed by systemic circulation [[
2]], nowadays, much attention is paid to targeted nanomaterial-mediated PS administration. However, some researchers still administer the free PSs directly without nanomaterials. The integration of coumarin into amphoteric nanocapsules is an example of the administration of the PS encapsulated in organic nanomaterials with the aim of optimal systemic navigation due to the amphiphilicity of the nanomaterial and disease accumulation due to hydrophobicity of the PS [[
3]].
Comparison of the in vitro efficacy of novel zinc (II) phthalocyanine-quinoline conjugate with Photofrin, on the other hand, illustrates the direct approach without incorporating the PS in any form of nanomaterial [[
4]]. In this study, the zinc (II) phthalocyanine-quinoline conjugate and the Photofrin were administered to the cell lines as solutions in N, N-dimethylacetamide containing 3.8% polyoxyethylene-35-castor oil, and phosphate-buffered saline, respectively. The advantages of using nanoparticles as carrier and delivery agents for the PS, compared to using the free PSs, which include the improvement of solubility, biodistribution, uptake, retention, targeted cancer cell delivery, the reduction of self-degradation, and applications in combination therapies involving PDT, have been elucidated with numerous examples of metal nanoparticles encapsulated in various shells containing the PS [[
5]]. In this regard, silver and gold nanoparticles are among the most widely studied [[
6]]. While metal oxide nanoparticles are widely studied as carriers and delivery nano-systems of the organic dye type of PSs for targeted anticancer PDT [[
7]], iron oxide nanoparticles have been highly prized for their potential for magnetic hyperthermia, magnetic tumor targeting, and magnetic resonance imaging-assisted anticancer PDT [[
8],[
9],[
10],[
11]]
Cancer cell-targeted, controlled, and stimulus-responsive release of the PS is seen as the ultimate gold prize of nanomaterial-mediated PDT, through which deep metastatic cancer cells can be isolated for destruction before they form tumors [[
12],[
13]]. Much research has therefore focused on metastatic cancer cell targeting for PDT. For example, photoimmunotherapy is a technology that combines PDT and immunotherapy, in which the PS is conjugated to a monoclonal antibody or biologically recognizable fragment thereof to actively and specifically target antigens for high-precision targeted drug delivery [[
14]]. Additionally, a peptide that is easily cleaved by the cysteine protease Cathepsin B was used as a linker for anchoring the PS Verteporfin onto the surface of gold nanoparticles so that upon cancer cell internalization of the nanoconjugate, the PS is released after cleavage of the peptide linker [[
15]].
Antimicrobial Photodynamic Therapy
From its origins as an anticancer therapeutic technology, PDT has evolved. It is now widely used against a variety of ailments, the top among which is antimicrobial PDT as applied against bacterial, fungal, and viral infections, as well as applications in the sanitization of the environment and pest control [[
16]]. Antimicrobial PDT against many viral infections, such as the recent SARS-CoV-2 viral pandemic [[
17]], bacterial [[
18]] and fungal [[
19]] infections, and environmental sanitation [[
20]] have been widely investigated. Examples of applications of antibacterial PDT include periodontal disease [[
21],[
22]], superficial wounds [[
23]], and burns [[
24]]. It is hailed as holding great promise for the developing world, where bacterial infections cause a large number of deaths, while the widespread use of antibiotics is fuelling the rapid emergence of bacterial resistance there [[
25]].
The formation of biofilms drives the development of resistance with some bacterial and fungal infections through a multi-stage process involving planktonic microbial quorum sensing [[
26]], surface adhesion, colony formation and maturation, biofilm formation, and microbial detachment from biofilm, leading to attachment elsewhere, starting the process all over again as the biofilm grows [[
27],[
28]]. Throughout its life cycle, the bacterial cells remain suspended in, and otherwise embedded in, the biofilm, which is made up of an extracellular polymeric substance matrix consisting of polysaccharides, proteins, peptides, nucleic acids, and lipids, making it difficult to reach the suspended bacterial cells [[
29],[
30],[
31]]. Antibacterial PDT has been shown to overcome the biofilm-mediated bacterial resistance strategy by breaking down the extracellular polymeric substance matrix, thereby providing access to the bacterial cells [[
32],[
33]].
Nanoconjugates incorporating the PS in their structure constitute the most widely reported antibacterial PDT strategy compared to the application of free PSs. The structure of these nanoconjugates is designed to ensure labile incorporation of the PS into the conjugate so that it retains the capability to generate reactive oxygen species. Several nanoparticles act as antibacterial PDT PSs capable of biofilm degradation and bacterial cell destruction on their own. The most widely reported include nanoparticles of porphyrins [[
34]] and phthalocyanines [[
35]], metallic silver [[
36]] and metallic gold [[
37]] copper sulphide [[
38]], as well as oxides of nanographene [
34], zinc [[
39]], and iron [[
40]]. For example, the three different morphologies of nanostructures of copper sulfide, including microspheres, nanosheets, and nanoparticles, were found to exhibit different antibacterial PDT activities against
E. coli, and this was largely attributed to their different concomitant photothermal conversion coefficients upon irradiation with normal sunlight [[
41]]. This study is in alignment with the more general observation that most of these nanomaterials exhibit the concomitant photothermal and magnetothermal conversion capability and are therefore used as agents for combination therapies involving photothermal therapy (PTT), MGH, and PDT. For example, iron oxide nanoparticles are used in MGH, PTT, and PDT combinations [
40]. Furthermore, studies have shown that nanographene oxide and copper sulfide nanoparticles are efficacious PTT and PDT agents [
34,
38]. Bacterial infection stimulus-responsive release of the PS, derived from cleverly designed nanoconjugates for antibacterial PDT exploits one or more of the characteristics of the biofilm microenvironment, such as lower pH, insufficient oxygenation, and the altered concentration of enzymes and hydrogen peroxide compared to normal tissue microenvironments [[
42],[
43]].
Table 1.
Some of the Applications of Photodynamic therapy.
Table 1.
Some of the Applications of Photodynamic therapy.
| |
Applications and description |
References |
| 1 |
Anticancer |
[4]-[15] |
| 2 |
Antimicrobial |
[16]-[42] |
| 3 |
Viral Herpes |
[56], [57] |
| 4 |
SARS-CoV-2 |
[17], [58] |
| 5 |
Bacterial Acne |
[46], [47] |
| 6 |
Wet age-related macular degeneration |
[48], [49] |
| 7 |
Atherosclerosis |
[50], [114,115] |
| 8 |
Psoriasis |
[52], [53] |
| 9 |
Environmental sanitization |
[16], [59], [97] |
| 10 |
Pest control |
[16], [45], [60] |
| 11 |
Dermatology |
[46], [53], [61,62,63] |
Other applications of Photodynamic Therapy
As the pathogenic cause of acne, bacterial and fungal growth that occurs when the hair follicles become plugged with oil and dead skin cells in the skin, especially on the face, may be treated with antibacterial PDT. Wet age-related macular degeneration occurs when abnormal blood vessels grow in the back of the eye and damage the macula. PDT destroys the macula blood vessels, after which new growth gives rise to normal blood vessels. An accumulation of plaque in the inner lining causes atherosclerosis as a thickening or hardening of arteries. Fast-growing skin cells cause an itchy scalp and flakes on the skin known as psoriasis. PDT is a proven treatment for fast-growing cells. The recent emergence of viral pandemics, such as the recent pandemic caused by the SARS-COV-2 virus, has triggered an avalanche of interest in antimicrobial PDT. PDT has been shown to destroy viruses, bacteria, and fungi [[
44]]. For this reason, the technology has been tested for sanitization of the environment, such as work surfaces, where the reactive oxygen species produced can kill microorganisms. It has also been tested as a pesticide against insect larvae [[
45]]. From the foregoing discussion, it is easy to see why the applications of PDT have gone beyond cancer and bacterial infections to include the treatment of acne [[
46],[
47]], wet age-related macular degeneration [[
48],[
49]], atherosclerosis [[
50],[
51]], psoriasis [[
52],[
53]], and anti-viral treatments [[
54],[
55]], including herpes [[
56],[
57]] and the SARS-COV-2 [[
58]] virus. Investigations for environmental sanitization [[
59]] and pest control [45,[
60]], and why it is among the most commonly used therapeutic techniques in dermatology [[
61],[
62]].
The Value Proposition of Combinations of Photodynamic Therapy
PDT has been described as a minimally invasive technology for treating an increasing range of ailments [[
63]]. Several limitations of PDT have been cited, including the poor solubility of the PSs, low depth of the tissue penetration by the light used to activate the PS, the rather low oxygenation levels in most of the microenvironments of the candidate diseases against which it is used, sub-optimal levels of the PS that reaches the disease after systemic circulation, and the poor selectivity for the disease at tissue and cellular levels. These challenges limit the efficacy in vivo of PDT even for candidate PSs that show potent efficacy in vitro. Therefore, combinations of PDT with a range of other minimally invasive therapies is one way that has been widely explored to enhance the efficacy in any of the identified areas where its efficacy is limited. The primary purpose of these combination therapies is to enhance efficacy in any way and therefore promote clinical translation.
Combination therapies have been evaluated on the basis of three outcome levels of efficacy enhancement. When the efficacy of the combination therapy exceeds the sum of their individual efficacies, the outcome is synergistic. When the two have equal efficacy, the outcome is additive. When the combination efficacy is less than that of the sum of the individual efficacies, the outcome might be antagonistic [[
64]]. For example, when the efficacy of the combination of PDT using meso-tetrakis(3-hydroxyphenyl)chlorin as the PS, and the platinum drugs carboplatin, cisplatin, and oxaliplatin, was evaluated in vitro, using the methyl thiazole tetrazolium essay, synergism was observed with oxaliplatin and three different cancer cell lines. In contrast, additivity and antagonism were observed with the other drugs [[
65]]. One of the mechanisms of drug resistance is the prevention of drug entry into target cells. A nanoconjugate consisting of a core of doxorubicin-loaded calcium carbonate and a shell of human serum albumin conjugated with the PS Rose Bengal was prepared so as to improve the endocytosis efficiency, thus overcoming the doxorubicin resistance. The enhancement of the anti-proliferation effect in vitro was synergistic [[
66]]. On the contrary, it was suggested more than two decades ago that the combination of radiotherapy with PDT is generally additive [[
67]]. Much of the subsequent evidence has supported this observation [[
68]]. An example of a sub-additive outcome was reported from the evaluation of the efficacy of the combination of PDT using the PS methylene blue with chemotherapy using the drug erlotinib against the human epidermoid carcinoma A431 cell lines in vitro [[
69]].
Table 2.
Outcomes of combinations of photodynamic therapy using meso-tetrakis(3-hydroxyphenyl)chlorin and chemotherapy against indicated cancer cell lines.
Nanomaterials as a Requirement for Combination Therapies
Before the rise of nanotechnology into its current state of prominence, therapeutic studies on the applications of PDT were conducted by directly applying the free form of the PS. After a period of time during which the free PS was in the systemic circulation, preferential accumulation generally leads to enhanced concentration in cancerous tissue. The preferential accumulation of the PS in disease tissue and the subsequent photosensitization reaction, which produces reactive oxygen species, is the essential basis of PDT because it means that there is a higher concentration of the PS at the disease site and in the disease cells, compared to cells in other sites of the body after the systemic circulation period. However, the degree of this preferential accumulation of the free form of the organic dye PSs soon proved to be insufficient for the required levels of disease targeting and selectivity for disease cells over host tissue cells as the technology translated to the clinic. The direct approach to combination therapies involving PDT involved the independent administration or direct combination of the free PS and the agent of the therapy that was being combined with PDT without the aid of nanomaterials. Examples of these abound [[
70],[
71]].
