Submitted:
17 April 2023
Posted:
18 April 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Methods
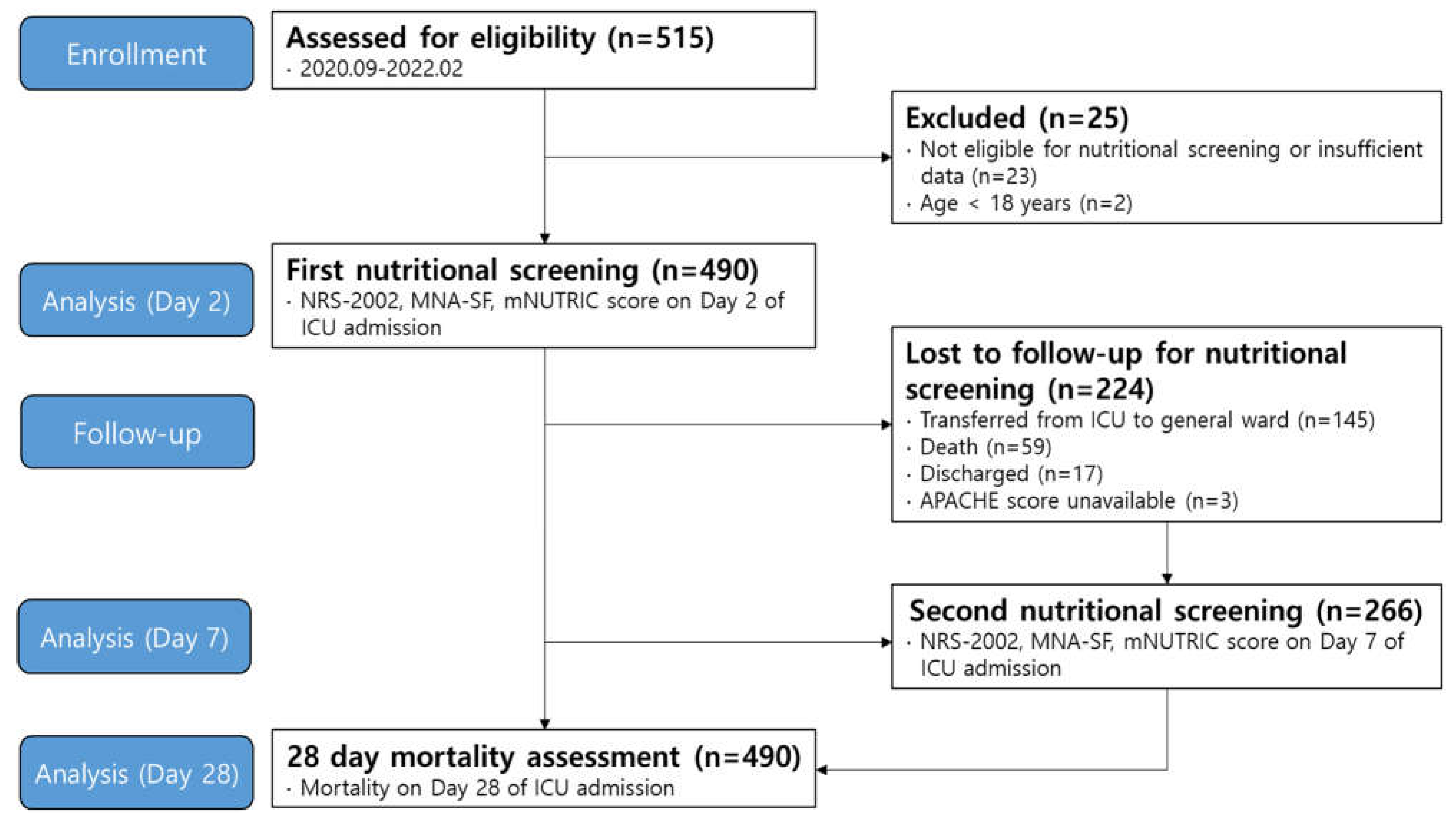
Study design and patient enrollment
Nutrition screening tools and data collection
Statistical analysis
Results
Discussion
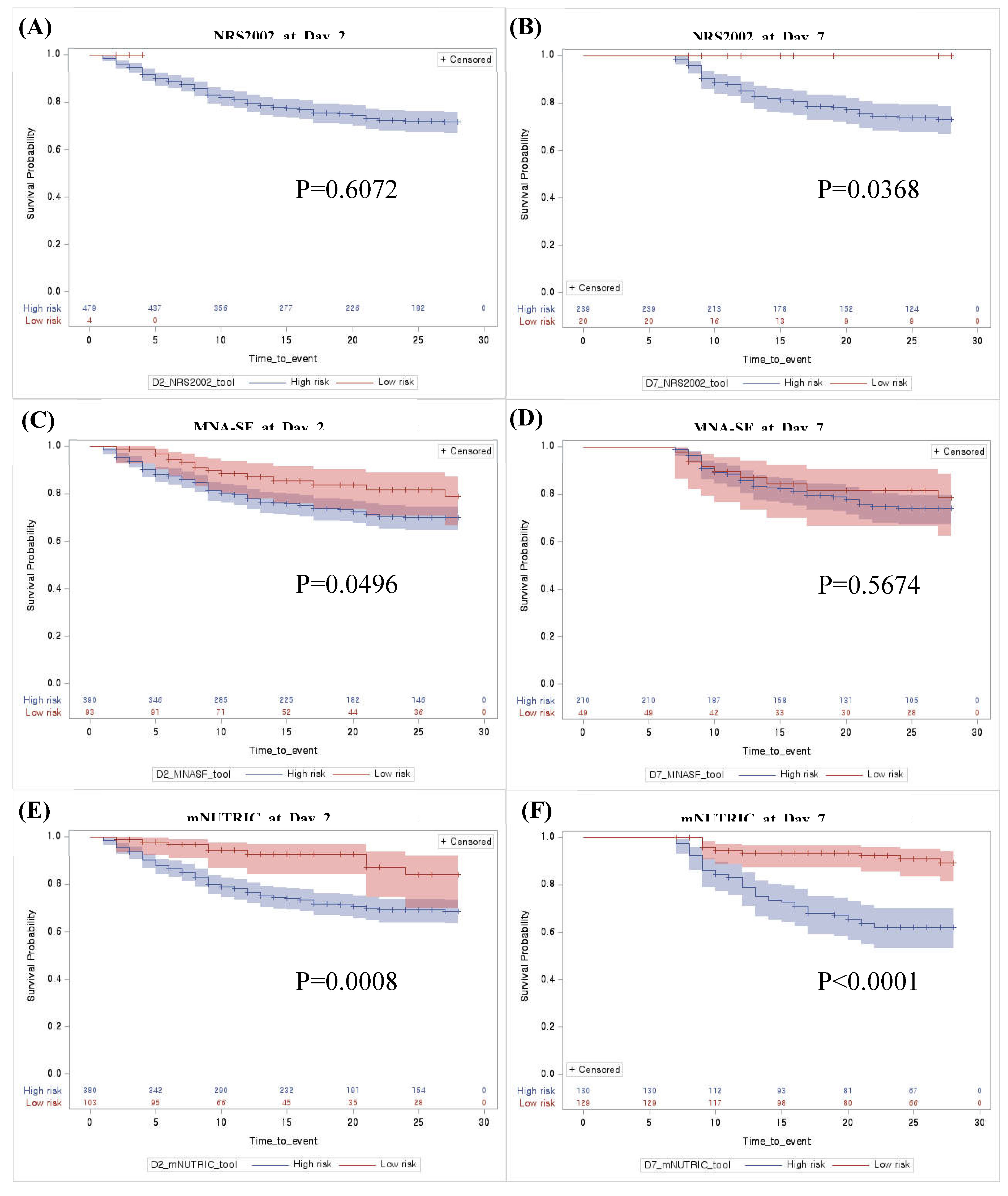
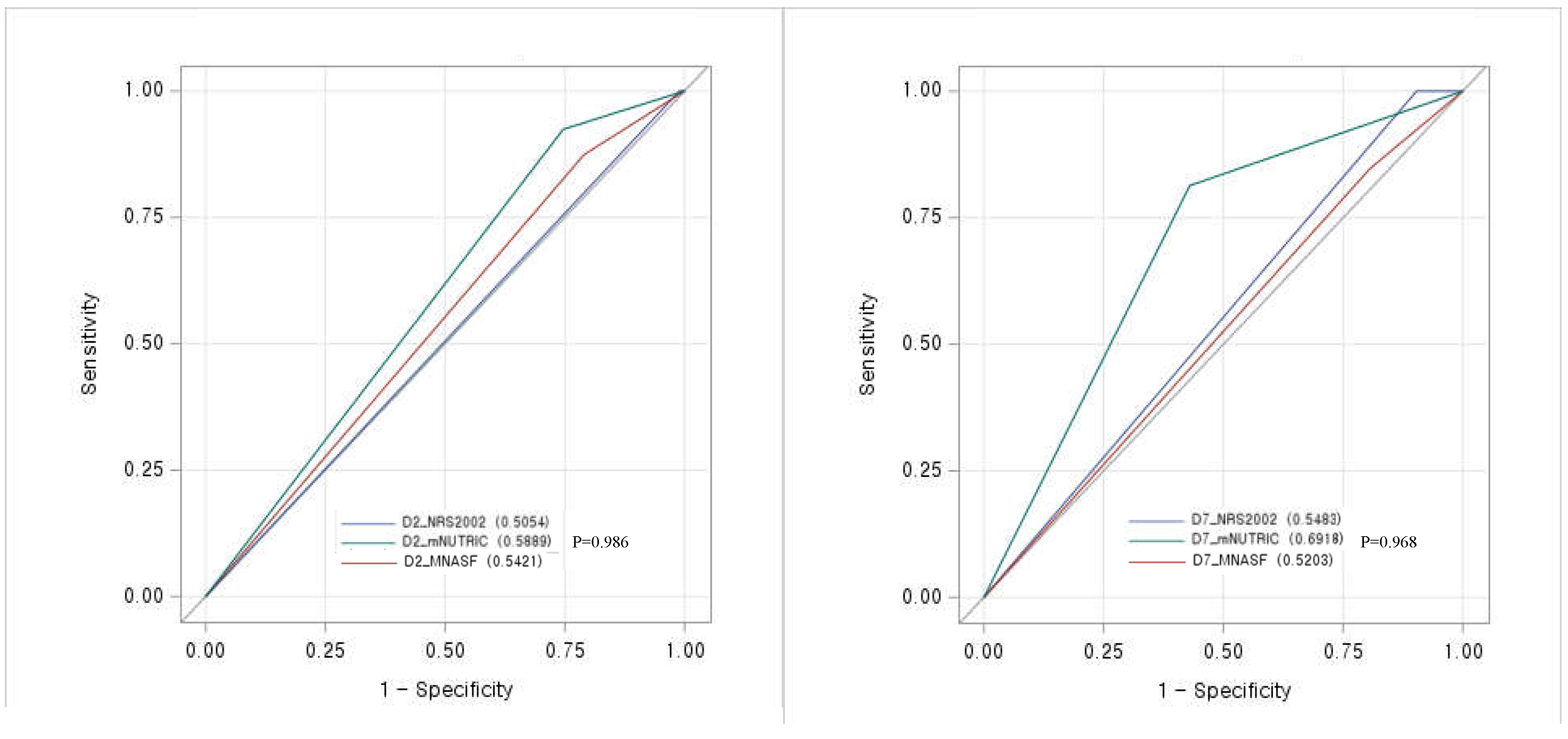
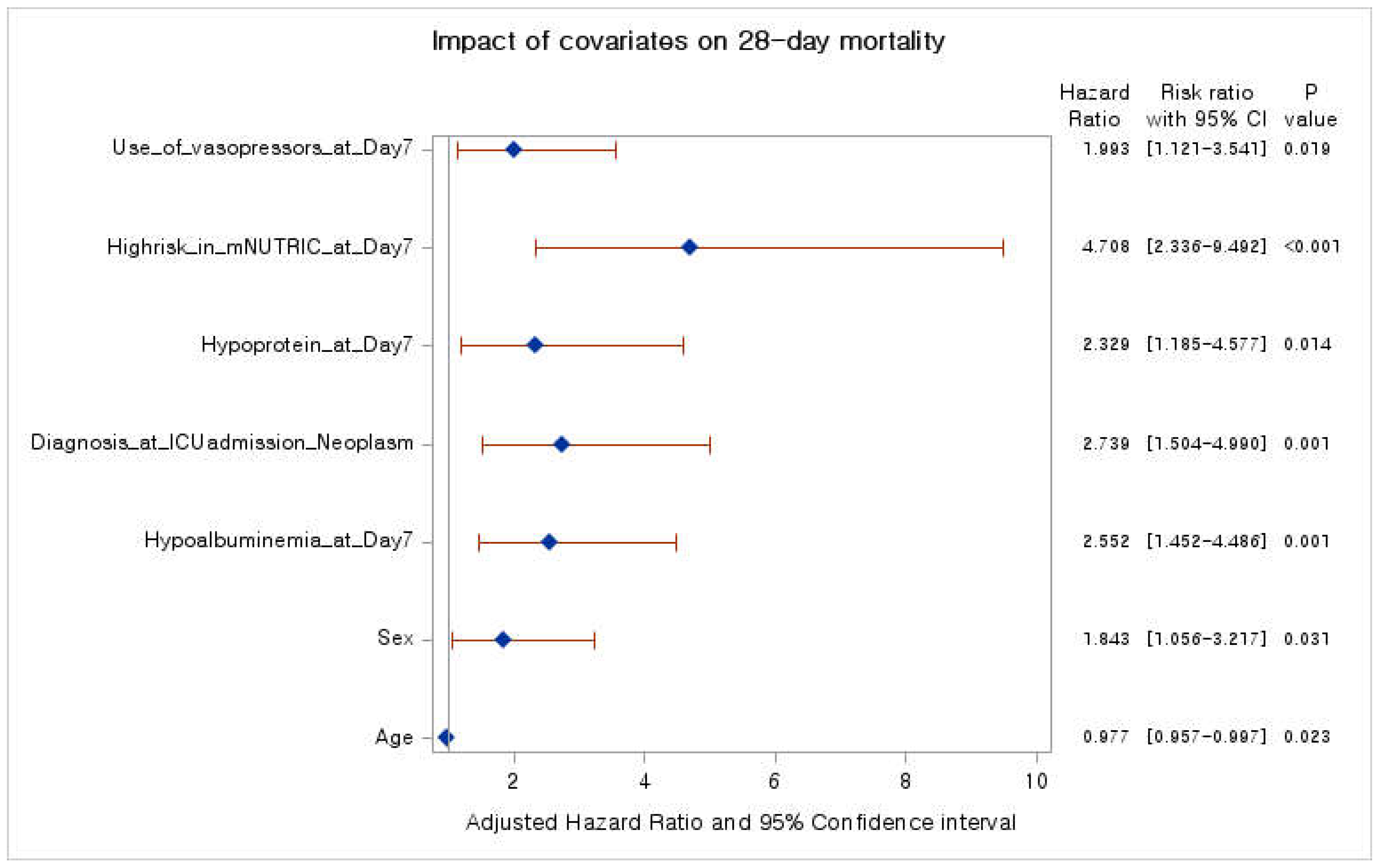
Prognostic performance of mNUTRIC score for 28-day mortality
Adequate timing to implement the mNUTRIC score in critically ill patients
Nutrition support strategy for improving 28-day mortality
New insights and limitations
Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Chada, R.R.; Chidrawar, S.; Goud, B.A.; Maska, A.; Medanki, R.; Nagalla, B. Association Between Nutrition Delivery, Modified Nutrition Risk In Critically III Score, and 28-Day Mortality. Nutr Clin Pract 2021, 36, 1020–1033. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.Y.; Heyland, D.K. Determination of Nutrition Risk and Status in Critically Ill Patients: What Are Our Considerations? Nutr Clin Pract 2019, 34, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, K.M.; Malone, A.; Becker, P.; Cutrell, S.; Frank, L.; Gonzales, K.; Hudson, L.; Miller, S.; Guenter, P. Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition Consensus Malnutrition Characteristics: Usability and Association With Outcomes. Nutr Clin Pract 2019, 34, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Lew, C.C.H.; Yandell, R.; Fraser, R.J.L.; Chua, A.P.; Chong, M.F.F.; Miller, M. Association Between Malnutrition and Clinical Outcomes in the Intensive Care Unit: A Systematic Review [Formula: see text]. JPEN J Parenter Enteral Nutr 2017, 41, 744–758. [Google Scholar] [CrossRef] [PubMed]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 2016, 40, 159–211. