Submitted:
12 April 2023
Posted:
12 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study site
2.2. Measurement of growth traits
2.3. Biomass and nutrient concentrations of understory vegetation
2.4. Soil analysis
2.5. Extraction and Amplification of Soil Bacterial DNA for Sequencing
2.6. Statistical analysis
3. Results
3.1. Growth of A. cincinnata in mixed and pure stands
3.2. Biomass and nutrient concentrations of understory vegetation
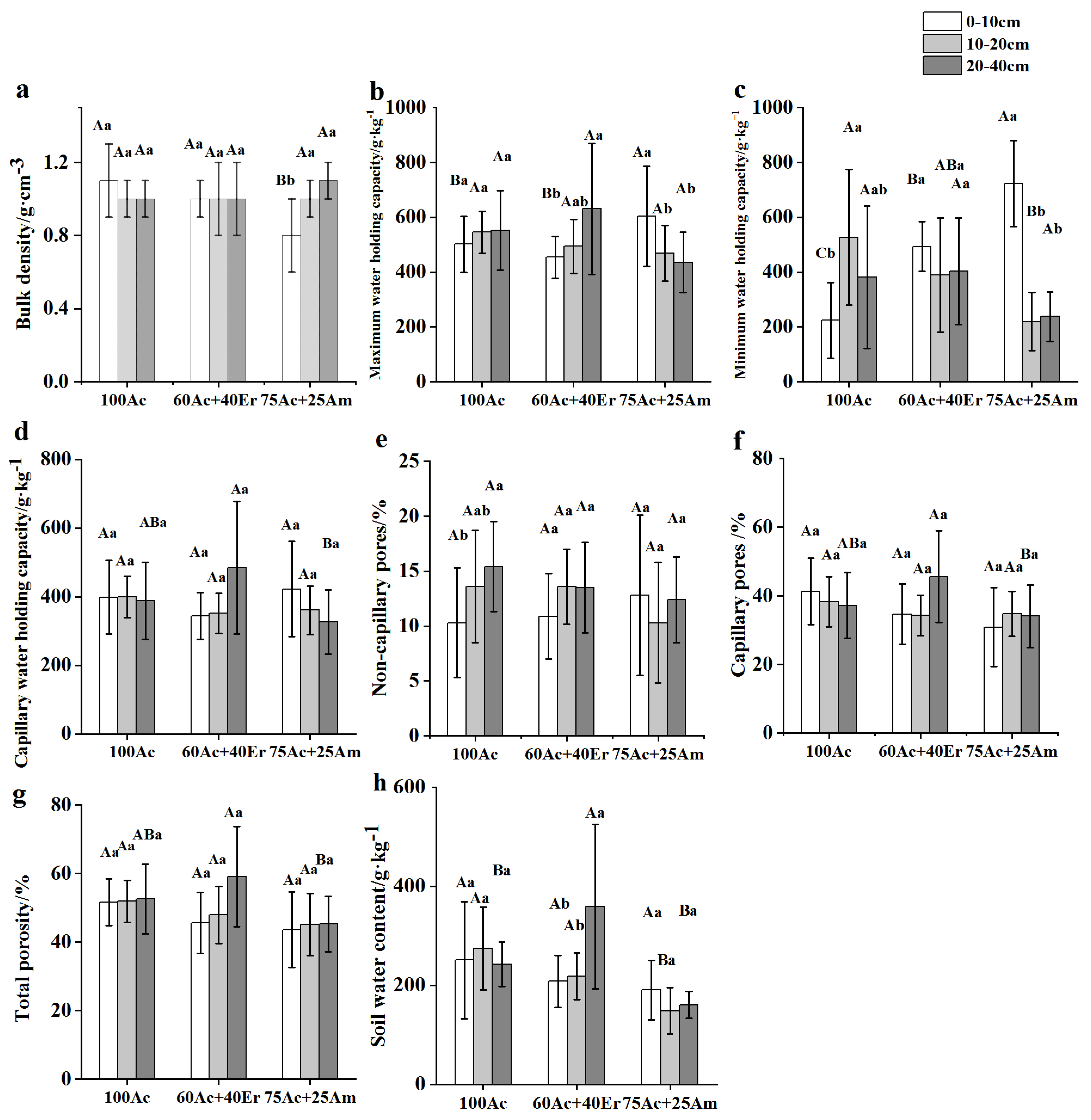
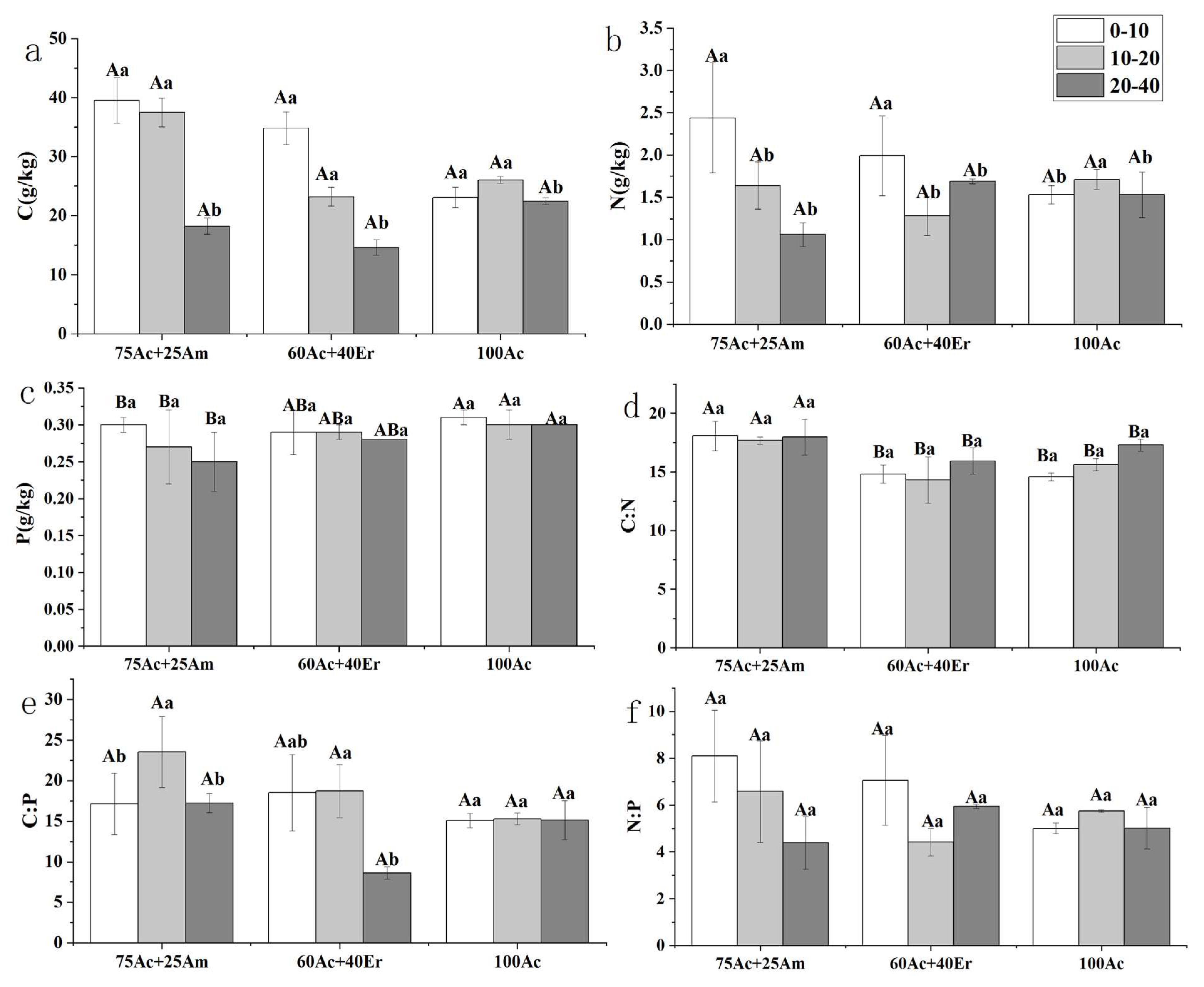
3.3. Soil physical properties and C:N:P stoichiometry
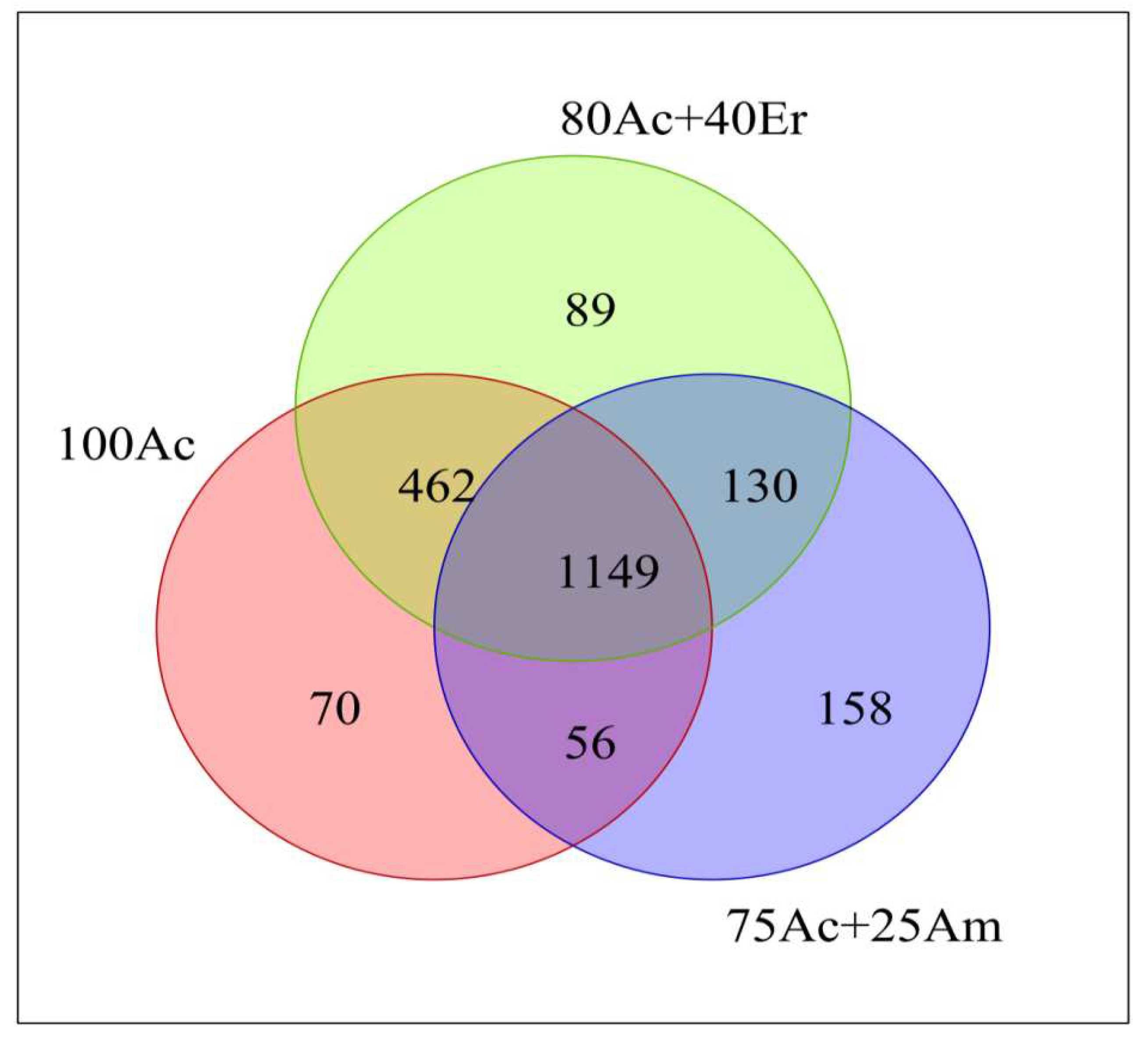
3.4. Soil bacterial diversity
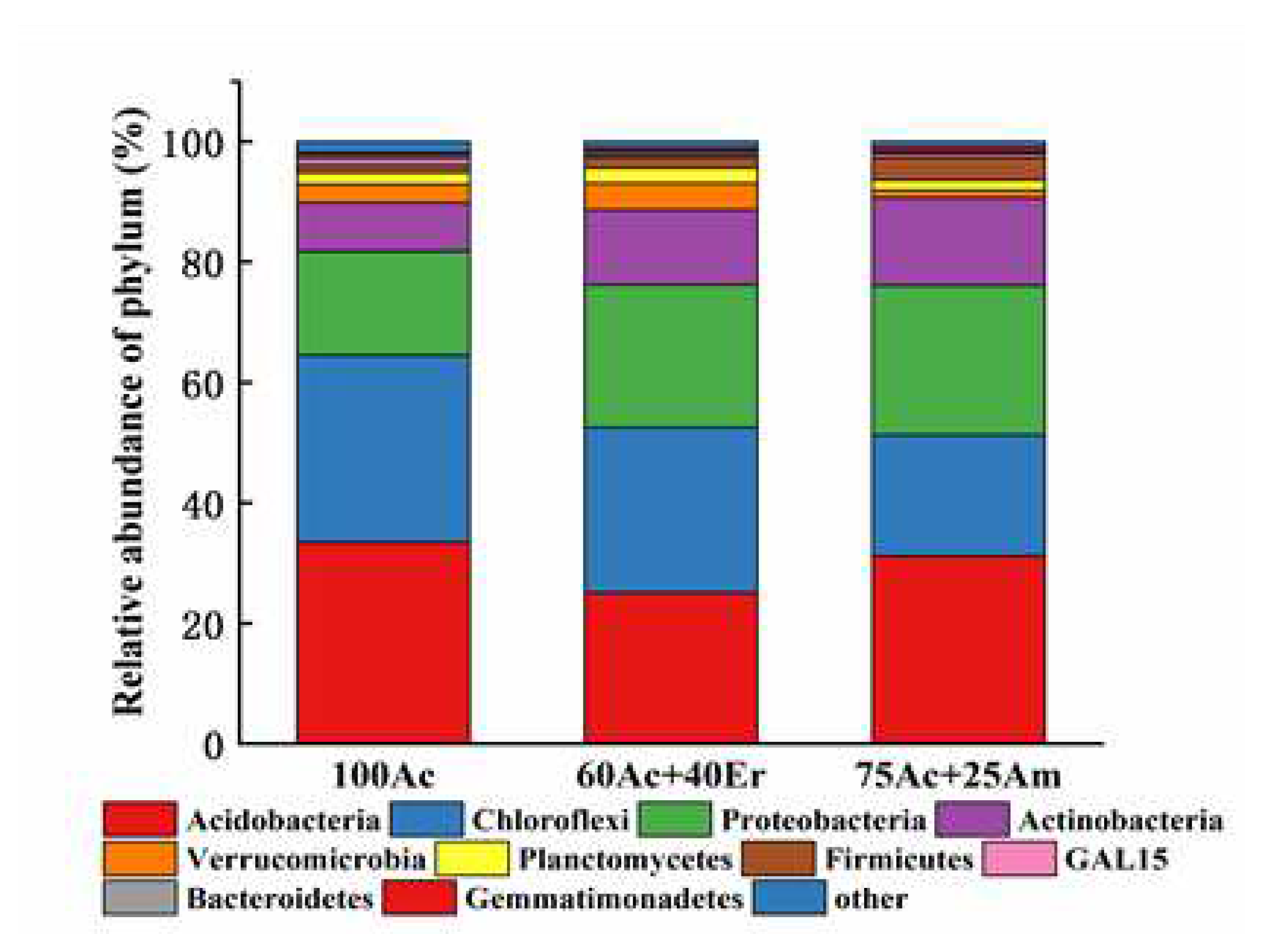
3.5. Composition of Soil Bacteria Community
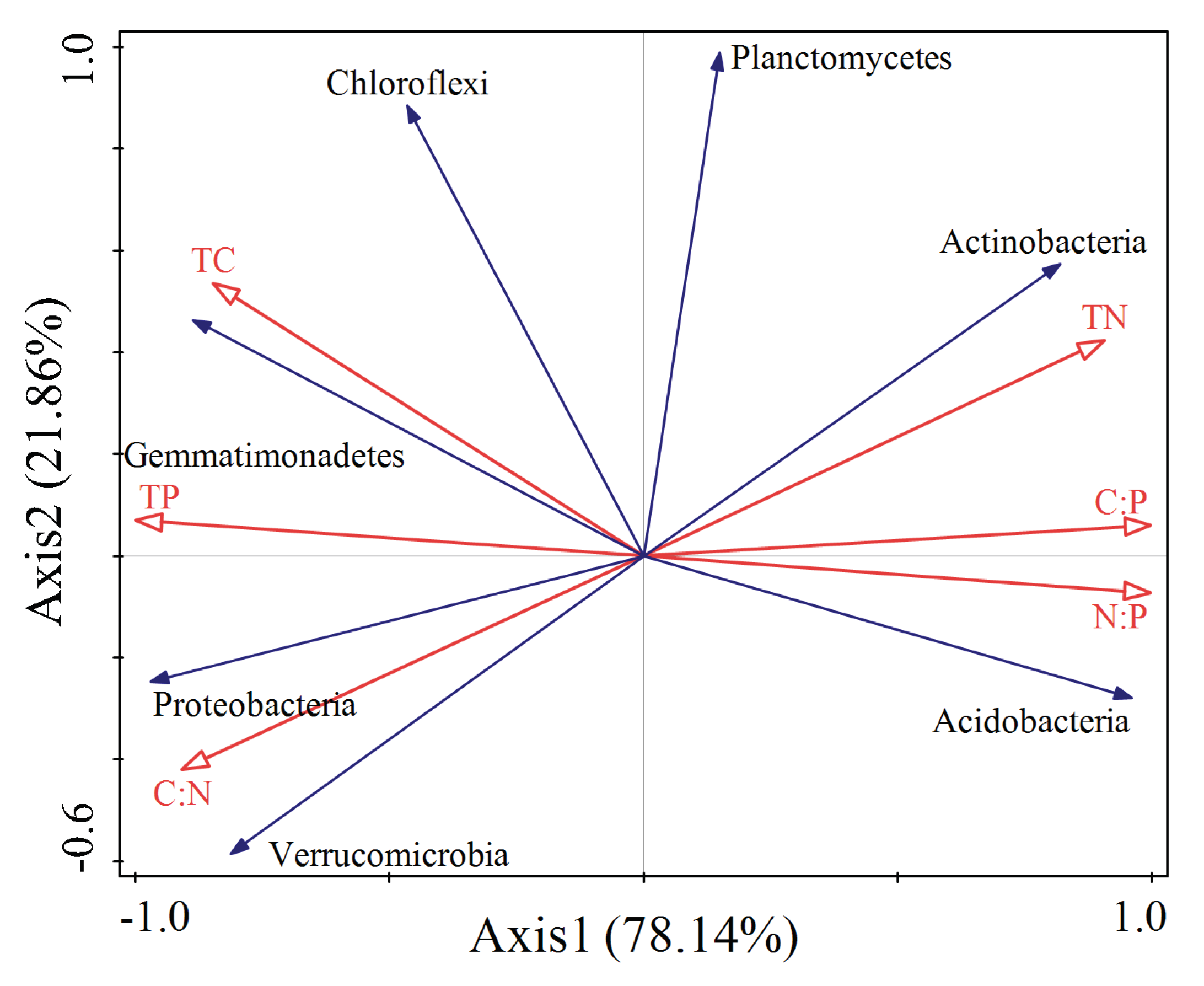
3.6. Relationship between soil bacteria and C, N and P stoichiometry
4. Discussion
4.1. Effects of species mixture on the growth of Acacia cincinnata
4.2. Effects of species mixture on composition and diversity of understory vegetation
4.3. Effects of species mixture on soil physical properties and C:N:P stoichiometry
4.4. Effect of species mixture on abundance and diversity of soil bacterial community
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| A. cincinnata stand | ||