In addition to the common applications for enhancing the selectivity of the PS for the disease over host tissue cells in PDT, nanotechnology is widely used to facilitate various combinations of PDT with other non-invasive therapeutic technologies, resulting in further enhancement of efficacy. Consequently, several innovations for target cell specificity started to emerge based on the elaborate design and fabrication of nanoconjugates that incorporate the PS. In any case, PTT and MGH are technologies commonly combined with PDT, which are already based on the use of nanomaterials. Another development of the application of nanotechnology in PDT was the expansion to applications on disease types other than cancers, including bacterial, fungal, and viral infections. The nanomaterial-mediated enhancement of target specificity in PDT, which implies reduced systemic dose requirements for copious PS delivery at the disease site and in the disease cells, shows great promise in research studies aimed at reducing the emergence of resistance, especially by microbial pathogens. Nanomaterial-mediated selectivity for disease cells over host tissue also facilitated various chemotherapy combinations with PDT. One spin-off from this is the possibility of repurposing chemotherapy drugs that had been rendered obsolete by the development of resistance against them.
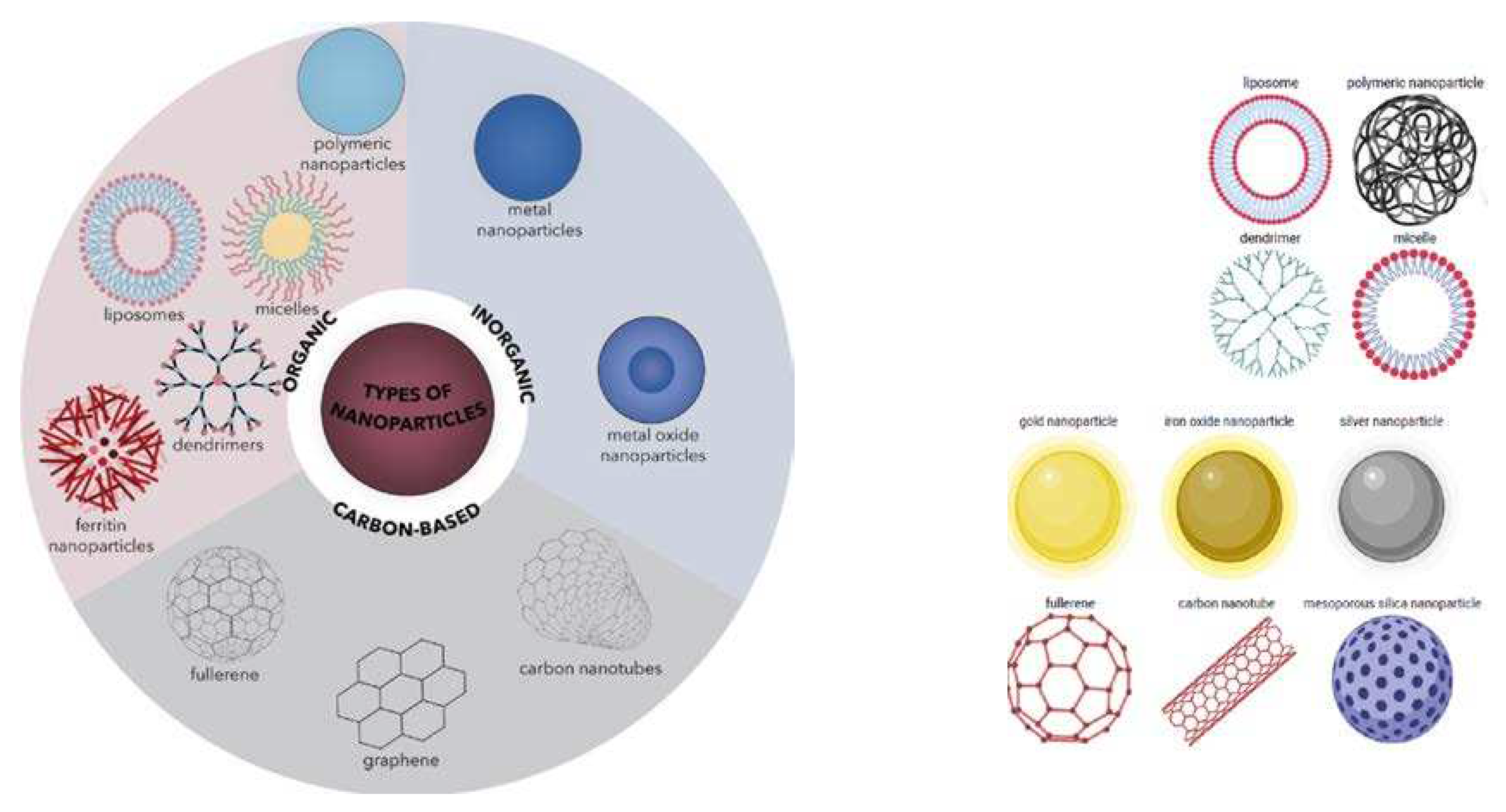
Figure 2.
Classification of organic and inorganic nanoparticles. Reproduced from Spirescu et al. (2021) [[
72]] and Greene et al. (2018) [[
73]], under the creative commons attribution license 4.0.
Figure 2.
Classification of organic and inorganic nanoparticles. Reproduced from Spirescu et al. (2021) [[
72]] and Greene et al. (2018) [[
73]], under the creative commons attribution license 4.0.
Although the innovations differ widely in design and fabrication, the two main divisions of nanomaterials used for PSs and other drug delivery are the organic and inorganic nanoparticles [[
74]]. In alignment with this classification, nanoparticles of organic compounds and those of entirely hydrocarbon materials are grouped under organic. In contrast, nanoparticles of metallic and metal chalcogenide materials are grouped under inorganic nanoparticles, as illustrated in
Figure 2 [[
75]]. Organic nanoparticles include all the nanomaterials fabricated from carbon compounds, whereas inorganic nanoparticles include all the nanomaterials fabricated from non-carbon-based compounds [[
76]]. In reality, however, there is a continuum between organic and inorganic nanoparticles because some researchers classify nanoparticles of hydrocarbon materials such as graphene, fullerenes, and carbon nanotubes as inorganic instead of organic [
74]. The incorporation of PSs into the nanoparticles can be accomplished by surface adsorption and absorption, covalent linking, and encapsulation. The incorporation of the PS into the nanoparticle can lead to enhancement of the preferential accumulation because nanoparticles are preferentially taken up and retained by disease cells due to the enhanced permeability and retention effect. In addition, the improvement of the stability and biocompatibility of the nanoconjugates in which the PS and other drugs are incorporated is used to ensure that during systemic circulation, nanoconjugates do not induce an immune response.
Combinations with Chemotherapy
The anticancer and antimicrobial therapeutic approach that uses chemical compounds to kill fast-growing cells in the body is generally referred to as chemotherapy. Chemotherapy drugs now in clinical use are mainly derived from basic research involving the prospecting of natural sources, followed by a series of standard chemical and biochemical research studies, including chemical synthesis and modification to facilitate subsequent structure-activity studies and enhancement of efficacy and selectivity. The candidate drug compounds translate to clinical applications and clinical case studies through preclinical studies and clinical trials. The rising incidence of resistance has triggered the search for alternate approaches, most of which are showing promising results in clinical trials. Chemical modification of known effective drugs is one of the methods used to overcome chemotherapy drug resistance. Recently, however, the combination of chemotherapy with PDT has emerged, offering great promise for overcoming drug resistance, enhancing efficacy, and improving selectivity. The chemotherapy approach generally includes antibiotic and anticancer chemotherapy, with antibiotic drugs used for antibiotic chemotherapy and anticancer drugs used in anticancer chemotherapy. Both have suffered different degrees of the development of resistance.
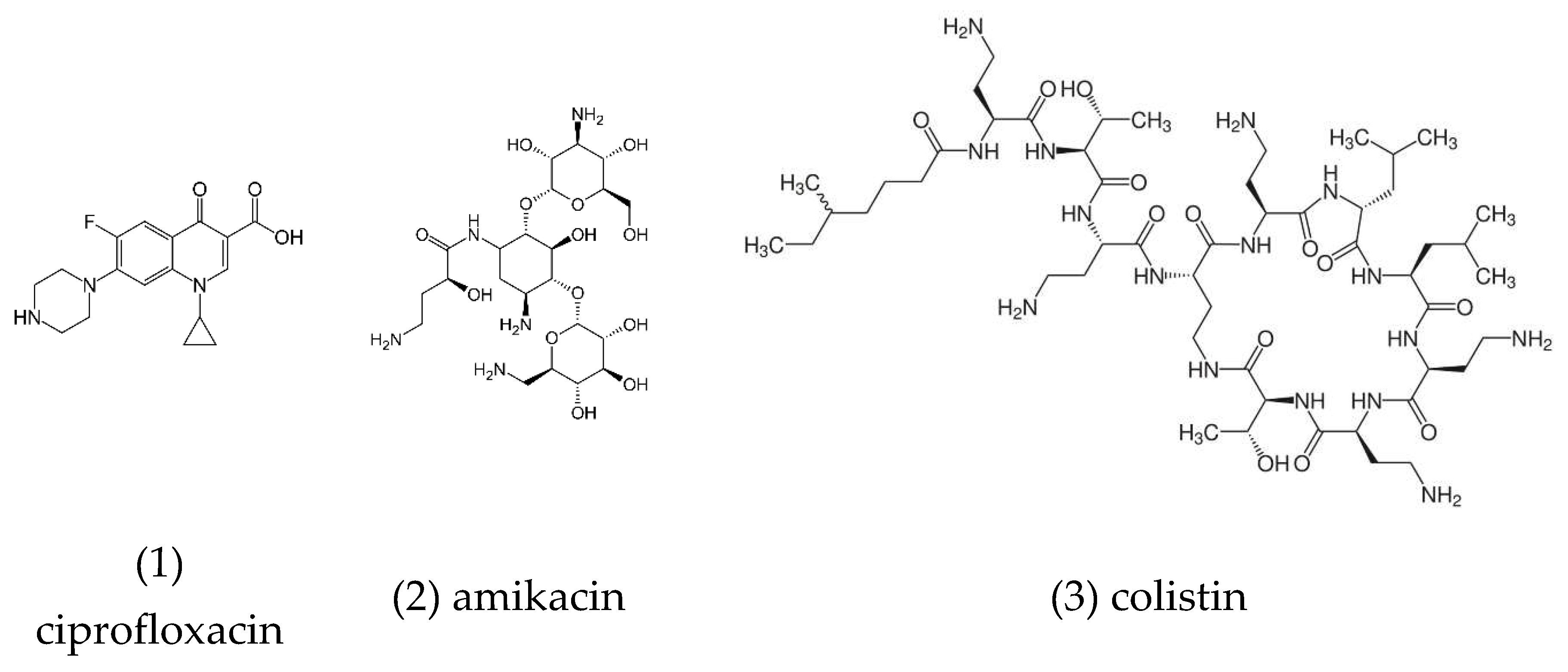
Figure 3.
chemical structures of the chemotherapy drugs ciprofloxacin, amikacin, colistin.
Figure 3.
chemical structures of the chemotherapy drugs ciprofloxacin, amikacin, colistin.
Combinations of anticancer chemotherapy with PDT have generally shown improvement in the efficacy against several microbial pathogens [[
77]]. This may be illustrated with the antimicrobial PDT study that was conducted on third-degree burn wounds in vivo using the PS protoporphyrin IX, combined with antibiotic chemotherapy using the broad-spectrum antibiotic drug ceftriaxone sodium. In this study, copious amounts of reactive oxygen species were produced upon photoactivation and synergistic inhibition of the growth of methicillin-resistant
S. aureus, E. coli, and
P. aeruginosa [
23]. Moreover, the impact of PDT using the PS Chlorin-e6, in combination with antimicrobial therapy using the antibiotic drug ciprofloxacin, improves the inhibition of bacterial growth and therefore offers a promising antibacterial approach against urinary tract infections [[
78]]. Optimization studies on antibacterial PDT in combination with chemotherapy, using the antibiotics ciprofloxacin, amikacin, and colistin (
Figure 3), found that the best conditions for optimal bactericidal activity of the combination therapy were 100 μg/mL of the PS Chlorin-e6, and a light energy dose of 120 J/cm2, for the quinolone antibiotic ciprofloxacin against
Escherichia coli suspensions [[
79]].
