Submitted:
15 April 2023
Posted:
17 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
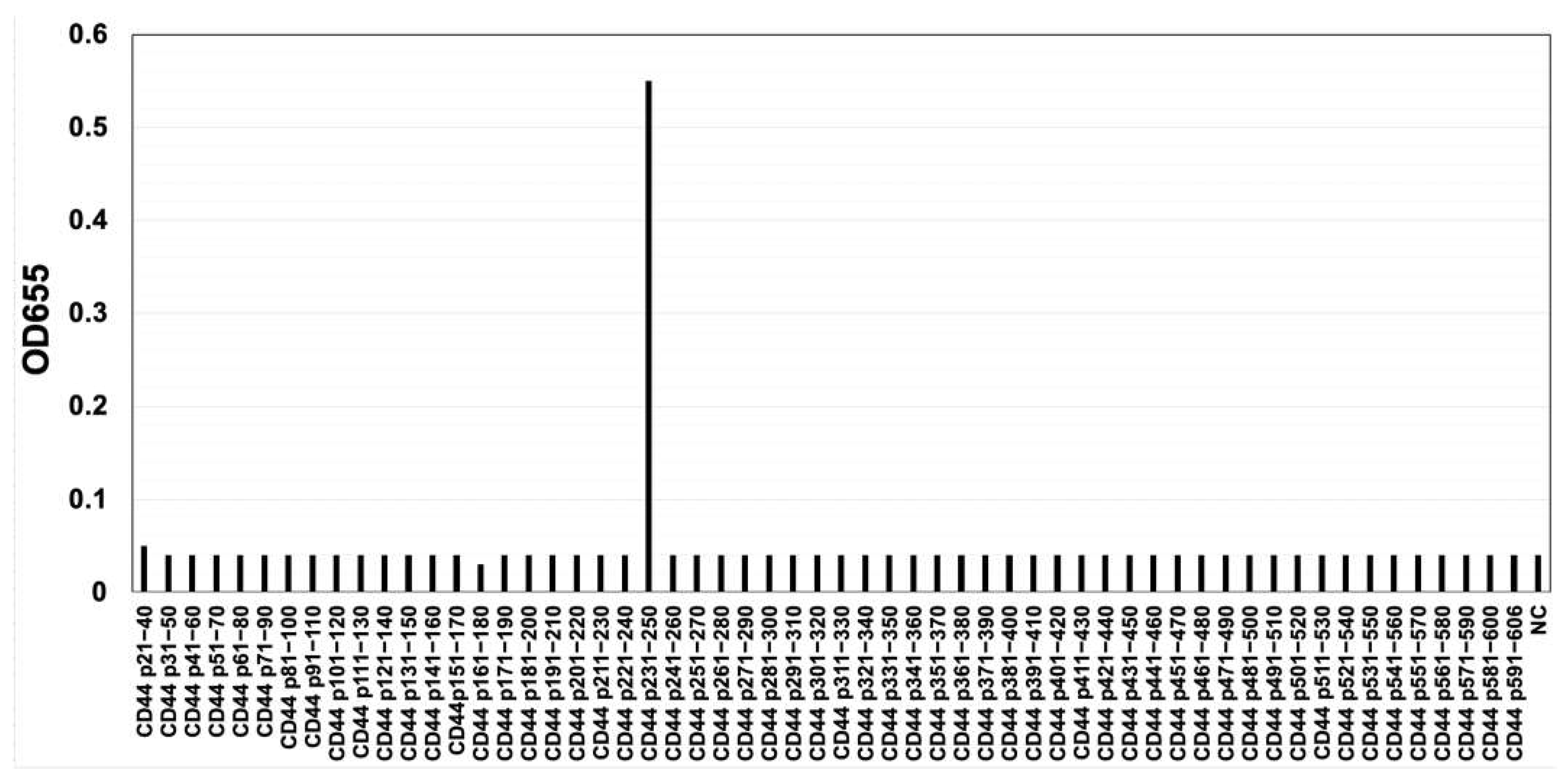
2.1. Development of C44Mab-6 as an anti-CD44v3 mAb
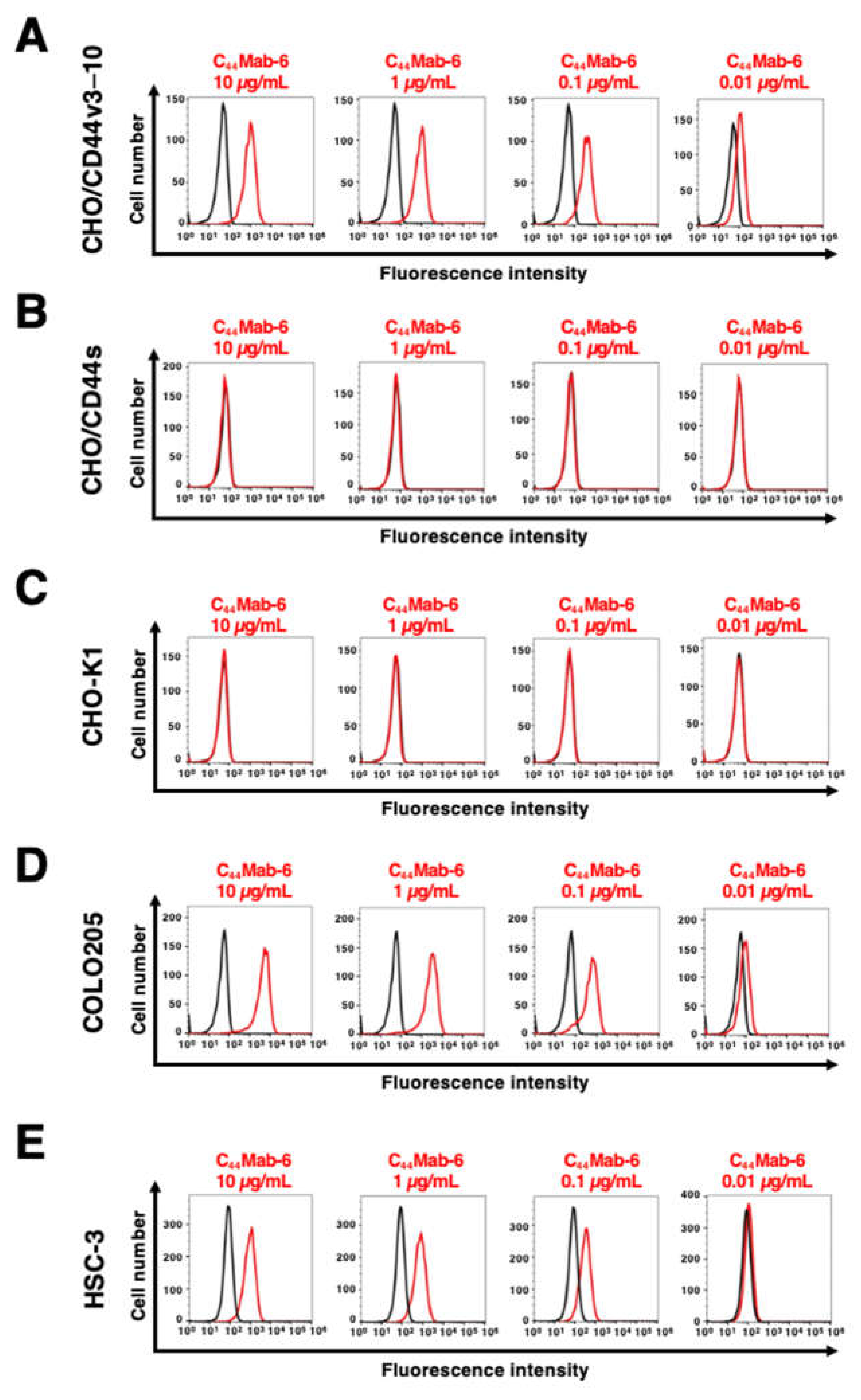
2.2. The reactivity of C44Mab-6 to CD44-Expressing Cells in Flow Cytometry
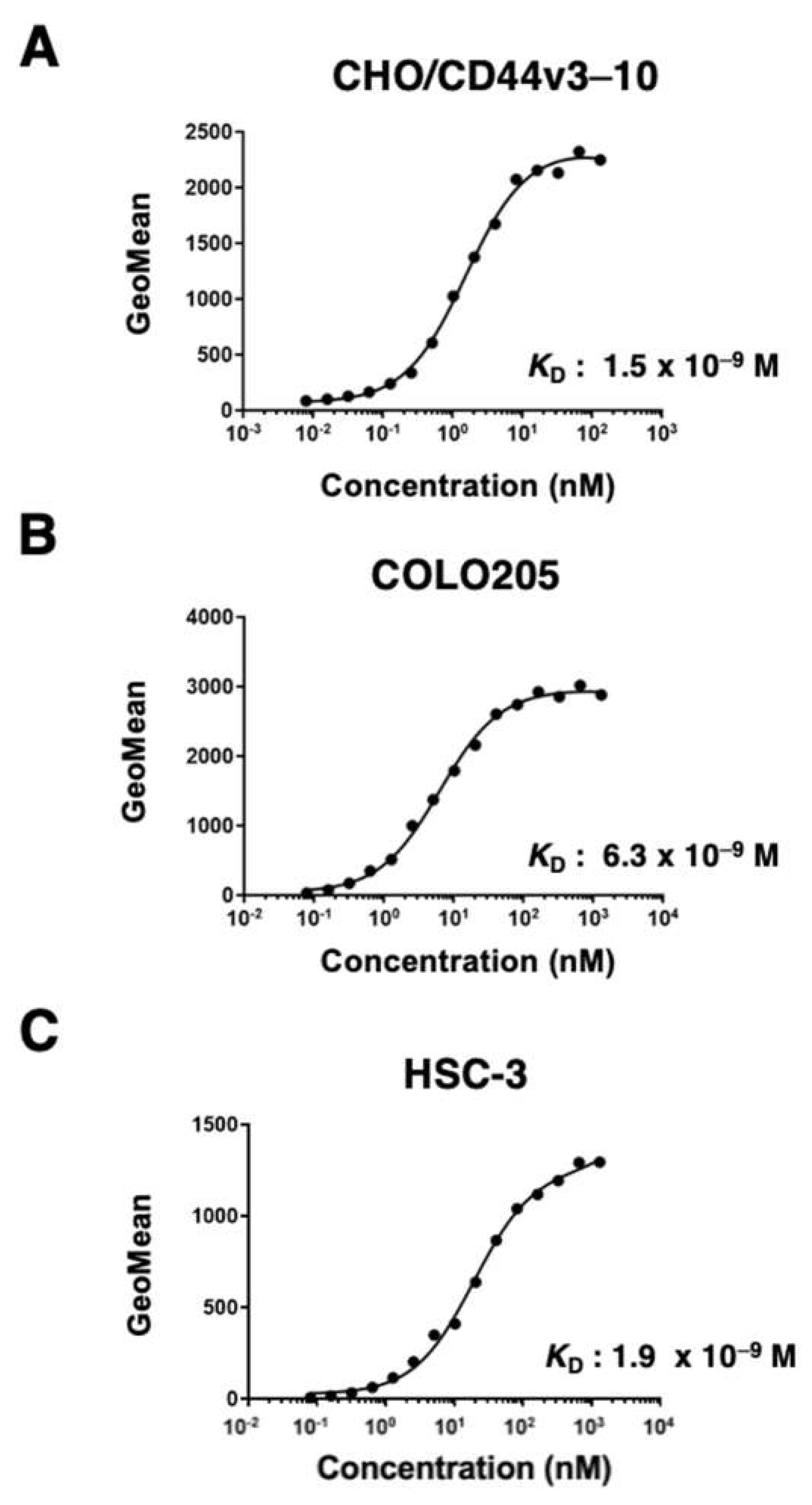
2.3. The binding affinity of C44Mab-6 to CD44-Expressing Cells
3.4. Western Blot Analysis
3.5. Immunohistochemical Analysis Using C44Mab-6 against Tumor Tissues
3. Discussion
4. Materials and Methods
4.1. Cell lines
4.2. Construction of expression plasmids and stable transfectants
4.3. Production of hybridomas
4.4. ELISA
4.5. Flow cytometry
4.6. Determination of dissociation constant (KD) via flow cytometry
4.7. Western blot analysis
4.8. Immunohistochemical analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ponta, H.; Sherman, L.; Herrlich, P.A. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol 2003, 4, 33-45. [CrossRef]
- Hassn Mesrati, M.; Syafruddin, S.E.; Mohtar, M.A.; Syahir, A. CD44: A Multifunctional Mediator of Cancer Progression. Biomolecules 2021, 11. [CrossRef]
- Zöller, M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer 2011, 11, 254-267. [CrossRef]
- Yan, Y.; Zuo, X.; Wei, D. Concise Review: Emerging Role of CD44 in Cancer Stem Cells: A Promising Biomarker and Therapeutic Target. Stem Cells Transl Med 2015, 4, 1033-1043. [CrossRef]
