1. Introduction
Under the increasing safety concerns, flame retardant adhesives attract enormous attention in the automotive, electronic, and construction industries. The adhesives are designed to provide thermal runaway protection and cell-to-cell bonding of battery systems in electric vehicles [
1,
2,
3]. Regarding the electronic assemblies such as power conversion units and cooling devices, fire blocking characteristics through system design or component materials are required [
4,
5,
6]. The thermal stability of organic resins determines the flame-retardant capability of adhesives. Although epoxy resins are the most widely used owing to their high strength and good dimensional stability, their flammable characteristic restricts their potential uses. Flame retardant adhesives in transportation and electronics allow composites to meet a V-0 rating during vertical burning tests (UL94). Benzoxazine possesses many desirable properties such as excellent thermal stability, low flammability, and near-zero shrinkage without releasing toxic products during cure [
7,
8,
9,
10,
11]. The aromatic rings and nitrogen element in polybenzoxazine structure, and crosslinkable feature give them high heat resistance and good flame retardancy. However, a glassy state at room temperature necessitates high curing temperature and additional reactive diluents to expand a processing window. When the epoxy is blended with benzoxazine, the mixture is easy to handle and additionally the interlocked homogeneous structures after copolymerization exhibit good thermos-mechanical properties [
12,
13,
14,
15,
16]. Despite many promises of the epoxy/benzoxazine blends, the flame retardancy is still unsatisfactory for industrial uses because of its intrinsic organic nature.
The use of flame-retardant additives is a simple and effective way of suppressing fire risk. Halogen-based substances reveal excellent fire resistance but generate toxic and corrosive gases during combustion. In the European Union (EU), halogenated flame retardants were prohibited in electronic display from March 2021. Recently, aluminum trihydrate (ATH) is widely used as a replacement because of its low cost and non-toxic gas emissions [
17,
18,
19,
20,
21,
22]. It undergoes endothermic dehydration upon heating above 180
oC, suppressing the flame spread and smoke emission]. In general, a considerable amount of ATH particles above 50 wt% needs to be added to meet the desired flame retardancy in industries due to relatively poor efficiency. Such high loading deteriorates the mechanical properties, processability, and long-term stability of the composites. Numerous attempts have been made to maximize flame retardancy without deteriorating mechanical properties, including the use of smaller ATH particles having large surface areas and combination with other flame retardants [
23,
24]. Surface modification is also a common approach to improving mechanical properties. Silane coupling agents having an organic and inorganic end is most frequently used [
25,
26,
27,
28].
In the present study, the epoxy resin was blended with the benzoxazine in order to enhance the fire and mechanical properties and then the ATH was added to achieve synergistic flammability. Physical property losses of the epoxy/benzoxazine/ATH composites have been ameliorated by surface modification of the ATH using three different silane coupling agents. Flammability and mechanical properties of epoxy/benzoxazine mixtures and epoxy/benzoxazine/ATH composites have been measured in relation to blending composition. UL94 burning test was carried out to determine the flame retardancies, while tensile and shear strength were measured for a mechanical test.
2. Materials and Methods
2.1. Materials and Sample Preparation
Epoxy with a reported viscosity of 250 mPa∙s at 25 oC manufactured from Daicel Chemical Industries were blended with benzoxazine (P-d type, Shikoku Chemicals, Kagawa, Japan) at various compositions. Pure epoxy and its mixture with benzoxazine were thermally cured in presence of 50 wt% of curing agent (RIKACID MH-T, New Japan Chemical, Osaka, Japan) and 5 wt% accelerator (2E4MZ-CN, Shikoku Chemicals, Kagawa, Japan), respectively. Aluminum trihydrate (SG-10LSA, Sibelco, Antwerpen, Belgium) having average particle size of 3.3 μm was used as a flame retardant. Three kinds of silane coupling agents containing epoxy (KBM403), amino (KBM603), mercapto (KBM803) functional group, were supplied by ShinEtsu, Japan.
The coupling agent (1 g) was dissolved in 200 g ethanol and then stirred at 150 rpm for 10 min. After 100 g of ATH was slowly added, the mixtures were homogenized by mechanical stirring at room temperature and then reacted at 60 oC. The product was filtered and then washed with deionized water several times. Silane-treated ATH was dried at 90 oC in a convection oven for 12 hrs. Uniform mixtures containing epoxy, benzoxazine, and ATH were achieved using a conventional three roll mill (EXAKT 80E, EXAKT, Norderstedt, Germany) and then rotated at 100 rpm for 30 min in a vacuum bath to remove the residual bubbles. The homogeneous mixtures were stored in a refrigerator at -40 oC prior to use. The epoxy/benzoxazine resins were thermally cured at 175 oC for 60min.