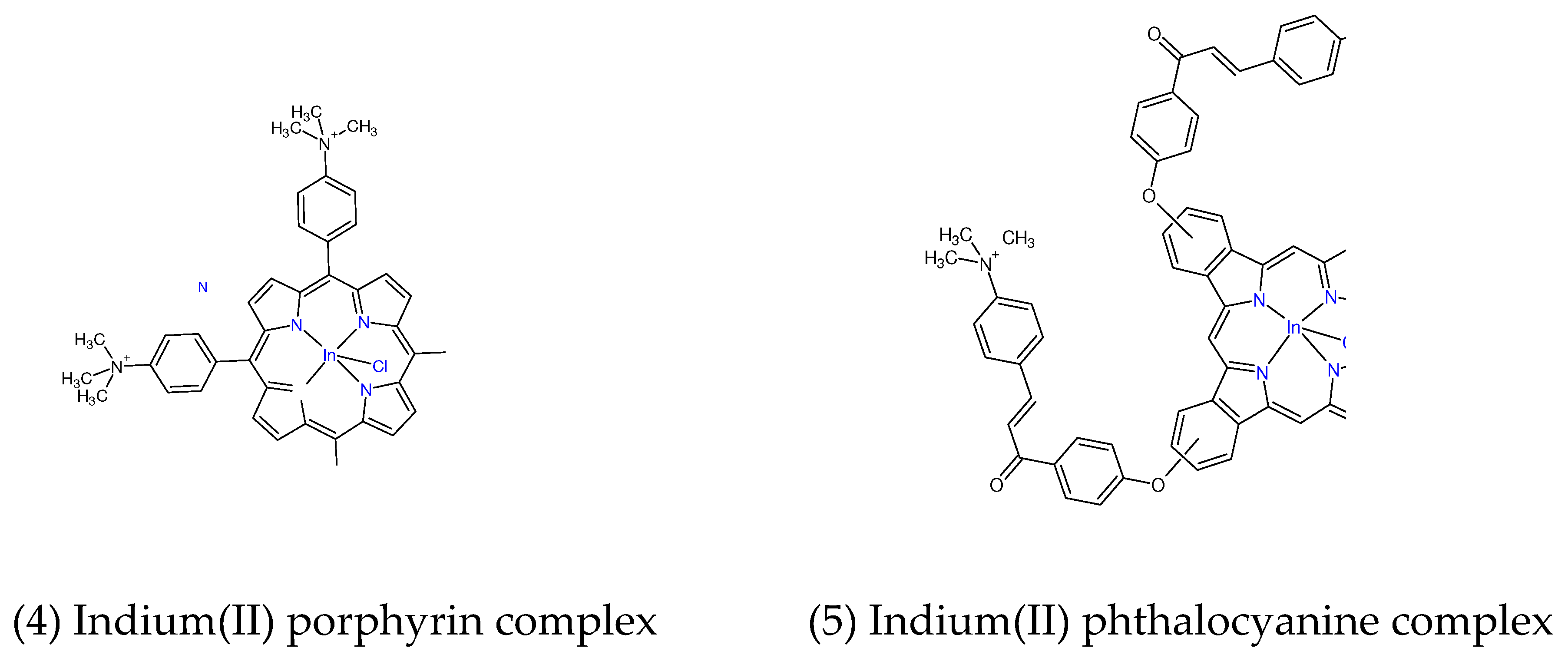
Figure 4.
chemical structures of the Indium (II) porphyrin and phthalocyanine complexes.
Figure 4.
chemical structures of the Indium (II) porphyrin and phthalocyanine complexes.
Synergistic effects of PDT and antibiotic therapy using the PS methylene blue and the antibiotic drug ciprofloxacin were observed with a PS concentration-independent and light dose-dependent biofilm reduction of 10-5.4 and 10-7 for
S. aureus and
E. coli respectively [[
80]]. Recently, the combination of antibiotic chemotherapy using the antibiotic drug ciprofloxacin
1 with antibacterial PDT using positively charged porphyrin
4, and phthalocyanine PS complexes
5 (
Figure 4) resulted in complete eradication of the bacterial biofilms of
S. aureus and
E. coli by log10 reductions of 7.05 and 7.20, respectively, at the PS-to-antibiotic concentration of 8 μM to 2 μg/mL, and 8 μM to 4 μg/mL, respectively [[
81]].
Combinations with Photothermal Therapy
As a result of the resonance of their low-lying surface electrons, nanoparticles can transform absorbed light energy into heat. This photothermal energy conversion is the fundamental basis for the photothermal hyperthermia therapeutic technology, which is often used against cancer and microbial infections. The technology is sometimes combined with PDT, enhancing the efficacy synergistically. When nanoconjugates are used as carriers and delivery agents, the photothermal energy conversion may be used as an external stimulus to trigger the release of the PDT PS and chemotherapy drug cargo from the nanoconjugate. Of the multitude of nanomaterials that exhibit this plasmonic resonance and photothermal energy conversion phenomenon, gold nanoparticles are among the most widely studied, and gold nanorods exhibit the highest photothermal conversion efficiency. All nanorods show two plasmonic resonance absorption bands in their electronic absorption spectra, an intense band on the near-infrared side and a lower intensity on the blue side. The near-infrared band is always the one used to activate the nanorods in photothermal conversion. Some metal chalcogenide nanostructures, such as copper sulfide nanoparticles, exhibit both the photothermal conversion and generation of reactive oxygen species and, for this reason, act as excellent agents for the combination of PTT and PDT. Because the challenge of tissue penetration depth of light is just as much of a limitation of PTT as it is for PDT, it is therefore, a limitation for the combination of PTT and PDT.
Therefore, loading nanoparticles that exhibit the photothermal energy conversion capability with PS molecules could potentially be used as a nanomaterial-mediated strategy for combining PTT and PDT. Superparamagnetic iron oxide nanoparticles were coated with 3-aminopropyl silane and loaded with indocyanine green as the PS to give a nanoconjugate with a superparamagnetic iron oxide core, and a shell composed of 3-aminopropyl silane and labile indocyanine green. Under conditions of the combination of PTT and PDT, in vitro studies showed that this nanoconjugate completely eradicated Gram-negative
P. aeruginosa and showed a 7-log reduction of Gram-negative
E. Coli. However, it was ineffective against Gram-negative
K. pneumoniae and Gram-positive
S. epidermis. In this study, the conditions of the combination of PTT and PDT were confirmed by temperature elevation measurements and the generation of reactive oxygen species [[
82]].
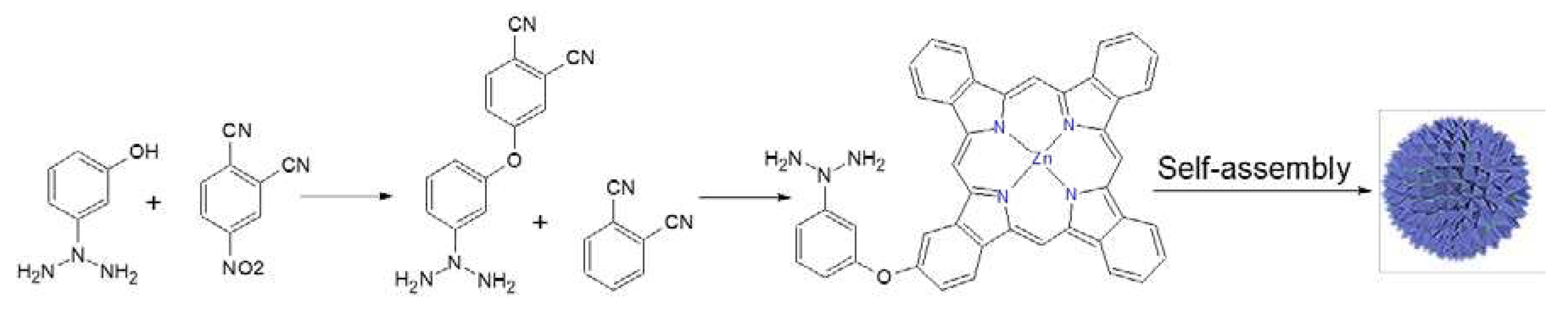
Figure 5.
The synthesis and self-assembly of 3-(dimethylamino)phenoxy-functionalized zinc (II) phthalocyanine 6 to give a phthalocyanine nanoparticle. Adapted from Wang et al. (2022) [
83].
Figure 5.
The synthesis and self-assembly of 3-(dimethylamino)phenoxy-functionalized zinc (II) phthalocyanine 6 to give a phthalocyanine nanoparticle. Adapted from Wang et al. (2022) [
83].
The self-assembly of a zinc (II) phthalocyanine functionalized with a 3-(dimethylamino) phenoxy group 6 to give a phthalocyanine nanoparticle is an example of nanomaterial constituted entirely from the organic dye PS, with plasmonic photothermal conversion capabilities. The reaction scheme is shown in
Figure 5. As a result, it acts as a PS as well as a photothermal conversion agent, thus enabling the potent combination of PTT and PDT, completely inhibiting the growth of methicillin-resistant
S. aureus at an ultralow nanomolar concentration under brief 655 nm laser irradiation [[
83]]. This study confirms the photothermal conversion capability of self-assembled nanostructures formed by organic dye PS self-assembly as a general phenomenon. In another study, for example, the self-assembled indocyanine green PS nanostructure, which was coated with poly2-(dimethylamino)ethyl methacrylate, in order to give a positively charged nanostructure, showed excellent photothermal and photodynamic antibacterial activity because it eradicated
P. gingivalis in both in vitro and in vivo studies [[
84]].
Combinations with Magnetic Hyperthermia
The application of a high-frequency alternating magnetic field on magnetic nanoparticles, known as magnetic hyperthermia, has the effect of increasing the temperature of the media in which the nanoparticles are embedded [[
85]]. Like photothermal hyperthermia, it has gained ground as a therapeutic technology against cancer [[
86]] and microbial infections [[
87]]. It has been investigated for potential applications against atherosclerosis and thrombosis [[
88]]. Unlike photothermal hyperthermia, however, magnetic hyperthermia does not have an energy penetration depth challenge because the alternating magnetic field radiates through biological materials with relative ease for more than 500 mm [[
89]]. It has been used in combination with PDT against cancer with promising results [[
90]]. Like the combination of photothermal hyperthermia therapy with PDT, the mechanism of the combination of MGH with PDT involves the generation of heat and reactive oxygen species. In both combinations, reactive oxygen species are generated from PDT, whereas heat is generated from photothermal hyperthermia in the former combination and magnetic hyperthermia in the latter. The apparent lack of research studies on the application of magnetic hyperthermia in combination with PDT against microbial infection compared to its anticancer and other applications is quite curious [
16]. It could be rationalized in terms of the complexity of the equipment and infrastructure requirements, where photothermal hyperthermia requirements are nearly trivial compared to the heavy investment requirements for magnetic hyperthermia.