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, L.Z.; Harker, J.O.; Salvà, A.; Guigoz, Y.; Vellas, B. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF). J Gerontol A Biol Sci Med Sci 2001, 56, M366–372. [Google Scholar] [CrossRef]
- Kondrup, J.; Rasmussen, H.H.; Hamberg, O.; Stanga, Z. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr 2003, 22, 321–336. [Google Scholar] [CrossRef]
- Stratton, R.J.; Hackston, A.; Longmore, D.; Dixon, R.; Price, S.; Stroud, M.; King, C.; Elia, M. Malnutrition in hospital outpatients and inpatients: prevalence, concurrent validity and ease of use of the 'malnutrition universal screening tool' ('MUST') for adults. Br J Nutr 2004, 92, 799–808. [Google Scholar] [CrossRef]
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M. ESPEN guidelines for nutrition screening 2002. Clin Nutr 2003, 22, 415–421. [Google Scholar] [CrossRef]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr 2019, 38, 48–79. [Google Scholar] [CrossRef]
- Heyland, D.K.; Dhaliwal, R.; Jiang, X.; Day, A.G. Identifying critically ill patients who benefit the most from nutrition therapy: the development and initial validation of a novel risk assessment tool. Crit Care 2011, 15, R268. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Hasan, R.M.; Agarwala, R.; Martin, C.; Day, A.G.; Heyland, D.K. Identifying critically-ill patients who will benefit most from nutritional therapy: Further validation of the "modified NUTRIC" nutritional risk assessment tool. Clin Nutr 2016, 35, 158–162. [Google Scholar] [CrossRef] [PubMed]