2.2. Characterization
Tensile and adhesion tests of the adhesive composites were performed using a Universal Testing Machine (AG-50KNX, Shimadzu) according to ASTM D638 and ASTM D1002 standard specifications, respectively. Tensile strength was measured at a crosshead speed of 5 mm/min, while the shear strength was measured by pulling the aluminum plates (114 mm in length × 20 mm in width × 3mm in thickness) at a rate of 1.27 mm/min in which the overlap length was 20 mm. The surface of aluminum is cleaned using ethanol prior to applying the adhesives. The flame retardance of cured epoxy/benzoxazine mixtures and their composites with ATH was evaluated by UL94 vertical test, where the dimension of specimen is 127 mm in length × 12.7 mm in width × 3.2 mm in thickness. Thermogravimetric analysis (TGA, Model Pyris 1, Perkin Elmer) was used to characterize the thermal stability of epoxy/benzoxazine blends. Approximately 10 mg of samples was heated from 30 oC to 600 oC at a rate of 10 oC/min under air environment. Coefficient of thermal expansion (CTE) of adhesives was estimated from the amount of thermal expansion versus temperature using TMA instrument (Model 2940, TA Instrument). A sample placed on the TMA cell was heated from 20 oC to 150 oC using a heating rate of 5 oC/min. The viscoelastic behavior was probed in a sinusoidal tension mode using Pyris Diamond DMA (Perkin Elmer) technique. The cured film (200 mm in length × 6 mm in width × 0.3 mm in thickness) was heated from 25 oC to 280 oC at a heating rate of 2 oC/min under nitrogen environment. The frequency of the dynamic mechanical measurement was fixed at 1Hz.
3. Results
3.1. Thermal, Mechanical, Flammable Properties of Epxoy/Benzoxazine Mixtures
The thermal and mechanical properties of epoxy/benzoxazine mixtures are shown in
Table 1. From the TGA measurement, pure epoxy reveals degradation temperature (
Td) at around 301
oC which corresponds to 5 wt% weight loss. Thermal degradation occurs at higher temperature with increasing the fraction of benzoxazine because of good thermal stability of benzoxazine. The glass transition temperature (
Tg) determined from tan δ peak of DMA results slightly increases when epoxy is copolymerization with benzoxazine. The
Tg of 50/50 epoxy/benzoxazine mixture is observed at around 163.0
oC. Thermal curing of epoxy/benzoxazine mixtures was conducted at 175
oC on the basis of the reaction peak of pure epoxy and thermal dehydration temperature of ATH. Considering the exothermic reaction of pure benzoxazine at around 250
oC, the
Tg of the mixtures can be shifted to the higher temperature upon elevating curing temperature.
The coefficient of thermal expansion (CTE) of pure epoxy below (α1) and above (α2) the Tg is found to be 95 ppm/oC and 162 ppm/oC, respectively. When benzoxazine is blended with epoxy, the CTE values tend to decrease. The thermal stress may be suppressed by lowering the thermal expansion mismatch between the adhesives and the die. The storage modulus of neat epoxy at room temperature increases with the amount of benzoxazine resin, i.e., 4 GPa for pure epoxy to 6.2 GPa for 70/30 epoxy/benzoxazine. The reduced modulus at high benzoxazine content above 40 wt% may be attributable to reduced crosslinking density. Viscosity is one of the crucial criteria to determine the processibility of the adhesives. The viscosity of pure epoxy having approximately 250 mPa∙s at 5 rpm increases significantly as benzoxazine content increases because pure benzoxazine is in a glassy state at room temperature. Generally, the viscosity of adhesives in the range of 8,000 to 30,000 cPs is recommended for dispensing purposes. Since the viscosity is expected to increase further upon adding ATH additives, the benzoxazine content in mixtures is limited.
