1. Introduction
Agarose is a natural polysaccharide, extracted from red seaweed, forming gel in aqueous medium. Agarose has a wide range of applications as gelling agent in food engineering to tissue engineering as porous scaffolds and biotechnologies for gel electrophoresis (especially for DNA) and agar gel plates as a growth medium for microorganisms. Being solubilised in water (or D
2O for NMR study) at high temperature (over the melting temperature T
m, i.e. over 80 to 90°C), it forms a strong homogeneous gel when temperature decreases (at temperature lower than the gelling temperature T
g) when concentration is larger than a critical concentration C
0 larger than the overlap concentration C
* [
1,
2]. Gel stabilisation is based on H-bond network involving -OH groups in associated double helical structure and water -agarose -OH groups. The sol-gel transition is reversible with a large hysteresis associated with the coil- helix conformation followed by helices packing as previously observed with κ-carrageenans [
3]. Then, agarose gel is formed by aggregation of double helices at lower temperature [
4,
5]. The physical characteristics (and specially positions of T
g and T
m) of such gels depend on the chemical structure of agarose chains which may contained few substituents which play a role on the helices stability and packing. Such substituents may be methyl, acetyl, sulphate or pyruvate usually determined by NMR [
6,
7]. Agarose under coiled conformation is soluble in DMSO allowing easy NMR analysis [
8]. On the opposite, ethanol is a non solvent and considered as a precipitant [
10].
Agarose is a linear polysaccharide made of repeating units of agarobiose that is extracted from boiled red algae
In a first part of this work, NMR spectrometry is used to establish the chemical structure of agarose and possible substituents on the main chain. Assignment of the different
1H and
13C signals were previously given in literature but not completely especially in D
2O [
4,
6,
7,
10]. The evolution of signals with temperature are also related to the intra- and inter-molecular H-bonds in relation agarose-agarose interaction but also related with the role of water interaction in agarose gel formation, using relaxation experiments [
11,
12,
13,
14,
15,
16].
In that respect, it was mentioned previously that gels look stable in acetone and ethanol, non-solvents of this polymer [
17]. Then, water and ethanol contents for gels stabilized in these two media were determined and their rheological behaviour as a function of temperature were examined in connection with NMR data. To our knowledge, it is the first time that such study is developed.
2. Materials and Methods
2.1. Agarose samples preparation
Agarose was provided by Sigma Chemicals (Agarose HGTP, N° A-3893/TYPEVI) having a high melting temperature. It was used without purification and solubilised in D2O for NMR or deionized water for rheology after heating 1 hour at 90°C. For agarose characterization, agarose was also solubilised directly in DMSO-d6 for NMR measurements.
For rheology, the solution was poured in cylindrical mold and the temperature was lowered to 15°C in order to obtain a stable gel with a cylindrical shape adapted for measurements. Samples were then stored at ambient temperature and used within 24 hours. In the conditions tested (especially the agarose concentration at 10mg/mL), no syneresis was observed. For rheology, water exchange with ethanol was performed by immersing the small cylindrical sample in ethanol for one week with exchange of the medium each day.
For NMR, the solution in D2O is introduced in the NMR tube at high temperature. The exchange of D2O against ethanol is performed directly in the NMR tube, covering the gel formed at low temperature, with ethanol during one week with exchange of ethanol each day.
2.2. NMR experiments.
The sample was dissolved in D2O (5 mg/mL) and in dimethylsulfoxide-d6 (7.6 mg in 0.6mL) for NMR characterization. The spectra were obtained at 80°C and analyzed with chemical shifts assignment for 13C and 1H. The temperature evolution of spectra in D2O was studied to compare with the hysteresis established by rheology. After stabilization at low temperature, temperature increase was imposed from 25°C up to 80°C at a rate 0.5°C/min. After 30 minutes at high temperature, temperature was decreased down to 25°C at the same rate. For this experiment, the agarose concentration in D2O is 10 mg/mL. In such conditions, evolution of 1H mobility was drawn to be related to the sol-gel transition in D20. To quantity this evolution a determined amount of dimethylsulfoxide (DMSO) was added to be able to calibrate the signal amplitudes of the agarose protons.
DEPTQ [
18] and proton NMR spectra were recorded with a Bruker Avance 400 spectrometer operating at a frequency of 100.618 MHz for
13C and 400.13 MHz for
1H. The solvent residual peaks of HOD and DMSO-
d6 were used as internal standard at 4.8 ppm at 298 K and 4.25 ppm at 353 K for D
2O, and at 2.5 ppm for DMSO-
d6 for
1H and 39.51 ppm for DMSO-
d6 for
13C NMR. Proton spectra were recorded with a 4000 Hz spectral width, 65536 data points, 8.19 s acquisition times, 1s relaxation delay, and 16 scans.
DEPTQ spectra were recorded using 90 degree pulses, 20161 Hz spectral width, 65536 data points, 1.62 s acquisition time, 2 s relaxation delay, and 6000 scans.
The 1H and 13C-NMR assignments were based on 1H-1H homo-nuclear and 1H-13C hetero-nuclear correlation experiments (correlation spectroscopy, COSY; hetero-nuclear multiple-bond correlation, HMBC; hetero-nuclear single quantum correlation, HSQC). They were performed with a 4000 Hz spectral width, 2048 data points, 0.255 s acquisition time, 1 to 1.5 s relaxation delay; 32 to 196 scans were accumulated.
2.3. Water regain and swelling degree
The degree of swelling was determined from the weight of swollen gel (Wh) in the solvent considered (H2O or ethanol) and the dried weight (Ws) expressed in mL solvent / g dried gel taking into account the ethanol density (ethanol d=0.79). The dried weight were obtained after 2 hours at 120°C.
2.4. Differential scanning calorimetry
To complete the analysis of regain water, the amount of freezing water was evaluated by Differential Scanning Calorimetry (DSC) using a Mettler Toledo DSC 821
e [
19,
20]. All experiments were carried out using the following protocol: (1) cooling the sample from 25°C to -50°C at -1°C/min, (2) isothermal at -50°C during 10 min and (3) heating from -50°C to 25°C at 1°C/min. A nitrogen flow atmosphere was imposed at 60mL/min to maintain a stable temperature and avoid fluctuations. After calibration with deionised water, it is shown that no residual water was left in the sample after ethanol exchange.
2.5. Rheology
Rheology was performed on a ARES-G2 rotational rheometer (TA Instruments) with plates geometries 25mm diameters in two configurations: one using the Advanced Peltier System, APS (-10°C to 150°C) and the other using the Forced Convection Oven, FCO (-150°C to 600°C).
During temperature increase, it is important to prevent solvent evaporation. As shown in
Figure 1 plates systems used where equipped a cup at the bottom in which a silicon oil paragon S3 (Paragon Scientific Ltd) where poured to cover the gel to prevent evaporation of water. The silicon oil viscosities, used to prevent water evaporation, change from 3.7 mPa.s at 20°C to 1.2 mPa.s at 80°C. No difference was observed on the elastic and viscous moduli of the gels measured at ambient temperature with and without silicon oil. Furthermore, no slip was observed on the sheared samples, thanks to the ribbed geometry. The temperature ramps applied to the samples were identical than that of the RMN ones with a polymer concentration of 10g/L and an imposed ramp rate of 0.5°C/min, in order to reduce the effect of thermal tool inertia.
The experiments were conducted keeping a particular attention to the contact between the gel and the plates. For this purpose, a small axial force between 0 to 0.1N was held perpendicular to the plate surface. During the temperature ramp, the water and ethanol gels behaved differently. For water gels no particular attention had to be made during experiments. However, for the ethanol gel during the experiment, a drastic change in the sample size when temperature increases is due to solvent evaporation. This makes rheometric measurements difficult for ethanol gels as the temperature is increased. Because of this difficulty in controlling the integrity of the ethanol gel, the present study is limited to 50°C for the ethanol gel.
3. Results and discussion
3.1. NMR study
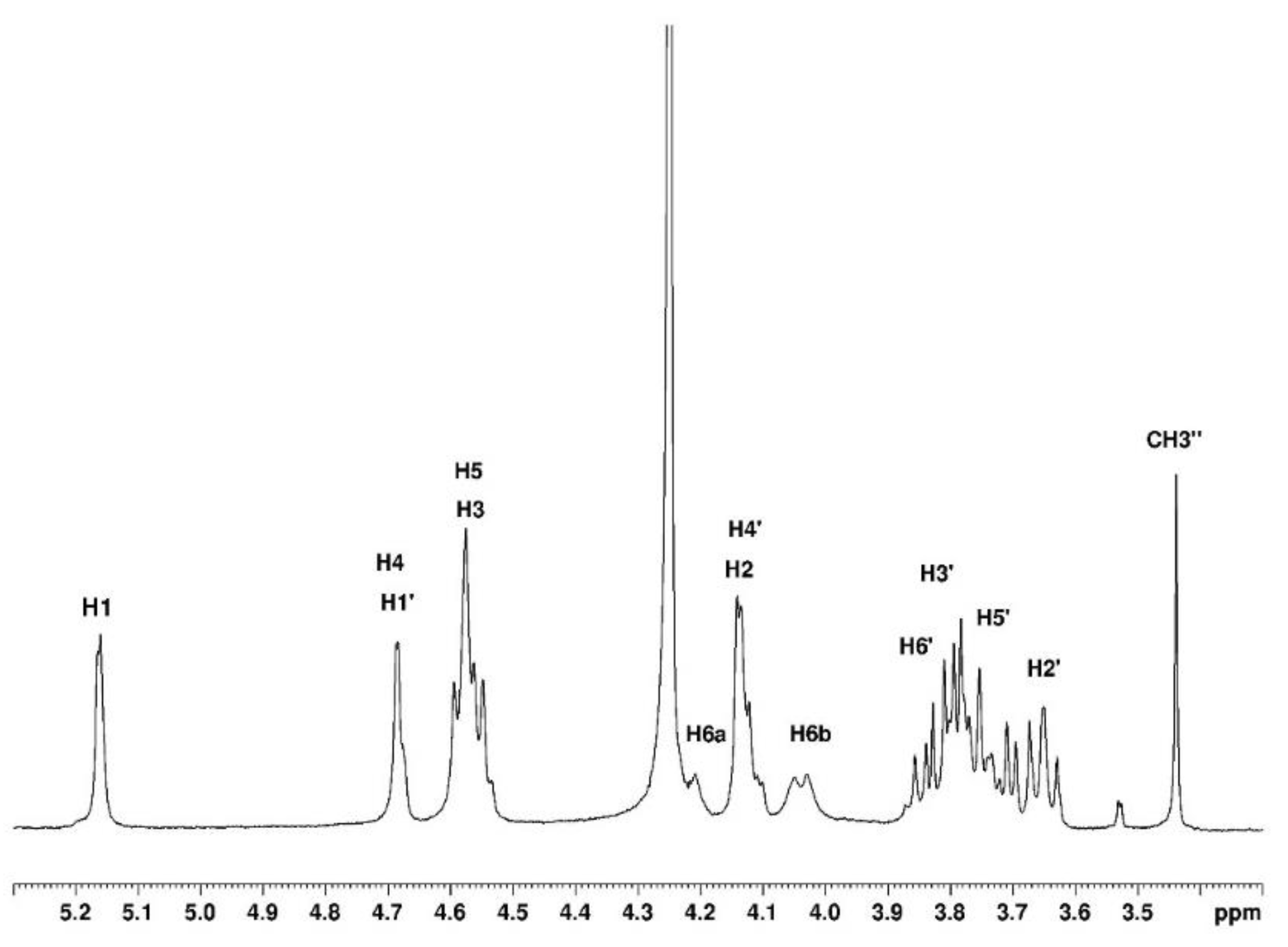
Analysis of the agarose sample is performed using 1D and 2D
1H and
13C NMR spectroscopies. Signal assignments are given in
Table 1 and
Table 2 for the solvents D
-O and DMSO-
d6. In the tables, G is used for the anhydrogalactose, G’ for the D-galactose unit and G’’ for the substituted D-galactose unit (
Figure 2).