| Species | Height (cm) | Coverage(%) |
| Rubus corchorifolius | 35 | 20 |
| Pteris dispar | 25 | 7 |
| Digitaria sanguinalis | 20 | 20 |
| Dryopteris chinensis | 10 | 10 |
| Dicranopteris dichotoma | 30 | 30 |
| Glochidion puberum | 50 | 25 |
| Adiantum flabellulatum | 5 | 0.5 |
| Smilax glabra | 10 | 0.4 |
| Lygodium japonicum | 5 | 4 |
| Clerodendrum cyrtophyllum | 40 | 5 |
| Mean ± SE | 23 ± 5 | 12.2 ± 3.4 |
| A. cincinnata+ E. robusta Stand | ||
| Dicranopteris dichotoma | 40 | 70 |
| Digitaria sanguinalis | 15 | 2 |
| Toxicodendron vernicifluum | 27 | 1 |
| Hedyotis hedyotidea | 6 | 1 |
| Urena lobate | 15 | 0.4 |
| Sapium discolor | 40 | 2 |
| Adiantum flabellulatum | 10 | 0.4 |
| Rubus corchorifolius | 8 | 0.4 |
| Smilaz china | 5 | 0.5 |
| Paederia cruddasiana | 60 | 16 |
| Embelia laeta | 37 | 50 |
| Gahnia tristis | 24 | 5 |
| Litsea rotundifolia var. Oblongifolia | 40 | 6 |
| Mean ± SE | 25.2 ± 4.8 | 11.9± 6.1 |
| A. cincinnata+ A. mangium stand | ||
| Adiantum flabellulatum | 15 | 4 |
| Pericampylus glaucus | 10 | 5 |
| Embelia ribes | 40 | 20 |
| Gahnia tristis | 90 | 15 |
| Pteris dispar | 10 | 6 |
| Dicranopteris dichotoma | 100 | 20 |
| Sapium discolor | 60 | 5 |
| Ficus hirta Vahl | 60 | 5 |