- Mereiter, S.; Martins Á, M.; Gomes, C.; Balmaña, M.; Macedo, J.A.; Polom, K.; Roviello, F.; Magalhães, A.; Reis, C.A. O-glycan truncation enhances cancer-related functions of CD44 in gastric cancer. FEBS Lett 2019, 593, 1675-1689. [CrossRef]
- Takahashi, K.; Stamenkovic, I.; Cutler, M.; Dasgupta, A.; Tanabe, K.K. Keratan sulfate modification of CD44 modulates adhesion to hyaluronate. J Biol Chem 1996, 271, 9490-9496. [CrossRef]
- Bennett, K.L.; Jackson, D.G.; Simon, J.C.; Tanczos, E.; Peach, R.; Modrell, B.; Stamenkovic, I.; Plowman, G.; Aruffo, A. CD44 isoforms containing exon V3 are responsible for the presentation of heparin-binding growth factor. J Cell Biol 1995, 128, 687-698. [CrossRef]
- Brown, T.A.; Bouchard, T.; St John, T.; Wayner, E.; Carter, W.G. Human keratinocytes express a new CD44 core protein (CD44E) as a heparan-sulfate intrinsic membrane proteoglycan with additional exons. J Cell Biol 1991, 113, 207-221. [CrossRef]
- Slevin, M.; Krupinski, J.; Gaffney, J.; Matou, S.; West, D.; Delisser, H.; Savani, R.C.; Kumar, S. Hyaluronan-mediated angiogenesis in vascular disease: uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biol 2007, 26, 58-68. [CrossRef]
- Guo, Q.; Yang, C.; Gao, F. The state of CD44 activation in cancer progression and therapeutic targeting. Febs j 2021. [CrossRef]
- Morath, I.; Hartmann, T.N.; Orian-Rousseau, V. CD44: More than a mere stem cell marker. Int J Biochem Cell Biol 2016, 81, 166-173. [CrossRef]
