Submitted:
12 May 2023
Posted:
19 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Electroretinogram
1.2. Analysis of the electroretinogram waveform
2. Materials and Methods
2.1. Study protocol and signals
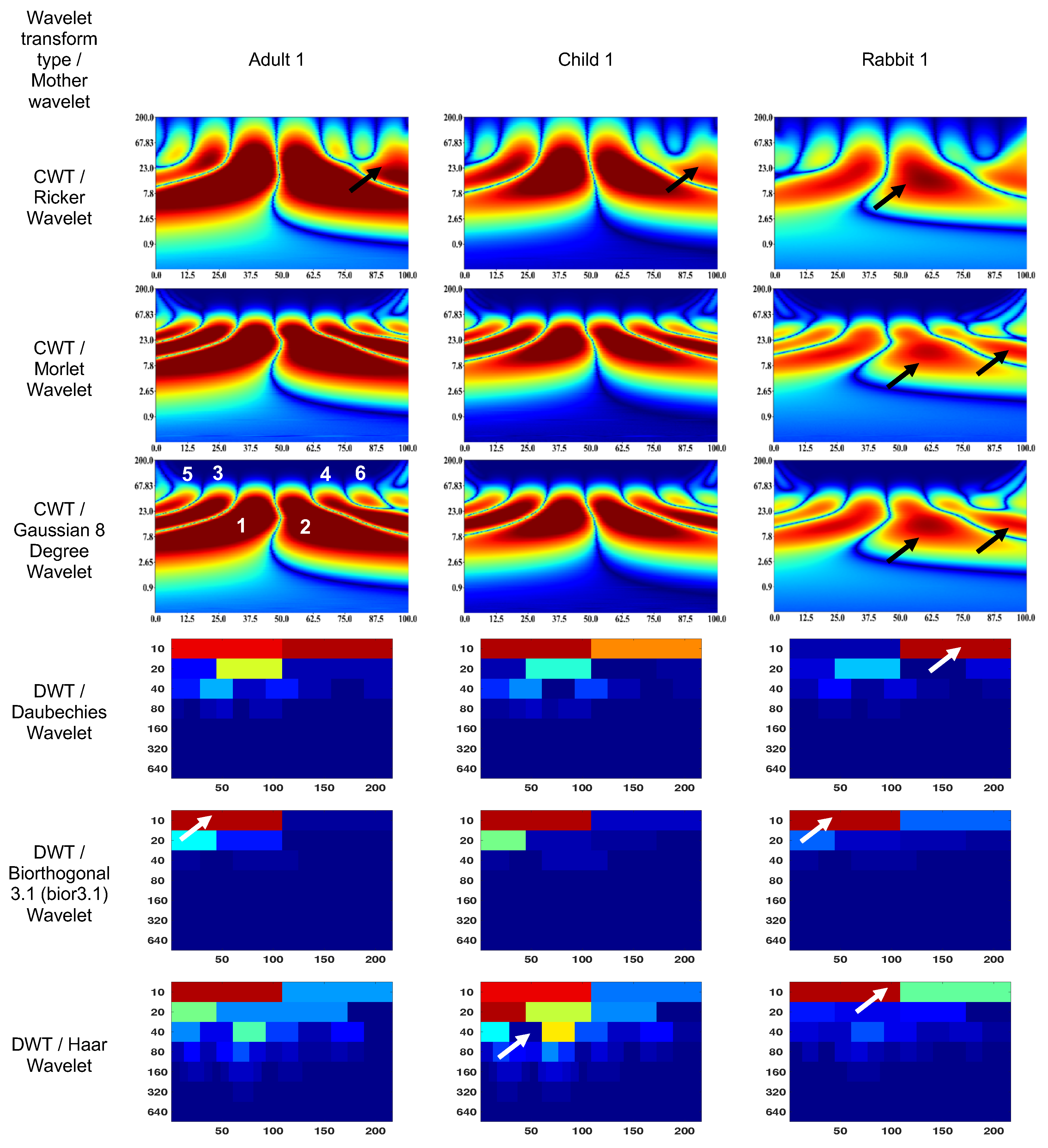
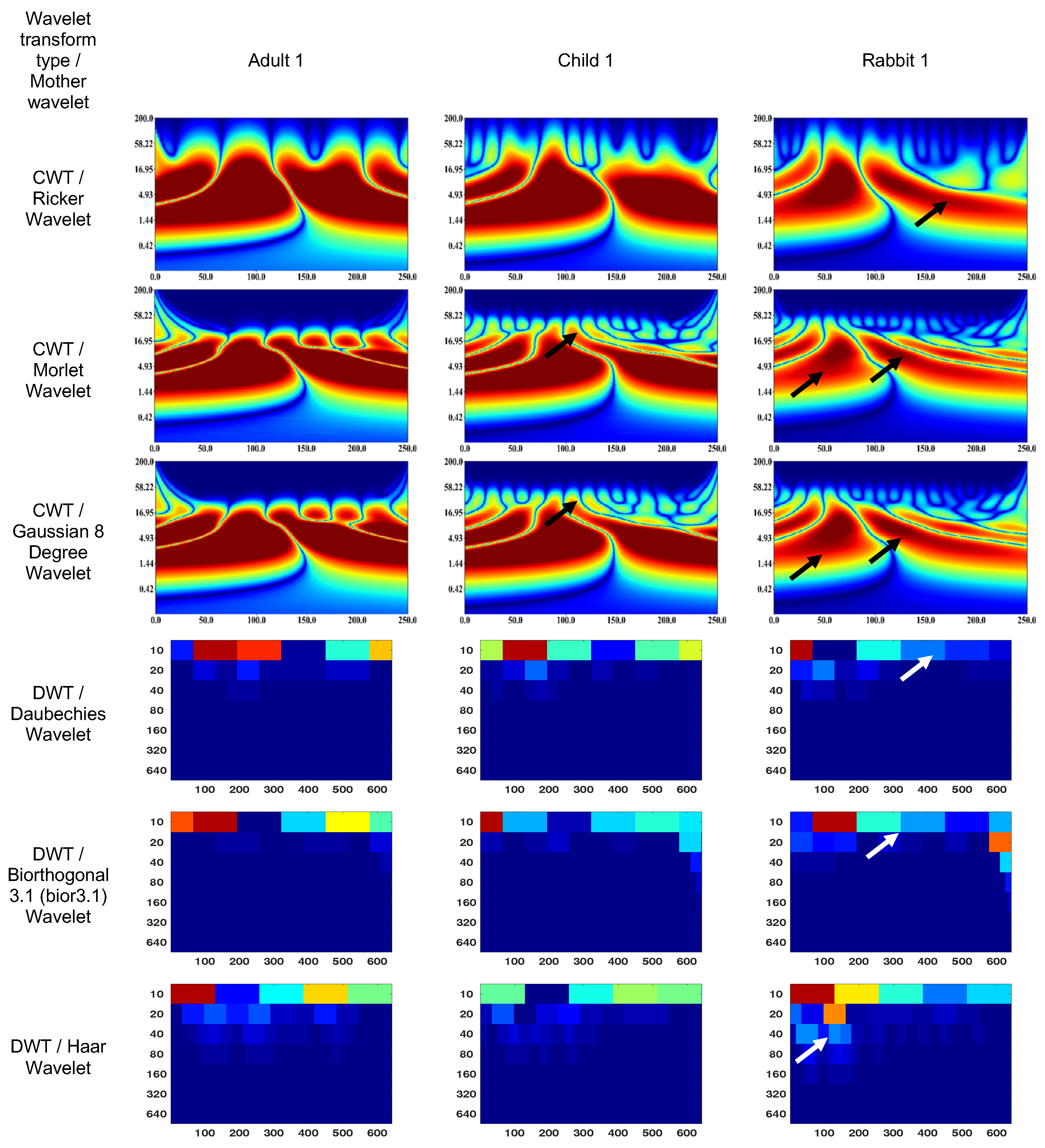
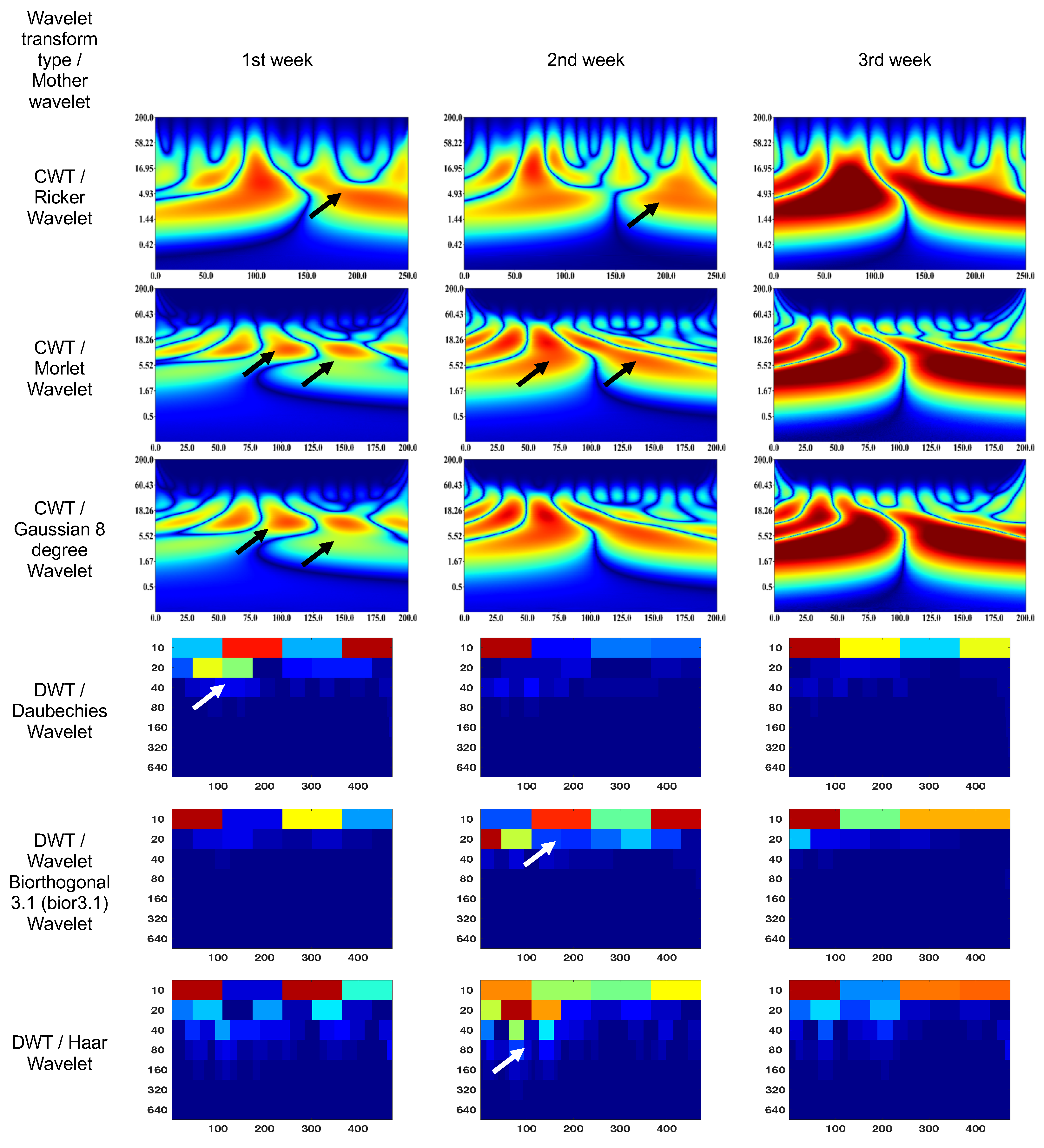
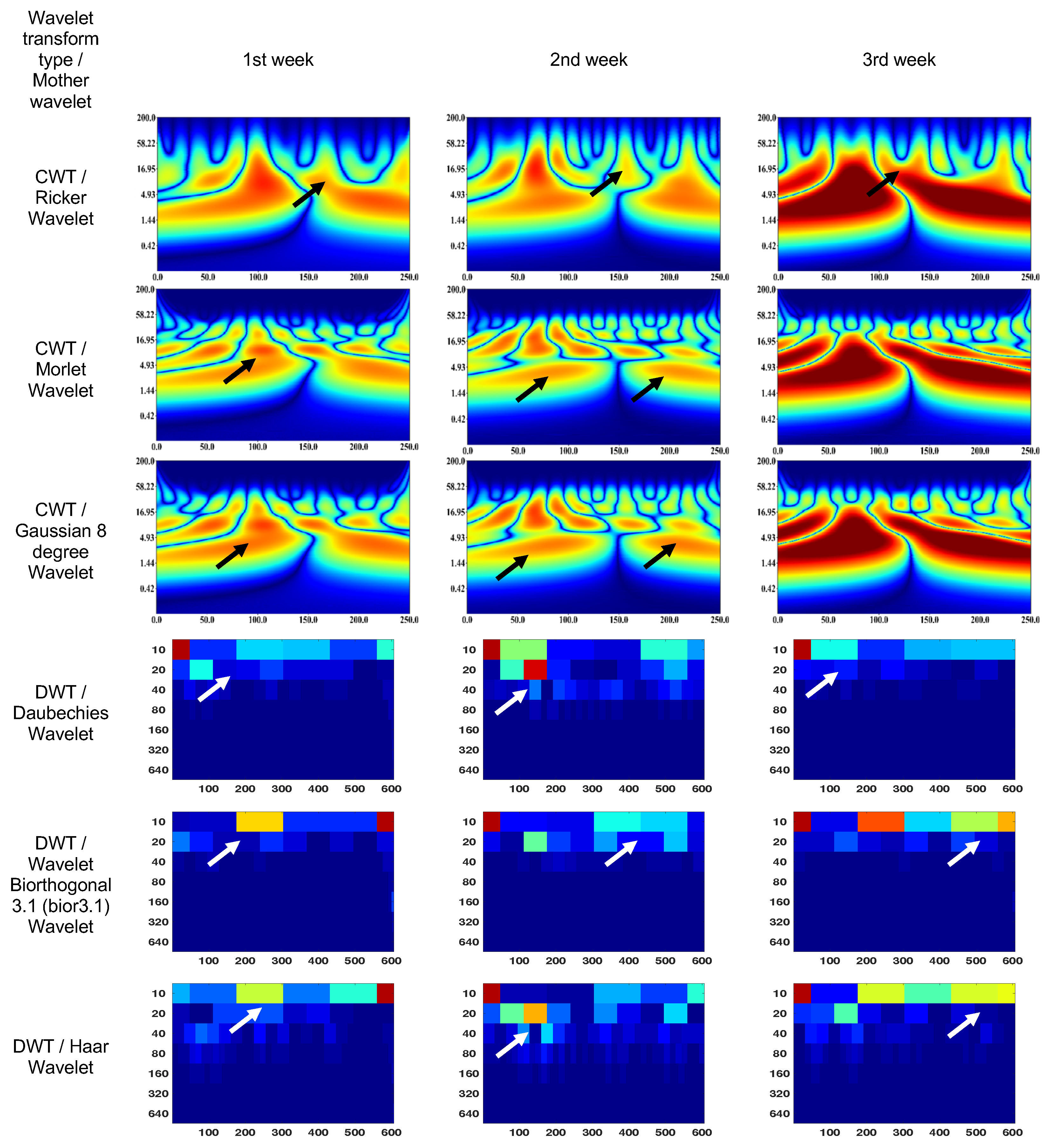
- Daubechies Wavelet
- Ricker Wavelet
- Wavelet Biorthogonal 3.1 (bior3.1)
- Morlet Wavelet
- Haar Wavelet
- Gaussian Wavelet
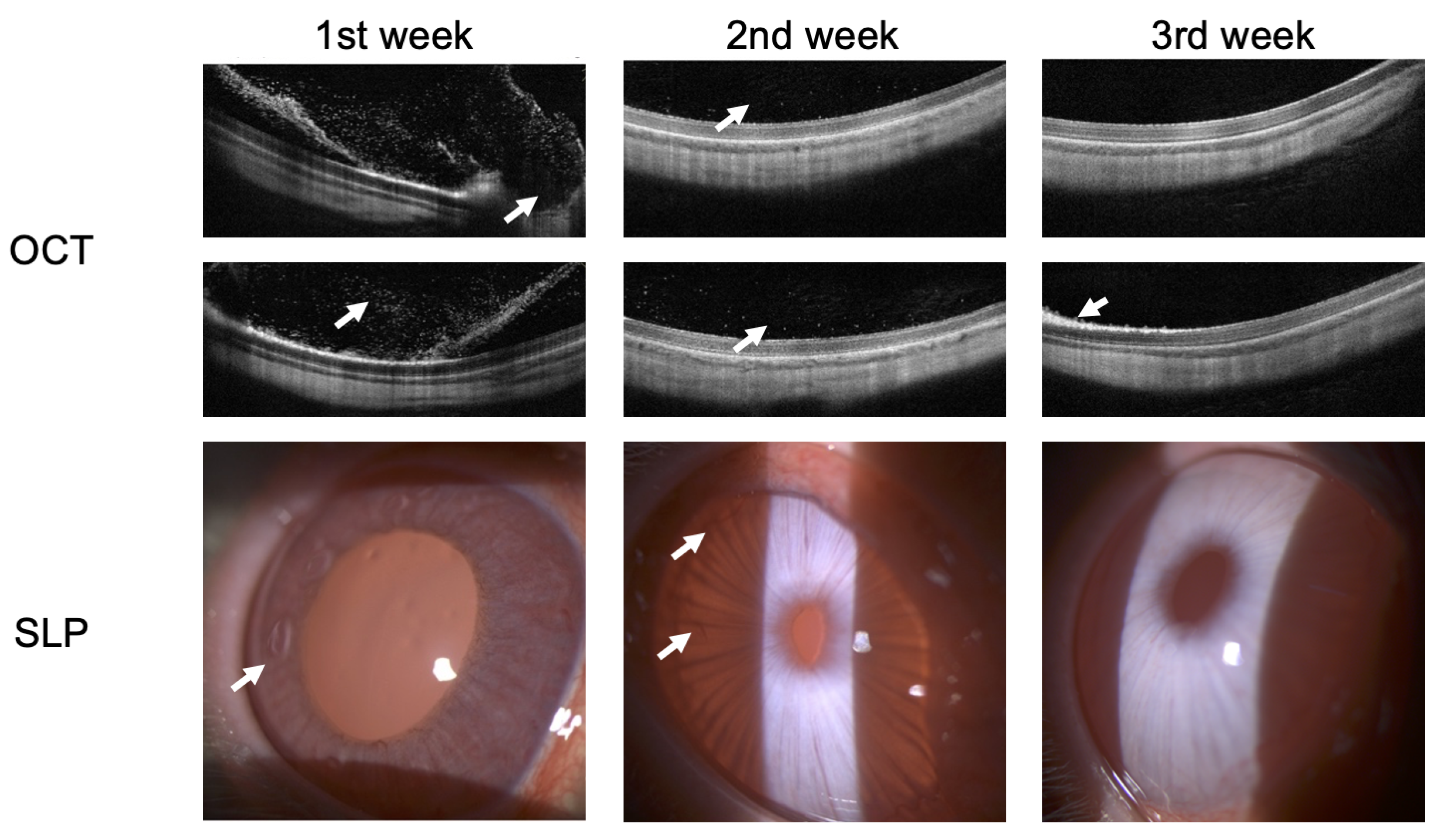
2.2. Endophthalmitis treatment in rabbit
3. Results
3.1. Wavelet scalograms of human and rabbit ERGs signals
3.2. Endophthalmitis treatment in rabbit
4. Discussion
5. Conclusions
5.1. Human and rabbit ERG signals
5.2. Endophthalmitis treatment in rabbit
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LA | Light Adapted |
| OPs | Oscillatory Potentials |
| DWT | Discrete Wavelet Transform |
| CWT | Continuous Wavelet Transform |
| SLP | Slit-lamp Photography |
| OCT | Optical Coherence Tomography |
References
- Robson, A.G., Frishman, L.J., Grigg, J., Hamilton, R., Jeffrey, B.G., Kondo, M., et al. (2022). ISCEV Standard for full-field clinical electroretinography (2022 update). Doc Ophthalmol 144(3), 165-177. [CrossRef]
- Robson, A.G., Nilsson, J., Li, S., Jalali, S., Fulton, A.B., Tormene, A.P., et al. (2018). ISCEV guide to visual electrodiagnostic procedures. Doc Ophthalmol 136(1), 1-26. [CrossRef]
- Granit, R. (1950). Physiology of Vision. Annu Rev Physiol 12, 485-502. [CrossRef]
- Baylor, D.A., Lamb, T.D., and Yau, K.W. (1979). The membrane current of single rod outer segments. J Physiol 288, 589-611. [CrossRef]
- Robson, J.G., Saszik, S.M., Ahmed, J., and Frishman, L.J. (2003). Rod and cone contributions to the a-wave of the electroretinogram of the macaque. J Physiol 547(Pt 2), 509-530. [CrossRef]
- Robson, J.G., and Frishman, L.J. (2014). The rod-driven a-wave of the dark-adapted mammalian electroretinogram. Prog Retin Eye Res 39, 1-22. [CrossRef]