Whereas a direct magnetic field has been used in combination with PDT using chlorin e6-laden mesoporous silica-capped iron oxide magnetic nanoparticles, without generating heat as in magnetic hyperthermia, it did push the magnetic nanoparticles to move deeper into the biofilm [[
91]]. After capping with the PS curcumin, a nanoconjugate fabricated from superparamagnetic iron oxide nanoparticles showed good magneto-thermal conversion in a high-frequency alternating magnetic field alone. The nanoconjugate also showed excellent PDT upon irradiation with blue light, eradicating planktonic
S. aureus. In spite of these experiments, no studies appear to have been conducted on the combination of magnetic hyperthermia with PDT using this nanoconjugate in this study. However, this research, conducted by Santana et al. (2020), shows all the preparatory studies in this direction because these researchers have successfully capped superparamagnetic iron oxide nanoparticles with the PS curcumin and measured both the PDT efficacy and magnetothermal conversion curves separately, thus paving the way to the coveted joint application of magnetic hyperthermia and PDT [[
92]].
Combinations with Cold Atmospheric Pressure Plasma Therapy
When a gas is passed between electrodes, with high direct or alternating voltage, a cold plasma discharge is generated with a high concentration of reactive gas species, including reactive oxygen species, the same as those generated by the photosensitization reaction of PDT. The cold plasma discharge can be applied directly in wound healing in vivo and in the clinic, the same way it is applied during experimental studies onto the surface of the growth medium containing bacterial test cell lines in vitro. CAP therapy has been combined with PDT in research studies and in the clinic. The rapidly increasing interest in this technology may be attributed to the fact that it generates reactive oxygen and reactive nitrogen species using plasma under ambient conditions at which the reactive gas species can be applied either directly against the disease sites or can be applied to appropriate stabilizing media and stored for subsequent administration. These are conditions for the combination of the CAP technology with other non-invasive technologies, such as PDT [[
93]], chemotherapy [[
94]], and electro-chemotherapy [[
95]], for antibacterial therapy in the healing of burns and wounds [[
96]] followed by the stimulation of the regeneration of tissues [[
97]], and in environmental antiseptic sanitization [[
98]].
The CAP technology has been reported as offering great promise for anticancer applications [[
99],[
100]]. Additionally, research studies have been increasing over the last two decades on the applications of the CAP technology against bacterial infection with excellent indications [[
101],[
102]]. PDT and CAP therapy have been projected by the World Health Organization to become top among the novel technologies for eradicating bacterial pathogens in the backdrop of the alarming incidence of bacterial drug resistance [[
103]]. New research studies have now emerged on the combination of PDT and CAP against various bacterial infections. For example, the adhesion and sealer penetration study using the push-out method showed that there was a positive effect of the combination of the CAP technology with PDT. Still, no correlation was found between adhesion and sealer penetration [[
104]].
The significance of infrastructure and devices in studying the combination of CAP and PDT against bacterial pathogens was highlighted with a recommendation for further study designs after a plasma device was found to be unsuitable in a comparative study of CAP and antimicrobial PDT [[
105]]. A more recent study under different conditions has shown that CAP and PDT improved the pushout bond strength [[
106]]. In a world-first and groundbreaking study, the combination of CAP and PDT against common skin and wound pathogens has been reported to have synergistic enhancement of efficacy in-vitro [[
107]].
Combinations with Sonodynamic Therapy
Apart from radiation therapy which generates ionizing radiation, magnetic hyperthermia and SDT are two interventions that have emerged in recent research reports as significantly responding to the light penetration depth challenge of PDT. Magnetic hyperthermia operates with a completely different set of mechanistic requirements compared to SDT, which is somewhat similar to PDT, although penetration to a much higher depth. SDT is based on ultrasonic activation of a SDT sensitizer to kill disease cells by production of reactive oxygen species, generating oxidative stress and overwhelming the cellular redox homeostasis biochemical mechanisms. A combination of the two technologies, therefore, is conceptually quite easily achieved by the simultaneous application of light and ultrasound after the accumulation of the sonosensitizer at the disease site because many of the compounds that have been identified as sonosensitizers also act as PSs [[
108]].
Several reports on the investigations of the combination of PDT with SDT against cancer and bacterial pathogens have appeared in the literature. Understandably, the combination is showing increasing research interest in bone and dark tissue disease such as osteosarcoma [[
109]], osteomyelitis [[
110]], periodontal [[
111]], pancreatic [[
112]], hepatic [[
113]], and brain [[
114]] disease. Like PDT, the mechanism of SDT involves the generation of reactive oxygen species. Sonoporation plays a key role in the pharmacokinetics, uptake, and retention of the sonosensitizer. The increasing number of patents and clinical case studies suggest that the translation to the clinic is underway.
Curcumin has been widely studied as an effective sonosensitizer against atherosclerosis [[
115],[
116]], and bacterial [[
117]] infection. A study of curcumin as a potential sono-PS for photo-SDT showed enhanced anti-biofilm efficacy and healing of wounds infected with
A. baumannii in mice [[
118]]. This study illustrates the application of a single sensitizer in the combination of SDT with PDT. Rose Bengal [[
119]] and Chlorin e6 [[
120]] both act as PSs and sonosensitizers albeit to different degrees. The combination of sonodynamic therapy and PDT using both Rose Bengal and Chlorin e6 at molar ratios of 1:1 and 1:3 showed synergistic enhancement of the generation of reactive oxygen species and efficacy against methicillin-resistant
S. aureus [[
121]]. This study illustrates the application of dual sono-PSs in the combination of SDT with PDT, with each responding mainly to one excitation method. However, both are excitable by both stimuli. These studies illustrate the synergistic enhancement of antibacterial efficacy and the production of reactive oxygen species.
Figure 6.
schematic showing the principle of the anticancer immuno-therapeutic technology known as checkpoint-blockade.
Figure 6.
schematic showing the principle of the anticancer immuno-therapeutic technology known as checkpoint-blockade.
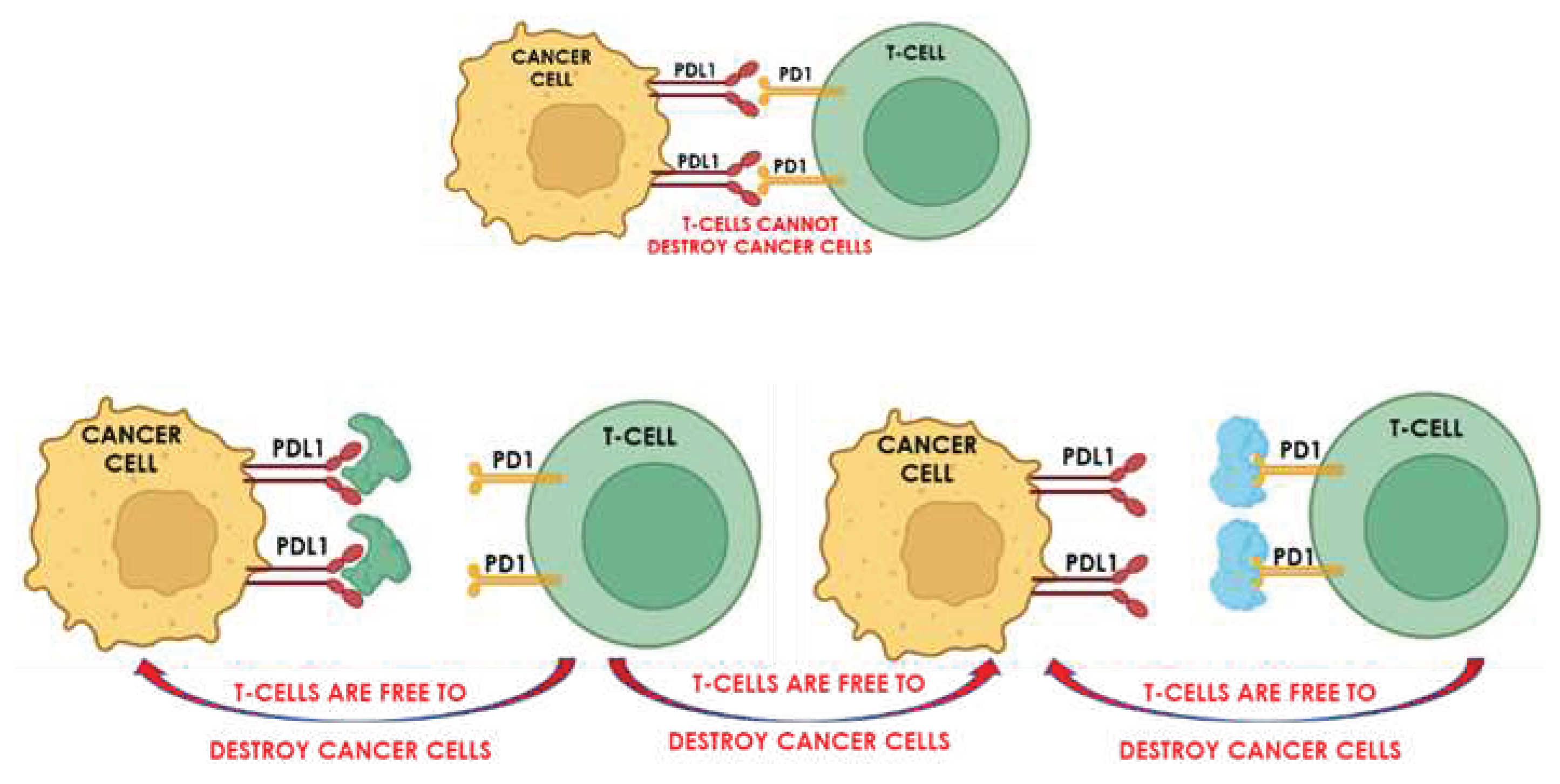
Combinations with Immunotherapy
SDT induces antitumor host immunity [[
122]] However, the immunity response is not always strong enough to inhibit tumor growth and metastasis [[
123]]. The immunity response is based on the binding of checkpoint proteins of cancer cells to proteins of the cell wall of T cells, thus preventing the T cells from attacking the cancer cells. Checkpoint blockade immunotherapy is based on preventing the cancer cell checkpoint proteins from binding to the proteins on T cells, thereby frustrating the induced immunity so that it fails to protect the cancer cells against the T cells. The technology uses inhibitors, which attach either to the checkpoint proteins or their T cell targets, thereby blocking the immune response. A schematic showing the principle of checkpoint-blockade immunotherapy is shown in
Figure 6.
The impact of combining SDT with checkpoint blockade immunotherapy is, therefore, to augment the antitumor immunity and improve efficacy. For example, the use of hematoporphyrin monomethyl ether as the sonosensitizer in combination with the immune modulator adjuvant R837, encapsulated in the same liposome, arrested the primary progression of Xenografts and prevented lung metastasis of breast and colorectal cancer cells [[
124]]. In another example, the use of a cell membrane-cloaked nano-metal-organic-framework sonosensitizer, combined with the R837 immune modulator adjuvant, showed similar synergistic efficacy enhancement [[
125]].
Pang et al. (2019) reported the first antibacterial application of such technology by the application of cell membrane nanovesicles encapsulating the sonosensitizer with antibodies that neutralize the alpha-toxin of methicillin-resistant
S. aureus genetically engineered onto their outer surface [[
126]]. Arguably, this research breaks new ground in the design of antibacterial immunotherapy in combination with SDT and other non-invasive therapeutic technologies. The authors refer to it as a bioinspired alternative approach, which aims to disarm as opposed to killing bacterial pathogens, in that it invokes the biology of bacterial immunity.
Combinations with Radiotherapy
Together with chemotherapy and surgery, ionizing radiation has been among the topmost tools used against cancer in the clinic for a long time [[
127]]. Possibly because both technologies exert their individual effects by generating radicals and, therefore, by inducing oxidative stress, PDT has been shown to enhance the effect of radiation therapy [[128]]. Unlike radiotherapy and SDT, which have higher penetration depths into human tissue, the light required to activate PDT PSs cannot penetrate normal tissue beyond a shallow depth of 8-10 mm. Therefore, the combination of PDT or SDT with radiation therapy was considered to offer potential enough for several researchers to conduct further investigations.