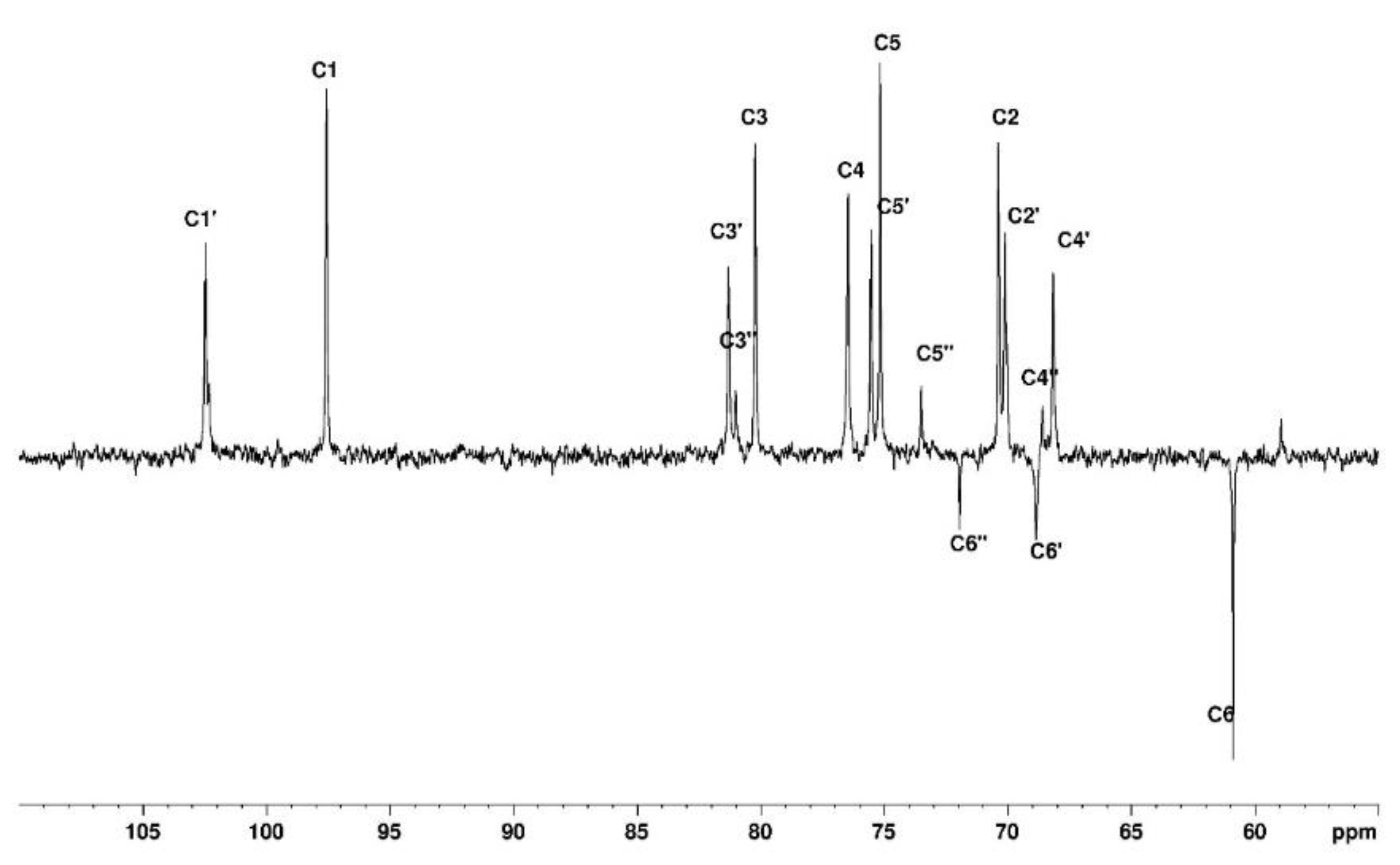
3.1.1. Assignement of 13C and 1H in D2O
The complete assignment for protons and carbons is given in
Figure 3 and
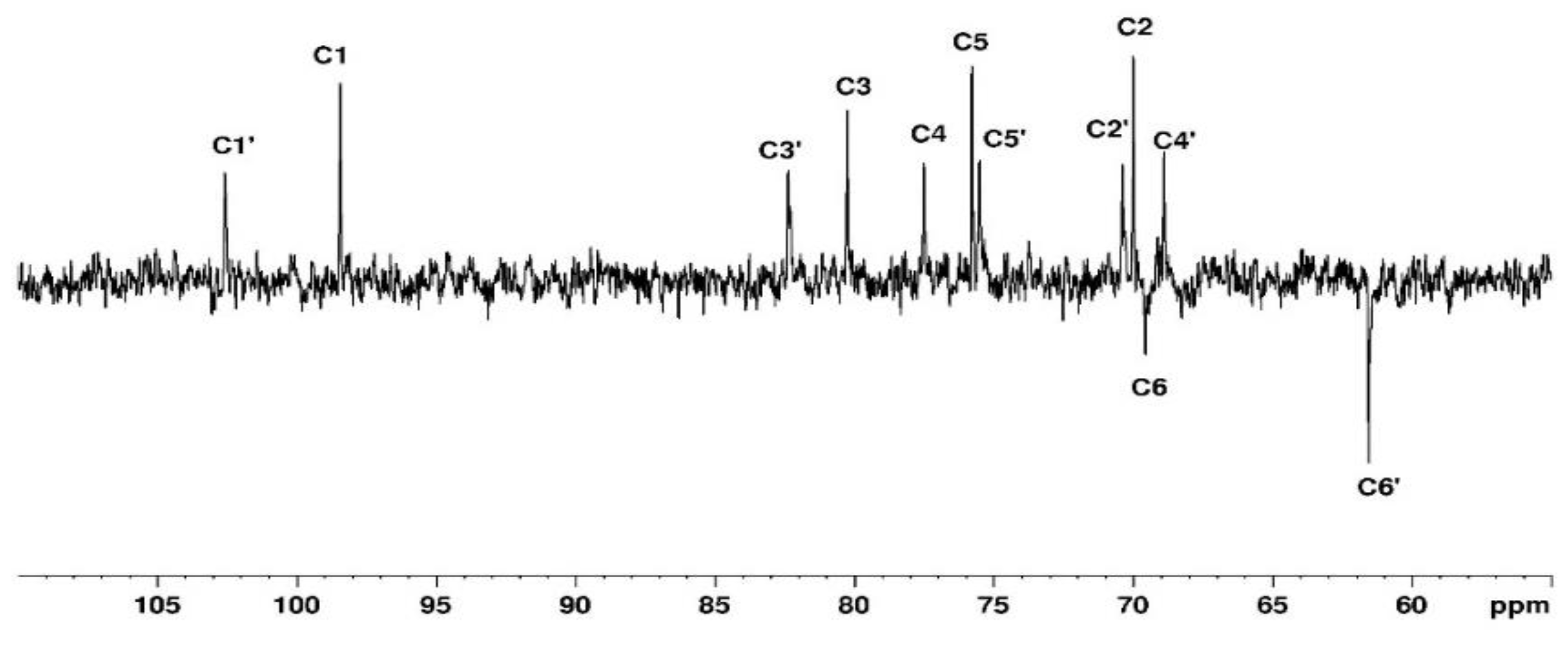
Figure 4. The DEPTQ permits to identify carbon when it is engaged in -CH
2 group (
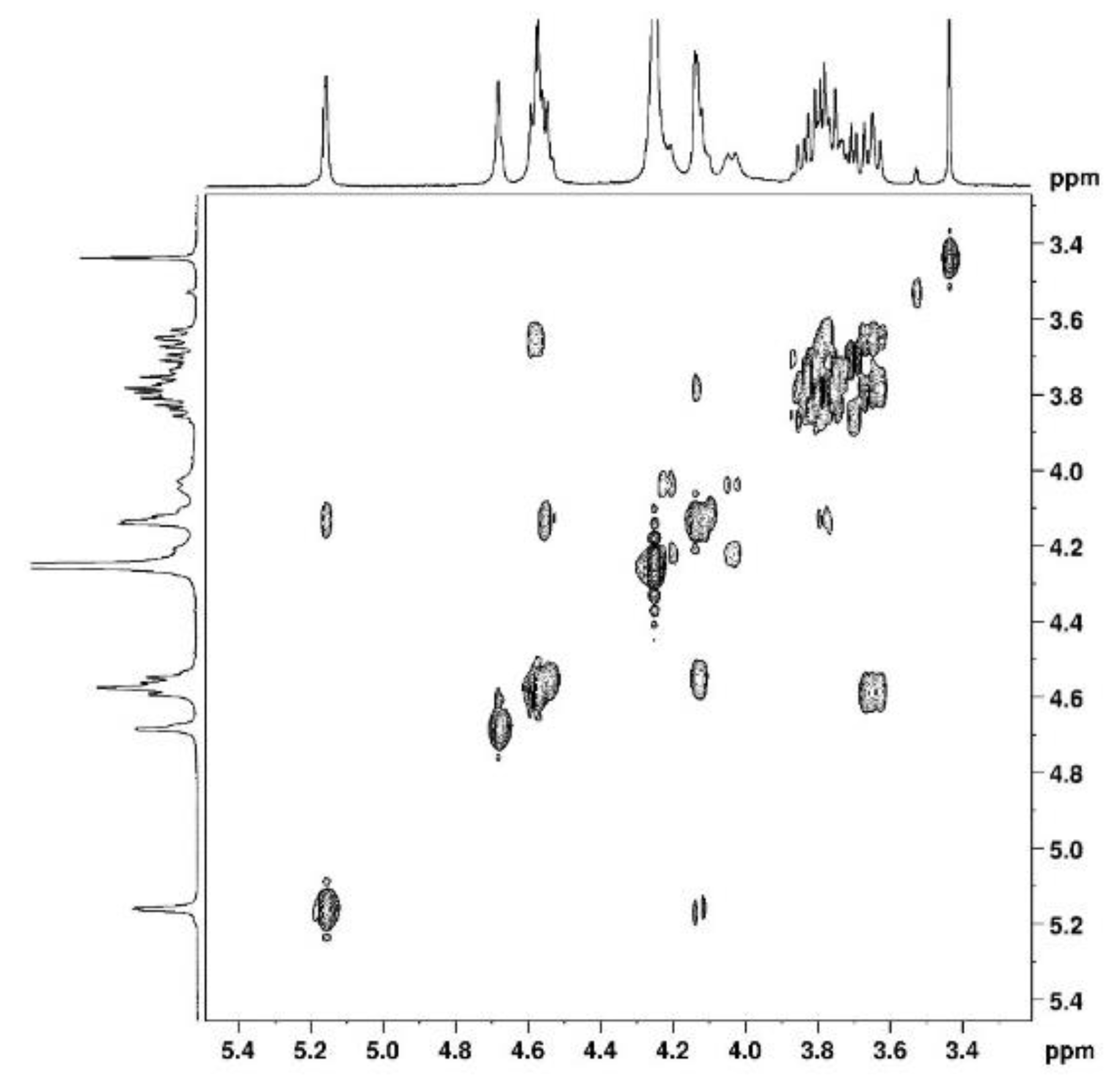
Figure 4). The attribution of the signals was permitted using other NMR techniques shown in the following. Use of COSY (
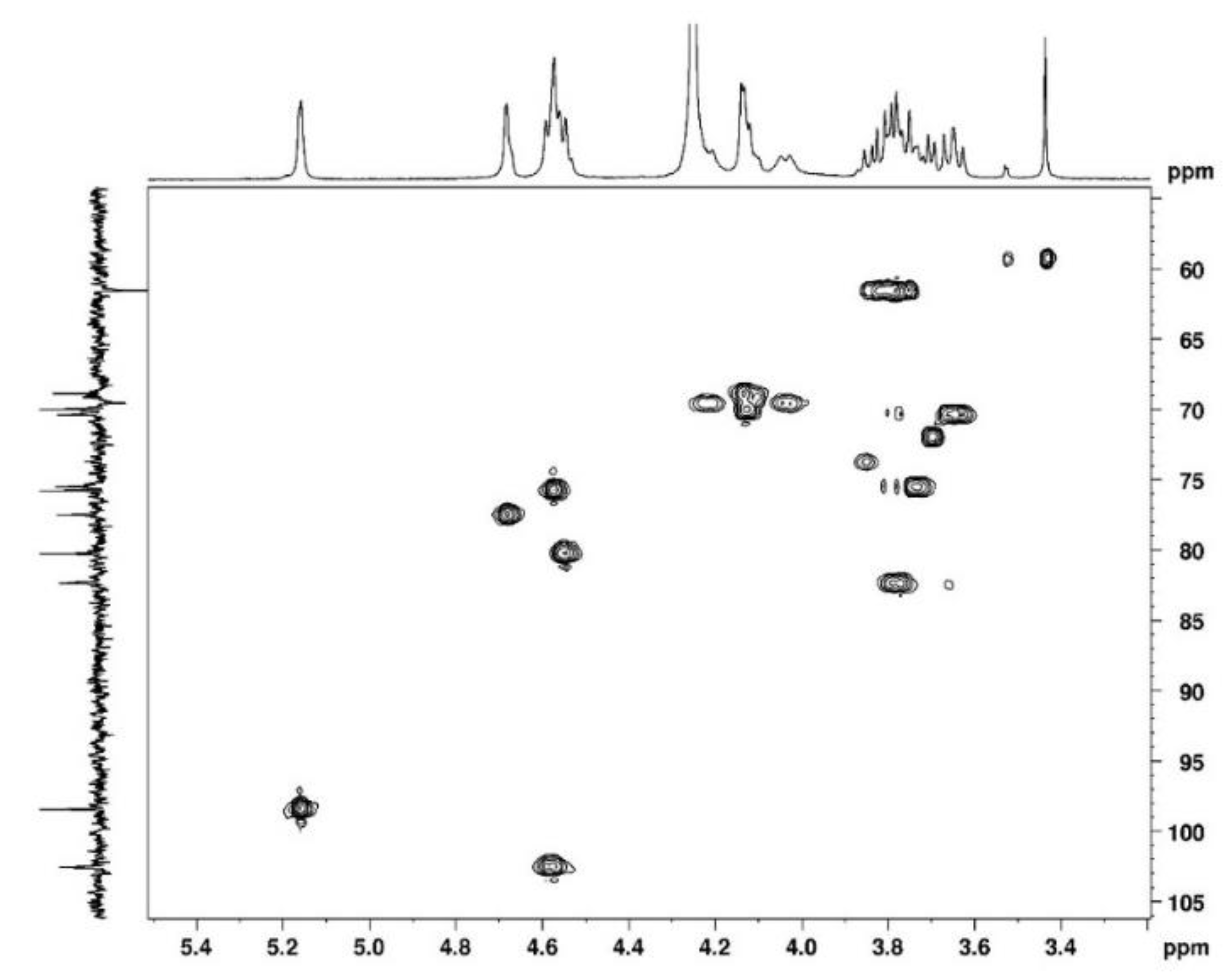
Figure 3) allows to assign protons and HSQC to attribute corresponding carbons (
Figure 4). The chemical shifts are summarised in
Table 1.
Figure 3.
Proton NMR of agarose in D2O at 80°C.
Figure 3.
Proton NMR of agarose in D2O at 80°C.
Figure 4.
DEPTQ spectrum of agarose in D2O at 80°C.
Figure 4.
DEPTQ spectrum of agarose in D2O at 80°C.
Figure 5.
2D COSY NMR spectrum of agarose in D2O at 80°C.
Figure 5.
