Submitted:
14 April 2023
Posted:
14 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
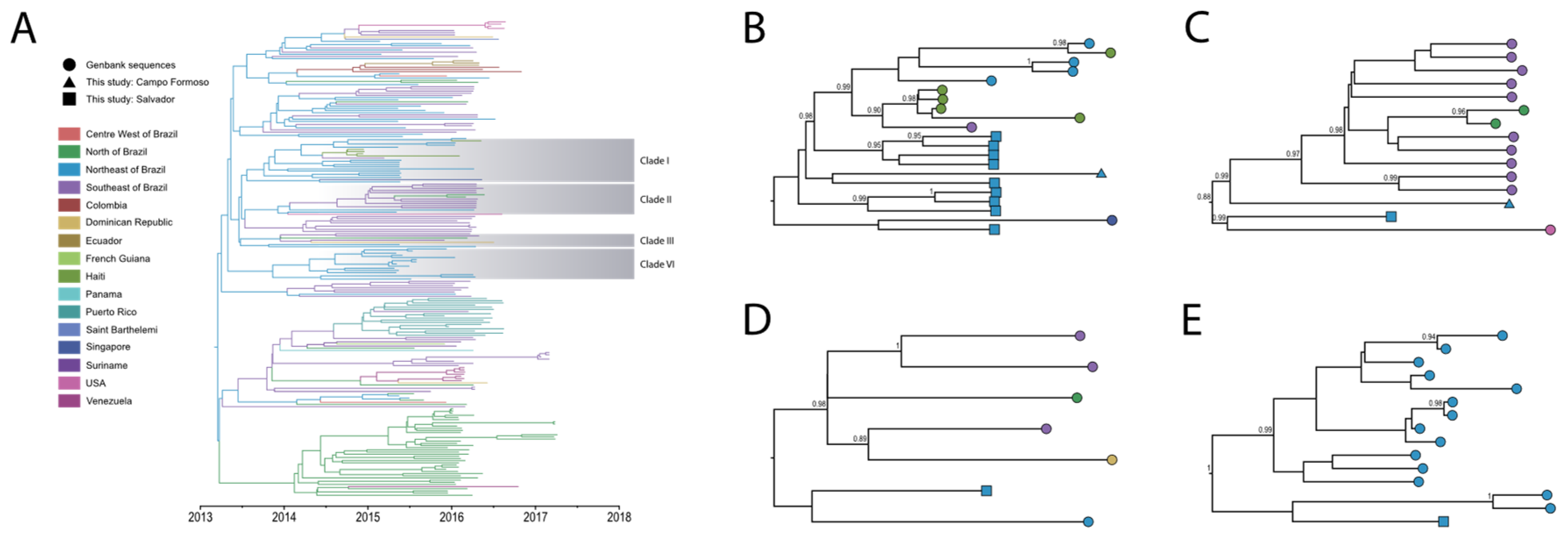
3. Results and Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Broutet, N.; Krauer, F.; Riesen, M.; Khalakdina, A.; Almiron, M.; Aldighieri, S.; Espinal, M.; Low, N.; Dye, C. Zika Virus as a Cause of Neurologic Disorders. N. Engl. J. Med. 2016, 374, 1506–1509. [Google Scholar] [CrossRef]
- Kuno, G.; Chang, G.-J.J.; Tsuchiya, K.R.; Karabatsos, N.; Cropp, C.B. Phylogeny of the Genus Flavivirus. J Virol. 1998, 72, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Kuno, G.; Chang, G.-J.J. Full-Length Sequencing and Genomic Characterization of Bagaza, Kedougou, and Zika Viruses. Arch Virol. 2007, 152, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Simón, D.; Fajardo, A.; Moreno, P.; Moratorio, G.; Cristina, J. An Evolutionary Insight into Zika Virus Strains Isolated in the Latin American Region. Viruses. 2018, 10, 698. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stanfield, S.M.; Duffy, M.R. Genetic and Serologic Properties of Zika Virus Associated with an Epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008, 14, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Aubry, M.; Teissier, A.; Huart, M.; Merceron, S.; Vanhomwegen, J.; Roche, C.; Vial, A.-L.; Teururai, S.; Sicard, S.; Paulous, S.; et al. Zika Virus Seroprevalence, French Polynesia, 2014–2015. Emerg. Infect. Dis. 2017, 23, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Zanluca, C.; Melo, V.C.A. de; Mosimann, A.L.P.; Santos, G.I.V. dos; Santos, C.N.D. dos; Luz, K. First Report of Autochthonous Transmission of Zika Virus in Brazil. Mem. Inst. Oswaldo Cruz. 2015, 110, 569–572. [Google Scholar] [CrossRef]
- Campos, G.S.; Bandeira, A.C.; Sardi, S.I. Zika Virus Outbreak, Bahia, Brazil. Emerg. Infect. Dis. 2015, 21, 1885–1886. [Google Scholar] [CrossRef]
- Grubaugh, N.D.; Ladner, J.T.; Kraemer, M.U.G.; Dudas, G.; Tan, A.L.; Gangavarapu, K.; Wiley, M.R.; White, S.; Thézé, J.; Magnani, D.M.; et al. Genomic Epidemiology Reveals Multiple Introductions of Zika Virus into the United States. Nature. 2017, 546, 401–405. [Google Scholar] [CrossRef]
- Grubaugh, N.D.; Saraf, S.; Gangavarapu, K.; Watts, A.; Tan, A.L.; Oidtman, R.J.; Ladner, J.T.; Oliveira, G.; Matteson, N.L.; Kraemer, M.U.G.; et al. Travel Surveillance and Genomics Uncover a Hidden Zika Outbreak during the Waning Epidemic. Cell. 2019, 178, 1057–1071.e11. [Google Scholar] [CrossRef]
- Chitti, S. V.; Prasad, A.K.; Saxena, S.K. Emerging Zika Virus Disease: A Public Health Emergency of Global Concern. VirusDisease. 2016, 27, 211–214. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Director-General Summarizes the Outcome of the Emergency Committee Regarding Clusters of Microcephaly and Guillain-Barré Syndrome. Available online: https://www.who.int/en/news-room/detail/01-02-2016-who-director-general-summarizes-the-outcome-of-the-emergency-committee-regarding-clusters-of-microcephaly-and-guillain-barré-syndrome (accessed on 01 April 2023).