The research by Browning et al. (2021) showed that SDT reduced the tumor perfusion and vascularization of the combination of chemotherapy and radiation treatment and improved the Kaplan–Meier survival curves, using gemcitabine as the anticancer chemotherapy drug [[129]]. The research [
69] and clinical studies [130] on the combination of PDT and radiotherapy have been reviewed. While these reviews have revealed that the combination has enhanced the efficacy against cancer, not much has been said about antibacterial applications. In any case, research is recommended since the reviews raise many questions such as light, ultrasound, radiation, and PS dosimetry, indicating that the successes are now overwhelmed by challenges.
Table 3.
Combination Therapies Involving Photodynamic Therapy.
Table 3.
Combination Therapies Involving Photodynamic Therapy.
| |
Free photosensitizer |
Example and nanoconjugate used |
Reference |
| 1 |
Anticancer |
photofrin and zinc (II) phthalocyanine-quinoline conjugate |
4 |
| photosensitizer-capped metal and metal chalcogenide nanoparticles |
5-6 |
| 3 |
Antibacterial |
porphyrins, phthalocyanines, metallic silver and gold, copper sulfide, and oxides of nanographene, zinc, and iron |
34-40 |
| |
Combinations |
Example and nanoconjugate used |
Reference |
| 4 |
chemotherapy |
protoporphyrin IX / ceftriaxone sodium |
77,78 |
| chlori-e6 / ciprofloxacin |
79 |
| chlori-e6 / ciprofloxacin, amikacin, and colistin |
80 |
| methylene blue / ciprofloxacin |
81 |
| positively charged porphyrin 4, and phthalocyanine photosensitizer complexes and 5 / ciprofloxacin |
82 |
| 5 |
photothermal therapy |
superparamagnetic iron oxide nanoparticles / indocyanine green |
83 |
| self-assembled zinc(II) phthalocyanine 6
|
84 |
| self-assembled indocyanine green coated with poly 2-(dimethylamino) ethyl methacrylate |
85 |
| 6 |
magnetic hyperthermia |
no research studies were found |
|
| 7 |
cold plasma therapy |
protoporphyrin IX-loaded polymersome- mediated photodynamic therapy / cold atmospheric pressure plasma |
100 |
| 5-Aminolevulinic acid photodynamic therapy / cold atmospheric pressure plasma |
101 |
| methylene blue photodynamic therapy / cold atmospheric pressure plasma |
105 |
| chlorine p6 photodynamic therapy / cold atmospheric pressure plasma |
107 |
| toluidine blue photodynamic therapy / cold atmospheric pressure plasma |
108 |
| 8 |
sonodynamic therapy |
curcumin photodynamic therapy / sonodynamic therapy |
110-113 |
| Rose Bengal and Chlorin e6 / sonodynamic therapy |
114-116 |
| 9 |
immunotherapy |
hematoporphyrin monomethyl ether / immune adjuvant R837 |
119 |
| membrane-cloaked nano-metal-organic-framework / R837 immune modulator |
120 |
| Sonosensitizer / cell membrane nanovesicles with antibodies that neutralize the alpha-toxin of MRSA genetically engineered onto their outer surface |
121 |
| 10 |
radiotherapy |
Rose Bengal-loaded oxygen microbubbles / radiotherapy |
124 |
Conclusions
To overcome each of the challenges of the direct application of PDT using free PSs, the technology has evolved to the currently predominant approach of conjugating the PS in nanomaterials and combining it with other minimally invasive therapeutic technologies. In this regard, examples of the research studies cited in this review are listed in
Table 1. These examples show the current trends in the evolution of what, after nearly 50 years, is still regarded as a novel technology because it is still translating to the clinic as it morphs during the current research and clinical practice. The most widely studied applications are still anticancer and antibacterial. Due to the frequency and threat to the life of viral diseases in recent times, antiviral applications have appeared among the dominant antimicrobial applications as the scope expands widely from the original neoplastic and infectious diseases to inflammation, neovascular diseases, and environmental applications.
Arguably, SDT has taken PDT and its combinations to greater depths. Similarly, greater depths are achieved from combinations with magnetic hyperthermia, where PDT affects mainly the superficial affliction and magnetic hyperthermia also affects the deeper-lying disease. This review only presents examples of the current research in anticancer and antibacterial PDT together with combinations with chemotherapy, PTT, MGH, CAP therapy, SDT, immunotherapy, and radiotherapy without being exhaustive. Although the combinations of PDT with each of these seven minimally invasive technologies offer alternatives to the current clinical tools, much research is still needed to evaluate their potential, elucidate their challenges, and obviate the development of clinical devices that will facilitate clinical translation.
Although much research has demonstrated the applicability of the combination of magnetic hyperthermia with PDT against cancer, there appears to be a notable paucity of similar research on the antibacterial applicability of this combination. This suggests a need to investigate this combination technology against bacterial pathogens in vitro initially followed by in vivo studies subject to the outcomes of the in vitro studies. The outcomes of the initial studies of the combination of PDT with CAP therapy conducted by Aly et al. (2021) suggest that this combination could be a useful addition to the antibacterial PDT combinations. Hence the ground-breaking research on the combination of CAP with PDT against common skin and wound pathogens promises to provide the much-needed proof-of-concept. Current studies and their progress towards clinical translation on the combinations of PDT or deep tissue SDT emulation with other minimally invasive technologies are at the cutting edge of the latest innovations that promise to be the latest anticancer and antimicrobial clinical tools.
References
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. ; Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer? Bioconjugate Chemistry 2016, 27, 10, 2225–2238. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Kaufman, J.E.; Goldfarb, A.; Weishaupt, K.R.; Boyle, D.; Mittleman, A. ; Photoradiation therapy for the treatment of malignant tumors. Cancer Research 1978, 38, 8, 2628–2635. [Google Scholar]
- Chen, J.; Wang, Y.; Fang, Y.; Jiang, Z.; Wang, A.; Xue, J. ; Improved photodynamic anticancer activity and mechanisms of a promising zinc(II) phthalocyanine-quinoline conjugate photosensitizer in vitro and in vivo. Biomedical Optics Express 2020, 11, 7, 3900–3912. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Zhou, X.; Zhang, J.; Shi, Y.; Zhong, L. ; Metal Nanoparticles for Photodynamic Therapy: A Potential Treatment for Breast Cancer. Molecules 2021, 26, 21, 6532. [Google Scholar] [CrossRef] [PubMed]
- Shariatzadeh, S.; Moghimi, N.; Khalafi, F.; Shafiee, S.; Mehrabi, M.; Ilkhani, S.; Tosan, F.; Nakhaei, P.; Alizadeh, A.; Varma, R.S. and Tahe Bonelli, J.; Ortega-Forte, E.; Rovira, A.; Bosch, M.; Torres, O.; Cuscó, C.; Rocas, J.; Ruiz, J. and Marchán, V.; Improving Photodynamic Therapy Anticancer Activity of a Mitochondria-Targeted Coumarin Photosensitizer Using a Polyurethane–Polyurea Hybrid Nanocarrier, Biomacromolecules 2022, 23, 7, 2900–2913. https://doi.org/10.1021/acs.biomac.2c00361.ri, M.; Metallic Nanoparticles for the Modulation of Tumor Microenvironment; A New Horizon. Frontiers in Bioengineering and Biotechnology 2022, 10, 847433. [CrossRef]
- Kritika, K. and Indrajit, R.; Therapeutic applications of magnetic nanoparticles: recent advances. Advanced Materials 2022, 3, 7425–7444. [CrossRef]
- Gu, H.; Xu, K.; Yang, Z.; Changa, C.K. and Xu, B.; Synthesis and cellular uptake of porphyrin decorated iron oxide nanoparticles—a potential candidate for bimodal anticancer therapy. Chemical Communications 2005, 4270–4272. [CrossRef]
- ang, Z.; Xu, K.; Zhang, B.; Xu, B.; Zhang, X. and Chang, C.K.; Photosensitizer decorated iron oxide nanoparticles: bimodal agent for combined hyperthermia and photodynamic therapy, in: Kessel, D. (ed), Optical Methods for Tumor Treatment and Detection: Mechanisms and Techniques in Photodynamic Therapy XV. Proceedings of SPIE 2006, 6139, 1605–7422. [CrossRef]
- Zhao, S.; Yu, X.; Qian, Y.; Chen, W.; Shen, J. ; Multifunctional magnetic iron oxide nanoparticles: an advanced platform for cancer theranostics. Theranostics 2020, 10, 14, 6278–6309. [Google Scholar] [CrossRef]
- Ansari, M.; Bigham, A.; Hassanzadeh-Tabrizi, S.A.; and Ahangar, H.A. ; Synthesis and characterization of CuO. 3ZnO.5MgO.2Fe2O4 nanoparticles as a magnetic drug delivery system, Journal of Magnetism and Magnetic Materials 2017, 439, 67–75. [Google Scholar] [CrossRef]
- Farjadian, F.; Ghasemi, S.; Akbarian, M.; Hoseini-Ghahfarokhi, M.; Moghoofei, M. and Doroudian, M.; Physically stimulus-responsive nanoparticles for therapy and diagnosis. Frontiers in Chemistry 2022, 10, 952675. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.A.; Jan, N.; Khan, S.; Shah, H.; Madni, A.; Khan, A.; Jabar, A.; Khan, S.; Abdelbary Elhissi, A.; Hussain, Z.; Aziz, H.C.; Sohail, M.; Khan, M. andThu, H.E.; Recent Advancements in Stimuli Responsive Drug Delivery Platforms for Active and Passive Cancer Targeting. Cancers (Basel) 2021, 13, 4, 670. [Google Scholar] [CrossRef]
