Submitted:
17 April 2023
Posted:
18 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
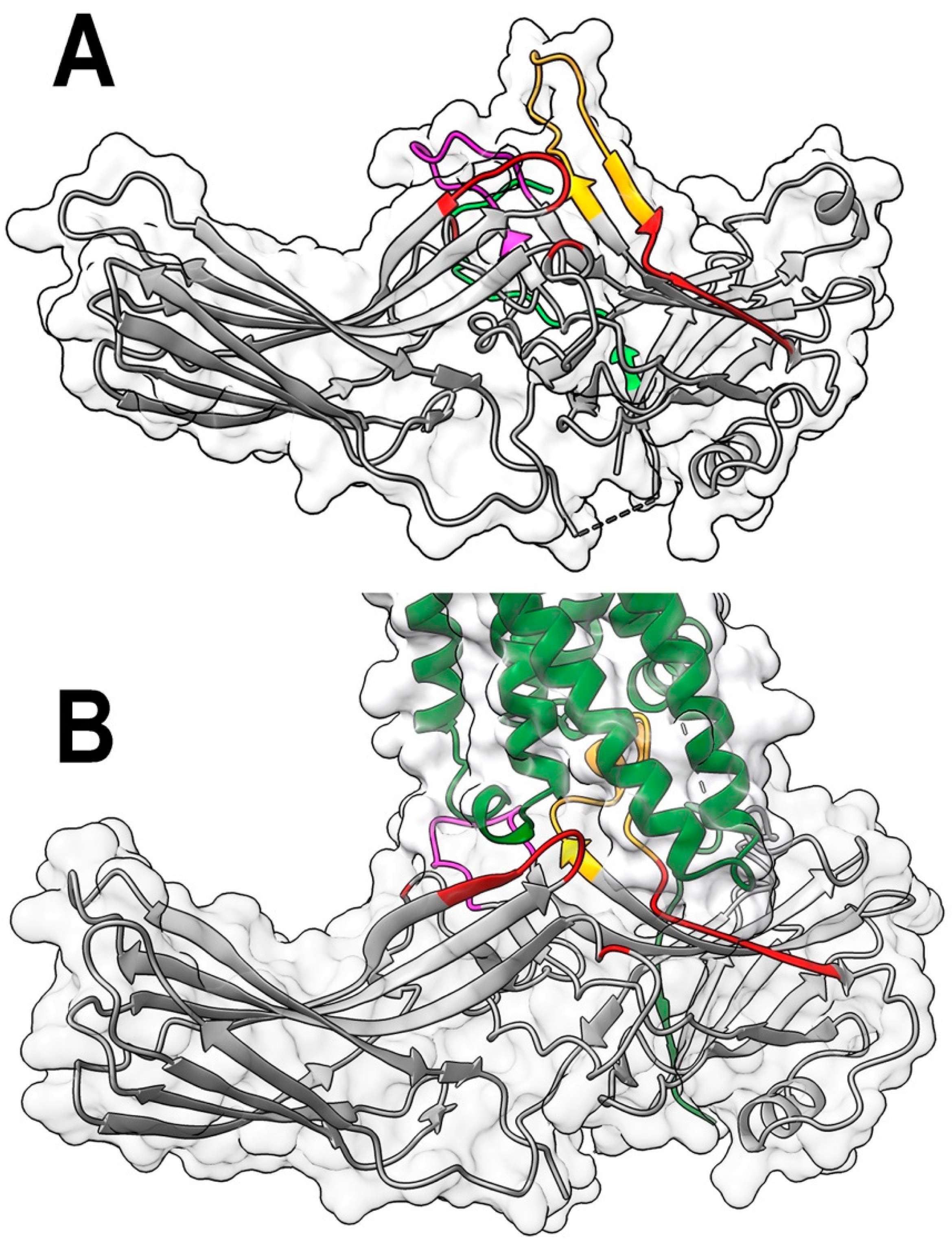
2. Results
β-strand VI
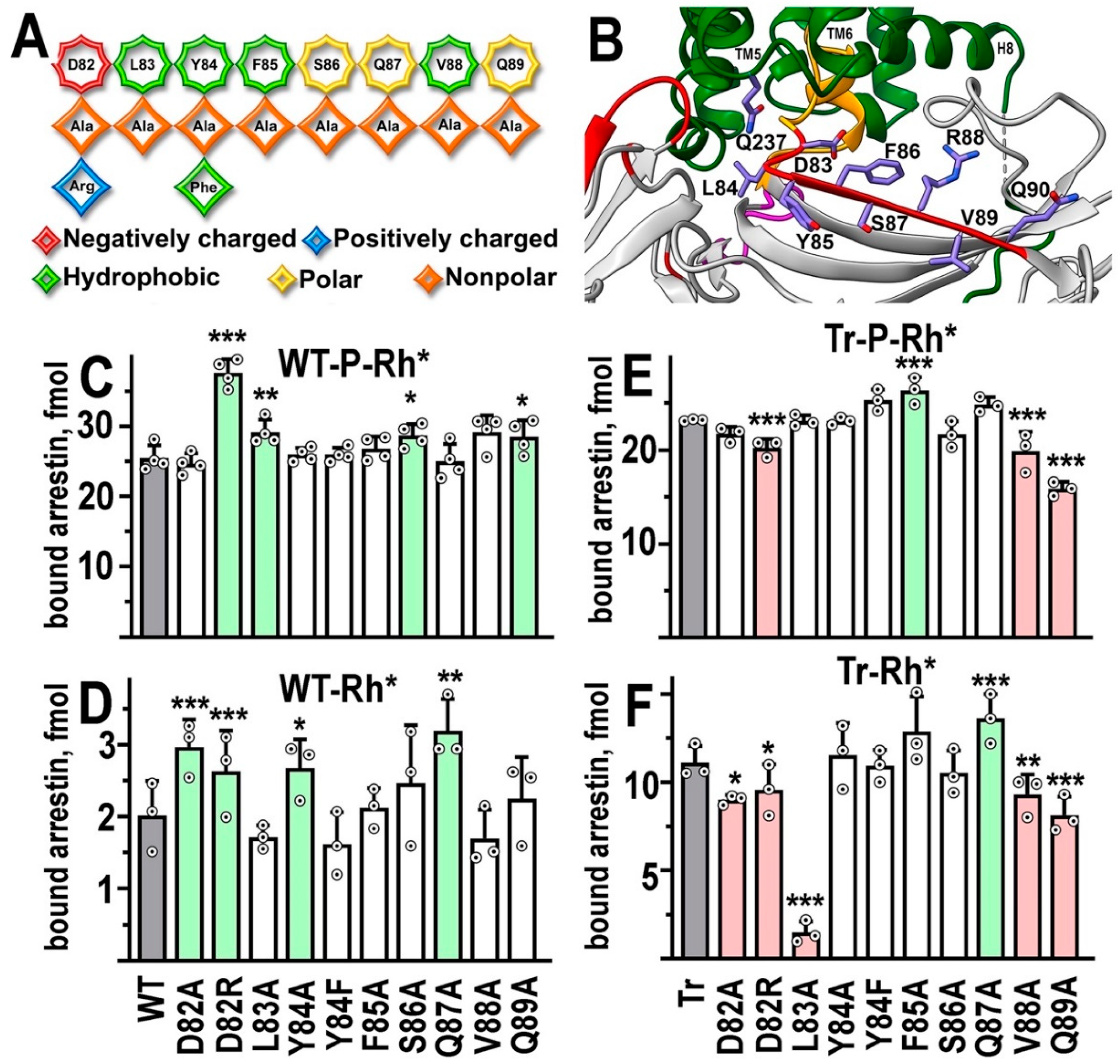
The C-loop
| Mutation | WT-P-Rh* | WT-Rh* | Tr-P-Rh* | Tr-Rh* |
|---|---|---|---|---|
| D82A | ↑ *** | ↓ * | ||
| D82R | ↑ *** | ↑ *** | ↓ *** | ↓ * |
| L83A | ↑ ** | ↓↓ *** | ||
| Y84A | ↑ * | |||
| Y84F | ||||
| F85A | ↑ *** | |||
| S86A | ↑ * | |||
| Q87A | ↑ ** | ↑ *** | ||
| V88A | ↓ *** | ↓ ** | ||
| Q89A | ↑ * | ↓ *** | ↓ *** | |
| L249A | ↓ * | ↓ ** | ↑ *** | ↓ *** |
| Y250A | ↓ * | ↓ *** | ↑ ** | ↓ *** |
| S251A | ↓ *** | ↓ ** | ||
| D253A | ↓ *** | ↓ *** | ||
| D253R | ↓ *** | ↓ *** | ||
| Y254A | ↓ * | |||
| Y254F | ||||
| R291A | ↑ *** | ↑ *** | ↑ ** | ↑ *** |
