Submitted:
13 April 2023
Posted:
14 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
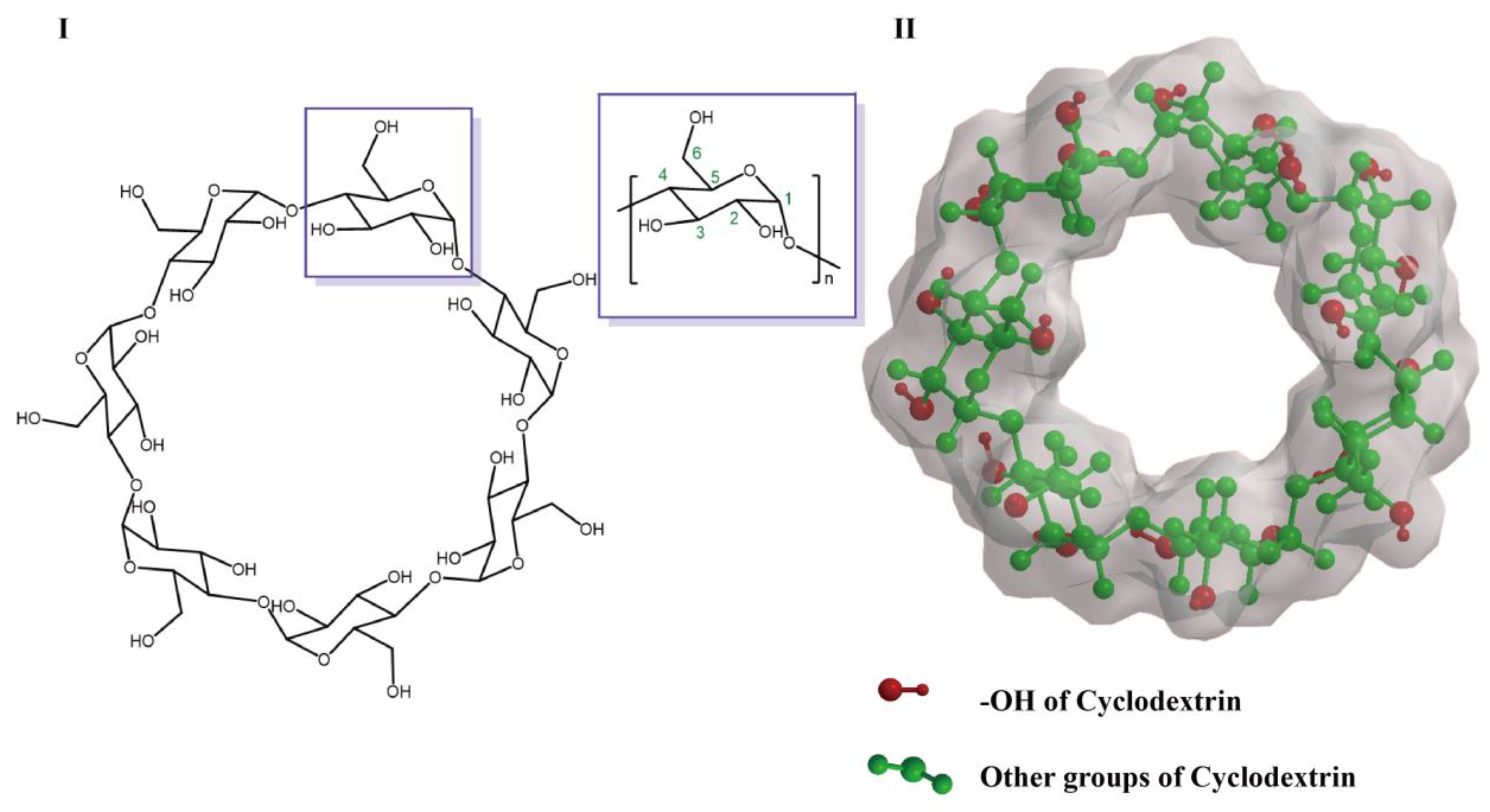
2. Structure
3. Function
3.1. Noncovalent interactions
3.2. Functional modification of the active hydroxyl groups
4. Application
4.1. Physicochemical characteristics alteration of the drugs
4.1.1. Without any modification
4.1.2. Pro or post modification of CyDs
4.2. Therapeutic talent
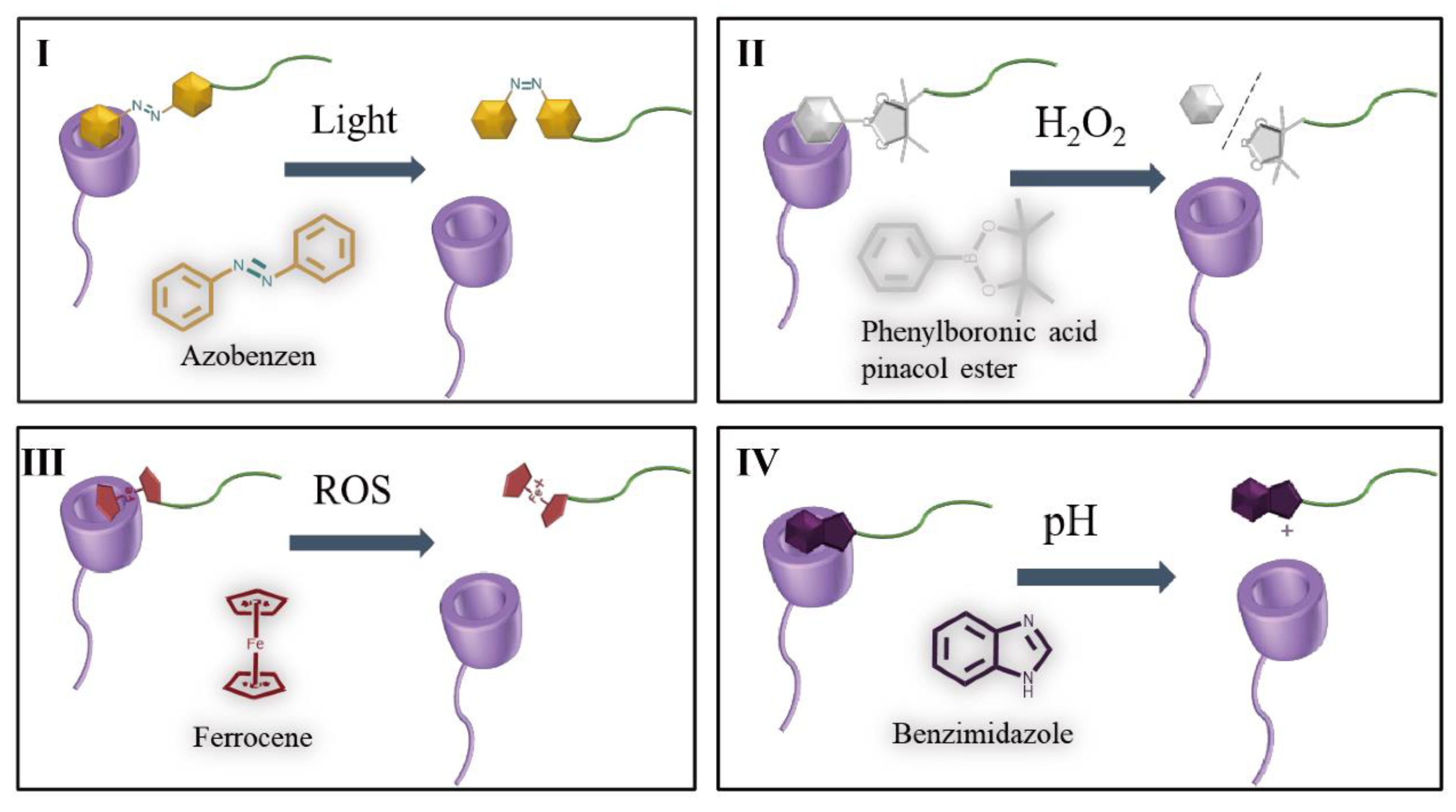
4.3. Stimulus responsive switch
4.4. Self-assemble capability
4.4.1. Self-assembly directed by hydrophilic-hydrophobic interactions
4.4.2. Self-assembly directed by charge interactions
4.4.3. Self-assembly directed by coordination interactions
4.4.4. CyDs-based supramolecular necklaces
4.5. Fiber formation
5. Summary and Outlook
- (1)
- Precise and regular structures are highly welcomed by biomaterial engineers so that the structure-property relationships could be tuned according to our needs 9. These systems are more possible to meet the plenty of challenges from complex organism owing to the unique structure-property relationship. The representatives of such kind of materials are MOF, covalent-organic frameworks (COF) and supramolecular necklaces [156].
- (2)
- Self-assembly has been widely applied to many fields, scientists have developed different kinds of materials and discovered the mechanism of self-assembly including charge interactions, hydrophilic-hydrophobic interactions, coordination interactions, etc. Benefit from controllability, self-assembly should be further developed by scientists in a long time.
- (3)
- Multifunction is another essential requirement for drug delivery. Regardless of the therapeutic effects of biomaterials, targeting capability, immune clearance avoidance and biocompatibility are essential characteristics for delivery system. Reactive hydroxyl group and cavity assigned to CyD are ideal candidates to meet the three needs at the same time. In-depth development of CyDs may help to increase the functions of drug delivery.
