Submitted:
12 April 2023
Posted:
13 April 2023
You are already at the latest version
Abstract
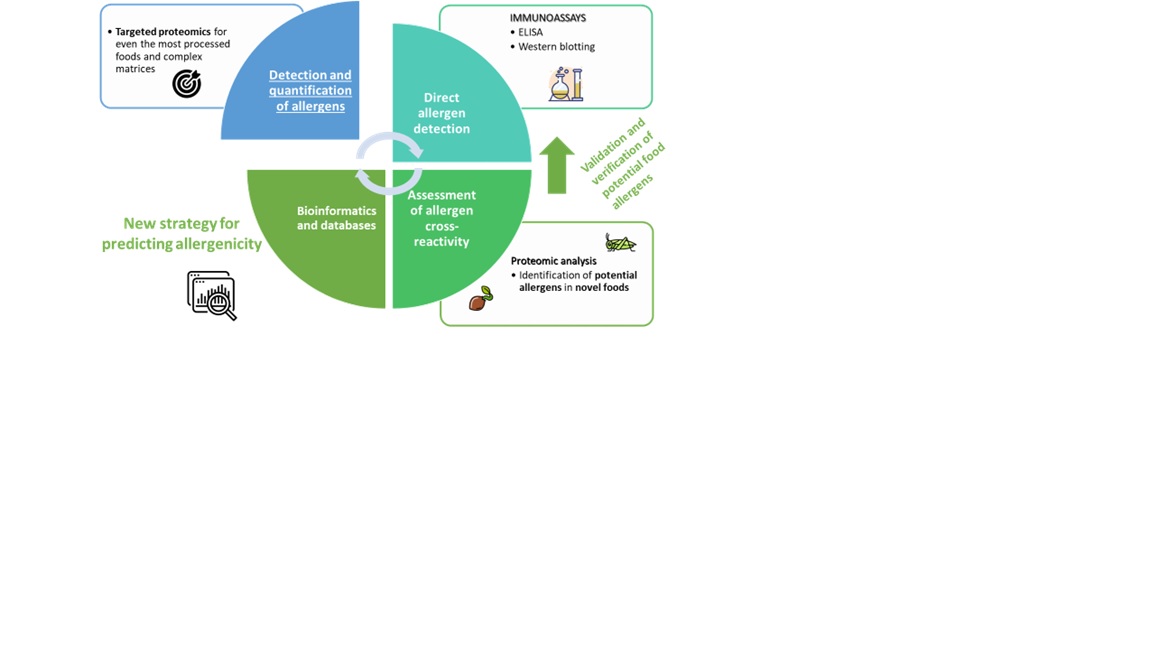
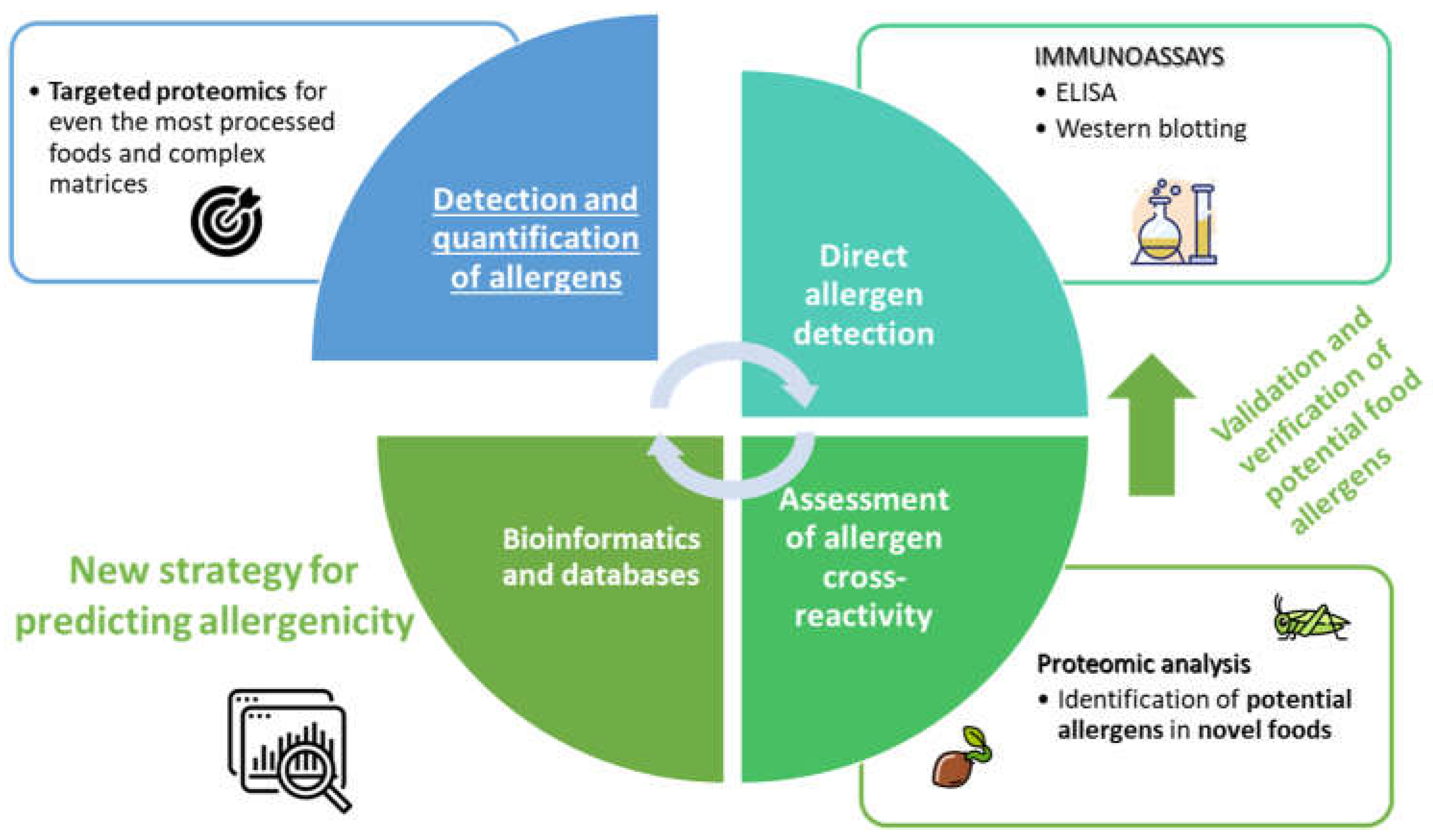
Keywords:
1. Introduction
2. Allergens of traditional food
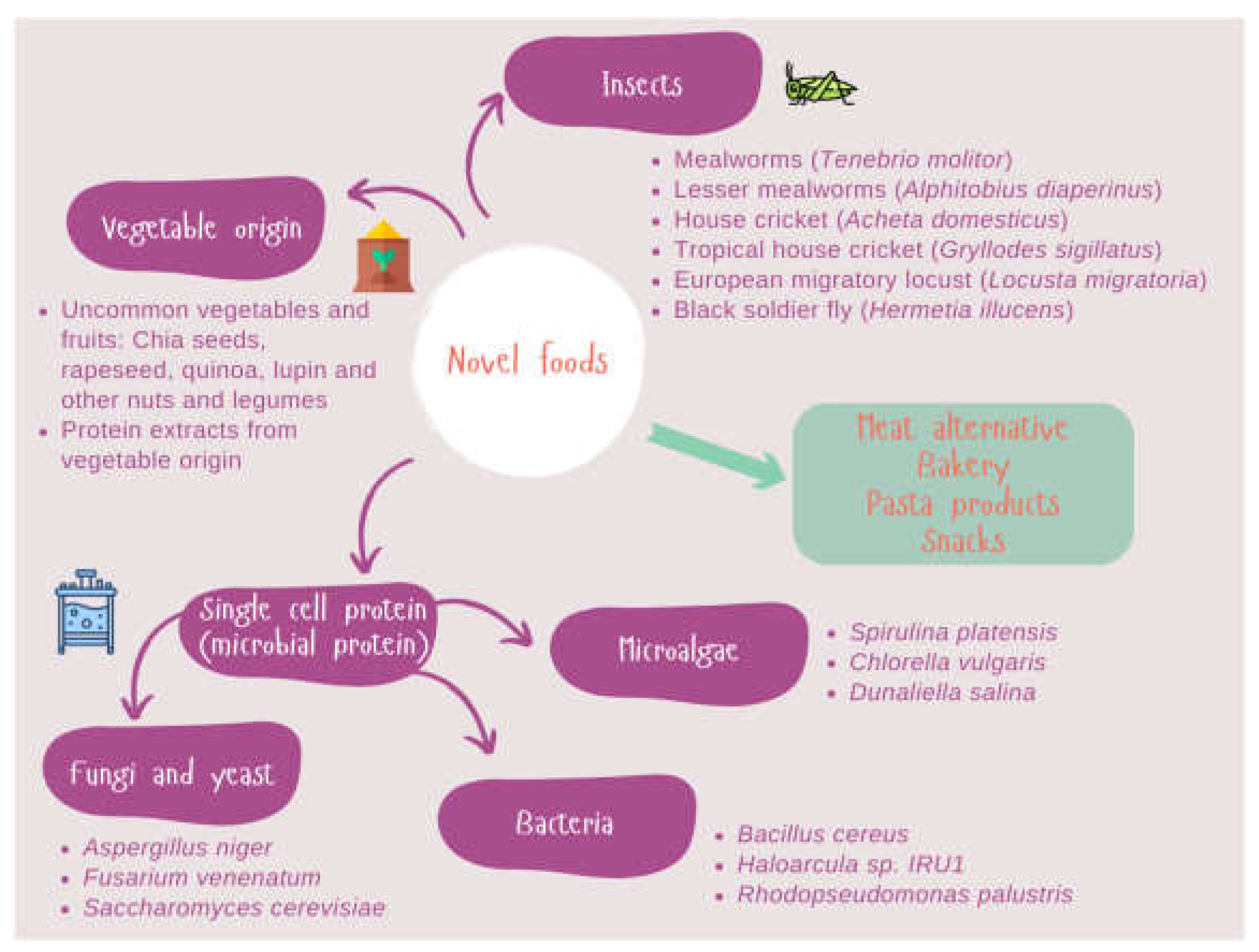
3. Allergens of novel foods
| Novel Food | Protein name/allergen | Specie | Ref. |
|---|---|---|---|
| Microalgae | C-phycocyanin Thioredoxins Superoxide dismutase Glyceraldehyde-3-phosphate dehydrogenase Triosephosphate isomerase |
Microalgae spirulina (A. platensis) | [35,37] |
| Microalgae | viz. calmodulin Fructose-bisphosphate aldolase |
Microalgae chlorella (C. vulgaris) | [37] |
| Insects | Tropomyosin, myosin, actin, troponin C (muscle proteins) Tubulin (cellular proteins) Hemocyanin, defensin (circulating proteins) Arginine kinase, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), triosephosphate isomerase, α-amylase, trypsin, phospholipase A, hyaluronidase (enzymes) |
[38] [39] |
4. Current prevalent methods used to assess the presence of allergens in foods
5. Proteomic approach to identify allergens in novel foods
6. Targeted proteomics for quantification of food allergens
7. Future trends
Author Contributions
Acknowledgments
Conflicts of Interest
References
- EFSA, Scientific Opinion on the evaluation of allergenic foods and food ingredients for labelling purposes, 2014. https://www.efsa.europa.eu/es/efsajournal/pub/3894.