| R291E | ↑ * | ↑ *** | ||
| T319A | ||||
| T319E | ↓ *** | ↓ *** | ↑ ** | ↓ *** |
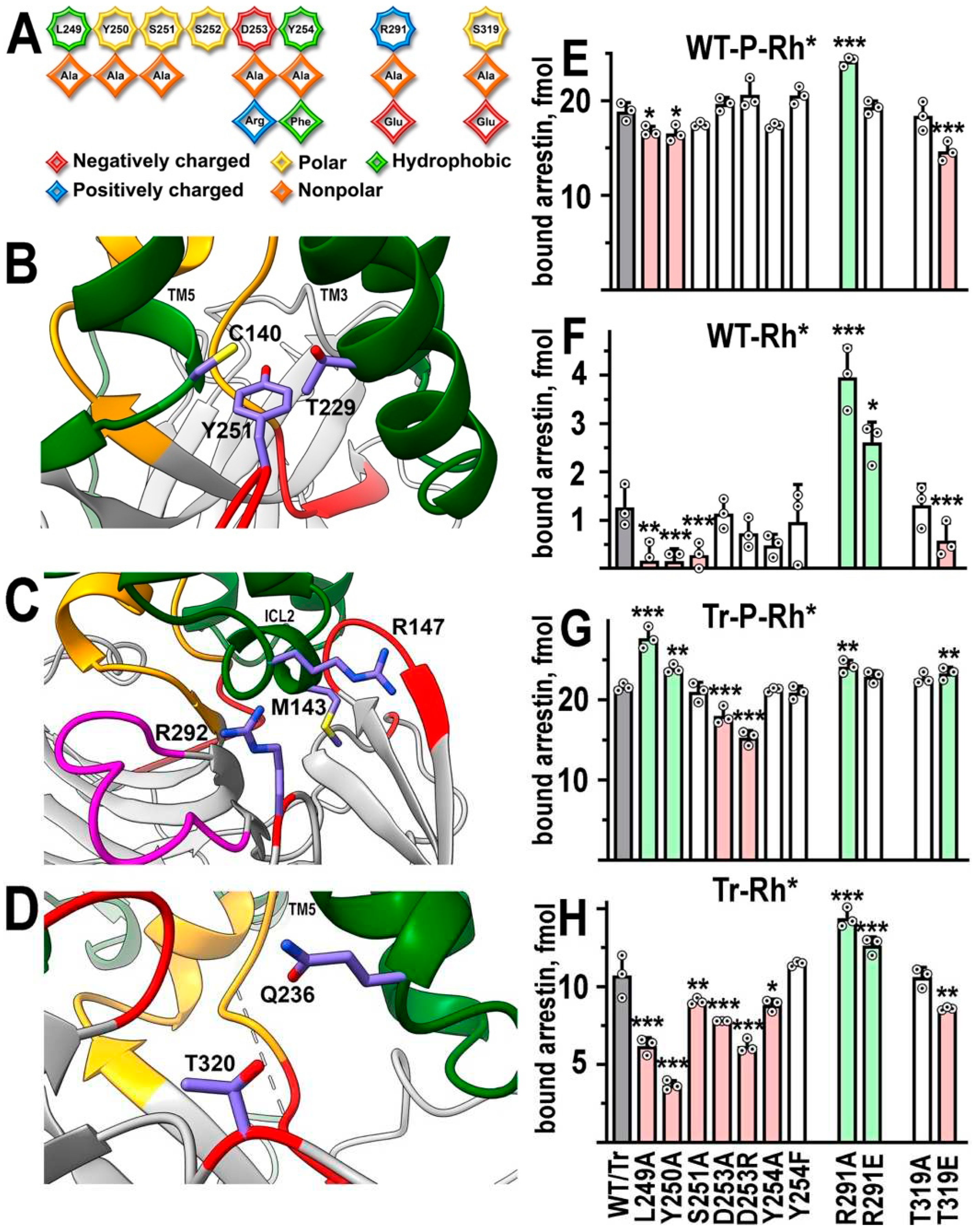
Back loop residues that contact rhodopsin in the structure
3. Discussion

4. Materials and Methods
Data Analysis and Statistics
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
| 1 | We use systematic names of arrestin proteins, where the number after the dash indicates the order of cloning: arrestin-1 (historic names S-antigen, 48 kDa protein, visual or rod arrestin), arrestin-2 (β-arrestin or β-arrestin1), arrestin-3 (β-arrestin2 or hTHY-ARRX), and arrestin-4 (cone or X-arrestin) |
References
- Gurevich, V. V.; Gurevich, E. V., The molecular acrobatics of arrestin activation. Trends Pharmacol Sci 2004, 25, 105-111. [CrossRef]
