1. Introduction
Multiple system atrophy (MSA) is a progressive degenerative process of the central and autonomic nervous systems. It usually happens in adulthood, with sporadic incidence. It causes severe disability in the medium term and leads to death in less than 10 years [
1,
2]. The disease affects (variably and in any combination) the nigrostriatal, olivopontocerebellar, autonomic, and corticospinal systems, along with lack of response to levodopa treatment [
1]. The involvement of the nigrostriatal neuronal pathway develops the parkinsonian syndrome consisting of tremor, bradykinesia/rigidity and postural instability [
3].
The diagnosis of MSA is based on widely accepted criteria that classify the disease as possible, probable or definite (depending on the patient’s symptoms) [
4]. These criteria were reviewed in 2008, including neuroimaging tests [
5]. One of them was brain SPECT with presynaptic dopamine transporters, which was included for possible MSA type C until the latest revision of the diagnostic criteria [
6].
This SPECT allows detecting “in vivo” changes at the molecular level that affect brain dopaminergic function, specifically the nigrostriatal pathway. One of the most widely used radiopharmaceuticals is [123I]Ioflupane, which makes it possible to visualize the density of presynaptic dopamine transporters in both striatal nuclei. The interpretation of this study consists of a qualitative assessment, where the uptake and distribution of the radiopharmaceutical in the striated nuclei are visualized. This visual analysis is sufficient for diagnosis. On the other hand, numerous image analysis software tools have emerged, which could help nuclear medicine physicians. However, there is currently no clear standardization in this respect and each company has its own quantification software.
The validity and usefulness of SPECT in the diagnosis of Parkinson’s disease (PD), as well as in clinically uncertain parkinsonian syndromes, even in early stages, has so far been demonstrated [
7,
8,
9]. The main indications for SPECT are: i) Differential diagnosis between neurodegenerative parkinsonian syndrome and essential tremor; ii) Distinguishing between Lewy body dementia and other dementias such as Alzheimer’s disease; iii) Differential diagnosis between parkinsonism with presynaptic dopamine deficit (such as PD) and secondary parkinsonism (psychogenic, drug-induced, or vascular); iv) Early detection of parkinsonian syndromes with presynaptic involvement [
10].
It has not been demonstrated that it is possible to reliably discriminate between PD and MSA by SPECT. There are a multitude of studies that address this question, seeking both qualitative and quantitative differences, with varying results [
11,
12,
13,
14]. The greatest diagnostic difficulty is found when analyzing the subtypes of MSA: parkinsonian (MSA-P) and cerebellar (MSA-C) and the usefulness of [
123I]Ioflupane SPECT. There is a certain consensus on its usefulness in MSA-P, being more questioned in the MSA-C subtype, given that, at the onset of the disease, SPECT can be completely normal [
15,
16,
17]. For this reason, a negative result does not exclude the diagnosis of MSA [
18]. In addition, it has been proposed to monitor the evolution of MSA by performing [
123I]Ioflupane SPECT. In fact, it is postulated that this test could be an important biomarker of disease progression, as well as the subtype of dopaminergic degeneration [
16,
19].
The aim of this work is to determine the diagnostic effectiveness of brain [123I]Ioflupane SPECT in both subtype of MSA, while addressing all the aforementioned controversies and lack of consensus. Possible differences between quantitative and quantitative analysis of this technique will also be analyzed.
2. Materials and Methods
2.1. Study design and subjects
Single-center observational cross-sectional study. Patients over 18 years of age, with clinical suspicion compatible with MSA according to Gilman’s criteria (probable/possible/definite), who had undergone [
123I]Ioflupane SPECT during the period 2004-2020 were included. All DAT-SPECT scans were requested by the Movement Disorders Unit of the Neurology Department of our hospital, after the initial interview with the patient. All of them had to be able to understand the diagnostic procedure and give informed consent. All patients who did not meet these requirements were excluded, as well as those who showed signs of “red flags” [
20] or were taking medication that could interfere with the SPECT result (qualitative and quantitative).
Demographic and clinical variables were obtained by means of a clinical interview conducted by the neurologist of the movement disorders unit, prior to SPECT.
2.2. SPECT of presynaptic dopamine transporters with [123I]Ioflupane
The SPECT tomographic study was performed with a Siemens® gamma camera, model Symbia®, equipped with a double head and a low energy and high resolution collimator. The images were obtained after a period of 3 to 4 hours of intravenous administration of 185 MBq of the radiotracer [123I]Ioflupane, after thyroid blockade with lugol solution.
A 360º circular orbit around the skull was performed, with 3º intervals, acquiring 60 images with a duration of 35 seconds per interval, and a 128×128 matrix. Images were reconstructed using filtered back projection algorithms without attenuation correction, with the application of a Hanning filter (frequency of 0.7) and images were obtained according to transaxial slices and orbito-meatal orientation.
This study was first evaluated by qualitative analysis by three nuclear medicine physicians, two of them with extensive experience in the field of movement disorders. Type of MSA and its diagnostic classification (possible/probable) were blinded. A kappa index of 100% was obtained. The studies were also evaluated by semi-quantitative analysis, establishing uptake indices (after the sum of the six most representative axial images) between regions of interest (ROI) of an area of specific activity (striatal nuclei) and an area of non-specific activity (occipital cortex), obtaining the striatal/occipital index (S/O) [
21]. This analysis can be performed independently of the gamma camera used and is highly reproducible.