- Jackson, D.G.; Bell, J.I.; Dickinson, R.; Timans, J.; Shields, J.; Whittle, N. Proteoglycan forms of the lymphocyte homing receptor CD44 are alternatively spliced variants containing the v3 exon. J Cell Biol 1995, 128, 673-685. [CrossRef]
- DiGabriele, A.D.; Lax, I.; Chen, D.I.; Svahn, C.M.; Jaye, M.; Schlessinger, J.; Hendrickson, W.A. Structure of a heparin-linked biologically active dimer of fibroblast growth factor. Nature 1998, 393, 812-817. [CrossRef]
- Spivak-Kroizman, T.; Lemmon, M.A.; Dikic, I.; Ladbury, J.E.; Pinchasi, D.; Huang, J.; Jaye, M.; Crumley, G.; Schlessinger, J.; Lax, I. Heparin-induced oligomerization of FGF molecules is responsible for FGF receptor dimerization, activation, and cell proliferation. Cell 1994, 79, 1015-1024. [CrossRef]
- Yayon, A.; Klagsbrun, M.; Esko, J.D.; Leder, P.; Ornitz, D.M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell 1991, 64, 841-848. [CrossRef]
- Orian-Rousseau, V.; Chen, L.; Sleeman, J.P.; Herrlich, P.; Ponta, H. CD44 is required for two consecutive steps in HGF/c-Met signaling. Genes Dev 2002, 16, 3074-3086. [CrossRef]
- Ishimoto, T.; Nagano, O.; Yae, T.; Tamada, M.; Motohara, T.; Oshima, H.; Oshima, M.; Ikeda, T.; Asaba, R.; Yagi, H.; et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 2011, 19, 387-400. [CrossRef]
- Maitland, N.J.; Collins, A.T. Cancer stem cells - A therapeutic target? Curr Opin Mol Ther 2010, 12, 662-673.