- J. Shroba, R. Das, L. Bilaver, E. Vincent, E. Brown, B. Polk, A. Ramos, A.F. Russell, J.A. Bird, C.E. Ciaccio, B.J. Lanser, K. Mudd, A. Sood, B.P. Vickery, R. Gupta, Food Insecurity in the Food Allergic Population: A Work Group Report of the AAAAI Adverse Reactions to Foods Committee, J. Allergy Clin. Immunol. Pract. 10 (2022) 81–90. [CrossRef]
- J. Huang, C. Liu, Y. Wang, C. Wang, M. Xie, Y. Qian, L. Fu, Application of in vitro and in vivo models in the study of food allergy, Food Sci. Hum. Wellness. 7 (2018) 235–243. [CrossRef]
- R. Valenta, H. Hochwallner, B. Linhart, S. Pahr, Food allergies: The basics, Gastroenterology. 148 (2015) 1120-1131.e4. [CrossRef]
- L. Tordesillas, M.C. Berin, H.A. Sampson, Immunology of Food Allergy, Immunity. 47 (2017) 32–50. [CrossRef]
- F.J. Salgado, J.J. Nieto-Fontarigo, F.J. González-Barcala, Proteomic analysis of food allergens, in: Food Proteomics, 2022: pp. 225–300.
- F. Zhou, S. He, H. Sun, Y. Wang, Y. Zhang, Advances in epitope mapping technologies for food protein allergens: A review, Trends Food Sci. Technol. 107 (2021) 226–239. [CrossRef]
- R.D.J. Huby, R.J. Dearman, I. Kimber, Why are some proteins allergens?, Toxicol. Sci. 55 (2000) 235–246. [CrossRef]
- L. Monaci, R. Pilolli, E. De Angelis, J.F. Crespo, N. Novak, B. Cabanillas, Food allergens: Classification, molecular properties, characterization, and detection in food sources, Adv. Food Nutr. Res. 93 (2020) 113–146. [CrossRef]
- D. Raheem, A. Raposo, O.B. Oluwole, M. Nieuwland, A. Saraiva, C. Carrascosa, Entomophagy: Nutritional, ecological, safety and legislation aspects, Food Res. Int. 126 (2019) 108672. [CrossRef]
- L. Yang, R. Zhu, Allergen Preparation in AIT, Now and in the Future, Curr. Treat. Options Allergy. 8 (2021) 120–132. [CrossRef]
- C. Villa, J. Costa, M.B.P.P. Oliveira, I. Mafra, Bovine Milk Allergens: A Comprehensive Review, Compr. Rev. Food Sci. Food Saf. 17 (2018) 137–164. [CrossRef]
- D.W. Dona, C. Suphioglu, Egg allergy: Diagnosis and immunotherapy, Int. J. Mol. Sci. 21 (2020) 1–35. [CrossRef]
- M.F. Sharp, A.L. Lopata, Fish allergy: In review, Clin. Rev. Allergy Immunol. 46 (2014) 258–271. [CrossRef]
- X. Dong, V. Raghavan, A comprehensive overview of emerging processing techniques and detection methods for seafood allergens, Compr. Rev. Food Sci. Food Saf. 21 (2022) 3540–3557. [CrossRef]
- P. Ozias-Akins, H. Breiteneder, The functional biology of peanut allergens and possible links to their allergenicity, Allergy Eur. J. Allergy Clin. Immunol. 74 (2019) 888–898. [CrossRef]
- M. Lu, Y. Jin, R. Cerny, B. Ballmer-Weber, R.E. Goodman, Combining 2-DE immunoblots and mass spectrometry to identify putative soybean (Glycine max) allergens, Food Chem. Toxicol. 116 (2018) 207–215. [CrossRef]
- S. Quirce, T. Boyano-Martínez, A. Diáz-Perales, Clinical presentation, allergens, and management of wheat allergy, Expert Rev. Clin. Immunol. 12 (2016) 563–572. [CrossRef]
- C. Villa, J. Costa, I. Mafra, Sesame as a source of food allergens: clinical relevance, molecular characterization, cross-reactivity, stability toward processing and detection strategies, Crit. Rev. Food Sci. Nutr. 0 (2022) 1–17. [CrossRef]
- L.H. Fasolin, R.N. Pereira, A.C. Pinheiro, J.T. Martins, C.C.P. Andrade, O.L. Ramos, A.A. Vicente, Emergent food proteins – Towards sustainability, health and innovation, Food Res. Int. 125 (2019) 108586. [CrossRef]
- J. Costa, S.L. Bavaro, S. Benedé, A. Diaz-Perales, C. Bueno-Diaz, E. Gelencser, J. Klueber, C. Larré, D. Lozano-Ojalvo, R. Lupi, I. Mafra, G. Mazzucchelli, E. Molina, L. Monaci, L. Martín-Pedraza, C. Piras, P.M. Rodrigues, P. Roncada, D. Schrama, T. Cirkovic-Velickovic, K. Verhoeckx, C. Villa, A. Kuehn, K. Hoffmann-Sommergruber, T. Holzhauser, Are Physicochemical Properties Shaping the Allergenic Potency of Plant Allergens?, Clin. Rev. Allergy Immunol. 62 (2022) 37–63. [CrossRef]
