1. Introduction
Methylxanthines are used for the therapy of a variety of respiratory disorders. Caffeine to treat neonates has been known for more than 60 years [
1]. Since 2000, it is in use as citrate salt of caffeine for treatment of apnea of prematurity [
2]. Newborns have special characteristics due to their physiological immaturity and their state of continuous development, resulting in unique medical conditions for this population. As consequence, a very small number of drugs are approved for neonates and data supporting the safety and efficacy are limited. Quite recently, considerable attention has been paid to clinical trials of caffeine citrate treatment for apnea of prematurity, the prevention of extubation failure, bronchopulmonary dysplasia, and the need for mechanical ventilation [
3,
4,
5,
6,
7,
8,
9,
10]. Current research focusses on effects on the central nervous system for apnea prevention.
Since caffeine citrate therapy for premature newborns is an effective therapy for several respiratory conditions, it is of great interest to investigate if caffeine citrate has also a direct function in the respiratory epithelium. Caffeine has several known mechanisms of actions: it is an adenosine receptor antagonist, releases calcium (Ca
2+) from intracellular stores and inhibits phosphodiesterases [
11]. Several of these pathways are known to be involved in regulation of the ciliary beating [
12,
13]. These pathways include signalling via adenosine receptors, Ca
2+ mediated events, phosphodiesterase, and guanylyl cyclase [
14,
15,
16,
17].
We hypothesized that caffeine citrate might constitute a potential pharmacological modulator for ciliary beat frequency (CBF) regulation in human respiratory cells. Therefore, we examined the effect of caffeine citrate on fully differentiated human primary respiratory epithelial cells (hRECs). Our results describe for the first time a therapeutic effect of caffeine by directly increasing the CBF of hRECs via ryanodine receptor (RYR) channels and might help to explain why caffeine citrate has been shown to be beneficial not only for apnea prevention but also for bronchiopulmonary dysplasia.
2. Materials and Methods
Cell isolation. Human respiratory epithelial cells were obtained by nasal brush biopsy of healthy donors and cystic fibrosis (CF) individuals. The study was approved by the Ethics Committee of the Westphalian Wilhelms University of Muenster (2013-512-f-S). Written informed consent to participate in this study was obtained from each individual. Cells were resuspended in DMEM/Ham´s F12 (1:1) (Invitrogen, Karlsruhe, Germany) supplemented with 2 % UltroserG (Pall Life Sciences, Germany) and Antibiotic-Antimycotic (100 x) (Invitrogen, Karlsruhe, Germany) and plated on collagen-coated tissue flasks. Cells were cultured in a humidified atmosphere with 5 % CO2 at 37 °C. Medium was replaced three times per week until 70 – 80 % confluence was reached. Ciliogenesis was induced in suspension culture or in Air-liquid interface (ALI) culture.
Culture of primary respiratory epithelial cells at Air-liquid Interface (ALI). ALI inserts (Costar™ Corning® 3470 Transwell™ Clear Polyester Membrane Inserts For 24-Well Plates, Corning Incorporated, USA) were collagen-coated using 250 µL diluted rat-tail collagen in acetic acid (1:5) per insert. After drying for 24 hours at room temperature, inserts were washed twice with PBS, before use. Cell layers of hRECs expanded on collagen-coated flasks were treated with collagenase type IV (200 U/mL; Worthington Biochemical Corporation, St. Katharinen, Germany) and cells were trypsinized for 5 min in 1x Trypsin-EDTA Solution (Sigma-Aldrich Co. LLC, St. Louis, USA). The cell pellet was resuspended in B-ALI Growth Basal Medium (Lonza Cologne GmbH, Germany) and seeded into collagen coated transwell inserts (100,000 cells per insert). Cells were incubated with 5 % CO2 at 37 °C in a humidified incubator and fed with B-ALI Growth Basal Medium from the basolateral and apical compartment every day. Once confluence was reached, air lift of the ALI culture was performed by aspirating the medium of the apical compartment and replacing medium of the basal compartment by B-ALI differentiation medium. Medium was replaced three times per week.
Video microscopy. ALI cultures of hRECs ensure steady experimental settings for CBF measurements over time. Sisson-Ammons Video Analysis (SAVA) (Ammons Engineering, Mt Morris, MI, USA) was used for CBF analyses. Videos of hRECs ALI were recorded using a Megaplus camera model ES 310 turbo (Redlake Inc., USA) attached to an inverted phase-contrast microscope (Nikon Eclipse Ti-S) equipped with an ELWD 40x S Plan Fluor objective. Digital image sampling was performed at 125 fps and 640×480-pixel resolution. All experiments were performed under physiological conditions, by maintaining the temperature at 37 °C by a Minitube SC300 heating system (Minitube International AG).