- Prince, M.E.; Ailles, L.E. Cancer stem cells in head and neck squamous cell cancer. J Clin Oncol 2008, 26, 2871-2875. [CrossRef]
- Ailles, L.E.; Weissman, I.L. Cancer stem cells in solid tumors. Curr Opin Biotechnol 2007, 18, 460-466. [CrossRef]
- Yu, S.S.; Cirillo, N. The molecular markers of cancer stem cells in head and neck tumors. J Cell Physiol 2020, 235, 65-73. [CrossRef]
- Tahmasebi, E.; Alikhani, M.; Yazdanian, A.; Yazdanian, M.; Tebyanian, H.; Seifalian, A. The current markers of cancer stem cell in oral cancers. Life Sci 2020, 249, 117483. [CrossRef]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 2003, 100, 3983-3988. [CrossRef]
- Lee, Y.; Shin, J.H.; Longmire, M.; Wang, H.; Kohrt, H.E.; Chang, H.Y.; Sunwoo, J.B. CD44+ Cells in Head and Neck Squamous Cell Carcinoma Suppress T-Cell-Mediated Immunity by Selective Constitutive and Inducible Expression of PD-L1. Clin Cancer Res 2016, 22, 3571-3581. [CrossRef]
- Prince, M.E.; Sivanandan, R.; Kaczorowski, A.; Wolf, G.T.; Kaplan, M.J.; Dalerba, P.; Weissman, I.L.; Clarke, M.F.; Ailles, L.E. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A 2007, 104, 973-978. [CrossRef]
- Davis, S.J.; Divi, V.; Owen, J.H.; Bradford, C.R.; Carey, T.E.; Papagerakis, S.; Prince, M.E. Metastatic potential of cancer stem cells in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg 2010, 136, 1260-1266. [CrossRef]
- Todaro, M.; Gaggianesi, M.; Catalano, V.; Benfante, A.; Iovino, F.; Biffoni, M.; Apuzzo, T.; Sperduti, I.; Volpe, S.; Cocorullo, G.; et al. CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell 2014, 14, 342-356. [CrossRef]
- Kimura, Y.; Goi, T.; Nakazawa, T.; Hirono, Y.; Katayama, K.; Urano, T.; Yamaguchi, A. CD44variant exon 9 plays an important role in colon cancer initiating cells. Oncotarget 2013, 4, 785-791. [CrossRef]
- Yamada, S.; Itai, S.; Nakamura, T.; Yanaka, M.; Kaneko, M.K.; Kato, Y. Detection of high CD44 expression in oral cancers using the novel monoclonal antibody, C(44)Mab-5. Biochem Biophys Rep 2018, 14, 64-68. [CrossRef]
- Goto, N.; Suzuki, H.; Tanaka, T.; Asano, T.; Kaneko, M.K.; Kato, Y. Development of a Novel Anti-CD44 Monoclonal Antibody for Multiple Applications against Esophageal Squamous Cell Carcinomas. Int J Mol Sci 2022, 23. [CrossRef]
- Takei, J.; Asano, T.; Suzuki, H.; Kaneko, M.K.; Kato, Y. Epitope Mapping of the Anti-CD44 Monoclonal Antibody (C44Mab-46) Using Alanine-Scanning Mutagenesis and Surface Plasmon Resonance. Monoclon Antib Immunodiagn Immunother 2021, 40, 219-226. [CrossRef]
- Asano, T.; Kaneko, M.K.; Takei, J.; Tateyama, N.; Kato, Y. Epitope Mapping of the Anti-CD44 Monoclonal Antibody (C44Mab-46) Using the REMAP Method. Monoclon Antib Immunodiagn Immunother 2021, 40, 156-161. [CrossRef]
- Asano, T.; Kaneko, M.K.; Kato, Y. Development of a Novel Epitope Mapping System: RIEDL Insertion for Epitope Mapping Method. Monoclon Antib Immunodiagn Immunother 2021, 40, 162-167. [CrossRef]
- Takei, J.; Kaneko, M.K.; Ohishi, T.; Hosono, H.; Nakamura, T.; Yanaka, M.; Sano, M.; Asano, T.; Sayama, Y.; Kawada, M.; et al. A defucosylated antiCD44 monoclonal antibody 5mG2af exerts antitumor effects in mouse xenograft models of oral squamous cell carcinoma. Oncol Rep 2020, 44, 1949-1960. [CrossRef]
- Kudo, Y.; Suzuki, H.; Tanaka, T.; Kaneko, M.K.; Kato, Y. Development of a Novel Anti-CD44 variant 5 Monoclonal An-tibody C44Mab-3 for Multiple Applications against Pancreatic Carcinomas. Antibodies 2023, in press.
- Ejima, R.; Suzuki, H.; Tanaka, T.; Asano, T.; Kaneko, M.K.; Kato, Y. Development of a Novel Anti-CD44 Variant 6 Monoclonal Antibody C(44)Mab-9 for Multiple Applications against Colorectal Carcinomas. Int J Mol Sci 2023, 24. [CrossRef]
- Suzuki, H.; Ozawa, K.; Tanaka, T.; Kaneko, M.K.; Kato, Y. Development of a Novel Anti-CD44 Variant 7/8 Monoclonal Antibody, C44Mab-34, for Multiple Applications against Oral Carcinomas. Biomedicines 2023, 11, 1099. [CrossRef]
- Suzuki, H.; Tanaka, T.; Goto, N.; Kaneko, M.K.; Kato, Y. Development of a Novel Anti-CD44 Variant 4 Monoclonal Antibody C44Mab-108 for Immunohistochemistry. Curr Issues Mol Biol 2023, 45, 1875-1888. [CrossRef]
- Fox, S.B.; Fawcett, J.; Jackson, D.G.; Collins, I.; Gatter, K.C.; Harris, A.L.; Gearing, A.; Simmons, D.L. Normal human tissues, in addition to some tumors, express multiple different CD44 isoforms. Cancer Res 1994, 54, 4539-4546.