- Knapp, A.G., and Schiller, P.H. (1984). The contribution of on-bipolar cells to the electroretinogram of rabbits and monkeys. A study using 2-amino-4-phosphonobutyrate (APB). Vision Res 24(12), 1841-1846. 1841. [CrossRef]
- Heynen, H.; van Norren, D. Origin of the electroretinogram in the intact macaque eye–II. Current source-density analysis. Vision Res 1985, 25, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.A.; Feather, S.; Stanescu, H.C.; Freudenthal, B.; Zdebik, A.A.; Warth, R.; et al. Altered electroretinograms in patients with KCNJ10 mutations and EAST syndrome. J Physiol 2011, 589 Pt 7, 1681–1689. [Google Scholar] [CrossRef] [PubMed]
- Diamond, J.S. Inhibitory Interneurons in the Retina: Types, Circuitry, and Function. Annu Rev Vis Sci 2017, 3, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Barnes, S.; Grove, J.C.R.; McHugh, C.F.; Hirano, A.A.; Brecha, N.C. Horizontal Cell Feedback to Cone Photoreceptors in Mammalian Retina: Novel Insights From the GABA-pH Hybrid Model. Front Cell Neurosci 2020, 14, 595064. [Google Scholar] [CrossRef] [PubMed]
- Bush, R.A.; Sieving, P.A. A proximal retinal component in the primate photopic ERG a-wave. Investigative Ophthalmology & Visual Science 1994, 35, 635–645. [Google Scholar]
- Gouras, P.; MacKay, C. A new component in the a-wave of the human cone electroretinogram. Doc Ophthalmol 2000, 101, 19–24. [Google Scholar] [CrossRef]
- Dang, T.M.; Tsai, T.I.; Vingrys, A.J.; Bui, B.V. Post-receptoral contributions to the rat scotopic electroretinogram a-wave. Doc Ophthalmol 2011, 122, 149–156. [Google Scholar] [CrossRef]
- Diamond, J.S. Inhibitory Interneurons in the Retina: Types, Circuitry, and Function. Annu Rev Vis Sci 2017, 3, 1–24. [Google Scholar] [CrossRef]
- Hirano, A.A.; Vuong, H.E.; Kornmann, H.L.; Schietroma, C.; Stella, S.L., Jr.; Barnes, S.; Brecha, N.C. Vesicular Release of GABA by Mammalian Horizontal Cells Mediates Inhibitory Output to Photoreceptors. Front Cell Neurosci 2020, 14, 600777. [Google Scholar] [CrossRef]
- Viswanathan, S.; Frishman, L.J.; Robson, J.G.; Walters, J.W. The photopic negative response of the flash electroretinogram in primary open angle glaucoma. Invest Ophthalmol Vis Sci 2001, 42, 514–522. [Google Scholar]
