1. Introduction
The food industry produces high volumes of byproducts, with considerable environmental impacts. However, in most cases, the nutritional content of such byproducts makes them an important subject for valorization [
1]. Cheese whey (CW), buttermilk (BM) and second cheese whey (SCW) are the byproducts resulting from the production of cheese, butter and whey cheeses respectively. Both CW and BM have excellent nutritional and techno-functional properties [
2,
3,
4,
5]. Due to its high organic load and with an estimated worldwide production of 190 million tons/year, CW represents an opportunity for bioenergy and biochemicals production [
6]. This byproduct represents 80-90% of the milk volume and contains more than 50% of milk nutrients with the following composition (% w/v) in the case of bovine CW: 4.5-5% lactose, 0.6-0.8% protein, 0.4-0.5% lipids, and 0.4-1% mineral salts [
7]. Lactose is responsible for the high Biochemical Oxigen Demand (BOD) of whey (
ca. 40-50 g/L). The values of total solids (lactose, proteins and fat) are even higher in ovine and caprine whey than in bovine whey [
8]. The potential environmental impact of CW represents a problem for the small/medium scale dairy companies that do not possess the capacity to produce whey powder (WP), whey protein concentrates (WPC) or other whey derivatives, such as whey protein isolates and hydrolysates (WPI, WPH, respectively). This problem is particularly relevant for producers of traditional cheeses based on sheep’s and/or goat’s milks. In the case of these producers, sheep’s and/or goat’s CW is often used to produce whey cheeses (WC). However, this option has some disadvantages, particularly the high energy input required to produce WC, its short shelf-life, and the fact that the byproduct of such process,
deproteinized whey, also known as SCW, presents the same environmental constraints of CW, which result from its high lactose content [
9].
Dairy industries manage whey mainly by membrane separation or fermentation [
10]. Using membrane technology, WPC, WPI and lactose concentrates, are segregated and dried in order to guarantee shelf-life. Pires et al. [
8] made an extensive revision of the po-tential alternatives to valorize CW or SCW. Several physical, chemical and biological pro-cesses are available to valorize CW by producing biofuels or chemical commodities [
2]. The adaptation of different processes for physicochemical treatment and fermentation of CW that are commonly used to obtain proteins, lactose and other compounds with different ac-tivities, can be integrated to achieve a complete recovery of this byproduct [
11]. Alongside with the solutions to recover and valorize CW and SCW components by membrane technologies, very promising developments have been made to convert these byproducts into value-added commodities such as biofuels, bioplastics, bacterial cellulose, food colors and flavors, bioactive peptides, and single-cell proteins, as reported by several authors [
12,
13,
14].
The recent growing interest in caprine products, attributed to their specific nutrition-al and nutraceutical characteristics, (i.e. lower allergenicity of their proteins and higher content of oligosaccharides), makes the recovery of goat CW an opportunity to transform this byproduct in value added novel dairy products [
15]. The results of cost–benefit analy-sis and sensitivity analysis show that an integrated process of ultrafiltration/nanofiltration (UF/NF) is economically viable in small/medium sized cheese dairies [
16]. Sheep’s dairy products other than cheese are also being developed and are looked at as an opportunity to valorize peripheral rural areas through the transformation of dairy products into competi-tive value-added commodities [
17,
18,
19].
Concomitantly, increasing awareness on health has increased demand for healthy food which is confirmed by raising market sales of functional foods. Functional foods are defined as foods that, in addition their nutritional value, contain ingredients that act spe-cifically on body functions associated with the control or the reduction of the risk of devel-oping some diseases. One of such foods is kefir. This product is a natural acidic-alcoholic fermented milk drink traditionally produced by milk fermentation using kefir grains. Kefir grains are composed of a complex population of bacteria and yeasts embedded in a poly-saccharide-protein matrix. Lactic acid bacteria, acetic acid bacteria and yeasts are the dominant microorganisms. Kefir can provide probiotic benefits, such as intestinal microe-cological balance regulation, antibacterial and anti-inflammatory activity [
20]. A system-atic review by Vieira et al. [
21] reports that the bioactive compounds more commonly found in kefir were exopolysaccharides, including kefiran, bioactive peptides, and organic acids, especially lactic acid. It has been indicated that the kefir peptides have therapeutic potential for the treatment of Alzheimer’s disease [
22]. It has also been reported the exist-ence of a relationship between the improvement in skin parameters and the changes of the intestinal microbial balance after kefir consumption [
23]. The bioconversion of whey by kefir lactic acid bacteria (LAB) may be effective in reducing obesity and obesity-related dis-eases [
24]. Besides, several authors [
25,
26,
27] report that milk kefir enhances bone microar-chitecture and metabolism, has osteoprotective effects and can be used as a nutritional supplement to accelerate fracture healing. The antimicrobial activity of kefir microorgan-isms derives from the microorganisms’ capacity to adhere to the intestinal epithelium, preventing the adhesion of pathogens, among other properties. Bacteria and yeast isolated from kefir have been shown to have in vivo and in vitro antimicrobial activity against en-teropathogenic bacteria and spoilage fungi [
28].
Some authors developed synbiotic drinks from milk fermented by kefir grains and supplemented by inulin, quinoa flour, or with the addition of specific probiotic bacteria [
20,
29,
30]. However, most available milk-based kefir products on the market are produced without kefir grains; they use commercial kefir cultures [
31]. This study will also aim to develop new synbiotic kefir products based on sheep’s and goat’s UF concentrated CW, fermented by kefir grains or commercial kefir starter, with and without the addition of a commercial probiotic culture, and supplemented with inulin.
2. Materials and Methods
2.1. Production of liquid whey concentrates
The sheep and goat CW were supplied by external dairy companies and processed at the dairy pilot plant of Escola Superior Agrária de Coimbra (Portugal). 500L of each type of whey were subjected to ultrafiltration (UF) in a pilot plant supplied by Proquiga Biotech SA (A Coruña, Spain), equipped with an organic UF membrane (3838 PVDF/polysulfone) with an effective area of filtration of 7 m2 and 10 kDa cut-off, supplied by FipoBiotech, Spain. The process was carried out at 40-45 °C, at a transmembrane pressure of 3-3.5 bar, aiming at a volumetric concentration factor (VCF=Vol. Feed/Vol. Retentate) of 20. The UF concentration step allowed for the obtention of 25 L of goat and 25 L of sheep liquid whey concentrate (LWC). The goat’s and sheep’s LWCs obtained were pasteurized (65 °C, 30 min) and then homogenized at 15 MPa using a Rannie™ model Bluetop homogenizer (Copenhagen, Denmark). LWCs were frozen at -25 ± 2 °C until they were used to produce goat and sheep kefir formulations.