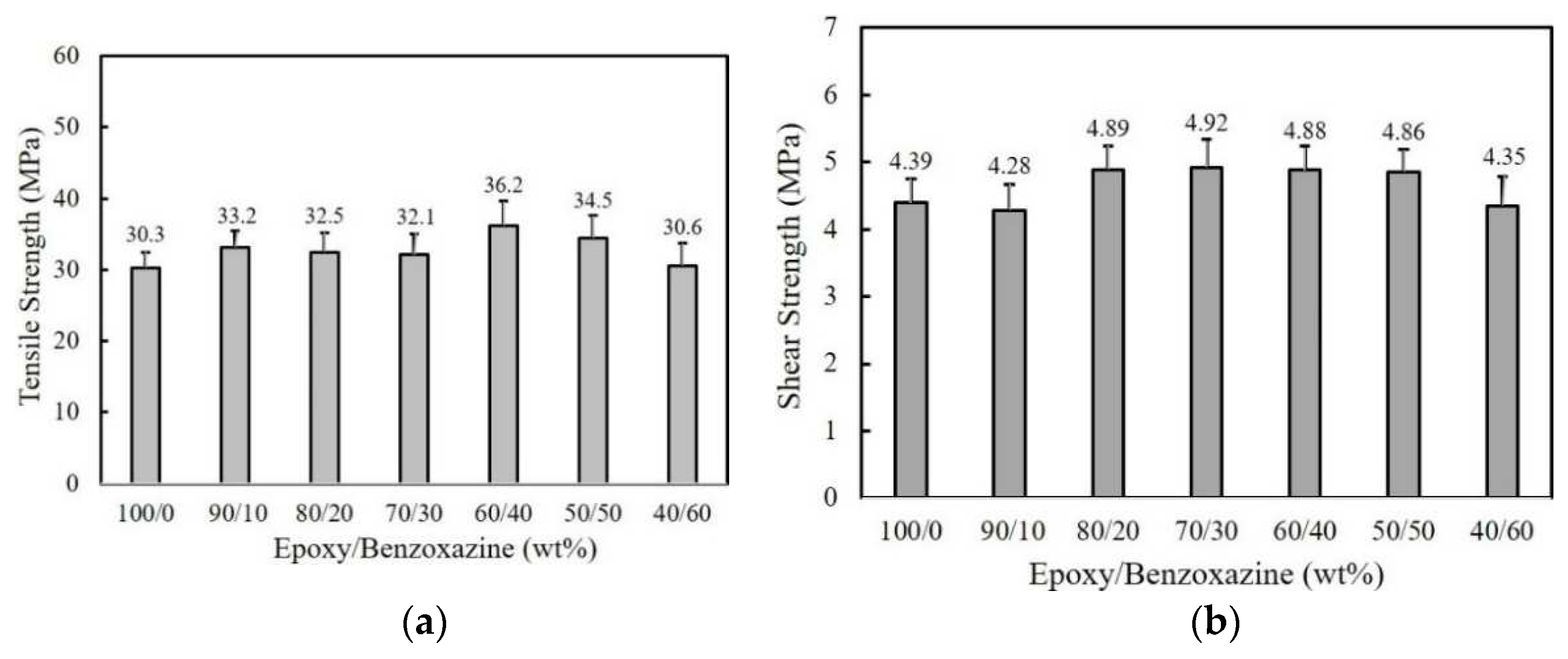
Figure 1(a) displays the tensile strength of pure epoxy and epoxy/benzoxazine blends. The benzoxazine loading is limited to 60 wt% due to high viscosity. The tensile strength of pure epoxy observed at around 30.3 MPa gradually increases upon the addition of benzoxazine and reaches the highest strength value of 36.2 MPa at 60/40 epoxy/benzoxazine composition. Tensile strength decreases to 34.5 MPa at 50 wt% and 30.6 MPa at 60wt%, respectively. At > 50 wt% benzoxazine, unreacted monomeric residues from benzoxazine increases at a given curing conditions. In addition, benzoxazine itself generally exhibits low crosslinking density compared to other thermoset resins. The lap shear strength results of various epoxy/benzoxazine mixtures on the aluminum (Al) substrates are shown in
Figure 1(b). The adhesion strength of pure epoxy showing at around 4.39 MPa increases above 4.8 MPa with the addition of benzoxazine in a range of 20-50 wt%. The adhesion strength is closely associated with the ability of the adhesive to wet and spread onto the substrate surface as well as mechanical properties of the resins. As the viscosity of the mixtures increases in proportional to benzoxazine content, the wettability on Al surface may be interfered. The crosslinking density of the mixtures may also affect the adhesion strength.
Table 2 presents the burning characteristics of epoxy/benzoxazine mixtures including a UL94 rating and dripping behavior. The mixtures containing less than 30 wt% benzoxazine completely burned after the first ignition and failed the flammability test. Highly flammable epoxy may have a dominant effect on the fire resistance at this composition. With further increase of benzoxazine content above 40 wt%, the combustion time, a sum of burning time after first and second ignition, is reduced to 175s for 60/40 and 71s for 50/50 epoxy/benzoxazine, indicative of having a V-1 rating. It is noticed that the fire resistance can be imparted when at least 40 wt% benzoxazine is added to epoxy. The epoxy/benzoxazine blend alone is still unsatisfactory in passing the commercial fire performance of a V-0 rating. The ATH is incorporated into the epoxy/benzoxazine mixtures.