| Melicope pteleifolia | 110 | 10 |
| Mean ± SE | 55 ± 13 | 10 ± 2.2 |
References
- Liu, S.R.; Yang, Y.J.; Wang, H. Development strategy and countermeasures of Chinese industrial and forestry management: from single-objective management pursuing wood yield to multi-objective management to improving the quality and efficiency of ecosystem services. Acta Ecologica Sinica 2018, 38, 1–10. [Google Scholar]
- Xu, C.; Lin, F.; Zhu, C. Li, C.; Cheng, B. Does Classification-Based Forest Management Promote Forest Restoration? Evidence from China’s Ecological Welfare Forestland Certification Program. Forests 2022, 13, 573. [Google Scholar] [CrossRef]
- Felton, A.; Nilsson, U.; Sonesson, J.; Felton, A.M.; Roberge, J.-M.; Ranius, T.; Ahlström, M.; Bergh, J.; Björkman, C.; Boberg, J.; Drössler, L.; Fahlvik, N.; Gong, P.; Holmström, E.; Keskitalo, E.C.H.; Klapwijk, M.J.; Laudon, H.; Lundmark, T.; Niklasson, M.; Nordin, A.; Pettersson, M.; Stenlid, J.; Sténs, A.; Wallertz, K. Replacing monocultures with mixed-species stands: Ecosystem service implications of two production forest alternatives in Sweden. Ambio 2016, 45 (Suppl 2), 124–139. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.J.; Zhang, J.X. Reflections on sustainable management of plantations. Science 2016, 68, 37–40. [Google Scholar]