2D COSY NMR spectrum of agarose in D2O at 80°C.
Figure 6.
2D HSQC NMR spectrum of agarose in D2O at 80°C.
Figure 6.
2D HSQC NMR spectrum of agarose in D2O at 80°C.
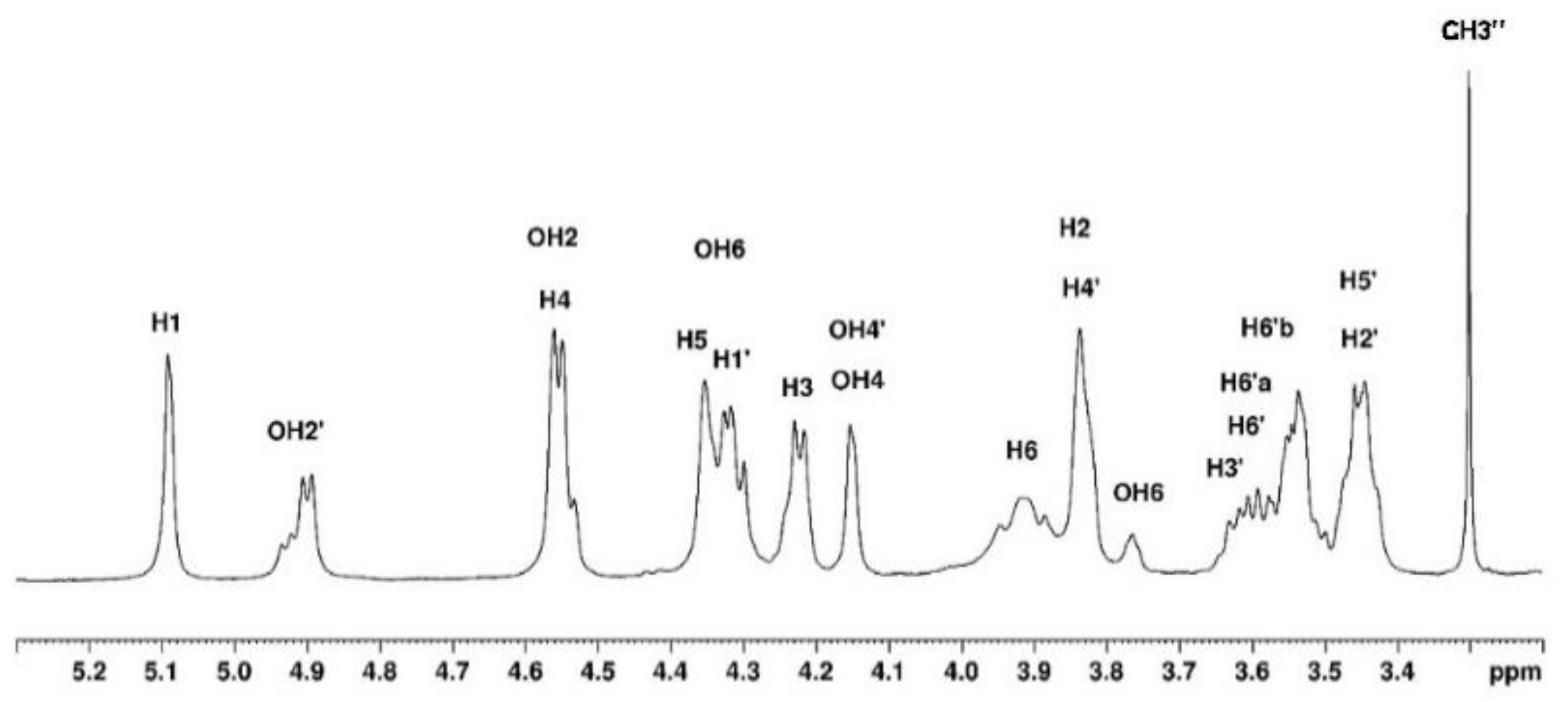
3.1.2. Assignement of 13C and 1H in DMSO-d6
In DMSO
-d6, agarose being in the coil conformation, the spectrum obtained shows the proton and carbon signals but also allows to assign the -OH groups (
Figure 7 and
Figure 8). The identification of
1H and
13C signals are summarised in
Table 2.
The assignments given in
Table 2 are in good agreement with literature data [
6,
8,
10,
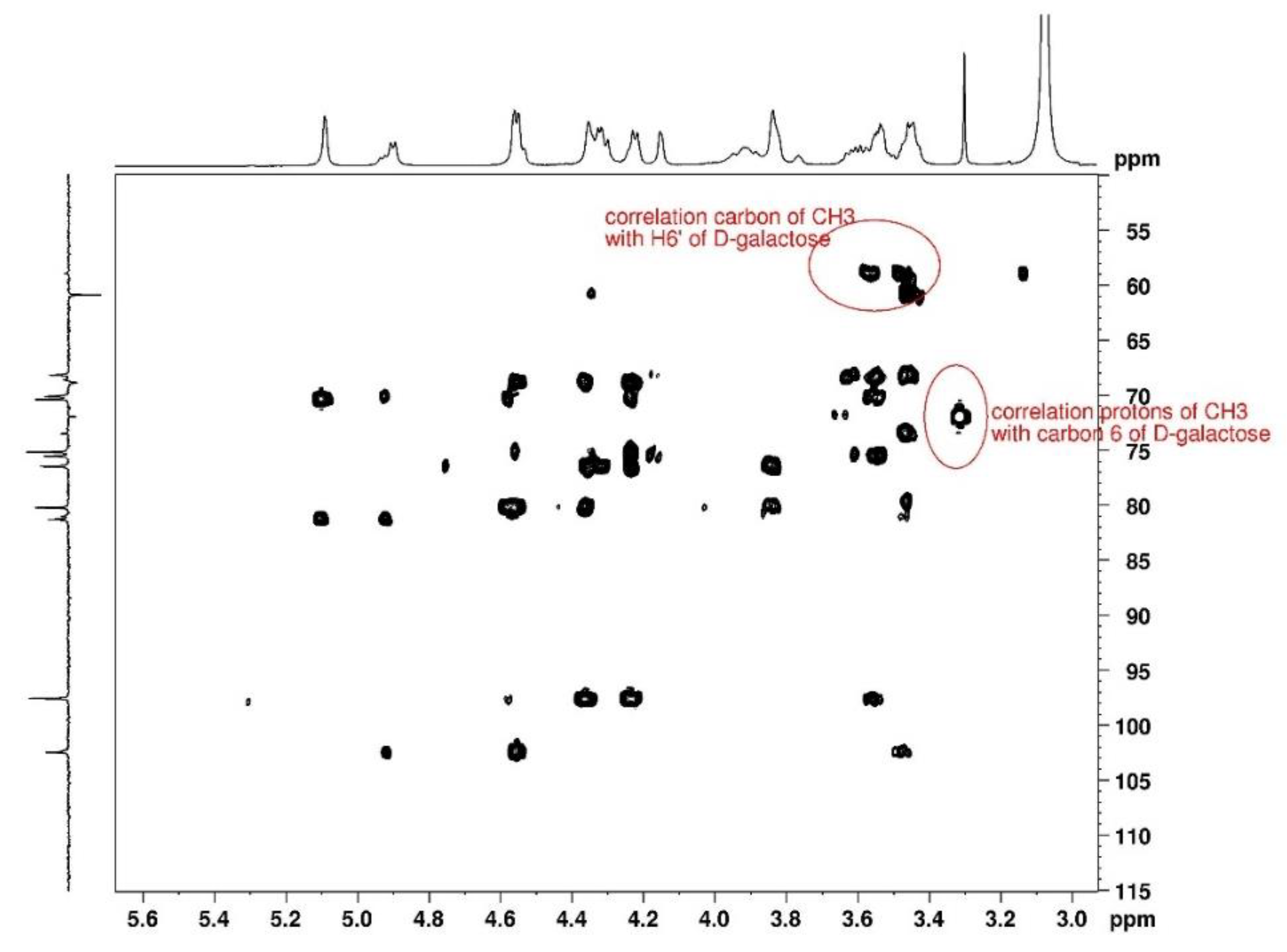
14]. 2D HMBC spectrum was used to locate the methyl substituent (
Figure 9). The methyl group is identified on D-galactose unit which shifts C-3 to C-6 signals (D-galactose G’’ in spectra) and the two H-6 (
Table 2). The substitution is discussed in the following paragraph.