- B.A. Albunni, H. Wessels, A. Paschke-Kratzin, M. Fischer, Antibody Cross-Reactivity between Proteins of Chia Seed (Salvia hispanica L.) and Other Food Allergens, J. Agric. Food Chem. 67 (2019) 7475–7484. [CrossRef]
- W. Xiong, M.A. McFarland, C. Pirone, C.H. Parker, Selection of tree nut allergen peptide markers: A need for improved protein sequence databases, J. AOAC Int. 102 (2019) 1263–1270. [CrossRef]
- J. Costa, C. Villa, K. Verhoeckx, T. Cirkovic-Velickovic, D. Schrama, P. Roncada, P.M. Rodrigues, C. Piras, L. Martín-Pedraza, L. Monaci, E. Molina, G. Mazzucchelli, I. Mafra, R. Lupi, D. Lozano-Ojalvo, C. Larré, J. Klueber, E. Gelencser, C. Bueno-Diaz, A. Diaz-Perales, S. Benedé, S.L. Bavaro, A. Kuehn, K. Hoffmann-Sommergruber, T. Holzhauser, Are Physicochemical Properties Shaping the Allergenic Potency of Animal Allergens?, Clin. Rev. Allergy Immunol. 62 (2021) 1–36.
- S. de Gier, K. Verhoeckx, Insect (food) allergy and allergens, Mol. Immunol. 100 (2018) 82–106. [CrossRef]
- C. Srinroch, C. Srisomsap, D. Chokchaichamnankit, P. Punyarit, P. Phiriyangkul, Identification of novel allergen in edible insect, Gryllus bimaculatus and its cross-reactivity with Macrobrachium spp. allergens, Food Chem. 184 (2015) 160–166. [CrossRef]
- H. Broekman, K.C. Verhoeckx, C.F. Den Hartog Jager, A.G. Kruizinga, M. Pronk-Kleinjan, B.C. Remington, C.A. Bruijnzeel-Koomen, G.F. Houben, A.C. Knulst, Majority of shrimp-allergic patients are allergic to mealworm, J. Allergy Clin. Immunol. 137 (2016) 1261–1263. [CrossRef]
- F. Francis, V. Doyen, F. Debaugnies, G. Mazzucchelli, R. Caparros, T. Alabi, C. Blecker, E. Haubruge, F. Corazza, Limited cross reactivity among arginine kinase allergens from mealworm and cricket edible insects, Food Chem. 276 (2019) 714–718. [CrossRef]
- C. Garino, H. Mielke, S. Knüppel, T. Selhorst, H. Broll, A. Braeuning, Quantitative allergenicity risk assessment of food products containing yellow mealworm (Tenebrio molitor), Food Chem. Toxicol. 142 (2020) 111460. [CrossRef]
- T.R. Murefu, L. Macheka, R. Musundire, F.A. Manditsera, Safety of wild harvested and reared edible insects: A review, Food Control. 101 (2019) 209–224. [CrossRef]
- I. Pali-Schöll, P. Meinlschmidt, D. Larenas-Linnemann, B. Purschke, G. Hofstetter, F.A. Rodríguez-Monroy, L. Einhorn, N. Mothes-Luksch, E. Jensen-Jarolim, H. Jäger, Edible insects: Cross-recognition of IgE from crustacean- and house dust mite allergic patients, and reduction of allergenicity by food processing, World Allergy Organ. J. 12 (2019). [CrossRef]
- F. Hall, P.E. Johnson, A. Liceaga, Effect of enzymatic hydrolysis on bioactive properties and allergenicity of cricket (Gryllodes sigillatus) protein, Food Chem. 262 (2018) 39–47. [CrossRef]
- S. Cardoso Alves, E. Díaz-Ruiz, B. Lisboa, M. Sharma, S.I. Mussatto, V.K. Thakur, D.M. Kalaskar, V.K. Gupta, A.K. Chandel, Microbial meat: A sustainable vegan protein source produced from agri-waste to feed the world, Food Res. Int. 166 (2023) 112596. [CrossRef]
- T.M. Le, A.C. Knulst, H. Röckmann, Anaphylaxis to Spirulina confirmed by skin prick test with ingredients of Spirulina tablets, Food Chem. Toxicol. 74 (2014) 309–310. [CrossRef]
- M. Petrus, R. Culerrier, M. Campistron, A. Barre, P. Rougé, First case report of anaphylaxis to spirulin: Identification of phycocyanin as responsible allergen, Allergy Eur. J. Allergy Clin. Immunol. 65 (2010) 924–925. [CrossRef]
- H.E. Yim, K.H. Yoo, W.H. Seo, N.H. Won, Y.S. Hong, J.W. Lee, Acute tubulointerstitial nephritis following ingestion of Chlorella tablets, Pediatr. Nephrol. 22 (2007) 887–888. [CrossRef]
- M. Bianco, G. Ventura, C.D. Calvano, I. Losito, T.R.I. Cataldi, A new paradigm to search for allergenic proteins in novel foods by integrating proteomics analysis and in silico sequence homology prediction: Focus on spirulina and chlorella microalgae, Talanta. 240 (2022) 123188. [CrossRef]