- de Vries, M.C.; Koekkoek, W.K.; Opdam, M.H.; van Blokland, D.; van Zanten, A.R. Nutritional assessment of critically ill patients: validation of the modified NUTRIC score. Eur J Clin Nutr 2018, 72, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Policarpo, S.; Fortuna, P.; Alves, M.; Virella, D.; Heyland, D.K. Nutritional risk assessment and cultural validation of the modified NUTRIC score in critically ill patients-A multicenter prospective cohort study. J Crit Care 2017, 37, 45–49. [Google Scholar] [CrossRef]
- Maday, K.R. The importance of nutrition in critically ill patients. Jaapa 2017, 30, 32–37. [Google Scholar] [CrossRef]
- Jensen, G.L. Inflammation as the key interface of the medical and nutrition universes: a provocative examination of the future of clinical nutrition and medicine. JPEN J Parenter Enteral Nutr 2006, 30, 453–463. [Google Scholar] [CrossRef]
- Singer, P.; Doig, G.S.; Pichard, C. The truth about nutrition in the ICU. Intensive Care Med 2014, 40, 252–255. [Google Scholar] [CrossRef]
- Wei, X.; Day, A.G.; Ouellette-Kuntz, H.; Heyland, D.K. The Association Between Nutritional Adequacy and Long-Term Outcomes in Critically Ill Patients Requiring Prolonged Mechanical Ventilation: A Multicenter Cohort Study. Crit Care Med 2015, 43, 1569–1579. [Google Scholar] [CrossRef]
- Alberda, C.; Gramlich, L.; Jones, N.; Jeejeebhoy, K.; Day, A.G.; Dhaliwal, R.; Heyland, D.K. The relationship between nutritional intake and clinical outcomes in critically ill patients: results of an international multicenter observational study. Intensive Care Med 2009, 35, 1728–1737. [Google Scholar] [CrossRef]
- Weijs, P.J.; Stapel, S.N.; de Groot, S.D.; Driessen, R.H.; de Jong, E.; Girbes, A.R.; Strack van Schijndel, R.J.; Beishuizen, A. Optimal protein and energy nutrition decreases mortality in mechanically ventilated, critically ill patients: a prospective observational cohort study. JPEN J Parenter Enteral Nutr 2012, 36, 60–68. [Google Scholar] [CrossRef]