The ciliary base frequency (CBFb) was determined over a time period of 20 minutes. The individual measurements were averaged to obtain the CBFb. CBF was measured over time, while treating the cells with defined drug concentrations. To test the dose response of caffeine citrate induced CBF excitation, CBF was measured over time, while treating the cells with 1 mM, 5 mM and 10 mM caffeine citrate (Peyona®, solution for infusion and oral solution). A stock solution of 10 mmol/l dantrolene IV (20 mg dantrolene sodium for injection) was prepared in Aqua ad injectablia (Norgine, NL).
Statistical analysis. The obtained maximal increase in CBF from CBF
b in percent was compared between standard dose effect curves of caffeine citrate. Statistical analysis was performed using the SPSS software (
www.ibm.com). Comparison was performed using the Wilcoxon rank-sum test. Means are reported ± s.e.m., with n values noted. Significance was assumed at P < 0.05.
3. Results
Caffeine citrate increases CBF in hRECs and shows a dose-dependent effect
Application of caffeine citrate to the apical compartment of the ALI culture (
Figure 1a,
Figure 1b) showed a short-term decrease followed by a sustained increase in CBF over CBF
b in the presence of caffeine citrate (
Figure 1c). We could assign the observed effect to caffeine, by ruling out any effect of citrate buffer only on CBF
b (n = 3, data not shown). We demonstrate a dose-dependent effect on hRECs by increasing exposure to 1, 5, and 10 mM caffeine citrate, resulting in highly significant CBF excitation of 14.16 ± 1.54 % (n = 6; P ≤ 0.0002), 18.4 ± 4.16 % (n = 6; P ≤ 0.0101), and 28.75 ± 2.74 % (n = 6; P ≤ 0.0002) over CBF
b respectively (
Figure 1d). Already a very low dose of 0.6 mM showed an increase in CBF by 7.99 ± 2.79% over CBF
b (n = 9; P ≤ 0.038) (data not shown).
In vitrocaffeine citrate application increases CBF in CFTR-mutant hRECS
To demonstrate that CBF can be also increased in hRECs with deficient mucociliary clearance capacity, we used hRECs cultured from three cystic fibrosis (CF) individuals with deleterious mutations in the cystic fibrosis transmembrane conductance regulator gene (
CFTR) (OS-277: c.1521_1523delCTT (p.Phe508del)/c.1007T>A (p.Ile336Lys) compound heterozygous; OS-271: c.1521_1523delCTT (p.Phe508del) homozygous) and OS-288: c.1521_1523delCTT (p.Phe508del)/ c.3849+10kbC->T compound heterozygous). As already demonstrated on cell cultures of hRECs of healthy donors (
Figure 1d), increasing concentrations of 1 mM, 5 mM, and 10 mM caffeine citrate result in highly significant CBF excitation of 12.83 ± 2.00 % (n = 9; P ≤ 0.008), 30.81 ± 2.26 % (n = 9; P ≤ 0.008), and 42.14 ± 2.06 % (n = 9; P ≤ 0.008) over CBF
b also in hRECs obtained from CF indivuduals (
Figure 1e).
