Introduction
Carbon exists in various configurations such as graphite, diamond, graphene, nanotube…etc. Graphene and its composites have been confirmed as promising adsorbents at high-temperature environments such as hydrogen, carbon dioxide, and methane in comparison to those traditional large surface areas activated carbons [
1]. The hydrogenated graphene contains sp
3 C-H bond on the basal plane made by chemical vapor deposition (CVD can be used as hydrogen storage [
1]. Apart from this, dramatic enhancement of the absorbing selective organic molecule is also observed in the reduced graphite oxide foams [
1]. Further modifying the structure into porous graphene oxide-like foam provides selective absorption of carbon dioxide over nitrogen, methane, hydrogen, and carbon monoxide at the conditions of 1x 10
6 Pascal and 300K with help of the aliphatic and aromatic domains with oxygen-rich functional groups on the surfaces [
1]. The molecular absorption in the porous carbon nanotubes is expected after the nanotubes are electrostatically charged in ionic solutions [
2] and the curvature-assisted effective atomic number Z is proposed in the thinner nanotube, which may offer an alternative way to improve the chemisorption [
3].
However, chemisorption is always paled at high temperatures. If the carbon nanomaterials exist in the presence of numerous local curvatures, it may bring hopes for effective chemisorption even at high temperatures due to the modified effective atomic number Z [
2,
3,
4]. The pure 1D form of carbon, the monoatomic carbon chain, becomes a hot research topic in recent years due to the giant elastic modulus, torsional-induced magnetism, and large electronic density of states at the Fermi level [
5,
6,
7]. The monoatomic carbon chain may generate numerous local curvatures in form of kinks owing to 1D atomic fluctuations, which may allow effective chemisorption. The linear chained carbon contains two phases [
8]. The metallic cumulene phase is likely more energetically favorable at low temperatures and the semiconducting polyyne phase should appear above the Peierls transition temperature at 500K [
8,
9]. However, the synthesis of the isolated infinite linear chain of carbon presents a huge challenge. Using the most modern technology could only obtain about 6000 atoms of carbon nanowire which required protection from the double-walled carbon nanotube unavoidably [
10]. As the Boltzmann excitation is dominated at high temperatures [
11], we are trying to investigate whether the kinks will be generated in the finite-length carbon nanowires upon heating. The disordered carbon chain associated with the kink structure may open an opportunity for designing next-generation materials for chemisorption.