- Mody, M.D.; Rocco, J.W.; Yom, S.S.; Haddad, R.I.; Saba, N.F. Head and neck cancer. Lancet 2021, 398, 2289-2299. [CrossRef]
- Bourguignon, L.Y.; Wong, G.; Earle, C.; Chen, L. Hyaluronan-CD44v3 interaction with Oct4-Sox2-Nanog promotes miR-302 expression leading to self-renewal, clonal formation, and cisplatin resistance in cancer stem cells from head and neck squamous cell carcinoma. J Biol Chem 2012, 287, 32800-32824. [CrossRef]
- Li, G.; Suzuki, H.; Ohishi, T.; Asano, T.; Tanaka, T.; Yanaka, M.; Nakamura, T.; Yoshikawa, T.; Kawada, M.; Kaneko, M.K.; et al. Antitumor activities of a defucosylated anti-EpCAM monoclonal antibody in colorectal carcinoma xenograft models. Int J Mol Med 2023, 51. [CrossRef]
- Nanamiya, R.; Takei, J.; Ohishi, T.; Asano, T.; Tanaka, T.; Sano, M.; Nakamura, T.; Yanaka, M.; Handa, S.; Tateyama, N.; et al. Defucosylated Anti-Epidermal Growth Factor Receptor Monoclonal Antibody (134-mG(2a)-f) Exerts Antitumor Activities in Mouse Xenograft Models of Canine Osteosarcoma. Monoclon Antib Immunodiagn Immunother 2022, 41, 1-7. [CrossRef]
- Kawabata, H.; Suzuki, H.; Ohishi, T.; Kawada, M.; Kaneko, M.K.; Kato, Y. A Defucosylated Mouse Anti-CD10 Monoclonal Antibody (31-mG(2a)-f) Exerts Antitumor Activity in a Mouse Xenograft Model of CD10-Overexpressed Tumors. Monoclon Antib Immunodiagn Immunother 2022, 41, 59-66. [CrossRef]
- Kawabata, H.; Ohishi, T.; Suzuki, H.; Asano, T.; Kawada, M.; Suzuki, H.; Kaneko, M.K.; Kato, Y. A Defucosylated Mouse Anti-CD10 Monoclonal Antibody (31-mG(2a)-f) Exerts Antitumor Activity in a Mouse Xenograft Model of Renal Cell Cancers. Monoclon Antib Immunodiagn Immunother 2022. [CrossRef]
- Asano, T.; Tanaka, T.; Suzuki, H.; Li, G.; Ohishi, T.; Kawada, M.; Yoshikawa, T.; Kaneko, M.K.; Kato, Y. A Defucosylated Anti-EpCAM Monoclonal Antibody (EpMab-37-mG(2a)-f) Exerts Antitumor Activity in Xenograft Model. Antibodies (Basel) 2022, 11. [CrossRef]
- Tateyama, N.; Nanamiya, R.; Ohishi, T.; Takei, J.; Nakamura, T.; Yanaka, M.; Hosono, H.; Saito, M.; Asano, T.; Tanaka, T.; et al. Defucosylated Anti-Epidermal Growth Factor Receptor Monoclonal Antibody 134-mG(2a)-f Exerts Antitumor Activities in Mouse Xenograft Models of Dog Epidermal Growth Factor Receptor-Overexpressed Cells. Monoclon Antib Immunodiagn Immunother 2021, 40, 177-183. [CrossRef]
- Takei, J.; Ohishi, T.; Kaneko, M.K.; Harada, H.; Kawada, M.; Kato, Y. A defucosylated anti-PD-L1 monoclonal antibody 13-mG(2a)-f exerts antitumor effects in mouse xenograft models of oral squamous cell carcinoma. Biochem Biophys Rep 2020, 24, 100801. [CrossRef]
- Xing, D.T.; Khor, R.; Gan, H.; Wada, M.; Ermongkonchai, T.; Ng, S.P. Recent Research on Combination of Radiotherapy with Targeted Therapy or Immunotherapy in Head and Neck Squamous Cell Carcinoma: A Review for Radiation Oncologists. Cancers (Basel) 2021, 13. [CrossRef]
- Muzaffar, J.; Bari, S.; Kirtane, K.; Chung, C.H. Recent Advances and Future Directions in Clinical Management of Head and Neck Squamous Cell Carcinoma. Cancers (Basel) 2021, 13. [CrossRef]
- Nagaya, T.; Nakamura, Y.; Okuyama, S.; Ogata, F.; Maruoka, Y.; Choyke, P.L.; Allen, C.; Kobayashi, H. Syngeneic Mouse Models of Oral Cancer Are Effectively Targeted by Anti-CD44-Based NIR-PIT. Mol Cancer Res 2017, 15, 1667-1677. [CrossRef]
- Zen, K.; Liu, D.Q.; Li, L.M.; Chen, C.X.; Guo, Y.L.; Ha, B.; Chen, X.; Zhang, C.Y.; Liu, Y. The heparan sulfate proteoglycan form of epithelial CD44v3 serves as a CD11b/CD18 counter-receptor during polymorphonuclear leukocyte transepithelial migration. J Biol Chem 2009, 284, 3768-3776. [CrossRef]
- Colgan, S.P.; Comerford, K.M.; Lawrence, D.W. Epithelial cell-neutrophil interactions in the alimentary tract: a complex dialog in mucosal surveillance and inflammation. ScientificWorldJournal 2002, 2, 76-88. [CrossRef]