- Gurevich, V. V.; Benovic, J. L., Cell-free expression of visual arrestin. Truncation mutagenesis identifies multiple domains involved in rhodopsin interaction. J Biol Chem 1992, 267, 21919-21923. [CrossRef]
- Gurevich, V. V.; Benovic, J. L., Visual arrestin interaction with rhodopsin: Sequential multisite binding ensures strict selectivity towards light-activated phosphorylated rhodopsin. J Biol Chem 1993, 268, 11628-11638. [CrossRef]
- Gurevich, V. V.; Benovic, J. L., Visual arrestin binding to rhodopsin: diverse functional roles of positively charged residues within the phosphorylation-recignition region of arrestin. J Biol Chem 1995, 270, (11), 6010-6016.
- Gurevich, V. V.; Benovic, J. L., Mechanism of phosphorylation-recognition by visual arrestin and the transition of arrestin into a high affinity binding state. Mol Pharmacol 1997, 51, 161-169. [CrossRef]
- Gray-Keller, M. P.; Detwiler, P. B.; Benovic, J. L.; Gurevich, V. V., Arrestin with a single amino acid sustitution quenches light-activated rhodopsin in a phosphorylation0independent fasion. Biochemistry 1997, 36, 7058-7063.
- Gurevich, V. V.; Pals-Rylaarsdam, R.; Benovic, J. L.; Hosey, M. M.; Onorato, J. J., Agonist-receptor-arrestin, an alternative ternary complex with high agonist affinity. J Biol Chem 1997, 272, 28849-28852. [CrossRef]
- Vishnivetskiy, S. A.; Schubert, C.; Climaco, G. C.; Gurevich, Y. V.; Velez, M.-G.; Gurevich, V. V., An additional phosphate-binding element in arrestin molecule: implications for the mechanism of arrestin activation. J Biol Chem 2000, 275, (52), 41049-41057.