2.2. Manufacture of goat and sheep kefir products
Approximately 12 L of sheep’s or goat’s LWCs were thawed under refrigeration at 0 °C for 24 h. Subsequently, the samples were heated to 80 ± 2 °C and 2.5% (w/v) inulin (Fibruline™, Cosucra, supplied by Induxtra de Suministros, Portugal) was added, being the mixture homogenized at 15 MPa. The mixtures were than cooled to 38 °C, divided in four portions (3 L each) and inoculated with one of the following cultures:
Commercial kefir culture: ExactTM Kefir 1 (CHR Hansen, Hoersholm, Denmark) mesophilic and thermophilic culture (Debaryomyces hansenii, Lactococcus lactis subsp. cremoris, L. lactis subsp. lactis biovar diacetylactis, L. lactis subsp. lactis, Leuconostoc and Streptococcus thermophilus) at a concentration of 0.01% (w/v) (EK);
A mixture of a commercial kefir culture (ExactTM Kefir 1, CHR Hansen, Hoersholm, Denmark) and a probiotic culture containing Bifidobacterium bifidus-BB12, Lactobacillus acidophilus-LA5 and S. thermophilus (ABT-5TM, CHR Hansen, Denmark) at 0.01% each one (EKABT5);
A traditional kefir culture at a concentration of 2.5% v/v (TK);
A mixture of the traditional kefir culture at 2.5% v/v and probiotic culture (ABT-5TM) at 0.01% (TKABT5).
The inoculated goat or sheep LWCs were placed in an incubation chamber (Jenogand™ Y 1000, Copenhagen, Denmark) at 37 °C and the pH and titratable acidity were monitored until the products reached a target pH of 4.5. The fermentation process was stopped by rapid cooling to 20 °C in less than 30 min. Afterwards, the fermented products were placed in the refrigeration chamber at 4 ± 2 °C for 12 hours, approximately. After this process, the goat and sheep kefir products were stored under refrigeration in a chamber at 0 ± 2 °C for 30 days. Products were evaluated at the 1st, 10th, 20th and 30th days of storage.
2.3. Physicochemical analysis
Total solids content of kefir products was determined by drying the samples in a Schutzart DIN 40050-IP20 Memmert™ oven, according to NP 703: 1982 for yoghurt [
32]. The ash content was determined by incineration of dry samples in a HD-23 Hobersal™ electric muffle furnace. The fat content was determined by the Gerber method (SuperVario-N Funke Gerber™ centrifuge) according to NP 2105:1983 [
33] for sheep kefir, and NP 1923:1987 for goat kefir [
34]. The total N content was determined by the Kjeldahl method in the Digestion System 6 1007 Digester Tecator™ following the AOAC (1997) standard [
35]. To calculate the percentage of protein the conversion factor of 6.38 was used. All analyzes were performed in triplicate.
2.3.1. pH and titratable acidity
The pH of products was directly determined with a HI 9025 HANNA Instruments pH meter, to monitor the evolution of the pH over fermentation, immediately after production and on the 10
th, 20
th and 30
th days of storage. The titratable acidity, expressed in % lactic acid, was determined by titration using a 0.1 N NaOH solution according to NP 701: 1982 for yoghurts [
36].
2.3.2. Color parameters
The color of kefir samples was determined with a Minolta™ Chroma Meter, model CR-200B colorimeter (Tokyo, Japan) calibrated with a white standard (CR-A47: Y = 94.7; x 0.313; y 0.3204). The following conditions were used: illuminant C, 1 cm diameter aperture, 10° standard observer. The color coordinates were measured in the CIEL*a*b* system.
Color difference (ΔEab*) was calculated as:
where L*
0, a*
0, and b*
0 and L*, a*, and b* were the values measured for the samples under comparison. A matrix of ΔEab* values between products was constructed. Three measurements were taken for each sample.
2.3.4. Rheological properties
The rheological properties of the kefir products were evaluated in a rheometer (Rheostress 1, ThermoHaake™) in oscillatory mode. The measurement system consisted of a cone and plate geometry, C60/Ti-0.052mm (35 mm diameter and 1° angle). Stress sweep tests were performed at 1 Hz to investigate the rheological linear viscoelastic behavior of the samples. The elastic modulus (G’), the viscous modulus (G’’) and the complex viscosity (η*) of the products were evaluated in the range of 0.3 to 6.5 rad/s at 3 Pa.
2.3.5. Texture parameters
A Stable Micro Systems™ texture analyzer, model TA.XT Express Enhanced, was used to perform the texture analysis of the sheep’s kefir samples and the results were calculated using the Specific Expression PC software. A TPA-type test was run with a penetration distance of 15 mm at 1 mm/s using an acrylic cylindrical probe with a diameter of 25.4 mm and a height of 38.1 mm.
2.4. Microbiological analysis
The microbial counts of lactic acid bacteria (LAB) of the genera Lactobacillus spp. and Lactococcus spp. were analyzed after production and over storage. Lactococci and lactoba-cilli were enumerated on plates at 37 °C for 48 h on M17 agar (in aerobiosis) and on MRS agar (in anaerobiosis) (Biokar Diagnostics, France), respectively, according to ISO 7889, IDF 117 (2003) [
37]. Yeasts were enumerated in plates at 25 °C according to ISO 6611 IDF 94 (2204) [
38]. Analyses were carried out in triplicate along with two controls for each medium and results are expressed as log CFU/g of product.
2.5. Sensory analysis
Consumer preference tests were conducted with an untrained panel within 7 days of storage. A hedonic test was performed for the kefir samples in order to evaluate the aroma, texture, flavor and global appreciation on a scale from 1 to 9 (1 = I don’t like it at all to 9 = I like it very much) using a non-trained panel with 30 members [
39]. The members of panel were also asked to rank the samples according to their preference, from 1-most preferred to 4-less preferred, according to ISO 8587 (1988) [
40].
2.6. Statistical analysis
Prior to statistical analysis, normal distribution was evaluated using the Kolmogorov–Smirnov test. The differences among formulations produced with the same LWC were analysed by one-way ANOVA and the means were compared by Tukey’s post hoc test. The same statistical treatment was done to study the effect of days of storage in the hardness of sheep kefir samples. The differences between each formulation produced with different LWC (sheep or goat) were compared by t-test for independent samples. For all mean evaluations, a significance level of p < 0.05 was used (IBM SPSS Statistics for Windows (version 27); 2021; IBM Corp, USA).