- (4)
- Chemical modification, polypeptide modification and biofilm functionalization are three powerful strategies in drug delivery. Among them, biofilm functionalization seems to be the most biocompatible one and has been demonstrated great potentials to clinical verification. However, biofilm functionalization is far from being explored in combining with CyDs.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Brewster, M.E.; Loftsson, T. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug. Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef] [PubMed]
- Wankar, J.; Kotla, N.G.; Gera, S.; Rasala, S.; Pandit, A.; and Rochev, Y.A. Recent advances in host-guest self-assembled cyclodextrin carriers: Implications for responsive drug delivery and biomedical engineering. Adv. Funct. Mater. 2020, 30, 1909049. [Google Scholar] [CrossRef]
- Mousazadeh, H.; Pilehvar-Soltanahmadi, Y.; Dadashpour, M.; Zarghami, N. Cyclodextrin based natural nanostructured carbohydrate polymers as effective non-viral siRNA delivery systems for cancer gene therapy. J. Control. Release 2021, 330, 1046–1070. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Jiang, M. Cyclodextrin-based inclusion complexation bridging supramolecular chemistry and macromolecular self-assembly. Chem. Soc. Rev. 2011, 40, 2254–2266. [Google Scholar] [CrossRef] [PubMed]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M. , Andaloussi, S.E., and Vader, P., Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Liu, Y.; Zhao, L.; Zhao, L.; Wang, C.; Liu, C.; Xu, B. , Construction of a pH-sensitive self-assembly in aqueous solutions based on a dansyl-modified β-cyclodextrin. Soft Matter 2021, 17, 7516–7523. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Chen, Y.; Sun, H.; and Liu, Y. Enzyme-responsive supramolecular nanoparticles based on carboxyl-modified cyclodextrins for dual substrate loading. Asian J. Org. Chem. 2018, 7, 870–874. [Google Scholar] [CrossRef]
- He, H.; Chen, S.; Zhou, J.; Dou, Y.; Song, L.; Che, L.; Zhou, X.; Chen, X.; Jia, Y.; Zhang, J.; Li, S.; Li, X. Cyclodextrin-derived pH-responsive nanoparticles for delivery of paclitaxel. Biomaterials 2013, 34, 5344–5358. [Google Scholar] [CrossRef]
- Webber, M.; Langer, R. Drug delivery by supramolecular design. Chem. Soc. Rev. 2017, 46, 6600–6620. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Garcia-Gonzalez, C.A.; Concheiro, A. Cyclodextrins as versatile building blocks for regenerative medicine. J. Control. Release 2017, 268, 269–281. [Google Scholar] [CrossRef]
- Ikuta, D.; Hirata, Y.; Wakamori, S.; Shimada, H.; Tomabechi, Y.; Kawasaki, Y.; Ikeuchi, K.; Hagimori, T.; Matsumoto, S.; Yamada, H. Conformationally supple glucose monomers enable synthesis of the smallest cyclodextrins. Science 2019, 364, 674–677. [Google Scholar] [CrossRef]
- Tian, B.; Hua, S.; Liu, J. Cyclodextrin-based delivery systems for chemotherapeutic anticancer drugs: A review. Carbohyd. Polym. 2020, 232, 115805. [Google Scholar] [CrossRef]
- Khan, A.; Forgo, P.; Stine, K.; Souza, V. D’; Methods for selective modifications of cyclodextrins. Chem. Rev. 1998, 98, 1977–1996. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dyer, M.; Yu, M. Cyclodextrin-mediated soft cutting of single-walled carbon nanotubes. J. Am. Chem. Soc. 2001, 123, 6201–6202. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.; Shende, P. Dual drug delivery of cyclodextrin cross-linked artemether and lumefantrine nanosponges for synergistic action using 23 full factorial designs. Colloids Surface. A 2020, 602, 125049. [Google Scholar] [CrossRef]
- Wang, L.; Kang, Y.; Xing, C.; Guo, K.; Zhang, X.; Ding, L.; Zhang, S.; Li, B. beta-Cyclodextrin based air filter for high-efficiency filtration of pollution sources. J. Hazard. Mater. 2019, 373, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.C.; Yang, C.Y.; Wu, T.H.; Tseng, C.H.; Yen, F.L. Myricetin nanofibers enhanced water solubility and skin penetration for increasing antioxidant and photoprotective activities. Pharmaceutics 2023, 15, 906. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, H.; Dai, X.; Chen, Y.; Liu, Y. Multivalent supramolecular assembly based on a triphenylamine derivative for near-infrared lysosome targeted imaging. ACS Appl. Mater. Interfaces 2022, 14, 4417–4422. [Google Scholar] [CrossRef]
- Escobar, K.; Garrido-Miranda, K.A.; Pulido, R.; Naveas, N.; Manso-Silván, M.; Hernandez-Montelongo, J. Coatings of cyclodextrin/ctric-acid biopolymer as drug delivery systems: a review. Pharmaceutics 2023, 15, 296. [Google Scholar] [CrossRef]
- Crini, G. A history of cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Rodal, S.; Skretting, G.; Garred, Ø.; Vilhardt, F.; Van Deurs, B.; Sandvig, K. Extraction of cholesterol with methyl-β-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol. Biol. Cell 1999, 10, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Fenton, O.; Olafson, K.; Pillai, P.; Mitchell, M.; Langer, R. Advances in biomaterials for drug delivery. Adv. Mater. 2018, 30, 1705328. [Google Scholar] [CrossRef]
- Chen, L.; Yang, H. Construction of stimuli-responsive functional materials via hierarchical self-assembly involving coordination interactions. Acc. Chem. Res. 2018, 51, 2699–2710. [Google Scholar] [CrossRef]
- Zheng, K.; Liu, X.; Liu, H.; Dong, D.; Li, L.; Jiang, L.; Huang, M.; Ding, C. Novel pH-triggered doxorubicin-releasing nanoparticles self-assembled by functionalized β-cyclodextrin and amphiphilic phthalocyanine for anticancer therapy. ACS Appl. Mater. Interfaces 2021, 13, 10674–10688. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; She, P.; Ma, B.; Zhao, Z.; Li, G.; Wang, Y. Cyclodextrin based natural nanostructured carbohydrate polymers as effective non-viral siRNA delivery systems for cancer gene therapy. Biomaterials 2022, 288, 121734. [Google Scholar]
- Xiu, K.M.; Yang, J.J.; Zhao, N.N.; Li, J.S.; Xu, F.J. Multiarm cationic star polymers by atom transfer radical polymerization from β-cyclodextrin cores: Influence of arm number and length on gene delivery. Acta Biomater. 2013, 9, 4726–4733. [Google Scholar] [CrossRef] [PubMed]
- Asim, M.; Nazir, I.; Jalil, A.; Laffleur, F.; Matuszczak, B.; Bernkop-Schnurch, A. Per-6-thiolated cyclodextrins: a novel type of permeation enhancing excipients for BCS class IV drugs. ACS Appl. Mater. Interfaces 2020, 12, 7942–7950. [Google Scholar] [CrossRef]