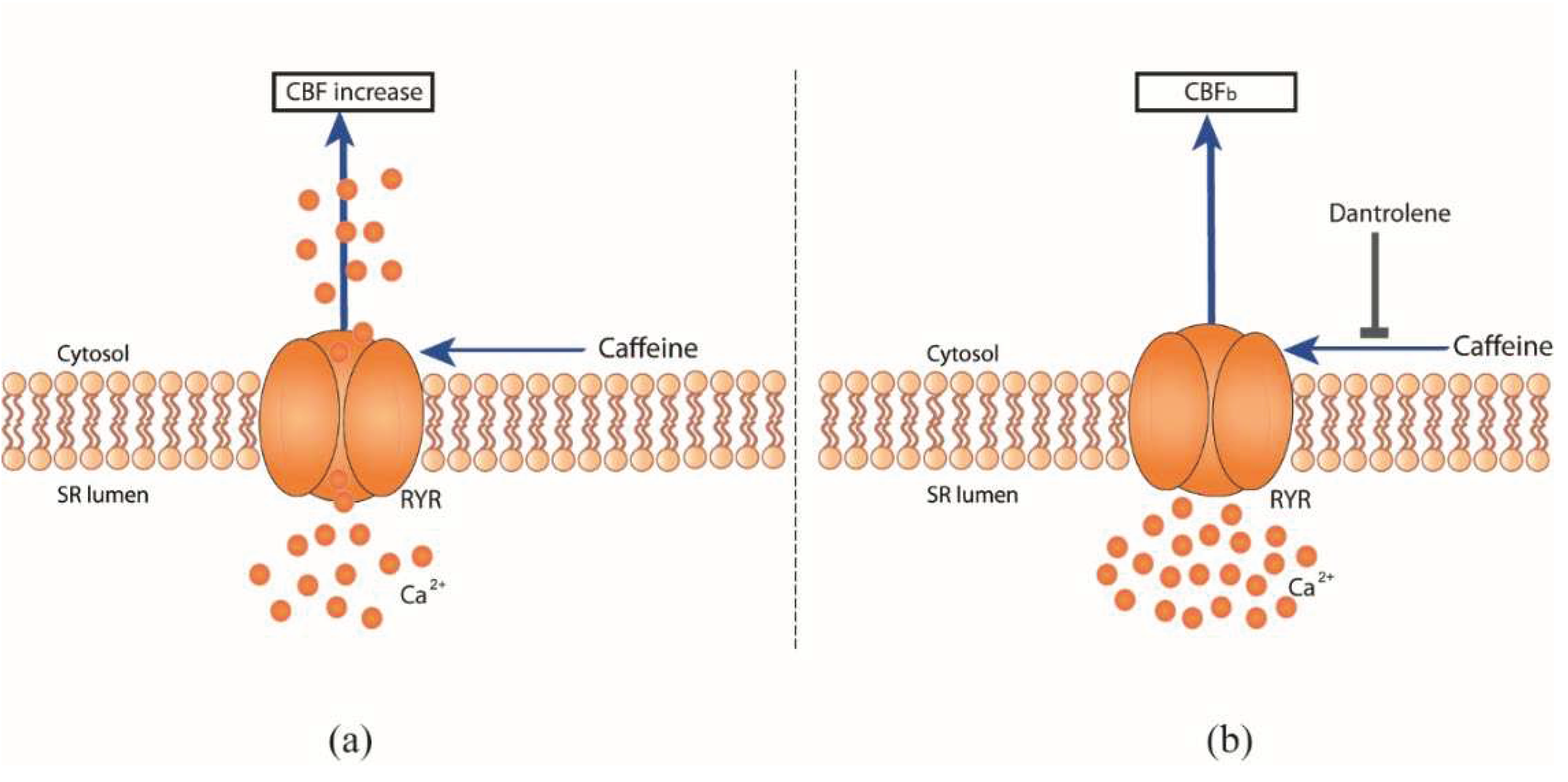
Caffeine citrate acts through RYR to modulate CBF
To determine the mechanisms of caffeine in CBF regulation, we performed additional pharmacological interventions. Caffeine is a known adenosine receptor antagonist, it releases Ca
2+ from intracellular stores, and inhibits phosphodiesterases, all of which can modulate CBF [
11]. In order to study Ca
2+-mediated events on the basis of intracellular Ca
2+ sources, Ca
2+ signaling pathways involved in CBF regulation via RYR channels were investigated using the cell permeable RYR channel blocker dantrolene [
18]. Here, dantrolene was found to inhibit the effect of caffeine-induced CBF excitation on hRECs (
Figure 1f,
Figure 2). A concentration of 10 mmol/l dantrolene applied to the basolateral compartment of the hREC transwell insert results in significant inhibition of the effect of caffeine induced CBF-increase, resulting in a reduction of CBF increase over CBF
b up to -10.39 ± 3.07 % (N = 4; P ≤ 0.0014; 10 mM caffeine).
4. Discussion
Our findings indicate that caffeine mediates CBF excitation via RYR channels in the human respiratory epithelium (
Figure 1f,
Figure 2), consistent with previous observations in the ciliated giant orange sea slug Tritonia Diomedea. In this model organism, Woodward et al. demonstrated a reduced effect of caffeine induced CBF excitation by dantrolene and concluded that caffeine is acting specifically at RYR channels to release Ca
2+ from intracellular sources [
12]. Thus, we demonstrate the evolutionary conservation of the functional role of caffeine in regulating beat frequency from gastropods, as previously demonstrated in the sea slug Tritonia Diomedea, to mammals (humans).