3. Results
3.1. Performance of UF process
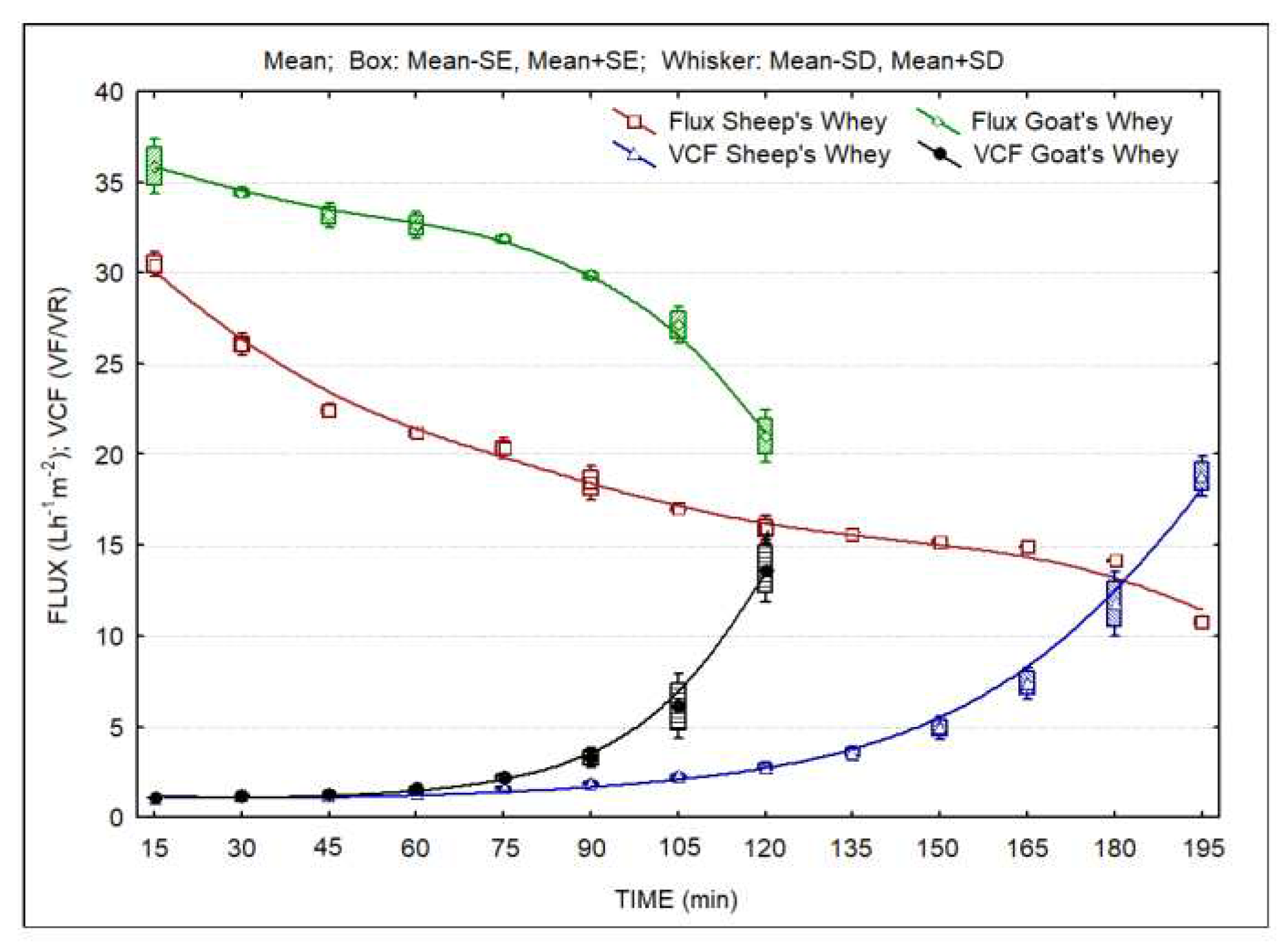
Figure 1 presents the evolution of the UF fluxes over time. The results are the average of two concentration trials. It is clear the higher filtration flux obtained during concentration of goat’s CW. In both cases, average fluxes are of the order of 30 and 20 Lm
-2h
-1, respectively for goat’s and sheep’s CW, which indicate the feasibility of the operation of ultrafiltration units in small scale cheese plants, processing volumes of
ca. 500-1000 L milk per day. The figure also presents the calculated evolution of the volumetric concentration factor during the concentration process. This information indicates that it is possible to concentrate whey to obtain high levels of protein and/or fat with the objective to obtain the desired levels of these nutritional components, namely protein, in the LWCs.
3.2. Physicochemical characteristics of LWC’s and kefir samples
The physicochemical characteristics of the original whey used in the trials and of the the LWCs used in the production of kefir samples are displayed in
Table 1. It is evident the higher protein content of the sheep’s LWC, while in the case of fat, goat’s LWC presented higher values when compared to sheep’s LWC. It has to be referred that, despite its higher fat content, goat’s cheese whey had higher UF fluxes than sheep’s whey. Hence, it can be concluded that protein level of the feed had the major impact on UF performance.
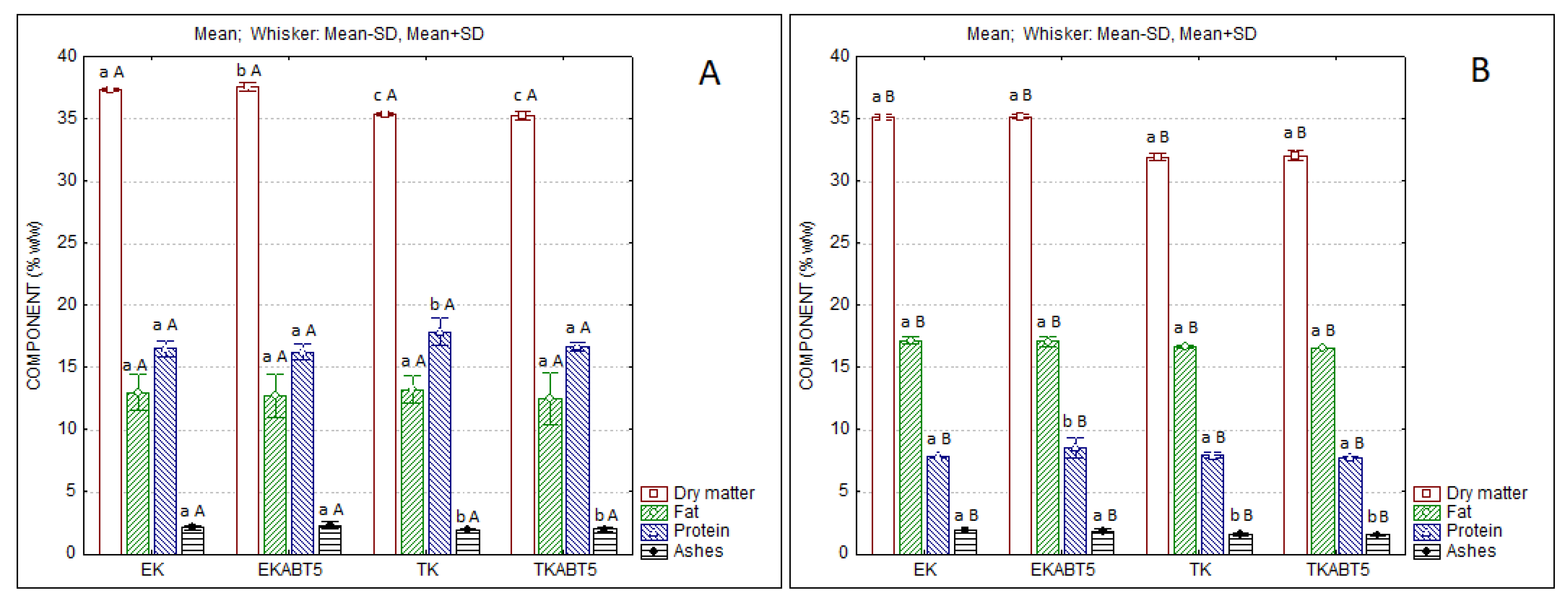
The physicochemical characteristics of the different kefir formulations produced are displayed in
Figure 2. As expected, the products originated with sheep’s LWCs present higher levels of protein which nearly double the ones of the products obtained from goat’s whey. However, goat’s whey products present higher levels of fat, which results from the higher fat level in the original goat’s CW.