- Wachtmeister, L.; Dowling, J.E. The oscillatory potentials of the mudpuppy retina. Invest Ophthalmol Vis Sci 1978, 17, 1176–1188. [Google Scholar]
- Wachtmeister, L. Further studies of the chemical sensitivity of the oscillatory potentials of the electroretinogram (ERG) I. GABA- and glycine antagonists. Acta Ophthalmol (Copenh) 1980, 58, 712–725. [Google Scholar] [CrossRef]
- Wachtmeister, L. Further studies of the chemical sensitivity of the oscillatory potentials of the electroretinogram (ERG). II. Glutamate-aspartate-and dopamine antagonists. Acta Ophthalmol (Copenh) 1981, 59, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Wachtmeister, L. Oscillatory potentials in the retina: what do they reveal. Prog Retin Eye Res 1998, 17, 485–521. [Google Scholar] [CrossRef] [PubMed]
- Diamond, J.S. Inhibitory Interneurons in the Retina: Types, Circuitry, and Function. Annu Rev Vis Sci 2017, 3, 1–24. [Google Scholar] [CrossRef]
- Knapp, A.G.; Schiller, P.H. The contribution of on-bipolar cells to the electroretinogram of rabbits and monkeys. A study using 2-amino-4-phosphonobutyrate (APB). Vision Res 1984, 24, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.J.; Agey, P.; Hare, W.A. Origins of the electroretinogram oscillatory potentials in the rabbit retina. Vis Neurosci 2004, 21, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Pi, Y.L.; Zhang, M.N. The effect of Vaccinium uliginosum on rabbit retinal structure and light-induced function damage. Chin J Integr Med 2012, 18(4), 299-303. [CrossRef]
- Myers, A.C.; Bruun, A.; Ghosh, F.; Adrian, M. L.; Andreasson, S.; Ponjavic, V. Intravitreal injection of triamcinolone acetonide into healthy rabbit eyes alters retinal function and morphology. Curr Eye Res 2013, 38, 649–661. [Google Scholar] [CrossRef]
- Pochop, P.; Darsova, D.; Uhlik, J.; Lestak, J.; Kukaka, J.; Kodetova, D.; et al. Retinal toxicity after repeated intravitreal carboplatin injection into rabbit eyes. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2014, 158, 552–556. [Google Scholar] [CrossRef]