- O. Schlüter, B. Rumpold, T. Holzhauser, A. Roth, R.F. Vogel, W. Quasigroch, S. Vogel, V. Heinz, H. Jäger, N. Bandick, S. Kulling, D. Knorr, P. Steinberg, K.H. Engel, Safety aspects of the production of foods and food ingredients from insects, Mol. Nutr. Food Res. 61 (2017) 1–14. [CrossRef]
- J.C. Ribeiro, L.M. Cunha, B. Sousa-Pinto, J. Fonseca, Allergic risks of consuming edible insects: A systematic review, Mol. Nutr. Food Res. 62 (2018) 1–12. [CrossRef]
- S.K. Vanga, M. Jain, V. Raghavan, Significance of fruit and vegetable allergens: Possibilities of its reduction through processing, Food Rev. Int. 34 (2018) 103–125. [CrossRef]
- X. Dong, J. Wang, V. Raghavan, Critical reviews and recent advances of novel non-thermal processing techniques on the modification of food allergens, Crit. Rev. Food Sci. Nutr. 62 (2020) 196–210.
- J. Wang, S.K. Vanga, C. McCusker, V. Raghavan, A Comprehensive Review on Kiwifruit Allergy: Pathogenesis, Diagnosis, Management, and Potential Modification of Allergens Through Processing, Compr. Rev. Food Sci. Food Saf. 18 (2019) 500–513. [CrossRef]
- P. Suriyamoorthy, A. Madhuri, S. Tangirala, K.R. Michael, V. Sivanandham, A. Rawson, A. Anandharaj, Comprehensive Review on Banana Fruit Allergy: Pathogenesis, Diagnosis, Management, and Potential Modification of Allergens through Food Processing, Plant Foods Hum. Nutr. 77 (2022) 159–171. [CrossRef]
- M. López-Pedrouso, J.M. Lorenzo, M. Gagaoua, D. Franco, Current trends in proteomic advances for food allergen analysis, Biology (Basel). 9 (2020) 1–13. [CrossRef]
- M. Koeberl, M.F. Sharp, R. Tian, S. Buddhadasa, D. Clarke, J. Roberts, Lupine allergen detecting capability and cross-reactivity of related legumes by ELISA, Food Chem. 256 (2018) 105–112. [CrossRef]
- T. Ruethers, A.C. Taki, J. Khangurha, J. Roberts, S. Buddhadasa, D. Clarke, C.E. Hedges, D.E. Campbell, S.D. Kamath, A.L. Lopata, M. Koeberl, Commercial fish ELISA kits have a limited capacity to detect different fish species and their products, J. Sci. Food Agric. 100 (2020) 4353–4363. [CrossRef]
- G. D’Auria, C. Nitride, M.A. Nicolai, G. Mamone, D. Montesano, E.N.C. Mills, P. Ferranti, Identification of allergen encoding sequences in a novel food ingredient from Moringa oleifera leaves, Food Chem. 401 (2023) 134185. [CrossRef]
- K.Y. Jeong, J.S. Lee, J.E. Yuk, H. Song, H.J. Lee, K.J. Kim, B.J. Kim, K.J. Lim, K.H. Park, J.H. Lee, J.W. Park, Allergenic characterization of Bomb m 4, a 30-kDa Bombyx mori lipoprotein 6 from silkworm pupa, Clin. Exp. Allergy. 52 (2022) 888–897. [CrossRef]
- Y. Tao, S. Yin, L. Fu, M. Wang, L. Meng, F. Li, X. Xue, L. Wu, Q. Li, Identification of allergens and allergen hydrolysates by proteomics and metabolomics: A comparative study of natural and enzymolytic bee pollen, Food Res. Int. 158 (2022) 111572. [CrossRef]
- T.L. Bai, X.Y. Han, M.S. Li, Y. Yang, M. Liu, N.R. Ji, C.C. Yu, D. Lai, M.J. Cao, G.M. Liu, Effects of the Maillard reaction on the epitopes and immunoreactivity of tropomyosin, a major allergen in: Chlamys nobilis, Food Funct. 12 (2021) 5096–5108. [CrossRef]
- J. Zhou, Q. Qi, C. Wang, Y. Qian, G. Liu, Y. Wang, L. Fu, Surface plasmon resonance (SPR) biosensors for food allergen detection in food matrices, Biosens. Bioelectron. 142 (2019) 111449. [CrossRef]
- J. Zimmermann, P. Hubel, J. Pfannstiel, M. Afzal, C.F.H. Longin, B. Hitzmann, H. Götz, S.C. Bischoff, Comprehensive proteome analysis of bread deciphering the allergenic potential of bread wheat, spelt and rye, J. Proteomics. 247 (2021) 104318. [CrossRef]
- S. Chen, M.L. Downs, Proteomic Analysis of Oil-Roasted Cashews Using a Customized Allergen-Focused Protein Database, J. Proteome Res. 21 (2022) 1694–1706.