Results and Discussion
In
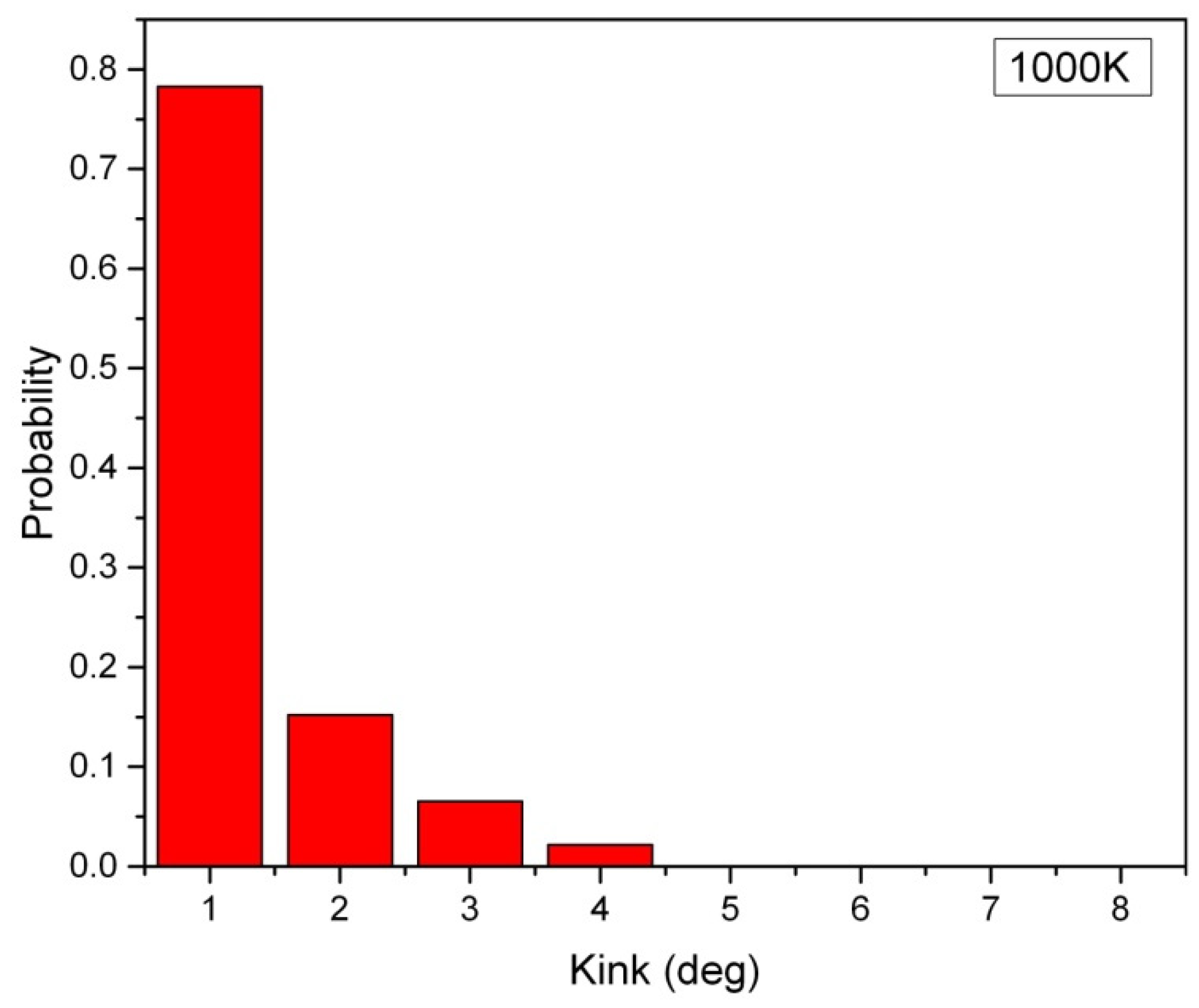
Figure 1, the distribution of the kink angle in a hexagonal array of carbon nanowires at 1000K is presented, where the carbon nanowires are composed of 2000 atoms, and they have a lateral chain-to-chain distance of 0.3nm. The most probable kink angle is observed to be 1 degree, while larger kink angles also appear with exponentially decreasing probability. The average kink angle of the entire sample, which can be considered linear, is only 2.9 degrees. However, in short carbon nanowires containing fewer than 250 atoms, the rise of kink angle becomes apparent.
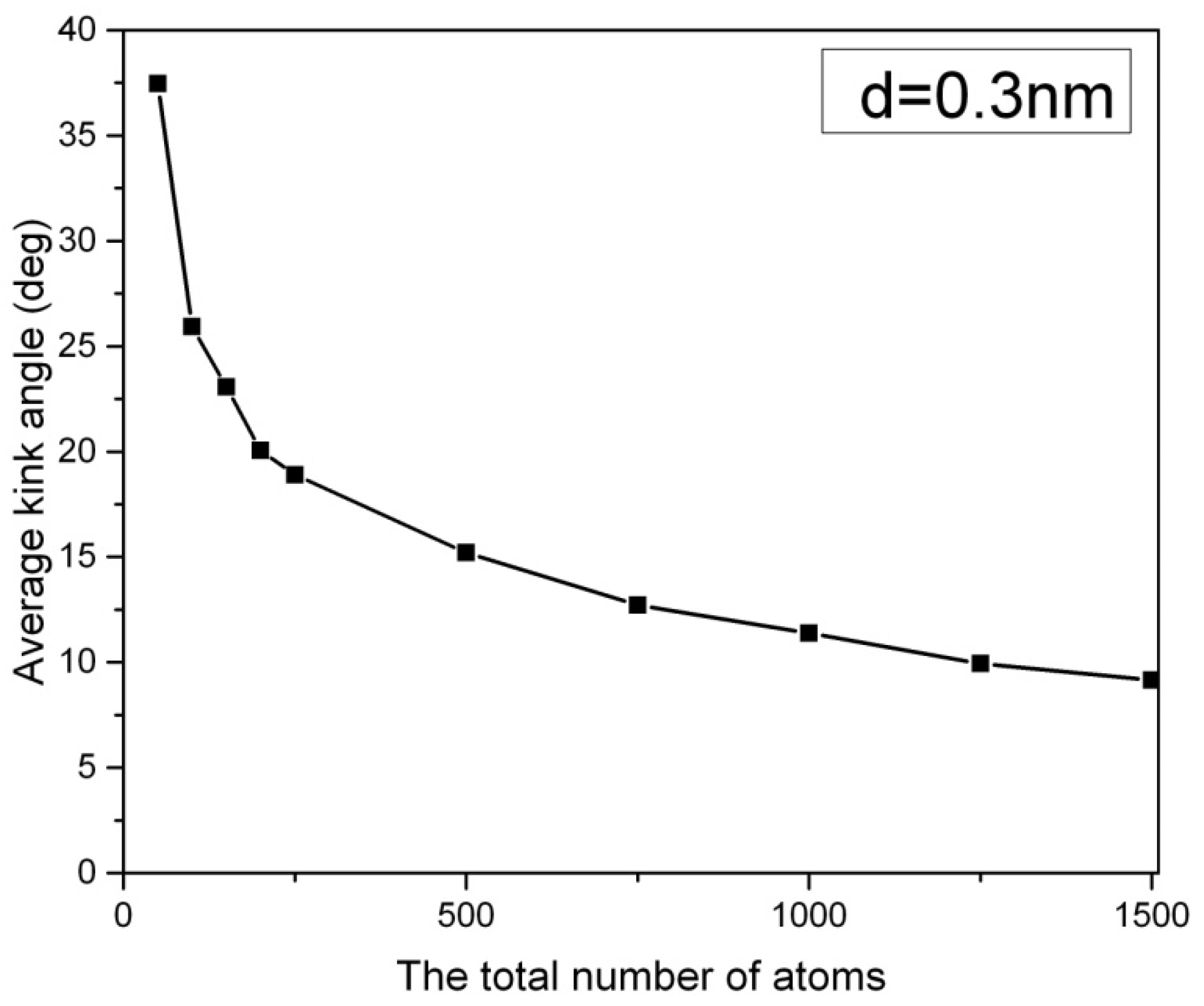
Figure 2 depicts the average kink angle of carbon nanowires at different chain lengths, with short nanowires showing an average kink angle of 38 degrees in 50-CNA. This phenomenon can be attributed to the lack of atomic spring constants in short carbon nanowires, making it difficult to control the kinematics of any rapidly moving atom at high temperatures. In contrast, atoms displaced from their equilibrium position along infinitely long carbon chains always contend with numerous serial atomic spring constants [
11].