2.3. Ethical considerations
Authorization for this study was obtained from the Biomedical Research Ethics Committee of the province of Malaga. At all times, the harmonized tripartite standards of the Helsinki declaration, the Organic Law on Biomedical Research of 15/1999 of July 3, the Organic Law on Personal Data Protection (LOPD) of December 13, 2018, the code of ethics of the Organización Médica Colegial (OMC), the basic regulatory law 41/2002 on patient autonomy and rights and obligations regarding clinical information and documentation, of November 14, as well as the standards of good clinical practice, were respected.
2.4. Statistical study
First, a descriptive study was performed, showing absolute and percentage frequencies for qualitative variables. Continuous quantitative variables were expressed as mean and standard deviation. The Shapiro-Wilks test was used to check whether the values of these variables followed a normal distribution.
To calculate the association between qualitative variables, the Chi-square test was applied with Fisher’s correction if appropriate. To analyze the differences between continuous quantitative variables, the Student t test (parametric) or Mann-Whitney U test (nonparametric) was used for two independent groups, and the ANOVA test (parametric) or Kruskal-Wallis test (nonparametric) in three or more independent groups. ROC curves were performed for different cut-off points according to quantitative SPECT analysis. A significance level was considered for p<0.05. Confidence intervals were established at 95%.
3. Results
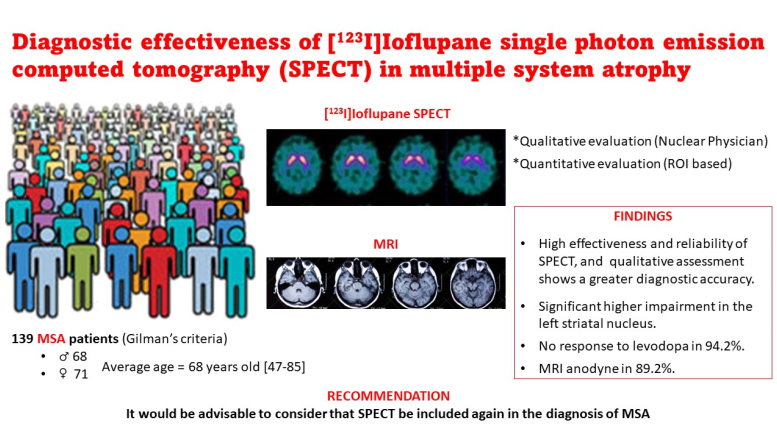
A total of 139 patients were studied: 68 males (48.9%) and 71 females (51.1%). The mean age was 68 years, with minimum and maximum ages of 47 and 85 years respectively and a standard deviation of 9. 104 cases were classified according to clinical criteria as MSA-probable (74.8%), 35 as MSA-possible (25.2%), and no subject was found with MSA-definite. The predominant subtype was MSA-P (62.6%) versus MSA-C (37.4%). Of the patients, 84.2% had parkinsonian syndrome, 40.3% had cerebellar symptomatology, 62.6% had dysautonomia and 20.1% had corticospinal symptoms. Some 94.2% showed no response to levodopa treatment, while the remaining 5.8% had a transient response.
Convectional MRI was anodyne in 89.2% of patients. The SPECT study with [123I]Ioflupane was compatible with nigrostriatal pathway involvement in 78.45%, with a non-pathological study in 21.55% (16.6% being normal and 5% inconclusive). The mean scores of the uptake indices obtained by SPECT were 1.35 ± 1.74 (0.9-1.88) for the global S/O index, 1.36 ± 1.8 (0.9-1.86) for the right S/O index and 1.34 ± 1.7 (0.91-1.89) for the left S/O.
3.1. Diagnostic validity study
Accuracy values were calculated for the entire MSA caseload (139 patients) and for both types of disease: MSA-P (87 patients) and MSA-C (52 patients), shown in
Table 1.
3.2. Qualitative vs. quantitative SPECT categorization
It is observed that there are significant differences in the scores of the three S/O indexes analyzed (global, right and left), in two of the groups defined by the qualitative assessment made by the nuclear medicine physician: pathological vs inconclusive group (p<0.05) and pathological vs normal group (p<0.001), as shown in
Table 2.
3.3. Relationship between SPECT categorizations (qualitative and quantitative) and clinical diagnosis (Gilman’s criteria, 2008)
We analyzed whether there are significant differences between the scores of the three quantitative indices, as well as between the three groups defined by visual assessment, according to the diagnostic classification (probable MSA, possible MSA), but no significant differences were obtained.