- S. Teixeira, I.M. Luís, M.M. Oliveira, I.A. Abreu, R. Batista, Goji berries superfood–contributions for the characterisation of proteome and IgE-binding proteins, Food Agric. Immunol. 30 (2019) 262–280. [CrossRef]
- J. Rost, S. Muralidharan, N.A. Lee, A label-free shotgun proteomics analysis of macadamia nut, Food Res. Int. 129 (2020) 108838. [CrossRef]
- M. Yakhlef, I. Giangrieco, M.A. Ciardiello, I. Fiume, A. Mari, L. Souiki, G. Pocsfalvi, Potential allergenicity of Medicago sativa investigated by a combined IgE-binding inhibition, proteomics and in silico approach, J. Sci. Food Agric. 101 (2021) 1182–1192. [CrossRef]
- N. Shaheen, O. Halima, K.T. Akhter, N. Nuzhat, R.S.P. Rao, R.S. Wilson, N. Ahsan, Proteomic characterization of low molecular weight allergens and putative allergen proteins in lentil (Lens culinaris) cultivars of Bangladesh, Food Chem. 297 (2019) 124936. [CrossRef]
- M. Hao, Xijiri, Z. Zhao, H. Che, Identification of Allergens in White-and Red-Fleshed Pitaya (Selenicereus undatus and Selenicereus costaricensis) Seeds Using Bottom-Up Proteomics Coupled with Immunoinformatics, Nutrients. 14 (2022). [CrossRef]
- M.S. Varunjikar, I. Belghit, J. Gjerde, M. Palmblad, E. Oveland, J.D. Rasinger, Shotgun proteomics approaches for authentication, biological analyses, and allergen detection in feed and food-grade insect species, Food Control. 137 (2022) 108888. [CrossRef]
- F.G. Hall, A.M. Liceaga, Isolation and proteomic characterization of tropomyosin extracted from edible insect protein, Food Chem. Mol. Sci. 3 (2021) 100049. [CrossRef]
- U. Bose, J.A. Broadbent, A. Juhász, S. Karnaneedi, E.B. Johnston, S. Stockwell, K. Byrne, V. Limviphuvadh, S. Maurer-Stroh, A.L. Lopata, M.L. Colgrave, Protein extraction protocols for optimal proteome measurement and arginine kinase quantitation from cricket Acheta domesticus for food safety assessment, Food Chem. 348 (2021). [CrossRef]
- G. Leni, T. Tedeschi, A. Faccini, F. Pratesi, C. Folli, I. Puxeddu, P. Migliorini, N. Gianotten, J. Jacobs, S. Depraetere, A. Caligiani, S. Sforza, Shotgun proteomics, in-silico evaluation and immunoblotting assays for allergenicity assessment of lesser mealworm, black soldier fly and their protein hydrolysates, Sci. Rep. 10 (2020) 1–10. [CrossRef]
- M. Kochanowski, J. Dąbrowska, M. Różycki, J. Karamon, J. Sroka, T. Cencek, Proteomic Profiling Reveals New Insights into the Allergomes of Anisakis simplex, Pseudoterranova decipiens, and Contracaecum osculatum, J. Parasitol. 106 (2020) 572–588. [CrossRef]
- I. Pali-Schöll, K. Verhoeckx, I. Mafra, S.L. Bavaro, E.N. Clare Mills, L. Monaci, Allergenic and novel food proteins: State of the art and challenges in the allergenicity assessment, Trends Food Sci. Technol. 84 (2019) 45–48. [CrossRef]
- A. Pomés, J.M. Davies, G. Gadermaier, C. Hilger, T. Holzhauser, J. Lidholm, A.L. Lopata, G.A. Mueller, A. Nandy, C. Radauer, S.K. Chan, U. Jappe, J. Kleine-Tebbe, W.R. Thomas, M.D. Chapman, M. van Hage, R. van Ree, S. Vieths, M. Raulf, R.E. Goodman, WHO/IUIS Allergen Nomenclature: Providing a common language, Mol. Immunol. 100 (2018) 3–13. [CrossRef]
- K. Kadam, R. Karbhal, V.K. Jayaraman, S. Sawant, U. Kulkarni-Kale, AllerBase: a comprehensive allergen knowledgebase, Database (Oxford). 2017 (2017) 1–12. [CrossRef]
- S. Maurer-Stroh, N.L. Krutz, P.S. Kern, V. Gunalan, M.N. Nguyen, V. Limviphuvadh, F. Eisenhaber, G. Frank Gerberick, AllerCatPro-prediction of protein allergenicity potential from the protein sequence, Bioinformatics. 35 (2019) 3020–3027. [CrossRef]
- R.E. Goodman, M. Ebisawa, F. Ferreira, H.A. Sampson, R. van Ree, S. Vieths, J.L. Baumert, B. Bohle, S. Lalithambika, J. Wise, S.L. Taylor, AllergenOnline: A peer-reviewed, curated allergen database to assess novel food proteins for potential cross-reactivity, Mol. Nutr. Food Res. 60 (2016) 1183–1198. [CrossRef]
- A. Mari, E. Scala, P. Palazzo, S. Ridolfi, D. Zennaro, G. Carabella, Bioinformatics applied to allergy: Allergen databases, from collecting sequence information to data integration. The Allergome platform as a model, Cell. Immunol. 244 (2006) 97–100. [CrossRef]
- R. Van Ree, D. Sapiter Ballerda, M.C. Berin, L. Beuf, A. Chang, G. Gadermaier, P.A. Guevera, K. Hoffmann-Sommergruber, E. Islamovic, L. Koski, J. Kough, G.S. Ladics, S. McClain, K.A. McKillop, S. Mitchell-Ryan, C.A. Narrod, L. Pereira Mouriès, S. Pettit, L.K. Poulsen, A. Silvanovich, P. Song, S.S. Teuber, C. Bowman, The COMPARE Database: A Public Resource for Allergen Identification, Adapted for Continuous Improvement, Front. Allergy. 2 (2021) 1–13. [CrossRef]