- Sheng, L.L. Research on sustainable management of ecological public welfare forests in China. Northeast Forestry University 2011. [Google Scholar]
- Zou, J.M.; Li, J.Z.; Yu, H.Q.; Qiao, J.D.; Liao, W.M. Research on the value assessment of ecosystem services of ecological public welfare forest: A case study of Jiangxi Province. China Forestry Economy 2022, 8–14. [Google Scholar]
- Lv, C.Q. Preliminary study on afforestation of Acacia curly pod in southern Fujian. Anhui Agricultural Science Bulletin 2021, 27, 70–71. [Google Scholar]
- Maire, G.L. Tree and stand light use efficiencies over a full rotation of single- and mixed-species Eucalyptus grandis and Acacia mangium plantations. Forest Ecology and Management 2013, 288, 31–42. [Google Scholar] [CrossRef]
- Forrester, D.I. Soil Organic Carbon is Increased in Mixed-Species Plantations of Eucalyptus and Nitrogen-Fixing Acacia. Ecosystems 2013, 16, 123–132. [Google Scholar] [CrossRef]
- Liao, J.L. Experimental study on afforestation of pure forest and mixed forest of Eucalyptus grandis x urophylla and Acacia mangium. Journal of South China Normal University (Natural Science Edition) 2000, 70–73.