- Arrais, A.; Bona, E.; Todeschini, V.; Caramaschi, A.; Massa, N.; Roncoli, M.; Minervi, A.; Perin, E.; Gianotti, V. Thymus vulgaris Eessential oil in beta-cyclodextrin for solid-state pharmaceutical applications. Pharmaceutics 2023, 15, 914. [Google Scholar] [CrossRef]
- Davis, M.; Brewster, M. Cyclodextrin-based pharmaceutics: past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: structure, physicochemical properties and pharmaceutical applications. Int. J. Pharmaceut. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Kang, Y.; Guo, K.; Li, B.; Zhang, S. Nanoassemblies driven by cyclodextrin-based inclusion complexation. Chem. Commun. 2014, 50, 11083–11092. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Kim, J.; Lee, Y.; Kim, J.; Choi, H.; Lee, J.; Park, H.; Kang, Y.; Kim, I.; Lee, B.; Hoffman, A.; Kim, W. Poly-paclitaxel/cyclodextrin-SPION nano-assembly for magnetically guided drug delivery system. J. Control. Release 2016, 231, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Ajiro, H. Preparation of stereocomplex and pseudo-polyrotaxane with various cyclodextrins as wheel components using triblock copolymer of poly (ethylene glycol) and polylactide. Soft Matter 2022, 18, 8885–8893. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.; Zhao, Y.; Sen, A.; Hui, S. Cyclodextrin enhanced transdermal delivery of piroxicam and carboxyfluorescein by electroporation. J. Control. Release 2004, 99, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, S.; Liu, H.; Yang, C.; Kang, Y.; Wang, M. Unimolecular micelles of amphiphilic cyclodextrin-core star-like block copolymers for anticancer drug delivery. Chem. Commun. 2015, 51, 15768–15771. [Google Scholar] [CrossRef] [PubMed]
- de Vries, W.; Niehues, M.; Wissing, M.; Würthwein, T.; Mäsing, F.; Fallnich, C.; Studer, A.; Ravoo, B. Photochemical preparation of gold nanoparticle decorated cyclodextrin vesicles with tailored plasmonic properties. Nanoscale 2019, 11, 9384–9391. [Google Scholar] [CrossRef] [PubMed]
- Widener, A.E.; Bhatta, M.; Angelini, T.E.; Phelps, E.A. A polysaccharide/ tetraphenylethylene- mediated blue-light emissive and injectable supramolecular hydrogel. Biomater. Sci. 2021, 9, 2480–2493. [Google Scholar] [CrossRef]
- Jia, S.; Xu, W.; Chen, Y.; Liu, Y. Pyrrole/macrocycle/MOF supramolecular co-assembly for flexible solid state supercapacitors. Chinese Chem. Lett. 2021, 32, 2773–2776. [Google Scholar] [CrossRef]
- Wdowiak, K.; Rosiak, N.; Tykarska, E.; Żarowski, M.; Płazińska, A.; Płaziński, W.; Cielecka-Piontek, J. Amorphous inclusion complexes: molecular interactions of hesperidin and hesperetin with HP-Β-CD and their biological effects. Int. J. Mol. Sci. 2022, 23, 4000. [Google Scholar] [CrossRef]
- Oka, N.; Matsubara, H.; Imai, T.; Yoshioka, Y.; Katsuki, J.; Fujii, S.; Nakamura, S.; Shimazawa, M.; Hara, H.; Sakurai, K. Protective effects of alpha-mangostin encapsulated in cyclodextrin-nanoparticle on cerebral ischemia. J. Controll. Release 2023, 353, 216–228. [Google Scholar] [CrossRef]
- Zarepour, A.; Zarrabi, A.; Larsen, K. Fabricating β-cyclodextrin based pH-responsive nanotheranostics as a programmable polymeric nanocapsule for simultaneous diagnosis and therapy. Int. J. Nanomedicine 2019, 14, 7017–7038. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Liu, Y.; Jiang, J.; Li, X.; Ye, F.; Fu, Y.; Zhao, L. Thiram/hydroxypropyl-β-cyclodextrin inclusion complex electrospun nanofibers for a fast dissolving water-based drug delivery system. Colloid. Surface. B 2021, 201, 111625. [Google Scholar] [CrossRef]
- Xu, C.; Lu, J.; Zhou, L.; Liang, J.; Fang, L.; Cao, F. Multifunctional nanocomposite eye drops of cyclodextrin complex@layered double hydroxides for relay drug delivery to the posterior segment of the eye. Carbohyd. Polym. 2021, 260, 117800. [Google Scholar] [CrossRef]
- Zeng, S.; Xing, C.; Chen, L.; Xu, L.; Li, B.; Zhang, S. Green flame-retardant flexible polyurethane foam based on cyclodextrin. Polym. Degrad. Stabil. 2020, 178, 109171. [Google Scholar] [CrossRef]
- Caizer, C.; Caizer, I.S. Study on maximum specific loss power in Fe3O4 nanoparticles decorated with biocompatible gamma-cyclodextrins for cancer therapy with superparamagnetic hyperthermia. Int. J. Mol. Sci. 2021, 22, 10071. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Liu, Y.; Liu, H.; Mao, Q.; Yu, Q.; Kitagishi, H.; Zhang, Y.; Xiao, L.; Liu, Y. Supramolecular dual polypeptides induced tubulin aggregation for synergistic cancer theranostics. J. Med. Chem. 2022, 65, 13473–13481. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.; Baek, Y.; Hahm, E.; Lee, S.; Pham, X.; Noh, M.; Kim, D.; Jun, B. Functionalized beta-cyclodextrin immobilized on Ag-Embedded silica nanoparticles as a drug carrier. Int. J. Mol. Sci. 2019, 20, 315–335. [Google Scholar] [CrossRef]
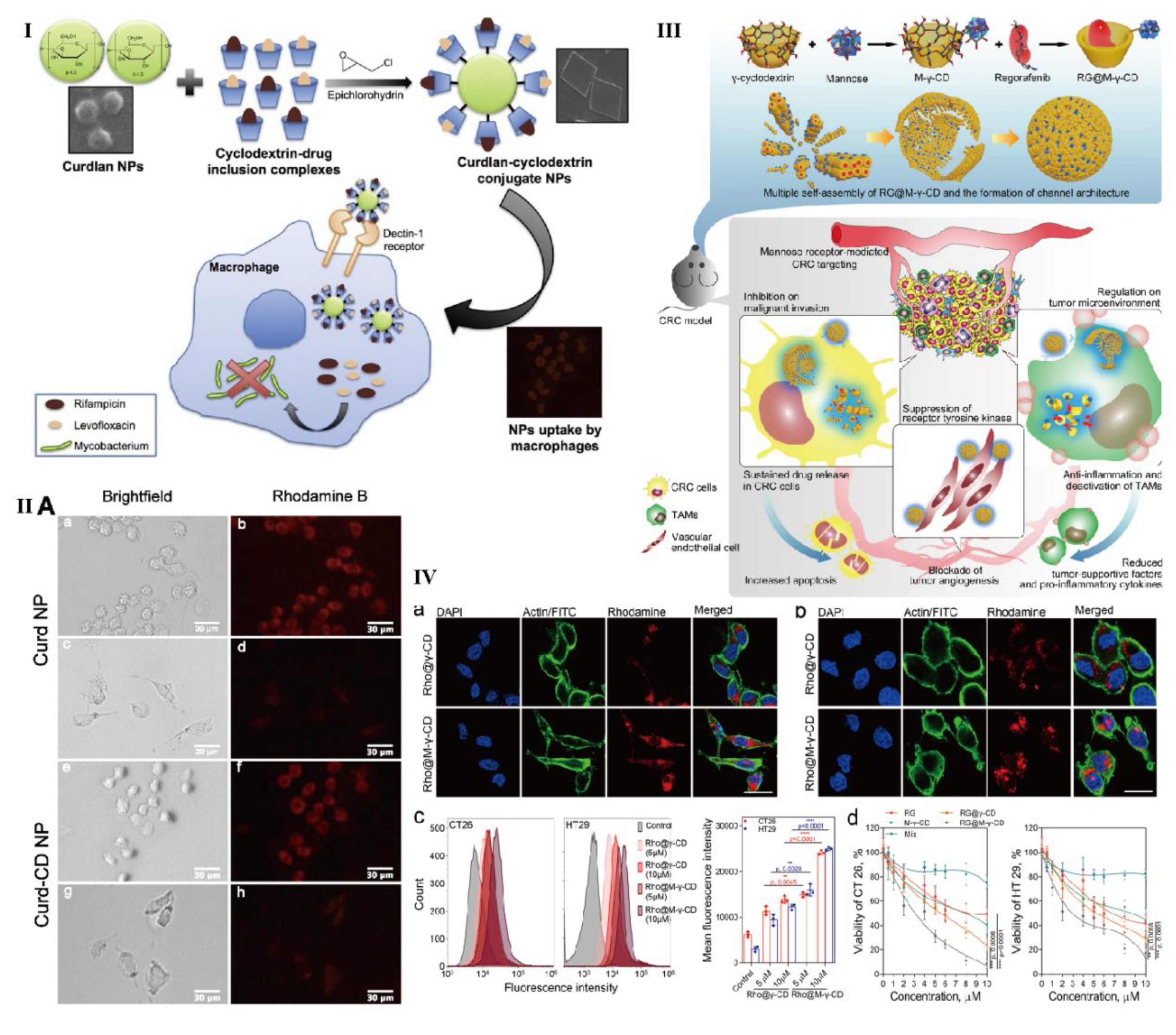
- Yunus Basha, R.; Doble, M. Dual delivery of tuberculosis drugs via cyclodextrin conjugated curdlan nanoparticles to infected macrophages. Carbohyd. Polym. 2019, 218, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, K.; Lee, H.; Kim, J.; Kim, Y.; Lee, J.; Kim, K.; Yun, J.; Hwang, B.Y.; Hong, J.; Kim, Y.; Han, S. Curdlan activates dendritic cells through dectin-1 and toll-like receptor 4 signaling. Int. Immunopharmacol. 2016, 39, 71–78. [Google Scholar] [CrossRef]
- Bai, H.; Wang, J.; Phan, C.; Chen, Q.; Hu, X.; Shao, G.; Zhou, J.; Lai, L.; Tang, G. Cyclodextrin-based host-guest complexes loaded with regorafenib for colorectal cancer treatment. Nat. Commun. 2021, 12, 759–777. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Liang, N.; Li, Q.; Yan, P.; Sun, S. Biotin and arginine modified hydroxypropyl-β-cyclodextrin nanoparticles as novel drug delivery systems for paclitaxel. Carbohyd. Polym. 2019, 216, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, P.; Lin, L.; Guo, F.; Wang, Q.; Piao, Y.; Diao, G. Study of a water-soluble supramolecular complex of curcumin and β-cyclodextrin polymer with electrochemical property and potential anti-cancer activity. Chinese Chem. Lett. 2022, 33, 4043–4047. [Google Scholar] [CrossRef]
- Juric, D.; Rohner, N.; von Recum, H. Molecular imprinting of cyclodextrin supramolecular hydrogels improves drug loading and delivery. Macromol. Biosci. 2019, 19, e1800246. [Google Scholar] [CrossRef] [PubMed]