- Rosenberg, W.M.; Prince, C.; Kaklamanis, L.; Fox, S.B.; Jackson, D.G.; Simmons, D.L.; Chapman, R.W.; Trowell, J.M.; Jewell, D.P.; Bell, J.I. Increased expression of CD44v6 and CD44v3 in ulcerative colitis but not colonic Crohn's disease. Lancet 1995, 345, 1205-1209. [CrossRef]
- Oliveira, G.; Wu, C.J. Dynamics and specificities of T cells in cancer immunotherapy. Nat Rev Cancer 2023. [CrossRef]
- Haslauer, T.; Greil, R.; Zaborsky, N.; Geisberger, R. CAR T-Cell Therapy in Hematological Malignancies. Int J Mol Sci 2021, 22. [CrossRef]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424-447. [CrossRef]
- Patel, J.P.; Gönen, M.; Figueroa, M.E.; Fernandez, H.; Sun, Z.; Racevskis, J.; Van Vlierberghe, P.; Dolgalev, I.; Thomas, S.; Aminova, O.; et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med 2012, 366, 1079-1089. [CrossRef]
- Tang, L.; Huang, H.; Tang, Y.; Li, Q.; Wang, J.; Li, D.; Zhong, Z.; Zou, P.; You, Y.; Cao, Y.; et al. CD44v6 chimeric antigen receptor T cell specificity towards AML with FLT3 or DNMT3A mutations. Clin Transl Med 2022, 12, e1043. [CrossRef]
- Holm, F.; Hellqvist, E.; Mason, C.N.; Ali, S.A.; Delos-Santos, N.; Barrett, C.L.; Chun, H.J.; Minden, M.D.; Moore, R.A.; Marra, M.A.; et al. Reversion to an embryonic alternative splicing program enhances leukemia stem cell self-renewal. Proc Natl Acad Sci U S A 2015, 112, 15444-15449. [CrossRef]
- Kato, Y.; Yamada, S.; Furusawa, Y.; Itai, S.; Nakamura, T.; Yanaka, M.; Sano, M.; Harada, H.; Fukui, M.; Kaneko, M.K. PMab-213: A Monoclonal Antibody for Immunohistochemical Analysis Against Pig Podoplanin. Monoclon Antib Immunodiagn Immunother 2019, 38, 18-24. [CrossRef]
- Furusawa, Y.; Yamada, S.; Itai, S.; Sano, M.; Nakamura, T.; Yanaka, M.; Fukui, M.; Harada, H.; Mizuno, T.; Sakai, Y.; et al. PMab-210: A Monoclonal Antibody Against Pig Podoplanin. Monoclon Antib Immunodiagn Immunother 2019, 38, 30-36. [CrossRef]
- Furusawa, Y.; Yamada, S.; Itai, S.; Nakamura, T.; Yanaka, M.; Sano, M.; Harada, H.; Fukui, M.; Kaneko, M.K.; Kato, Y. PMab-219: A monoclonal antibody for the immunohistochemical analysis of horse podoplanin. Biochem Biophys Rep 2019, 18, 100616. [CrossRef]
- Furusawa, Y.; Yamada, S.; Itai, S.; Nakamura, T.; Takei, J.; Sano, M.; Harada, H.; Fukui, M.; Kaneko, M.K.; Kato, Y. Establishment of a monoclonal antibody PMab-233 for immunohistochemical analysis against Tasmanian devil podoplanin. Biochem Biophys Rep 2019, 18, 100631. [CrossRef]
- Kato, Y.; Kaneko, M.K.; Kuno, A.; Uchiyama, N.; Amano, K.; Chiba, Y.; Hasegawa, Y.; Hirabayashi, J.; Narimatsu, H.; Mishima, K.; et al. Inhibition of tumor cell-induced platelet aggregation using a novel anti-podoplanin antibody reacting with its platelet-aggregation-stimulating domain. Biochem Biophys Res Commun 2006, 349, 1301-1307. [CrossRef]
- Chalise, L.; Kato, A.; Ohno, M.; Maeda, S.; Yamamichi, A.; Kuramitsu, S.; Shiina, S.; Takahashi, H.; Ozone, S.; Yamaguchi, J.; et al. Efficacy of cancer-specific anti-podoplanin CAR-T cells and oncolytic herpes virus G47Delta combination therapy against glioblastoma. Mol Ther Oncolytics 2022, 26, 265-274. [CrossRef]
- Ishikawa, A.; Waseda, M.; Ishii, T.; Kaneko, M.K.; Kato, Y.; Kaneko, S. Improved anti-solid tumor response by humanized anti-podoplanin chimeric antigen receptor transduced human cytotoxic T cells in an animal model. Genes Cells 2022, 27, 549-558. [CrossRef]
- Tamura-Sakaguchi, R.; Aruga, R.; Hirose, M.; Ekimoto, T.; Miyake, T.; Hizukuri, Y.; Oi, R.; Kaneko, M.K.; Kato, Y.; Akiyama, Y.; et al. Moving toward generalizable NZ-1 labeling for 3D structure determination with optimized epitope-tag insertion. Acta Crystallogr D Struct Biol 2021, 77, 645-662. [CrossRef]