3.1.3. Degree of substitution on agarose
Considering long distance correlation for 1H -13C performed in DMSO-d6, it is shown that the proton with a thin signal at 3.3 ppm is assigned to a methyl sustituent correlated with C -6 of D- galactose unit. In the same way, the C from methyl group correlates with the proton H-6 of D-galactose. Taking into account the integral of this signal in reference with H-1 of anhydrogalactose, it comes DS=0.24±0.01, indicating the degree of substitution of D-galactose units.
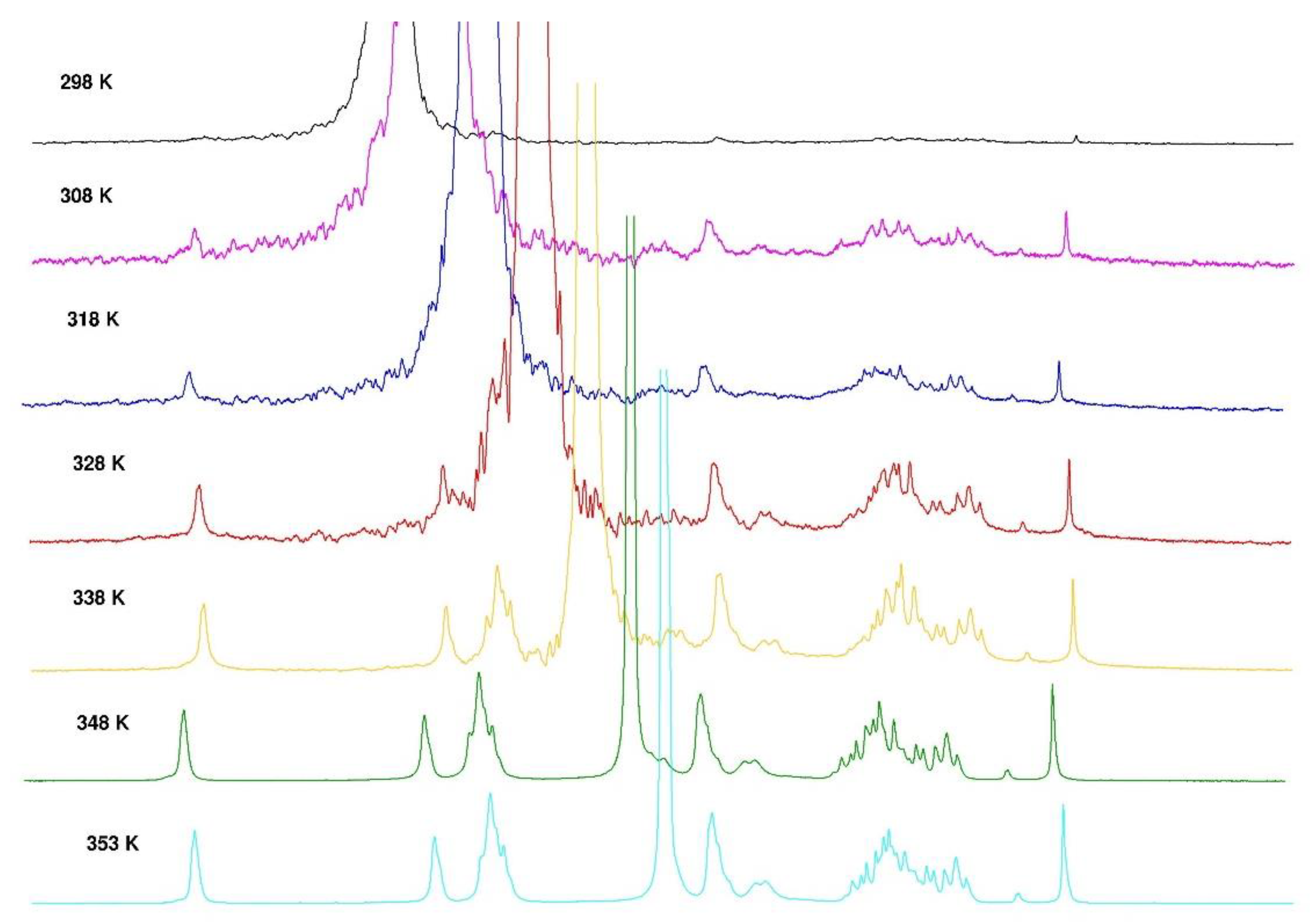
3.1.4. Thermal hysteresis
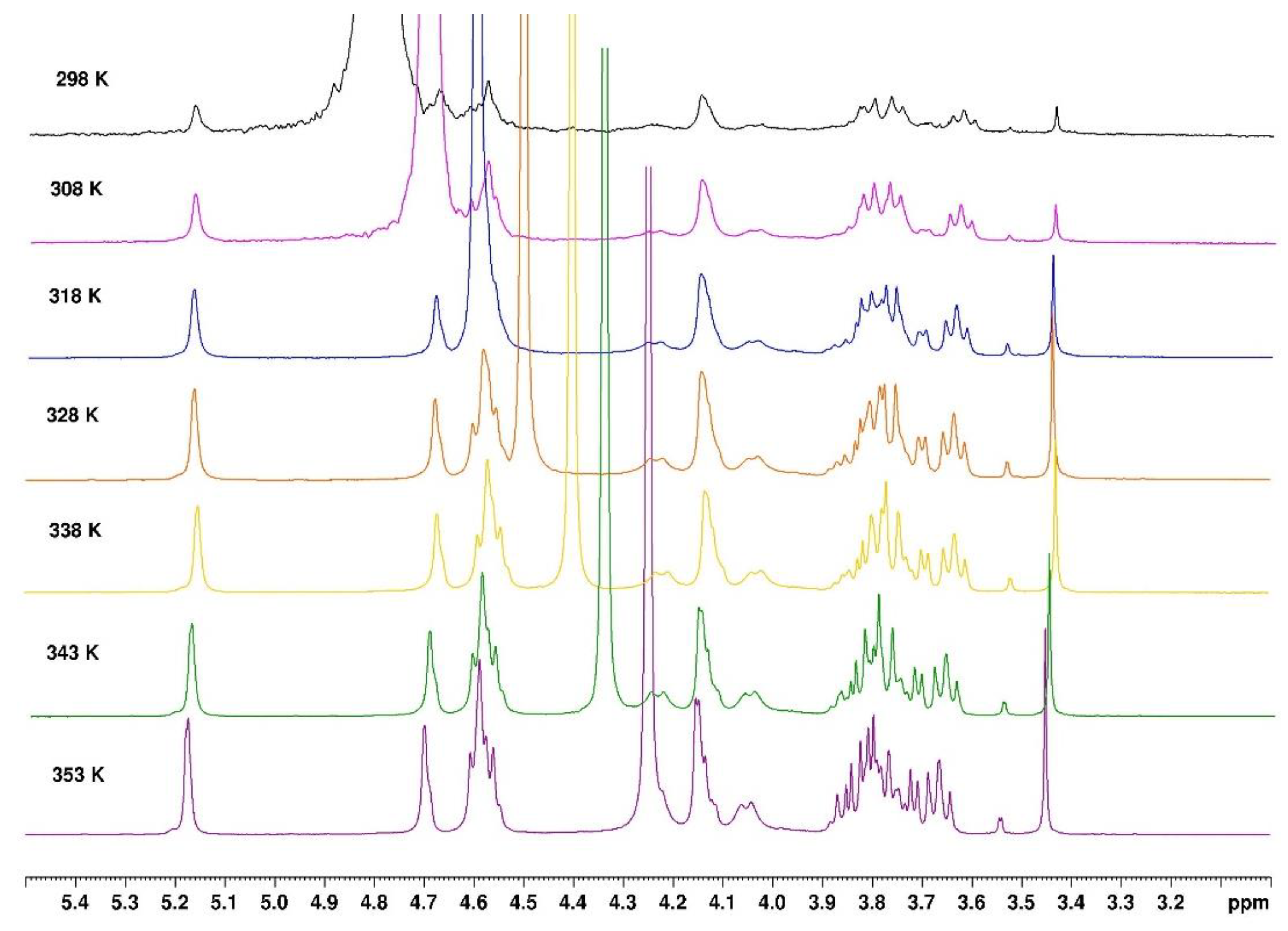
NMR is an interesting technique to investigate the local mobility of molecules. The spectra given in
Figure 10 and
Figure 11 show the evolution of the signal amplitudes as well as the chemical shift of HOD (from agarose -OH exchanged to -OD in D
2O) used as reference for temperature from 25 to 80°C and from 80 to 25°C.