- Chaterji, S.; Kwon, I.; Park, K. Smart polymeric gels: redefining the limits of biomedical devices. Prog. Polym. Sci. 2007, 32, 1083–1122. [Google Scholar] [CrossRef] [PubMed]
- Pooresmaeil, M.; Namazi, H. Surface modification of graphene oxide with stimuli-responsive polymer brush containing beta-cyclodextrin as a pendant group: Preparation, characterization, and evaluation as controlled drug delivery agent. Colloids Surf. B Biointerfaces 2018, 172, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, D.; Liao, C.; Fang, Y.; Guo, B. Development of a promising drug delivery for formononetin: Cyclodextrin-modified single-walled carbon nanotubes. J. Drug Deliv. Sci. Tec. 2018, 43, 461–468. [Google Scholar] [CrossRef]
- Yamada, Y.; Daikuhara, S.; Tamura, A.; Nishida, K.; Yui, N.; Harashima, H. Enhanced autophagy induction via the mitochondrial delivery of methylated beta-cyclodextrin-threaded polyrotaxanes using a MITO-Porter. Chem. Commun. 2019, 55, 7203–7206. [Google Scholar] [CrossRef]
- Hammoud, Z.; Khreich, N.; Auezova, L.; Fourmentin, S.; Elaissari, A.; Greige-Gerges, H. Cyclodextrin-membrane interaction in drug delivery and membrane structure maintenance. Int. J. Pharm. 2019, 564, 59–76. [Google Scholar] [CrossRef]
- Mehta, S.; Bongcaron, V.; Nguyen, T.; Jirwanka, Y.; Maluenda, A.; Walsh, A.; Palasubramaniam, J.; Hulett, M.; Srivastava, R.; Bobik, A.; Wang, X.; Peter, K. An ultrasound-responsive theranostic cyclodextrin-loaded nanoparticle for multimodal imaging and therapy for atherosclerosis. Small 2022, 18, 2200967. [Google Scholar] [CrossRef]
- Collins, C.; Loren, B.; Alam, M.; Mondjinou, Y.; Skulsky, J.; Chaplain, C.; Haldar, K.; Thompson, D. Pluronic based beta-cyclodextrin polyrotaxanes for treatment of Niemann-Pick Type C disease. Sci. Rep. 2017, 7, 46737. [Google Scholar] [CrossRef]
- Tamura, A.; Ohashi, M.; Nishida, K.; Yui, N. Acid-induced intracellular dissociation of beta-cyclodextrin-threaded polyrotaxanes directed toward attenuating phototoxicity of bisretinoids through promoting excretion. Mol. Pharmaceut. 2017, 14, 4714–4724. [Google Scholar] [CrossRef]
- Jana, B.; Mohapatra, S.; Mondal, P.; Barman, S.; Pradhan, K.; Saha, A.; Ghosh, S. α-Cyclodextrin interacts close to vinblastine site of tubulin and delivers curcumin preferentially to the tubulin durface of cancer cell. ACS Appl. Mater. Interfaces 2016, 8, 13793–13803. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Gong, X.; Kang, Y.; Jiang, Z.; Zhang, S.; Li, B. Light-, pH- and thermal-responsive hydrogels with the triple-shape memory effect. Chem. Commun. 2016, 52, 10609–10612. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Cao, Y.; Han, X.; Fan, M.; Yuan, Q.; Wang, Y.; Li, B.; Zhang, S. Photoreversible polymer-surfactant micelles using the molecular recognition of alpha-cyclodextrin. Langmuir 2013, 29, 3188–3194. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Barner-Kowollik, C. Dynamic macromolecular material design-the versatility of cyclodextrin-based host-guest chemistry. Angew. Chem. Int. Ed. Engl. 2017, 56, 8350–8369. [Google Scholar] [CrossRef]
- Erdogar, N.; Esendagli, G.; Nielsen, T.; Sen, M.; Oner, L.; Bilensoy, E. Design and optimization of novel paclitaxel-loaded folate-conjugated amphiphilic cyclodextrin nanoparticles. Int. J. Pharm. 2016, 509, 375–390. [Google Scholar] [CrossRef]
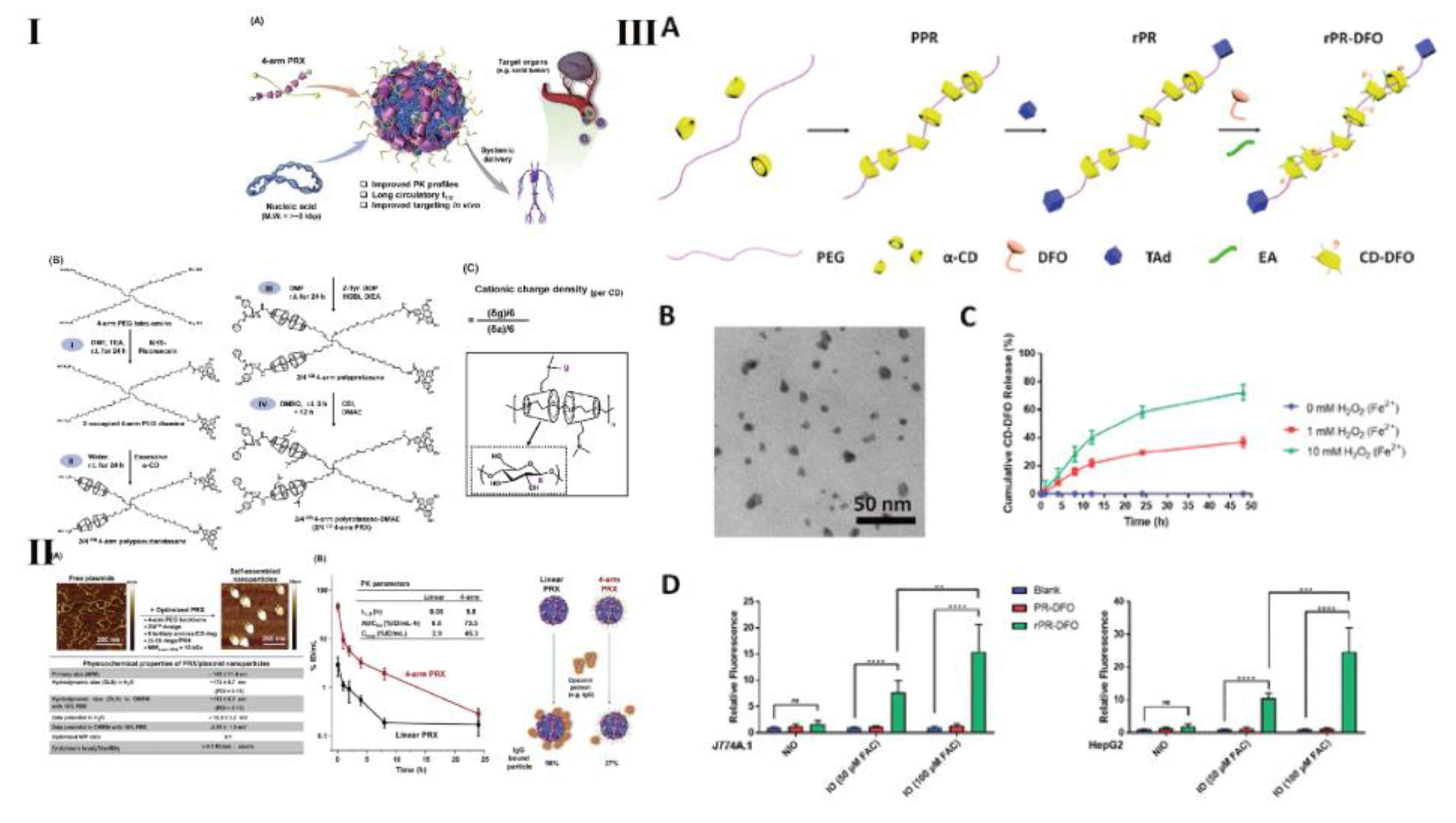
- Chu, H.; Meng, X.; Liu, B.; Liu, C.; Cheng, Y.; Sun, Z.; Wang, Y. Reactive oxygen species-triggered dissociation of a polyrotaxane-based nanochelator for enhanced clearance of systemic and hepatic iron. Biomater. Sci. 2022, 10, 3569–3574. [Google Scholar] [CrossRef]
- Kang, Y.; Ju, X.; Ding, L.S.; Zhang, S.; Li, B.J. Reactive oxygen species and glutathione dual redox-responsive supramolecular assemblies with controllable release capability. ACS Appl. Mater. Interfaces 2017, 9, 4475–4484. [Google Scholar] [CrossRef]
- Asfiya, R.; Maiti, B.; Kamra, M.; Karande, A.A.; Bhattacharya, S. Enzyme-responsive polysaccharide supramolecular nanoassembly for enhanced DNA encapsulation and controlled release. Biomater. Sci. 2021, 9, 7636–7647. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, N.; Xiao, K.; Yu, Q.; Liu, Y. Photo-controlled reversible microtubule assembly mediated by paclitaxel-modified cyclodextrin. Angew. Chem. Int. Ed. Engl. 2018, 130, 8649–8653. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, Y.; Yu, Q.; Li, P.; Chen, X.; Liu, Y. Photo-responsive cyclodextrin/anthracene/ Eu3+ supramolecular assembly for a tunable photochromic multicolor cell label and fluorescent ink. Chem. Sci. 2019, 10, 3346–3352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Liu, Y. Cyclodextrin-based multistimuli-responsive supramolecular assemblies and their biological functions. Adv. Mater. 2020, 32, e1806158. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y. Highly effective gene delivery based on cyclodextrin multivalent assembly in target cancer cells. J. Mater. Chem. B 2022, 10, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, H.; Wang, H.; Han, Y.; Zheng, Z.; Liu, X.; Feng, B.; Zhang, H. Light-responsive dual-functional biodegradable mesoporous silica nanoparticles with drug delivery and lubrication enhancement for the treatment of osteoarthritis. Nanoscale 2021, 13, 6394–6399. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wu, R.; Li, X.; Wang, X.; Tang, X.; Tan, K.; Wan, M.; Mao, C.; Xu, X.; Jiang, H.; Li, J.; Zhou, M.; Shi, D. Multi-pathway microenvironment regulation for atherosclerosis therapy based on beta-cyclodextrin/l-arginine/Au nanomotors with dual-mode propulsion. Small 2022, 18, 2104120. [Google Scholar] [CrossRef] [PubMed]
- Engel, S.; Moller, N.; Stricker, L.; Peterlechner, M.; Ravoo, B.J. A Modular System for the Design of stimuli-responsive multifunctional nanoparticle aggregates by use of host-guest chemistry. Small 2018, 14, e1704287. [Google Scholar]
- Zhao, M.; Li, B.; Wang, P.; Lu, L.; Zhang, Z.; Liu, L.; Wang, S.; Li, D.; Wang, R.; Zhang, F. Supramolecularly engineered NIR-II and upconversion nanoparticles in vivo assembly and disassembly to improve bioimaging. Adv. Mater. 2018, 30, e1804982. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, M.; Yang, J.; Zeng, X.; Zhou, Y.; Yang, S.; Yang, R.; Yuan, Q.; Zheng, J. A modular system for the design of stimuli-responsive multifunctional nanoparticle aggregates by use of host-guest chemistry. Small 2021, 17, e2100243. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Gou, K.; Kang, W.; Guo, X.; Zhu, K.; Li, S.; Li, H. pH/H2O2 Dual-responsive chiral mesoporous silica nanorods coated with a biocompatible active targeting ligand for cancer therapy. ACS Appl. Mater. Interfaces 2021, 13, 35397–35409. [Google Scholar] [CrossRef]