Figure 3 presents the evolution of the pH and titratable acidity of kefir products over 30 days of refrigerated storage. It is clear the lower pH and the higher acidity of goat’s kefir products. The sheep’s kefir products produced with commercial kefir starter without and with the addition of the probiotic culture (EK and EKABT5) presented the highest pH and the lowest acidity values, while the traditional kefir without or with the addition of the probiotic culture (TK and TKABT5) presented pH below 4.5 and acidity values of the order of 1.3% lactic acid immediately after production. The acidity increased to values higher than 1.5 on days 10 and 20 and presented a sharp increase from the 20
th to the 30
th day of storage. In the case of goat’s kefir samples, only TKABT5 surpassed values of 1.5% lactic acid. The use of the probiotic culture had impacts on these parameters, decreasing the pH and increasing the titratable acidity, both in sheep’s and in goat’s kefir products.
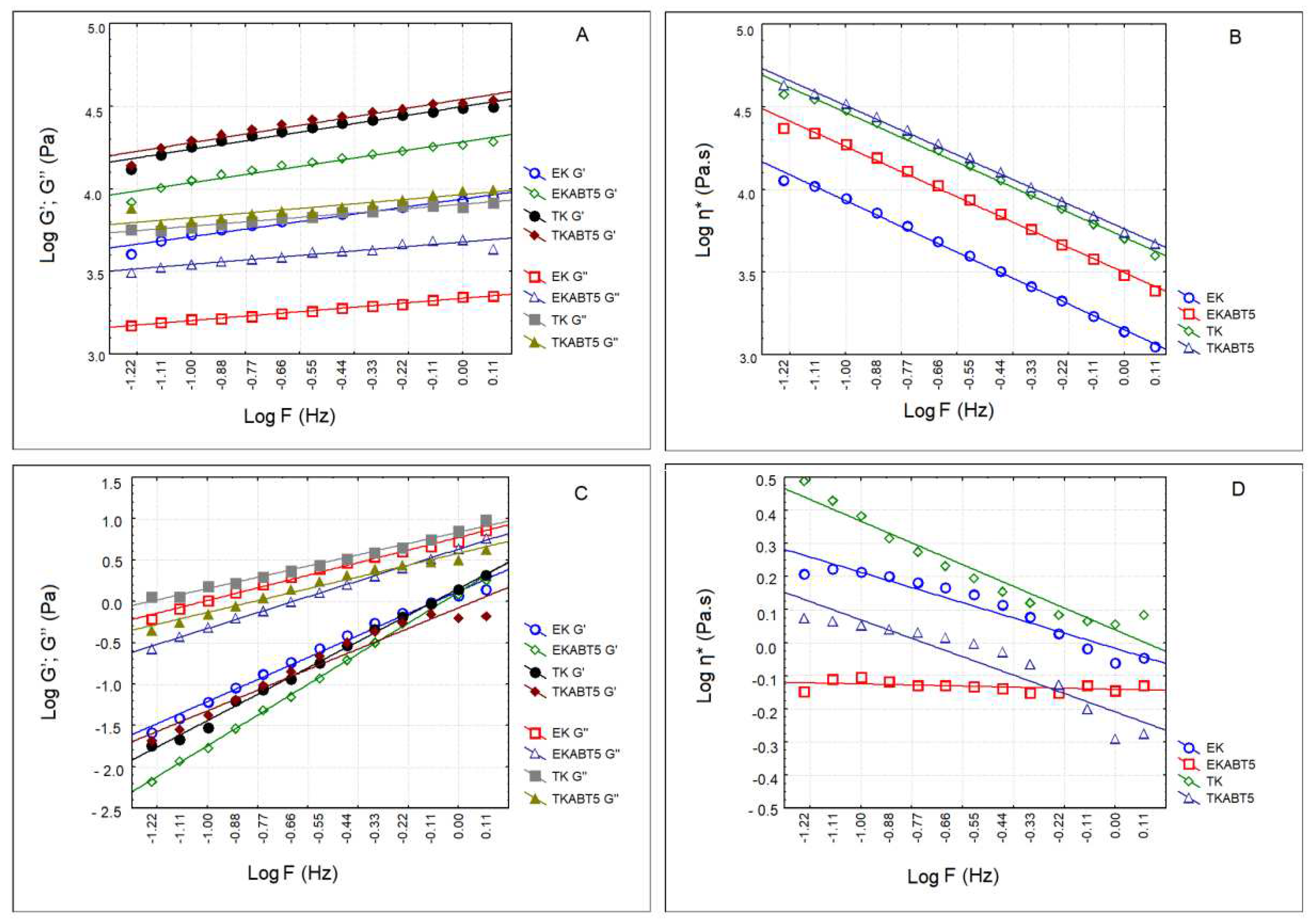
Figure 4 shows the elastic (G’) and viscous (G’’) moduli as well as the complex viscosity of sheep’s and goat’s kefir products. Sheep’s kefir products presented a solid like texture while goat’s kefir products were liquid. Regarding sheep’s kefir products, the highest values of G’ were obtained for TK and TKABT5 (
Figure 4A). EK and EKABT5 presented G’ values
ca. one log cycle lower as compared to the ones produced with the traditional kefir. These observations are confirmed by the evaluation of the complex viscosity (
Figure 4B). In the case of goat’s kefir products, in all cases, the viscous modulus (G’’) was higher than the elastic modulus (G’) (
Figure 4C), indicating the liquid nature of the products, which is also confirmed by their complex viscosity (
Figure 4D).
The texture evaluation performed on sheep’s kefir products is displayed in
Figure 5. It is clear the tendency for the increase in the hardness of sheep’s kefir products between the first and the 30
th days of storage. Most probably, this result could be due to the increased links in the protein matrix of the products and/or to separation of water from the protein matrix (syneresis), which increased the hardness of the protein/lipid matrix.