- Crous, A. and Abrahamse, H; Targeted Photodynamic Therapy for Improved Lung Cancer Treatment. InTech 2018. [Google Scholar] [CrossRef]
- Demiral, A.; S. Goralı, I.; Hülya Yılmaz, H.; Verimli, N.; Çulha, M.; Erdem, S.S.; Stimuli-responsive theranostic system: A promising approach for augmented multimodal imaging and efficient drug release. European Journal of Pharmaceutics and Biopharmaceutics 2022, 177, 9–23. [CrossRef] [PubMed]
- Songca, S.P. and Adjei, Y.; Applications of Antimicrobial Photodynamic Therapy against Bacterial Biofilms. International Journal of Molecular Sciences 2022, 23, 6, 3209. [CrossRef]
- Svyatchenko, V.A.; Nikonov, S.D.; Mayorov, A.P.; Gelfond, M.L.; Loktev, V.B. Antiviral photodynamic therapy: Inactivation and inhibition of SARS-CoV-2 in vitro using methylene blue and Radachlorin. Photodiagnosis and Photodynamic Therapy 2021, 33, 102112. [Google Scholar] [CrossRef]
- Ghorbani, J.; Rahban, D.; Aghamiri, S.; Teymouri, A.; Bahador, A. Photosensitizers in antibacterial photodynamic therapy: An overview. Laser Therapy 2018, 27, 293–302. [Google Scholar] [CrossRef]
- Liang, Y.I.; Lu, L.M.; Chen, Y.; Lin, Y.K. Photodynamic therapy as an antifungal treatment. Experimental and Therapeutic Medicine 2016, 12, 23–27. [Google Scholar] [CrossRef]
- Brovko, L.Y.; Meyer, A.; Tiwana, A.S.; Chen, W.; Liu, H.; Filipe, C.D.; Griffiths, M.W. Photodynamic treatment: A novel method for sanitation of food handling and food processing surfaces. Journal of Food Protection 2009, 72, 1020–1024. [Google Scholar] [CrossRef]
- Theodoro, L.H.; Lopes, A.B.; Nuernberg, M.; Cláudio, M.M.; Miessi, D.; Alves, M.; Duque, C.; Mombelli, A.; Garcia, V.G. Comparison of repeated applications of aPDT with amoxicillin and metronidazole in the treatment of chronic periodontitis: A short-term study. Journal of Photochemistry and Photobiology B Biology 2017, 174, 364–369. [Google Scholar] [CrossRef]
- Hokari, T.; Morozumi, T.; Komatsu, Y.; Shimizu, T.; Yoshino, T.; Tanaka, M.; Tanaka, Y.; Nohno, K.; Kubota, T.; Yoshie, H. Effects of Antimicrobial Photodynamic Therapy and Local Administration of Minocycline on Clinical, Microbiological, and Inflammatory Markers of Periodontal Pockets: A Pilot Study. International Journal of Dentistry 2018, 1748584. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ogawa, R.; Xiao, B.H.; Feng, Y.X.; Wu, Y.; Chen, L.H.; Gao, X.H. and Hong-Duo ChenH.D.; Antimicrobial photodynamic therapy in skin wound healing: A systematic review of animal studies. International Wound Journal 2020, 17, 2, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ma, J.; Wang, Y.; Xu, Z.; Zhao, L.; Zhao, J.; Hong, G.; Liu, T. Antimicrobial Photodynamic Therapy Combined with Antibiotic in the Treatment of Rats with Third-Degree Burns. Frontiers in Microbiology 2021, 12, 622410. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qin, R.; Zaat, S.A.J.; Breukink, E.; Heger, M. ; Antibacterial photodynamic therapy: overview of a promising approach to fight antibiotic-resistant bacterial infections. Journal of Clinical and Translational Research 2015, 1, 3, 140–167. [Google Scholar] [CrossRef]
- Dickschat, J.S. ; Quorum sensing and bacterial biofilms. Natural Product Reports 2010, 27, 343–369. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B. Biofilm dispersal: Mechanisms, clinical implications, and potential therapeutic uses. Journal of Dental Research 2010, 89, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, W.; Huang, S.; Li, J.; Zhang, Y.; Gao, Y.; Cheng, Y.; Wu, Z.; Zhang, X. A targeted nanozyme based on multiple porphyrins for enhanced photodynamic antibacterial application. Chemical Engineering Journal 2022, 431, 133704. [Google Scholar] [CrossRef]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Frontiers in Microbiology 2020, 11, 928. [Google Scholar] [CrossRef]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends in Microbiology 2020, 28, 668–681. [Google Scholar] [CrossRef]
- Amankwah, S.; Abdella, K.; Kassa, T. Bacterial Biofilm Destruction: A Focused Review On The Recent Use of Phage-Based Strategies With Other Antibiofilm Agents. Nanotechnology, Science and Applications 2021, 14, 161–177. [Google Scholar] [CrossRef]
- Kikuchi, T.; Mogi, M.; Okabe, I.; Okada, K.; Goto, H.; Sasaki, Y.; Fujimura, T.; Fukuda, M.; Mitani, A. Adjunctive Application of Antimicrobial Photodynamic Therapy in Nonsurgical Periodontal Treatment: A Review of Literature. International Journal of Molecular Sciences 2015, 16, 24111–24126. [Google Scholar] [CrossRef] [PubMed]
- Sharab, L.; Baier, R.E.; Ciancio, S.; Mang, T. Influence of Photodynamic Therapy on Bacterial Attachment to Titanium Surface. Journal of Oral Implantology 2021, 47, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.P.; Marangoni, V.S.; de Faria, C.G.; Leite, I.S.; Silva, C.C.C.; Maroneze, C.M.; Pereira-da-Silva, M.A.; Bagnato, V.S.; Inada, N.M. Graphene Oxide Mediated Broad-Spectrum Antibacterial Based on Bimodal Action of Photodynamic and Photothermal Effects. Frontiers in Microbiology 2019, 10, 2995. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Niu, L.; Qiao, Z.Y.; Cheng, D.B.; Wang, J.; Zhong, Y.; Bai, F.; Wang, H.; Fan, H. Synthesis of Self-Assembled Porphyrin Nanoparticle Photosensitizers. ACS Nano 2018, 24, 3796–3803. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Surface-Coating-Dependent Dissolution, Aggregation, and Reactive Oxygen Species (ROS) Generation of Silver Nanoparticles under Different Irradiation Conditions. Environmental Science & Technology 2013, 47, 10293–10301. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, D.Y. Near-Infrared-Responsive Cancer Photothermal and Photodynamic Therapy Using Gold Nanoparticles. Polymers 2018, 10, 961. [Google Scholar] [CrossRef]
- Wang, S.; Riedinger, A.; Li, H.; Fu, C.; Liu, H.; Li, L.; Liu, T.; Tan, L.; Barthel, M.J.; Pugliese, G.; de Donato, F.; D'Abbusco, M.S.; Meng, X.; Manna, L.; Meng, H.; Pellegrino, T. Plasmonic copper sulfide nanocrystals exhibiting near-infrared photothermal and photodynamic therapeutic effects. ACS Nano 2015, 24, 1788–1800. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Bahador, A. Enhanced reduction of polymicrobial biofilms on the orthodontic brackets and enamel surface remineralization using zeolite-zinc oxide nanoparticles-based antimicrobial photodynamic therapy. BMC Microbiology 2021, 21, 273. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Y.; Hong, W.; Chen, Z.; Peng, P.; Yuan, S.; Qu, J.; Xiao, M.; Xu, L. Glucose oxidase and polydopamine functionalized iron oxide nanoparticles: Combination of the photothermal effect and reactive oxygen species generation for dual-modality selective cancer therapy. Journal of Materials Chemistry B Biology 2019, 7, 2190–2200. [Google Scholar] [CrossRef]
- Mutalik, C.; Okoro, G.; Krisnawati, D.I.; Jazidie, A.; Rahmawati, E.Q.; Rahayu, D.; Hsu, W.T.; Kuo, T.R.; Copper sulfide with morphology-dependent photodynamic and photothermal antibacterial activities. Journal of Colloid and Interface Science 2022, 607, 2, 1825–1835. [CrossRef]
- Munir, M.U.; Ahmed, A.; Usman, M.; Salman, S. ; Recent Advances in Nanotechnology-Aided Materials in Combating Microbial Resistance and Functioning as Antibiotics Substitutes. International Journal of Nanomedicine 2020, 15, 7329–7358. [Google Scholar] [CrossRef]
- Lv, X.; Zhang, J.; Yang, D.; Shao, J.; Wang, W.; Zhang, Q.; Dong, X. Recent advances in pH-responsive nanomaterials for anti-infective therapy. Journal of Materials Chemistry B: Biology 2020, 8, 10700–10711. [Google Scholar] [CrossRef] [PubMed]
- Demidova TN, Hamblin MR. Photodynamic therapy targeted to pathogens. International Journal of Immunopathology and Pharmacology 2004, 17, 3, 245–254. [Google Scholar] [CrossRef]
- Shiao, S.H.; Weng, S.C.; Luan, L.; Vicente, M.G.H.; Ng, D.K.P.; Kolli, B.K.; Chang, K.P. ; Novel phthalocyanines activated by dim light for mosquito larva- and cell-inactivation with inference for their potential as broad-spectrum photodynamic insecticides. PLoS One 2019, 14, 5–e0217355. [Google Scholar] [CrossRef] [PubMed]
- Boen, M; Brownell, J; Patel, P; Tsoukas, M. M.; The Role of Photodynamic Therapy in Acne: An Evidence-Based Review. American Journal of Clinical Dermatology 2017, 18, 3, 311–321. [Google Scholar] [CrossRef]
- Wojewoda, K.; Gillstedt, M.; Tovi, J.; Salah, L.; Larkö, A.M.W.; Sjöholm, A.; Sandberg, C. ; Optimizing treatment of acne with photodynamic therapy (PDT) to achieve long-term remission and reduce side effects. A prospective randomized controlled trial, Journal of Photochemistry and Photobiology B: Biology 2021, 223, 112299. [Google Scholar] [CrossRef]
- Meads, C.; Salas, C.; Roberts, T.; Moore, D.; Fry-Smith, A. and Hyde, C.; Clinical effectiveness and cost-utility of photodynamic therapy for wet age-related macular degeneration: a systematic review and economic evaluation. 2003. In: NIHR Health Technology Assessment programme: Executive Summaries. Southampton (UK): NIHR Journals Library; 2003-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK62326/.