- C. Radauer, M. Bublin, S. Wagner, A. Mari, H. Breiteneder, Allergens are distributed into few protein families and possess a restricted number of biochemical functions, J. Allergy Clin. Immunol. 121 (2008). [CrossRef]
- B. Peters, J. Sidney, P. Bourne, H.H. Bui, S. Buus, G. Doh, W. Fleri, M. Kronenberg, R. Kubo, O. Lund, D. Nemazee, J. V. Ponomarenko, M. Sathiamurthy, S. Schoenberger, S. Stewart, P. Surko, S. Way, S. Wilson, A. Sette, The immune epitope database and analysis resource: From vision to blueprint, PLoS Biol. 3 (2005) 0379–0381. [CrossRef]
- O. Ivanciuc, C.H. Schein, W. Braun, SDAP: Database and computational tools for allergenic proteins, Nucleic Acids Res. 31 (2003) 359–362. [CrossRef]
- L. Monaci, E. De Angelis, N. Montemurro, R. Pilolli, Comprehensive overview and recent advances in proteomics MS based methods for food allergens analysis, TrAC - Trends Anal. Chem. 106 (2018) 21–36. [CrossRef]
- V. Marzano, B. Tilocca, A.G. Fiocchi, P. Vernocchi, S. Levi Mortera, A. Urbani, P. Roncada, L. Putignani, Perusal of food allergens analysis by mass spectrometry-based proteomics, J. Proteomics. 215 (2020) 103636. [CrossRef]
- R.C. Aalberse, Structural biology of allergens, J. Allergy Clin. Immunol. 106 (2000) 228–238. [CrossRef]
- M. Abdelmoteleb, C. Zhang, B. Furey, M. Kozubal, H. Griffiths, M. Champeaud, R.E. Goodman, Evaluating potential risks of food allergy of novel food sources based on comparison of proteins predicted from genomes and compared to www.AllergenOnline.org, Food Chem. Toxicol. 147 (2021) 111888. [CrossRef]
- H. Bagdonas, C.A. Fogarty, E. Fadda, J. Agirre, The case for post-predictional modifications in the AlphaFold Protein Structure Database, Nat. Struct. Mol. Biol. 28 (2021) 869–870. [CrossRef]
- W. Kang, J. Zhang, H. Li, N. Yu, R. Tang, X. Sun, L. Wei, J. Sun, Y. Chen, Quantification of major allergens in peach based on shotgun proteomics using liquid chromatography-tandem mass spectrometry, Lwt. 160 (2022) 113234. [CrossRef]
- S. Chen, C. Yang, M. Downs, Targeted mass spectrometry quantification of total soy protein residues from commercially processed ingredients for food allergen management, J. Proteomics. 239 (2021) 104194. [CrossRef]
- M. Bianco, C.D. Calvano, G. Ventura, I. Losito, T.R.I. Cataldi, Determination of hidden milk allergens in meat-based foodstuffs by liquid chromatography coupled to electrospray ionization and high-resolution tandem mass spectrometry, Food Control. 131 (2022) 108443. [CrossRef]
| Food | Protein name | Specie | Allergen | Ref. |
|---|---|---|---|---|
| Milk |
Caseins α S1-casein (23.6 kDa) α S2-casein (25.2 kDa) β -casein (24 kDa) κ-casein (19 kDa) β-lactoglobulin (18.3 kDa) α-lactalbumin (14.2 kDa) Serum albumin (66.3 kDa) Immunoglobulin (160 kDa) |
Bos taurus |
Bos d 9 Bos d 10 Bos d 11 Bos d 12 Bos d 5 Bos d 4 Bos d 6 Bos d 7 |
[12] |
| Eggs |
Ovomucoid (28 kDa) Ovalbumin (44 kDa) Ovotransferrin (78 kDa) Lysozyme (14 kDa) α-livetin (69 kDa) YGP42 (35 kDa) |
Gallus domesticus | Gal d 1 Gal d 2 Gal d 3 Gal d 3 Gal d 5 Gal d 6 |
[13] |
| Fish |
Parvalbumin α-parvalbumin (13 kDa) β-parvalbumin (11.6 kDa) |
Gadius callarias (Baltic cod) |
Gad p 2 Gad p 1 |
[14] |
| Shellfish |
Tropomyosin (34 kDa) | Metapenaeus ensis (Shrimp) | Met e 1 | [15] |
| Peanuts /tree nuts | 7S seed storage globulin, vicilins (64 kDa) 2S albumin (17 kDa) Nonspecific lipid transfer proteins Oleosins Defensins Profilins Plant pathogenesis-related proteins PR-10 |
Arachis hypogaea | Ara h 1 Ara h 2, Ara h 6, Ara h 7 Ara h 9, Ara h 16, Ara h 17 Ara h 10, Ara h 11, Ara h 14, Ara h 15 Ara h 12, Ara h 13 Ara h 5 Ara h 8 |
[16] |
| Soy | 7S seed storage globulin, β-conglycinin 11S seed storage globulin, glycinin |
Glycine max | Gly m 5 Gly m 6 |
[17] |
| Wheat |
α-amylase inhibitor (13 kDa) Gamma gliadin (88 kDa) Elongation factor 1 |
Triticum aestivum | Tri a 28 Tri a 20 Tri a 45 |
[18] |
| Sesame | 2S albumins 7S vicilin-type globulin (45 kDa) Oleosins 11S globulin, legumins Profilin |
Sesamum indicum | Ses i 1, Ses i 2 Ses i 3. Ses i 4, Ses i 5 Ses i 6, Ses i 7 Ses i 8 |
[19] |
| Novel Food | Bioinformatic tool | Goal/main achievements | Ref. |