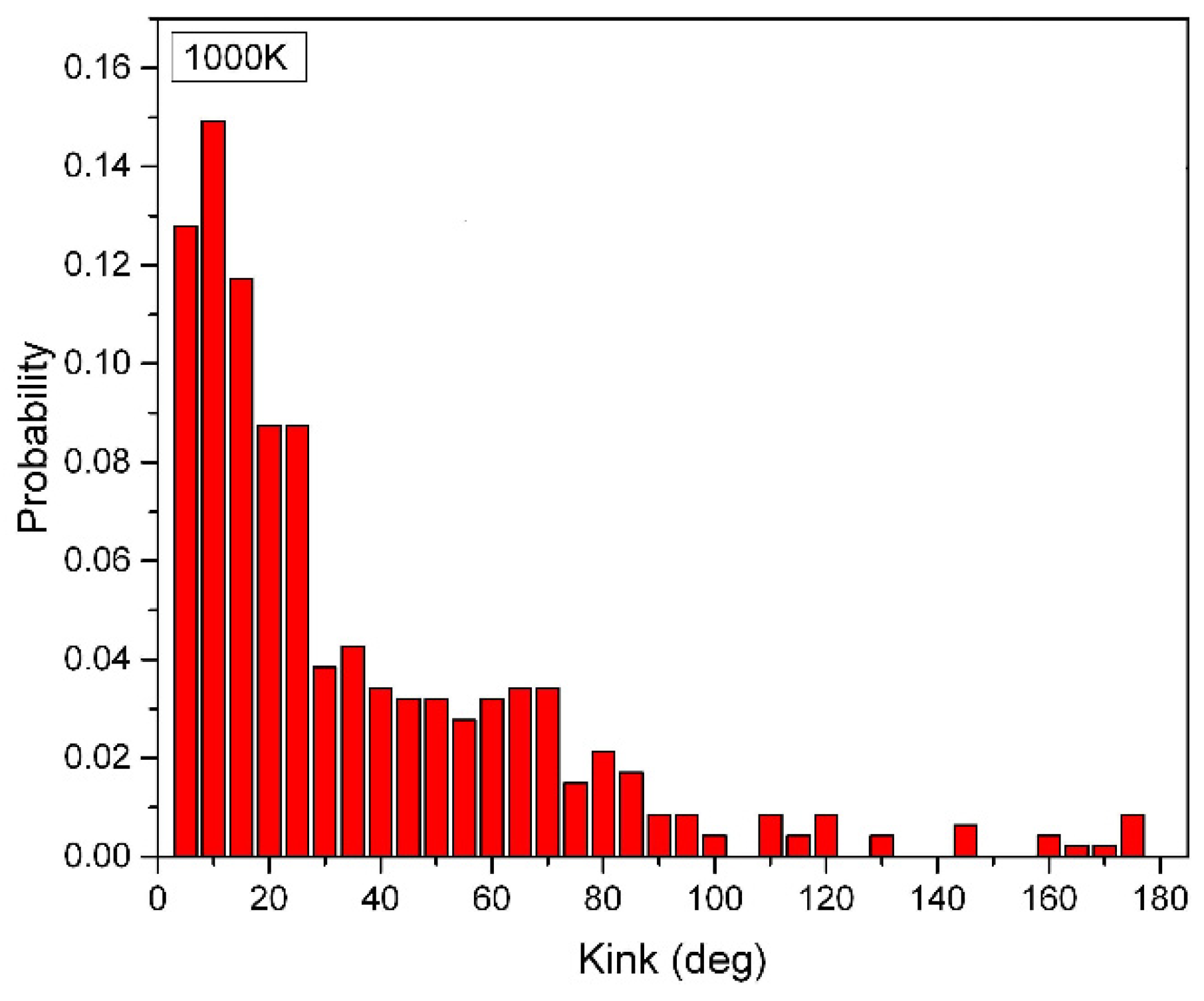
Figure 3 depicts the ten 50-CNA structures at a high temperature of 1000K. Unlike the 2000-CNA, the 50-CNA exhibits a diverse range of kink angles that extend beyond 100 degrees, although these are relatively rare due to electrostatic repulsion. At such high temperatures, thermal energy induces significant lateral vibrations in the atoms, preventing the perfect linear alignment of carbon atoms.