- Kaneko, M.K.; Ohishi, T.; Nakamura, T.; Inoue, H.; Takei, J.; Sano, M.; Asano, T.; Sayama, Y.; Hosono, H.; Suzuki, H.; et al. Development of Core-Fucose-Deficient Humanized and Chimeric Anti-Human Podoplanin Antibodies. Monoclon Antib Immunodiagn Immunother 2020, 39, 167-174. [CrossRef]
- Fujii, Y.; Matsunaga, Y.; Arimori, T.; Kitago, Y.; Ogasawara, S.; Kaneko, M.K.; Kato, Y.; Takagi, J. Tailored placement of a turn-forming PA tag into the structured domain of a protein to probe its conformational state. J Cell Sci 2016, 129, 1512-1522. [CrossRef]
- Abe, S.; Kaneko, M.K.; Tsuchihashi, Y.; Izumi, T.; Ogasawara, S.; Okada, N.; Sato, C.; Tobiume, M.; Otsuka, K.; Miyamoto, L.; et al. Antitumor effect of novel anti-podoplanin antibody NZ-12 against malignant pleural mesothelioma in an orthotopic xenograft model. Cancer Sci 2016, 107, 1198-1205. [CrossRef]
- Kaneko, M.K.; Abe, S.; Ogasawara, S.; Fujii, Y.; Yamada, S.; Murata, T.; Uchida, H.; Tahara, H.; Nishioka, Y.; Kato, Y. Chimeric Anti-Human Podoplanin Antibody NZ-12 of Lambda Light Chain Exerts Higher Antibody-Dependent Cellular Cytotoxicity and Complement-Dependent Cytotoxicity Compared with NZ-8 of Kappa Light Chain. Monoclon Antib Immunodiagn Immunother 2017, 36, 25-29. [CrossRef]
- Ito, A.; Ohta, M.; Kato, Y.; Inada, S.; Kato, T.; Nakata, S.; Yatabe, Y.; Goto, M.; Kaneda, N.; Kurita, K.; et al. A Real-Time Near-Infrared Fluorescence Imaging Method for the Detection of Oral Cancers in Mice Using an Indocyanine Green-Labeled Podoplanin Antibody. Technol Cancer Res Treat 2018, 17, 1533033818767936. [CrossRef]
- Tamura, R.; Oi, R.; Akashi, S.; Kaneko, M.K.; Kato, Y.; Nogi, T. Application of the NZ-1 Fab as a crystallization chaperone for PA tag-inserted target proteins. Protein Sci 2019, 28, 823-836. [CrossRef]
- Shiina, S.; Ohno, M.; Ohka, F.; Kuramitsu, S.; Yamamichi, A.; Kato, A.; Motomura, K.; Tanahashi, K.; Yamamoto, T.; Watanabe, R.; et al. CAR T Cells Targeting Podoplanin Reduce Orthotopic Glioblastomas in Mouse Brains. Cancer Immunol Res 2016, 4, 259-268. [CrossRef]
- Kuwata, T.; Yoneda, K.; Mori, M.; Kanayama, M.; Kuroda, K.; Kaneko, M.K.; Kato, Y.; Tanaka, F. Detection of Circulating Tumor Cells (CTCs) in Malignant Pleural Mesothelioma (MPM) with the "Universal" CTC-Chip and An Anti-Podoplanin Antibody NZ-1.2. Cells 2020, 9. [CrossRef]
- Nishinaga, Y.; Sato, K.; Yasui, H.; Taki, S.; Takahashi, K.; Shimizu, M.; Endo, R.; Koike, C.; Kuramoto, N.; Nakamura, S.; et al. Targeted Phototherapy for Malignant Pleural Mesothelioma: Near-Infrared Photoimmunotherapy Targeting Podoplanin. Cells 2020, 9. [CrossRef]
- Fujii, Y.; Kaneko, M.; Neyazaki, M.; Nogi, T.; Kato, Y.; Takagi, J. PA tag: a versatile protein tagging system using a super high affinity antibody against a dodecapeptide derived from human podoplanin. Protein Expr Purif 2014, 95, 240-247. [CrossRef]
- Kato, Y.; Kaneko, M.K.; Kunita, A.; Ito, H.; Kameyama, A.; Ogasawara, S.; Matsuura, N.; Hasegawa, Y.; Suzuki-Inoue, K.; Inoue, O.; et al. Molecular analysis of the pathophysiological binding of the platelet aggregation-inducing factor podoplanin to the C-type lectin-like receptor CLEC-2. Cancer Sci 2008, 99, 54-61. [CrossRef]
- Kato, Y.; Vaidyanathan, G.; Kaneko, M.K.; Mishima, K.; Srivastava, N.; Chandramohan, V.; Pegram, C.; Keir, S.T.; Kuan, C.T.; Bigner, D.D.; et al. Evaluation of anti-podoplanin rat monoclonal antibody NZ-1 for targeting malignant gliomas. Nucl Med Biol 2010, 37, 785-794. [CrossRef]



| No. | Age | Sex | Anatomic Site | Pathology diagnosis | TNM | Grade | Type | C44Mab-6 | C44Mab-46 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 78 | M | Tongue | SCC of tongue | T2N0M0 | 1 | Malignant | ++ | + |
| 2 | 40 | M | Tongue | SCC of tongue | T2N0M0 | 1 | Malignant | ++ | ++ |
| 3 | 75 | F | Tongue | SCC of tongue | T2N0M0 | 1 | Malignant | + | + |