3.4. Cut-off proposition for the overall S/O index in relation to the qualitative categorization performed by the nuclear medicine physician
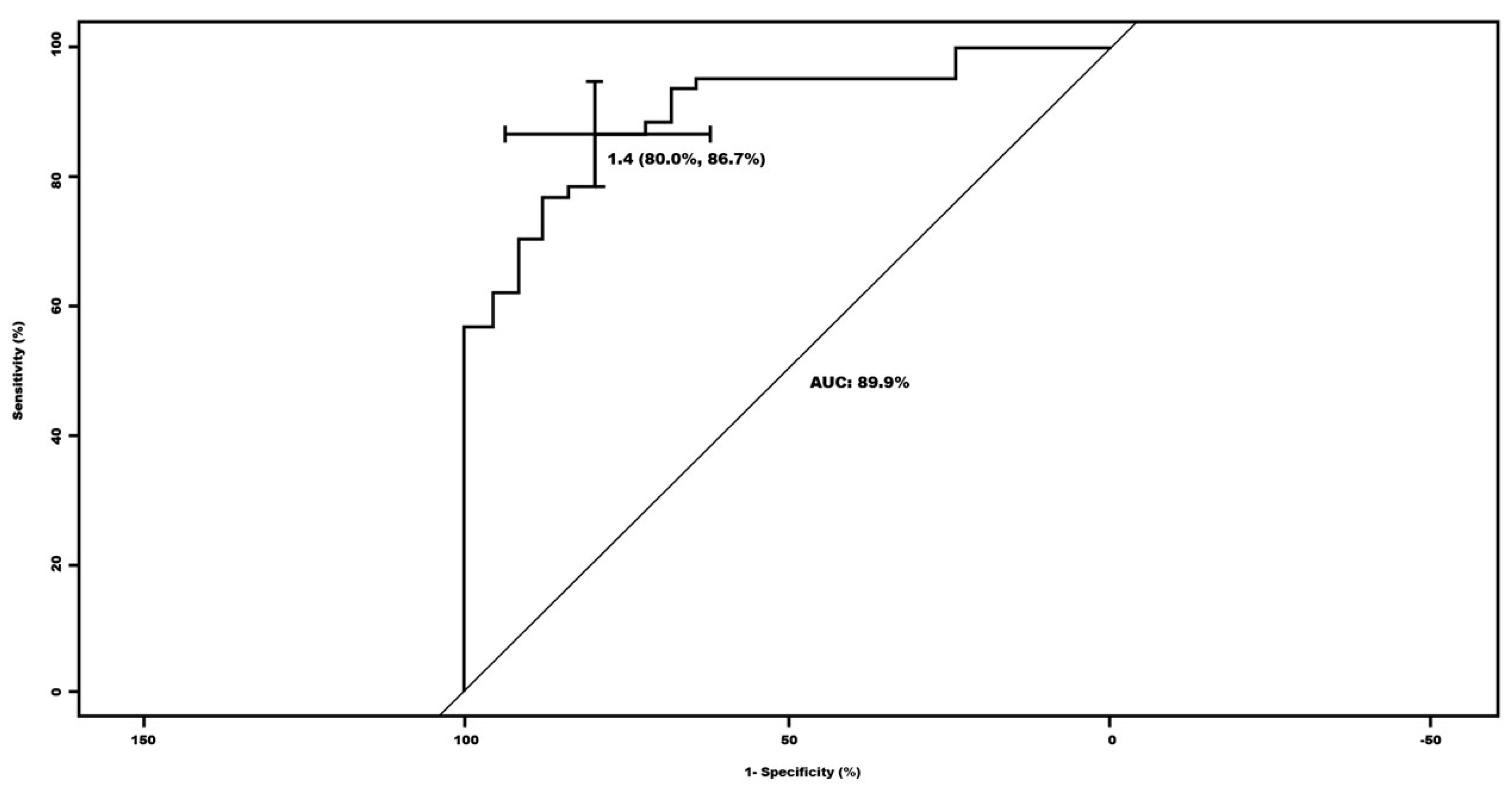
The optimal cut-off for the global S/O index in relation to the qualitative categorization for the normal vs. pathological groups was established at 1.4. Based on this, a sensitivity of 80% and a specificity of 86.7% were obtained. The area under the curve (AUC) was 89.9%, range 83.3-96.5% (
Figure 1).