- Compher, C.; Chittams, J.; Sammarco, T.; Nicolo, M.; Heyland, D.K. Greater Protein and Energy Intake May Be Associated With Improved Mortality in Higher Risk Critically Ill Patients: A Multicenter, Multinational Observational Study. Crit Care Med 2017, 45, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Henry, J.; Ong, V.; Leong, C.S.; Teh, A.L.; van Dam, R.M.; Kowitlawakul, Y. Association of modified NUTRIC score with 28-day mortality in critically ill patients. Clin Nutr 2017, 36, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Canales, C.; Elsayes, A.; Yeh, D.D.; Belcher, D.; Nakayama, A.; McCarthy, C.M.; Chokengarmwong, N.; Quraishi, S.A. Nutrition Risk in Critically Ill Versus the Nutritional Risk Screening 2002: Are They Comparable for Assessing Risk of Malnutrition in Critically Ill Patients? JPEN J Parenter Enteral Nutr 2019, 43, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Basile-Filho, A.; Lago, A.F.; Menegueti, M.G.; Nicolini, E.A.; Rodrigues, L.A.B.; Nunes, R.S.; Auxiliadora-Martins, M.; Ferez, M.A. The use of APACHE II, SOFA, SAPS 3, C-reactive protein/albumin ratio, and lactate to predict mortality of surgical critically ill patients: A retrospective cohort study. Medicine (Baltimore) 2019, 98, e16204. [Google Scholar] [CrossRef]
- Petros, S.; Horbach, M.; Seidel, F.; Weidhase, L. Hypocaloric vs Normocaloric Nutrition in Critically Ill Patients: A Prospective Randomized Pilot Trial. JPEN J Parenter Enteral Nutr 2016, 40, 242–249. [Google Scholar] [CrossRef]