Caffeine citrate therapy has been shown to reduce the risk for bronchopulmonary dysplasia in neonates. Increased mucus production as well as mucus plugging plays a role in the pathophysiology of bronchopulmonary dysplasia. Our data indicate that the beneficial effect of caffeine citrate to reduce frequency of bronchopulmonary dysplasia in preterm infants might also result from increased ciliary beat frequency and improved cleaning of airways as mucus propelling velocity and thereby efficiency of mucociliary clearance capacity (MCC) is linearly dependent on CBF [
19].
Already low concentrations (0.6 mM) of caffeine citrate induce a significant increase in CBF. Those concentrations are predicted to be present in preterm infants treated with caffeine citrate to prevent central apnea. We propose that this direct function of caffeine citrate on the respiratory epithelium may be a promising approach to treat mucociliary clearance disorders as asthma (MIM 600807), chronic obstructive pulmonary disease (COPD; MIM 606963), or cystic fibrosis (CF; MIM 219700). Mucociliary dysfunction can result in substantial morbidity characterized by upper and lower respiratory tract infections, dyspnea, and chronic cough [
20,
21]. Persistent infections and immune response can result in damage of the airways, bronchiectasis and chronic lung failure [
22]. In consequence, improving MCC by increasing the CBF presents a promising strategy to treat lung diseases.
5. Conclusions
The direct action of caffeine citrate on airway cells, together with its excellent bioavailability and licensed application even in premature infants, demonstrate great potential of methylxanthines for further research in this field. Various methylxanthines such as caffeine, theophylline, theobromine, aminophylline, or synthetic xanthines are already commonly used in respiratory disorders [
11]. To emphasize, caffeine citrate has certain advantages over other methylxanthines, as it was shown to have a good performance relating to stable plasma concentration and longer half-life, and is better tolerated than e.g. theophylline [
23]. Based on our findings, we conclude that caffeine citrate therapy for respiratory diseases with compromised mucociliary clearance should further be investigated.
Author Contributions
All authors contributed to the study conception and design. Data were collected by S.C. and L.B. Respiratory epithelial cells were collected by C.E, E.R.F. and H.O. Data analysis and interpretation was performed by S.C., L.B., R.H., N.T.L., P.P. and H.O. The first draft of the manuscript was written by S.C. and H.O. All authors commented on previous versions of the manuscript and approved the final manuscript.
Funding
This specific research received no external funding. Work in the laboratory of H. Omran was funded by the Deutsche Forschungsgemeinschaft (Om6/7, Om6/8, OM6/10, OM6/14 and DFG KFO 326 (OM6/11)), the Interdisziplinäres Zentrum fur Klinische Forschung Muenster (Om2/015/16), Eva Luise Köhler Forschungspreis and Care-for-Rare Foundation.
Institutional Review Board Statement
Published research complied with the guidelines for human studies and was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The study was approved by the Ethics Committee of the Westphalian Wilhelms University of Muenster (2013-512-f-S).
Informed Consent Statement
Written informed consent to participate in this study was obtained from each subject involved in the study.
Data Availability Statement
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Acknowledgments
The authors thank all individuals providing cells for analysis. The authors also thank members of the laboratory for technical support and fruitful discussions about the project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Roberts, C.L. Intravenous caffeine in prevention of neonatal asphyxia and narcosis. Obstet. Gynecol. 1954, 4, 545-547.