3.5. Cut-off proposition for the global S/O index in relation to clinical diagnosis
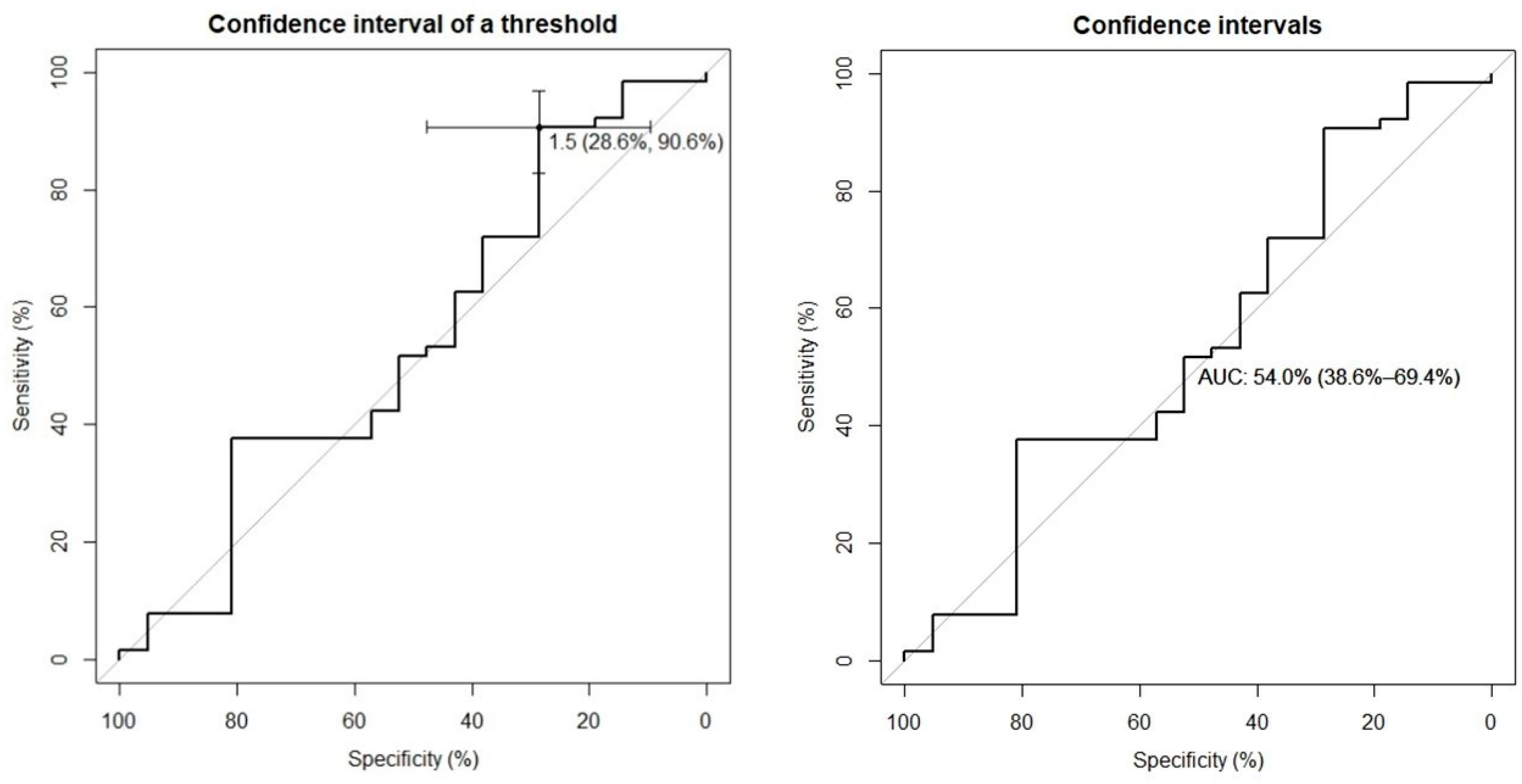
When comparing this index with the diagnostic classification made by the neurologist (Gilman’s criteria) in both groups mentioned (normal vs. pathological), an optimal cut-off of 1.5 was obtained, according to which we have a sensitivity of 28.6% and a specificity of 90.6%. The area under the ROC curve (AUC) was 54%, range 38.6-69.4% (
Figure 2).
3.6. Relationship between MSA subtypes, SPECT categorization (qualitative and quantitative) and clinical diagnosis
Significant differences were found between MSA subtype (MSA-C or MSA-P) and both SPECT categorizations (both with p<0.01), but not so with clinical diagnosis (p= 0.11) (
Table 3).
3.7. Lateralization in striatal involvement of MSA
We studied whether one cerebral hemisphere is more affected than the other at the onset or during the development of the disease and whether this preponderance has an impact on other variables. In this regard, according to the quantitative SPECT assessment, we observed that there are significant differences between the mean values of the striatal nuclei of both cerebral hemispheres, obtained from the right S/O index and left S/O index (mean 1.4 (SD 0.2) vs mean 1.3 (SD 0.2), p<0.05). Similarly, according to the visual assessment performed by the nuclear medicine physician, it was observed that 57.65% of the patients had greater involvement of the left striate nucleus.
When trying to find associations between the predominant hemisphere with other variables (qualitative SPECT assessment, MSA subtype and diagnostic classification), no significant differences were obtained (
Table 4).
3.8. Response to levodopa
We analyzed the responsiveness to levodopa in MSA as a diagnostic resource and compared it with other diagnostic or classification strategies. We found no association between the response to this treatment and the different variables used (diagnostic classification, SPECT categorizations and type of MSA) (
Table 5).
3.9. Relationship between parkinsonian clinic, SPECT findings and MSA subtype
Significant differences were found between the presence of clinical parkinsonism and the SPECT result (for both qualitative and quantitative assessments), as well as with the MSA subtype (MSA-C or MSA-P). Significant differences were found in all of them, except for clinical diagnosis (
Table 6).
4. Discussion
According to our study, we can observe a high reliability of the diagnosis issued by the nuclear medicine physician at initial clinical suspicion of MSA. This is demonstrated when we analyze the relationships between the visual (qualitative) diagnostic classification of [123I]Ioflupane SPECT and quantitative assessment (with predefined ROIs method). There is a parallelism between this visual assessment and the quantitative value of SPECT. Currently there is a multitude of specific software to quantitatively assess the SPECT image, but there is no diagnostic standardization. This fact, in conjunction with the results of our study, allows us to propose our semi-quantification method because of its simplicity and high reproducibility.