|---|---|---|---|
| Vegetables | |||
| Bread wheat spelt and rye | Database of Allergen Families-AllFam AllergenOnline Allergome |
Comparison of allergenicity in cereal products | [52] |
| Cashews | BLASTP Search against AllergenOnline sequence | Analysis of allergen stability under heat treatment | [53] |
| Goji berries | AlgPred software hybrid approach | Identification of 11 IgE-binding proteins | [54] |
| Macadamia nut | AllergenOnline Immune Epitope Database Analysis Resource (IEDB) |
Analysis of homology and linear epitope similarities to known allergens | [55] |
| Medicago sativa | COMPARE allergen database | Identification of three allergenic protein families | [56] |
| Lentil (Lens culinaris) | Blast2GO - Functional Annotation and Genomics | Quantification of major allergen proteins | [57] |
| White- and red-fleshed pitaya seeds | AllermatchTM webtool Algpred 2.0 AllerCatPro web server |
Identification of five potential allergens | [58] |
| Seaweeds | |||
| Spirulina and chlorella microalgae | AllergenOnline | Six proteins exhibit significant homology with food allergens | [37] |
| Insects | |||
| Black soldier fly, yellow mealworm, lesser mealworm, house cricket and Morio Worms | Allergen nomenclature (WHO/IUIS) |
Detection of arginine kinase and tropomyosin | [59] |
| Cricket | Allermatch TM webtool AlgPred 2.0 ABCPred Bepipred |
Description of the impact of processing on allergenic reactivity of insect proteins. | [60] |
| Cricket Acheta domesticus | Database of Allergen Families-AllFam Allergen nomenclatura (WHO/IUIS) CLC Genomics Workbench 20.0.4. AllerCatPro web server |
Identification of 20 putative allergens | [61] |
| Lesser mealworms, black soldier flies and their protein hydrolysate | AllermatchTM webtool | Identification of potential allergens by similarity to known allergens | [62] |
| Parasites | |||
| Anisakis simplex, Pseudoterranova decipiens, and Contracaecum osculatum | Blast2GO - Functional Annotation and Genomics AllergenOnline AllerTOP web server ver. 2.0 PREAL web server |
Prediction of 53 probable allergens in three species | [63] |
| Name | Link (Website) | Description | Ref. |
|---|---|---|---|
| Allergen nomenclature | http://www.allergen.org | Official site for the systematic allergen nomenclature provided by the World Health Organization and International Union of Immunological Societies (WHO/IUIS) | [65] |
| AllerBase | http://bioinfo.unipune.ac.in/AllerBase/Home.html | Database of allergens detected as IgE-binding epitopes, IgE antibodies and cross-reactivity. Allergen data such as experimental information on its allergenic activity and food source is compiled, resulting in a curated database. | [66] |
| AllerCatPro | https://allercatpro.bii.a-star.edu.sg/ | Provides protein allergenicity potential prediction based on the similarity of amino acid sequence and 3D protein structure | [67] |
| AllergenOnline | http://www.allergenonline.org | Provides sequence database of allergens to identify proteins and assess the potential risk of allergenic cross-reactivity. This database offers 2233 peer-reviewed sequences from 912 taxonomic protein groups (February 2021) | [68] |
| Allergome | http://www.allergome.org | A website with detailed information on Allergenic Molecules (Allergens) causing an IgE-mediated (allergic, atopic) disease (anaphylaxis, asthma, atopic dermatitis, conjunctivitis, rhinitis, urticaria). | [69] |
| Comprehensive protein allergen resource (COMPARE allergen database) | https://comparedatabase.org/ | A database comprised of protein sequences of known allergens | [70] |
| Database of Allergen Families-AllFam | http://www.meduniwien.ac.at/allfam/ | Comprises a resource for classifying allergens into protein families as well as biochemical properties and allergology significance | [71] |
| Immune Epitope Database and analysis resource (IEDB) | https://www.iedb.org | Provides experimental data on antibody and T cell epitopes to identify allergens and to assist in the prediction and analysis of allergenicity | [72] |
| Structural Database of Allergenic Proteins (SDAP) | https://fermi.utmb.edu | Tool for testing the FAO/WHO allergenicity rules in new proteins and investigating cross-reactivity, also offering information about protein sequence and structure | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