- Smith, N.P.; Lamb, T.D. The a-wave of the human electroretinogram recorded with a minimally invasive technique. Vision Res 1997, 37, 2943–2952. [Google Scholar] [CrossRef]
- Friedburg, C. , Allen, C.P., Mason, P.J., and Lamb, T.D. Contribution of cone photoreceptors and post-receptor mechanisms to the human photopic electroretinogram. J Physiol 2004, 556 Pt 3, 819–834. [Google Scholar] [CrossRef] [PubMed]
- Mahroo, O.A.; Ban, V.S.; Bussmann, B.M.; Copley, H.C.; Hammond, C.J.; Lamb, T.D. Modeling the initial phase of the human rod photoreceptor response to the onset of steady illumination. Doc Ophthalmol 2012, 124, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Forte, J.D.; Bui, B.V.; Vingrys, A.J. Wavelet analysis reveals dynamics of rat oscillatory potentials. J Neurosci Methods 2008, 169, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Gauvin, M.; Dorfman, A.L.; Trang, N.; Gauthier, M.; Little, J.M.; Lina, J.M.; et al. Assessing the Contribution of the Oscillatory Potentials to the Genesis of the Photopic ERG with the Discrete Wavelet Transform. Biomed Res Int 2016, 2016, 2790194. [Google Scholar] [CrossRef] [PubMed]
- Rufiange, M.; Dassa, J.; Dembinska, O.; Koenekoop, R.K.; Little, J.M.; Polomeno, R.C.; et al. The photopic ERG luminance-response function (photopic hill): method of analysis and clinical application. Vision Res 2003, 43, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, R.; Bees, M.A.; Chaplin, C.A.; McCulloch, D.L. The luminance-response function of the human photopic electroretinogram: a mathematical model. Vision Res 2007, 47, 2968–2972. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.A.; Jeffrey, B.G.; Messias, A.M.V.; Robson, A.G. ISCEV extended protocol for the stimulus–response series for the dark-adapted full-field ERG b-wave. Doc Ophthalmol 2019, 138, 217–227. [Google Scholar] [CrossRef]
- Garon, M.L.; Dorfman, A.L.; Racine, J.; Koenekoop, R.K.; Little, J.M.; Lachapelle, P. Estimating ON and OFF contributions to the photopic hill: normative data and clinical applications. Doc Ophthalmol 2014, 129, 9–16. [Google Scholar] [CrossRef]