- WHO. Fifth Meeting of the Emergency Committee under the International Health Regulations (2005) Regarding Microcephaly, Other Neurological Disorders and Zika Virus. Available online: https://www.who.int/en/news-room/detail/18-11-2016-fifth-meeting-of-the-emergency-committee-under-the-international-health-regulations-(2005)-regarding-microcephaly-other-neurological-disorders-and-zika-virus (accessed on 01 April 2023).
- Faria, N.R.; Azevedo, R. do S. da S.; Kraemer, M.U.G.; Souza, R.; Cunha, M.S.; Hill, S.C.; Thézé, J.; Bonsall, M.B.; Bowden, T.A.; Rissanen, I.; et al. Zika Virus in the Americas: Early Epidemiological and Genetic Findings. Science. 2016, 352, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Lednicky, J.; Beau De Rochars, V.M.; El Badry, M.; Loeb, J.; Telisma, T.; Chavannes, S.; Anilis, G.; Cella, E.; Ciccozzi, M.; Rashid, M.; et al. Zika Virus Outbreak in Haiti in 2014: Molecular and Clinical Data. PLoS Negl. Trop. Dis. 2016, 10, e0004687. [Google Scholar] [CrossRef] [PubMed]
- Campos, T.D.L.; Durães-Carvalho, R.; Rezende, A.M.; de Carvalho, O.V.; Kohl, A.; Wallau, G.L.; Pena, L.J. Revisiting Key Entry Routes of Human Epidemic Arboviruses into the Mainland Americas through Large-Scale Phylogenomics. Int. J. Genomics. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Villabona-Arenas, C.J.; Hanage, W.P.; Tully, D.C. Phylogenetic Interpretation during Outbreaks Requires Caution. Nat. Microbiol. 2020, 5, 876–877. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Rasche, A.; Baronti, C.; Aldabbagh, S.; Cadar, D.; Reusken, C.B.; Pas, S.D.; Goorhuis, A.; Schinkel, J.; Molenkamp, R.; et al. Assay Optimization for Molecular Detection of Zika Virus. Bull. World Health Organ. 2016, 94, 880–892. [Google Scholar] [CrossRef]
- Rathore, A.P.S.; St. John, A.L. Cross-Reactive Immunity Among Flaviviruses. Front. Immunol. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Cardoso, C.W.; Paploski, I.A.D.; Kikuti, M.; Rodrigues, M.S.; Silva, M.M.O.; Campos, G.S.; Sardi, S.I.; Kitron, U.; Reis, M.G.; Ribeiro, G.S. Outbreak of Exanthematous Illness Associated with Zika, Chikungunya, and Dengue Viruses, Salvador, Brazil. Emerg. Infect. Dis. 2015, 21, 2274–2276. [Google Scholar] [CrossRef]
- Silva, M.M.O.; Tauro, L.B.; Kikuti, M.; Anjos, R.O.; Santos, V.C.; Gonçalves, T.S.F.; Paploski, I.A.D.; Moreira, P.S.S.; Nascimento, L.C.J.; Campos, G.S.; et al. Concomitant Transmission of Dengue, Chikungunya, and Zika Viruses in Brazil: Clinical and Epidemiological Findings From Surveillance for Acute Febrile Illness. Clin. Infect. Dis. 2019, 69, 1353–1359. [Google Scholar] [CrossRef]