3.2. Mechanical and Flammable Properties of Epoxy/Benzoxazine/ATH Composites
Table 3 shows the variation of thermal and mechanical properties of the 60/40 epoxy/benzoxazine mixture when ATH is incorporated. The T
g of epoxy/benzoxazine mixtures remains almost invariant regardless of ATH content, showing at around 160 ~ 162
oC. It implies that the crosslinking density of the mixture is not affected by the ATH. The coefficient of thermal expansion (CTE) values, α
1 and α
2, gradually decreases with the increase in ATH content because of the low CTE of inorganic ATH (15 ppm/
oC), whereby the expansion of the matrix is constrained. As the average inter-particle distance decreases with the incorporation of more particles, the loosely bound network gradually gets transformed into a tightly bound network. The reduction of α
2 value is more apparent, 141 ppm/
oC for epoxy/benzoxazine mixtures and 92 ppm/
oC for the mixture containing 40 wt% ATH. The storage modulus of epoxy/benzoxazine mixtures increases up to 8.4 GPa at 25
oC and 0.081 GPa at 250
oC, respectively when 40 wt% ATH is loaded. It is observed that the addition of inorganic materials improves the storage modulus but causes a decrease in tensile and adhesion properties.
From the thermal and mechanical behaviors, the 60/40 epoxy/benzoxazine mixture was selected as the optimal composition.
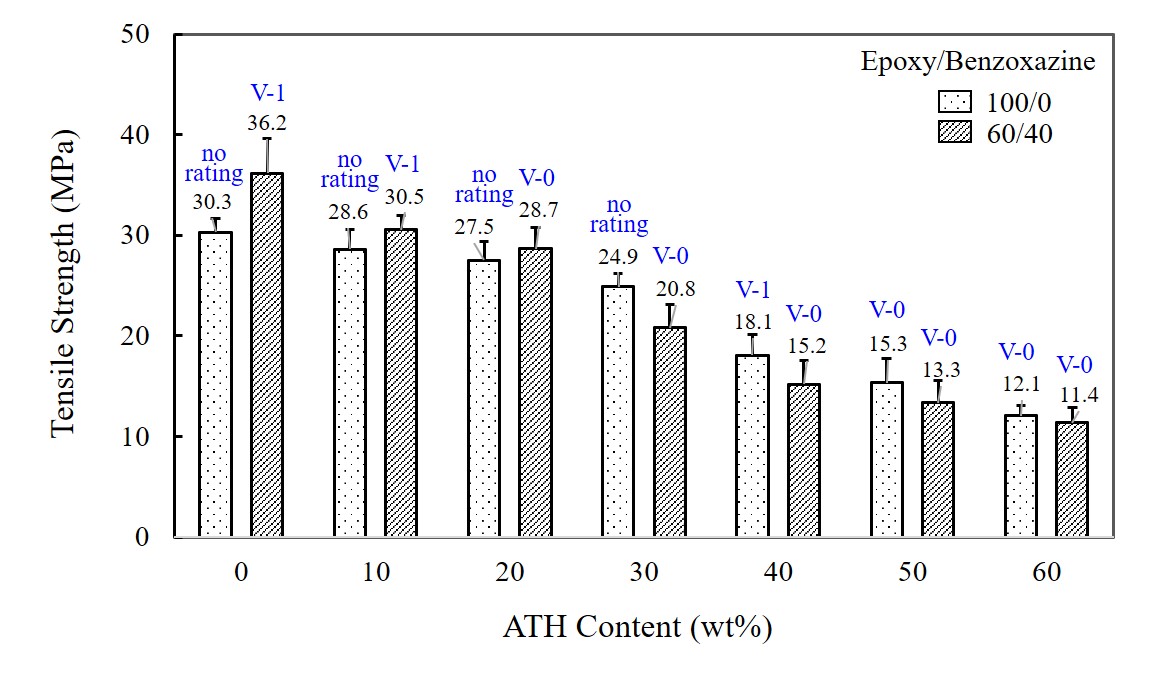
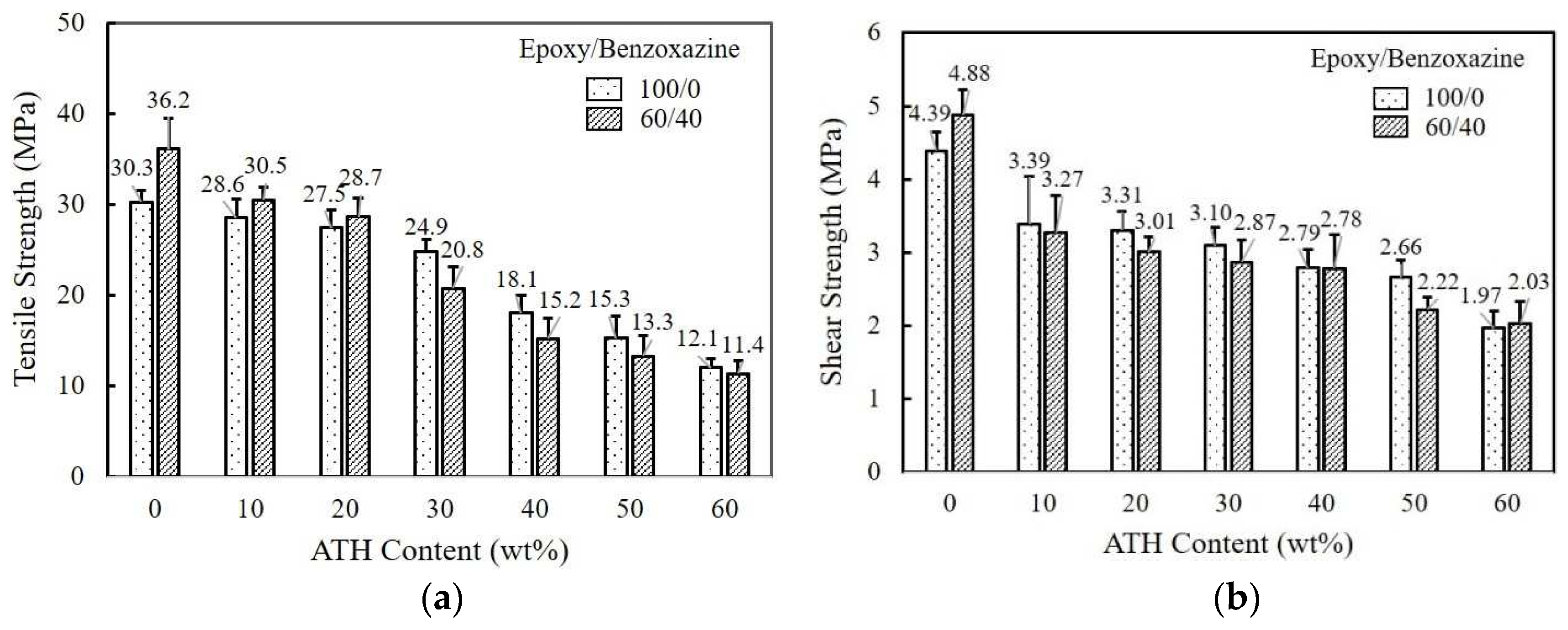
Figure 2(a) shows the variation of tensile strength of neat epoxy and 60/40 epoxy/benzoxazine mixture when the ATH in different amount is added. Tensile strength of pure epoxy and epoxy/benzoxazine mixture exhibiting 30.3 and 36.2 MPa is reduced dramatically as ATH content increases, exhibiting 12.1 and 11.4 MPa at 60 wt% ATH. No reinforcement effect is observed for both epoxy and epoxy/benzoxazine mixtures. Reduced strength value may be attributable to the incompatibility between adhesive resin and ATH additive. Adhesives become harder as the amount of ATH increases, resulting in low elongation at the break.