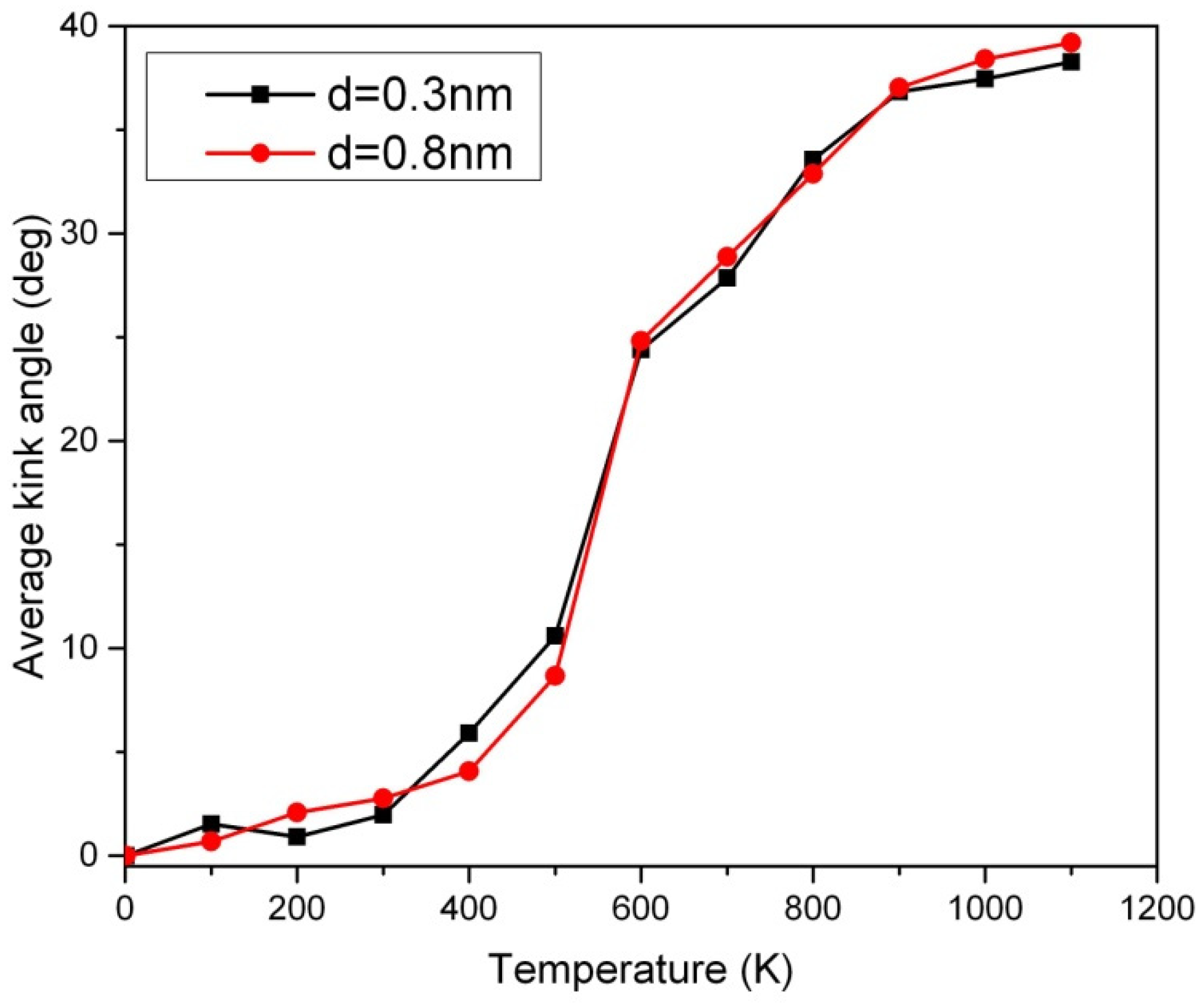
The Peierls transition of short carbon chains has been probed using a new method involving temperature sweep analysis. The slope or inflection point in
Figure 4 can be used for the detection of the Peierls transition. Below the Peierls transition temperature, no significant disorder in the 50-CNA was observed, as indicated by an average kink angle of less than 10 degrees. This is attributed to insufficient Boltzmann excitation at low-temperature regimes. However, a disordered kink structure is observed above the Peierls transition temperature [
8,
9] as evidenced by a sharper upturn at ~500K in
Figure 4. Interestingly, using the Boltzmann factor alone is not sufficient in explaining the rapid increase of the average kink angle across the phase transition. The Peierls instability appears to actuate the changes of the period in the one-dimensional nanowire that presumably forms kinks. Therefore, the rigorous atomic motion due to the Boltzmann excitation in combination with the increase of entropy across the Peierls transition causes carbon atoms to align more chaotically. The disorder in the 50-CNA starts to saturate above 800K due to the stabilization of thermal oscillations [
11]. It is worth noting that Van der Waal’s force is too weak to influence the Peierls transition temperature in
Figure 4. Our findings suggest that the kink structure is non-negligible in short carbon nanowires above the Peierls transition temperature.