| 4 | 35 | F | Tongue | SCC of tongue | T2N0M0 | 1 | Malignant | ++ | ++ |
| 5 | 61 | M | Tongue | SCC a of tongue | T2N0M0 | 1 | Malignant | +++ | +++ |
| 6 | 41 | F | Tongue | SCC of tongue | T2N0M0 | 1 | Malignant | + | + |
| 7 | 64 | M | Tongue | SCC of right tongue | T2N2M0 | 1 | Malignant | ++ | ++ |
| 8 | 76 | M | Tongue | SCC of tongue | T1N0M0 | 1 | Malignant | ++ | ++ |
| 9 | 50 | F | Tongue | SCC of tongue | T2N0M0 | 1 | Malignant | ++ | ++ |
| 10 | 44 | M | Tongue | SCC of tongue | T2N1M0 | 1 | Malignant | +++ | +++ |
| 11 | 53 | F | Tongue | SCC of tongue | T1N0M0 | 1 | Malignant | ++ | ++ |
| 12 | 46 | F | Tongue | SCC of tongue | T2N0M0 | 1 | Malignant | + | + |
| 13 | 50 | M | Tongue | SCC of root of tongue | T3N1M0 | 1 | Malignant | +++ | + |
| 14 | 36 | F | Tongue | SCC of tongue | T1N0M0 | 1 | Malignant | +++ | +++ |
| 15 | 63 | F | Tongue | SCC of tongue | T1N0M0 | 1 | Malignant | ++ | + |
| 16 | 46 | M | Tongue | SCC of tongue | T2N0M0 | 1 | Malignant | +++ | - |
| 17 | 58 | M | Tongue | SCC of tongue | T2N0M0 | 1 | Malignant | + | + |
| 18 | 64 | M | Lip | SCC of lower lip | T1N0M0 | 1 | Malignant | +++ | +++ |
| 19 | 57 | M | Lip | SCC of lower lip | T2N0M0 | 1 | Malignant | +++ | +++ |
| 20 | 61 | M | Lip | SCC of lower lip | T1N0M0 | 1 | Malignant | ++ | ++ |
| 21 | 60 | M | Gum | SCC of gum | T3N0M0 | 1 | Malignant | + | + |
| 22 | 60 | M | Gum | SCC of gum | T1N0M0 | 1 | Malignant | +++ | +++ |
| 23 | 69 | M | Gum | SCC of upper gum | T3N0M0 | 1 | Malignant | ++ | ++ |
| 24 | 53 | M | Bucca cavioris | SCC of bucca cavioris | T2N0M0 | 1 | Malignant | + | + |
| 25 | 55 | M | Bucca cavioris | SCC of bucca cavioris | T1N0M0 | 1 | Malignant | ++ | + |
| 26 | 58 | M | Tongue | SCC of base of tongue | T1N0M0 | 1 | Malignant | ++ | ++ |
| 27 | 63 | M | Oral cavity | SCC | T1N0M0 | 1 | Malignant | ++ | ++ |
| 28 | 48 | F | Tongue | SCC of tongue | T1N0M0 | 1--2 | Malignant | ++ | + |
| 29 | 80 | M | Lip | SCC of lower lip | T1N0M0 | 1--2 | Malignant | +++ | +++ |
| 30 | 77 | M | Tongue | SCC of base of tongue | T2N0M0 | 1--2 | Malignant | +++ | ++ |
| 31 | 59 | M | Tongue | SCC of tongue | T2N0M0 | 2 | Malignant | + | - |
| 32 | 77 | F | Tongue | SCC of tongue | T1N0M0 | 2 | Malignant | ++ | ++ |
| 33 | 56 | M | Tongue | SCC of root of tongue | T2N1M0 | 2 | Malignant | + | + |
| 34 | 60 | M | Tongue | SCC of tongue | T2N1M0 | 2 | Malignant | ++ | ++ |
| 35 | 62 | M | Tongue | SCC of tongue | T2N0M0 | 2 | Malignant | +++ | ++ |
| 36 | 67 | F | Tongue | SCC of tongue | T2N0M0 | 2 | Malignant | +++ | ++ |
| 37 | 47 | F | Tongue | SCC of tongue | T2N0M0 | 2 | Malignant | +++ | +++ |
| 38 | 37 | M | Tongue | SCC of tongue | T2N1M0 | 2 | Malignant | - | - |
| 39 | 55 | F | Tongue | SCC of tongue | T2N0M0 | 2 | Malignant | ++ | + |
| 40 | 56 | F | Bucca cavioris | SCC of bucca cavioris | T2N0M0 | 2 | Malignant | +++ | + |
| 41 | 49 | M | Bucca cavioris | SCC of bucca cavioris | T1N0M0 | 2 | Malignant | - | - |
| 42 | 45 | M | Bucca cavioris | SCC of bucca cavioris | T2N0M0 | 2 | Malignant | - | - |
| 43 | 42 | M | Bucca cavioris | SCC of bucca cavioris | T3N0M0 | 2 | Malignant | +++ | ++ |
| 44 | 44 | M | Jaw | SCC of right drop jaw | T1N0M0 | 2 | Malignant | ++ | +++ |
| 45 | 40 | F | Tongue | SCC of base of tongue | T2N0M0 | 2 | Malignant | - | ++ |
| 46 | 49 | M | Bucca cavioris | SCC of bucca cavioris | T1N0M0 | 2 | Malignant | +++ | +++ |
| 47 | 56 | F | Tongue | SCC of base of tongue | T2N0M0 | 3 | Malignant | - | + |
| 48 | 42 | M | Bucca cavioris | SCC of bucca cavioris | T3N0M0 | 3 | Malignant | +++ | +++ |
| 49 | 87 | F | Face | SCC of left face | T2N0M0 | 3 | Malignant | + | + |
| 50 | 50 | M | Gum | SCC of gum | T2N0M0 | 3 | Malignant | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