- Constable, P.A.; Gaigg, S.B.; Bowler, D.M.; Jagle, H.; Thompson, D.A. Full-field electroretinogram in autism spectrum disorder. Doc Ophthalmol 2016, 132, 83–99. [Google Scholar] [CrossRef]
- Constable, P.A.; Marmolejo-Ramos, F.; Gauthier, M.; Lee, I.O.; Skuse, D.H.; Thompson, D.A. Discrete Wavelet Transform Analysis of the Electroretinogram in Autism Spectrum Disorder and Attention Deficit Hyperactivity Disorder. Frontiers in Neuroscience 2022, 16. [Google Scholar] [CrossRef]
- Gauvin, M.; Lina, J.M.; Lachapelle, P. Advance in ERG analysis: from peak time and amplitude to frequency, power, and energy. Biomed Res Int 2014, 2014, 246096. [Google Scholar] [CrossRef]
- Gauvin, M.; Little, J.M.; Lina, J.M.; Lachapelle, P. Functional decomposition of the human ERG based on the discrete wavelet transform. J Vis 2015, 15. [Google Scholar] [CrossRef]
- Gauvin, M.; Sustar, M.; Little, J.M.; Brecelj, J.; Lina, J.M.; Lachapelle, P. Quantifying the ON and OFF Contributions to the Flash ERG with the Discrete Wavelet Transform. Transl. Vis. Sci. Technol. 2017, 6, 3. [Google Scholar] [CrossRef]
- Brandao, L.M.; Monhart, M.; Schötzau, A.; Ledolter, A.A.; Palmowski-Wolfe, A.M. Wavelet decomposition analysis in the two-flash multifocal ERG in early glaucoma: a comparison to ganglion cell analysis and visual field. Doc. Ophthalmol. 2017, 135, 29–42. [Google Scholar] [CrossRef]
- Dorfman, A.L.; Gauvin, M.; Vatcher, D.; Little, J.M.; Polomeno, R.C.; Lachapelle, P. Ring analysis of multifocal oscillatory potentials (mfOPs) in cCSNB suggests near-normal ON-OFF pathways at the fovea only. Doc. Ophthalmol. 2020, 141, 99–109. [Google Scholar] [CrossRef]
- Mohammad-Manjur, S.; Hossain, M.-B.; Constable, P.A.; Thompson, D.A.; Marmolejo-Ramos, F.; Lee, I.O.; et al. Detecting Autism Spectrum Disorder Using Spectral Analysis of Electroretinogram and Machine Learning: Preliminary results. IEEE Trans. Biomed. Eng.