Can the kink-structuring carbon nanowire perform chemisorption effectively? The preliminary research on the carbon nanotube shows that the effective nuclear charge Z is increased due to curvature [
3]. The kink structure in the short carbon chain creates numerous local curvatures and eventually, the effective Z is expected to be enhanced. The average kink angle of about 40 degrees in the 10 parallel 50-CNA at 1000K causes about a 30% increase in the linear charge density from the positively charged lattice [
14]. It may increase the efficiency of absorbing negatively charged atoms at kinks which opens an opportunity for absorbing disastrous gases from the environment [
15,
16]. DFT simulations are desired to predict the atomic fluctuations at 0K. Unlike DFT simulations focusing on ground states, our Monte Carlo method is capable of creating thermal disorder of carbon nanowires in excited states at high temperatures, which allows the designing of carbon nanowire-based devices for high-temperature environments.
Computational Method
Based on the Monte Carlo simulation of 10 parallel monoatomic carbon chains in form of a hexagonal array, the distribution of the bond angles will be computed in a series of chain lengths in the presence of weak Van der Waal’s force via the Hamiltonian
H below.
where
,
,
,
are the total number of chains, the total number of carbon in each chain, double bond energy, and temperature respectively. The formation in single, double, and triple bond corresponds to
and
respectively. The
is a stochastic variable in the simulation. The
is computed in Cartesian coordinate and
is equilibrium position. The temperature to break the covalent bond
is determined by
where the Boltzmann constant equals to
. The Van der Waals energy
is the only interaction between the adjacent carbon chains with the sample length
. The
and
are
and
respectively [
11]. The algorithm and other simulation parameters can be found in C.H.Wong et al [
12]. The Van der Waal’s distance d is 0.3nm unless otherwise specified. Three adjacent carbon atoms form one pivot angle with the appearance of the kink structure eventually where the pivot angle in the linear carbon chain is defined as zero degree. The carbon chain carrying M atoms is named M-CNA. For example, the carbon nanowire made of 50 atoms is named 50-CNA.
Conclusion
In summary, we have investigated the size effect on the kink structure in the monoatomic carbon chains via the Monte Carlo method. Our results indicate that the alignment of the carbon atoms along the chain becomes more chaotic above the Peierls transition temperature. Our simulation system opens a possibility to refine the atomic coordinates of the short carbon nanowire in the DFT simulation in order to achieve a more accurate theoretical prediction in high-temperature environments. It will be a valuable mission to keep investigating the thermal-induced kink structure in the carbon nanowire that may be used for chemisorption in the future.
Author contributions
Conceptualization, C.H.W and A.F.Z; Methodology, C.H.W.; Validation, C.H.W., A.F.Z. and M.B.G; Formal Analysis, C.H.W., E.A.B., A.F,Z ; Investigation, C.H.W., E.A.B., A.F.Z., W.S.Y., S.T., M.B.G; Resources, A.F.Z., W.S.Y., S.T.; Data Curation, C.H.W., E.A.B; Writing – Original Draft Preparation, C.H.W., E.A.B.; Writing – Review & Editing, C.H.W., E.A.B., A.F.Z., W.S.Y., S.T., M.B.G.; Visualization, X.X.; Supervision, A.F.Z., E.A.B; Funding Acquisition, A.F.Z, W.S.Y,
Acknowledgments
This study was supported by the Ministry of Education and Science of the Russian Federation (Government Task No. 3.1485.2017/4.6) and by Act 211 Government of the Russian Federation (contract no. 02.A03.21.0006).