- Vishnivetskiy, S. A.; Zheng, C.; May, M. B.; Karnam, P. C.; Gurevich, E. V.; Gurevich, V. V., Lysine in the lariat loop of arrestins does not serve as phosphate sensor. J Neurochem 2021, 156, (4), 435-444. [CrossRef]
- Vishnivetskiy, S. A.; Paz, C. L.; Schubert, C.; Hirsch, J. A.; Sigler, P. B.; Gurevich, V. V., How does arrestin respond to the phosphorylated state of rhodopsin? J Biol Chem 1999, 274, 11451-11454.
- Zhou, X. E.; He, Y.; de Waal, P. W.; Gao, X.; Kang, Y.; Van Eps, N.; Yin, Y.; Pal, K.; Goswami, D.; White, T. A.; Barty, A.; Latorraca, N. R.; Chapman, H. N.; Hubbell, W. L.; Dror, R. O.; Stevens, R. C.; Cherezov, V.; Gurevich, V. V.; Griffin, P. R.; Ernst, O. P.; Melcher, K.; Xu, H. E., Identification of Phosphorylation Codes for Arrestin Recruitment by G protein-Coupled Receptors. Cell 2017, 170, (3), 457–469. [CrossRef]
- Pan, L.; Gurevich, E. V.; Gurevich, V. V., The nature of the arrestin x receptor complex determines the ultimate fate of the internalized receptor. J Biol Chem 2003, 278, 11623-11632.
- Kovoor, A.; Celver, J.; Abdryashitov, R. I.; Chavkin, C.; Gurevich, V. V., Targeted construction of phosphorylation-independent b-arrestin mutants with constitutive activity in cells. J Biol Chem 1999, 274, 6831-6834.
- Celver, J.; Vishnivetskiy, S. A.; Chavkin, C.; Gurevich, V. V., Conservation of the phosphate-sensitive elements in the arrestin family of proteins. J Biol Chem 2002, 277, (11), 9043-9048. [CrossRef]
- Hirsch, J. A.; Schubert, C.; Gurevich, V. V.; Sigler, P. B., The 2.8 A crystal structure of visual arrestin: a model for arrestin’s regulation. Cell 1999, 97, (2), 257-269.
- Vishnivetskiy, S. A.; Huh, E. K.; Gurevich, E. V.; Gurevich, V. V., The finger loop as an activation sensor in arrestin. J Neurochem 2021, 157, (4), 1138-1152. [CrossRef]
- Chen, Q.; Perry, N. A.; Vishnivetskiy, S. A.; Berndt, S.; Gilbert, N. C.; Zhuo, Y.; Singh, P. K.; Tholen, J.; Ohi, M. D.; Gurevich, E. V.; Brautigam, C. A.; Klug, K. S.; Gurevich, V. V.; Iverson, T. M., Structural basis of arrestin-3 activation and signaling. Nat Commun 2017, 8, (1), 1427. [CrossRef]
- Zheng, C.; Tholen, J.; Gurevich, V. V., Critical role of the finger loop in arrestin binding to the receptors. PLoS One 2019. [CrossRef]
- Peterhans, C.; Lally, C. C.; Ostermaier, M. K.; Sommer, M. E.; Standfuss, J., Functional map of arrestin binding to phosphorylated opsin, with and without agonist. Sci Rep 2016, 6, 28686.