- Sarossy, M.; Crowston, J.; Kumar, D.; Weymouth, A.; Wu, Z. Time-Frequency Analysis of ERG With Discrete Wavelet Transform and Matching Pursuits for Glaucoma. Transl. Vis. Sci. Technol. 2022, 11, 19. [Google Scholar] [CrossRef]
- Hamilton, R. (2021). Clinical electrophysiology of vision-commentary on current status and future prospects. Eye (Lond), 35(9), 2341-2343. [CrossRef]
- Yip, Y. W. Y. , Man, T. C., Pang, C. P., & Brelen, M. E. (2018). Improving the quality of electroretinogram recordings using active electrodes. E. ( 176, 46–52.
- Zhdanov, A. E. , Dolganov, A. Y., Zanca, D., Borisov, V. I., Lucian, E., & Dorosinskiy, L. G. (2023). Evaluation of the effectiveness of the decision support algorithm for physicians in retinal dystrophy using machine learning methods. G. ( 47(2), 272–277.
- Behbahani, S. , Ahmadieh, H., & Rajan, S. (2021). Feature Extraction Methods for Electroretinogram Signal Analysis: A Review. IEEE Access, 9, 116879-116897. [Google Scholar]
- Ponomarev, V.O., Kazaykin, V.N., Lizunov, A.V., Vokhmintsev, A.S., Vainshtein, I.A., Dezhurov, S.V., & Marysheva, V.V. (2021). Evaluation of the Ophthalmotoxic Effect of Quantum Dots InP/ZnSe/ZnS 660 and Bioconjugates Based on Them in Terms of the Prospects for the Treatment of Resistant Endophthalmitis. experimental research. Part 2 (Stage 1). Ophthalmology in Russia, 18(4), 876-884.
- Ozkan, J., Majzoub, M. E., Coroneo, M., Thomas, T., & Willcox, M. (2021). Comparative analysis of ocular surface tissue microbiome in human, mouse, rabbit, and guinea pig. Experimental Eye Research, 207, 108609.
- Famiglietti, E. V., & Sharpe, S. J. (1995). Regional topography of rod and immunocytochemically characterized “blue” and “green” cone photoreceptors in rabbit retina. Visual neuroscience, 12(6), 1151-1175.
- Williams, D. L. (2012). The rabbit eye. In J. E. Cooper, & M. R. L. Thomas (Eds.), Ophthalmology of Exotic Pets (pp. 15-55). Wiley-Blackwell.
- Zhdanov, A., Dolganov, A., Zanca, D., Borisov, V., & Ronkin, M. (2022). Advanced Analysis of Electroretinograms Based on Wavelet Scalogram Processing. Applied Sciences 2022, 12(23), 12365.