- Zusman, O.; Theilla, M.; Cohen, J.; Kagan, I.; Bendavid, I.; Singer, P. Resting energy expenditure, calorie and protein consumption in critically ill patients: a retrospective cohort study. Crit Care 2016, 20, 367. [Google Scholar] [CrossRef]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Donini, L.M.; Poggiogalle, E.; Molfino, A.; Rosano, A.; Lenzi, A.; Rossi Fanelli, F.; Muscaritoli, M. Mini-Nutritional Assessment, Malnutrition Universal Screening Tool, and Nutrition Risk Screening Tool for the Nutritional Evaluation of Older Nursing Home Residents. J Am Med Dir Assoc 2016, 17, 959–e911. [Google Scholar] [CrossRef]
- Ata Ur-Rehman, H.M.; Ishtiaq, W.; Yousaf, M.; Bano, S.; Mujahid, A.M.; Akhtar, A. Modified Nutrition Risk in Critically Ill (mNUTRIC) Score to Assess Nutritional Risk in Mechanically Ventilated Patients: A Prospective Observational Study from the Pakistani Population. Cureus 2018, 10, e3786. [Google Scholar] [CrossRef]
- Mart, M.F.; Girard, T.D.; Thompson, J.L.; Whitten-Vile, H.; Raman, R.; Pandharipande, P.P.; Heyland, D.K.; Ely, E.W.; Brummel, N.E. Nutritional Risk at intensive care unit admission and outcomes in survivors of critical illness. Clin Nutr 2021, 40, 3868–3874. [Google Scholar] [CrossRef]
- Majari, K.; Imani, H.; Hosseini, S.; Amirsavadkouhi, A.; Ardehali, S.H.; Khalooeifard, R. Comparison of Modified NUTRIC, NRS-2002, and MUST Scores in Iranian Critically Ill Patients Admitted to Intensive Care Units: A Prospective Cohort Study. JPEN J Parenter Enteral Nutr 2021, 45, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhang, Y.; Zhang, Q.; Wu, B. Modified Nutrition Risk in Critically ill is an effective nutrition risk screening tool in severely burned patients, compared with Nutrition Risk Screening 2002. Front Nutr 2022, 9, 1007885. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, D.; Guenter, P.; Howell, W.H.; Kochevar, M.E.; Roth, J.; Seidner, D.L. Definition of terms, style, and conventions used in A.S.P.E.N. guidelines and standards. Nutr Clin Pract 2005, 20, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Lochs, H.; Allison, S.P.; Meier, R.; Pirlich, M.; Kondrup, J.; Schneider, S.; van den Berghe, G.; Pichard, C. Introductory to the ESPEN Guidelines on Enteral Nutrition: Terminology, definitions and general topics. Clin Nutr 2006, 25, 180–186. [Google Scholar] [CrossRef]
- Im, K.M.; Kim, E.Y. Identification of ICU Patients with High Nutritional Risk after Abdominal Surgery Using Modified NUTRIC Score and the Association of Energy Adequacy with 90-Day Mortality. Nutrients 2022, 14. [Google Scholar] [CrossRef]
- Ishibashi, N.; Plank, L.D.; Sando, K.; Hill, G.L. Optimal protein requirements during the first 2 weeks after the onset of critical illness. Crit Care Med 1998, 26, 1529–1535. [Google Scholar] [CrossRef]
| Nutrition screening group on Day 2 (N=490) | Nutrition screening group on Day 7 (N=266) | P value | |
|---|---|---|---|
| Age (years) | 67.9 ± 15.0 | 68.8 ± 14.5 | 0.408 |
| Sex (N, %) | 0.685 | ||
| Male | 317 (64.7%) | 176 (66.2%) | |
| Female | 173 (35.3%) | 90 (33.8%) | |
| Body mass index (kg/m2) | 23.5 ± 5.6 | 23.8 ± 6.6 | 0.509 |
| Weight at ICU admission (kg) | 62.1 ± 15.2 | 62.6 ± 17.4 | 0.673 |
| Days from hospital to ICU (days) | 5.4 ± 11.4 (1, 0-6) | 6.9 ± 13.2 (1, 0-8) | 0.110 |
| Source of admission to ICU (N, %) | 0.346 | ||
| Ward | 189 (38.6%) | 104 (39.1%) | |
| Emergency room | 254 (51.8%) | 128 (48.1%) | |
| ICU | 47 (9.6%) | 34 (12.08) | |
| Comorbidities ≥ 2 (N, %) | 411 (83.88%) | 233 (87.59%) | 0.170 |
| APACHE II score | 28.6 ± 8.9 (29, 22-35) | 17.1 ± 7.8 (16, 12-22) | <0.001 |
| SOFA score | 7.5 ± 3.7 (8, 5-10) | 6.8 ± 3.5 (6.5, 4-9) | 0.012 |
| Vasopressors (N, %) | 335 (68.5%) | 135 (51.1%) | <0.001 |