- Yip, P.P.; Woo, C.F.; Tang, H.H.Y. and Ho, C.K.; Triple therapy for neovascular age-related macular degeneration using single-session photodynamic therapy combined with intravitreal bevacizumab and triamcinolone. British Journal of Ophthalmology 2009, 93, 6, 754–758. [CrossRef]
- Houthoofd, S.; Vuylsteke, M.; Mordon, S.; Fourneau, I.; Photodynamic therapy for atherosclerosis. The potential of indocyanine green. Photodiagnosis and Photodynamic Therapy 2020, 29, 101568. [Google Scholar] [CrossRef]
- Jain, M.; Zellweger, M.; Wagnières, G.; van den Bergh, H.; Cook, S. and Giraud, MN.; Photodynamic therapy for the treatment of atherosclerotic plaque: Lost in translation? Cardiovascular Therapeutics 2017, 35, 2. [Google Scholar] [CrossRef]
- Choi, Y.M.; Adelzadeh, L.; Wu, J.J. ; Photodynamic therapy for psoriasis. Journal of Dermatological Treatment 2015, 26, 3, 202–207. [Google Scholar] [CrossRef]
- Tandon, Y.K.; Yang, M.F.; Baron, E.D. ; Role of photodynamic therapy in psoriasis: a brief review. Photodermatology, Photoimmunology and Photomedicine 2008, 24, 5, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Praena, B.; Mascaraque, M.; Andreu, S.; Bello-Morales, R.; Abarca-Lachen, E.; Rapozzi, V.; Gilaberte, Y.; González, S.; López-Guerrero, J.A.; Juarranz, Á. Potent Virucidal Activity In Vitro of Photodynamic Therapy with Hypericum Extract as Photosensitizer and White Light against Human Coronavirus HCoV-229E. Pharmaceutics 2022, 14, 2364. [Google Scholar] [CrossRef] [PubMed]
- Mfouo-Tynga, I.S.; Mouinga-Ondeme, A.G. ; Photodynamic Therapy: A Prospective Therapeutic Approach for Viral Infections and Induced Neoplasia. Pharmaceuticals 2022, 15, 1273. [Google Scholar] [CrossRef] [PubMed]
- La Selva, A.; Negreiros, R.M.; Bezerra, D.T.; Rosa, E.P.; Pavesi, V.C.S.; Navarro, R.S.; Bello-Silva, M.S.; Ramalho, K.M.; Aranha, A.C.C.; Braz-Silva. ; P.H.; Fernandes, K.P.S.; Bussadori, S.K. and Horliana, A.C.R.T.; Treatment of herpes labialis by photodynamic therapy: Study protocol clinical trial (SPIRIT compliant). Medicine (Baltimore) 2020, 99, 12, e19500. [Google Scholar] [CrossRef] [PubMed]
- Marotti, J.; Aranha, A.C.; Eduardo, C.P.; Ribeiro, M.S. ; Photodynamic therapy can be effective as a treatment for herpes simplex labialis. Photomedicine and Laser Surgery 2009, 27, 2, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Conrado, P.C.V.; Sakita, K.M.; Arita, G.S.; Galinari, C.B.; Gonçalves, R.S.; Lopes, L.D.G.; Lonardoni, M.V.C.; Teixeira a, J.J.V.; Bonfim-Mendonça, P.S.; Kioshima, E.S. ; A systematic review of photodynamic therapy as an antiviral treatment: Potential guidance for dealing with SARS-CoV-2. Photodiagnosis and Photodynamic Therapy 2021, 34, 102221. [Google Scholar] [CrossRef]
- Bartolomeu, M.; Oliveira, C.; Pereira, C.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. ; Antimicrobial Photodynamic Approach in the Inactivation of Viruses in Wastewater: Influence of Alternative Adjuvants. Antibiotics (Basel) 2021, 10, 7, 767. [Google Scholar] [CrossRef]
- Abivardi, C. ; Photodynamic Action in Pest Control and Medicine. In: Encyclopedia of Entomology 2004, Springer, Dordrecht. [CrossRef]
- Kalka, K.; Merk, H.; Mukhtar, H. ; Photodynamic therapy in dermatology. Journal of the American Academy of Dermatology 2000, 42, 3, 389–416. [Google Scholar] [CrossRef]
- Wan, M.T.; Lin, J.Y. ; Current evidence and applications of photodynamic therapy in dermatology. Clinical, Cosmetic and Investigational Dermatology 2014, 7, 145–163. [Google Scholar] [CrossRef]
- Benov, L. ; Photodynamic therapy: current status and future directions. Medical Principles and Practice 2015, 24, 1, 14–28. [Google Scholar] [CrossRef]
- Yuan, S.; and Chen, H. Mathematical rules for synergistic, additive, and antagonistic effects of multi-drug combinations and their application in research and development of combinatorial drugs and special medical food combinations. Food Science and Human Wellness 2019, 8, 2, 136–141. [Google Scholar] [CrossRef]
- Lange, C. and Bednarski, P.J.; Evaluation for Synergistic Effects by Combinations of Photodynamic Therapy (PDT) with Temoporfin (mTHPC) and Pt(II) Complexes Carboplatin, Cisplatin or Oxaliplatin in a Set of Five Human Cancer Cell Lines. International Journal of Molecular Sciences 2018, 19, 10, 3183. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cui, W. and Zhao, J.; Synergistic treatment of doxorubicin-resistant breast cancer by the combination of chemotherapy and photodynamic therapy. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2022, 648, 129167. [CrossRef]
- Luksiene, Z.; Kalvelyte, A.; Supino, R. ; On the combination of photodynamic therapy with ionizing radiation. Journal of Photochemistry and Photobiology B Biology 1999, 52, 1-3, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Gao, J. and Wei, Q.; Combination of Photodynamic Therapy with Radiotherapy for Cancer Treatment, Journal of Nanomaterials. Nanomaterials for Cancer Phototheranostics 2016, 8507924. [Google Scholar] [CrossRef]
- Gontijo, S.M.L.; Felizali, R.C.; Guimarães, P.P.G.; Santos, R.A.S.; Sinisterra, R.D.; Cortés, M.E.; Araújo, P.V. ; Sub-additive effects of photodynamic therapy combined with erlotinib for the treatment of epidermoid carcinoma: An in vitro study. Photodiagnosis and Photodynamic Therapy 2017, 18, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, R.; Khorsandi, K.; Jahanshiri, M. ; Combination photodynamic therapy of human breast cancer using salicylic acid and methylene blue. Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy 2017, 184, 198–203. [Google Scholar] [CrossRef]
- Khorsandi, K.; Hosseinzadeh, R.; Chamani, E. ; Molecular interaction and cellular studies on combination photodynamic therapy with rutoside for melanoma A375 cancer cells: an in vitro study. Cancer Cell International 2020, 20, 525. [Google Scholar] [CrossRef]
- Adeoye, A.O.M.; Fatoba, S.O.; Bodunde, O.P.; Ikumapayi, O.M.; Akinlabi, E.T.; Oladapo, B.I. ; A futuristic insight into a ‘‘nano-doctor”, A clinical review on medical diagnosis and devices using nanotechnology. Materials Today 2021, 44, 1, 1144–1153. [Google Scholar] [CrossRef]
- Greene, M.K.; Johnston, M.C.; Scott, C.J. Nanomedicine in Pancreatic Cancer: Current Status and Future Opportunities for Overcoming Therapy Resistance. Cancers 2021, 13, 6175. [Google Scholar] [CrossRef]
- Estelrich, J. and Busquets M.A.; Iron Oxide Nanoparticles in Photothermal Therapy. Molecules 2018, 23, 7–1567. [CrossRef]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Vasile, B. S,.; Andronescu, E. Inorganic Nanoparticles and Composite Films for Antimicrobial Therapies. International Journal of Molecular Sciences 2021, 22, 4595. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, B.; Naveed, M.; Makhdoom, S.I.; Jabeen, K.; Farrukh Asif, M.F.; Batool, H.; Ahmed, N. and Chan, Y.Y.; A Comparison Between Organic and Inorganic Nanoparticles: Prime Nanoparticles for Tumor Curation. Nano 2021, 16, 13, 2130011. [CrossRef]
- Feng, Y.; Tonon, C.C.; Ashraf, S.; T.; Hasan, T.; Photodynamic and antibiotic therapy in combination against bacterial infections: efficacy, determinants, mechanisms, and future perspectives. Advanced Drug Delivery Reviews 2021, 177, 113941, ISSN 0169-409X. [CrossRef]
- Tichaczek-Goska, D.; Wojnicz, D.; Symonowicz, K.; Ziółkowski, P. and Hendrich, A.B.; Photodynamic enhancement of the activity of antibiotics used in urinary tract infections. Lasers in medical science 2019, 34, 8, 1547. [CrossRef]
- Pérez-Laguna, V.; García-Luque, I.; Ballesta, S.; Rezusta, A. and Gilaberte, Y.; Photodynamic Therapy Combined with Antibiotics or Antifungals against Microorganisms That Cause Skin and Soft Tissue Infections: A Planktonic and Biofilm Approach to Overcome Resistances. Pharmaceuticals (Basel) 2021, 14, 7, 603. [Google Scholar] [CrossRef] [PubMed]
- Ronqui, M.R.T.; de Aguiar Coletti, M.; de Freitas, L. M. Miranda, E. T. and Fontana, C. R. Synergistic antimicrobial effect of photodynamic therapy and ciprofloxacin. Journal of Photochemistry and Photobiology B: Biology 2016, 158, 122–129. [Google Scholar] [CrossRef]
- Openda, Y.I.; Nyokong, T. ; Combination of photodynamic antimicrobial chemotherapy and ciprofloxacin to combat S. aureus and E. coli resistant biofilms, Photodiagnosis and Photodynamic Therapy 2023, 42, 103142. [Google Scholar] [CrossRef]
- Bilici, K.; Atac, N.; Muti, A.; Baylam, I.; Dogan, O.; Sennaroglu, A.; Can, F. and Yagci Acar, H.; Broad spectrum antibacterial photodynamic and photothermal therapy achieved with indocyanine green loaded SPIONs under near infrared irradiation. Biomaterials Science 2020, 8, 16, 4616–4625. [Google Scholar] [CrossRef]
- Wang, R.; Kim, D.; Yang, M.; Li, X.; and Yoon, J.; Phthalocyanine-Assembled “One-For-Two” Nanoparticles for Combined Photodynamic–Photothermal Therapy of Multidrug-Resistant Bacteria. ACS Applied Materials & Interfaces 2022, 14, 6, 7609–7616. [CrossRef]
- Shi, E.; Bai, L.; Mao, L. Wang, H.; Yang, X.; Wang, Y.; Zhang, M.; Li C. and Wang, Y.; Self-assembled nanoparticles containing photosensitizer and polycationic brush for synergistic photothermal and photodynamic therapy against periodontitis. Journal of Nanobiotechnology 2021, 19, 413. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Wang, Y.; Zhu, W.; Li, G.; Ma, X.; Zhang, Y.; Chen, S.; Tiwari, S.; Shi, K.; Zhang, S.; Fan, H.M.; Zhao, Y.X.; and Liang, X.J. ; Comprehensive understanding of magnetic hyperthermia for improving antitumor therapeutic efficacy. Theranostics 2020, 10, 8, 3793–3815. [Google Scholar] [CrossRef] [PubMed]
- Vilas-Boas, V.; Carvalho, F.; Espiña, B. ; Magnetic Hyperthermia for Cancer Treatment: Main Parameters Affecting the Outcome of In Vitro and In Vivo Studies. Molecules 2020, 25, 12, 2874. [Google Scholar] [CrossRef] [PubMed]
- Alumutairi, L.; Yu, B.; Filka, M.; Nayfach, J.; Kim, M.H. ; Mild magnetic nanoparticle hyperthermia enhances the susceptibility of Staphylococcus aureus biofilm to antibiotics. International Journal of Hyperthermia 2020, 37, 1, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Guo, X.; Zhao, Z.; Li, B.; Qin, J.; Peng, Z.; He, G.; Brett, D.J.L. Wang, R.; Lu, X.; Fe3S4 nanoparticles for arterial inflammation therapy: Integration of magnetic hyperthermia and photothermal treatment. Applied Materials Today 2020, 18, 100457. [CrossRef]
- Włodarczyk, A.; Gorgo´ n, S.; Rado´ n, A.; Bajdak-Rusinek, K. Magnetite Nanoparticles in Magnetic Hyperthermia and Cancer Therapies: Challenges and Perspectives. Nanomaterials 2022, 12, 1807. [Google Scholar] [CrossRef] [PubMed]
- Bienia, A.; Wiecheć-Cudak, O.; Murzyn, A.A.; Krzykawska-Serda, M. ; Photodynamic Therapy and Hyperthermia in Combination Treatment-Neglected Forces in the Fight against Cancer. Pharmaceutics 2021, 13, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Balhaddad, A.A.; Xia, Y.; Lan, Y.; Mokeem, L.; Ibrahim, M.S.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. Magnetic-Responsive Photosensitizer Nanoplatform for Optimized Inactivation of Dental Caries-Related Biofilms: Technology Development and Proof of Principle. ACS Nano 2021, 15, 19888–19904. [Google Scholar] [CrossRef]
- de Santana, W.M.O.S.; Caetano, B.L.; de Annunzio, S.R.; de Annunzio, S.R.; Pulcinelli, S.H.; Ménager, C.; Fontana, C.R.; Santilli, C.V. Conjugation of superparamagnetic iron oxide nanoparticles and curcumin photosensitizer to assist in photodynamic therapy. Colloids and Surfaces B: Biointerfaces 2020, 196, 111297. [Google Scholar] [CrossRef]
- Wang, M.; Geilich, B.M.; Keidar, M.; Webster, T.J. ; Killing malignant melanoma cells with protoporphyrin IX-loaded polymersome-mediated photodynamic therapy and cold atmospheric plasma. International Journal of Nanomedicine 2017, 12, 4117–4127. [Google Scholar] [CrossRef]
- Brullé, L.; Vandamme, M.; Riès, D.; Martel, E.; Robert, E.; Lerondel, S.; Trichet, V.; Richard, S.; Pouvesle, J.M. and Le Pape, A.; Effects of a Non Thermal Plasma Treatment Alone or in Combination with Gemcitabine in a MIA PaCa2-luc Orthotopic Pancreatic Carcinoma Model. PLoS one 2012, 7, 12, e52653. [Google Scholar] [CrossRef] [PubMed]
- Daeschlein, G.; Scholz, S.; Lutze, S.; Arnold, A.; von Podewils, S.; Kiefer, T.; Tueting, T.; Hardt, O.; Haase, H.; Grisk, O.; Langner, S.; Ritter, C.; von Woedtke, T. and Jünger, M.; Comparison between cold plasma, electrochemotherapy and combined therapy in a melanoma mouse model. Experimental dermatology 2013, 22, 9, 582. [Google Scholar] [CrossRef] [PubMed]
- Betancourt-Ángeles, M.; Peña-Eguiluz, R.; López-Callejas, R.; Domínguez-Cadena, N.A.; Antonio Mercado-Cabrera, A.; Muñoz-Infante, J.; Rodríguez-Méndez, B.G.; Valencia-Alvarado, R.; and Moreno-Tapia, J.A. ; Treatment in the healing of burns with a cold plasma source. International Journal of Burns and Trauma 2017, 7, 7, 142–146. [Google Scholar]
- Boeckmann, L.; Schäfer, M.; Bernhardt, T.; Semmler, M.L.; Jung, O.; Ojak, G.; Fischer, T.; Peters, K.; Nebe, B.; Müller-Hilke, B.; Seebauer, C.; Bekeschus, S. and en Emmert, S.; Cold Atmospheric Pressure Plasma in Wound Healing and Cancer Treatment. Applied Sciences 2020, 10, 6898. [Google Scholar] [CrossRef]
- Klebes, M.; Ulrich, C.; Kluschke, F.; Patzelt, A.; Vandersee, S.; Richter, H.; Bob, A.; von Hutten, J.; Krediet, J.T.; Kramer, A.; Lademann, J. and Lange-Asschenfeld, B.; Combined antibacterial effects of tissue-tolerable plasma and a modern conventional liquid antiseptic on chronic wound treatment. Journal of biophotonics 2015, 8, 5–382. [Google Scholar] [CrossRef]
- Wang, M.; Geilich, B.M.; Keidar, M.; Webster, T.J. Killing malignant melanoma cells with protoporphyrin IX-loaded polymersome- mediated photodynamic therapy and cold atmospheric plasma. International Journal of Nanomedicine 2017, 12, 4117–4127. [Google Scholar] [CrossRef]
- Karami-Gadallo, L.; Ghoranneviss, M.; Ataie-Fashtami, L.; Pouladian, M.; Sardari, D. Enhancement of cancerous cells treatment by applying cold atmospheric plasma and photo dynamic therapy simultaneously. Clinical Plasma Medicine 2017, 7, 46–51. [Google Scholar] [CrossRef]
- Jungbauer, G.; Moser, D.; Müller, S.; Pfister, W.; Sculean, A.; Eick, S. The Antimicrobial Effect of Cold Atmospheric Plasma against Dental Pathogens-A Systematic Review of In-Vitro Studies. Antibiotics 2021, 10, 211. [Google Scholar] [CrossRef]
- Theinkom, F.; Singer, L.; Cieplik, F.; Cantzler, S.; Weilemann, H.; Cantzler, M.; Hiller, K.A.; Maisch, T.; Zimmermann, J.L. Antibacterial efficacy of cold atmospheric plasma against Enterococcus faecalis planktonic cultures and biofilms in vitro. Plos One 2019, 14, e0223925. [Google Scholar] [CrossRef]
- Maisch, T. Photodynamic inactivation of multi-resistant bacteria, Session 4: Photodynamic inactivation of microorganisms, 4.01, Short reports from the First Sino-German Symposium, 23–28 March 2015, Fuzhou, China.