References
- Srinivas Gadipelli, Zheng Xiao Guo, Graphene-based materials: Synthesis and gas sorption, storage and separation, Progress in Materials Science, Volume 69, Pages 1-60 (2015). [CrossRef]
- Irena Yzeiri, Niladri Patra, and Petr Král, Porous carbon nanotubes: Molecular absorption, transport, and separation, The Journal of Chemical Physics 140, 104704 (2014). [CrossRef]
- L. X. Benedict, V. H. Crespi, S. G. Louie, M. L. Cohen, Static conductivity and superconductivity of carbon nanotubes: Relations between tubes and sheets, Phys. Rev. B 52, 14935 (1995). [CrossRef]
- Bruce H. Mahan, Rollie J. Myers, University Chemistry, Benjamin-Cummings Publishing Company, ISBN-13: 978-0201058338 (2000).
- Mingjie Liu, Vasilii I. Artyukhov, Hoonkyung Lee, Fangbo Xu, and Boris I. Yakobson, Carbyne from First Principles: Chain of C Atoms, a Nanorod or a Nanorope, ACS Nano, 7, (11), pp 10075–10082 (2013). [CrossRef]
- Ivano E. Castelli, Paolo Salvestrini, and Nicola Manini, Mechanical properties of carbynes investigated by ab initio total-energy calculations, Phy Rev B, 85, 214110 (2012). [CrossRef]
- C.H. Wong, J.Y. Dai, M.B. Guseva, V.N. Rychkov, E.A. Buntov, A.F. Zatsepin, Effect of symmetry on the electronic DOS, charge fluctuations and electron-phonon coupling in carbon chains, arXiv:1611.05584 (2016). [CrossRef]
- Xiangjun Liu, Gang Zhang and Yong-Wei Zhang, Tunable Mechanical and Thermal Properties of One-Dimensional Carbyne Chain: Phase Transition and Microscopic Dynamics, J. Phys. Chem. C 119, 24156−24164 (2015). [CrossRef]
- Alberto Milani, Matteo Tommasini, Daniele Fazzi, Chiara Castiglioni, Mirella Del Zoppo and Giuseppe Zerbi, First-principles calculation of the Peierls distortion in an infinite linear carbon chain: the contribution of Raman spectroscopy, Journal of Raman Spectroscopy, Volume 39, Issue 2, pp 164–168 (2008). [CrossRef]
- L.Shi, P. Rohringer, K. Suenaga, Y. Niimi, J. Kotakoski, J.C. Meyer, H.M. Wanko, S. Cahangirov, A. Rubio, Z. J. Lapin, L. Novotny, P. Ayala & T. Pichler, Confined linear carbon chains as a route to bulk carbyne, Nature Materials, Vol 15, pp 634-640 (2016). [CrossRef]
- J. R. Christman, Fundamentals of solid state physics, Wiley (1988).
- C.H.Wong, E.A. Buntov, V.N. Rychkov, M.B. Guseva, A.F. Zatsepin, Simulation of chemical bond distributions and phase transformation in carbon chains, Carbon, Volume 114, Pages 106-110 (2017). [CrossRef]
- Jean-PaulPouget, The Peierls instability and charge density wave in one-dimensional electronic conductors, Comptes Rendus Physique Volume 17, Issues 3–4, Pages 332-356 (2016). [CrossRef]
- John David Jackson, Classical Electrodynamics, Wiley, ISBN-13: 978-0471309321 (1998).
- Xinxing Shan,Yong Qian, Lifeng Zhu, Xingcai Lu, Effects of EGR rate and hydrogen/carbon monoxide ratio on combustion and emission characteristics of biogas/diesel dual fuel combustion engine, Fuel, Volume 181, 1 Pages 1050-1057 (2016). [CrossRef]
- V.Arul Mozhi Selvan, R.B.,Anand, M.Udayakumar, Effect of Cerium Oxide Nanoparticles and Carbon Nanotubes as fuel-borne additives in Diesterol blends on the performance, combustion and emission characteristics of a variable compression ratio engine, Fuel, Volume 130, 15 Pages 160-167 (2014). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).