| Renal dialysis (N, %) | 120 (24.5%) | 56 (21.1%) | 0.286 |
| Antibiotics (N, %) | 408 (83.4%) | 214 (80.5%) | 0.304 |
| Route of administration § | <0.001 | ||
| NPO | 35 (7.2%) | 3 (1.1%) | |
| EN | 91 (18.7%) | 68 (25.6%) | |
| PN | 214 (43.9%) | 54 (20.3%) | |
| EN+PN | 148 (30.3%) | 141 (53.0%) | |
| Calorie ENPN (kcal) | 587.5 ± 505.4 | 1074.5 ± 589.8 | <0.001 |
| Hypocaloric§§ | 299 (61.0%) | 99 (37.2%) | <0.001 |
| Protein Supply (g/d) | 0.4 ± 0.5 | 0.8 ± 0.5 | <0.001 |
| Hypo-protein (< 1.0g/kg/d) | 441 (90.0%) | 166 (62.4%) | <0.001 |
| Hypo-protein (< 1.3g/kg/d) | 463 (94.5%) | 220 (82.7%) | <0.001 |
| Diagnosis at ICU admission | 0.734 | ||
| Respiratory system | 123 (25.1%) | 74 (27.8%) | |
| Circulatory system | 104 (21.2%) | 56 (21.1%) | |
| Neoplasm | 76 (15.5%) | 37 (13.9%) | |
| Digestive system | 38 (7.8%) | 15 (5.6%) | |
| Infectious (Including covid-19) | 33 (6.8%) | 23 (8.8%) | |
| Others | 116 (23.7%) | 61 (22.9%) | |
| Albumin (mg/dL) | 2.9 ± 0.9 (2.8, 2.4-3.2) | 2.7 ± 0.5 (2.7, 2.5-3.0) | 0.013 |
| CRP (mg/L) | 11.6 ± 9.1 (9.0, 4.0-17.6) | 8.5 ± 7.2 (6.3, 3.3-11.2) | <0.001 |
| Lactate (mg/dL) | 3.4 ± 4.2 (2.0, 1.3-3.3) | 2.1 ±2.6 (1.4, 1.1-2.2) | <0.001 |
| WBC (/mm3) | 13.6 ± 12.6 (11.1, 7.8-15.7) | 12.4 ± 8.0 (10.8, 7.5-15.2) | 0.163 |
| mNUTRIC | <0.001 | ||
| Low risk | 103 (21.0%) | 129 (48.5%) | |
| High risk | 387 (79.0%) | 137 (51.5%) | |
| NRS2002 | <0.001 | ||
| Low risk | 4 (0.8%) | 20 (7.5%) | |
| High risk | 486 (99.2%) | 246 (92.5%) | |
| MNA-SF | 0.851 | ||
| Low risk | 93 (19.0%) | 49 (18.4%) | |
| High risk | 397 (81.0%) | 217 (81.6%) | |
| 28-day mortality | 0.515 | ||
| Death (N, %) | 119 (24.3%) | 59 (22.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