The effect of ATH content on the adhesion (or shear) strength of pure epoxy and 60/40 epoxy/benzoxazine blends was investigated using aluminum (Al) adherend. The shear strength is shown in
Figure 2(b). The shear strength of pure epoxy and epoxy/benzoxazine mixtures indicates that the addition of ATH lowers the strength of the matrix-Al interfacee, showing about 55% and 58% reduction at 60 wt% ATH loading. The strength of an adhesive joint is closely related to the ability of the adhesive to wet and spontaneously spread onto the Al surface. The addition of ATH particles increases the viscosity of the resin, which in turn hampers the flowability and wetting in a bond.
The flame retardancy of the epoxy/benzoxazine mixtures with respect to ATH content is summarized in
Table 4. Pure epoxy is highly combustible and thus the flame spreads to the holding clamp rapidly after ignition. When ATH is added to epoxy, the cotton indicator is still ignited by melt drips up to 30 wt%. At 40 wt% ATH, the fire stops within the 60s, and ignition of the cotton by dripping is no longer observed, exhibiting a V-1 rating. At 50 wt%, the composite finally reaches a V-0 rating. The flammability behavior clearly indicates that pure epoxy needs at least 50 wt% ATH to pass the required fire performance. However, such high ATH loading deteriorates tensile and adhesion strength. When 60/40 epoxy/benzoxazine mixture exhibiting a V-1 rating is combined with ATH, the burning time lessened to 70s at 10 wt%, 35s at 20 wt%, and 25s at 30 wt% ATH. This result indicates that the mixture can pass the V-0 rating even at 20 wt% ATH loading. The mixture containing 50 wt% benzoxazine shows a V-0 rating at 10 wt% of ATH with a reduced combustion time of 40s, and no ignition occurs at 30 wt% ATH. This result clearly indicates that the flame can be retarded effectively by the combined use of benzoxazine resin and ATH filler.