- Chen, J.B.; Liang, J.P.; Li, G.M.; Huang, D.Y.; Huang, R.L.; Zou, J.Y.; Hu, Y.G.; Zeng, J.J. Study on the effect of mixed forest on soil nutrients in. Guangxi Forestry Science 2004, 1-5+14. [Google Scholar]
- Zang, M.H.; Huang, B.L.; Cheng, L.; Lv, C.Q.; Li, Z.X.; Wei. L.X. Study on soil microbial characteristics of mixed forest land of Eucalyptus robusta - Acacia. Guangxi Agricultural Sciences 2009, 40, 681–685. [Google Scholar]
- Chen, W.l. Cultivation technology of timber forest in short rotation period of Acacia curly pod in southern Fujian mountain. Forestry Investigation and Design 2015, 13–17. [Google Scholar]
- Zhan, N.; Huang, L.J. ; Review of research progress of acacia tree species in China. Tropical Forestry 2015, 43, 41–45. [Google Scholar]
- Yang, H.G. Experimental study on artificial mixed management technology of Eucalyptus robusta - Acacia cincinnata. Forestry Investigation and Planning 2015, 40, 91–95. [Google Scholar]
- Zhao, W.D.; Li, K.; Wang, J.; He, Z.M. Current stock and water-holding characteristics of five kinds of artificial Acacia forest litter. Journal of Sichuan Agricultural University 2020, 38, 677–684. [Google Scholar]
- Hartemink A, E. Soil fertility decline in the tropics: with case studies on plantations. Cabi 2003. [Google Scholar]
- Chen, X.Z. Analysis of ecological forest supply problems and countermeasures in Yanping District, Fujian. China forestry economics 2017, 33–35. [Google Scholar]
- Guo, D.Q.; Lu, L.F.; Deng, Z.Y.; Lan, C.Z.; Mo, K.Z.; Chen, J.B. Research progress of eucalyptus mixed forest in China. Eucalyptus. Science and Technology 2018, 35, 27–32. [Google Scholar]
- Nigussie, M.B.; Habeteyohannes, L.; Teshome, G. The Effect of Mixed Plantation on the Stand Yield and Soil Attributes of Eucalyptus globulus and Acacia decurrens in North Shewa Zone, Ethiopia. J Forest Res 2021, 10, 282. [Google Scholar]
- Zagatto, M.R.G. Interactions between mesofauna, microbiological and chemical soil attributes in pure and intercropped Eucalyptus grandis and Acacia mangium plantations. Forest Ecology and Management 2019, 433, 240–247. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, M.; Qi, L.; Zhao, C.; Zhang, W.; Zhang, Y.; Wen, W.; Yuan, J. Coupled Relationship between Soil Physicochemical Properties and Plant Diversity in the Process of Vegetation Restoration. Forests 2022, 13, 648. [Google Scholar] [CrossRef]
- Begon, M.; Harper, J.L.; Townsend, C.R. Ecology, Individuals, Populations and Communities, 3rd ed.; Blackwell Science: London, UK, 1996. [Google Scholar]
- Paula, R.R.; Bouillet, J-P.; de M. Gonçalves, J.L.; Trivelin, P. C. O.; Balieiro, F. de C.; Nouvellon, Y.; Oliveira, J. de C.; Júnior, J.C. de D.; Bordron, B.; Laclau, J-P. Nitrogen fixation rate of Acacia mangium Wild at mid rotation in Brazil is higher in mixed plantations with Eucalyptus grandis Hill ex Maiden than in monocultures. Annals of Forest Science 2018, 75, 14.
- Hoogmoed, M.; Cunningham, S.C.; Baker, P.; Beringer, J.; Cavagnaro, T.R. N-fixing trees in restoration plantings: Effects on nitrogen supply and soil microbial communities. Soil Biology & Biochemistry 2014, 77, 203–212. [Google Scholar]
- Oliveira, I.R.; Bordron, B.; Laclau, J-P.; Paula, R.R.; Ferraz, A.V.; Gonçalves, J.L.M.; le Maire, G.; Bouillet, J.P. Nutrient deficiency enhances the rate of short-term belowground transfer of nitrogen from Acacia mangium to Eucalyptus trees in mixed-species plantations. Forest Ecology and Management 2021, 491, 119192.