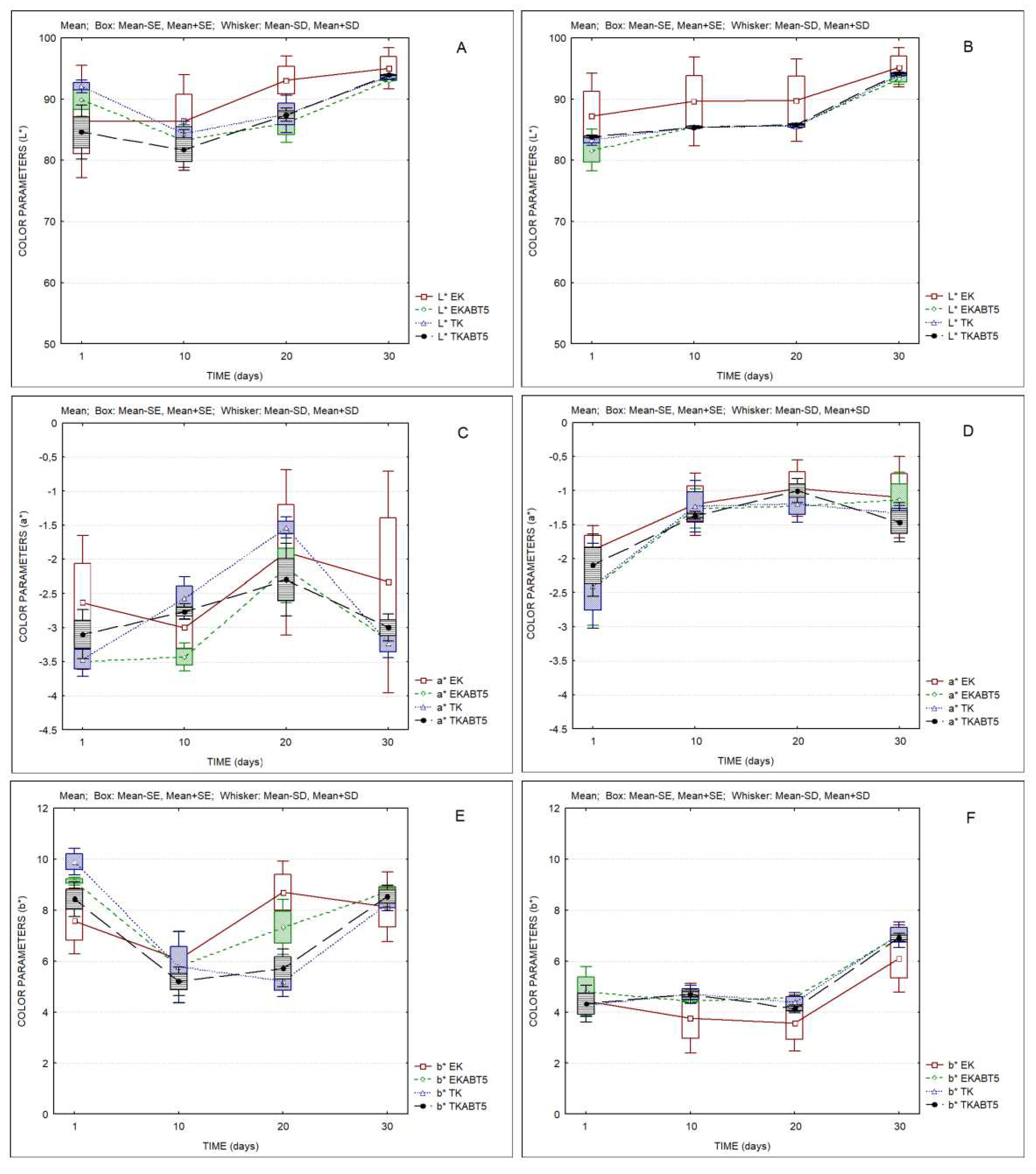
Figure 6 shows the evolution of the color parameters of all kefir samples over storage. It can be observed that both sheep’s and goat’s kefir products show a slight increase in the lightness parameter L* over time (
Figure 6 A,B). Goat’s kefir products presented higher values for parameter a* (red-green axis) as compared to sheep’s kefir products (
Figure 6 B,C). However, the main difference between sheep’s and goat’s kefir products was observed in parameter b* (blue-yellow axis), with goat’s products presenting values of the order of 4, increasing to 6 at the 30
th day of storage, while the lowest value registered for sheep’s samples was of the order of 6, indicating thus a higher yellow color for these samples.
Table 1 and
Table 2 of supplementary materials indicate the values of ΔEab* between different products, and for the same product over storage, which, in most cases, are higher than 1, indicating that color differences can be detected by a common observer. Less differences were observed between kefir products based on goat’s LWC (ΔEab* values <1).
3.3. Microbiological characteristics of kefir products
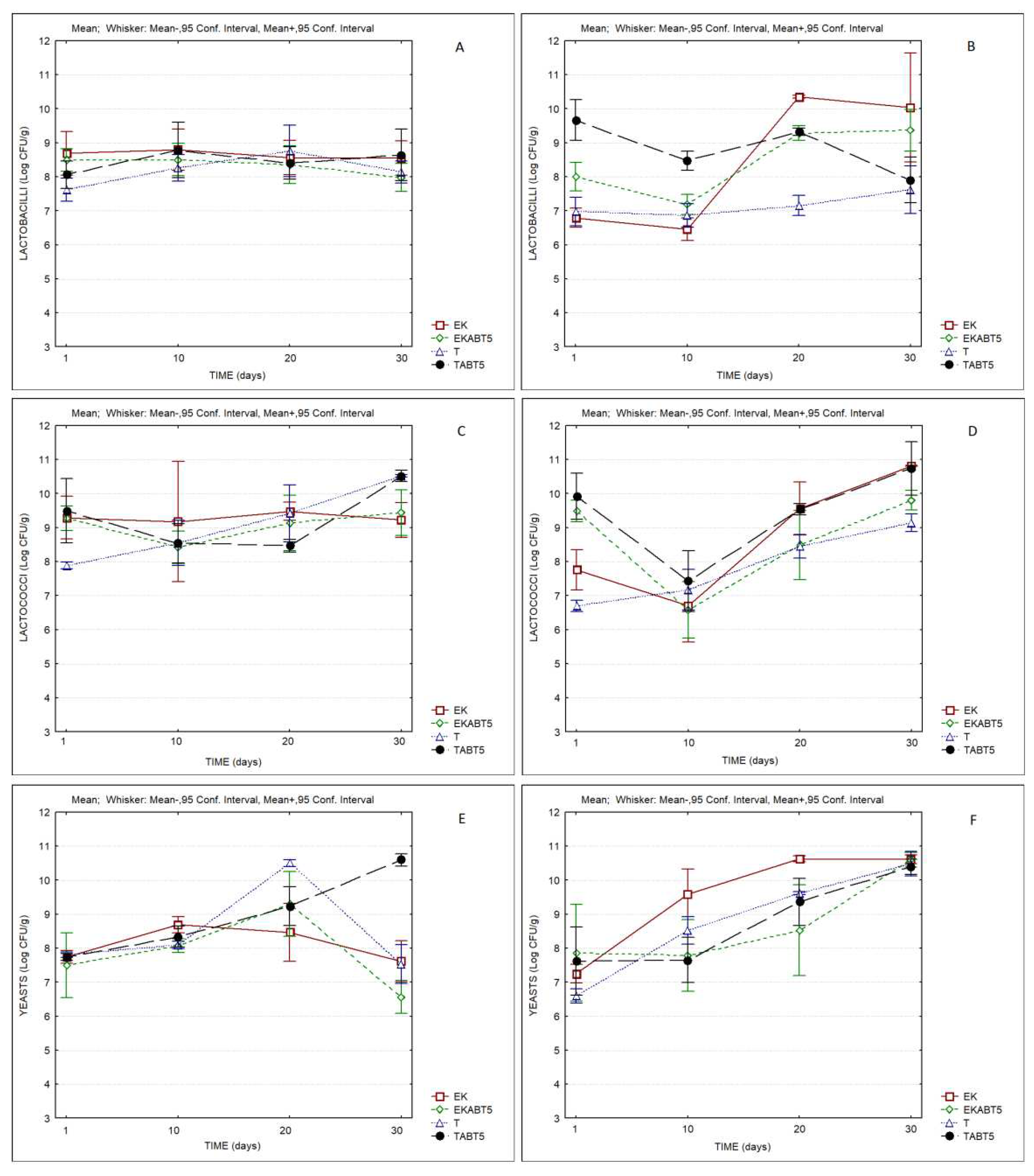
Regarding the evolution of the microbial composition of the different kefirs,
Figure 7 compares the counts of lactobacilli, lactococci and yeasts of the different products during storage. In all cases, the products presented counts of lactobacilli, lactococci and yeasts of the order of, or higher than, log 7 CFU/g, indicating the good adaptation of LAB and yeasts to the LWC, and the probiotic potential of the products over the storage period.