The SPECT result (qualitative and quantitative) was related to the MSA subtype. This is to be expected from a pathophysiological point of view, given that SPECT detects nigrostriatal pathway involvement even in the prodromal phase of the disease [
7,
8]. This affectation is the origin of the parkinsonian clinic (which is mostly present in the MSA-P form) [
1,
2].
However, there was no relationship between the presence of parkinsonian symptoms and the diagnostic classification of the disease (probable or possible), the closest to a true MSA without postmortem confirmatory study (according to diagnostic criteria) [
5]. Nevertheless, the previous clinical diagnostic classification (probable/possible) does not fully correspond with the SPECT assessment (both qualitative and quantitative), despite the fact that most of our patients (74%) were probable MSA [
4,
5]. Also, for cases not confirmed by autopsy (possible MSA/probable MSA), [
123I]Ioflupane SPECT can be an accurate and non-invasive in vivo study.
Since there is still no clear consensus within quantitative assessment to establish a cut-off to consider a study normal or pathological, we propose, based on our results (probably the first study with such a large number of patients), that this cut-off can be set at 1.4, since it provides good values of sensitivity (80%) and specificity (86%).
On the other hand, we have not been able to establish a useful cut-off in the with the diagnostic classification (probable/possible). For a cut-off of 1.5, we obtained a good specificity (90.6%), although with a low sensitivity and area under the curve (28.6% and 50.4%, respectively). Therefore, it does not appear that clinical classification has an impact on the SPECT quantitative assessment.
In the present study, the proportion of patients with MSA was similar in men and women, which is consistent with the scientific literature [
22]. Although initially a higher percentage of males may present for consultation due to the clinical manifestations of erectile dysfunction [
23].
The age distribution of the patients is also consistent with the literature [
3,
24,
25,
26,
27]. It should be added that the age of our patients does not refer to the clinical onset, but to the timing of SPECT, which is usually requested after a first or second consultation in the movement disorders unit. The time elapsed between the clinical debut and the performance of the imaging test could be of importance in older subjects, as it could be due to a delay in clinical suspicion. We recommend further studies with this subgroup of patients, as it would be interesting to analyze their clinical particularities (coexistence of multiple signs and symptoms, other concomitant pathologies, greater difficulty in using current diagnostic criteria, etc.) [
20]. Such particularities may mean that the care of these elderly patients with suspected MSA requires earlier care and/or closer follow-up.
The most frequent subtype of MSA was MSA-P, with a 3:1 ratio with respect to MSA-C. These data coincide with those published by the European MSA study group (EMSA group) where, although a higher percentage of MSA-C was obtained in Spain, the overall results show a predominance of MSA-P in Europe, with a homogeneous distribution [
26,
28,
29,
30].
In this aspect, [
123I]Ioflupane SPECT is useful to diagnose both subtypes, since we found significant differences for both qualitative and quantitative assessment. This finding is interesting, since the vast majority of our patients had parkinsonian syndrome and less than half of them had cerebellar symptomatology. Another important element seems to be the manifestation of dysautonomia, also very frequent in our patients, but not the presence of corticospinal symptoms. These results are in agreement with the diagnostic criteria regarding the clinical characteristics of this disease and their impact on establishing the diagnosis [
5,
6].
The scarce or null response to levodopa treatment in practically all of our patients coincides with that previously described by other authors [
1,
31]. In this context and given that there does not seem to be a clear association between the response to levodopa and the SPECT result (qualitative or quantitative), we questioned whether in the presence of a pathological SPECT result in suspected MSA, we could dispense with this drug. This would imply a reduction in costs and would avoid potential adverse effects for patients [
32,
33].
Similarly, conventional MRI did not prove useful in the diagnosis of MSA in the majority of patients (89.2%). Since SPECT is performed at the same time as MRI upon initial suspicion of MSA, perhaps its usefulness lies in the detection of other pathologies that could be confused with MSA. It has been reported that this imaging technique usually shows nonspecific signs in atypical parkinsonism, with structural alterations appearing when the disease is advanced [
34,
35].
In contrast, [
123I]Ioflupane SPECT was positive in most patients (78.45%). This is similar to previously published studies, given that there may be integrity of the nigrostriatal pathway in early cases of MSA, especially in the MSA-C subtype [
17]. In this context, it would be interesting to perform an evolutionary study in this subgroup of patients, since the rapidly progressive deterioration of the disease, it could confirm or rule out the existence of MSA at a later stage. In the literature, we found hardly any research on this aspect [
16,
19,
36].
We can confirm the high sensitivity of SPECT for the diagnosis of MSA (82%), especially in patients with MSA-P (97%) with respect to MSA-C. On the other hand, it has a high positive predictive value (PPV >85%) in both subtypes, so we believe that in the clinical suspicion of MSA, SPECT is a very useful tool. These results correspond to those of other authors [
9]. This tool could be considered for those incipient cases, perhaps even as diagnostic support in the prodromal phases (as the new category published) [
6]. Furthermore, it is a widely available technique in our setting compared to others, such as PET-CT, for example, which is not available in all hospitals. For all these reasons, we propose that the role of [
123I]Ioflupane SPECT can be considered in the current diagnostic criteria.