- Edberg, S.; Tigabu, M.; Odén, P.C. Commercial Eucalyptus Plantations with Taungya System: Analysis of Tree Root Biomass. Forests 2022, 13, 1395. [Google Scholar] [CrossRef]
- Leuschner, C.; Hertel, D.; Coners, H.; Büttner, V. Root competition between beech and oak: a hypothesis. Oecologia 2001, 126, 276–284. [Google Scholar] [CrossRef]
- Brassard, B.W.; Chen, H.Y.H.; Cavard, X.; Laganière, J.; Reich, P.B.; Bergeron, Y.; Paré, D.; Yuan, Z.; Chen, H. Tree species diversity increases fine root productivity through increased soil volume filling. J. Ecol. 2013, 2013, 210–219. [Google Scholar] [CrossRef]
- Ruiz-Blandon, B.A.; Salcedo-Pérez, E.; Rodríguez-Macías, R.; Hernández-Álvarez, E.; Campo, J.; Merino, A. Growth, biomass, carbon and nutrient pools in Gmelina arborea established in pure and mixed forest stand production systems in Mexico. New Forests 2022, 53, 735–757. [Google Scholar] [CrossRef]
- Viera M, Schumacher MV, Liberalasso E (2011) Crescimento e produtividade de povoamentos monoespecíficos e mistos de eucalipto e acácia-negra. Pesqui Agropecu Trop 41:415–421. [CrossRef]
- Tang, G.; Li, K.; Zhang, C.; Gao, C.; Li, B. Accelerated nutrient cycling via leaf litter, and not root interaction, increases growth of Eucalyptus in mixed-species plantations with Leucaena. For Ecol Manag 2013, 310, 45–53. [Google Scholar] [CrossRef]
- Forrester, D.I. The spatial and temporal dynamics of species interactions in mixed-species forests: From pattern to process. For Ecol Manag 2014, 312, 282–292. [Google Scholar] [CrossRef]
- Vezzani, F.M.; Tedesco, M.J.; Barros, N.F. Alterações dos nutrientes no solo e nas plantas em consórcio de eucalipto e acácia negra. Rev Bras Ciênc Solo 2001, 25, 225–231. [Google Scholar] [CrossRef]
- Barbier, S.; Gosselin, F.; Balandier, P. Influence of tree species on understory vegetation diversity and mechanisms involved – a critical review for temperate and boreal forests. For. Ecol. Manage. 2008, 254, 1–15. [Google Scholar] [CrossRef]
- Kumar, P.; Chen, H.Y.H.; Thomas, S.C.; Shahi, C. Linking resource availability and heterogeneity to understorey species diversity through succession in boreal forest of Canada. J. Ecol. 2018, 106, 1266–1276. [Google Scholar] [CrossRef]
- Messier, C.; Parent, S.; Bergeron, Y. Effects of overstory and understory vegetation on the understory light environment in mixed boreal forests. J. Veg. Sci. 1998, 9, 511–520. [Google Scholar] [CrossRef]
- Augusto, L.; Dupoey, J.-L.; Ranger, J. Effects of tree species on understory vegetation and environmental conditions in temperate forests. Ann. For. Sci. 2003, 60, 823–831. [Google Scholar] [CrossRef]
- Lindroos, A.-J.; Derome, J.; Derome, K.; Smolander, A. The effect of Scots pine, Norway spruce and silver birch on the chemical composition of stand throughfall and upper soil percolation water in northern Finland. Boreal Env. Res. 2011, 16, 240–250. [Google Scholar]
- Macdonald, S.E.; Fenniak, T.E. Understory plant communities of boreal mixed wood forests in western Canada: Natural patterns and response to variable-retention harvesting. For. Ecol. Manage. 2007, 242, 34–48. [Google Scholar] [CrossRef]
- Jonsson, M.; Bengtsson, J.; Gamfeldt, L.; Moen, J.; Snäll, T. Levels of forest ecosystem services depend on specific mixtures of commercial tree species. Nat. Plants 2019, 5, 141–147. [Google Scholar] [CrossRef]
- Hedwall, P.-O.; Holmström, E.; Lindbladh, M.; Felton, A. Concealed by darkness: How stand density can override the biodiversity benefits of mixed forests. Ecosphere 2019, 10, e02835. [Google Scholar] [CrossRef]
- Ampoorter, E.; Selvi, F.; Auge, H.; Baeten, L.; Berger, S.; Carrari, E.; Coppi, A.; Fotelli, M.; Radoglou, K.; Setiawan, N.N.; Vanhellemont, M.; Verheyen, K. Driving mechanisms of overstorey – understorey diversity relationships in European forests. Perspect. Plant Ecol. 2016, 19, 21–29. [Google Scholar]
- Guo, J.; Feng, H.; McNie, P.; Liu, Q.; Xu, X.; Pan, C.; Yan, K.; Feng, L.; Goitom, E.A.; Yu, Y. Species mixing improves soil properties and enzymatic activities in Chinese fir plantations: A meta-analysis. Catena 2023, 220, 106723. [Google Scholar] [CrossRef]
- López-Vicente, M.; Álvarez, S. Stability and patterns of topsoil water content in rainfed vineyards, olive groves, and cereal fields under different soil and tillage conditions. Agric. Water Manag. 2018, 201, 167–176. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, L.; Gong, W.; Yang, X.; Kang, Y. Evaluation of the Water Conservation Function of Different Forest Types in Northeastern China. Sustainability 2019, 11, 4075. [Google Scholar] [CrossRef]