Concerning the sensory evaluation of the different products, it is clear that further work must be undertaken in order to improve the acceptability of the products. Taking into account the results present in
Table 2, one can conclude that sheep’s kefir products were below an acceptable score of 6, regarding aroma and taste of samples S-TK and S-TKABT5. Concerning goat’s products G-EK and G-EKABT5 presented unacceptable scores for aroma, taste and texture. The ranking test allowed to separate samples in different groups. Regarding sheep’s products, samples S-EK and S-EKABT5 were ranked 1
st and 2
nd respectively, being S-TK and S-TKABT5 ranked 3
rd and 4
th. The opposite pattern was observed with goat’s kefir samples which were ranked in the following order: 1
st G-TKABT5; 2
nd G-TK; 3
rd G-EKABT5; 4
th G-EK.
4. Discussion
Considering the performance of the UF process, the direct concentration of sheep’s or goat’s CW can lead to the production of LWCs with interesting nutritional properties, which can subsequently be used on the production of fermented synbiotic dairy products with excellent nutritional characteristics. As expected, at higher volumetric concentration factors, the increased solids concentration of retentates started to affect UF filtrate fluxes negatively. This was particularly evident in the case of sheep’s whey due to its higher protein content. One must also point out the fact that the total solids concentration factors of the retentates do not reflect the VCF 20 aimed. This is due to the dilution of the volume of product with approximately 15 L of water retained in dead volumes at the beginning of operation and, the need to push the final volume of retentate with the introduction of water in the system. Both these situations originate a certain amount of dilution of the feed and of the retentate, with higher impacts when working in batch mode and when low feed volumes are used. This was particularly evident in the case of goat’s whey. The characteristics of the UF processing unit used can fit the needs of small-scale dairy industries that, currently, do not possess the capacity to valorize whey. Macedo and coworkers [
16] had already reported that an integrated process of ultrafiltration (UF)/nanofiltration (NF) is economically viable in small/medium sized cheese dairies processing of
ca. 3500 L milk/day. The efficiency of the process performed in the present work allows us to consider that the process is feasible even in smaller scale units (
i.e. processing
ca. 500 L milk/day).
Regarding the physicochemical characteristics of the kefir samples obtained, one can refer that they reflect the composition of the LWCs obtained. The higher values of protein reached in sheep‘s LWC in relation to goat‘s LWC influenced the rheological properties (G’, G’’, η*) of the kefir. The lower levels of protein in goat‘s kefir caused were the cause for their liquid nature.
Some differences in kefir from sheep and goat have also been reported. Guangsen et al. [
41] compared the kefir produced with ovine and caprine milk and observed significantly higher values of pH and in the parameter b* of ovine kefir.
The high counts of lactobacilli, lactococci and yeasts (> log 7 CFU/g) indicate the good adaptation of microorganisms to the ovine´s and goat´s LWCs. Commercial and traditional kefir did not show great differences regarding their microbial counts. Several studies compared the microbiological characteristics of kefir produced by commercial cultures or grains [41-44]. Guangsen et al. [
41] report that the counts of lactic acid bacteria were higher in the sheep kefir than in goat kefir. These authors indicated that the sheep kefir was found better than goat kefir due to the microbiological characteristics, volatile compounds and sensory profile. Biçer et al. [
42] evaluated the bacterial microbiota of five commercial and one traditional kefir and observed that the microbial diversity in traditional kefir is higher than in commercial kefir, being
Lactobacillaceae and
Streptococaccceae, the most important families in traditional kefir. Guclu et al. [
43] studied industrial kefirs (plain kefirs (without fruits) and fruit kefirs) and a kefir fermented using kefir grains. They observed counts of total aerobic mesophilic bacteria in the order of log 5.5-8.0 CFU/mL in plain kefir and of log 6.2-8.5 CFU/mL in fruit kefir; higher counts were found in kefir fermented with grains. They did not find any differences in the counts of lactic acid bacteria in plain and fruit kefir measured at two different times of storage. Wang et al. [
44] observed that the microbiological and sensory characteristics of the kefir fermented by a compound strain starter and by kefir grains was similar.
Kef & Arslan [
29] observed in cow’s milk kefir and goat’s milk kefir a decrease of streptococci and lactobacilli counts during the storage of the products, slowly until the 7
th day and quickly until the 14
th day of storage. These results are different than those observed in our work since it was not observed a decrease in the microbiological counts during the 30 days of storage.
In relation to sensory analysis, it has been reported that the ovine kefir is more appreciated than the goat kefir [
31]. The sensory scores of our ovine and goat kefirs showed significant differences but some sensorial aspects of both kefirs can be improved. It must be referred that the composition of the LWCs can be tailored according to the needs, particularly concerning the levels of solids, protein and fat desired for the fermented products. It has also to be referred that, other ingredients such as fruit syrups or jams can be used in the formulations according to consumer’s preferences. Therefore, there is a large margin to improve the sensory acceptability of the products. The improvement of the acceptability of the products will largely increase the feasibility of this approach as a means to valorize CW in small-scale dairy plants through the production of fermented dairy products with potential positive health effects. Besides, these products can have a shelf-life of
ca. 30 days, which is larger than the one of conventional whey cheeses traditionally produced by such companies.