- Li, J.; Cheng, Q.; Yue, L.; Gao, C.; Wei, J.; Ding, Y.; Wang, Y.; Zheng, Y.; Wang, R. Macrophage-hitchhiking supramolecular aggregates of CuS nanoparticles for enhanced tumor deposition and photothermal therapy. Nanoscale Horiz. 2021, 6, 907–912. [Google Scholar] [CrossRef]
- Adeli, F.; Abbasi, F.; Babazadeh, M.; Davaran, S. Thermo/pH dual-responsive micelles based on the host-guest interaction between benzimidazole-terminated graft copolymer and β-cyclodextrin-functionalized star block copolymer for smart drug delivery. J. nanobiotechnol. 2022, 20, 1–24. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, J.; Zuo, T.; Ma, S.; Xokrat, N.; Hu, Z.; Wang, Z.; Xu, R.; Wei, Y.; Shen, Q. Heparanase-driven sequential released nanoparticles for ferroptosis and tumor microenvironment modulations synergism in breast cancer therapy. Biomaterials 2021, 266, 120429. [Google Scholar] [CrossRef]
- Zhang, Y.; Gong, F.; Wu, Y.; Hou, S.; Xue, L.; Su, Z.; Zhang, C. Poly-β-cyclodextrin supramolecular nanoassembly with a pH-Sensitive switch removing lysosomal cholesterol crystals for antiatherosclerosis. Nano Lett. 2021, 21, 9736–9745. [Google Scholar] [CrossRef]
- Dong, Z.; Kang, Y.; Yuan, Q.; Luo, M.; Gu, Z. H2O2-responsive nanoparticle based on the supramolecular self-assemble of cyclodextrin. Front. Pharmacol. 2018, 9, 552–562. [Google Scholar] [CrossRef]
- Xu, X.; Shang, H.; Zhang, T.; Shu, P.; Liu, Y.; Xie, J.; Zhang, D.; Tan, H.; Li, J. A stimuli-responsive insulin delivery system based on reversible phenylboronate modified cyclodextrin with glucose triggered host-guest interaction. Int. J. Pharm. 2018, 548, 649–658. [Google Scholar] [CrossRef]
- Chen, W.; Li, X.; Liu, C.; He, J.; Qi, M.; Sun, Y.; Shi, B.; Sepehrpour, H.; Li, H.; Tian, W.; Stang, P. β-Cyclodextrin modified Pt(II) metallacycle-based supramolecular hyperbranched polymer assemblies for DOX delivery to liver cancer cells. Proc. Natl. Acad. Sci. USA 2020, 117, 30942–30948. [Google Scholar] [CrossRef]
- Yin-Ku, L.; Shiu-Wei, W.; Ren-Shen, L. Photo and redox dual-stimuli-responsive β-cyclodextrin-ferrocene supramolecules for drug delivery. J. Macromol. Sci. 2020, 58, 8–21. [Google Scholar] [CrossRef]
- Peng, J.; Liu, Y.; Zhang, M.; Liu, F.; Ma, L.; Yu, C.; Wei, H. One-pot fabrication of dual-redox sensitive, stabilized supramolecular nanocontainers for potential programmable drug release using a multifunctional cyclodextrin unit. J. Controll. Release 2021, 334, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Chen, H.; Guan, Y.; Zhang, S.; Tong, T.; Ding, N.; Luo, T.; Kang, Y.; Pang, J. Cyclodextrin-based supramolecular nanoparticles break the redox balance in chemodynamic therapy-enhanced chemotherapy. J. Colloid Interface Sci. 2022, 628, 864–876. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Yu, G.; Yang, Z.; Yue, L.; Zhang, X.; Sun, C.; Wei, J.; Rao, L.; Chen, X.; Wang, R. Supramolecular polymerization-induced nanoassemblies for self-augmented cascade chemotherapy and chemodynamic therapy of tumor. Angew. Chem. Int. Ed. 2021, 60, 17570–17578. [Google Scholar] [CrossRef] [PubMed]
- Xue, E.; Yang, C.; Fong, W.; Ng, D. Site-specific displacement-driven activation of supramolecular photosensitizing nanoassemblies for antitumoral photodynamic therapy. ACS Appl. Mater. Interfaces 2022, 14, 14903–14915. [Google Scholar] [CrossRef]
- Peng, L.; Liu, S.; Feng, A.; Yuan, J. Polymeric nanocarriers based on cyclodextrins for drug delivery: host-guest interaction as dtimuli responsive linker. Mol. Pharmaceut. 2017, 14, 2475–2486. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Ju, X.; Wang, L.; Ding, L.; Liu, G.; Zhang, S.; Li, B. pH and glutathione dual-triggered supramolecular assemblies as synergistic and controlled drug release carriers. Polym. Chem. 2017, 8, 7260–7270. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; Li, M.; Li, Y.; Xiong, H.; Jiang, D.; Li, L.; Huang, H.; Kang, Y.; Pang, J. Reactive oxygen species and glutathione dual responsive nanoparticles for enhanced prostate cancer therapy. Mater. Sci. Eng. C 2021, 123, 111956. [Google Scholar] [CrossRef] [PubMed]
- Perret, F.; Marminon, C.; Zeinyeh, W.; Nebois, P.; Bollacke, A.; Jose, J.; Parrot-Lopez, H.; Le Borgne, M. Preparation and characterization of CK2 inhibitor-loaded cyclodextrin nanoparticles for drug delivery. Int. J. Pharm. 2013, 441, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Boiani, M.; González, M.; Imidazole and benzimidazole derivatives as chemotherapeutic agents. Mini-Rev. Med. Chem. 2005, 5, 409–424. [Google Scholar]
- De Luca, L.; Ferro, S.; Buemi, M.; Monforte, A.; Gitto, R.; Schirmeister, T.; Maes, L.; Rescifina, A.; Micale, N. Discovery of benzimidazole-based Leishmania mexicana cysteine protease CPB 2.8 Δ CTE inhibitors as potential therapeutics for leishmaniasis. Chem. Biol. Drug Des. 2018, 92, 1585–1596. [Google Scholar] [CrossRef]
- Beaulieu, P.; Bös, M.; Bousquet, Y.; Fazal, G.; Gauthier, J.; Gillard, J.; Goulet, S.; LaPlante, S.; Poupart, M.; Valois, S.; Kukoij, G. Non-nucleoside inhibitors of the hepatitis C virus NS5B polymerase: discovery and preliminary SAR of benzimidazole derivatives. Bioorg. Med. Chem. Lett. 2004, 14, 967–971. [Google Scholar] [CrossRef]
- Akhtar, W.; Khan, M.; Verma, G.; Shaquiquzzaman, M.; Rizvi, M.; Mehdi, S.; Akhter, M.; Alam, M. Therapeutic evolution of benzimidazole derivatives in the last quinquennial period. Eur. J. Med. Chem. 2017, 126, 705–753. [Google Scholar] [CrossRef]
- Simoes, S.; Rey-Rico, A.; Concheiro, A.; Alvarez-Lorenzo, C. Supramolecular cyclodextrin- based drug nanocarriers. Chem. Commun. 2015, 51, 6275–6289. [Google Scholar] [CrossRef]