- Wen, X.F.; Li, Y.; Yang, W.F. Study on growth and soil physicochemical properties of mixed forest of Pinus koraiensis and Alnus sibirica. Liaoning Forestry Science and Technology 2012, 19+34. [Google Scholar]
- Singh, K.; Singh, B.; Singh, R.R. Changes in physico-chemical, microbial and enzymatic activities during restoration of degraded sodic land: Ecological suitability of mixed forest over monoculture plantation. Catena 2012, 96, 57–67. [Google Scholar] [CrossRef]
- Huang, W.Y. Analysis of the effect of Acacia cincinnata in the second generation budding of Eucalyptus grandis x urophylla. Collection 2019, 17. [Google Scholar]
- Cotrufo, M.F.; Soong, J.L.; Horton, A.J.; Campbell, E.E.; Haddix, M.L.; Wall, D.H.; Parton, W.J. Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat. Geosci. 2015, 8. [Google Scholar] [CrossRef]
- Awad, Y.M.; Blagodatskaya, E.; Yong, S.O.; Kuzyakov, Y. Effects of polyacrylamide, biopolymer, and biochar on decomposition of soil organic matter and plant residues as determined by C and enzyme activities. Eur. J. Soil Biol. 2012, 48, 1–10. [Google Scholar] [CrossRef]
- Berger, T.W.; Berger, P. Greater accumulation of litter in spruce (Picea abies) compared to beech (Fagus sylvatica) stands is not a consequence of the inherent recalcitrance of needles. Plant Soil 2012, 358, 349e369. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, L.; Wen, D.; Yu, K. . Soil potential labile but not occluded phosphorus forms increase with forest succession. Biol. Fertil. Soils 2016, 52, 41e51. [Google Scholar] [CrossRef]
- Xie, H.; Tang, Y.; Yu, M.; Wang, G.G. The effects of afforestation tree species mixing on soil organic carbon stock, nutrients accumulation, and understory vegetation diversity on reclaimed coastal lands in Eastern China. Global Ecology and Conservation 2021, 26, e01478. [Google Scholar] [CrossRef]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Pienaar, L.V.; Shiver, B.D. Early results from an old-field loblolly pine spacing study in the Georgia Piedmont with competition control. South J. Appl. For. 1993, 17, 193–196. [Google Scholar] [CrossRef]
- Xie, Y.; Zhou, Z. Research advance on adaptation mechanism of forest tree to low-phosphorus stress and genetics of phosphorus efficiency. Forest Research 2002, 15, 734–740. [Google Scholar]
- Zhang, W.; Li, J.J.; Xiang, M.Z.; Huang, H.M.; Li, C.H.; Yan, J.L.; Gao, G.N.; Su, X.Y.; You, Y.M.; Huang, X.M. Effects of nitrogen-fixing tree Acacia mangium on particle size distribution and stability of soil aggregates in Eucalyptus grandis plantations. Flora of Guangxi 1–18.
- Huang, P.; Wang, N.; Zhou, Z.Y.; Wang, T.; Yuan, Z.L.; Chang, J.T.; Ye, Y.Z. Correlation analysis of soil bacterial community structure and environmental factors in deciduous broad-leaved forest in Baiyun Mountain. Journal of Henan Agricultural University 2020, 54, 415–421. [Google Scholar]
- Dukunde, A.; Schneider, D.; Schmidt, M.; Veldkamp, E.; Daniel, R. Tree Species Shape Soil Bacterial Community Structure and Function in Temperate Deciduous Forests. Front. Microbiol. 2019, 10, 1519. [Google Scholar] [CrossRef] [PubMed]
- Koutika, L-S.; Fiore, A.; Tabacchioni, S.; Aprea, G.; de Araujo Pereira, A. P.; Bevivino, A. Influence of Acacia mangium on Soil Fertility and Bacterial Community in Eucalyptus Plantations in the Congolese Coastal Plains. Sustainability 2020, 12, 8763. [CrossRef]
- Li, J.G.; Shen, M.C.; Hou, J.F.; Li, L.; Wu, J.X.; Dong, Y.H. Effect of Different Levels of Nitrogen on Rhizosphere Bacterial Community Structure in Intensive Monoculture of Greenhouse Lettuce. Sci. Rep. 2016, 6, 25305. [Google Scholar] [CrossRef]
- Zhu, H.; He, X.; Wang, K. Interactions of vegetation succession, soil bio-chemical properties and microbial communities in a Karst ecosystem. European Journal of Soil Biology 2012, 51, 1–7. [Google Scholar] [CrossRef]
- Zhang, Z.F. Effects of mixed breeding of precious native tree species and Eucalyptus on biomass, carbon storage and soil physicochemical properties. Guangxi University 2020. [Google Scholar]
- Alves, L.F.; Vieira, S.A.; Scaranello, M.A. Forest structure and live aboveground biomass variation along an elevational gradient of tropical Atlantic moist forest (Brazil). Forest ecology and management 2010, 260, 679–691. [Google Scholar] [CrossRef]
- Mercado-Blanco, J.; Abrantes, I.; Barra Caracciolo, A.; Bevivino, A.; Ciancio, A.; Grenni, P.; Hrynkiewicz, K.; Kredics, L.; Proença, D.N. Belowground Microbiota and the Health of Tree Crops. Front. Microbiol. 2018, 9, 1006. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N. Embracing the Unknown: Disentangling the Complexities of the Soil Microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-Based Assessment of Soil PH as a Predictor of Soil Bacterial Community Structure at the Continental Scale. Appl. Environ. Microbiol. 2009, 75, 5111–5120. [Google Scholar] [CrossRef]