5. Conclusions
Considering the feasibility of the use of UF concentration of CW in small/medium scale cheese plants processing small ruminant’s milk, the present work proves that this operation can allow for the valorization of such byproducts, reducing therefore the environmental impact of the operation and allowing for the development of functional foods with proven health benefits. Despite the need to improve the sensory acceptability of the kefir products developed, one can consider that this approach represents an excellent opportunity for such companies, which in most cases face the production of CW as a problem and do not take advantage of the potential opportunities to valorize it.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org., Table S1: Color difference values (ΔEab*) between different sheep’s kefir products, and for the same product over storage. Table S2: Color difference values (ΔEab*) between different goat’s’s kefir products, and for the same product over storage.
Author Contributions
Conceptualization C.P., A.P., O.D., A.C.; methodology C.P, A.P.; validation, C.P., S.D., O.D., A.C.; formal analysis, A.P., G.T.; investigation, A.P., G.T, D.G.; resources, C.P.; data curation, C.P, O.D., A.C; writing—original draft preparation, A.P., C.P.; writing—review and editing, C.P., O.D., A.C., S.D.; supervision, C.P.; project administration, C.P.; funding acquisition, C.P. All authors have read and agreed to the published version of the manuscript.
Funding
(1) Government of Portugal: PDR2020-101-030768: LACTIES: Inovação, Ecoeficiência e Segurança em PME´s do Setor dos Laticínios.; (2) Fundação para a Ciência e Tecnologia: UID/AMB/00681/2013.
Data Availability Statement
Data is included on the manuscript or in supplementary materials.
Acknowledgments
The authors would like to thank, Adélia Vaz, Lurdes Pires and Jorge Arede for technical support in the dairy pilot plant.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Banaszewska, A.; Cruijssen, F.; Claassen, G. D. H.; & van der Vorst, J. G. A. J.; & van der Vorst, J. G. A. J. Effect and key factors of byproducts valorization: The case of dairy industry. J. Dairy Sci. 2014, 97, 1893–1908. [Google Scholar] [CrossRef]
- Barukčić, I.; Jakopović, K. L.; Božanić, R. Valorisation of whey and buttermilk for production of functional beverages - An overview of current possibilities. Food Tech. Biotech. 2019, 57, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Henriques, M.; Gomes, D.; Pereira, C. Liquid whey protein concentrates produced by ultrafiltration as primary raw materials for thermal dairy gels. Food Tech. Biotech. 2017, 55, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Henriques, M.H.F.; Gomes, D.; Borges, A.R.; Pereira, C.J.D. Liquid whey protein concentrates as primary raw material for acid dairy gels. Food Sci. Tech. 2019, 40, 361–369. [Google Scholar] [CrossRef]
- Borges, A.R.; Pires, A.F.; Marnotes, N.G.; Gomes, D.G.; Henriques, M.F.; Pereira, C.D. Dairy by-products concentrated by ultrafiltration used as ingredients in the production of reduced fat washed curd cheese. Foods 2020, 9, 9–1020. [Google Scholar] [CrossRef]
- Asunis, F.; De Gioannis, G.; Dessì, P.; Isipato, M.; Lens, P. N. L.; Muntoni, A.; Polettini, A.; Pomi, R.; Rossi, A.; Spiga, D. dairy biorefinery: Integrating treatment processes for cheese whey valorisation. J. Env. Man. 2020, 276, 111240. [Google Scholar] [CrossRef] [PubMed]
- Yadav, J. S. S.; Yan, S.; Ajila, C. M.; Bezawada, J.; Tyagi, R. D.; Surampalli, R. Y. Food-grade single-cell protein production, characterization and ultrafiltration recovery of residual fermented whey proteins from whey. Food Bioprod Process. 2016, 99, 156–165. [Google Scholar] [CrossRef]
- Pires, A.F.; Marnotes, N.G.; Rubio, O.D.; Garcia, A.C.; Pereira, C.D. Dairy by-Products: A review on the valorization of whey and second cheese whey. Foods 2021, 10, 10–1067. [Google Scholar] [CrossRef]
- Pires, A.; Gomes, D.; Noronha, J.; Díaz, O.; Cobos, A.; Pereira, C.D. Evaluation of the characteristics of sheep’s and goat’s ice cream, produced with UF concentrated second cheese whey and different starter cultures. Foods 2022, 11, 4091. [Google Scholar] [CrossRef]
- Zotta, T.; Solieri, L.; Iacumin, L.; Picozzi, C.; Gullo, M. Valorization of cheese whey using microbial fermentations. Appl. Microbiol. Biotechnol. 2020, 104, 2749–2764. [Google Scholar] [CrossRef]
- Barba, F. J. An integrated approach for the valorization of cheese whey. Foods 2021, 10. [Google Scholar] [CrossRef]
- Sebastián-Nicolás, J. L.; González-Olivares, L. G.; Vázquez-Rodríguez, G. A.; Lucho-Constatino, C. A.; Castañeda-Ovando, A.; Cruz-Guerrero, A. E. Valorization of whey using a biorefinery. Biofuels, Biopr. Bioref. 2020, 14, 1010–1027. [Google Scholar] [CrossRef]
- Osorio-González, C. S.; Gómez-Falcon, N.; Brar, S. K.; Ramírez, A. A. Cheese whey as a potential feedstock for producing renewable biofuels: A review. Energies 2022, 15, 6828. [Google Scholar] [CrossRef]
- Zou, J.; Chang, X. Past, Present, and Future Perspectives on Whey as a Promising Feedstock for Bioethanol Production by Yeast. J. of Fungi 2022, 8. [Google Scholar] [CrossRef]
- Macedo, A.; Azedo, D.; Duarte, E.; Pereira, C. Valorization of goat cheese whey through an integrated process of ultrafiltration and nanofiltration. Membranes 2021, 11, 477. [Google Scholar] [CrossRef] [PubMed]
- Macedo, A.; Bilau, J.; Cambóias, E.; Duarte, E. Integration of membrane processes for by-product valorization to improve the eco-efficiency of small/medium size cheese dairy plants. Foods 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Tulla, A.F. Sustainable rural development requires value-added activities linked with comparative advantage: The case of the Catalan Pyrenees. Eur. Countrys 2019, 11, 229–256. [Google Scholar] [CrossRef]