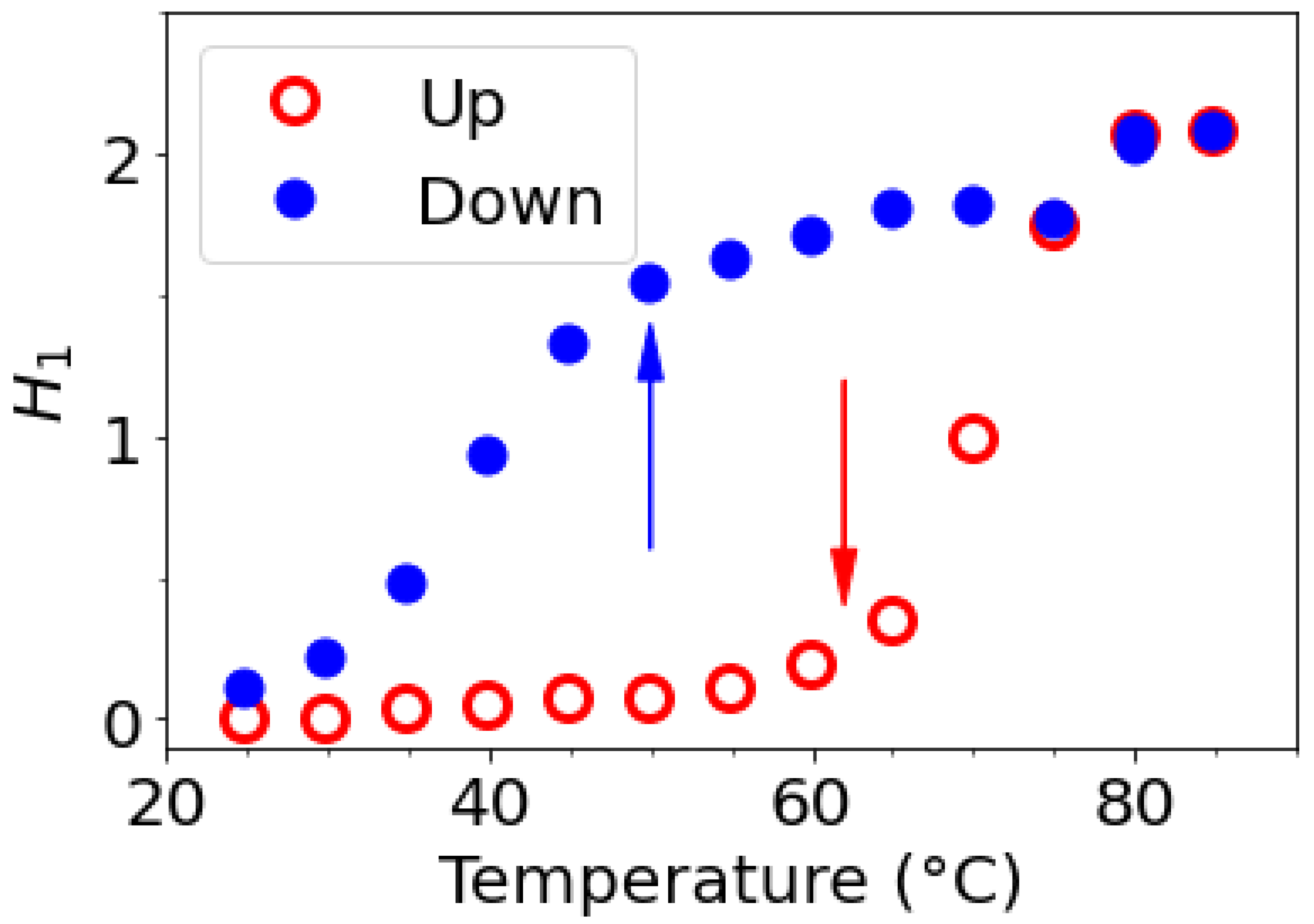
The H-1 from anhydrogalactose at 5.16 ppm and the methyl group on galactose at 3.3 ppm were selected to draw the temperature dependence. The hysteresis obtains may be discussed in relation with the rheological data (
Figure 12). For this experiment, a small defined amount of DMSO is added to be able to compare the amplitude of the two protons selected signals.
The integrals of the signals corresponding to H-1 of anhydrogalactose unit (or -CH
3 substituent on galactose unit, not shown) are plotted as a function of temperature in
Figure 12 reflecting the modification of mobility with a large hysteresis: it shows a transition over 60°C on increasing temperature and another one under 50°C on decreasing temperature.
Papers concerning NMR of agarose in aqueous medium are based mainly on
1H and
2H magnetic relaxation study (T
1 and T
2) allowing to deduce bound and free water [
12,
13] without no exact estimation of the fraction of bound water molecules. Interactions is related to the existence of H-bonds in the gel state or double helices aggregates involving agarose-agarose and water-agarose interactions. It was proposed that the large magnetic relaxation dispersion is due to internal water molecules located in the central cavity of agarose double helix which stabilize the conformation [
11]. Slower self-diffusion of water was also related to obstructive effect of the agarose network [
16]. Dependence of NMR spectra with temperature was mainly investigated in few papers in relation with gelation [
4,
14].
Our data clearly show a hysteresis between heating and cooling that it is related with rheology as discussed later. In addition, a small chemical shift (0.023 ppm) is obtained around 65°C on heating steps attributed to conformational change i.e. probably, helix-coil transition associated with aggregates dissociation. The signal related to HOD is also larger in the temperature range up to 65°C probably due to gel presence as suggested previously [
16]. This transition was also described by Chavez et al. around 55°C and mentioned at 55°C by Ed-Daoui et al. [
11,
21]. Our data at high temperature are interpreted as a consequence of the high mobility of the coiled chains, at melting temperature T
m around 80°C. On cooling, the chemical shift located around 70°C is smaller, interpreted as the helix formation, with a smaller chemical shift of 0.011 and a thin signal for HOD down to 45°C. From this temperature, aggregates formed in liquid phase from 65°C are cross-linked down to gelling temperature around T
g= 20°C. It is known that H-bond type interactions are formed in D
2O and that there are stronger than in H
2O. Then, the double helical conformation is more stable in D2O as obtained for K-carrageenan [
22]. This may justify small increase of the characteristic temperatures given by NMR compared to rheology.
3.1.5. Thermal behaviour of gel in ethanol
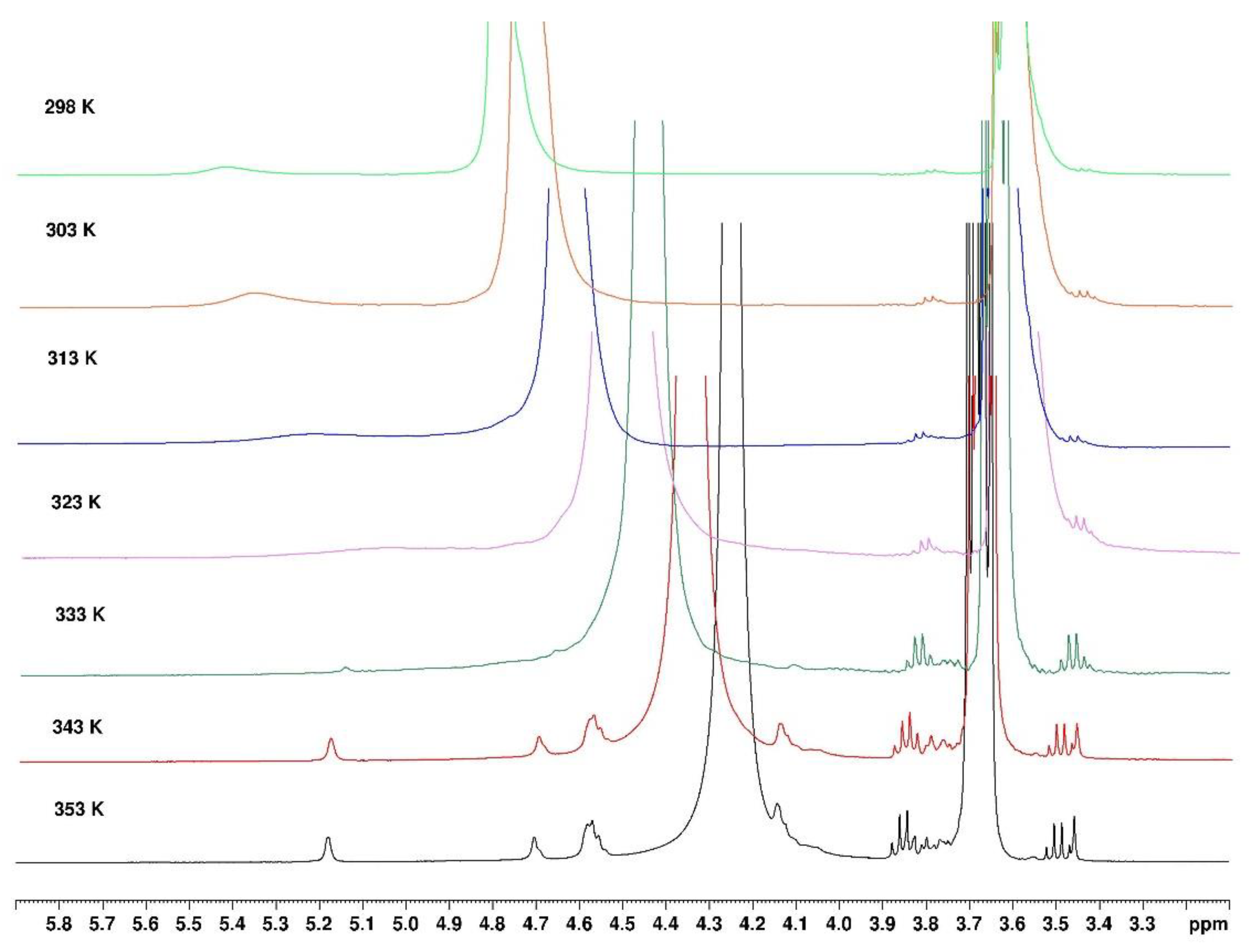
From the influence of increasing temperature on NMR spectrum, it is shown that the small large signal around 5.2 ppm decreases progressively up to 65°C follows by appearance of a thinner signal observed over 65°C (
Figure 13). It seems those signals appearing are related to H-1 of the anhydrogalactose unit based on the chemical shifts obtained in D
2O. This indicates that only a small fraction of agarose is mobilized when temperature increases over 65°C (if the signals are compared with those obtained in D
2O in the same conditions). It is probably related to the release of few H-bonds in the loose junction zones connecting the stiff aggregates. In these spectra, the signal of HOD is related to the exchange on agarose -OH due to the addition of a small amount of D
20 necessary to lock the sample, the conditions to perform the experiment. This signal remains large indicating a cross-linked system.
In a separate experiment, it was shown that the gel never melts up to 80°C when immersed in ethanol. This indicates that in absence of H2O or D2O inserted inside the cavity of double helices or between them, the cooperative H-bonds are more stable as confirmed by rheological experiment in the following.
3.1. Degree of solvation
The first step was to determine the degree of swelling on the gel in water and after the exchange in presence of ethanol. In water, (Wh - Ws)/Ws= 101g/ g dried gel or 101 mL/ g dried gel in agreement with the weight concentration of the solution prepared at 10g/L. In ethanol, the content in ethanol is given by (Wh-Ws)x 0.79/ Ws = 95 mL/ g dried gel. On the same sample, DSC showed that there is no water molecules remaining in the ethanol gel. It is concluded that the degree of swelling (and consequently the porosity) is nearly the same in both solvent conditions and confirms our previous results [
17], even if ethanol is a non-solvent of agarose. Hence during the process, ethanol replaces water inducing a very little shrinkage. This indicates clearly that there is no gel collapse as described on chemically cross-linked polymers by Tanaka [
23,
24].
3.2. Rheology of agarose gels
3.3.1. Comparison for gels in water and in ethanol
Gels stabilised in D
2O and ethanol were tested as a function of temperature in rheological experiments.