- Liu, C.L.; Zuo, W.Y.; Zhao, Z.Y.; Qiu, L.H. Diversity of forest soil bacteria at different succession stages in Dinghu Mountain. Acta Microbiol 2012, 52, 1489–1496. [Google Scholar]
- Liu, J.; Yang, Z.; Dang, P.; Zhu, H.; Gao, Y.; Ha, V.N.; Zhao, Z. Response of Soil Microbial Community Dynamics to Robinia Pseudoacacia, L. Afforestation in the Loess Plateau: A Chronosequence Approach. Plant Soil 2018, 423, 327–338. [Google Scholar] [CrossRef]
- Li, Y.; Tian, D.; Wang, J.; Niu, S.; Tian, J.; Ha, D.; Qu, Y.; Jing, G.; Kang, X.; Song, B. Differential Mechanisms Underlying Responses of Soil Bacterial and Fungal Communities to Nitrogen and Phosphorus Inputs in a Subtropical Forest. PeerJ 2019, 7, e7631. [Google Scholar] [CrossRef]
- Chen, W.F.; Shi, Y.X. Microbial distribution characteristics of soils of different vegetation types in the new wetlands of the Yellow River Delta. Journal of Grassland 2010, 18, 859–864. [Google Scholar]
- Ding, X.J.; Huang, Y.L.; Jing, R.Y.; Ma, F.Y.; An, R.; Tian, Q.; Chen, B.J. Study on soil bacterial structure and diversity of four plantations in the Yellow River Delta based on high-throughput sequencing. Acta Ecologica Sinica 2018, 38, 5857–5864. [Google Scholar]
- Ding, X.J.; Xie, G.L.; Jing, R.Y.; Ma, F.Y.; Liu, F.C.; Ma, H.L. Decomposition characteristics of litter in different artificial Robinia pseudoacacia mixed forests in the Yellow River Delta. Journal of Soil and Water Conservation 2016, 30, 249–253+307. [Google Scholar]
- Zou, L.; Tang, Q.M.; Wang, Y. Community distribution characteristics of soil microorganisms in pure and mixed forests of Larix gmelinii and Pinus sylvestris. Journal of Northeast Forestry University 2010, 38, 63–64+79. [Google Scholar]
| Stand type* | Species | Diameter (cm) | Height (m) | Volume per tree (m3) | Stand volume (m3/hm2) |
Total stock (m3/hm2) |
|---|---|---|---|---|---|---|
| 75Ac+ 25Am | Acacia cincinnata | 9.6 ± 0.7a | 10.1 ± 0.2a | 1.06 ± 0.2a | 18.34 ± 3.03a | 109.6 ± 14.9b |
| A. mangium | 11.3 ± 0.1b | 11.9 ± 0.1bc | 1.62 ± 0.04b | 91.23 ± 15.27c | ||
| 60Ac + 40Er | Acacia cincinnata | 9.9 ± 0.4a | 10.5 ± 0.1a | 1.12 ± 0.1a | 28.37 ± 12.56a | 84.6 ± 23.9ab |
| E. robusta | 13.4 ± 2.3b | 14.9 ± 2.16c | 2.80 ± 1.01b | 56.20 ± 18.75b | ||
| 100Ac | Acacia cincinnata | 10.4 ± 0.1a | 10.8 ± 0.01a | 1.24 ± 0.04a | 61.12 ± 12.95b | 61.1 ± 9.8a |
| Forest stand type* | Herbal biomass(Kg/ha) | Shrub biomass (Kg/ha) | |||||
|---|---|---|---|---|---|---|---|
| Above ground | Under ground | Total biomass | root | branch | leaf | Total biomass | |
| 75Ac + 25Am | 820.1±200.4a | 429.9 ± 71.2a | 1250.0 ± 251.0a | 285.3 ± 186.5a | 378.7 ± 171.7a | 151.9 ± 40.7a | 815.8 ± 392.4a |
| 60Ac + 40Er | 254.4±103.7b | 258.1 ± 12.2b | 512.5 ± 106.9b | 54.6 ± 12.4b | 141.4 ± 52.3b | 88.0 ± 30.0ab | 284.1 ± 79.1b |
| 100Ac | 752.8±123.4a | 386.6 ± 87.7a | 1139.4 ± 184.1a | 109.8 ± 31.5a | 112.6 ± 37b | 76.9 ± 26.5b | 299.2 ± 81.7b |
| Vegetation | Plant Parts | Nutrient | Forest stand type* | ||
|---|---|---|---|---|---|
| 75Ac + 25Am | 60Ac + 40Er | 100Ac | |||
| Herbal biomass | Above ground | N | 9.1 ± 1.1a | 11.6 ± 1.1ab | 13.7 ± 1.3b |
| C | 363.7 ± 61.6a | 402.3 ± 28.8a | 420.8 ± 14.6a | ||
| P | 1.0 ± 0.1a | 0.8 ± 0.3a | 1.2 ± 0.3a | ||
| Under ground | N | 8.1 ± 0.7b | 7.1 ± 1.2b | 5.7 ± 0.3a | |
| C | 244.8 ± 26.4b | 282.4 ± 40.9b | 185.8 ± 3.2a | ||
| P | 0.8 ± 0.1a | 0.7 ± 0.3a | 1.5 ± 0.0b | ||
| Shrub biomass | root | N | 15.5 ± 1.5c | 10.3 ± 0.1b | 7.6 ± 0.0a |
| C | 404.5 ± 18.4a | 420.2 ± 5.6a | 420.5 ± 0a | ||
| P | 0.6 ± 0.1b | 0.3 ± 0.1a | 0.5 ± 0.0b | ||
| branch | N | 11.4 ± 1.3a | 13.1 ± 4.01a | 12.7 ± 0.0a | |
| C | 453.6 ± 3.9a | 445.0 ± 9.2a | 450.5 ± 0.0a | ||
| P | 0.6 ± 0.1a | 0.5 ± 0.2a | 0.9 ± 0.0b | ||
| leaf | N | 22.5 ± 0.6c | 17.3 ± 1.9b | 14.3 ± 0.0a | |
| C | 466.0 ± 1.3c | 431.7 ± 5.0a | 449.6 ± 0.0b | ||
| P | 0.5 ± 0.0a | 0.5 ± 0.3a | 0.7 ± 0.0a | ||
| Forest stand* | |||
|---|---|---|---|
| Bacterial diversity index | 100Ac | 60Ac + 40Er | 75Ac + 25Am |
| Chao1 | 1605.5 ± 49.9a | 1546.4 ± 45.3a | 1297.4 ± 86.0b |
| Observed_ species | 1327.8 ± 47.3a | 1315.9 ± 76.26a | 1109.3 ± 55.1b |
| PD_whole_tree | 85.8 ± 2.9a | 84.8 ± 4.6a | 71.3 ± 3.1b |
| Shannon | 8.1 ± 0.2a | 8.3 ± 0.1a | 7.8 ± 0.1a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