- Hamilton, R. (2021). Clinical electrophysiology of vision—commentary on current status and future prospects. Eye, 35(9), 490, 2341-2343.
- Mahroo, O. A. (2023). Visual electrophysiology and “the potential of the potentials.” Eye, 1-10.
- Kondo, M. I. N. E. O. (2010). Animal models of human retinal and optic nerve diseases analyzed using electroretinography. Nippon Ganka Gakkai Zasshi, 114(3), 248-78.
- Ciulla, T. A., Criswell, M. H., Danis, R. P., Hill, T. E., & Introne, W. J. (2000). Endothelin-1-mediated retinal artery vasospasm and the rabbit electroretinogram. Journal of ocular pharmacology and therapeutics, 16(4), 393-398.
- Ponomarev, V.O., Kazaykin, V.N., Lizunov, A.V., Vokhmintsev, A.S., Vainshtein, I.A., Dezhurov, S.V., & Marysheva, V.V. (2021). Evaluation of the Ophthalmotoxic Effect of Quantum Dots InP/ZnSe/ZnS 660 and Bioconjugates Based on Them in Terms of the Prospects for the Treatment of Resistant Endophthalmitis. Experimental Research. Part 2 (Stage 1). Ophthalmology in Russia, 18(4), 876-884.
| Scotopic 2.0 ERG Response | Maximum 2.0 ERG Response | |
|---|---|---|
| Flash intensity, cd·s·m-2 | 2 | 2 |
| Background light, cd·s·m-2 | 0 | 0 |
| Flash duration, ms | 0.5 | 3 |
| Flash frequency, kHz | 20 | 20 |
| Stimulus interval, s (cps) | 2.5 (0.4) | 10 or 13 (0.1) |