- Ostermaier, M. K.; Peterhans, C.; Jaussi, R.; Deupi, X.; Standfuss, J., Functional map of arrestin-1 at single amino acid resolution. Proc Natl Acad Sci USA 2014, 111, (5), 1825-30. [CrossRef]
- Vishnivetskiy, S. A.; Huh, E. K.; Karnam, P. C.; Oviedo, S.; Gurevich, E. V.; Gurevich, V. V., The Role of arrestin-1 middle loop in rhodopsin binding. Int J Mol Sci 2022, 23, 13887. [CrossRef]
- Kang, Y.; Zhou, X. E.; Gao, X.; He, Y.; Liu, W.; Ishchenko, A.; Barty, A.; White, T. A.; Yefanov, O.; Han, G. W.; Xu, Q.; de Waal, P. W.; Ke, J.; Tan, M. H. E.; Zhang, C.; Moeller, A.; West, G. M.; Van Eps, N.; Caro, L. N.; Vishnivetskiy, S. A.; Lee, R. J.; Suino-Powell, K. M.; Gu, X.; Pal, K.; Ma, J.; Zhi, X.; Boutet, S.; Williams, G. J.; Messerschmidt, M.; Gati, C.; Zatsepin, N. A.; Wang, D.; James, D.; Basu, S.; Roy-Chowdhuty, S.; Conrad, S.; Coe, J.; Liu, H.; Lisova, S.; Kupitz, C.; Grotjohann, I.; Fromme, R.; Jiang, Y.; Tan, M.; Yang, H.; Li, J.; Wang, M.; Zheng, Z.; Li, D.; Zhao, Y.; Standfuss, J.; Diederichs, K.; Dong, Y.; Potter, C. S.; Carragher, B.; Caffrey, M.; Jiang, H.; Chapman, H. N.; Spence, J. C. H.; Fromme, P.; Weierstall, U.; Ernst, O. P.; Katritch, V.; Gurevich, V. V.; Griffin, P. R.; Hubbell, W. L.; Stevens, R. C.; Cherezov, V.; Melcher, K.; Xu, H. E., Crystal structure of rhodopsin bound to arrestin determined by femtosecond X-ray laser. Nature 2015, 523, (7562), 561-7.
- Yin, W.; Li, Z.; Jin, M.; Yin, Y. L.; de Waal, P. W.; Pal, K.; Yin, Y.; Gao, X.; He, Y.; Gao, J.; Wang, X.; Zhang, Y.; Zhou, H.; Melcher, K.; Jiang, Y.; Cong, Y.; Zhou, X. E.; Yu, X.; Xu, H. E., A complex structure of arrestin-2 bound to a G protein-coupled receptor. Cell Res 2019, 29, (12), 971-983. [CrossRef]
- Staus, D. P.; Hu, H.; Robertson, M. J.; Kleinhenz, A. L. W.; Wingler, L. M.; Capel, W. D.; Latorraca, N. R.; Lefkowitz, R. J.; Skiniotis, G., Structure of the M2 muscarinic receptor-β-arrestin complex in a lipid nanodisc. Nature 2020, 579, (7798), 297-302. [CrossRef]
- Lee, Y.; Warne, T.; Nehmé, R.; Pandey, S.; Dwivedi-Agnihotri, H.; Chaturvedi, M.; Edwards, P. C.; García-Nafría, J.; Leslie, A. G. W.; Shukla, A. K.; Tate, C. G., Molecular basis of β-arrestin coupling to formoterol-bound β(1)-adrenoceptor. Nature 2020, 583, (7818), 862-866.
- Huang, W.; Masureel, M.; Qianhui, Q.; Janetzko, J.; Inoue, A.; Kato, H. E.; Robertson, M. J.; Nguyen, K. C.; Glenn, J. S.; Skiniotis, G.; Kobilka, B. K., Structure of the neurotensin receptor 1 in complex with β-arrestin 1. Nature 2020, 579, (7798), 303-308. [CrossRef]
- Bous, J.; Fouillen, A.; Orcel, H.; Trapani, S.; Cong, X.; Fontanel, S.; Saint-Paul, J.; Lai-Kee-Him, J.; Urbach, S.; Sibille, N.; Sounier, R.; Granier, S.; Mouillac, B.; Bron, P., Structure of the vasopressin hormone-V2 receptor-β-arrestin1 ternary complex. Sci Adv 2022, 8, (35), eabo7761.
- Cao, C.; Barros-Álvarez, X.; Zhang, S.; Kim, K.; Dämgen, M. A.; Panova, O.; Suomivuori, C. M.; Fay, J. F.; Zhong, X.; Krumm, B. E.; Gumpper, R. H.; Seven, A. B.; Robertson, M. J.; Krogan, N. J.; Hüttenhain, R.; Nichols, D. E.; Dror, R. O.; Skiniotis, G.; Roth, B. L., Signaling snapshots of a serotonin receptor activated by the prototypical psychedelic LSD. Neuron 2022, 110, (19), 3154-3167.