3.3. Effect of Surface Modification on Mechanical Properties of Epoxy/Benzoxazine/ATH Composites
It was observed that the incorporation of ATH into the 60/40 epoxy/benzoxazine mixture led to a significant reduction in tensile and adhesion strength. The ATH was modified using three different silane coupling agents containing epoxy (EP), amino (AM), and mercapto (MC) functional groups.
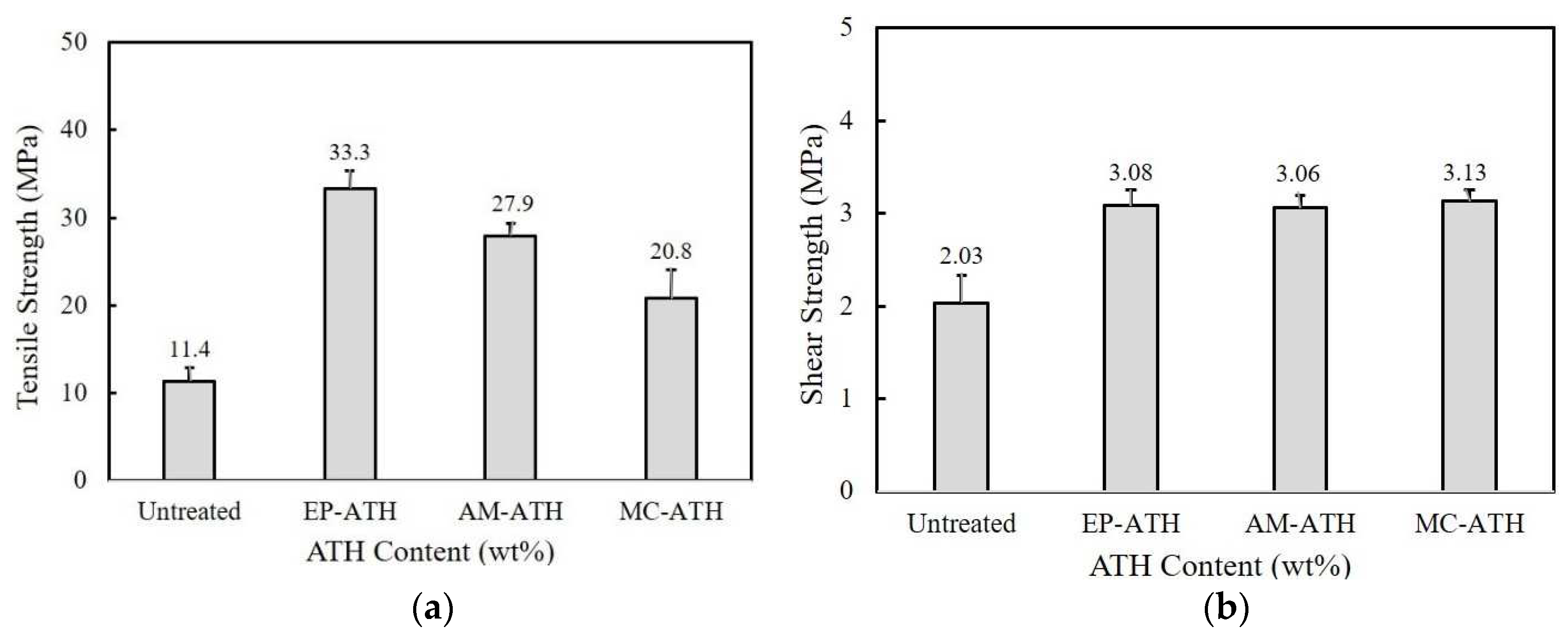
Figure 3(a) shows the tensile strength of 60/40 epoxy/benzoxazine blends when 60 wt% ATH is incorporated. All the coupling agents show high tensile strength compared to untreated ATH, in the order of EP-ATH > AM-ATH > MC-ATH. The strength value of the composite filled with EP-ATH is observed at around 33.3 MPa, which is about three times higher than that of the untreated ATH.
The composites containing surface-modified ATH also exhibit higher adhesion strength (Fig. 3b). Regardless of the functional groups of coupling agents, the composites prepared using modified ATH show the shear strength above 3 MPa, about 1 MPa higher than that of untreated ATH composites. From the tensile and adhesion strength results, it is inferred that surface modification using silane coupling agents is an effective way to prevent the deterioration of the mechanical properties of adhesives. The improvement is mostly attributed to a better dispersion of surface-modified ATH and strong adhesion between the ATH and matrix. The introduction of a coupling agent on the ATH surface may increase an affinity to the matrix and suppress the generation of voids and cracks at the interface.