- Messner, M.; Kurkov, S.; Maraver Palazon, M.; Alvarez Fernandez, B.; Brewster, M.; Loftsson, T. Self-assembly of cyclodextrin complexes: effect of temperature, agitation and media composition on aggregation. Int. J. Pharm. 2011, 419, 322–328. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Kasprzak, A.; Poplawska, M.; Ruzycka, M.; Grudzinski, I.P.; Nowicka, A.M. Controlled drug release and cytotoxicity studies of beta-lapachone and doxorubicin loaded into cyclodextrins attached to a polyethyleneimine matrix. Int. J. Mol. Sci. 2020, 21, 5832. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, V.; Gervaise, C.; Djedaini-Pilard, F.; Furlan, A.; Sarazin, C. Cyclodextrin nanoassemblies: a promising tool for drug delivery. Drug Discov. Today 2015, 20, 1120–1126. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y. Construction and functions of cyclodextrin-based 1D supramolecular strands and their secondary assemblies. Adv. Mater. 2015, 27, 5403–5409. [Google Scholar] [CrossRef]
- You, X.; Gu, Z.; Huang, J.; Kang, Y.; Chu, C.; Wu, J. Arginine-based poly(ester amide) nanoparticle platform: From structure-property relationship to nucleic acid delivery. Acta Biomater. 2018, 74, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, C.; Li, H.; Wang, X.; Goh, S.; Ding, J.; Wang, D.; Leong, K. Cationic supramolecules composed of multiple oligoethylenimine-grafted β-cyclodextrins threaded on a polymer chain for efficient gene delivery. Adv. Mater. 2006, 18, 2969–2974. [Google Scholar] [CrossRef]
- Varan, G.; Benito, J.; Mellet, C.; Bilensoy, E. Development of polycationic amphiphilic cyclodextrin nanoparticles for anticancer drug delivery. Beilstein J. Nanotech. 2017, 8, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Verza, B.; van den Beucken, J.; Brandt, J.; Jafelicci Junior, M.; Barao, V.; Piazza, R.; Tagit, O.; Spolidorio, D.; Vergani, C.; de Avila, E. A long-term controlled drug-delivery with anionic beta cyclodextrin complex in layer-by-layer coating for percutaneous implants devices. Carbohyd. Polym. 2021, 257, 117604. [Google Scholar] [CrossRef]
- Badwaik, V.; Aicart, E.; Mondjinou, Y.; Johnson, M.; Bowman, V.; Thompson, D. Structure-property relationship for in vitro siRNA delivery performance of cationic 2-hydroxypropyl-beta- cyclodextrin: PEG-PPG-PEG polyrotaxane vectors. Biomaterials 2016, 84, 86–98. [Google Scholar] [CrossRef]
- Radhakrishna, M.; Sing, C. Charge correlations for precise, coulombically driven self assembly. Macromol. Macromol. Chem. Phys. 2016, 217, 126–136. [Google Scholar] [CrossRef]
- Seripracharat, C.; Sinthuvanich, C.; Karpkird, T. Cationic cyclodextrin-adamantane poly (vinyl alcohol)-poly (ethylene glycol) assembly for siRNA delivery. J. Drug. Deliv. Sci. Tec. 2022, 68, 103052. [Google Scholar] [CrossRef]
- Monfared, Y.; Mahmoudian, M.; Cecone, C.; Caldera, F.; Haiaty, S.; Heidari, H.; Rahbarghazi, R.; Matencio, A.; Zakeri-Milani, P.; Trotta, F. Hyper-branched cationic cyclodextrin polymers for improving plasmid transfection in 2D and 3D spheroid cells. Pharmaceutics 2022, 14, 2690–2710. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Liu, Y.; Li, X.; Wang, W.; Yuan, Z. NIR light-driven Bi2Se3-based nanoreactor with “three in one” hemin-assisted cascade catalysis for synergetic cancer therapy. Adv. Func. Mater. 2020, 30, 2006883. [Google Scholar] [CrossRef]
- Hardy, A.; Seguin, C.; Brion, A.; Lavalle, P.; Schaaf, P.; Fournel, S.; Bourel-Bonnet, L.; Frisch, B.; De Giorgi, M. β-Cyclodextrin-functionalized chitosan/alginate compact polyelectrolyte complexes (CoPECs) as functional biomaterials with anti-inflammatory properties. ACS Appl. Mater. Interfaces 2018, 10, 29347–29356. [Google Scholar] [CrossRef]
- Chen, M.; Perez, R.; Du, P.; Bhattarai, N.; McDonough, K.; Ravula, S.; Kumar, R.; Mathis, J.; Warner, I. Tumor-targeting NIRF nanoGUMBOS with cyclodextrin-enhanced chemo/ photothermal antitumor activities. ACS Appl. Mater. Interfaces 2019, 11, 27548–27557. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, L.; Shi, L.; Yuan, Y.; Fu, D.; Ye, Z.; Li, Q.; Deng, Y.; Liu, X.; Lv, Q.; Cheng, Y.; Xu, Y.; Jiang, X.; Wang, G.; Wang, L.; Wang, Z. A sequentially responsive nanosystem breaches cascaded bio-barriers and suppresses P-glycoprotein function for reversing cancer drug resistance. ACS Appl. Mater. Interfaces 2020, 12, 54343–54355. [Google Scholar] [CrossRef]
- Neva, T.; Carbajo-Gordillo, A.; Benito, J.; Lana, H.; Marcelo, G.; Ortiz Mellet, C.; Tros de Ilarduya, C.; Mendicuti, F.; Garcia Fernandez, J. Tuning the topological landscape of DNA-cyclodextrin nanocomplexes by molecular design. Chem. 2020, 26, 15259–15269. [Google Scholar] [CrossRef] [PubMed]
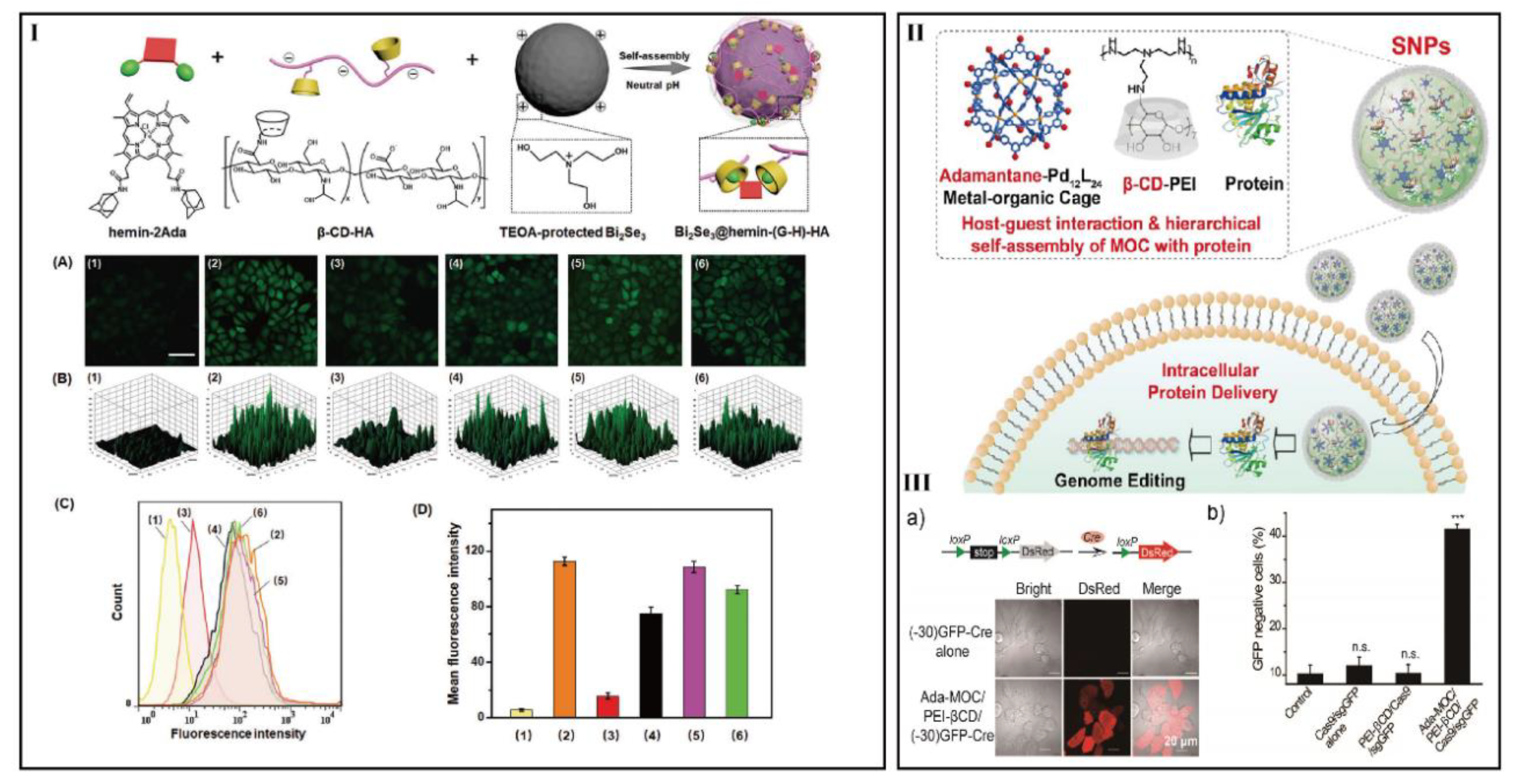
- Liu, J.; Luo, T.; Xue, Y.; Mao, L.; Stang, P.; Wang, M. Hierarchical self-assembly of discrete metal-organic cages into supramolecular nanoparticles for intracellular protein delivery. Angew. Chem. Int. Ed. 2021, 133, 5489–5495. [Google Scholar] [CrossRef]
- Taharabaru, T.; Yokoyama, R.; Higashi, T.; Mohammed, A.; Inoue, M.; Maeda, Y.; Niidome, T.; Onodera, R.; Motoyama, K. Genome editing in a wide area of the brain using dendrimer-based ternary polyplexes of Cas9 ribonucleoprotein. ACS Appl. Mater. Interfaces 2020, 12, 21386–21397. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Liu, Y.; Qi, D.; Yang, L.; Chen, H.; Wang, C.; Feng, X. Nucleus-targeted delivery of multi-protein self-assembly for combined anticancer therapy. Small 2021, 17, e2101219. [Google Scholar] [CrossRef] [PubMed]
- Belbekhouche, S.; Oniszczuk, J.; Pawlak, A.; El Joukhar, I.; Goffin, A.; Varrault, G.; Sahali, D.; Carbonnier, B. Cationic poly(cyclodextrin)/alginate nanocapsules: From design to application as efficient delivery vehicle of 4-hydroxy tamoxifen to podocyte in vitro. Colloid. Surface. B 2019, 179, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, L.; Chen, J.; Deng, M.; Feng, X.; Chen, L. Supramolecular nanoplatforms via cyclodextrin host-guest recognition for synergistic gene-photodynamic therapy. Eur. Polym. J. 2019, 118, 222–230. [Google Scholar] [CrossRef]
- Ahmadi, D.; Zarei, M.; Rahimi, M.; Khazaie, M.; Asemi, Z.; Mir, S.; Sadeghpour, A.; Karimian, A.; Alemi, F.; Rahmati-Yamchi, M.; Salehi, R.; Jadidi-Niaragh, F.; Yousefi, M.; Khelgati, N.; Majidinia, M.; Safa, A.; Yousefi, B. Preparation and in-vitro evaluation of pH-responsive cationic cyclodextrin coated magnetic nanoparticles for delivery of methotrexate to the Saos-2 bone cancer cells. J. Drug. Deliv. Sci. Tec. 2020, 57, 101584. [Google Scholar] [CrossRef]
- Lakkakula, J.; Matshaya, T.; Krause, R.; Cationic cyclodextrin/alginate chitosan nanoflowers as 5-fluorouracil drug delivery system. Mat. Sci. eng. C- Mater. 2017, 70, 169–177.