- Palczewski, K.; Buczyłko, J.; Imami, N. R.; McDowell, J. H.; Hargrave, P. A., Role of the carboxyl-terminal region of arrestin in binding to phosphorylated rhodopsin. J Biol Chem 1991, 266, (23), 15334-9. [CrossRef]
- Kim, M.; Vishnivetskiy, S. A.; Van Eps, N.; Alexander, N. S.; Cleghorn, W. M.; Zhan, X.; Hanson, S. M.; Morizumi, T.; Ernst, O. P.; Meiler, J.; Gurevich, V. V.; Hubbell, W. L., Conformation of receptor-bound visual arrestin. Proc Nat Acad Sci USA 2012, 109, (45), 18407-18412. [CrossRef]
- Hanson, S. M.; Francis, D. J.; Vishnivetskiy, S. A.; Kolobova, E. A.; Hubbell, W. L.; Klug, C. S.; Gurevich, V. V., Differential interaction of spin-labeled arrestin with inactive and active phosphorhodopsin. Proc Natl Acad Sci USA 2006, 103, 4900-4905. [CrossRef]
- Vishnivetskiy, S. A.; Francis, D. J.; Van Eps, N.; Kim, M.; Hanson, S. M.; Klug, C. S.; Hubbell, W. L.; Gurevich, V. V., The role of arrestin alpha-helix I in receptor binding. J. Mol. Biol. 2010, 395, 42-54. [CrossRef]
- Gurevich, V. V., The selectivity of visual arrestin for light-activated phosphorhodopsin is controlled by multiple nonredundant mechanisms. J Biol Chem 1998, 273, 15501-15506. [CrossRef]
- Vishnivetskiy, S. A.; Gimenez, L. E.; Francis, D. J.; Hanson, S. M.; Hubbell, W. L.; Klug, C. S.; Gurevich, V. V., Few residues within an extensive binding interface drive receptor interaction and determine the specificity of arrestin proteins. J Biol Chem 2011, 286, 24288-24299. [CrossRef]
- Vishnivetskiy, S. A.; Hosey, M. M.; Benovic, J. L.; Gurevich, V. V., Mapping the arrestin-receptor interface: structural elements responsible for receptor specificity of arrestin proteins. J Biol Chem 2004, 279, (2), 1262-1268.
- Indrischek, H.; Prohaska, S. J.; Gurevich, V. V.; Gurevich, E. V.; Stadler, P. F., Uncovering missing pieces: duplication and deletion history of arrestins in deuterostomes. BMC Evol Biol 2017, 17, (1), 163. [CrossRef]
- Gurevich, V. V.; Dion, S. B.; Onorato, J. J.; Ptasienski, J.; Kim, C. M.; Sterne-Marr, R.; Hosey, M. M.; Benovic, J. L., Arrestin interaction with G protein-coupled receptors. Direct binding studies of wild type and mutant arrestins with rhodopsin, b2-adrenergic, and m2 muscarinic cholinergic receptors. J Biol Chem 1995, 270, 720-731.
- Jacobson, S. G.; Kemp, C. M.; Cideciyan, A. V.; Macke, J. P.; Sung, C. H.; Nathans, J., Phenotypes of stop codon and splice site rhodopsin mutations causing retinitis pigmentosa. Invest Ophthalmol Vis Sci 1994, 35, (5), 2521-34.