Quantitative SPECT analysis showed that both striatal nuclei were significantly impaired, being more frequent the initial involvement of the left striatal nucleus. This lateralization has not been previously described, so we believe it is a finding that should be taken into account when diagnosing the disease. Moreover, it could be a distinctive feature to differentiate MSA from other entities. Therefore, it would be interesting to know how this condition evolves, by means of follow-up studies. In this regard, a baseline study could be performed (either at the onset of the disease or at any stage of the disease), in order to corroborate this deterioration at a later time. Previous publications point out the usefulness of [
123I]Ioflupane SPECT to monitor the evolution of this disease [
16,
19].
5. Conclusions
In conclusion, we could state: i) [123I]Ioflupane SPECT is a useful and reliable tool in the diagnosis of MSA (especially upon initial suspicion), with high diagnostic effectiveness (in terms of sensitivity and specificity) and higher accuracy than other conventional imaging techniques; ii) The qualitative assessment shows a net superiority in discerning between healthy-sick categories, as well as between MSA-P and MSA-C subtypes, which denotes that the assessment of the nuclear medicine physician has a relevant point in the diagnosis. There is also a good correspondence between the nuclear medicine physician’s assessment and the quantitative assessment of [123I]Ioflupane SPECT using predefined ROIs and our proposed cut-off; iii) Treatment with levodopa does not seem to provide benefits in the diagnosis of patients with MSA, so in the presence of a positive [123I]Ioflupane SPECT study, we believe that this drug could be omitted in terms of cost-effectiveness and cost-efficiency; iv) Most of our patients showed an initial predominant involvement of the left striatal nucleus. This finding could be interesting for its possible usefulness in the diagnosis of MSA, especially to identify incipient cases of this disease.
Author Contributions
Concept and study design: J.V.-S., S.J.O.-L. and J.C.-V. Qualitative assessment of brain images and data acquisition: J.V.-S., S.J.O.-L. and T.A.-R. Drafting the manuscript and figures: J.V.-S. and J.C.-V. Supervision and correction of the manuscript: E.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Research Ethics Committee of Málaga on June 24th, 2021 (123-J-9).
Informed Consent Statement
All participants gave written informed consent before data collection began.
Acknowledgments
To the Movement Disorders Unit (Department of Neurology) and the Department of Nuclear Medicine, Virgen de la Victoria University Hospital, Malaga. To Rita Pérez from the Andalusian Public Foundation for Research of Malaga in Biomedicine and Health (FIMABIS) and Institute of Biomedical Research of Malaga (IBIMA), for statistical advice.
Conflicts of Interest
This study has no sponsors or incentives, nor does it require funding for its development. Neither the participating investigators nor the hospital center have received any financial compensation. The authors declare that they have no conflicts of interest.
References
- Stefanova, N.; Bücke, P.; Duerr, S.; Wenning, G.K. Multiple System Atrophy: An Update. Lancet Neurol. 2009, 8, 1172–1178. [Google Scholar] [CrossRef]
- Fanciulli, A.; Stankovic, I.; Krismer, F.; Seppi, K.; Levin, J.; Wenning, G.K. Multiple System Atrophy. Int. Rev. Neurobiol. 2019, 149, 137–192. [Google Scholar] [CrossRef] [PubMed]
- Geser, F.; Seppi, K.; Stampfer-Kountchev, M.; Köllensperger, M.; Diem, A.; Ndayisaba, J.P.; Ostergaard, K.; Dupont, E.; Cardozo, A.; Tolosa, E.; et al. The European Multiple System Atrophy-Study Group (EMSA-SG). Proceedings of the Journal of Neural Transmission;. J Neural Transm. 2005, 112, 1677–1686. [Google Scholar] [CrossRef]