- de Moraes, L.; Cerqueira-Silva, T.; Nobrega, V.; Akrami, K.; Santos, L.A.; Orge, C.; Casais, P.; Cambui, L.; Rampazzo, R. de C.P.; Trinta, K.S.; et al. A Clinical Scoring System to Predict Long-Term Arthralgia in Chikungunya Disease: A Cohort Study. PLoS Negl. Trop. Dis. 2020, 14, e0008467. [Google Scholar] [CrossRef]
- Quick, J.; Grubaugh, N.D.; Pullan, S.T.; Claro, I.M.; Smith, A.D.; Gangavarapu, K.; Oliveira, G.; Robles-Sikisaka, R.; Rogers, T.F.; Beutler, N.A.; et al. Multiplex PCR Method for MinION and Illumina Sequencing of Zika and Other Virus Genomes Directly from Clinical Samples. Nat. Protoc. 2017, 12, 1261–1276. [Google Scholar] [CrossRef] [PubMed]
- Ho, Z.J.M.; Hapuarachchi, H.C.; Barkham, T.; Chow, A.; Ng, L.C.; Lee, J.M.V.; Leo, Y.S.; Prem, K.; Lim, Y.H.G.; de Sessions, P.F.; et al. Outbreak of Zika Virus Infection in Singapore: An Epidemiological, Entomological, Virological, and Clinical Analysis. Lancet Infect. Dis. 2017, 17, 813–821. [Google Scholar] [CrossRef] [PubMed]
- MASSAD, E.; BURATTINI, M.N.; KHAN, K.; STRUCHINER, C.J.; COUTINHO, F.A.B.; WILDER-SMITH, A. On the Origin and Timing of Zika Virus Introduction in Brazil. Epidemiol. Infect. 2017, 145, 2303–2312. [Google Scholar] [CrossRef] [PubMed]
- Metsky, H.C.; Matranga, C.B.; Wohl, S.; Schaffner, S.F.; Freije, C.A.; Winnicki, S.M.; West, K.; Qu, J.; Baniecki, M.L.; Gladden-Young, A.; et al. Zika Virus Evolution and Spread in the Americas. Nature. 2017, 546, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Faria, N.R.; Sabino, E.C.; Nunes, M.R.T.; Alcantara, L.C.J.; Loman, N.J.; Pybus, O.G. Mobile Real-Time Surveillance of Zika Virus in Brazil. Genome Med. 2016, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Faria, N.R.; Quick, J.; Claro, I.M.; Thézé, J.; de Jesus, J.G.; Giovanetti, M.; Kraemer, M.U.G.; Hill, S.C.; Black, A.; da Costa, A.C.; et al. Establishment and Cryptic Transmission of Zika Virus in Brazil and the Americas. Nature. 2017, 546, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Iani, F.C.M.; Giovanetti, M.; Fonseca, V.; Souza, W.M.; Adelino, T.E.R.; Xavier, J.; Jesus, J.G.; Pereira, M.A.; Silva, M.V.F.; Costa, A.V.B.; et al. Epidemiology and Evolution of Zika Virus in Minas Gerais, Southeast Brazil. Infect. Genet. Evol. 2021, 91, 104785. [Google Scholar] [CrossRef] [PubMed]
- Giovanetti, M.; Pereira, L.A.; Adelino, T.É.R.; Fonseca, V.; Xavier, J.; de Araújo Fabri, A.; Slavov, S.N.; da Silva Lemos, P.; de Almeida Marques, W.; Kashima, S.; et al. A Retrospective Overview of Zika Virus Evolution in the Midwest of Brazil. Microbiol. Spectr. 2022, 10. [Google Scholar] [CrossRef]