- Concepcion, F.; Chen, J., Q344ter mutation causes mislocalization of rhodopsin molecules that are catalytically active: a mouse model of Q344ter-induced retinal degeneration. PLoS One 2010, 5, (6), e10904. [CrossRef]
- Khani, S. C.; Nielsen, L.; Vogt, T. M., Biochemical evidence for pathogenicity of rhodopsin kinase mutations correlated with the oguchi form of congenital stationary night blindness. Proc Natl Acad Sci U S A 1998, 95, (6), 2824-7. [CrossRef]
- Cideciyan, A. V.; Zhao, X.; Nielsen, L.; Khani, S. C.; Jacobson, S. G.; Palczewski, K., Null mutation in the rhodopsin kinase gene slows recovery kinetics of rod and cone phototransduction in man. Proc Natl Acad Sci U S A 1998, 95, (1), 328-33. [CrossRef]
- Yamamoto, S.; Sippel, K. C.; Berson, E. L.; Dryja, T. P., Defects in the rhodopsin kinase gene in the Oguchi form of stationary night blindness. Nat Genet 1997, 15, 175-8. [CrossRef]
- Gurevich, V. V., Use of bacteriophage RNA polymerase in RNA synthesis. Methods Enzymol 1996, 275, 382-397.
- Vishnivetskiy, S. A.; Lee, R. J.; Zhou, X. E.; Franz, A.; Xu, Q.; Xu, H. E.; Gurevich, V. V., Functional role of the three conserved cysteines in the N domain of visual arrestin-1. J Biol Chem 2017, 292, (30), 12496-12502. [CrossRef]
- Vishnivetskiy, S. A.; Chen, Q.; Palazzo, M. C.; Brooks, E. K.; Altenbach, C.; Iverson, T. M.; Hubbell, W. L.; Gurevich, V. V., Engineering visual arrestin-1 with special functional characteristics. J Biol Chem 2013, 288, (17), 11741-11750. [CrossRef]
- Vishnivetskiy, S. A.; Sullivan, L. S.; Bowne, S. J.; Daiger, S. P.; Gurevich, E. V.; Gurevich, V. V., Molecular Defects of the Disease-Causing Human Arrestin-1 C147F Mutant. Invest Ophthalmol Vis Sci 2018, 59, (1), 13-20. [CrossRef]
- Lee, K. B.; Ptasienski, J. A.; Pals-Rylaarsdam, R.; Gurevich, V. V.; Hosey, M. M., Arrestin binding to the M2 muscarinic acetylcholine receptor is precluded by an inhibitory element in the third intracellular loop of the receptor. J Biol Chem 2000, 275, 9284-9289. [CrossRef]
- Vishnivetskiy, S. A.; Baameur, F.; Findley, K. R.; Gurevich, V. V., Critical role of the central 139-loop in stability and binding selectivity of arrestin-1. J Biol Chem 2013, 288, (17), 11741-11750. [CrossRef]
- Zhuo, Y.; Vishnivetskiy, S. A.; Zhan, X.; Gurevich, V. V.; Klug, C. S., Identification of receptor binding-induced conformational changes in non-visual arrestins. J Biol Chem 2014, 289, (30), 20991-1002. [CrossRef]
- Zhuang, T.; Chen, Q.; Cho, M.-K.; Vishnivetskiy, S. A.; Iverson, T. I.; Gurevich, V. V.; Hubbell, W. L., Involvement of Distinct Arrestin-1 Elements in Binding to Different Functional Forms of Rhodopsin. Proc Nat Acad Sci USA 2013, 110, (3), 942-947. [CrossRef]
- Böttke, T.; Ernicke, S.; Serfling, R.; Ihling, C.; Burda, E.; Gurevich, V. V.; Sinz, A.; Coin, I., Exploring GPCR-arrestin interfaces with genetically encoded crosslinkers. EMBO Rep 2020, 21, (11), e50437. [CrossRef]
- Aydin, Y.; Böttke, T.; Lam, J. H.; Ernicke, S.; Fortmann, A.; Tretbar, M.; Zarzycka, B.; Gurevich, V. V.; Katritch, V.; Coin, I., Structural details of a class B GPCR-arrestin complex revealed by genetically encoded crosslinkers in living cells. Nat Commun 2023, 14, (1), 1151.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