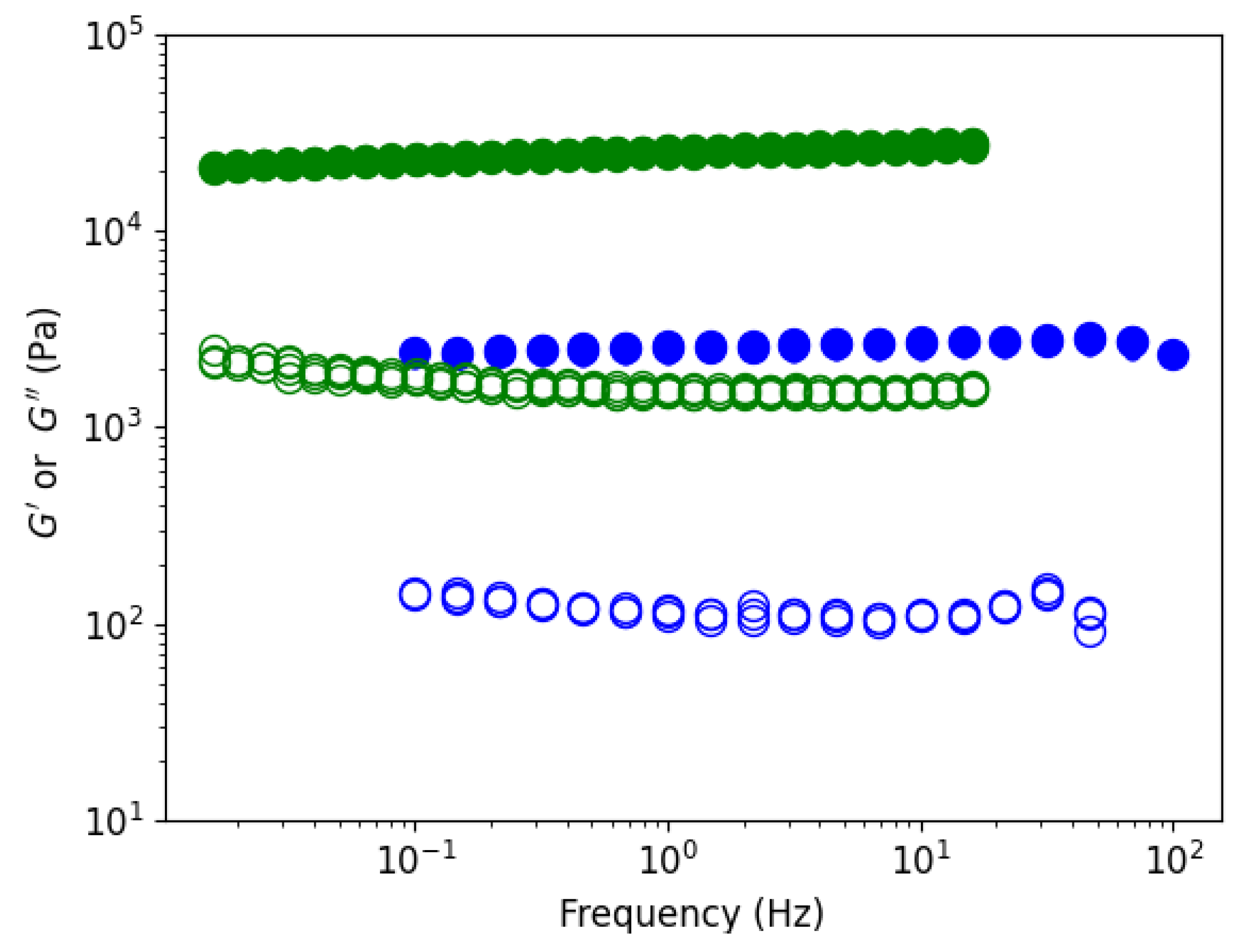
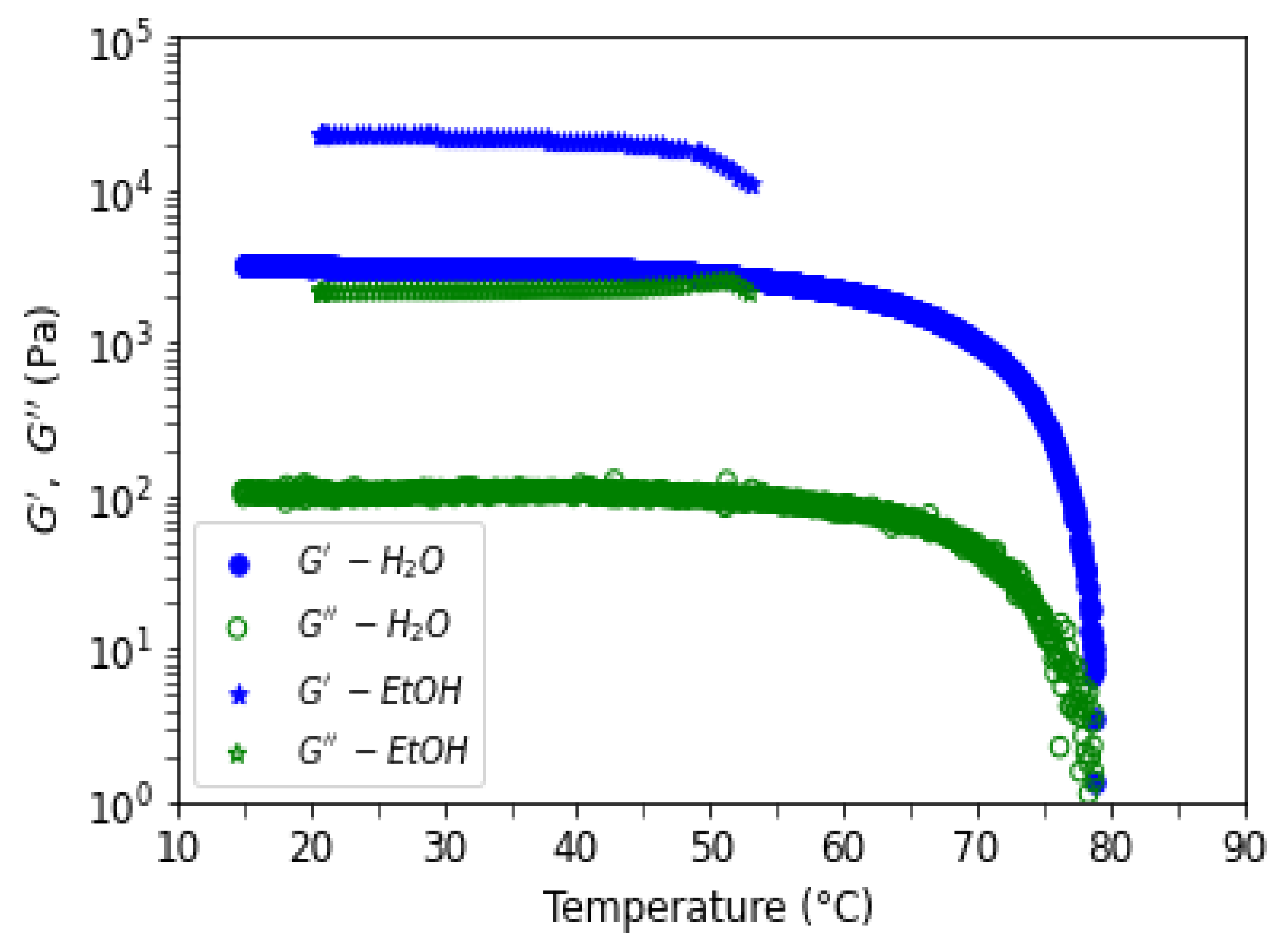
Figure 14 shows elastic and viscous moduli as a function of the frequency on formed gels in water (blue) and in ethanol (green) for a concentration of 10 mg/mL at T=20°C. Elastic moduli are well above viscous moduli, with a ratio around 10, and independent of the investigated frequencies, as expected for stiff gels. Ethanol gel is 10 times stronger than water gel even if, the swelling volume change in the non-solvent is nearly the same as in water. The characteristic strength of the ethanol gel remains even when the temperature is increased to 50°C (
Figure 15). Moduli at highest temperature are not shown due to a repeatable experimental problem (see materiel and methods).
To our knowledge, this is a first time that an ethanol gel is studied. In fact, few experiments are described in literature about agarose behaviour in the presence of ethanol. For a diluted agarose solution in water containing 6% of ethanol, mesocopic aggregates observed are more compact in morphology due to modification of the balance between H-bonds and hydrophobic interactions [
2]. On another way, ethanol is used for coagulation of agarose /DMSO for fibers production [
9] and in isoelectric focusing technique was applied for characterisation of gliadins [
25] in 45% ethanol for a better resolution. The authors precise that this composition is the highest possible ethanol concentration not causing precipitation of agarose.
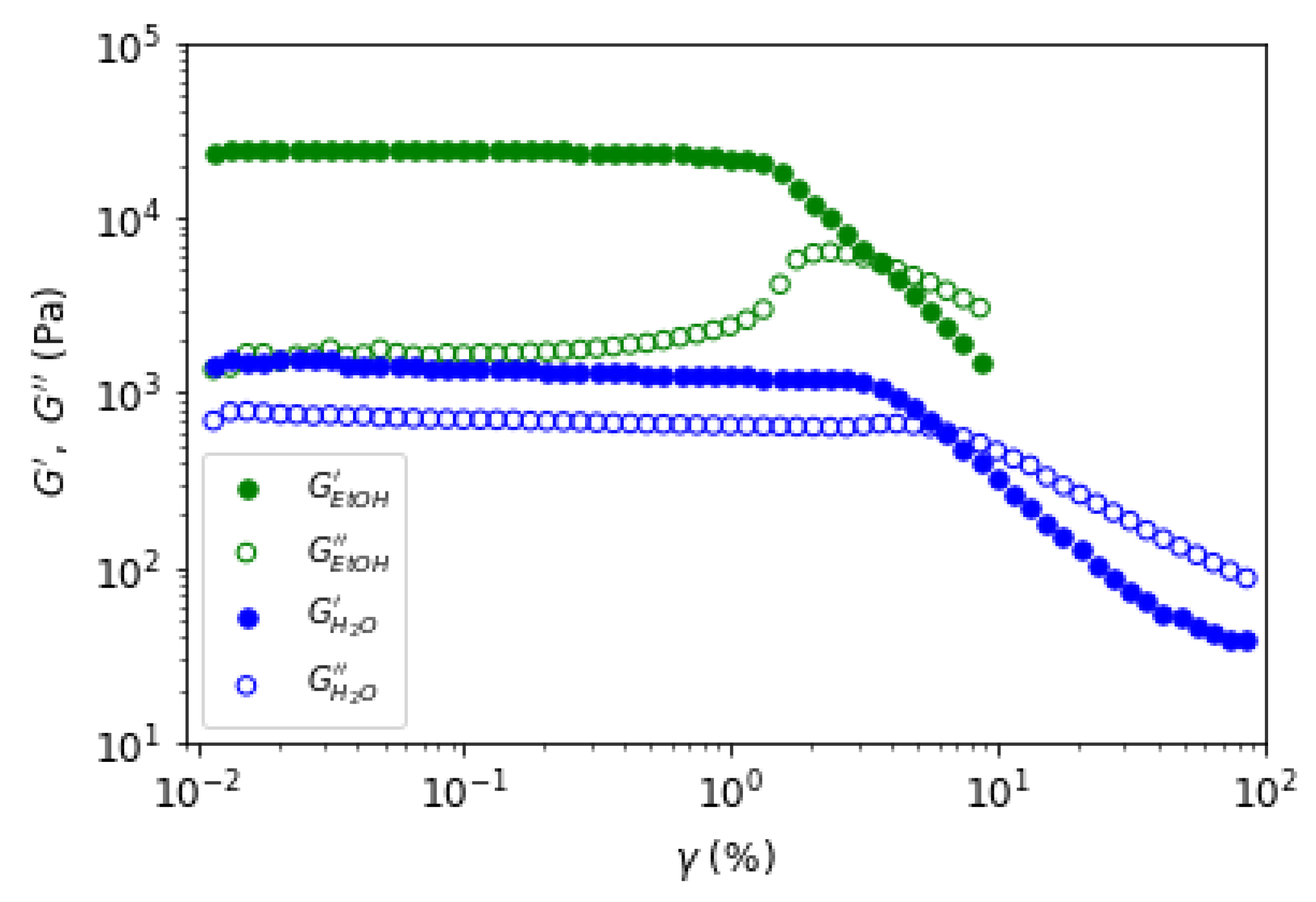
To complete the gel study,
Figure 16 shows the loss and viscous moduli as function of the applied strain for a constant frequency of f=1Hz. From the amplitude sweep, a weaker linear regime is observed for the ethanol gel (1 %) than for the water gel (3 %). For both gels, a reduction in moduli at high amplitude of oscillation is present but with a difference in the loss modulus. Indeed, there is an overshoot in the loss modulus for the ethanol gel but not for the water gel. We interpret this difference as a modification of the structural organization for the water gel and a destruction of the structure for the ethanol gel. This is confirmed by the subsequent experiments done on the ethanol gel where, the viscous and the elastic moduli drop by a factor 10 and where the oscillatory stress and strain curves do no resemble anymore to sinusoidal curves, even in the linear regime of the gel identified initially in
Figure 16. This interpretation is in agreement with literature [
26] and it is in favor of the plasticizing role of water in agarose gel. Due to the absence of water in the ethanol gel, it behaves as a brittle gel.