| Flash color | white | white |
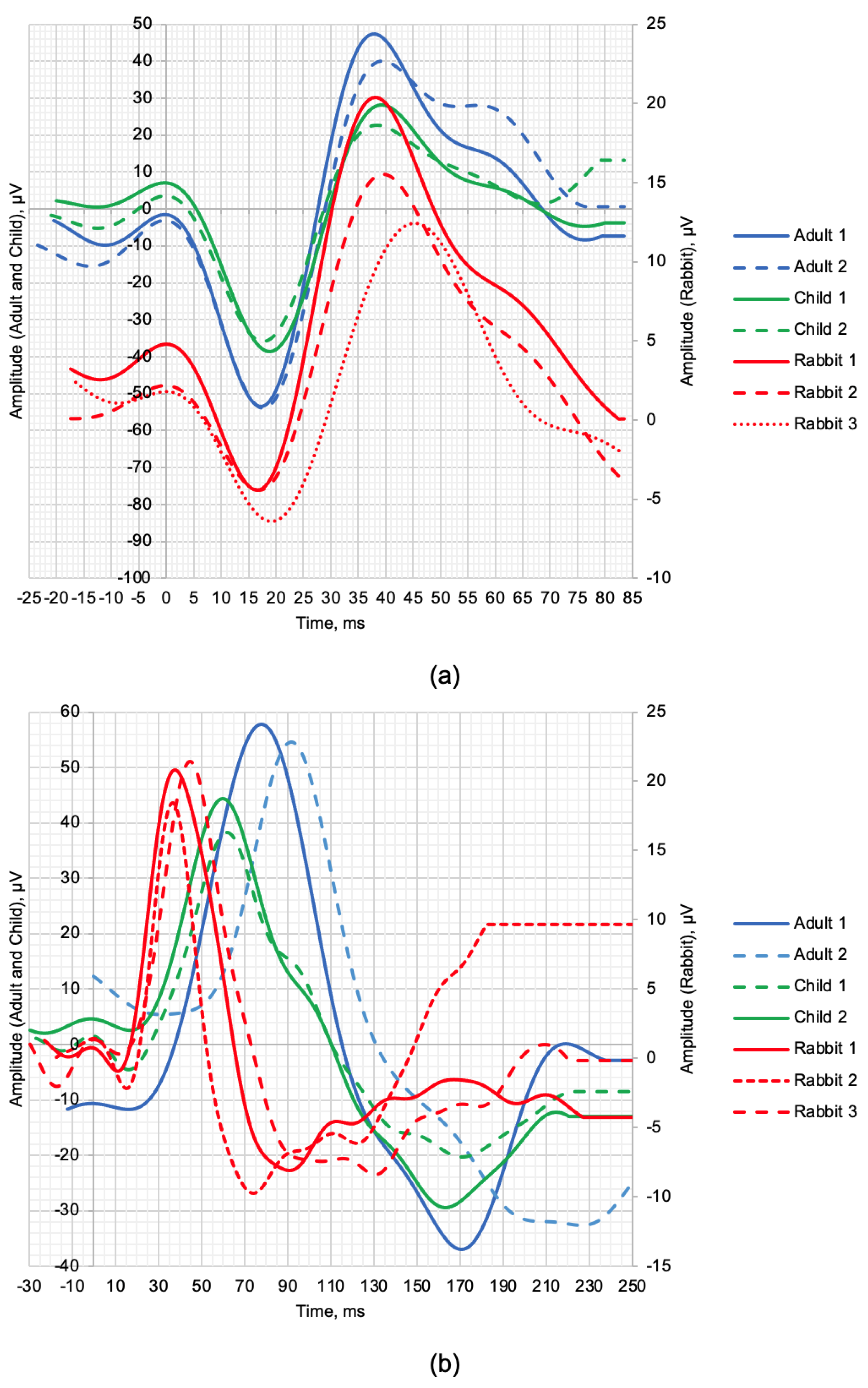
| Adult 1 | Adult 2 | Child 1 | Child 2 | Rabbit 1 | Rabbit 2 | Rabbit 3 | |
|---|---|---|---|---|---|---|---|
| Age | 20.5 y.o. | 27.8 y.o. | 10.9 y.o. | 7.5 y.o. | 2-3.5 mos. | ||
| Maximum 2.0 ERG Response | |||||||
| a, µV | 52.32 | 50.69 | 45.65 | 39.28 | 9.18 | 6.59 | 8.2 |
| b, µV | 101.22 | 93.78 | 66.74 | 58.35 | 24.75 | 19.92 | 18.86 |
| la, ms | 17.5 | 18 | 19 | 18 | 16.5 | 17 | 19.5 |
| lb, ms | 38 | 39.5 | 39.5 | 38.5 | 38 | 39.5 | 45.5 |
| Scotopic 2.0 ERG Response | |||||||
| a, µV | 1.04 | - | 6.05 | 2.03 | 1.82 | 3.47 | 1.04 |
| b, µV | 69.53 | 60.01 | 42.77 | 46.91 | 21.87 | 20.47 | 21.12 |
| la, ms | 17 | - | 16 | 16.5 | 10.5 | 14.5 | 12.5 |
| lb, ms | 78 | 92.5 | 62.5 | 60.5 | 38.5 | 37.5 | 44.5 |
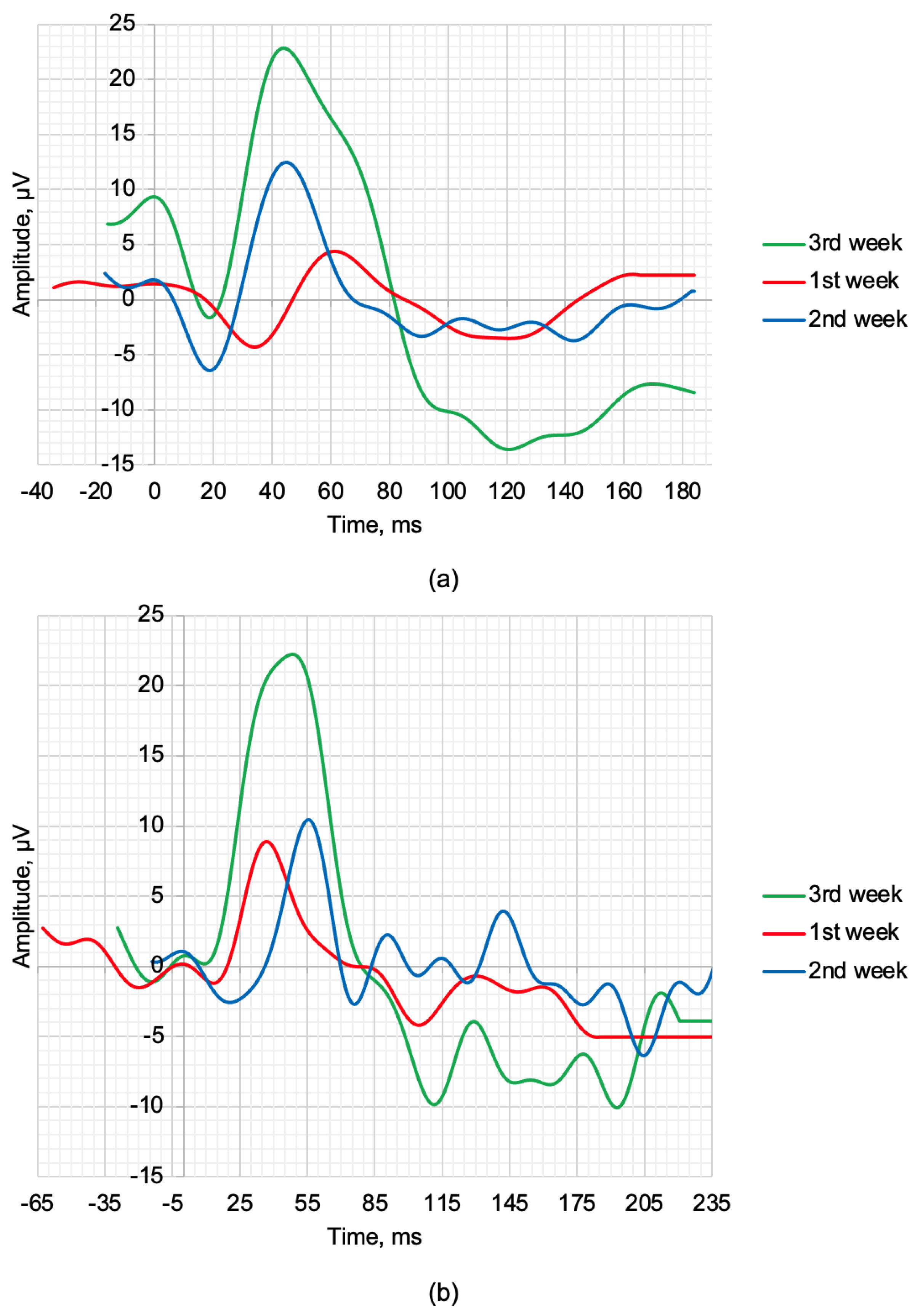
| 1st week | 2nd week | 3rd week | |
|---|---|---|---|
| Maximum 2.0 ERG Response | |||
| a, µV | 5.61 | 8.03 | 10.81 |
| b, µV | 16.48 | 18.6 | 24.14 |
| la, ms | 33 | 17.5 | 20 |
| lb, ms | 47 | 46.5 | 43 |
| Scotopic 2.0 ERG Response | |||
| a, µV | 1.81 | 3.43 | 0.75 |
| b, µV | 10.26 | 12.72 | 21.92 |
| la, ms | 13 | 22.5 | 8.5 |
| lb, ms | 39.5 | 57.5 | 50.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