4. Conclusions
The benzoxazine and ATH were added to the epoxy and then the thermal, mechanical, and flame-retardant behaviors of the composites have been examined. The blends of epoxy and benzoxazine exhibited high degradation temperature, low CTE, high tensile strength, and storage modulus. The mixtures containing 40 wt% benzoxazine passed the UL94 V-1 rating while maintaining good tensile and adhesion strength. The amount of benzoxazine in mixtures was limited because of the narrow processing window and low crosslinking density. The ATH provided a satisfactory flame retardancy. The effective amount of ATH required to achieve a V-0 rating for pure epoxy was 50 wt%. Higher ATH loading led to a pronounced decrease in tensile and shear strength of the composites. When ATH was added to the 60/40 epoxy/benzoxazine, a V-0 rating could be achieved even at 20 wt% without much sacrificing the mechanical properties. The combined use of ATH and benzoxazine showed noteworthy synergism in terms of flame retardance and mechanical properties. The lower tensile and shear strength of the ATH composites can be improved by surface modification using a silane coupling agent.
Author Contributions
Conceptualization, N.I. Kim; Methodology, K.S. Sung and N.I. Kim; Formal Analysis, K.S. Sung and N.I. Kim; Investigation, K.S. Sung and N.I. Kim; Writing Review & Editing, N. Kim; Visualization, K.S. Sung; Supervision, N.I. Kim.
Funding
This research was funded by 2022 Hannam University Research Fund.
Acknowledgments
This work was supported by 2022 Hannam University Research Fund.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fulik, N.; Hofmann, A.; Nötzel, D.; Müller, M.; Reuter, I.; Müller, F.; Smith, A.; Hanemann, T. Effect of Flame Retardants and Electrolyte Variations on Li-Ion Batteries. Batteries 2023, 9, 82. [Google Scholar] [CrossRef]
- Kovács, Z.; Polmázi, Á.; Toldy, A. Development of Multifunctional Flame-Retardant Gel Coatings for Automotive Applications. Coatings 2023, 13, 345. [Google Scholar] [CrossRef]
- Xiong, X.; Niu, Y.; Zhou, Z.; Ren, J. Development and Application of a New Flame-Retardant Adhesive. Polymers 2020, 12, 2007. [Google Scholar] [CrossRef]
- Mariappan, T.; Wilkie, C.A. Flame retardant epoxy resin for electrical and electronic applications. Fire Mater. 2014, 38, 588–598. [Google Scholar] [CrossRef]
- Rakotomalala, M.; Wagner, S.; Döring, M. Recent Developments in Halogen Free Flame Retardants for Epoxy Resins for Electrical and Electronic Applications. Materials 2010, 3, 4300–4327. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, F.; Moon, K.S.; Wong, C.P. Novel Curing Agent for Lead-Free Electronics: Amino Acid. J. Polym. Sci., Part A: Polym. Chem. 2006, 44, 1020–1027. [Google Scholar] [CrossRef]
- Machado, I.; Shaer, C.; Hurdle, K.; Calado, V.; Ishida, H. Towards the Development of Green Flame Retardancy by Polybenzoxazines. Prog. Polym. Sci. 2021, 121, 101435. [Google Scholar] [CrossRef]
- Zeng, M.; Zhu, W.; Feng, Z.; Chen, J.; Huang, Y.; Xu, Q.; Wang, J. Two novel halogen-free, phosphorus-free, and intrinsically flame-retardant benzoxazine thermosets containing electron-withdrawing bridge groups. J. Appl. Polym. Sci. 2020, 137, e49300. [Google Scholar] [CrossRef]
- Lyu, Y.; Zhang, Y.; Ishida, H. Intrinsically noncombustible polymers without flame retardant additives: Sulfur-containing and bio-based benzoxazines. Eur. Polym. J. 2020, 133, 109770. [Google Scholar] [CrossRef]
- Lyu, Y.; Ishida, H. Natural-sourced benzoxazine resins, homopolymers, blends and composites: A review of their synthesis, manufacturing and applications. Prog. Polym. Sci. 2019, 99, 101168. [Google Scholar] [CrossRef]
- Chen, C.-H.; Lin, C.-H.; Hon, J.-M.; Wang, M.-W.; Juang, T.-Y. First halogen and phosphorus-free, flame-retardant benzoxazine thermosets derived from main-chain type bishydroxydeoxybenzoin-based benzoxazine polymers. Polymer 2018, 154, 35–41. [Google Scholar] [CrossRef]