As observed in all measurements of G’ and G’’, regardless of temperature, G’ is higher than G’’ at the observed frequencies, which is a consequence of the behavior of an elasto-viscous fluid in a concentrated regime of high molar mass polymers.
3.3.2. Hysteresis for sol-gel transition in water
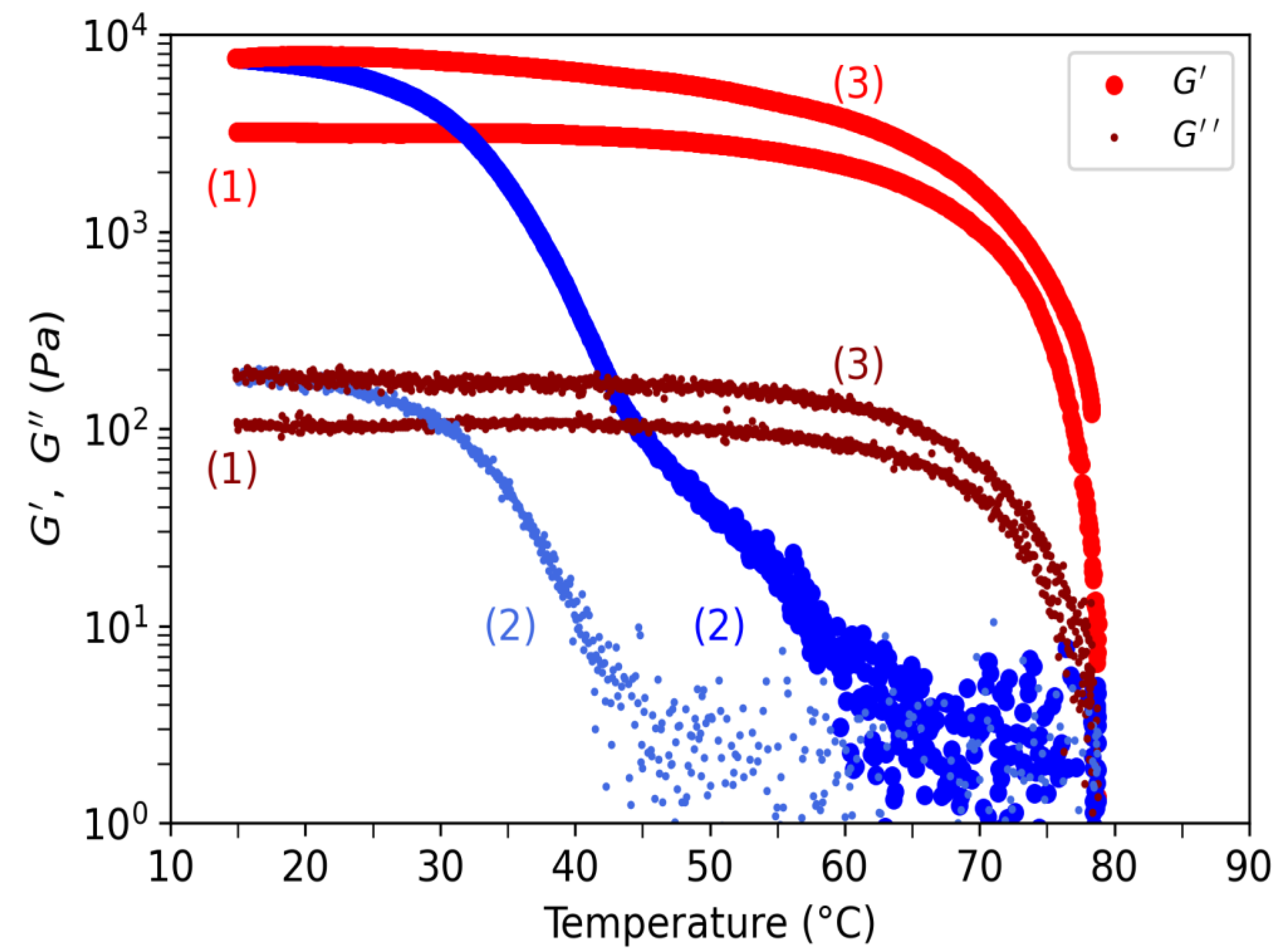
In
Figure 17, elastic and loss moduli are tracked between 15°C to 80°C at 0.5°C/min for the water gel. In that figure, three temperature ramps are shown. First increasing ramp after loading at 20°C (1) the sample was brought at 20°C before being measured in an increasing ramp where the moduli of elasticy and loss slowly decrease before starting to drop at around 60°C, showing a solid-gel transition at about 78°C, attributed to Tm. These values confirm those obtained in NMR. During this ramp, G’~3.10
3Pa and G’’~10
2Pa confirming the 10 ratio of G’/G’’. (2) first decreasing ramp, G’ is constant until about 62°C (probably at the coil-helix transition) where it increases exponentially with temperature before to reach a constant value of G’=8.10
3Pa highest that the initial value at first load. At G~45°C, there is a change of concavity corresponding to the abrupt fall in
Figure 12 in NMR. This behavior is interpreted as a consequence of the beginning cross-linkage of helix aggregates accompanied with the formation of a non-continous bulk gel. Interestingly, G’’ does not change too much until this same temperature of 62°C where it starts to increase during the decreasing temperature ramp. This behavior is interpreted as the starting point of the gel continuum formation. At T~20°C corresponding to Tg, G’’~2.10
2Pa which is also a highest value than at the initial load. (3) for this last increasing ramp, G’ and G’’ follow a similar behavior as for the first ramp but with higher values confirming a strongest strengthening of the gel at low temperature rate. This increase in G’ at low temperature is due to the temperature hystory of the sample firstly prepared without control of the temperature. The physical properties of the gels at lower temperature are in fact depending on the kinetic of gelation playing on the degree of double helices packing and of that of junction zones formation.
Conclusion
Agarose is a well-known polysaccharide extracted from red algae. After alkaline treatment, it becomes soluble in aqueous medium at high temperature (over a melting temperature Tm characterising the gel-sol transition). Then, it forms strong physical gels at low temperature (lower then Tg corresponding to the sol-gel transition). Agarose is mainly used in biotechnologies taking advantage of the large rigid pores in the gel state.
Nevertheless, the physical properties of agarose are directly related to their chemical structure and specially to the presence of substituents on the main chains. Then, in a first part of this paper, the chemical structure of agarose is perfectly determined by 1H and 13C NMR spectroscopies. Presence of a methyl substituent is determined on the D-galactose repeat unit with a degree of substitution of 0.24.
Secondly, the influence of temperature on 1H NMR spectra during heating and cooling is investigated allowing to draw a hysteresis able to help analysis of the rheological behaviour. From those results, it is suggested that on heating helix-coil transition occurs around 60°C followed by melting at Tm~80°C. This helix-coil transition is also associated with a chemical shift of the H-1 signal but also of the other protons. On cooling, a smaller chemical shift is observed but the signal indicates a slow decrease down to around 50°C corresponding to the progressive formation of double helix aggregates. Then, rapid decrease of the signal down to the gelling temperature Tg~25°C corresponds to the mobility decrease during the network formation. It is necessary to mention that there is a large dynamic process of crosslinkage between the double helix aggregates which modifies progressively the packing of aggregates and the gel properties at low temperature.
In a next step, the homogeneous gel formed at the concentration tested (10g/L in water) is charaterised by its degree of swelling and then the water is exchange by ethanol, a non-solvent of agarose. This original gel has nearly the same porosity than the gel formed in water. This will allow to extend the domains of application of agarose as gelling polymer. The NMR indicates that this gel does not melt until 80°C (limit in ethanol) as confirmed in a separate experiment. This means that there is no collapse in ethanol as previously mentioned [
17].
The last part of this study concerns rheology of agarose gel forms in water and ethanol. At ambient temperature, the ethanol gel is stronger but clearly more brittle than the gel in water identified in oscillatory strain sweep experiments. This result is related to the plasticizing effect of water in aqueous medium which is suppressed after ethanol exchange. Then, the influence of temperature on the G’ and G’’ moduli in the linear regime is study particularly on gel formed in water. In the increasing ramps water gel shows a clear melting temperature Tm=78°C in agreement with NMR. During the decreasing ramp, G’ and G’’ behaviour are correlated with two temperatures T=62°C and T=45°C interpreted respectively as the beginning of the coil to helix transition and aggregate formation followed by bulk gel formation. To conclude, it is demonstated that NMR and rheology techniques give complementary and usefull informations.
Aknowledgements
The authors thank Vincent Verdoot, Didier Bleses and Mohamed Karouch, research engineers at the Laboratoire Rheologie et Proocédés (UGA) for their technical assistance.
Conflicts of Interest
The authors declare no conflict of interest. The role of all the authors consists in collection of data, analysis and interpretation of experimental results and writing of the manuscript for publication.