- Menezes, M.; Prado, M.; Gomes, B.; Gusman, H.; Simão, R. Effect of photodynamic therapy and non-thermal plasma on root canal filling: Analysis of adhesion and sealer penetration. Journal of Applied Oral Science 2017, 25, 396–403. [Google Scholar] [CrossRef]
- Abdulwahed, A. Canal pretreatment using cold atmospheric argon plasma, chitosan and Chlorine p6 activated by photodynamic therapy with EDTA as a final irrigant on t Hafner, S.; Ehrenfeld, M.; Neumann, A.C. and Wieser, A.; Comparison of the bactericidal effect of cold atmospheric pressure plasma (CAPP), antimicrobial photodynamic therapy (aPDT), and polihexanide (PHX) in a novel wet surface model to mimic oral cavity application. Journal of Cranio-Maxillofacial Surgery he pushout bond strength of fiber post. Photodiagnosis and Photodynamic Therapy 2023, 42, 103517. 10.1016/j.pdpdt.2023.103517. 2018, 46, 12, 2197. [Google Scholar] [CrossRef]
- Aly, F. ; Synergistic Antimicrobial Effects of Cold Atmospheric Pressure Plasma and Photodynamic Therapy against Common Skin and Wound Pathogens In-Vitro. Ph.D. Thesis, niversität Greifswald, Greifswald, Germany, 2021. Available online: https://epub.ub.unigreifswald.de/frontdoor/index/index/docId/5680 (accessed on 16 January 2022).
- Harris, F,; Dennison, S. R. and David A. Phoenix, Sounding the death knell for microbes? Trends in Molecular Medicine 2014, 20, 7, 363–367. [Google Scholar] [CrossRef]
- Wan, G.Y.; Liu, Y.; Chen, B.W.; Liu, Y.Y.; Wang, Y.S.; Zhang, N. Recent advances of sonodynamic therapy in cancer treatment. Cancer Biology & Medicine 2016, 13, 3, 325–338. [Google Scholar] [CrossRef]
- Youf, R, Müller, M, Balasini, A, Thétiot, F. ; Müller, M.; Hascoët, A.; Jonas, U.; Schönherr, H.; Lemercier, G.; Montier, T. and Le Gall, T. Antimicrobial Photodynamic Therapy: Latest Developments with a Focus on Combinatory Strategies. Pharmaceutics 2021, 13, 12, 1995. [Google Scholar] [CrossRef]
- Pang, X.; Li, D.; Zhu, J.; Cheng, J.; Liu, G. Beyond Antibiotics: Photo/Sonodynamic Approaches for Bacterial Theranostics. Nano-Micro Letters 2020, 12, 144. [Google Scholar] [CrossRef] [PubMed]
- Karimnia, V,; Slack, F. J. and Celli, J.P. Photodynamic Therapy for Pancreatic Ductal Adenocarcinoma. Cancers (Basel) 2021, 13, 17, 4354. [Google Scholar] [CrossRef]
- Yang, H.; Jing, H.; Han, X.; Tan, H. and Cheng, W. Synergistic Anticancer Strategy of Sonodynamic Therapy Combined with PI-103 Against Hepatocellular Carcinoma. Drug Design, Development and Therapy 2021, 15, 531–542. [Google Scholar] [CrossRef]
- Borah, B.M.; Cacaccio, J.; Durrani, F.A.; Bshara, W.; Turowski, S.G.; Spernyak, J.A. and Pandey, R.K. Sonodynamic therapy in combination with photodynamic therapy shows enhanced long-term cure of brain tumor. Scientific Reports 2020, 10, 1, 21791. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gao, Q.; Guo, S.; Cheng, J.; Sun, X.; Li, Q,; Wang, T. ; Zhang, Z.; Cao, W. and Tian, Y. The sonodynamic effect of curcumin on THP-1 cell-derived macrophages. BioMed Research International 2013, 2013, 737264. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Jiang, J.; Zhang, C.; Zhao, M.; Chen, Z.; Wang, N.; Hu, D.; Liu, X.; Peng, H. and Lian, M. Sonodynamic therapy in atherosclerosis by curcumin nanosuspensions: Preparation design, efficacy evaluation, and mechanisms analysis. European Journal of Pharmaceutics and Biopharmaceutics 2020, 146, 101–110. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Esboei, B.R.; Hodjat, M.; Bahador, A. ; Sonodynamic excitation of nanomicelle curcumin for eradication of Streptococcus mutans under sonodynamic antimicrobial chemotherapy: Enhanced anti-caries activity of nanomicelle curcumin. Photodiagnosis and Photodynamic Therapy 2020, 30, 101780. [Google Scholar] [CrossRef] [PubMed]
- Pourhajibagher, M.; Pourakbari, B. and Bahador, A. Contribution of antimicrobial photo-sonodynamic therapy in wound healing: an in vivo effect of curcumin-nisin-based poly (L-lactic acid) nanoparticle on Acinetobacter baumannii biofilms. BMC Microbiology 2022, 22, 28. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Z.; Wang, C.; You, J.; Jin, B.; Mo, F.; Chen, J.; Zheng, Y.; and Chen, H. ; Self-assembled chitosan/rose bengal derivative nanoparticles for targeted sonodynamic therapy: preparation and tumor accumulation. RSC Advances 2015, 5, 17915–17923. [Google Scholar] [CrossRef]
- Xu, C.; Dong, J.; Ip, M.; Wang, X. and Leung, A.W. Sonodynamic action of chlorin e6 on Staphylococcus aureus and Escherichia coli. Ultrasonics 2016, 64, 54–57. [Google Scholar] [CrossRef]
- Ziental, D.; Wysocki, M.; Michalak, M.; Dlugaszewska, J.; Güzel, E.; Sobotta, L. The Dual Synergy of Photodynamic and Sonodynamic Therapy in the Eradication of Methicillin-Resistant Staphylococcus aureus. Applied Sciences 2023, 13, 3810. [Google Scholar] [CrossRef]
- Zhang, Q.; Bao, C.; Cai, X.; Jin, L.; Sun, L.; Lang, Y. and Li, L. Sonodynamic therapy-assisted immunotherapy: A novel modality for cancer treatment. Cancer Science 2018, 109, 5, 1330–1345. [Google Scholar] [CrossRef]
- Whiteside, T.L. ; Immune responses to malignancies. Journal of Allergy and Clinical Immunology 2010, 125, 2, S272–S283. [Google Scholar] [CrossRef]
- Yue, W, Chen, L, Yu L, Zhou, B. ; Yin, H.; Ren, W.; Liu, C.; Guo, L.; Zhang, Y.; Sun, L.; Zhang, K.; Xu, H. and Yu Chen, Y. Checkpoint blockade and nanosonosensitizer-augmented noninvasive sonodynamic therapy combination reduces tumour growth and metastases in mice. Nature Communications 2019, 10, 1, 2025. [Google Scholar] [CrossRef]
- Luo, J.; Wang, X.; Shi, Z.; Zeng, Y.; He, L.; Cao, J.; Sun, Y.; Zhang, T. and Huang, P. Enhancement of antitumor immunotherapy using mitochondria-targeted cancer cell membrane-biomimetic MOF-mediated sonodynamic therapy and checkpoint blockade immunotherapy. Journal of Nanobiotechnology 2022, 20, 228. [Google Scholar] [CrossRef]
- Pang, X, Liu, X, Cheng, Y, Zhang, C. ; Ren, E.; Liu, C.; Zhang, Y.; Zhu, J.; Chen, X. and Liu, G. Sono-Immunotherapeutic Nanocapturer to Combat Multidrug-Resistant Bacterial Infections. Advanced Materials 2019, 31, 35, e1902530. [Google Scholar] [CrossRef]
- Debela, D.T. , Muzazu, S.G., Heraro, K.D., Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K. and Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Medicine 2021, 9, 20503121211034366. [Google Scholar] [CrossRef] [PubMed]
- Heckenkamp, J. Leszczynski, D, Schiereck, J, Kung, J, LaMuraglia, G.M. Different effects of photodynamic therapy and gamma-irradiation on vascular smooth muscle cells and matrix : implications for inhibiting restenosis. Arteriosclerosis, Thrombosis, and Vascular Biology 1999, 19, 9, 2154–2161. [Google Scholar] [CrossRef] [PubMed]
- Browning, R.J.; Able, S.; Ruan, J.L.; Bau, L.; Allen, P.D.; Kersemans, V.; Wallington, S.; Kinchesh, P.; Smart, S.; Kartsonaki, C.; Kamila, S.; Logan, K.; Taylor, M.A.; McHale, A.P.; Callan, J.F. Stride, E. and Vallis, K.A. Combining sonodynamic therapy with chemoradiation for the treatment of pancreatic cancer. Journal of Controlled Release 2021, 337, 371–377. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).