- Gilman, S.; Low, P.; Quinn, N.; Albanese, A.; Ben-Shlomo, Y.; Fowler, C.; Kaufmann, H.; Klockgether, T.; Lang, A.; Lantos, P.; et al. Consensus Statement on the Diagnosis of Multiple System Atrophy. Clin. Auton. Res. 1998, 8, 359–362. [Google Scholar] [CrossRef]
- Gilman, S.; Wenning, G.K.; Low, P.A.; Brooks, D.J.; Mathias, C.J.; Trojanowski, J.Q.; Wood, N.W.; Colosimo, C.; Dürr, A.; Fowler, C.J.; et al. Second Consensus Statement on the Diagnosis of Multiple System Atrophy. Neurology 2008, 71, 670–676. [Google Scholar] [CrossRef]
- Wenning, G.K.; Stankovic, I.; Vignatelli, L.; Fanciulli, A.; Calandra-Buonaura, G.; Seppi, K.; Palma, J.A.; Meissner, W.G.; Krismer, F.; Berg, D.; et al. The Movement Disorder Society Criteria for the Diagnosis of Multiple System Atrophy. Mov. Disord. 2022, 37. [Google Scholar] [CrossRef]
- Ortega Lozano, S.J.; Martínez Del Valle Torres, M.D.; Jiménez-Hoyuela García, J.M.; Delgado García, A.; Campos Arillo, V. Aplicación Del FP-CIT-I-123 En La Práctica Clínica de Pacientes Con Parkinsonismo. Rev. Esp. Med. Nucl. 2005, 24, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Ortega Lozano, S.J.; Martínez Del Valle Torres, M.D.; Jiménez-Hoyuela García, J.M.; Delgado García, A.; Molero Campos, E.; Campos Arillo, V. Validez Diagnóstica de La SPECT Con FP-CIT En La Evaluación de Pacientes Con Síndrome Parkinsoniano Clínicamente Incierto. Neurologia 2007, 22, 86–92. [Google Scholar] [PubMed]
- Arbizu, J.; Luquin, M.R.; Abella, J.; de la Fuente-Fernández, R.; Fernandez-Torrón, R.; García-Solís, D.; Garrastachu, P.; Jiménez-Hoyuela, J.M.; Llaneza, M.; Lomeña, F.; et al. Neuroimagen Funcional En El Diagnóstico de Pacientes Con Síndrome Parkinsoniano: Actualización y Recomendaciones Para El Uso Clínico. Rev. Esp. Med. Nucl. Imagen Mol. 2014, 33, 215–226. [Google Scholar] [CrossRef]
- Morbelli, S.; Esposito, G.; Arbizu, J.; Barthel, H.; Boellaard, R.; Bohnen, N.I. EANM Practice Guideline / SNMMI Procedure Standard for Dopaminergic Imaging in Parkinsonian Syndromes 1.0. 2020, 1885–1912. [Google Scholar] [CrossRef]
- El Fakhri, G.; Habert, M.O.; Maksud, P.; Kas, A.; Malek, Z.; Kijewski, M.F.; Lacomblez, L. Quantitative Simultaneous 99mTc-ECD/123I-FP-CIT SPECT in Parkinson’s Disease and Multiple System Atrophy. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 87–92. [Google Scholar] [CrossRef]
- Dethy, S.; Hambÿe, A.S. Apport de La Scintigraphie Par 123I-FP-CIT (DaTSCANTM) Au Diagnostic Différentiel Des Troubles Du Mouvement. Rev. Med. Brux. 2008, 29, 238–247. [Google Scholar] [PubMed]
- Brooks, D.J.; Tambasco, N. Imaging Synucleinopathies. Mov. Disord. 2016, 31, 814–829. [Google Scholar] [CrossRef] [PubMed]
- Iwabuchi, Y.; Kameyama, M.; Matsusaka, Y.; Narimatsu, H.; Hashimoto, M.; Seki, M.; Ito, D.; Tabuchi, H.; Yamada, Y.; Jinzaki, M. A Diagnostic Strategy for Parkinsonian Syndromes Using Quantitative Indices of DAT SPECT and MIBG Scintigraphy: An Investigation Using the Classification and Regression Tree Analysis. Eur. J. Nucl. Med. Mol. Imaging 2021. [Google Scholar] [CrossRef] [PubMed]
- Joling, M.; Vriend, C.; Van Den Heuvel, O.A.; Raijmakers, P.G.H.M.; Jones, P.A.; Berendse, H.W.; Booij, J. Analysis of Extrastriatal 123I-FP-CIT Binding Contributes to the Differential Diagnosis of Parkinsonian Diseases. J. Nucl. Med. 2017, 58, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Brumberg, J.; Isaias, I.U. SPECT Molecular Imaging in Atypical Parkinsonism. In International Review of Neurobiology; Elsevier Inc., 2018, 142, 37–65. ISBN 9780128151419.