- Erenberg, A.; Leff, R.D.; Haack, D.G.; Mosdell, K.W.; Hicks, G.M.; Wynne, B.A. Caffeine citrate for the treatment of apnea of prematurity: a double-blind, placebo-controlled study. Pharmacotherapy 2000, 20, 644–652. [CrossRef]
- Alansari, K.; Toaimah, F.H.; Khalafalla, H.; El Tatawy, L.A.; Davidson, B.L.; Ahmed, W. Caffeine for the Treatment of Apnea in Bronchiolitis: A Randomized Trial. J. Pediatr. 2016, 177, 204-211.e3. [CrossRef]
- Belén Rivas, A.; Arruza, L.; Pacheco, E.; Portoles, A.; Diz, J.; Vargas, E. Adverse drug reactions in neonates: a prospective study. Arch. Dis. Child. 2016, 101, 371–376. [CrossRef]
- Hand, I.; Zaghloul, N.; Barash, L.; Parris, R.; Aden, U.; Li, H.-L. Timing of Caffeine Therapy and Neonatal Outcomes in Preterm Infants: A Retrospective Study. Int. J. Pediatr. 2016, 2016, 9478204. [CrossRef]
- Katheria, A.C.; Sauberan, J.B.; Akotia, D.; Rich, W.; Durham, J.; Finer, N.N. A Pilot Randomized Controlled Trial of Early versus Routine Caffeine in Extremely Premature Infants. Am. J. Perinatol. 2015, 32, 879–886. [CrossRef]
- Lista, G.; Fabbri, L.; Polackova, R.; Kiechl-Kohlendorfer, U.; Papagaroufalis, K.; Saenz, P.; Ferrari, F.; Lasagna, G.; Carnielli, V.P. The Real-World Routine Use of Caffeine Citrate in Preterm Infants: A European Postauthorization Safety Study. Neonatology 2016, 109, 221–227. [CrossRef]
- Mohammed, S.; Nour, I.; Shabaan, A.E.; Shouman, B.; Abdel-Hady, H.; Nasef, N. High versus low-dose caffeine for apnea of prematurity: a randomized controlled trial. Eur. J. Pediatr. 2015, 174, 949–956. [CrossRef]
- Yu, T.; Balch, A.H.; Ward, R.M.; Korgenski, E.K.; Sherwin, C.M.T. Incorporating pharmacodynamic considerations into caffeine therapeutic drug monitoring in preterm neonates. BMC Pharmacol. Toxicol. 2016, 17, 22. [CrossRef]
- McPherson, C.; Neil, J.J.; Tjoeng, T.H.; Pineda, R.; Inder, T.E. A pilot randomized trial of high-dose caffeine therapy in preterm infants. Pediatr. Res. 2015, 78, 198–204. [CrossRef]
- Oñatibia-Astibia, A.; Martínez-Pinilla, E.; Franco, R. The potential of methylxanthine-based therapies in pediatric respiratory tract diseases. Respir. Med. 2016, 112, 1–9. [CrossRef]
- Woodward, O.M.; Willows, A.O.D. Nervous control of ciliary beating by Cl(-), Ca(2+) and calmodulin in Tritonia diomedea. J. Exp. Biol. 2006, 209, 2765–2773. [CrossRef]
- Salathe, M. Regulation of mammalian ciliary beating. Annu. Rev. Physiol. 2007, 69, 401–422. [CrossRef]
- Chen, J.-F.; Eltzschig, H.K.; Fredholm, B.B. Adenosine receptors as drug targets--what are the challenges? Nat. Rev. Drug Discov. 2013, 12, 265–286. [CrossRef]
- Bruce, C.; Yates, D.H.; Thomas, P.S. Caffeine decreases exhaled nitric oxide. Thorax 2002, 57, 361–363. [CrossRef]
- Varani, K.; Portaluppi, F.; Gessi, S.; Merighi, S.; Ongini, E.; Belardinelli, L.; Borea, P.A. Dose and time effects of caffeine intake on human platelet adenosine A(2A) receptors : functional and biochemical aspects. Circulation 2000, 102, 285–289. [CrossRef]
- Zagoory, O.; Braiman, A.; Priel, Z. The mechanism of ciliary stimulation by acetylcholine: roles of calcium, PKA, and PKG. J. Gen. Physiol. 2002, 119, 329–339. [CrossRef]
- Zhao, F.; Li, P.; Chen, S.R.; Louis, C.F.; Fruen, B.R. Dantrolene inhibition of ryanodine receptor Ca2+ release channels. Molecular mechanism and isoform selectivity. J. Biol. Chem. 2001, 276, 13810–13816. [CrossRef]
- Braiman, A.; Priel, Z. Efficient mucociliary transport relies on efficient regulation of ciliary beating. Respir. Physiol. Neurobiol. 2008, 163, 202–207. [CrossRef]
- Marthin, J.K.; Petersen, N.; Skovgaard, L.T.; Nielsen, K.G. Lung function in patients with primary ciliary dyskinesia: a cross-sectional and 3-decade longitudinal study. Am. J. Respir. Crit. Care Med. 2010, 181, 1262–1268. [CrossRef]
- Munkholm, M.; Mortensen, J. Mucociliary clearance: pathophysiological aspects. Clin. Physiol. Funct. Imaging 2014, 34, 171–177. [CrossRef]
- Wallmeier, J.; Nielsen, K.G.; Kuehni, C.E.; Lucas, J.S.; Leigh, M.W.; Zariwala, M.A.; Omran, H. Motile ciliopathies. Nat. Rev. Dis. Primers 2020, 6, 77. [CrossRef]
- Gannon, B.A. Theophylline or caffeine: which is best for apnea of prematurity? Neonatal Netw. 2000, 19, 33–36. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).