- Xu, J.; Zhang, G.; Luo, X.; Wang, D.; Zhou, W.; Zhang, Y.; Zhang, W.; Chen, J.; Meng, Q.; Chen, E.; Chen, H.; Song, Z. Co-delivery of 5-fluorouracil and miRNA-34a mimics by host-guest self-assembly nanocarriers for efficacious targeted therapy in colorectal cancer patient-derived tumor xenografts. Theranostics 2021, 11, 2475–2489. [Google Scholar] [CrossRef]
- Liu, J.; Song, Y.; Wang, Y.; Han, M.; Wang, C.; Yan, F. Cyclodextrin-functionalized gold nanorods loaded with meclofenamic acid for improving N6-methyladenosine-mediated second near-infrared photothermal immunotherapy. ACS Appl. Mater. Interfaces 2022, 14, 40612–40623. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xing, C.; Ke, D.; Chen, L.; Qiu, Z.; Zeng, S.; Li, B.; Zhang, S. Amino-functionalized beta-cyclodextrin to construct green metal-organic framework materials for CO2 capture. ACS Appl. Mater. Interfaces 2020, 12, 3032–3041. [Google Scholar] [CrossRef]
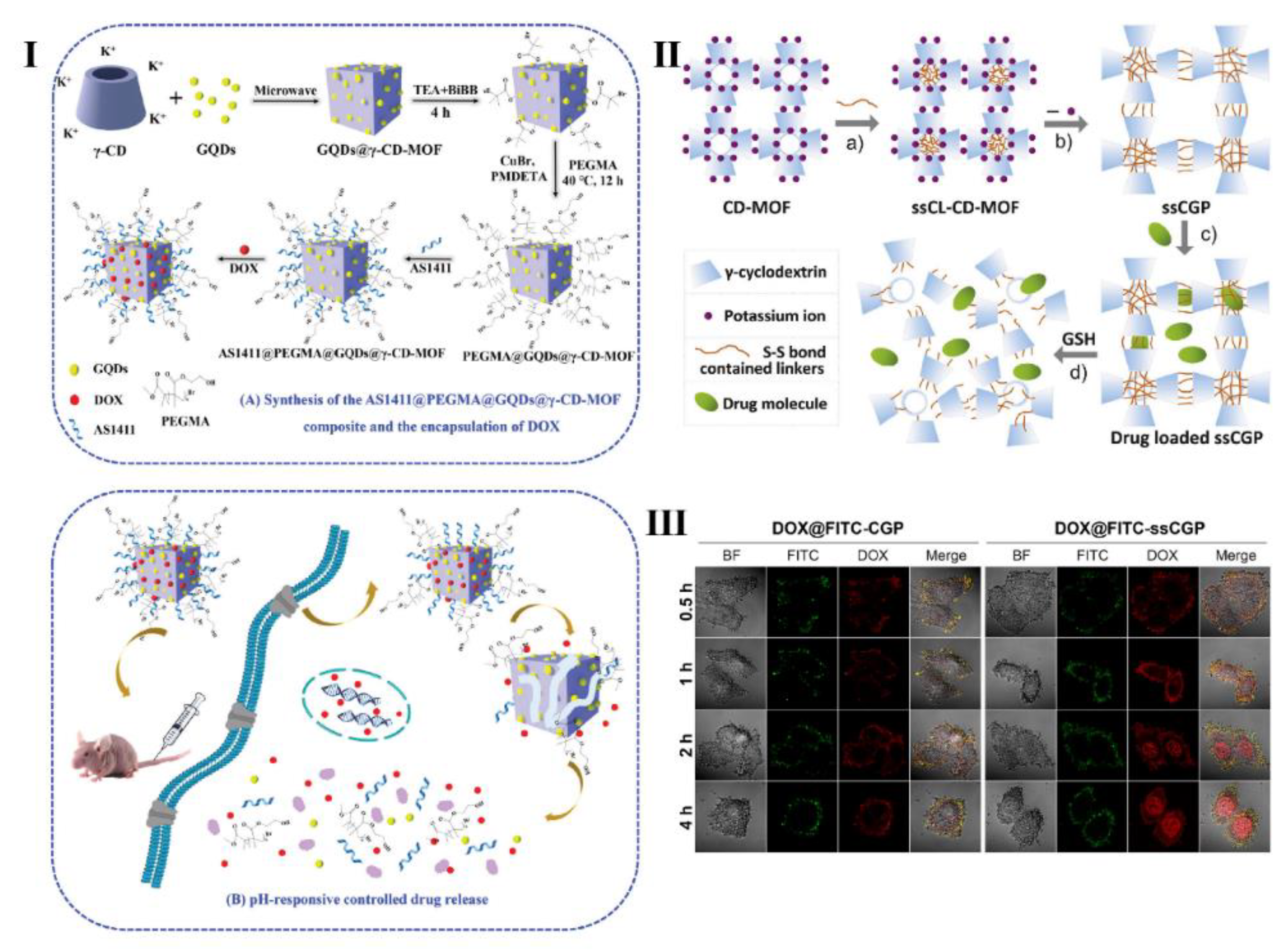
- Jia, Q.; . Li, Z.; Guo, C.; Huang, X.; Song, Y.; Zhou, N.; Wang, M.; Zhang, Z.; He, L.; Du, M. A γ-cyclodextrin-based metal-organic framework embedded with graphene quantum dots and modified with PEGMA via SI-ATRP for anticancer drug delivery and therapy. Nanoscale 2019, 11, 20956–20967. [Google Scholar] [CrossRef]
- Xue, Q.; Ye, C.; Zhang, M.; Hu, X.; Cai, T. Glutathione responsive cubic gel particles cyclodextrin metal-organic frameworks for intracellular drug delivery. J. Colloid Interf. Sci. 2019, 551, 39–46. [Google Scholar] [CrossRef]
- Sivakumar, P.; Peimanfard, S.; Zarrabi, A.; Khosravi, A.; Islami, M. Cyclodextrin-based nanosystems as drug carriers for cancer therapy. Anticancer Agent. Me. 2020, 20, 1327–1339. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xia, B.; Branham, M.; Ha, W.; Wu, H.; Peng, S.; Ding, L.; Li, B.; Zhang, S. Self-assembly of carboxymethyl konjac glucomannan-g-poly(ethylene glycol) and (α-cyclodextrin) to biocompatible hollow nanospheres for glucose oxidase encapsulation. Carbohyd. Polym. 2011, 86, 120–126. [Google Scholar] [CrossRef]
- Qin, J.; Meng, X.; Li, B.; Ha, W.; Yu, X.; Zhang, S. Self-assembly of beta-cyclodextrin and pluronic into hollow nanospheres in aqueous solution. J. Colloid Interf. Sci. 2010, 350, 447–452. [Google Scholar] [CrossRef]
- Harada, A.; Li, J.; Kamachi, M. The molecular necklace: a rotaxane containing many threaded α-cyclodextrins. Nature 1992, 356, 325–327. [Google Scholar] [CrossRef]
- Taveira, S.; Varela-Garcia, A.; Dos Santos Souza, B.; Marreto, R.; Martin-Pastor, M.; Concheiro, A.; Alvarez-Lorenzo, C. Cyclodextrin-based poly(pseudo)rotaxanes for transdermal delivery of carvedilol. Carbohyd. Polym. 2018, 200, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Marreto, R.; Cardoso, G.; Dos Santos Souza, B.; Martin-Pastor, M.; Cunha-Filho, M.; Taveira, S.; Concheiro, A.; Alvarez-Lorenzo, C. Hot melt-extrusion improves the properties of cyclodextrin-based poly(pseudo)rotaxanes for transdermal formulation. Int. J. Pharm. 2020, 586, 119510. [Google Scholar] [CrossRef]
- Di Donato, C.; Iacovino, R.; Isernia, C.; Malgieri, G.; Varela-Garcia, A.; Concheiro, A.; Alvarez-Lorenzo, C. Polypseudorotaxanes of pluronic (R) F127 with combinations of alpha- and beta-cyclodextrins for topical formulation of acyclovir. Nanomaterials 2020, 10, 613–628. [Google Scholar] [CrossRef]
- Ji, Y.; Liu, X.; Huang, M.; Jiang, J.; Liao, Y.; Liu, Q.; Chang, C.; Liao, H.; Lu, J.; Wang, X.; Spencer, M.; Meng, H. Development of self-assembled multi-arm polyrotaxanes nanocarriers for systemic plasmid delivery in vivo. Biomaterials 2019, 192, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Takashima, Y.; Yamaguchi, H. Cyclodextrin-based supramolecular polymers. Chem. Soc. Rev. 2009, 38, 875–882. [Google Scholar] [CrossRef]
- Zhang, C.; Bu, W.; Ni, D.; Zhang, S.; Li, Q.; Yao, Z.; Zhang, J.; Yao, H.; Wang, Z.; Shi, J. Synthesis of iron nanometallic glasses and their application in cancer therapy by a localized fenton reaction. Angew. Chemie. Int. Ed. Engl. 2016, 128, 2101–2106. [Google Scholar] [CrossRef]
- Nishida, K.; Tamura, A.; Kang, T.; Masuda, H.; Yui, N. An antibody-supermolecule conjugate for tumor-specific targeting of tumoricidal methylated beta-cyclodextrin-threaded polyrotaxanes. J. Mater. Chem. B 2020, 8, 6975–6987. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Simchick, G.; Qiao, J.; Ashcraft, M.; Cui, S.; Nagy, T.; Zhao, Q.; Xiong, M. Reactive oxygen species-triggered dissociation of a polyrotaxane-based nanochelator for enhanced clearance of systemic and hepatic iron. ACS Nano 2021, 15, 419–433. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Q.; Jia, S.; Lin, K.; Fan, G.; Yuan, J.; Yu, S.; Shi, J. Specific modification with TPGS and drug loading of cyclodextrin polyrotaxanes and the enhanced antitumor activity study in vitro and in vivo. ACS Appl. Mater. Interfaces 2019, 11, 46427–46436. [Google Scholar] [CrossRef]
- Fan, M.; Yu, Z.; Luo, H.; Zhang, S.; Li, B. Supramolecular network based on the delf-sssembly of gamma-cyclodextrin with poly(ethylene glycol) and its shape memory effect. Macromol. Rapid Comm. 2009, 30, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Chen, G.; Liu, X.; Chen, W.; Chen, F.; Jiang, M. Photoresponsive pseudopolyrotaxane hydrogels based on competition of host-guest interactions. Angew. Chem. Int. Ed. Engl. 2010, 49, 4409–4413. [Google Scholar] [CrossRef]
- Wang, J.; Williamson, G.; Yang, H. Branched polyrotaxane hydrogels consisting of alpha- cyclodextrin and low-molecular-weight four-arm polyethylene glycol and the utility of their thixotropic property for controlled drug release. Colloid. Surface. B 2018, 165, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.; Lee, S.; Kim, H.; Lee, K.; Lee, J.; Kim, D.; Cho, H. Polypseudorotaxane and polydopamine linkage-based hyaluronic acid hydrogel network with a single syringe injection for sustained drug delivery. Carbohyd. Polym. 2021, 266, 118104. [Google Scholar] [CrossRef]
- Higashi, T.; Ohshita, N.; Hirotsu, T.; Yamashita, Y.; Motoyama, K.; Koyama, S.; Iibuchi, R.; Uchida, T.; Mieda, S.; Handa, K.; Kimoto, T.; Arima, H. Stabilizing effects for antibody formulations and safety profiles of cyclodextrin polypseudorotaxane hydrogels. J. Pharm. Sci. 2017, 106, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y. Supramolecular assembly-enhanced chiroptical properties of pyrene-modified cyclodextrins. Chinese Chem. Lett. 2022, 34, 107836. [Google Scholar] [CrossRef]