- Marnotes, N.G.; Pires, A.F.; Díaz, O.; Cobos, A.; Pereira, C.D. Sheep’s and goat’s frozen yoghurts produced with ultrafiltrated whey concentrates. Appl. Sci. 2021, 11, 6568. [Google Scholar] [CrossRef]
- Silva, T.; Pires, A.; Gomes, D.; Viegas, J.; Pereira-Dias, S.; Pintado, M.E.; Henriques, M.; Pereira, C.D. Sheep’s butter and correspondent buttermilk produced with sweet cream and cream fermented by aromatic starter, kefir and probiotic culture. Foods 2023, 12, 331. [Google Scholar] [CrossRef]
- Lv, T.; Huang, X.; Zhang, C.; Chen, D.; Gu, R.; Wa, Y.; Peng, K.; Zong, L.; Chen, X. Enhancement of the antibacterial properties of kefir by adding lactobacillus fermentum grx08. Journal of Food Protection 2021, 84, 1463–1471. [Google Scholar] [CrossRef]
- Vieira, C.P.; Rosario, A.I.L.S.; Lelis, C.A.; Rekowsky, B.S.S.; Carvalho, A.P.A.; Rosário, D.K.A.; Elias, T.A.; Costa, M.P.; Foguel, D.; Conte-Junior, C.A. Bioactive compounds from Kefir and their potential benefits on health: A systematic review and meta-analysis. Oxidative Med. Cell. Longevity 2021, 9081738. [Google Scholar] [CrossRef]
- Malta, S.M.; Batista, L.L.; Silva, H.C.G.; Franco, R.R.; Silva, M.H.; Rodrigues, T.S.; Correia, L.I.V.; Martins, M.M.; Venturini, G.; Espindola, F.S.; da Silva, M.V.; Ueira-Vieira, C. Identification of bioactive peptides from a Brazilian kefir sample, and their anti-Alzheimer potential in Drosophila melanogaster. Scientific Reports 2022, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.; Gregório, J.; Rijo, P.; Rosado, C.; Monteiro Rodrigues, L. Kefir and the gut–skin axis. Int. J. Environ. Res. Pub. Health 2022, 19, 19–13791. [Google Scholar] [CrossRef]
- Youn, H.Y.; Seo, K.H.; Kim, H.J.; Kim, Y.S.; Kim, H. Effect of postbiotics derived from kefir lactic acid bacteria-mediated bioconversion of citrus pomace extract and whey on high-fat diet-induced obesity and gut dysbiosis. Food Res. Int. 2022, 162, 111930. [Google Scholar] [CrossRef] [PubMed]
- de Vasconcelos, R.F.; Costa, V.; Araujo, B.; Maia, T.A.C.; Dias, R.; Vasconcelos, L.; Silveira, H.; Carneiro, B.; Thiers, D.; Costa, F.W.G.; Kurita, L.; Ayala, A.; Leitão, R.; Pereira, K.M.A.; Gondim, D.V.; Goes, P. Milk kefir therapy improves the skeletal response to resistance exercise in rats submitted to glucocorticoid-induced osteoporosis. Exp. Gerontology 2022, 167. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.R.L.; Lin, W.Y.; Fan, H.C.; Tu, M.Y.; Liu, Y.H.; Yen, C.C.; Cidem, A.; Chen, W.; Chen, C.M. Kefir peptides ameliorate osteoporosis in AKR1A1 knockout mice with vitamin C deficiency by promoting osteoblastogenesis and inhibiting osteoclastogenesis. Biomed. and Pharmacotherapy 2022, 156, 113859. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.C.; Li, H.P.; Chang, G.R.L.; Lan, Y.W.; Chen, Y.H.; Tseng, Y.S.; Tu, M.Y.; Chen, C.F.; Chen, H.L.; Chen, C.M. Kefir peptides promote osteogenic differentiation to enhance bone fracture healing in rats. Life Sciences 2022, 310, 121090. [Google Scholar] [CrossRef]
- González-Orozco, B.D.; García-Cano, I.; Jiménez-Flores, R.; Alvárez, V.B. Invited review: Milk kefir microbiota—Direct and indirect antimicrobial effects. J. Dairy Sci. 2022, 105, 3703–3715. [Google Scholar] [CrossRef]
- Kef, S.; Arslan, S. The effects of different dietary fiber use on the properties of kefir produced with cow’s and goat’s milk. J. Food Proc. Pres. 2021, 45, 45–11. [Google Scholar] [CrossRef]
- Sebayang, F.; Sinaga, M.Z.E.; Kahiri, A.; Tarigan, J.B.; Sitepu, E.K. Synbiotic Functional Drink From Cow Milk Fermented With Kefir and Supplemented With Inulin. Rasayan J. Chemistry 2022, 15, 395–401. [Google Scholar] [CrossRef]
- Agarbati, A.; Ciani, M.; Canonico, L.; Galli, E.; Comitini, F. Exploitation of Yeasts with Probiotic Traits for Kefir Production: Effectiveness of the Microbial Consortium. Fermentation 2022, 8, 9. [Google Scholar] [CrossRef]
- 32. NP 703:1982. Iogurte. Determinação do resíduo seco e do resíduo seco isento de gordura. Comissão Técnica-32, 1º Edição, Portugal.
- 33. NP 2105:1983. Queijos. Determinação do teor de matéria gorda. Técnica de Van Gulick. Processo corrente. Direcção Geral da Qualidade.
- 34. NP 1923:1987. Iogurte. Determinação do teor de matéria gorda. Técnica de Gerber. Processo corrente. Direcção Geral da Qualidade.
- AOAC (1997). Official methods of analysis of Association of Official Analytical Chemists. 16th ed., Volume II. 33 Dairy Products USA.
- 36. NP 701:1982. Iogurte. Determinação da acidez. Comissão Técnica- 32, 1º Edição. Direcção Geral da Qualidade.
- IDF 117:2003. Yoghurt-Enumeration of characteristic microorganisms-Colony-count technique at 37 ºC (1st ed.).
- IDF 94:2004. Milk and milk products-Enumeration of colony-forming units of yeasts and/or moulds - Colony-count technique at 25 °C (2nd ed.).
- Stone, H.; Sidel, J. Sensory Evaluation Practices, 3rd ed.; Food Science and Technology; Academic Press: New York, NY, USA, 2004. [Google Scholar]
- ISO 8587:1988 Sensory analysis. Methodology. Ranking. Technical Committee ISO/TC 34/SC 12 Sensory analysis.
- Guangsen, T.; Xiang, L.; Jiahu, G. Microbial diversity and volatile metabolites of kefir prepared by different milk types. CYTA - Journal of Food 2021, 19, 399–407. [Google Scholar] [CrossRef]
- Biçer, Y.; Telli, A.E.; Sönmez, G.; Turkal, G.; Telli, N.; Uçar, G. Comparison of commercial and traditional kefir microbiota using metagenomic analysis. Int. J. Dairy Tech. 2021, 74, 528–534. [Google Scholar] [CrossRef]
- Guclu, A.U.; Yesil, E.; Kocak, A.A.; Saka, M.; Mirza, H.C.; Dinc, B.; Basustaoglu, A. Quantitative probiotic analysis of various Kefir samples. Jordan J. Biol. Sci. 2021, 14, 799–804. [Google Scholar] [CrossRef]
- Wang, L.; Deng, K.; Zhang, Y. Isolation and screening of high-quality lactic acid bacteria and yeast strains in kefir grains and preparation of kefir compound fermentation starter. J. Food Proc. Pres. 2022, 1–13. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).