References
- Djabourov, M.; Nishinari, K.; Ross-Murphy, S.B. Helical structures from neutral biopolymers in Physical gels from biological and synthetic polymers; Cambridge University Press: Cambridge, UK, 2013; pp. 208–221. [Google Scholar]
- Bulone, D.; Newman, J.; San Biagio, P.L. Mesoscopic gels at low agarose concentration: perturbation effects of ethanol. Biophysical journal 1997, 72, 388–394. [Google Scholar] [CrossRef]
- Rochas, C.; Rinaudo, M. Mechanism of gel formation in κ-carrageenan. Biopolymers: Original Research on Biomolecules 1984, 23, 735–745. [Google Scholar] [CrossRef]
- Dai, B.; Matsukawa, S. Elucidation of gelation mechanism and molecular interactions of agarose in solution by 1H NMR. Carbohydrate Research 2013, 365, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Belton, P.S.; Hills, B.P.; Raimbaud, E.R. The effects of morphology and exchange on proton NMR relaxation in agarose gels. Molecular Physics 1988, 63, 825–842. [Google Scholar] [CrossRef]
- Minghou, J.; Lahaye, M.; Yaphe, W. Structure of agar from Gracilaria spp (Rhodophyta) collected in the People’s Republic of China. Botanica Marina 1985, 28, 521–528. [Google Scholar] [CrossRef]
- Rodríguez, M.C.; Matulewicz, M.C.; Noseda, M.D.; Ducatti, D.R.B.; Leonardi, P.I. Agar from Gracilaria gracilis (Gracilariales, Rhodophyta) of the Patagonic coast of Argentina–Content, structure and physical properties. Bioresource technology 2009, 100, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Rochas, C.; Lahaye, M. Average molecular weight and molecular weight distribution of agarose and agarose-type polysaccharides. Carbohydrate polymers 1989, 10, 289–298. [Google Scholar] [CrossRef]
- Bao, X.; Hayashi, K.; Li, Y.; Teramoto, A.; Abe, K. Novel agarose and agar fibers: Fabrication and characterization. Materials Letters 2010, 64, 2435–2437. [Google Scholar] [CrossRef]
- Lahaye, M.; Rochas, C. Chemical structure and physico-chemical properties of agar. Hydrobiologia 1991, 221, 137–148. [Google Scholar] [CrossRef]
- Vaca Chávez, F.; Persson, E.; Halle, B. Internal water molecules and magnetic relaxation in agarose gels. Journal of the American Chemical Society 2006, 128, 4902–4910. [Google Scholar] [CrossRef]
- Andrasko, J. Water in agarose gels studied by nuclear magnetic resonance relaxation in the rotating frame. Biophysical Journal 1975, 15, 1235–1243. [Google Scholar] [CrossRef]
- Aşkin, M.; Yilmaz, A. The Calculation of Correlation Time (τ) for T 1 Spin–Lattice and T 2 Spin–Spin Relaxation Times in Agar Solutions. Spectroscopy letters 2004, 37, 217–224. [Google Scholar] [CrossRef]
- Gamini, A.; Toffanin, R.; Murano, E.; Rizzo, R. Hydrogen-bonding and conformation of agarose in methyl sulfoxide and aqueous solutions investigated by 1H and 13C NMR spectroscopy. Carbohydrate Research 1997, 304, 293–302. [Google Scholar] [CrossRef]
- Nicolaisen, F.M.; Meyland, I.N.G.E.; Schaumberg, K. 13C-NMR Spectra at 67.9 MHz of aqueous solutions of agarose and partly 6-O-methylated agarose at 95° C. Acta chem. scand. B 1980, 34, 103–107. [Google Scholar] [CrossRef]
- Davies, E.; Huang, Y.; Harper, J.B.; Hook, J.M.; Thomas, D.S.; Burgar, I.M.; Lillford, P.J. Dynamics of water in agar gels studied using low and high resolution 1H NMR spectroscopy. International journal of food science & technology 2010, 45, 2502–2507. [Google Scholar]
- Rinaudo, M.; Landry, S. On the volume change on non covalent gels in solvent-non solvent mixtures. Polymer Bulletin 1987, 17, 563–565. [Google Scholar] [CrossRef]
- Burger, R.; Bigler, P. DEPTQ: distorsionless enhancement by polarization transfer including the detection of quaternary nuclei. Journal of Magnetic Resonance 1998, 135, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Jouon, N.; Rinaudo, M.; Milas, M.; Desbrieres, J. Hydration of hyaluronic acid as a function of the counterion type and relative humidity. Carbohydrate polymers 1995, 26, 69–73. [Google Scholar] [CrossRef]
- Yoshida, H.; Hatakeyama, T.; Hatakeyama, H. Characterization of water in polysaccharide hydrogels by DSC. Journal of Thermal Analysis and Calorimetry 1993, 40, 483–489. [Google Scholar] [CrossRef]
- Ed-Daoui, A.; Snabre, P. Poroviscoelasticity and compression-softening of agarose hydrogels. Rheologica Acta 2021, 60, 327–351. [Google Scholar] [CrossRef]
- Cardoso, M.V.C.; Sabadini, E. The gelling of κ-carrageenan in light and heavy water. Carbohydrate research 2010, 345, 2368–2373. [Google Scholar] [CrossRef]
- Tanaka, T. Collapse of gels and the critical endpoint. Physical review letters 1978, 40, 820. [Google Scholar] [CrossRef]
- Tanaka T. Phase transition of gels. In Polyelectrolyte gels; properties, preparation and applications ACS Symposium Series No. 480, pp.1-21. Edited by R. A. Harland and R. K. Prud’homme American Chemical Society, Washington, 1991.
- Stenram, U.; Acevedo, F.; Larsson-Raznikiewicz, M. Agarose gel isoelectric focusing of gliadins in the presence of ethanol. Food chemistry 1990, 36, 305–310. [Google Scholar] [CrossRef]
- Donley, G.J.; Singh, P.K.; Shetty, A.; Rogers, S.A. Elucidating the G ″overshoot in soft materials with a yield transition via a time-resolved experimental strain decomposition. Proceedings of the National Academy of Sciences 2020, 117, 21945–21952. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Photographs of the two plates geometry used for the gel rheology. The photograph on the left shows the smooth plate-plate mounted onto the Peltier system control. The photograph on the right shows the geometry of the radial ribbed plate. Both geometries have a diameter of 25mm consist of a cup on the bottom part and a plate on the upper part between which the gel is placed before being covered by a silicon oil to prevent evaporation of water.
Figure 1.
Photographs of the two plates geometry used for the gel rheology. The photograph on the left shows the smooth plate-plate mounted onto the Peltier system control. The photograph on the right shows the geometry of the radial ribbed plate. Both geometries have a diameter of 25mm consist of a cup on the bottom part and a plate on the upper part between which the gel is placed before being covered by a silicon oil to prevent evaporation of water.
Figure 2.
Representation of the repeat unit in agarose.
Figure 2.
Representation of the repeat unit in agarose.
Figure 7.
Figure 7. Proton NMR spectrum of agarose in DMSO-d6 at 80°C
Figure 7.
Figure 7. Proton NMR spectrum of agarose in DMSO-d6 at 80°C
Figure 8.
DEPTQ spectrum for agarose in DMSO-d6 at 80°C.
Figure 8.
DEPTQ spectrum for agarose in DMSO-d6 at 80°C.
Figure 9.
2D HMBC NMR spectrum of agarose in DMSO-d6 at 80°C used for location of the methyl substituent.
Figure 9.
2D HMBC NMR spectrum of agarose in DMSO-d6 at 80°C used for location of the methyl substituent.
Figure 10.
Temperature increase on 1H NMR spectrum of agarose in D2O from 298°K to 353°K.
Figure 10.
Temperature increase on 1H NMR spectrum of agarose in D2O from 298°K to 353°K.
Figure 11.
Temperature decrease for 1H NMR spectrum of agarose in D2O from 353°K to 298°K.
Figure 11.
Temperature decrease for 1H NMR spectrum of agarose in D2O from 353°K to 298°K.
Figure 12.
Evolution of the H-1 integrals as a function of temperature on heating from 25 to 85°C and cooling from 85 to 25°C. Open red circle corresponds to the heating and filled blue circles to the cooling.
Figure 12.
Evolution of the H-1 integrals as a function of temperature on heating from 25 to 85°C and cooling from 85 to 25°C. Open red circle corresponds to the heating and filled blue circles to the cooling.
Figure 13.
1H NMR agarose gel in ethanol. Evolution during temperature increase from 298°K to 353°K.
Figure 13.
1H NMR agarose gel in ethanol. Evolution during temperature increase from 298°K to 353°K.
Figure 14.
Rheological characteristic G’ (●) and G’’(○) as a function of the frequency on gels formed in water (blue) and in ethanol (green). Concentration 10g/L at 20°C for 01% applied strain.
Figure 14.
Rheological characteristic G’ (●) and G’’(○) as a function of the frequency on gels formed in water (blue) and in ethanol (green). Concentration 10g/L at 20°C for 01% applied strain.
Figure 15.
Rheological moduli G’ and G’’, at 1 Hz and 0.1 % strain, for agarose gels in water ( ●, ○) and in ethanol (★, ☆) as a function of temperature. The imposed temperature rate ramp is equal to 0.5°C/min.
Figure 15.
Rheological moduli G’ and G’’, at 1 Hz and 0.1 % strain, for agarose gels in water ( ●, ○) and in ethanol (★, ☆) as a function of temperature. The imposed temperature rate ramp is equal to 0.5°C/min.
Figure 16.
Elastic and viscous moduli as a function of the strain for a constant frequency of 1Hz for the water et ethanol gels.
Figure 16.
Elastic and viscous moduli as a function of the strain for a constant frequency of 1Hz for the water et ethanol gels.
Figure 17.
Elastic (big dots) and viscous (small dots) moduli as a function of the applied temperature for the water gel. Increasing temperatures correspond to the red symbols and decreasing temperatures to the blue symbols. Numbers indicate the time sequence of the applied temperature ramp, (1) first increase, (2) first decrease, (3) second increase.
Figure 17.
Elastic (big dots) and viscous (small dots) moduli as a function of the applied temperature for the water gel. Increasing temperatures correspond to the red symbols and decreasing temperatures to the blue symbols. Numbers indicate the time sequence of the applied temperature ramp, (1) first increase, (2) first decrease, (3) second increase.
Table 1.
Chemical shifts of proton and carbon signals for agarose in D2O at 80°C.
Table 1.
Chemical shifts of proton and carbon signals for agarose in D2O at 80°C.
| |
C1 |
C2 |
C3 |
C4 |
C5 |
C6 |
CH3 |
| G’ |
102.63 |
70.38 |
82.33 |
68.93 |
75.51 |
61.56 |
|
| G” |
102.63 |
70.38 |
82.33 |
68.93 |
75.51 |
71.89 |
59.2 |
| G |
98.52 |
70.05 |
80.31 |
77.6 |
75.7 |
69.64 |
|
| |
H1 |
H2 |
H3 |
H4 |
H5 |
H6 |
CH3 |
| G’ |
4.58 |
3.65 |
3.78 |
4.14 |
3.73 |
3.80 |
|
| G” |
4.58 |
3.65 |
3.78 |
4.14 |
3.73 |
3.70 |
3.43 |
| G |
5.16 |
4.13 |
4.54 |
4.68 |
4.57 |
4.22a-4.04b
|
|
Table 2.
Chemical shifts of proton and carbon signals for agarose in DMSO-d6 at 80°C.
Table 2.
Chemical shifts of proton and carbon signals for agarose in DMSO-d6 at 80°C.
| |
C1 |
C2 |
C3 |
C4 |
C5 |
C6 |
CH3 |
| G’ |
102.5 |
70.13 |
81.35 |
68.11 |
75.54 |
60.8 |
|
| G” |
102.5 |
70.13 |
80.99 |
68.63 |
73.52 |
71.96 |
58.9 |
| G |
97.54 |
70.37 |
80.23 |
76.53 |
75.12 |
68.9 |
|
| |
H1 |
H2 |
H3 |
H4 |
H5 |
H6 |
CH3 |
| G’ |
4.31 |
3.45 |
3.54 |
3.83 |
3.44 |
3.55 |
|
| G” |
4.31 |
3.45 |
3.47 |
3.76 |
3.63 |
3.54a-3.46b
|
3.3 |
| G |
5.09 |
3.83 |
4.22 |
4.34 |
4.35 |
3.91 |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).