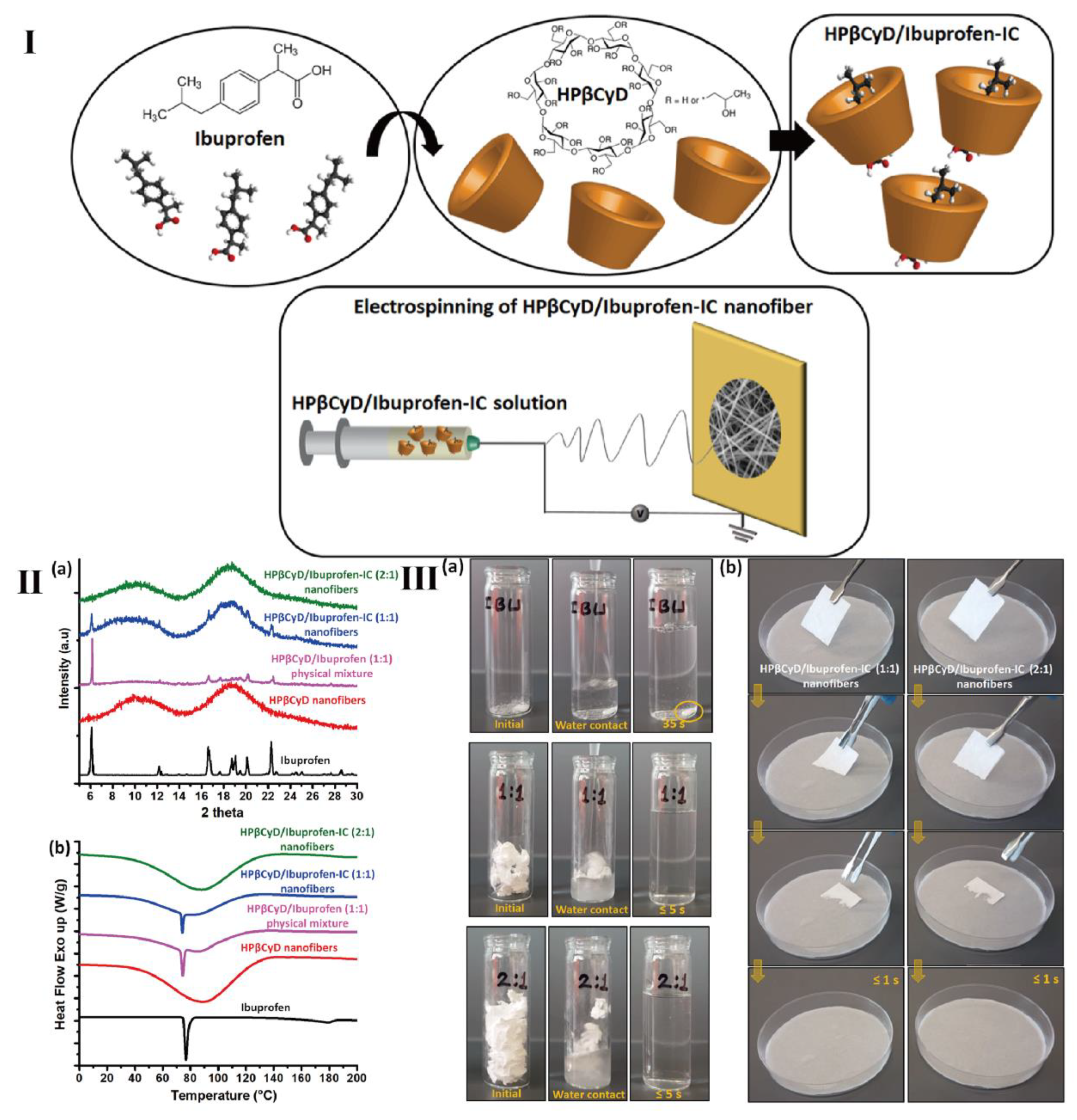
- Celebioglu, A.; Uyar, T. Fast dissolving oral drug delivery system based on electrospun nanofibrous webs of cyclodextrin/ibuprofen inclusion complex nanofibers. Mol. Pharm. 2019, 16, 4387–4398. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Metronidazole/hydroxypropyl-β-cyclodextrin inclusion complex nanofibrous webs as fast-dissolving oral drug delivery system. Int. J. Pharmaceut. 2019, 572, 118828. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Development of ferulic acid/cyclodextrin inclusion complex nanofibers for fast-dissolving drug delivery system. Int. J. Pharmaceut. 2020, 584, 119395. [Google Scholar] [CrossRef]
- Topuz, F.; Kilic, M.E.; Durgun, E.; Szekely, G. Tuning mechanical properties of biobased polymers by supramolecular chain entanglement. Macromolecules 2019, 52, 8967–8975. [Google Scholar]
- Celebioglu, A.; Uyar, T. Cyclodextrin nanofibers by electrospinning. Chem. Comm. 2010, 46, 6903–6905. [Google Scholar] [CrossRef] [PubMed]
- Hsiung, E.; Celebioglu, A.; Chowdhury, R.; Kilic, M.; Durgun, E.; Altier, C.; Uyar, T. Antibacterial nanofibers of pullulan/tetracycline-cyclodextrin inclusion complexes for fast-disintegrating oral drug delivery. J. Colloid Interface Sci. 2022, 610, 321–333. [Google Scholar] [CrossRef]
- Topuz, F.; Kilic, M.; Durgun, E.; Szekely, G. Fast-dissolving antibacterial nanofibers of cyclodextrin/antibiotic inclusion complexes for oral drug delivery. J. Colloid. Interface. Sci. 2021, 585, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Wang, W.; Hou, H.; Liu, Y.; Liu, Z.; Geng, L.; Sun, L.; Luo, A. A β-cyclodextrin covalent organic framework used as a chiral stationary phase for chiral separation in gas chromatography. Chinese Chem. Lett. 2022, 33, 898–902. [Google Scholar] [CrossRef]
| CyD type | stimuli | Responsive guest molecules | drug | cells | Ref |
|---|---|---|---|---|---|
| β-CyD | Light | Azo | DOX | MCF-7 | [6] |
| β-CyD | NIR irradiation and reductase | Arylazopyrazole | siRNA | A549, HeLa, and 293T | [73] |
| β-CyD | light | Azo | Diclofenac sodium | MC3T3-E1 | [74] |
| β-CyD | ROS | 6-(Mercaptohexyl) ferrocene | β-Cyclodextrin/L-Arginine/Au Nanomotors | RAW264.7, and HUVECs | [75] |
| β-CyD | Light | Arylazopyrazole | Gold, iron oxide, and lanthanide-doped LiYF4 NPs | [76] | |
| β-CyD | Light | Azo | Azo modified lanthanide upconversion NPs and β CD modified downconversion nanoprobes | HEK293T and CaOV3 | [77] |
| β-CyD | Hypoxia | Azo | Rho-TP | MCF-7 | [78] |
| β-CyD | H2O2 | Phenylboronic acid pinacol ester | DOX | 4T1 | [79] |
| β-CyD | ROS | Fc | CuS | B16 | [80] |
| β-CyD | pH | Benzimidazole | DOX | MCF-7 | [81] |
| β-CyD | ROS | Fc | DOX | 4T1 | [82] |
| β-CyD | pH | benzimidazole | β-CyD | [83] | |
| β-CyD | H2O2 | Fc | Glucose oxidase | CT26 | [84] |
| β-CyD | Glucose | Phenylboronic acid | Insulin | L929 | [85] |
| β-CyD | ROS | Fc | DOX | HepG2 | [86] |
| β-CyD | ROS | Fc | DOX | HeLa | [87] |
| β-CyD | ROS | Fc | DOX | Bel-7402 and L02 | [88] |
| β-CyD | ROS | Fc | CPT | HEK-293T and PC3 | [89] |
| β-CyD | ROS | Fc | Platinum (IV) | 4T1 | [90] |
| β-CyD | ROS | Fc | Carboxy phthalocyanine | HT29 and A431 | [91] |
| Strategy | Carrier | Drug | Target | cells | Ref |
|---|---|---|---|---|---|
| Dual stimulus responsive of NIR irradiation and reductase under anaerobic conditions | Upconversion NPs encapsulated by β-cyclodex- trin-grafted hyaluronic acid /spermine modified with arylazopyrazoles-IC | siRNA | CD44 | A549, HeLa, and 293T | [76] |
| layer-by-layer coating | Anionic-β-CyD and poly(acrylic acid) and poly(l-lysine) | Tetracycline | HGF and S. aureus | [108] | |
| Host-guest inclusion forms the cationic supramolecular polymer | Cationic β-CyD/ adamantane (Ad)-poly (vinyl alcohol) (PVA)-poly(ethylene glycol) (PEG) -IC | siRNA | A549 and A549/GFP | [111] | |
| Improving Plasmid Transfection in 2D and 3D Spheroid Cells | Cationic hyper-branched cyclodextrin-based polymers | Plasmid DNA | EGFP | HT-29 | [112] |
| Chemodynamic therapy photodynamic therapy | β-CyD-HA/ Bi2Se3 NPs | Bi2Se3 and hemin-2Ada | NIR light assisted tumor targeting | HepG2 | [113] |
| Compact polyelectrolyte complexes | β-CyD-functionalized chitosan/ alginate |
Piroxicam | pH |
RAW | [114] |
| Templated synthesis | CyD-nanoGUMBOS | IR780 | MDA-MB-231, Hs578T, and MCF-7 | [115] | |
| Individually over coming bio-barriers at each delivery stage | PEG-b-PLLDA/MSNs-SS-Py/CyD-PEI | DOX | P-glycoprotein | MCF7/ADR | [116] |
| Doubly linked aromatic clip-polycationic CyD hybrids | CyD-aromatic hybrid/ plasmid DNA | Plasmid DNA | COS-7, and HepG2 | [117] | |
| Hierarchical self-assembly | β-CyD-conjugated polyethylenimine with damantane-functionalized M12L24 MOC-IC | Proteins | HeLa, HeLa-DsRed, and HEK-GFP | [118] | |
| CyD conjugates with dendrimer | Glucuronylglucosyl-β-CyD conjugate | Cas9/single-guide RNA complex | Mouse brain | SHSY5Y | [119] |
| Protein co-assembly | Wind chime-like lysine modified CyD | Ribonuclease A and deoxyribo- nuclease I |
Nucleus | Hela | [120] |
| Layer-by-layer self-assemble | Cationic poly(CyD)/ alginate | 4-Hydroxy- tamoxifen |
Pyrene and 4- hydroxy-tamoxifen | Immortalized mouse podocytes | [121] |
| Combinational therapy | Cationic poly (L-lysine) modified by β-CyD / PEGylated tetraphenyl- porphyrin (TPP)-IC | TPP-PEG and DNA | HeLa | [122] | |
| Cationic moieties for targeted delivery and enhanced uptake | Cationic CyD magnetic nanocarrier | MTX | Magnetic | Saos-2 and human red blood cells | [123] |
| Intracellular protein delivery with fluorescent microscopy imaging | Tetraphenylethylene-featured metal-organic cages (MOCs) and β-CyD-conjugated polyethylenimine | Tetraphenyle- thylene and protein | MAPK/ERK signaling | Neural cells | [124] |
| Synergistic therapy | Multiple β-CyD-attached QD NPs/Ad-modified TCP1 peptide-targeting ligand | 5-Fluorouracil and miRNA- 34a mimics | Colorectal cancer | DLD1 | [125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