- Giovanetti, M.; Faria, N.R.; Lourenço, J.; Goes de Jesus, J.; Xavier, J.; Claro, I.M.; Kraemer, M.U.G.; Fonseca, V.; Dellicour, S.; Thézé, J.; et al. Genomic and Epidemiological Surveillance of Zika Virus in the Amazon Region. Cell Rep. 2020, 30, 2275–2283.e7. [Google Scholar] [CrossRef]
- Balm, M.N.D.; Lee, C.K.; Lee, H.K.; Chiu, L.; Koay, E.S.C.; Tang, J.W. A Diagnostic Polymerase Chain Reaction Assay for Zika Virus. J. Med. Virol. 2012, 84, 1501–1505. [Google Scholar] [CrossRef]
- Black, A.; Moncla, L.H.; Laiton-Donato, K.; Potter, B.; Pardo, L.; Rico, A.; Tovar, C.; Rojas, D.P.; Longini, I.M.; Halloran, M.E.; et al. Genomic Epidemiology Supports Multiple Introductions and Cryptic Transmission of Zika Virus in Colombia. BMC Infect. Dis. 2019, 19, 963. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and Accurate Long-Read Alignment with Burrows–Wheeler Transform. Bioinformatics. 2010, 26, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The Variant Call Format and VCFtools. Bioinformatics. 2011, 27, 2156–2158. [Google Scholar] [CrossRef] [PubMed]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve Years of SAMtools and BCFtools. Gigascience. 2021, 10, 1–4. [Google Scholar] [CrossRef]
- Vilsker, M.; Moosa, Y.; Nooij, S.; Fonseca, V.; Ghysens, Y.; Dumon, K.; Pauwels, R.; Alcantara, L.C.; Vanden Eynden, E.; Vandamme, A.-M.; et al. Genome Detective: An Automated System for Virus Identification from High-Throughput Sequencing Data. Bioinformatics. 2019, 35, 871–873. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Katoh, K. MAFFT: A Novel Method for Rapid Multiple Sequence Alignment Based on Fast Fourier Transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
| ID | Municipality | Collection Date | Cq Value | No. of Mapped Reads |
Avg. Depth Coverage |
Reference Covered |
|---|---|---|---|---|---|---|
| TRDP173 | Salvador | 2015-05-06 | 30.96 | 39,617 | 1,207.66 | 94.03 |
| TRDP238 | Salvador | 2015-05-18 | 31.16 | 44,842 | 1258.69 | 90.22 |
| TRDP252 | Salvador | 2015-05-19 | 28.64 | 38,452 | 1,197.17 | 88.76 |
| TRDP256 | Salvador | 2015-05-19 | 29.95 | 39,530 | 1,170.28 | 89.10 |
| TRDP257 | Salvador | 2015-05-19 | 37.96 | 58,214 | 1,372.15 | 83.82 |
| TRDP274 | Salvador | 2015-05-20 | 37.84 | 35,944 | 1,116.41 | 84.62 |
| TRDP282 | Salvador | 2015-05-21 | 35.49 | 21,026 | 727.62 | 88.89 |
| TRDP300 | Salvador | 2015-05-22 | 30.03 | 19,670 | 678.25 | 81.89 |
| TRDP309 | Salvador | 2015-05-26 | 33.87 | 20,459 | 705.72 | 87.94 |
| TRDP317 | Salvador | 2015-05-26 | 33.60 | 20,414 | 704.03 | 87.99 |
| TRDP333 | Salvador | 2015-05-27 | 39.07 | 11,078 | 377.35 | 80.62 |
| TRDP433 | Salvador | 2015-07-09 | 27.63 | 60,626 | 1,438.09 | 96.64 |
| ZK0110 | Campo Formoso | 2016-04-08 | 21.91 | 40,452 | 1,301.48 | 97.18 |
| ZK0152 | Campo Formoso | 2016-04-09 | 34.94 | 17,820 | 562.33 | 84.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