- Bornosuz, N.V.; Korotkov, R.F.; Shutov, V.V.; Sirotin, I.S.; Gorbunova, I.Y. Benzoxazine Copolymers with Mono- and Difunctional Epoxy Active Diluents with Enhanced Tackiness and Reduced Viscosity. J. Compos. Sci. 2021, 5, 250. [Google Scholar] [CrossRef]
- Yue, J.; Wang, H.; Zhou, Q.; Zhao, P. Reaction-Induced Phase Separation and Morphology Evolution of Benzoxazine/Epoxy/Imidazole Ternary Blends. Polymers 2021, 13, 2945. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, J.; Zhou, J.; Zhang, D.; Zhang, A. Dramatic toughness enhancement of benzoxazine/epoxy thermosets with a novel hyperbranched polymeric ionic liquid. Chem. Eng. J. 2018, 334, 1371–1382. [Google Scholar] [CrossRef]
- Chow, W.S.; Grishchuk, S.; Burkhart, T.; Karger-Kocsis, J. Gelling and curing behaviors of benzoxazine/epoxy formulations containing 4,4’-thiodiphenol accelerator. Thermochim. Acta 2012, 543, 172–177. [Google Scholar] [CrossRef]
- Ishida, H.; Allen, D.J. Mechanical characterization of copolymers based on benzoxazine and epoxy. Polymer 1996, 37, 4487–4495. [Google Scholar] [CrossRef]
- Yang, X.; Shen, A.; Su, Y.; Zhao, W. Effects of alumina trihydrate (ATH) and organic montmorillonite (OMMT) on asphalt fume emission and flame retardancy properties of SBS-modified asphalt. Constr. Build. Mater. 2020, 236, 117576. [Google Scholar] [CrossRef]
- Xi, W.; Qian, L.; Li. L. Flame Retardant Behavior of Ternary Synergistic Systems in Rigid Polyurethane Foams. Polymers 2019, 11, 207–1. [Google Scholar] [CrossRef]
- Vaari, J.; Paajanen, A. Evaluation of the reactive molecular dynamics method for Research on flame retardants: ATH-filled polyethylene. Comput. Mater. Sci. 2018, 153, 103–112. [Google Scholar] [CrossRef]
- Elbasuney, S. Novel multi-component flame retardant system based on nanoscopic aluminium-trihydroxide (ATH). Powder Technol. 2017, 305, 538–545. [Google Scholar] [CrossRef]
- Han, Z.; Wang, Y.; Dong, W.; Wang, P. Enhanced fire retardancy of polyethylene/alumina trihydrate composites by graphene nanoplatelets. Mater. Lett. 2014, 128, 275–278. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.-M.; Dubois, Ph. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng.: R 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Zhang, L.; Bian, Y.; Kuai, D. Preparation and flame retardant property of nano-aluminum hydroxide foam for preventing spontaneous coal combustion. Fuel 2021, 304, 121494. [Google Scholar] [CrossRef]
- Qin, Z.; Li, D.; Li. Q.; Yang, R. Effect of nano-aluminum hydroxide on mechanical properties, flame retardancy and combustion behavior of intumescent flame retarded polypropylene. Mater. Des. 2016, 89, 988–995. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Irska, I.; Taraghi, I.; Piesowicz, E.; Sieminski, J.; Zawisza, K.; Pypeć, K.; Dobrzynska, R.; Terelak-Tymczyna, A.; Stateczny, K.; Szymczak, B. Halloysite Nanotubes and Silane-Treated Alumina Trihydrate Hybrid Flame Retardant System for High-Performance Cable Insulation. Polymers 2021, 13, 2134. [Google Scholar] [CrossRef]
- Wu, B.; Kong, W.; Hu, K.; Fu, X.; Lei, J.; Zhou, C. Synergistic effect of phosphorus-containing silane coupling agent with alumina trihydrate in ethylene-vinyl acetate composites. Adv. Polym. Technol. 2018, 37, 1456–1468. [Google Scholar] [CrossRef]
- Lin, H.; Yan, H.; Liu, B.; Wei, L.; Xu, B. The influence of KH-550 on properties of ammonium polyphosphate and polypropylene flame retardant composites. Polym. Degrad. Stab. 2011, 96, 1382–1388. [Google Scholar] [CrossRef]
- Zhang, L.; Li, C.Z.; Zhou, Q.; Shao, W. Aluminum hydroxide filled ethylene vinyl acetate (EVA) composites: effect of the interfacial compatibilizer and the particle size. J. Mater. Sci. 2007, 42, 4227–4232. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).