- Bu, L.L.; Liu, F.T.; Jiang, C.F.; Guo, S.S.; Yu, H.; Zuo, C.T.; Wu, P.; Wang, J. Patterns of Dopamine Transporter Imaging in Subtypes of Multiple System Atrophy. Acta Neurol. Scand. 2018, 138, 170–176. [Google Scholar] [CrossRef]
- Vergnet, S.; Hives, F.; Foubert-Samier, A.; Payoux, P.; Fernandez, P.; Meyer, M.; Dupouy, J.; Brefel-Courbon, C.; Ory-Magne, F.; Rascol, O.; et al. Dopamine Transporter Imaging for the Diagnosis of Multiple System Atrophy Cerebellar Type. Park. Relat. Disord. 2019, 63, 199–203. [Google Scholar] [CrossRef]
- Sakakibara, S.; Hashimoto, R.; Katayama, T.; Kenjyo, M.; Yokokawa, Y.; Saito, Y.; Hirakawa, A.; Ito, M.; Nakamura, T.; Hara, K.; et al. Longitudinal Change of DAT SPECT in Parkinson’s Disease and Multiple System Atrophy. J. Parkinsons. Dis. 2020, 10, 123–130. [Google Scholar] [CrossRef]
- Köllensperger, M.; Geser, F.; Seppi, K.; Stampfer-Kountchev, M.; Sawires, M.; Scherfler, C.; Boesch, S.; Mueller, J.; Koukouni, V.; Quinn, N.; et al. Red Flags for Multiple System Atrophy. Mov. Disord. 2008, 23, 1093–1099. [Google Scholar] [CrossRef]
- Ortega Lozano, S.J.; Martinez del Valle Torres, M.D.; Ramos Moreno, E.; Sanz Viedma, S.; Amrani Raissouni, T.; Jiménez-Hoyuela, J.M. Valoración Cuantitativa Del SPECT Con FP-CIT. Importancia Del Área de Referencia. Rev. Española Med. Nucl. 2010, 29, 246–250. [Google Scholar] [CrossRef]
- Wenning, G.K.; Shlomo, Y. Ben; Magalhães, M.; Danie, S.E.; Quinn, N.P. Clinical Features and Natural History of Multiple System Atrophy. An Analysis of 100 Cases. Brain 1994, 117 Pt 4, 835–845. [Google Scholar] [CrossRef]
- Vanacore, N. Epidemiological Evidence on Multiple System Atrophy. In Proceedings of the Journal of Neural Transmission. J Neural Transm. 2005, 112, 1605–1612. [Google Scholar] [CrossRef]
- Ben-Shlomo, Y.; Wenning, G.K.; Tison, F.; Quinn, N.P. Survival of Patients with Pathologically Proven Multiple System Atrophy: A Meta-Analysis. Neurology 1997, 48, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Wüllner, U.; Schmitz-Hübsch, T.; Abele, M.; Antony, G.; Bauer, P.; Eggert, K. Features of Probable Multiple System Atrophy Patients Identified among 4770 Patients with Parkinsonism Enrolled in the Multicentre Registry of the German Competence Network on Parkinson’s Disease. J. Neural Transm. 2007, 114, 1161–1165. [Google Scholar] [CrossRef]
- Köllensperger, M.; Geser, F.; Ndayisaba, J.P.; Boesch, S.; Seppi, K.; Ostergaard, K.; Dupont, E.; Cardozo, A.; Tolosa, E.; Abele, M.; et al. Presentation, Diagnosis, and Management of Multiple System Atrophy in Europe: Final Analysis of the European Multiple System Atrophy Registry. Mov. Disord. 2010, 25, 2604–2612. [Google Scholar] [CrossRef] [PubMed]
- Wenning, G.K.; Geser, F.; Krismer, F.; Seppi, K.; Duerr, S.; Boesch, S.; Köllensperger, M.; Goebel, G.; Pfeiffer, K.P.; Barone, P.; et al. The Natural History of Multiple System Atrophy: A Prospective European Cohort Study. Lancet. Neurol. 2013, 12, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Saito, Y.; Terao, S.; Ando, T.; Kachi, T.; Mukai, E.; Aiba, I.; Abe, Y.; Tamakoshi, A.; Doyu, M.; et al. Progression and Prognosis in Multiple System Atrophy: An Analysis of 230 Japanese Patients. Brain 2002, 125, 1070–1083. [Google Scholar] [CrossRef]
- Quinn, N.P. How to Diagnose Multiple System Atrophy. Mov. Disord. 2005, 20 Suppl 1, S5–S10. [Google Scholar] [CrossRef]
- Jellinger, K.A. Multiple System Atrophy: An Oligodendroglioneural Synucleinopathy. J. Alzheimer’s Dis. 2018, 62, 1141–1179. [Google Scholar] [CrossRef]
- Ozawa, T.; Paviour, D.; Quinn, N.P.; Josephs, K.A.; Sangha, H.; Kilford, L.; Healy, D.G.; Wood, N.W.; Lees, A.J.; Holton, J.L.; et al. The Spectrum of Pathological Involvement of the Striatonigral and Olivopontocerebellar Systems in Multiple System Atrophy: Clinicopathological Correlations. Brain 2004, 127, 2657–2671. [Google Scholar] [CrossRef]
- Bhidayasiri, R.; Ling, H. Multiple System Atrophy. Neurologist 2008, 14, 224–237. [Google Scholar] [CrossRef]
- Ishida, C.; Takahashi, K.; Kato-Motozaki, Y.; Tagami, A.; Komai, K. Effectiveness of Levodopa in Patients with Multiple System Atrophy and Associated Clinicopathological Features. Intern. Med. 2021, 60, 367–372. [Google Scholar] [CrossRef]
- Ramli, N.; Nair, S.R.; Ramli, N.M.; Lim, S.Y. Differentiating Multiple-System Atrophy from Parkinson’s Disease. Clin. Radiol. 2015, 70, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, N.; Valentina, G.; Burkhard Pierre, R. The Role of Molecular Imaging in Assessing Degenerative Parkinsonism – An Updated Review. Swiss Med. Wkly. 2018, 148, 1–9. [Google Scholar] [CrossRef]
- Martínez-Valle Torres, M.D.; Ortega Lozano, S.J.; Gómez Heredia, M.J.; Amrani Raissouni, T.; Ramos Moreno, E.; Moya Espinosa, P.; Jiménez-Hoyuela, J.M. Evaluación Longitudinal Con FP-CIT En Pacientes Con Parkinsonismo. Neurologia 2014, 29, 327–333. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).